Abstract
Unresolved inflammation is key in linking metabolic dysregulation and the immune system in type 2 diabetes. Successful regulation of acute inflammation requires biosynthesis of specialized pro-resolving lipid mediators, such as resolvin E1 (RvE1), and activation of cognate g-protein coupled receptors (GPCR). RvE1 binds to BLT-1 on neutrophils and ERV-1/ChemR23 on monocyte/macrophages. We show novel actions of RvE1 and expression patterns of neutrophil receptors in type 2 diabetes. Neutrophils from healthy subjects express functional BLT-1, low levels of minimally functional ERV-1 and inversed co-expression in type2 diabetes. Stimulation with TNFα or LPS increased expression of ERV-1 by healthy and diabetic neutrophils. RvE1 has counteracted LPS and TNFα induction of ERV1 overexpression and diabetic overexpression activating phagocytosis and resolution signals. Functional ERV-1 was determined by phosphorylation of the signaling protein, ribosomal S6 (rS6). Receptor antagonism experiments revealed that the phospho-rS6 increase was mediated by BLT-1 in healthy subject neutrophils and ERV1 in diabetes. Metalolipidomics reveal a pro-inflammatory profile in diabetic serum. Cell phagocytosis is impaired in type 2 diabetes and requires RvE1 for activation. The dose of RvE1 required to activate resolution signals in type 2 diabetic neutrophils was significantly higher than healthy controls. RvE1 rescued aberrant neutrophil receptor profile and, following a therapeutic dosage, activates phagocytosis and resolution in type 2 diabetes. These findings reveal the importance of resolution receptors in health, disease and dysregulation of inflammation in type 2 diabetes.
Keywords: Neutrophils, Diabetes, Resolvin E1, GPCR, ERV1, Inflammation, Resolution
INTRODUCTION
Over the past decades, the global prevalence of type 2 diabetes has drastically increased. According to the International Diabetes Federation roughly 415 million adults currently have diabetes, and the incidence of type 2 diabetes is estimated to increase by 2040 (1, 2). This represents a global problem, heavily impacting quality of life, lifespan, and overall healthcare costs. In the natural course of type 2 diabetes it typically takes 15–20 years for hyperglycemia to progress to the establishment of disease. Uncontrolled inflammation plays an essential role in the pathogenesis of diabetes and its associated pathologies. Over the long-term, uncontrolled type-2 diabetes can cause a cluster of diseases which are linked through inflammatory pathways, such as obesity, cardiovascular diseases, blindness, chronic kidney diseases and periodontal diseases (3, 4).
Inflammation can play a protective role against injury and infections, but prolonged or excessive inflammation can lead to pathology (5). The resolution phase of inflammation is activated temporally, after an acute challenge, and involves eicosanoid class switching from pro-inflammatory to pro-resolution lipid mediators (LM). The failure of inflammation to resolve leads to chronic oxidative stress, tissue damage, scar formation and fibrosis (6–10). Continuous unresolved inflammation establishes a pathological response, leading to chronic disease (11).
A central characteristic of functional, acute inflammation is a rapid return to homeostasis. Protective acute inflammatory response is tightly regulated by a genus of specialized pro-resolving lipid mediators (SPM). SPM are produced via the actions of specific lipoxygenases on different substrates, including arachidonic acid-derived lipoxins (LXA4 and LXB4), eicosapentaenoic acid (EPA)-derived E-series resolvins (RvE1-3), docosahexaenoic acid (DHA)-derived D-series resolvins (RvD1-6), maresins and protectins (10, 12–24). Activation of resolution signals occurs when specific SPMs interact with cognate G-protein coupled receptors (GPCRs) present on the cell surface (25, 26). Lipoxin A4 (LXA4) binds its receptor, ALX/FPR2, transducing signals with potent actions in experimentally induced animal disease models (27). Resolvin E1 (5S, 18R-trihydroxy-6E, 8Z, 11Z, 14Z, 16E eicosapentaenoic acid, RvE1) binds to at least two GPCRs, BLT1 (a leukotriene B4 receptor) and ERV1 (formerly chemR23), limiting neutrophil migration and accumulation and stimulating non-phlogistic recruitment of monocyte/macrophages for phagocytosis of apoptotic neutrophils and bacteria, which are eventually cleared through the lymphatic’s (16, 17). RvE1 binds to ERV-1 on monocyte/macrophages with high affinity (Kd = 11.3 ± 5.4 nM) and with lower affinity to BLT-1 on neutrophils (Kd =48.3 nM) (1, 2). RvE1 treatment positively influences the outcome of inflammatory disease in animal models, such as murine colitis, periodontitis and type 2 diabetes (28–37). Many studies have focused on these lipid mediator ligands and their downstream functions, but there remains a dearth of research addressing how the receptors of resolution behave in chronic human diseases.
In type 2 diabetes, uncontrolled inflammation has detrimental effects, and dysregulation of resolution is a possible link to the severity of the disease presentation and therapy (38). In our previous work, using a monogenic murine model of obesity and type-2 diabetes, db/db mice were shown to exhibit decreased neutrophil chemotaxis, delayed wound healing, deficient phagocytosis, delayed neutrophil apoptosis and defective clearance of inflammatory lesions (39). Deficient phagocyotsis of pathogens promote chronic diseases, for example phagocytosis of phorphyromans gingivalis, a key pathogen in periodontal disease(40), is deficient in diabetes (41). The reason diabetic immune cells fail to resolve inflammation remains unknown. As RvE1 increases cell functions in db/db mice, we investigated its cognate receptors, ERV1 and BLT1, on human neutrophils from volunteers with and without diabetes.
