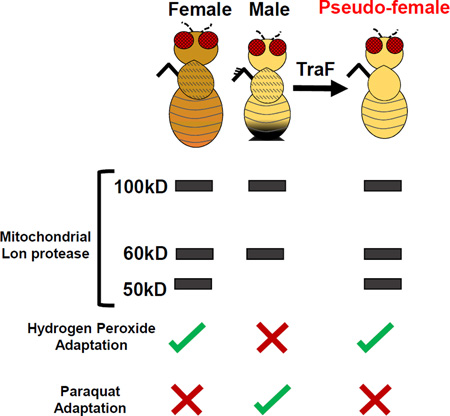
Summary
Multiple human diseases involving chronic oxidative stress show a significant sex bias, including neurodegenerative diseases, cancer, immune dysfunction, diabetes, and cardiovascular disease. However a possible molecular mechanism for the sex-bias in physiological adaptation to oxidative stress remains unclear. Here we report that Drosophila melanogaster females but not males adapt to hydrogen peroxide stress, whereas males but not females adapt to paraquat (superoxide) stress. Stress adaptation in each sex required the conserved mitochondrial Lon protease and was associated with sex-specific expression of Lon protein isoforms and proteolytic activity. Adaptation to oxidative stress was lost with age in both sexes. Transgenic expression of transformer gene during development transformed chromosomal males into pseudo-females, and conferred the female-specific pattern of Lon isoform expression, Lon proteolytic activity induction and H2O2 stress adaptation; these effects were also observed using adult-specific transformation. Conversely, knockdown of transformer in chromosomal females eliminated the female-specific Lon isoform expression, Lon proteolytic activity induction and H2O2 stress adaptation, and produced the male-specific paraquat (superoxide) stress adaptation. Sex-specific expression of alternative Lon isoforms was also observed in mouse tissues. The results develop Drosophila melanogaster as a model for sex-specific stress adaptation regulated by the Lon protease, with potential implications for understanding sexual-dimorphism in human disease.
Graphical Abstract
Introduction
Stress adaptation is the phenomenon in which a mild stress enables cells, tissues or whole organisms to withstand future toxic levels of that stress [1, 2]. Stress adaptation is thought to result from up-regulation of specific stress response factors, including proteases, molecular chaperones and heat shock proteins (Hsps) [3–6]. Adaptation to hydrogen peroxide (H2O2) stress has been studied in cultured mammalian cells, C. elegans, and D. melanogaster, and in each case requires increased expression of proteasome subunits, regulated by the conserved CnC-C/Nrf2 transcription factor [1, 5, 7, 8].
The mitochondrial electron transport chain (ETC) is a primary source of intracellular superoxide and H2O2. The proximity of the ETC makes mitochondrial proteins highly susceptible to oxidative damage [9]. To counteract the accumulation of damaged and mis-folded proteins, mitochondria rely upon the highly conserved Lon protease [10]. Lon is responsible for the turnover of several mitochondrial proteins, and preferentially degrades oxidatively-damaged proteins in an ATP-dependent manner [11]. Discovered in E. coli, Lon is a critical stress response protease conserved in humans [12]. In eukaryotes Lon is encoded in the nucleus and targeted to the mitochondrial matrix. When cultured human cells are treated with exogenous H2O2, Lon is required both for stress resistance and stress adaptation, indicating that mitochondria and the mitochondrial proteome are critical targets for H2O2 toxicity [12].
Several diseases involve chronic oxidative stress and show a marked sex bias. For example, heart failure involves maladaptive hypertrophy and altered mitochondrial turnover regulated by insulin/IGF-1-like signaling (IIS) and mTOR, and mitochondrial metabolic reprogramming regulated by p53; these pathways can be activated by H2O2 and superoxide [13]. Men are generally more susceptible to cardiovascular disease, however diabetic cardiomyopathy preferentially affects women [14]. Mechanisms for sex-dimorphism in maladaptive tissue remodeling remain unclear, however studies in mice and in Drosophila indicate that females may have greater baseline activity of both IIS and p53 [15–18]. Physiological studies also indicate relatively greater sensitivity to IIS in women than in men [18]. Sex-bias in the cellular response to acute oxidative stress has been reported for humans and mice, where female cells generally show greater stress resistance than do male cells [18]. In contrast, the potential for sex-specific oxidative stress adaptation of cells and animals is less studied. We have previously shown that Drosophila females but not males are capable of H2O2 stress adaptation [1]. Here we show that Drosophila males but not females are capable of paraquat stress adaptation, and that stress adaptation in each sex is regulated by Lon.
Results
H2O2 Stress Adaptation Does Not Alter Lon mRNA Levels
lon mRNA induction in response to mild H2O2 pre-treatments [10µM and 100µM] was analyzed in adult flies. Three day old flies were selected to represent young [1, 19], and 60 day old flies to represent the old time point, because at 60 days >80% of the population had survived, thereby reducing potential selection bias and cohort effects [20]. Virgin male and female flies were examined to avoid any potential confounding effects of mating on stress responses [21].
The young and old male and female flies were treated with an adaptive dose of H2O2 [10µM or 100µM] for 8 hours, followed by 16 hour recovery. Total mRNA was isolated from whole bodies, and lon mRNA levels determined using qPCR. The Glutathione S transferase D1 (GstD1) gene was chosen as positive control, because it is robustly induced in male and female flies in response to toxic H2O2 stress [20]. GstD1 was induced in 3 day females in response to the mild 10µM and 100µM H2O2 pre-treatment (Figure S1A,B), however GstD1 was not detectably induced in males. The lack of transcriptional response in males to the mild stress may underlie the inability of males to adapt to H2O2. The lack of H2O2 adaptation in males was not simply due to insufficient level of pre-treatment exposure, as greater concentrations H2O2 (to 1,000uM) caused increased toxicity, yet did not yield stress adaptation ([1] and data not shown).
lon mRNA showed no change following H2O2 pre-treatment, irrespective of sex or age (Figure 1A,B; Figure S1E), consistent with previous observations that Lon is not induced at RNA level in mammalian cells [22, 23] or adult flies [20] in response to H2O2.
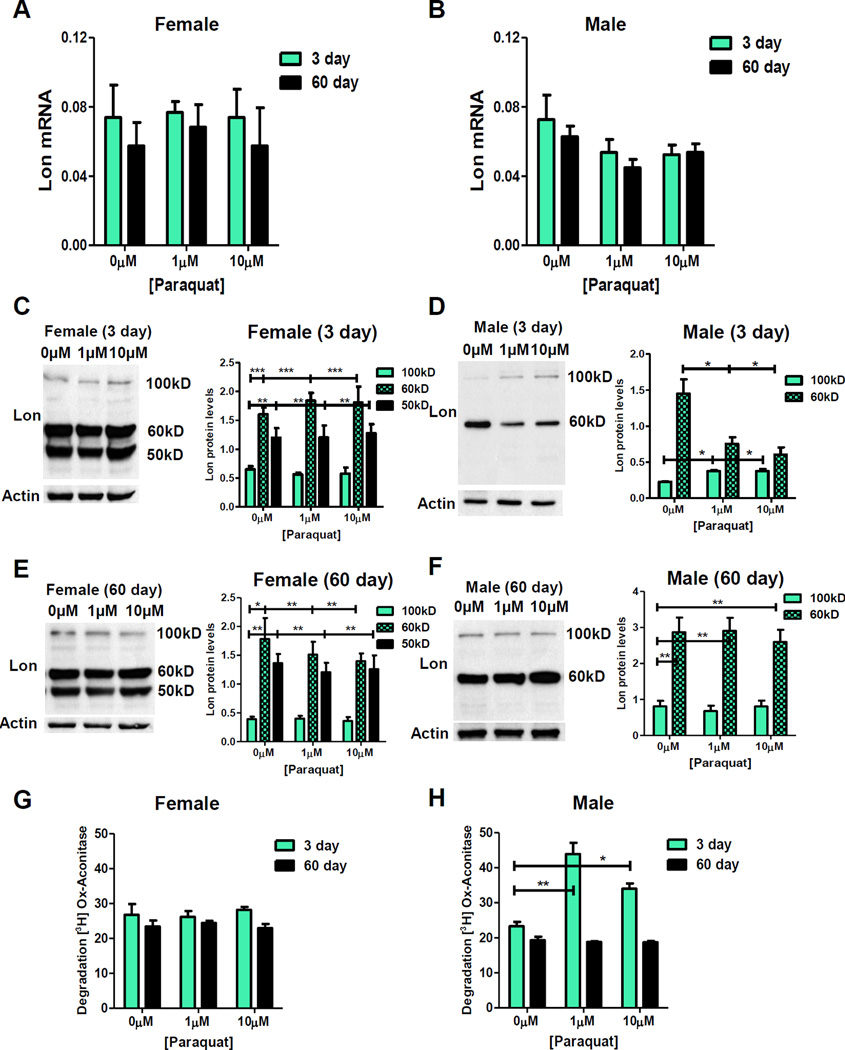
Figure 1. H2O2 Pre-treatment Induces Lon in a Female-specific Manner that Diminishes With Age.