The goal of this study was to investigate the basis for inflammatory dysregulation in Type 2 diabetes. We found that the expression and function of a key receptor in resolution, ERV1, was upregulated in T2D neutrophils. Signaling via ERV1, not BLT1, was predominant for RvE1 actions. TNFα and LPS induced ERV1 expression was modulated by RvE1. Deficient phagocytosis of diabetic neutrophils was rescued by higher RvE1 doses, when compared to healthy.
MATERIALS & METHODS
SUBJECT SAMPLES
Subjects were recruited to the Center for Clinical and Translational Research at the Forsyth Institute and samples were obtained under consent approved by the Forsyth Institute Review Board (protocol #11-03). Peripheral venous blood (~60 ml) was collected from patients diagnosed with T2D and healthy, non-diabetic controls (39). The diagnosis of T2D was made by the subjects primary care physician following American Association of Diabetes guidelines (42). HbA1c was used to determine 3 month historic glycemic levels (Supplementary Table 1). All blood donors were uncontrolled diabetics or healthy controls. Subjects were excluded when taking insulin sensitizers, non-steroidal anti-inflammatory drugs (NSAIDs) or antimicrobials within 3 months prior to the experiment.
ISOLATION & CULTURE OF HUMAN NEUTROPHILS
Human neutrophils were isolated from human whole blood by Ficoll-Histopaque density-gradient centrifugation (Histopaque 107 1077& 1119, Sigma-Aldrich). Neutrophils were isolated after isotonic lysis of red blood cells, followed by two washes in phosphate buffered saline (Sigma-Aldrich). In culture experiments, neutrophils were obtained and incubated with RPMI 1640 medium (Sigma-Aldrich) supplemented with 10% FBS (v/v) (Life Technologies) at 37°C.
To confirm isolated cells were neutrophils, cells were stained with Wright-Giemsa. After centrifugation at 2000 × g for 5 min, cell pellets were suspended in PBS (200 μL), counted with a hemocytometer, and 50 μL of each cell suspension was mixed with 150 μL of 30% BSA in PBS and centrifuged onto microscope slides at 500 rpm for 5 min using a cytospin centrifuge, air-dried, and stained with Wright-Giemsa to identify individual cell type.
After cell isolation, neutrophils from subjects with T2D and normal controls were incubated with stimulants, including LPS (10ng/ml), RvE1 (0.1nM–100nM), TNF-α (10ng/ml) and LTB4 (10nM). Various combinations of the agonists were explored. When two compounds were used, the first was incubated for 15 min at 37° C in 5% CO2 before addition of the second.
In order to understand whether ERV1 is functional in type 2 diabetes, peripheral blood neutrophils were isolated and treated with RvE1 (10nM) in the presence and absence of the BLT1 receptor antagonist, U230495 (1ng/ml) and a RvE1 receptor antagonist antibody (1ng/ml). Various combinations of the RvE1 and antagonists were explored. When two compounds were used, the first was incubated for 15min at 37°C in 5% CO2 before addition of the second. The total stimulation time was 30 minutes.
RIBOSOMAL S6 MEDIATED SIGNALING
To determine phosphorylation of rS6, cells were plated into 96-well plates (100,000 cells/well). RvE1 (1nM) was incubated with neutrophils for 30 min at 37°C, followed by cellular permebilization (Cytofix/Cytoperm solution Kit; BD). Cells were labeled with APC-conjugated anti-phospho-rS6 antibody (BD Biosciences) for 30 min at 37°C. Quantification of phosphorylation was determined by gating ERV-1 positive cells only (FACS Aria II, BD Biosciences).
CELL LINES
Recombinant Human ERV1 was transfected into Chinese hamster ovarian cells (CHO ERV1 +, Genscript). ERV1 receptor CHO ERV1− cells were obtained from Sigma. Cells were cultured in Ham’s 12 medium supplemented with 10% fetal bovine serum (GIBCO), 100U/ml of Zeocin and Hydromycin antimicrobials and maintained 37°C in 5% CO2. Cells were incubated with RvE1 for one hour before total RNA was extracted.
RESOLVIN
RvE1 was prepared by total organic synthesis (16, 43). The structural integrity of RvE1 was monitored using UV tandem LC-MS/MS. Immediately before use, RvE1 was diluted in phosphate-buffered saline to final ethanol concentration of <1%.
SURFACE MARKER EXPRESSION
Peripheral blood neutrophils were isolated from healthy and type 2 diabetic adult individuals. Isolated cells were incubated with anti-Fc receptor (BD) blocking antibody (5 μg/ml × 106 cells, 15 min) and then labeled with anti-human ERV1 alexafluor 488-conjugated antibody (10 μg/ml × 106 cells, 1 hour at RT) or anti-IgG alexafluor 488 (isotype control, R&D Systems). Expression of ERV1 on neutrophils was evaluated by immunofluorescence and quantified by flow cytometry. Cells were also stained with PE-conjugated anti-human CD11b, FITC-conjugated anti-human CD14 and APC-conjugated CD18 antibodies (10 μg/ml × 106 cells, 1 hour at RT) (BD Biosciences). Expression levels of the proteins were monitored by flow cytometry (FACS Aria II, BD Biosciences) and analyzed with FlowJo (Tree Star).
qPCR
Total RNA was isolated from human neutrophils with TRIzol (Life Technologies, Carlsbad, CA, USA) (M. Portillo, 2006) and purity was confirmed using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific). RNA was stored for later use at −80° C in RNAlater. RNA was then reverse transcribed using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). mRNA expression levels were quantified by real-time PCR using SYBR Select Master Mix (Applied Biosystems) on a Light Cycler 480 (Roche Diagnostics). Reactions were performed under the following conditions: preheating 10 minutes at 95°C, followed by 45 cycles of denaturation for 5 seconds at 95°C, annealing 10 seconds at 60°C, and extension for 6 seconds at 72°C. Relative gene expression was normalized to GAPDH. Data is expressed as DeltaCT of mRNA levels (Figure 1G), primers related to resolution and inflammatory genes are listed on Supplementary Table 2. Human ERV1 primer sequence for CHO experiments: forward primer 5′-ATAGAATGGAGGATGAAGATTACAACACT-3′, reverse 5′-TCCCGAGGAAGCAGACGATG-3′. Table. Data is expressed as fold change.
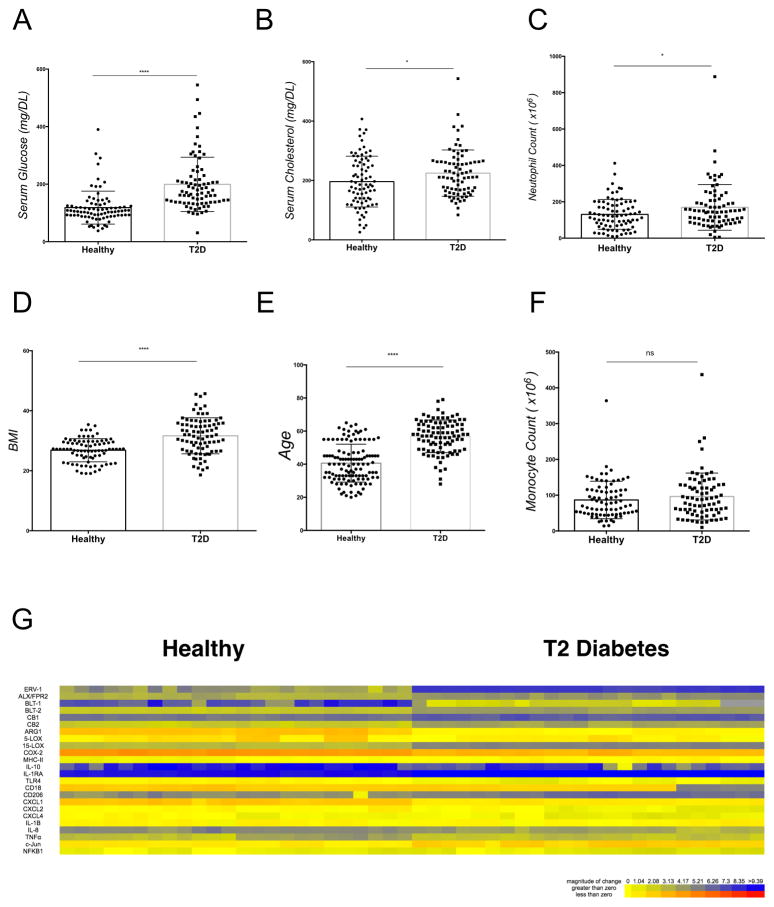
Figure 1. Clinical characteristics of study subjects.
(A) Serum glucose; (B) serum cholesterol; (C) total neutrophil count;) (D) BMI, (E) Age (F) total monocyte count (total number of individuals n=166, healthy, n=83, T2D, n=83). Statistical significance was evaluated by Wilcoxon test (* p<0.05, **** p<0.0001, ns = non-significant). (G) mRNA expression of resolution and inflammation gene expression of isolated neutrophils (healthy, n=24, T2D, n=24). DCT data is expressed as a heat map.
LC-MS/MS–BASED LIPID MEDIATOR (LM) METABOLOLIPIDOMICS
Blood samples were collected, centrifuged at 2,300 rpm, serum was isolated and frozen at −80° C until analysis. Methanol (4 volumes, 4° C, 30 min) containing 500 pg of deuterated internal standards d4-LTB4, d8-5S-HETE, d5-LXA4, and d4-PGE2 to facilitate lipid mediator identification and quantification were added to the serum. Lipid mediators were extracted using C18-silica reverse-phase cartridges and Biotage Rapid Trace (44). Mediators were eluted using 6 mL methylformate and brought to dryness using TurboVap L.V. and suspended in methanol/water for LC-MS-MS. The liquid chromatography-UV coupled with tandem mass spectrometry system includes QTrap 6500 equipped with a Shimadzu SIL-20AC auto-injector and LC-20AD binary pump. An Agilent Eclipse Plus C18 column (100 mm × 4.6 mm × 1.8 μm) was used with a gradient of methanol/water/acetic acid of 60:40:0.01 (v/v/v) to 100:0:0.01 at a 0.5-mL/min flow rate. To monitor and quantify the levels of the specific lipid mediators, a multiple reaction–monitoring (MRM) method with signature ion fragments for each molecule was used. Identification was conducted using published criteria (44). Calibration curves were obtained using synthetic and authentic LM mixtures, including d8–5S-HETE, d4-LTB4, d4-PGE2, LXA4, LXB4, LTB4, PGE2, PGD2, PGF2α, TxB2, RvE1, RvE2, RvD1, RvD2, RvD3, RvD5, PD1, and MaR1 at 12.5, 25, 50, and 100 pg. Linear calibration curves for each were obtained with r2 values in the range of 0.98 to 0.99. Quantification was based on peak area of the MRM transition and the linear calibration curve for each compound (44).