(A–F) Control flies assayed for lon mRNA and protein following H2O2 pre-treatment. lon mRNA in (A) 3 day and 60 day females. (B) 3 day and 60 day males. (C) Lon protein increased upon various concentrations of H2O2 in 3 day females. (D) Lon protein unchanged in 3 day, pretreated males. (E) Lon protein not inducible upon H2O2 pre-treatment in 60 day females. (F) Lon protein unchanged in 60 day, pre-treated males. Western blots performed in triplicate, normalized to anti-Actin-HRP, quantified using Image J. Quantification is 100kD band, additional bands (60kD and 50kD) quantification presented in Figure S1C,D. (G,H) Proteolytic capacity to degrade oxidized [3H] aconitase in presence of 5mM ATP measured in mitochondria from 3 day and 60 day control flies following H2O2 pre-treatment. (G) Females. (H) Males. Samples normalized to 3 day and 60 day 0µM controls. Statistical significance (p < 0.05) calculated here and below using one-way ANOVA and indicated with asterisk. See also Figure S1 and S2.
Female-specific Lon Protein Isoforms and Protease Activity
Human Lon protein is expressed as a short-lived ~107kD precursor, including a mitochondrial targeting motif at the amino-terminus. Upon uptake by mitochondria the targeting motif is cleaved to yield mature protein of ~100kDa [24]. To analyze Lon in adult flies, total protein was isolated from young, whole adult control flies, and analyzed by western blot using D. melanogaster-specific Lon polyclonal antibody. Three distinct bands were observed in females (100kD, 60kD, and 50kD), and two in males (100kD and 60kD) (Figure S2A). To ensure the additional bands were not a result of non-specific cross-reactivity, immunoprecipitation was used to isolate the bands for mass spectrometry and protein sequence analysis. Each band detected by the Lon antibody was confirmed to represent regions of Lon protein (Figure S2B).
In D. melanogaster, Lon transcript is alternatively spliced into two isoforms, Lon RA and Lon RC (Figure S2C), with a predicted difference of ~3kD between resulting proteins, and both these proteins are expected to represent the 100kD band. qPCR analysis confirmed both Lon RA and Lon RC splice forms to be present in males and females in similar ratio (Figure S2D). In addition, re-analysis of whole-body transcriptome of 12 day-old virgin females, mated females, and males found no sex-related variation in expression of Lon exons (Figure S2E) [21]. Therefore the 60kD and 50kD Lon protein isoforms do not appear to result from alternative splice variants of lon mRNA.
Young females exhibited an increase in the 100kD Lon protein isoform upon pre-treatment with 10µM and 100µM H2O2 (Figure 1C), whereas no changes were detected in the 60kD or 50kD protein isoforms (Figure S1C). No change in Lon protein expression following H2O2 pre-treatment was observed in old females (Figure 1E; Figure S1C). The lack of response in old females was not due to a decline in lon RNA levels, as both young and aged females had equivalent expression of lon mRNA (Figure 1A; Figure S1E). Male flies showed no change in Lon protein expression, regardless of pre-treatment, sex, or age (Figure 1D,F; Figure S1D).
To determine if changes in Lon protein levels represented a functional protease, Lon proteolytic activity was assayed in extracts of mitochondria isolated from whole adult flies. Prior studies have shown that Lon activity is most robust in presence of ATP and oxidized substrates, and that result was confirmed here (Figure S1I,J) [11]. Mitochondrial extracts were incubated with ATP and oxidized aconitase. Interestingly, both aged female and male flies exhibited a decrease in basal Lon protease activity compared to their young counterparts (Figure S1H), despite Lon protein levels being similar between young and old flies of each sex (Figure S1F,G). Mitochondrial extracts from young females that had been pre-treated with H2O2 showed increased proteolytic activity, whereas mitochondrial extracts from pre-treated old females did not (Figure 1G). The age-related loss of proteolytic induction, evident in aged females, is not a result of diminished basal amounts of Lon, as both young and old females showed equivalent levels of Lon protein expression (Figure S1F). This finding is consistent with previous analyses of young and old mouse liver tissue extracts, where basal Lon protein expression was similar but basal proteolytic capacity was decreased in old animals [25], although mouse skeletal muscles exhibit significant loss of both Lon protein and Lon activity [11]. Mitochondrial extracts prepared from male flies showed no induction in Lon proteolytic capacity, regardless of pre-treatment or age (Figure 1H).
Females But Not Males Adapt to H2O2 Stress
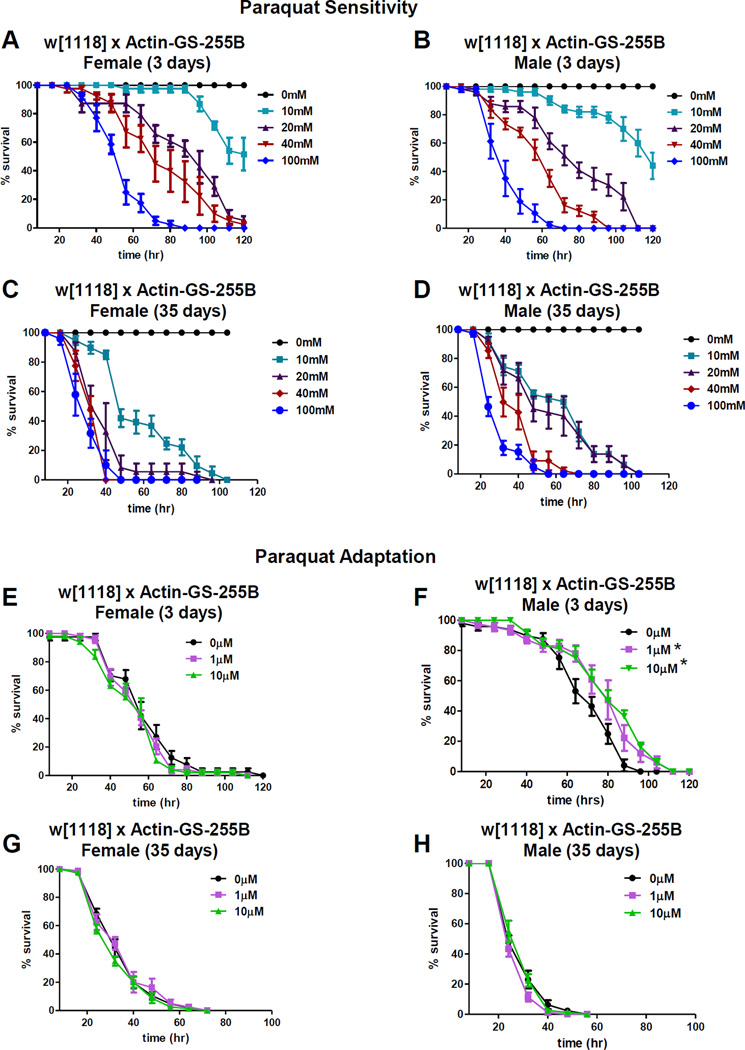
In adult Drosophila, dietary H2O2 caused decreased survival associated with ERK pathway activation and apoptosis in central nervous tissue [26]. To assess age-related changes in H2O2 toxicity, 3 day old and 35 day old male and female progeny of the control cross (Actin-GS-255B driver strain crossed to w[1118]; see SI Methods), were fed various concentrations of H2O2 [1M–8M] and survival scored every 8 hours (Figure S2F,G,I,J). H2O2 toxicity was similar for males and females, with slightly greater sensitivity observed in males, and increased with age in both sexes.
To assess the age-associated change in adaptive response, H2O2 [10µM or 100µM] pre-treatment of 3 day old females conferred increased survival upon 4.4M H2O2 challenge, which was lost with age (Figure S2H; Table S1). Pretreatment in males, irrespective of age, produced no adaptive response (Figure S2K; Table S1), consistent with our previous observations [1].
Lon Is Required for H2O2 Stress Adaptation In Females
To investigate the role of Lon in adaptation during oxidative stress, the mifepristone (RU486)-activated “Gene-Switch” system was used to modulate Lon expression (Figure S3A) [27]. The Gene-Switch transcription factor was expressed in a tissue-general pattern using the cytoplasmic actin Actin5C gene promoter in “driver” line Actin-GS-255B [27]. Target constructs contained Lon RNAi sequences (Lon RNAi strains R1 and R2) under control of a UAS-based promoter. Adult flies were fed mifepristone in media for 9 days, causing activation of Gene-Switch and expression of the target construct. Virgin females and males were assayed to avoid any potential confounding effects that might be caused by the ability of mifepristone to block the negative effect of mating on life span in females [21]. Control flies contained the Actin-GS-255B driver construct but not the Lon RNAi construct, and were assayed to confirm that mifepristone itself did not cause changes in lon mRNA or protein expression (Figure S3B–E). For LonR1 RNAi strain, both females and males exhibited 50% or greater decrease in basal lon mRNA (Figure S4A,B). Similar findings were observed in Lon R2 RNAi strain (Figure S4C,D). Conditional expression of Lon R1 resulted in decreased protein expression for all three Lon protein isoforms in females (Figure S4E) and a decrease in only the 100kD isoform in males (Figure S4F), which may relate to differences in Lon processing between males and females. Similar results were obtained with Lon R2 (Figure S4G,H).
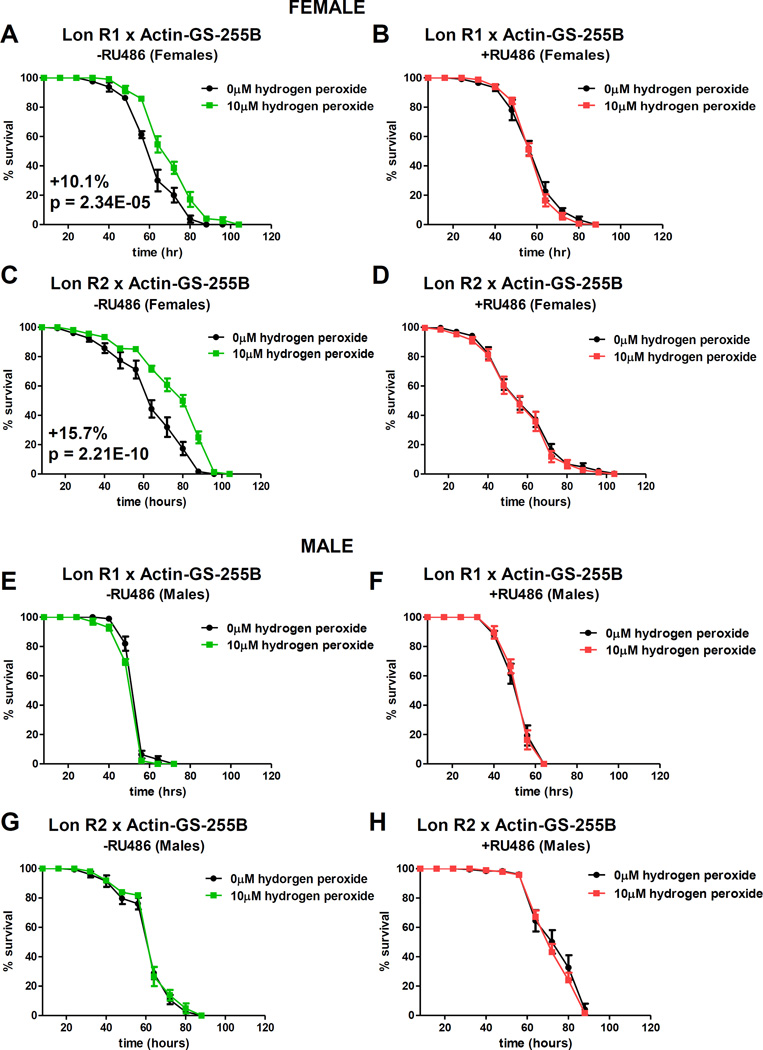
Control and experimental flies were fed ±mifepristone for 9 days, then pre-treated with H2O2 for 8 hours, allowed to recover for 16 hours, and then subjected to lethal H2O2 challenge. As expected, control females, but not males, could adapt to H2O2, as indicated by increased survival time upon toxic challenge (Figure S3F–I; Table S2). For both Lon R1 and R2 RNAi, females raised in absence of mifepristone also adapted to H2O2 (Figure 2A,C; Table S3). However, females fed mifepristone to knock-down Lon expression were not capable of adaptation (Figure 2B,D; Table S3). Males of Lon R1 and R2 RNAi showed no ability to adapt to H2O2, both with and without knockdown of Lon (Figure 2E–H; Table S3).
Figure 2. Lon Is Required for H2O2 Adaptation in Females.
Actin-GS-255B crossed to Lon R1 (A,B,E,F) or Lon R2 (C,D,G,H) to knock-down Lon expression. All progeny cultured ±RU486 for 9 days prior to pre-treatment. At day 10, flies were cultured in presence or absence of adaptive dose of H2O2 before being fed H2O2 challenge dose [4.4M]. (A, C) Females without RU486. (B, D) Females with RU486. (E,G) Males without RU486. (F,H) Males with RU486. Statistically significant difference in survival (p < 0.05) calculated here and below using Log-Rank test, and indicated by p-value and percent change median. Statistical summary in Table S3. See also Figures S1–6, Tables S2 and S4.
Over-expression of Lon Increases H2O2 Stress Adaptation In Females But Not Males
Over-expression of Lon was generated by crossing Actin-GS-255B driver to Lon over-expression strains Lon OE1 and Lon OE2. Progeny were fed ±mifepristone for 9 days, and basal lon mRNA and protein were measured. Both male and female progeny of Lon OE2 cross exhibited approximately four-fold increase in lon mRNA, whereas Lon OE1 progeny exhibited more modest increase (Figure S4I–L). This difference may be attributable to different chromosomal insertion sites for Lon OE1 and OE2 transgenes. In females, abundance of 100kD Lon isoform increased in Lon OE2, whereas 60kD and 50kD bands were not detectably altered (Figure S4O). Similarly, overexpression of Lon in males with Lon OE2 also produced increase in 100kD Lon isoform with no detectable change in 60kD isoform (Figure S4P). No significant changes in Lon protein abundance were observed in either females or males for Lon OE1, consistent with relatively smaller increase in transcript levels produced by this strain (Figure S4M,N). Both males and females showed increased proteolytic capacity upon Lon over-expression, consistent with the increase in Lon protein (Figure S4M–P). Males showed no induction of proteolytic capacity by H2O2 pre-treatment, irrespective of Lon over-expression or strain (Figure S4R,T). Proteolytic capacity in females was increased in both Lon OE1 and OE2 upon mifepristone treatment, and was further induced upon H2O2 pre-treatment (Figure S4Q,S).
Next effects of Lon over-expression on H2O2 stress adaptation was assayed. Progeny of Lon OE1 and OE2 crosses were treated ±mifepristone for 9 days to induce over-expression. Flies were then pre-treated with H2O2 [10uM] for 8 hours, allowed to recover for 16 hours, then subjected to lethal H2O2 challenge [4.4M]. In absence of mifepristone, Lon OE1 pre-treated females showed trend towards increased survival, whereas Lon OE2 females were unaffected (Figure S5A,C; Table S4). The lack of adaptive response in Lon OE2 females raised in absence of mifepristone is potentially attributed to variation in genetic background of the parental strain. However, upon mifepristone-induced Lon overexpression, both Lon OE1 and OE2 females exhibited robust H2O2 stress adaptation (Figure S5B,D; Table S4), consistent with the positive role of Lon in conferring H2O2 stress adaptation in females. In contrast, male flies showed no H2O2 stress adaptation with or without Lon over-expression (Figure 5E–H; Table S4). Therefore, tissue-general over-expression of Lon (100kD) in males was not sufficient to confer H2O2 stress adaptation.