PHAGOCYTOSIS ASSAY
Human neutrophils were cultured in RPMI 1640 medium (Gibco) supplemented with 10% heat-inactivated FBS, 5.5 mM glucose, 1% DMSO (Sigma) and incubated at 37° C. Porphyromonas gingivalis strain A7436 was cultured on 2% trypticase soy agar (TSA) supplemented with 2.6% Brain Heart Infusion Agar (BD BBL), 1% (w/v) yeast extract (BD BACTO), 5% defibrinated sheep RBC (Northeast Laboratory Services), 5μg/ml of hemin, and 0.5 μg/ml vitamin K (Sigma-Aldrich). All cultures were placed in an anaerobic chamber (85% N2, 10% CO2, 5% H2 at 37° C). Colonies were transferred from the plate to Wilkin’s broth (OXOID) and grown for 4 days. Bacterial titers were determined at 600 nm using a spectrometer (SmartSpec 3000, Bio-Rad) and adjusted to OD = 1.0 (approximately 130 CFU/ml) prior to experiments. Human neutrophils were then counted and aliquot in 1 × 106 increments. Bacteria was labeled with BacLight Green (Molecular Probes) for 20 m at RT with gentle agitation and washed twice with PBS. Labeled bacteria were opsonized in heat inactivated normal serum (Sigma) for 30 minutes at room temperature and incubated with human neutrophils (1:20 ratio) for 1h in serum and antibiotic-free medium at 37°C. Cells were gently washed, extracellular fluorescence was quenched by Trypan blue and phagocytosis was determined by flow cytometry (FACS Aria II, BD Biosciences). A similar protocol was followed for labeled zymosan fluorescent bioparticles (Life sciences). Data is expressed as a phagocytic index: Phagocytic index = % phagocytic neutrophils × mean fluorescence intensity.
STATISTICAL ANALYSIS
Results are expressed as mean ± SEM. Statistical analysis was performed using Prism 6 (GraphPad). Wilcoxon Test and Student unpaired t test were used to compare measurements. Values of P ≤ 0.05 were considered statistically significant.
RESULTS
CLINICAL CHARACTERISTICS OF THE RESEARCH SUBJECTS
To investigate the role of the RvE1/ERV1 axis in type 2 diabetes, peripheral whole blood was collected from subjects enrolled in the study (healthy subjects: n = 83; T2D subjects: n=83) after obtaining informed consent. Experimental protocol for this study was approved by the Institutional Review Board of the Forsyth Institute (IRB#13-07). Elevated serum glucose was evident in patients with type 2 diabetes when compared to controls (p<0.0001, Figure 1A), and HbA1c levels confirmed serum measurements (Supplementary Figure 1). Serum cholesterol, BMI and age were elevated in subjects with type 2 diabetes (p<0.05, Figure 1B and p<0.0001, Figure 1D, p<0.0001, Figure 1E, respectively). Total neutrophil counts were increased in subjects with type 2 diabetes (p<0.05, Figure 1C); no significant difference was found in total monocyte counts (p= 0.176, Figure 1F). Healthy gender and race matched volunteers served as controls. Demographic parameters are reported in Supplementary Table 1. Correlation analysis showed positive association between age and cholesterol (p=0.006). Negative associations were found when evaluating ERV-1 and BLT-1 receptor, BMI and, glucose levels. Sub-group analysis of BMI and age by Pearson coefficeint no association of BMI or age (p>0.05). Gene expression profile of neutrophil mRNA was evaluated. Resolution and inflammatory genes were plotted as a heatmap of individual samples (Figure 1G). 1. A table of primers and respective genes investigated in this study are listed in Supplementary Table 2.
ERV1 IS UPREGULATED ON HUMAN NEUTROPHILS IN TYPE 2 DIABETES
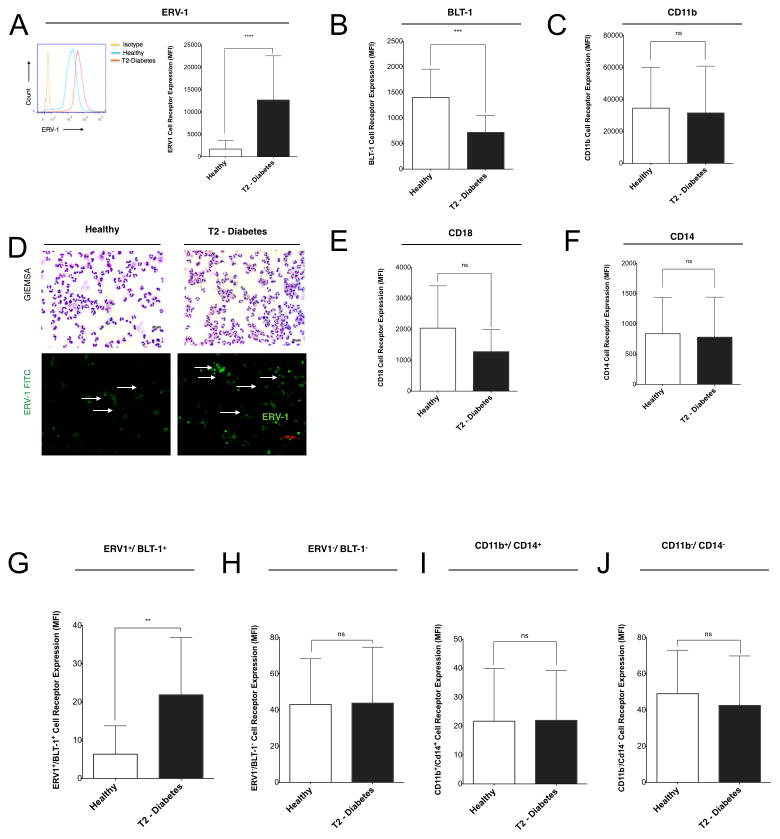
In order to elucidate the expression profile or ERV-1, mRNA and protein levels were evaluated. mRNA expression levels of ERV-1 was quantified by PCR (Figure 1G) and protein expression of ERV1 on neutrophils from individuals with type 2 diabetes; cells were isolated and quantified by flow cytometry (Figure 2A). The ERV1 receptor was only known to be active on monocytes/macrophages and the BLT1 receptor on neutrophils (29). To determine cell surface ERV1 receptor expression patterns of human neutrophils in type 2 diabetes, isolated cells were labeled with anti-ERV1 antibody for quantification by flow cytometry and immunofluorescence (Figure 2A). ERV1 expression was significantly increased on neutrophils isolated from type 2 diabetics (Figure 2A, p<0.0001 and IF panel 2D). The distinct co-expression pattern of neutrophil ERV1 and BLT1 receptors demonstrates that, in type 2 diabetes, there is high expression of ERV1 and low expression of BLT1 (Figure 2B, p<0.001), while in healthy individuals there is a lower expression of ERV1 and a higher expression of BLT1 (Figure 2 A–B). CD11b, CD14, CD18 expression by neutrophils from type 2 diabetes and healthy controls is not statistically different (Figure 2C–F), demonstrating a specific regulation of resolvin E1 receptors. Double positive (ERV1+/BLT1+) neutrophils were significantly increased in type 2 diabetes, whereas double negative neutrophils were not (Figure 2G, H). CD11b/CD14 double positive or double negative neutrophils were not different between groups (Figure 2I, J).
Figure 2. Human ERV-1 receptor is upregulated on type 2 diabetic neutrophils.
(A–C) The expression of ERV-1, BLT-1 and CD11b on human neutrophils was quantified by flow cytometry. (D) GIEMSA staining of isolated neutrophils showed similar morphology between the groups (upper panels) and immunofluorescence staining with anti-human ERV1 antibody (green) showed increased staining for ERV1 receptors. (E–F) CD18, CD14 receptor expression of healthy and diabetic neutrophils was quantified by flow cytometry. Results are expressed as mean fluorescence intensity (MFI). Statistical significance was evaluated by Wilcoxon test (n=82, healthy, n=41, T2D, n=41; ***p<0.001**** p<0.0001, ns = non-significant). Co-expression profile of ERV-1/BLT-1 receptor profile was quantified. (G) Double positive (ERV-1+/BLT-1+ neutrophils plotted and quantified by flow cytometry. (I) Double negative (ERV1−/BLT1−) neutrophils. (I–J) Double positive and negative CD11b/CD14 were quantified by flow cytometry. Results of marker co-expression are expressed as mean fluorescence intensity (MFI). Statistical significance was evaluated by Wilcoxon test (n=20, healthy, n=10, T2D, n=10; ***p<0.001**** p<0.0001, ns = non-significant).