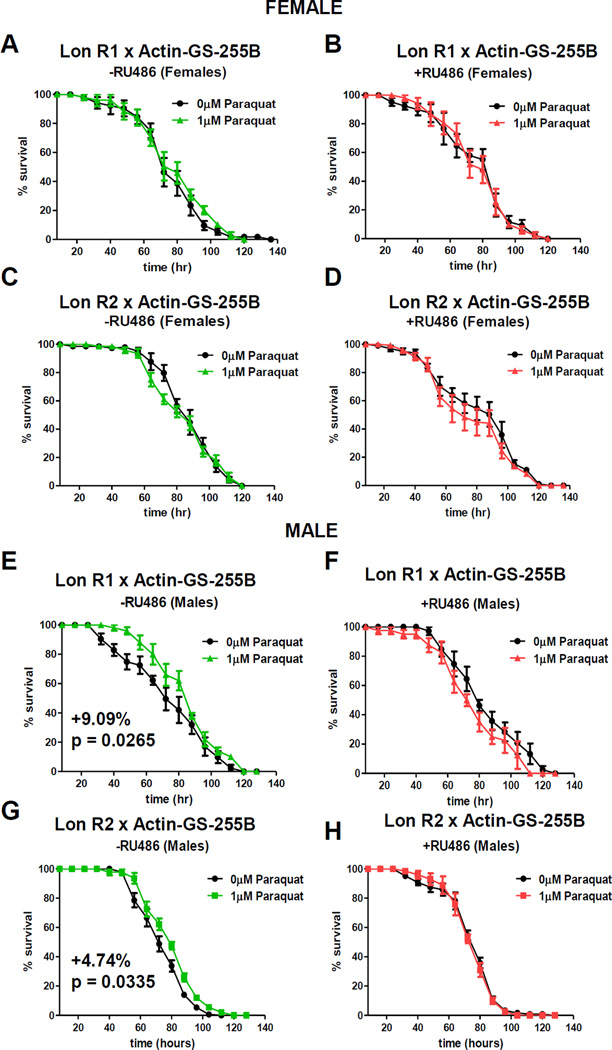
Figure 5. Lon Is Required for PQ Adaptation in Males.
Lon R1 and Lon R2 males were crossed to virgin females of Actin-GS-255B, and progeny cultured ±RU486 for 9 days prior to pre-treatment. (A,C) Females without RU486. (B,D) Females with RU486. (E,G) Males without RU486. (F,H) Males with RU486. Statistical Summary in Table S5. See also Figures S2–4 and S6.
Dietary Paraquat (PQ) Is Toxic to Both Males and Females
In mammalian cells paraquat (PQ) reacts with NADPH oxidase enzymes in the mitochondria and at the cell membrane to produce PQ radical [28, 29]. PQ radical in turn interacts with molecular oxygen to form superoxide, and PQ resistance is positively regulated by Nrf2 stress-response transcription factor [30]. To test for possible sex-specific PQ toxicity, young male and female progeny from control cross were fed various concentrations of PQ [10mM-100mM] and survival assayed. Males and females exhibited similar sensitivity to PQ, which increased with age (Figure 3A–D).
Figure 3. Equal Sex Sensitivity to PQ, But Only Males Adapt.
(A–D) Survival for control flies with increasing concentrations PQ. (A) 3 day females. (B) 3 day males. (C) 35 day females. (D) 35 day males. (E–H) Survival for control flies fed adaptive doses PQ [0µM-10µM] prior to challenge dose [30mM]. (E) 3 day females. (H). 3 day males. (I) 35 day females. (J) 35 day males. Statistical Summary in Table S1. See also Figure S2 and Table S6.
Males But Not Females Adapt to Paraquat Stress
Next, adaptation to PQ was assessed. 3 day and 35 day old male and female control cross progeny were pre-treated with low concentrations PQ [1µM and 10µM] for 8 hours prior to administration of toxic dose [30mM]. Young pre-treated males exhibited robust adaptation as evidenced by increased survival upon toxic challenge, which was lost with age (Figure 3F,H; Table S1). In contrast, pre-treated females showed no adaptation, irrespective of age (Figure 3E,G; Table S1). lon mRNA and protein expression following PQ pre-treatment were also measured. No change in lon mRNA levels were detected, irrespective of pretreatment, age, or sex (Figure 4A,B). Females showed no change in any of the three Lon protein isoforms, irrespective of pre-treatment or age (Figure 4C,E). In contrast, pre-treated young males showed increased amounts of 100kD Lon isoform, coupled with a decrease in 60kD Lon isoform (Figure 4D), and these effects diminished with age (Figure 4F). Similarly, proteolytic capacity remained unchanged in females (Figure 4G), but increased in pretreated males, and was lost with age (Figure 4H).
Figure 4. PQ Pre-treatment Induces Lon in a Male-specific Manner that Diminishes with Age.
(A,B) Control flies assayed for lon mRNA expression following PQ pre-treatment. lon mRNA in (A) 3 day and 60 day females. (B) 3 day and 60 day males. (C–F) Lon protein following PQ pretreatment. (C) 3 day females. (D) 3 day males. (E) 60 day females. (F) 60 day males. Western blots performed in triplicate, quantified using Image J. (G,H) Proteolytic capacity to degrade oxidized [3H] aconitase in presence of 5mM ATP measured in mitochondria from 3 day and 60 day control flies following PQ pre-treatment. (G) Females. (H) Males.
Lon Is Required for Paraquat Stress Adaptation in Males
To address the potential role of Lon in PQ stress adaptation, Gene-Switch was again used to knock-down expression of Lon. Flies were fed ±mifepristone for 9 days, then pre-treated with 1uM PQ for 8 hours, allowed to recover for 16 hours, and then subjected to lethal PQ challenge [30mM]. As expected, control males, but not females, showed PQ adaptation both with and without mifepristone treatment (Figure S3J–M). Similarly, in absence of mifepristone, males of both Lon R1 and Lon R2 RNAi showed PQ adaptation (Figure 5E,G; Table S5). However, knock-down of Lon by mifepristone eliminated PQ adaptation in males (Figure 5F,H; Table S5). Females showed no adaptation to PQ, regardless of mifepristone treatment and knock-down of Lon (Figure 5A–D, Table S5).
Constitutive Over-expression or RNAi Knockdown of Lon Is Detrimental to Lifespan
Partial knock-down of Lon using RNAi in late L4 stage C. elegans was reported to extend lifespan [31]. However, that finding may be unique to the nematode and/or to partial knock-down, as Lon null mutation in mice (Lon−/−) caused embryonic lethality and accelerated cellular senescence [32]. Here, Lon RNAi and over-expression were used to test impact of increased or decreased Lon on adult Drosophila lifespan. The tissue-general Actin-GS-255B driver was used to activate Lon OE and Lon RNAi throughout adulthood. Constitutive feeding of mifepristone had no detectable impact on lifespan in control flies (Figure S6A,B; Table S6). Knock-down of Lon using Lon R1 and Lon R2 had negative effect on lifespan for both males and females (Figure S6C–F; Table S6), indicating a requirement for Lon for normal longevity. Remarkably, constitutive over-expression of Lon using Lon OE2 strain resulted in dramatic decline in lifespan in both males and females (Figure S6G,H; Table S6), indicating that abnormally high levels of Lon are also toxic. Lon OE1 flies showed no difference in lifespan upon over-expression in either sex (Figure S6I,J; Table S6), however this may be attributable to relatively weaker Lon expression produced by this strain (Figure S4M,N). Taken together the results indicate that normal levels of Lon expression are required for optimal longevity in Drosophila males and females.