ERV1 SIGNAL TRANSDUCTION
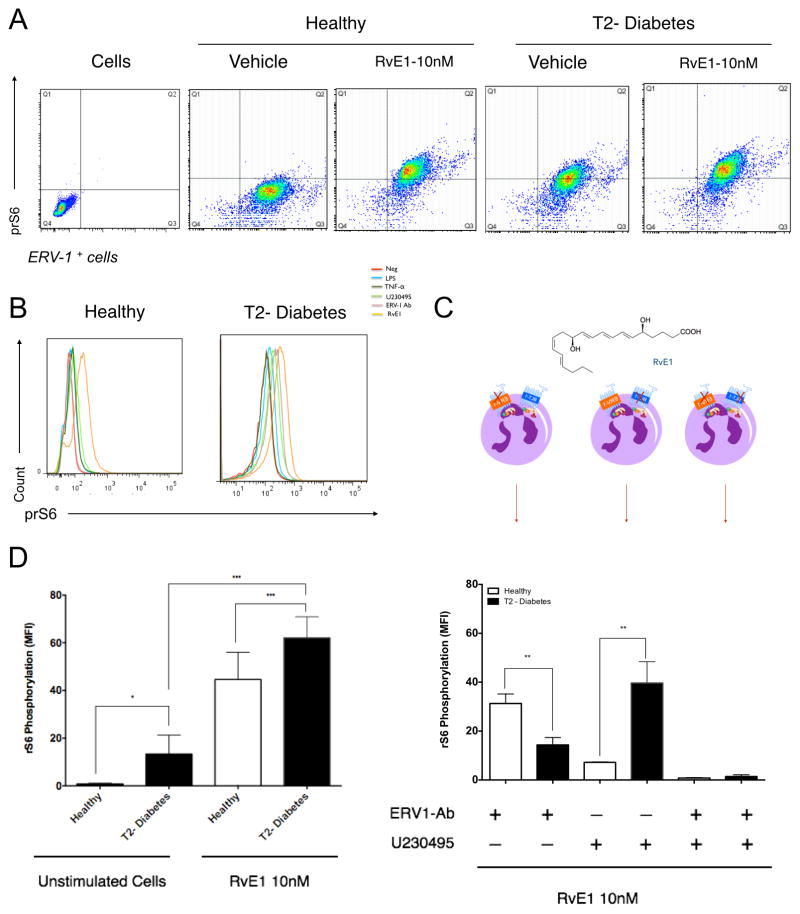
Stimulation of human macrophages transduces ERV1 signals through the Akt/ribosomal protein S6 (rS6)/mTOR pathway (36). In this study, we demonstrate that neutrophils are also stimulated with RvE1. ERV-1 and BLT-1 signal transduction through an identical pathway. We monitored phosphorylation of rS6 protein after alternatively blocking each receptor in order to determine which receptor(s) is active in T2D neutrophils. We found that, when ERV1+ neutrophils are incubated with resolvin E1 (10nM), intracellular rS6 signaling increases compared to baseline controls (Figure 3A). Due to the overexpression of the ERV1 receptor and rS6 phosphorylation in type 2 diabetes, we investigated the baseline rS6 phosphorylation levels of unstimulated cells. We quantified levels of phospho-rS6 in neutrophils from T2D compared to healthy controls (p<0.05, Figure 3B). RvE1 treatment significantly increased rS6 phosphorylation in both T2D and healthy neutrophils (p<0.001, Figure 3B) with T2D expression found to be significantly greater (p<0.001, Figure 3B, D). Neutrophils were stimulated with TNFα or LPS and blocking agents (ERV1 blocking antibody and the BLT1 antagonist U230495) to assess functional transduction of signals (Figure 3C). Our findings indicate that RvE1 activates rS6 phosphorylation through BLT1 in neutrophils from healthy donors and ERV1 in neutrophils from T2D subjects. Treating neutrophils with both blockers simultaneously completely obliterated rS6 phosphorylation in both T2D and healthy subject neutrophils, suggesting that BLT1 and ERV1 are the key receptors transducing RvE1 signals.
Figure 3. Signaling by rS6 phosphorylation is regulated through RvE1/ERV1 axis.
Activation of ERV1 receptor by RvE1 is through rS6 signaling. (A) RvE1 induces rS6 phosphorylation of healthy and diabetic neutrophils as demonstrated by gating ERV-1 positive cells (10nM, 30min). (B) To quantify phospho-rS6 expression of healthy and diabetic populations, unstimulated cells were evaluated and compared to RvE1 treated cells. (C) Phosphorylation of rS6 was measured after blocking ERV1 and BLT1 receptors. Cells were treated with RvE1 (10nM) alone, or in combination with BLT1 receptor antagonist (U-230495) or ERV1 receptor antagonist (ERV1/Ab) or both. (D) Cells were treated with various stimuli to investigate the specificity of rS6 phosphorylation LPS (10ng/ml), TNFα (10ng/ml), U-230495 (10nM), ERV1/Ab (10ng/ml), RvE1 (10nM). Results are expressed as mean fluorescence intensity (MFI, mean ± SEM). Statistical significance was evaluated by Wilcoxon test (n=16; healthy n=8, T2D, n=8, * p<0.05, ** p<0.01, *** p<0.001, ns = non-significant).