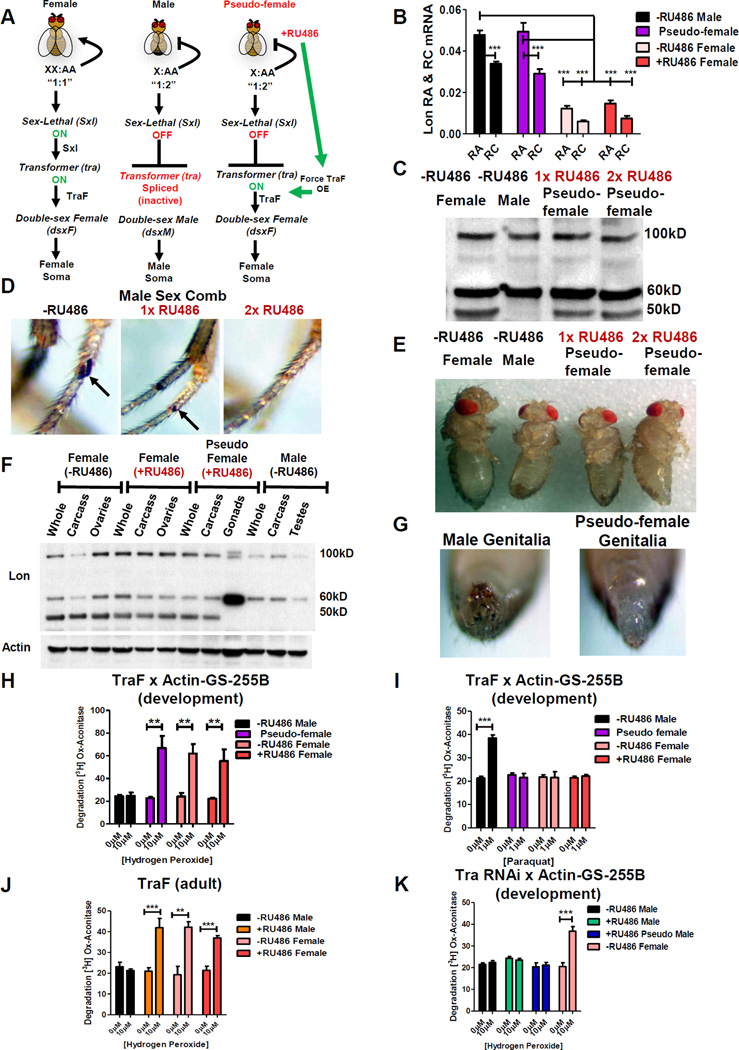
Transformation of Males to Pseudo-females Confers the Female Pattern of Lon Expression and H2O2 Adaptation
A large part of Drosophila somatic sexual differentiation is determined by expression of either male-specific or female-specific transcripts of transformer gene [33]. To further investigate the sex-specific nature of Lon expression, Gene-Switch was used to transform chromosomal males into phenotypic females (“pseudo-females”), by forcing expression of transformer femalespecific transcript (“TraF”) in chromosomal males (Figure 6A). Expression of TraF in pseudo-females caused no change in ratio of Lon RA to Lon RC compared to normal chromosomal males (Figure 6B), indicating no detectable effect of TraF expression on lon mRNA splicing patterns. Western blot revealed that pseudo-females recapitulated the female-specific pattern of Lon isoform expression, as indicated by presence of 50kD band (Figure 6C). Efficient phenotypic transformation was evidenced by development of female external genitalia and pigmentation patterns, and absence of male sex combs (Figure 6D,E,G). Pseudo-female gonads showed the male-specific Lon protein pattern (Figure 6F), consistent with the role of TraF being limited to feminization of the soma [34].
Figure 6. The Female Lon Banding Pattern Is Recapitulated in Pseudo-females.
(A) UAS-TraF x Actin-GS-255B Gene-Switch pseudo-female formation. RU486 activates TraF in males, producing female morphology (‘pseudo-females’). (B–I) UAS-TraF crossed to Actin-GS-255B, with resulting larvae cultured in either 160µg/mL (1x) or 320µg/mL (2x) RU486. (B) qPCR analysis of transcript variants lon RA and lon RC from progeny cultured in 2x ±RU486. (C) Western blot using anti-Lon antibody. (D) Male sex comb. Upon increasing RU486, the sex comb shrinks (1x) until no longer present (2x) RU486. (E) Whole flies. (F) Western blot of whole fly, dissected gonads, and carcass minus gonads. (G) Male genitalia in progeny grown without RU486. Formation of female genitalia in pseudo-females grown in (2x) RU486. (H–J) Proteolytic capacity of pretreated males without RU486, 2x RU486 pseudo-females, and 2x ±RU486 females to degrade [3H] aconitase (H) H2O2 pretreatment. (I) PQ pretreatment. (J) H2O2 pretreatment in RU486-fed adults. (K) UAS-Tra RNAi crossed to Actin-GS-255B, larvae cultured in 2x RU486. Proteolytic activity assayed following H2O2 pretreatment. See also Figure S7.
Pseudo-females also exhibited increased Lon expression and proteolytic capacity in mitochondrial extracts after H2O2 pre-treatment (Figure S7B; Figure 6H), analogous to results presented above for normal chromosomal females. Mifepristone feeding had no effect on Lon expression in chromosomal males in absence of transformer over-expression, and neither mifepristone nor transformer over-expression had effect on Lon expression in chromosomal females (Figure S7A). Next, pseudo-females were treated with PQ and no increase in Lon expression or proteolytic capacity was observed (Figure S7C; Figure 6I). Adult specific over-expression of TraF was also sufficient to confer H2O2 adaptation to chromosomal males: After 10 days of TraF over-expression, H2O2 pretreatment produced increased expression of Lon, expression of 50kD female-specific Lon isoform, as well as increased proteolytic activity (Figure S7D; Figure 6J). Together, these findings indicate that both presence of 50kD isoform and increased expression of 100kD isoform are necessary for H2O2 stress adaptation. Finally, expression of Tra RNAi in chromosomal females caused transformation to pseudo-males, based on production of male-like genitalia and sex combs, as expected [33, 35, 36] (data not shown), and this eliminated induction of proteolytic activity in response to H2O2 pretreatment (Figure S7E; Figure 6K), analogous to normal chromosomal males. In contrast, expression of Tra RNAi in chromosomal males had no effect on proteolytic activity.
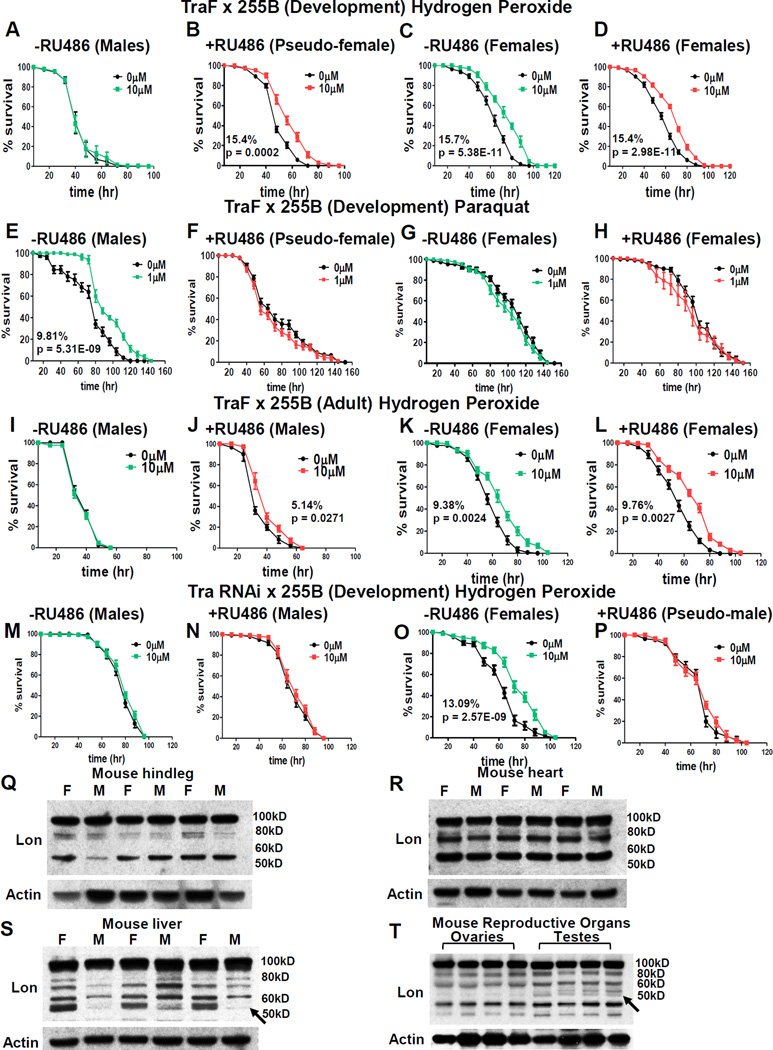
The Lon banding pattern in pseudo-females provided a model to determine if sex-specific expression of Lon contributes to adaptation. Consistent with results presented above, normal chromosomal males were unable to adapt to H2O2 stress (Figure 7A; Table S7). In contrast, pseudo-females showed robust H2O2 stress adaptation (Figure 7B; Table S7), equivalent to normal chromosomal females (Figure 7C,D; Figure S7F–I; Table S7). In contrast, normal chromosomal males were capable of PQ adaptation (Figure 7E; Figure S7J–M; Table S7), whereas pseudo-females, like normal chromosomal females, were unable to adapt to PQ (Figure 7F–H; Table S7). As expected mifepristone had no effect in absence of TraF transgene (Figure 7I; Table S7). Strikingly, adult-specific expression of TraF in chromosomal males was sufficient to confer H2O2 adaptation, consistent with transformation of adult male tissues to pseudo-female state (Figure 7J; Table S7), however adaptation was not as strong as normal chromosomal females (Figure 7K,L; Table S7), or pseudo-females generated by developmental expression of TraF (Figure 7B). Lastly, pseudo-males, like normal chromosomal males, were incapable of H2O2 adaptation (Figure 7M,N,P; Table S7), whereas normal chromosomal females showed adaptive response as expected (Figure 7O; Table S7). These results indicate that female-specific Lon isoform expression and female-specific H2O2 adaptation are regulated by sex-specific expression of transformer.