ERV1 FUNCTION
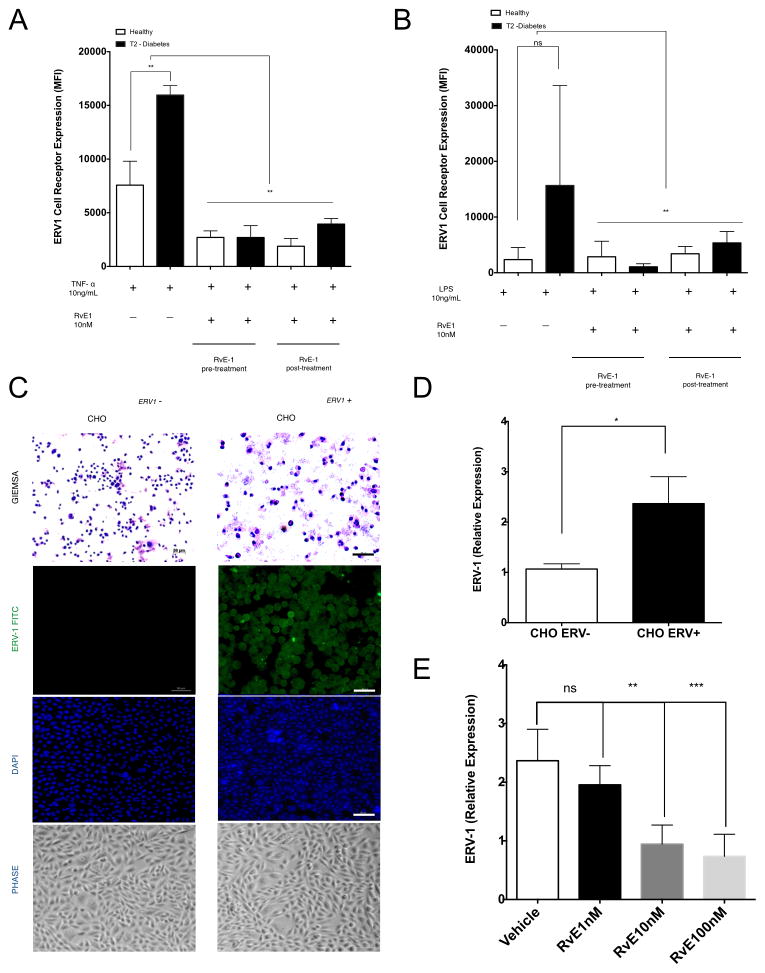
RvE1 stimulated healthy neutrophils have been shown to both signal via BLT1 and inhibit LTB4 function through competitive inhibition (29). Since surface BLT1 is downregulated and ERV1 is upregulated in T2D, we performed experiments to determine how ERV1 expression is regulated. We first exposed T2D and control neutrophils to the pro-inflammatory cytokine TNFα or bacterial LPS and then measured surface expression of ERV1 with FACS. When neutrophils are treated with TNFα (10ng/mL) for 1 hour, ERV1 protein expression increased in both T2D and healthy controls; T2D displayed significantly greater expression than controls (p<0.01, Figure 4A).
Figure 4. ERV1 receptor upregulation on neutrophils is stimulated by TNF-α and LPS, and reversed by RvE1.
(A) ERV1 expression was evaluated on neutrophils treated with TNFα (10ng/ml) alone or in combination with pre- and post- RvE1 treatment (10nM, 15 minutes before or after TNFα treatment). (B) ERV-1 was evaluated on LPS treated neutrophils (10ng/ml) alone or in combination with RvE1 (10nM) pre- and post- treatment (15 minutes before or after LPS treatment). Expression of ERV1 was quantified by immunofluorescence and flow cytometry. (C) ERV1 gene was transfected into Chinese hamster ovarian (CHO) cells (CHO ERV1+) and compared by PCR to mock transfection (CHO ERV−).(D) Human ERV1 transfected with cells (CHO ERV1+) were stained with Giemsa, FITC labeled anti-ERV1 and DAPI to demonstrate successful transfection compared to un-transfected (CHO ERV−). (E) mRNA levels of ERV1 cells were quantified by real time PCR. CHO ERV1+ and CHO ERV− were treated RvE1 (1–100nM) for 1 hour. Cells treated with RvE1 (10nM) or RvE1 (100nM) exhibited downregulation of ERV1. Cells were stained with alexafluor 488 labeled anti-human ERV1 or anti-IgG alexafluor 488 (isotype control). Results are expressed as mean fluorescence intensity (MFI, mean ± SEM) and mRNA levels (fold change). Statistical significance was evaluated by Wilcoxon test (n=20; healthy n=10, T2D, n=10 for A, B and n=5 for D, E, * p<0.05, ** p<0.01, *** p<0.001, ns = non-significant).
Interestingly, LPS stimulation did not upregulate ERV1 on healthy neutrophils (a slight reduction was observed), but significantly upregulated ERV1 on T2D neutrophils (p<0.01, Figure 4B). Neutrophils were also exposed to RvE1 15 minutes before or 15 minutes after TNFα or LPS. Under each condition, RvE1 treatment of neutrophils returned ERV1 surface expression to the levels of unstimulated healthy neutrophils (Figure 4A, 4B). When cells were treated with leukotriene B4 (LTB4), patterns remained similar to baseline, with a slight increase in healthy neutrophils. Fifteen minute pre-treatment or 15 minute post-treatment with RvE1 decreased ERV1 expression (Supplementary Figure 2). Since the kinetics of ERV1 clearance and re-expression are known (16, 29, 36, 45, 46) and not likely to account for downregulation of the receptor, we investigated transcriptional regulation to determine whether expression was controlled at the RNA level. We transfected Chinese Hamster Ovarian cells (CHO) with the human ERV1 receptor (designated CHO ERV1 +). Expression of ERV1 protein was confirmed compared to CHOERV1− by immunofluorescence (Figure 4C) and by qPCR (Figure 4D). We further confirmed that regulation was directly in response to RvE1 in a dose-response experiment (1–100nM RvE1). Lower concentrations of RvE1 (1nM) reduced the overexpression of ERV1 on CHO ERV1 + cells, (ns, Figure 4E). 10 nM RvE1 significantly reduced ERV1 expression (p<0.01, Figure 4E), as did 100nM RvE1 (p<0.01, Figure 4E).