Figure 7. Adaptation in Transformed Flies and Sex-biased Lon Isoform Expression Across Species.
(A–H) UAS-TraF crossed to Actin-GS-255B, resulting larvae cultured in 2x RU486. (A–D) Survival following H2O2 pretreatment (A) Males raised without RU486. (B) Pseudo-females. Female progeny cultured (C) without RU486 and (D) with 2x RU486 prior to H2O2 pretreatment. (E–H) Survival following PQ pretreatment. (E) Males raised without RU486. (F) Pseudo females. Females cultured (G) without RU486 or (H) with 2x RU486. (I–L) UAS-TraF crossed to Actin-GS-255B, adult progeny cultured ±2x RU486 prior to H2O2 pretreatment. Males without RU486 (I) or with RU486 (J). Females without RU486 (K) or with RU486 (L). (M–P) Survival following H2O2 pretreatment in progeny of Tra RNAi crossed to Actin-GS-255B. (M) Males raised without RU486. (N) Males raised with RU486. (O) Females raised without RU486. (P) Pseudo-males raised without RU486. Statistical summary in Table S7. (Q–T) Western blots using mouse anti- Lon antibody in female (F) and male (M) tissue from 3 month black C57Bl/6 strain. (Q) Hindleg. (R) Heart. (S) Liver. (T) Ovaries and testes. Anti-actin-HRP loading control. See also Figure S7.
Sex-dimorphic Expression of Lon Protein Isoforms in Mammalian Tissues
To test if sex-specific differences in Lon protein isoforms might extend to mammals, tissues from 3 month-old black C57BI/6 male and female mice were analyzed. Western blot of muscle tissue from hindleg revealed a Lon banding pattern that was consistent in both sexes: a 100kD band, as expected for full-length Lon, as well as 80kD and 55kD bands (Figure 7Q). Similarly, analysis of cardiac tissue revealed a similar pattern of Lon isoforms in both sexes (Figure 7R). In contrast, mouse liver, which is a highly sexually-dimorphic tissue [37], had abundant expression of 55kD band in females, with only slight detection in males (Figure 7S). Analysis of mouse gonadal tissue revealed an additional 55kD Lon isoform in testes that was not present in ovaries (Figure 7T). These results indicate that sexual dimorphism in expression of Lon protein isoforms is observed in mouse tissues, including increased abundance of an ~55kD Lon species in female liver.
Discussion
Here we identify multiple isoforms of mitochondrial Lon protease expressed in sex-specific patterns. Adult Drosophila males expressed Lon 100kD and 60kD isoforms, whereas Drosophila females expressed 100kD, 60kD and 50kD isoforms. Males and females showed similar sensitivity to both H2O2 and PQ toxicity, which increased in an age-dependent manner, demonstrating that both sexes efficiently absorbed the drugs and experienced a toxic stress. Surprisingly, females but not males adapted to H2O2 stress, whereas males but not females adapted to PQ (superoxide) stress. Males consume less media, which could conceivably result in smaller drug effects, however, males also have smaller body size, so per unit intake should generally have greater effect. The fact that PQ led to male-specific stress-adaptation indicates the results are not simply due to sex differences in ingestion of pre-treatment drug. The adaptive response was associated with induction of 100kD Lon protein isoform in females, and 100kD and 60kD isoforms in males, without changes in lon mRNA, consistent with sex-specific posttranscriptional up-regulation of Lon expression. Over-expression of Lon increased H2O2 stress adaptation in females, however, over-expression of Lon was not sufficient to confer H2O2 stress adaptation to males, suggesting that both Lon induction and the female-specific pattern of Lon isoform expression are required for H2O2 stress adaptation. Consistent with this, transformation of males to pseudo-females using transformer produced female-specific Lon isoform expression and H2O2 stress adaptation.
Previous studies of cultured human female-derived lung fibroblasts and rhabdomyosarcoma cells exposed to H2O2 also revealed rapid induction of Lon 100kD form, with lesser or no increase in mRNA, consistent with post-transcriptional up-regulation of Lon [22, 23, 38]. Smaller size Lon protein isoforms were not previously noted in those female-derived human cell lines [22], however, here smaller Lon isoforms were observed in mouse liver and gonad tissues, suggesting possible evolutionary conservation of tissue-specific and sex-specific expression of alternative Lon protein isoforms. The possible mechanisms for sex-specific, post-transcriptional up-regulation of Lon expression and the sex-specific generation and function of alternative Lon protein isoforms will be important areas for future studies.
The increased expression of Lon protein and proteolytic activity in Drosophila females upon H2O2 pre-treatment and in Drosophila males upon PQ pre-treatment provides a likely mechanism contributing to stress adaptation, as increased Lon activity is expected to allow animals to more efficiently degrade oxidatively-damaged mitochondrial proteins produced during subsequent toxic challenges. Transgenic over-expression of Lon produced greater magnitude H2O2 stress adaptation in females, providing further evidence in support of the specific role of Lon. Lon was required for adaptation to oxidative stress in each sex, yet both increased and decreased expression of Lon was detrimental for longevity. These results suggest a trade-off, wherein transient oxidative stress adaptation produced by increased Lon is beneficial for survival upon near-term stress, but is ultimately costly for longevity. Consistent with this conclusion, repeated H2O2 pre-treatments of females reduced longevity [19].
Transformer was initially characterized as a regulator of alternative splicing, however our data indicate that TraF does not regulate expression of the novel 60kD and 50kD Lon isoforms through regulation of splicing. One possibility is that TraF regulates expression of a sex-specific Lon protein processing machinery. The protein sequencing data indicates that both 60kD and 50kD Lon protein isoforms correspond to the central region of the primary 100kD Lon, suggesting that Lon may be cleaved at both amino and carboxyl ends. Moreover, the female-specific 50kD isoform might have a regulatory or chaperone-like role, as Lon has been previously shown to regulate assembly of protein complexes in the mitochondria and to bind to mitochondrial DNA, independent of function as a protease [39].
Recently TraF was reported to regulate female-specific expression of many genes through mechanisms that may involve direct or indirect transcriptional regulation by Tra [40]. Normal TraF expression in female fat body caused increased IIS in females relative to males [35]. Up-regulation of IIS by TraF may help explain the female-specific induction of Lon and adaptation to H2O2 stress. Several lines of evidence suggest that females have greater baseline IIS than do males in D. melanogaster, mice and humans [18]. In mammals, a localized burst of H2O2 produced by membrane-associated NADPH oxidase stimulates IIS by increasing receptor tyrosine phosphorylation and activity of downstream signaling kinases including AKT [41]. Therefore one possibility is that Drosophila females are physiologically better equipped to respond to low-concentration H2O2 pre-treatment because of greater expression and sensitivity of IIS.
Because mitochondria are inherited almost exclusively from the mother, natural selection can only optimize mitochondrial gene function and nuclear-mitochondrial gene interactions in females, and therefore mitochondrial function is expected to be better optimized for the female [18, 42]. This may be one explanation for the presence of a beneficial female-specific 50kD Lon isoform. One possibility is that females have evolved to better handle H2O2 because this is a normal signaling molecule generated by mitochondria [7]. Consistent with this, female mammalian cardiomyocytes were more resistant to H2O2 toxicity [43], and females have greater survival following cardiac ischemia-reperfusion, which is characterized by a burst of mitochondrial H2O2 [44]. In human tumor cells Lon is positively correlated with glycolytic metabolism and more aggressive cancer [39]. Women are marked by both greater insulin sensitivity and reduced cancer incidence relative to men. IIS negatively regulates the Foxo tumor suppressor by stimulating AKT phosphorylation of Foxo. In turn, Foxo positively regulates insulin-like receptor expression and insulin sensitivity in mammals and flies [45]. Potentially, increased IIS pathway expression and sensitivity in women may create more robust regulation of Foxo (negative and positive), thereby facilitating interactions with p53 and Lon to reduce tumor incidence.
The observation that Drosophila males but not females were capable of PQ adaptation likely results from the different oxidative toxicity caused by PQ. PQ reacts with NADPH oxidase enzymes and molecular oxygen in mitochondria and at the cell membrane to generate PQ radical and superoxide [28, 29]. Superoxide is converted to H2O2 by superoxide-dismutase, and in presence of iron superoxide and H2O2 can combine to create highly-toxic hydroxyl radical. These free radicals can in turn damage cellular macromolecules, including proteins, consistent with the beneficial effect of up-regulated Lon protease activity upon PQ stress adaptation. The fact that Lon is required for PQ stress adaptation in Drosophila indicates the mitochondrial proteome is one critical target of PQ-induced damage. Studies in Drosophila and mice reveal dopaminergic neurons are particularly susceptible to PQ toxicity, and implicate membrane NADPH oxidase as an important target for superoxide generation [46, 47]. Male Drosophila are more sensitive to behavioral disruptions by PQ, and mutations that increase dopamine levels (Catsup) conferred increased resistance, whereas mutations that decreased dopamine increased sensitivity [47, 48]. Notably, increased expression of dopamine receptor DAMB increased PQ sensitivity [48], and DAMB is expressed at higher levels in males. Therefore, one possibility is that Drosophila males are physiologically better-equipped to respond to PQ pre-treatment than females because of greater basal expression of PQ/superoxide sensitive pathways such as DAMB, while at the same time, this creates a greater target for toxic levels of PQ/superoxide stress. Intriguingly, men are at greater risk for Parkinson’s Disease (PD) than women, and PD preferentially targets dopaminergic neurons [49]. One possibility is that greater expression and activity of dopamine signaling in men confers greater sensitivity to PQ/superoxide stress, yielding more effective stress adaptation at low stress, such as observed here for male Drosophila, but at the same time provides a greater target for toxic stress, such as in development of PD.
Experimental Procedures
Drosophila Culture
Control flies were progeny of males of w[1118] crossed to virgin females of Actin-‘Geneswitch’-255B (Actin-GS-255B) driver strain. Lon RNAi and over-expression strains were obtained from Bloomington Drosophila Stock Center and Vienna Drosophila RNAi Center. Males of Lon strains were mated to virgin females of the Actin-GS-255B driver strain. Virgin progeny were collected following eclosion. Fly maintenance and additional strains described in SI Materials and Methods.
Adaptation with H2O2 and PQ
Control studies used 3 day, 35 day, or 60 day old flies prior to oxidant exposure. Transgenic and RNAi strains were cultured in ±RU486 (mifepristone) for 9 days prior to H2O2 or PQ pretreatment. Flies underwent 8-hour pretreatment with 0µM-100µM H2O2 or 0µM-10µM PQ, prior to 16-hour recovery. Flies were then fed a semi-lethal dose of oxidant, [4.4M] H2O2 or [30mM] PQ. Detailed treatment description in SI Materials and Methods.
mRNA, Protein, and Proteolytic Capacity
For mRNA and western blots, flies were homogenized using electric pestle. mRNA extracted using Trizol and quantified by qPCR (primers in SI Materials and Methods). For western blot, protein was quantified using Bicinchoninic acid assay (BCA) reducing agent kit and 10µg lysate loaded on 10% SDS Page. 200 flies were used for mitochondrial isolation, and proteolytic activity measured with addition of ±5mM ATP with tritium-labeled aconitase ± oxidation. Detailed description in SI Experimental Procedures.
Supplementary Material
Acknowledgments
We thank S. Kaguni for D. melanogaster Lon antibody, V. Longo for mouse tissue, and R. Raynes for technical advice. This work was supported by NSF grant (DGE-1418060) to L.C.D.P., NIH/NIEHS grant (ES03598) to K.J.A.D., and NIH/NIA grant (AG011833) and pilot funding from SCEHSC (5P30ES007048) to J.T.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
L.C.D.P., K.J.A.D., and J.T. conceived and designed the study. L.C.D.P., C.C., B.S., S.W., & K.W., performed survival and adaptation experiments. L.C.D.P and C.C. performed lifespan studies. L.C.D.P and B.S. performed RNA extraction and qPCR. L.C.D.P, B.S., & S.W. performed western blots. M.P.S. analysed of RNAseq data. L.C.D.P performed proteolytic activity experiments. L.C.D.P., K.J.A.D. and J.T. performed data interpretation and analysis. L.C.D.P, K.J.A.D. and J.T. wrote the manuscript.
References
- 1.Pickering AM, Staab TA, Tower J, Sieburth D, Davies KJA. A conserved role for the 20S proteasome and Nrf2 transcription factor in oxidative stress adaptation in mammals, Caenorhabditis elegans and Drosophila melanogaster. J. Exp. Biol. 2013;216:543–553. doi: 10.1242/jeb.074757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiese AG, Pacifici RE, Davies KJ. Transient adaptation to oxidative stress in mammalian cells. Arch. Biochem. Biophys. 1995;318:231–240. doi: 10.1006/abbi.1995.1225. [DOI] [PubMed] [Google Scholar]
- 3.Tower J. Heat shock proteins and Drosophila aging. Exp. Gerontol. 2011;46:355–362. doi: 10.1016/j.exger.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tower J. Hsps and aging. Trends Endocrinol. Metab. 2009;20:216–222. doi: 10.1016/j.tem.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pickering AM, Koop AL, Teoh CY, Ermak G, Grune T, Davies KJ. The immunoproteasome, the 20S proteasome and the PA28αβ proteasome regulator are oxidative-stress-adaptive proteolytic complexes. Biochem. J. 2010;432:585–595. doi: 10.1042/BJ20100878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grune T, Catalgol B, Licht A, Ermak G, Pickering AM, Ngo JK, Davies KJ. HSP70 mediates dissociation and reassociation of the 26S proteasome during adaptation to oxidative stress. Free Radic. Biol. Med. 2011;51:1355–1364. doi: 10.1016/j.freeradbiomed.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 8.Pickering AM, Linder RA, Zhang H, Forman HJ, Davies KJ. Nrf2-dependent induction of proteasome and Pa28αβ regulator are required for adaptation to oxidative stress. J. Biol. Chem. 2012;287:10021–10031. doi: 10.1074/jbc.M111.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson BW. The human mitochondrial proteome: oxidative stress, protein modifications and oxidative phosphorylation. Int. J. Biochem. Cell Biol. 2005;37:927–934. doi: 10.1016/j.biocel.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Ngo JK, Pomatto LC, Davies KJ. Upregulation of the mitochondrial Lon Protease allows adaptation to acute oxidative stress but dysregulation is associated with chronic stress, disease, and aging. Redox Biol. 2013;1:258–264. doi: 10.1016/j.redox.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bota DA, Davies KJ. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat. Cell Biol. 2002;4:674–680. doi: 10.1038/ncb836. [DOI] [PubMed] [Google Scholar]
- 12.Bota DA, Ngo JK, Davies KJ. Downregulation of the human Lon protease impairs mitochondrial structure and function and causes cell death. Free Radic. Biol. Med. 2005;38:665–677. doi: 10.1016/j.freeradbiomed.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Battiprolu PK, Lopez-Crisosto C, Wang ZV, Nemchenko A, Lavandero S, Hill JA. Diabetic cardiomyopathy and metabolic remodeling of the heart. Life Sci. 2013;92:609–615. doi: 10.1016/j.lfs.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat. Rev. Endocrinol. 2016;12:144–153. doi: 10.1038/nrendo.2015.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen J, Landis GN, Tower J. Multiple Metazoan Life-span Interventions Exhibit a Sex-specific Strehler-Mildvan Inverse Relationship Between Initial Mortality Rate and Age-dependent Mortality Rate Acceleration. J. Gerontol. A Biol. Sci. Med. Sci. 2016 doi: 10.1093/gerona/glw005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waskar M, Landis GN, Shen J, Curtis C, Tozer K, Abdueva D, Skvortsov D, Tavare S, Tower J. Drosophila melanogaster p53 has developmental stage-specific and sex-specific effects on adult life span indicative of sexual antagonistic pleiotropy. Aging (Albany NY) 2009;1:903–936. doi: 10.18632/aging.100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen J, Tower J. Drosophila foxo acts in males to cause sexual-dimorphism in tissue-specific p53 life span effects. Exp. Gerontol. 2010;45:97–105. doi: 10.1016/j.exger.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tower J. Mitochondrial maintenance failure in aging and role of sexual dimorphism. Arch. Biochem. Biophys. 2015;576:17–31. doi: 10.1016/j.abb.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pickering AM, Vojtovich L, Tower J, A Davies KJ. Oxidative stress adaptation with acute, chronic, and repeated stress. Free Radic. Biol. Med. 2013;55:109–118. doi: 10.1016/j.freeradbiomed.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landis G, Shen J, Tower J. Gene expression changes in response to aging compared to heat stress, oxidative stress and ionizing radiation in Drosophila melanogaster. Aging (Albany NY) 2012;4:768–789. doi: 10.18632/aging.100499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landis GN, Salomon MP, Keroles D, Brookes N, Sekimura T, Tower J. The progesterone antagonist mifepristone/RU486 blocks the negative effect on life span caused by mating in female Drosophila. Aging (Albany NY) 2015;7:53–69. doi: 10.18632/aging.100721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ngo JK, Davies KJ. Mitochondrial Lon protease is a human stress protein. Free Radic. Biol. Med. 2009;46:1042–1048. doi: 10.1016/j.freeradbiomed.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ngo JK, Pomatto LC, Bota DA, Koop AL, Davies KJ. Impairment of lon-induced protection against the accumulation of oxidized proteins in senescent wi-38 fibroblasts. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:1178–1185. doi: 10.1093/gerona/glr145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang N, Maurizi MR, Emmert-Buck L, Gottesman MM. Synthesis, processing, and localization of human Lon protease. J. Biol. Chem. 1994;269:29308–29313. [PubMed] [Google Scholar]
- 25.Delaval E, Perichon M, Friguet B. Age-related impairment of mitochondrial matrix aconitase and ATP-stimulated protease in rat liver and heart. Eur. J. Biochem. 2004;271:4559–4564. doi: 10.1111/j.1432-1033.2004.04422.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee SY, Lim IA, Kang GU, Cha SJ, Altanbyek V, Kim HJ, Lee S, Kim K, Yim J. Protective effect of Drosophila glutathione transferase omega 1 against hydrogen peroxide-induced neuronal toxicity. Gene. 2015;568:203–210. doi: 10.1016/j.gene.2015.05.058. [DOI] [PubMed] [Google Scholar]
- 27.Ford D, Hoe N, Landis GN, Tozer K, Luu A, Bhole D, Badrinath A, Tower J. Alteration of Drosophila life span using conditional, tissue-specific expression of transgenes triggered by doxycyline or RU486/Mifepristone. Exp. Gerontol. 2007;42:483–497. doi: 10.1016/j.exger.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cocheme HM, Murphy MP. Complex I is the major site of mitochondrial superoxide production by paraquat. J. Biol. Chem. 2008;283:1786–1798. doi: 10.1074/jbc.M708597200. [DOI] [PubMed] [Google Scholar]
- 29.Bonneh-Barkay D, Reaney SH, Langston WJ, Di Monte DA. Redox cycling of the herbicide paraquat in microglial cultures. Mol. Brain Res. 2005;134:52–56. doi: 10.1016/j.molbrainres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev. Cell. 2008;14:76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reis-Rodrigues P, Czerwieniec G, Peters TW, Evani US, Alavez S, Gaman EA, Vantipalli M, Mooney SD, Gibson BW, Lithgow GJ, et al. Proteomic analysis of age-dependent changes in protein solubility identifies genes that modulate lifespan. Aging Cell. 2012;11:120–127. doi: 10.1111/j.1474-9726.2011.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quirós Pedro M, Español Y, Acín-Pérez R, Rodríguez F, Bárcena C, Watanabe K, Calvo E, Loureiro M, Fernández-García MS, Fueyo A, et al. ATP-Dependent Lon Protease Controls Tumor Bioenergetics by Reprogramming Mitochondrial Activity. Cell Rep. 2014;8:542–556. doi: 10.1016/j.celrep.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 33.Verhulst EC, van de Zande L, Beukeboom LW. Insect sex determination: it all evolves around transformer. Curr. Opin. Genet. Dev. 2010;20:376–383. doi: 10.1016/j.gde.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Casper AL, Van Doren M. The establishment of sexual identity in the Drosophila germline. Development. 2009;136:3821–3830. doi: 10.1242/dev.042374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rideout EJ, Narsaiya MS, Grewal SS. The Sex Determination Gene transformer Regulates Male-Female Differences in Drosophila Body Size. PLoS Genet. 2015;11:e1005683. doi: 10.1371/journal.pgen.1005683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rideout EJ, Dornan AJ, Neville MC, Eadie S, Goodwin SF. Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nat. Neurosci. 2010;13:458–466. doi: 10.1038/nn.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang X, Schadt EE, Wang S, Wang H, Arnold AP, Ingram-Drake L, Drake TA, Lusis AJ. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006;16:995–1004. doi: 10.1101/gr.5217506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hori O, Ichinoda F, Tamatani T, Yamaguchi A, Sato N, Ozawa K, Kitao Y, Miyazaki M, Harding HP, Ron D, et al. Transmission of cell stress from endoplasmic reticulum to mitochondria: enhanced expression of Lon protease. J. Cell Biol. 2002;157:1151–1160. doi: 10.1083/jcb.200108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinti M, Gibellini L, Liu Y, Xu S, Lu B, Cossarizza A. Mitochondrial Lon protease at the crossroads of oxidative stress, ageing and cancer. Cell. Mol. Life Sci. 2015;72:4807–4824. doi: 10.1007/s00018-015-2039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hudry B, Khadayate S, Miguel-Aliaga I. The sexual identity of adult intestinal stem cells controls organ size and plasticity. Nature. 2016;530:344–348. doi: 10.1038/nature16953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol. Cell Biol. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gemmell NJ, Metcalf VJ, Allendorf FW. Mother's curse: the effect of mtDNA on individual fitness and population viability. Trends. Ecol. Evol. 2004;19:238–244. doi: 10.1016/j.tree.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Magkos F, Wang X, Mittendorfer B. Metabolic actions of insulin in men and women. Nutrition. 2010;26:686–693. doi: 10.1016/j.nut.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaha VG, Qi D, Su KN, Palmeri M, Lee HY, Hu X, Wu X, Shulman GI, Rabinovitch PS, Russell RR, 3rd, et al. AMPK is critical for mitochondrial function during reperfusion after myocardial ischemia. J. Mol. Cell. Cardiol. 2016;91:104–113. doi: 10.1016/j.yjmcc.2015.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puig O, Tjian R. Nutrient availability and growth: regulation of insulin signaling by dFOXO/FOXO1. Cell Cycle. 2006;5:503–505. doi: 10.4161/cc.5.5.2501. [DOI] [PubMed] [Google Scholar]
- 46.Cristovao AC, Choi DH, Baltazar G, Beal MF, Kim YS. The role of NADPH oxidase 1-derived reactive oxygen species in paraquat-mediated dopaminergic cell death. Antioxid. Redox Signal. 2009;11:2105–2118. doi: 10.1089/ars.2009.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaudhuri A, Bowling K, Funderburk C, Lawal H, Inamdar A, Wang Z, O'Donnell JM. Interaction of genetic and environmental factors in a Drosophila parkinsonism model. J. Neurosci. 2007;27:2457–2467. doi: 10.1523/JNEUROSCI.4239-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cassar M, Issa AR, Riemensperger T, Petitgas C, Rival T, Coulom H, Iche-Torres M, Han KA, Birman S. A dopamine receptor contributes to paraquat-induced neurotoxicity in Drosophila. Hum. Mol. Genet. 2015;24:197–212. doi: 10.1093/hmg/ddu430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gillies GE, Pienaar IS, Vohra S, Qamhawi Z. Sex differences in Parkinson's disease. Front. Neuroendocrinol. 2014;35:370–384. doi: 10.1016/j.yfrne.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.