LIPID MEDIATOR METABOLOLIPIDOMICS
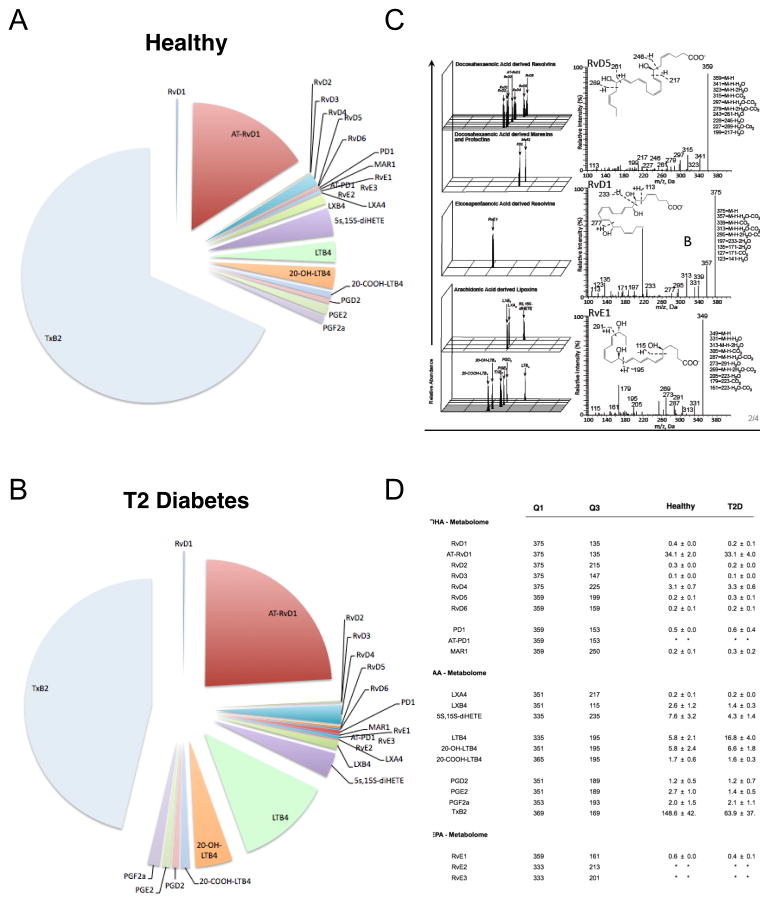
Sera were subjected to LC-MS-MS profiling in order to understand the profile of LM and SPM in peripheral blood from T2D vs. healthy subjects. Selected bioactive metabolomes of DHA, EPA and AA were analyzed (Figure 5A,B). MS-MS fragmentation spectra were employed for identification (Figure 5C). Lipid mediators from the DHA bioactive metabolome (RvD1-6, aspirin-triggered (AT)-RvD1, protectin D1 and maresin 1, MaR1) and the EPA bioactive metabolome (RvE1-3) were identified and quantified following established criteria (Colas et al., 2014). In these sera, we also identified and quantified mediators from the arachidonic acid (AA) bioactive metabolome including lipoxin (LX) A4, B4, leukotriene B4 (LTB4), and its further metabolites 20-OH-LTB4, 20-COOH-LTB4, prostaglandin (PG) D2, E2 and F2α, and thromboxane (TxB)2. Quantification of mediators from the EPA metabolome from healthy sera revealed: RvE1 at 0.6 ± .001 pg/ml; RvE2 and RvE3 were below limits of detection (Figure 5). In type 2 diabetic sera, a slight decrease in RvE1 levels was observed (0.4± 0.1 pg/ml), and RvE2 and RvE3 were similarly below limits of detection. Since endogenous RvE1 is a key target of this study, we further measured RvE1 by ELISA (Supplementary Figure 1). Results demonstrated a decrease of the lipid mediator in T2D (p<0.001, Figure 5D). The AA bioactive metabolome gave a remarkable increase in LTB4 in serum from T2D (16.8 ± 4.0 pg/ml) compared to healthy controls (5.8 ± 2.1 pg/ml, p<0.05, Figure 5B). TxB2 was higher in healthy serum compared to T2D (148.5 ± 63.9 pg/ml and 63.9± 37.7 pg/ml, respectively, p<0.001, Figure 5D). An increase of inflammatory mediators and a slight decrease in resolution mediators was found in the sera from T2D subjects, compared to control subjects (Figure 5).
Figure 5. Lipid mediator metabololipidomic profiling of healthy and diabetic serum lipid mediators of subjects with and without type 2 diabetes were evaluated.
(A–B) Proportions of lipid mediators from the bioactive AA, EPA and DHA metabolomes identified in Type 2 diabetes patient and healthy volunteer serum. (C) Representative MRM chromatograms of identified mediators in healthy and diabetic serum; left panel: fragmentation spectra, right panel: MS-MS fragmentation spectra employed in the identification of RvD5, RvE1 and RvE1. (D) Quantification of lipid mediators, where: Q1, M–H (parent ion); and Q3, diagnostic ion in the MS-MS (daughter ion), along with mean ± SEM values for healthy and diabetic mediators identified from serum. The detection limit was < 1 pg and * represents below detection limits. Results are expressed as mean ± SEM; n = 5 distinct serum preparations.
NEUTROPHIL PHAGOCYTOSIS
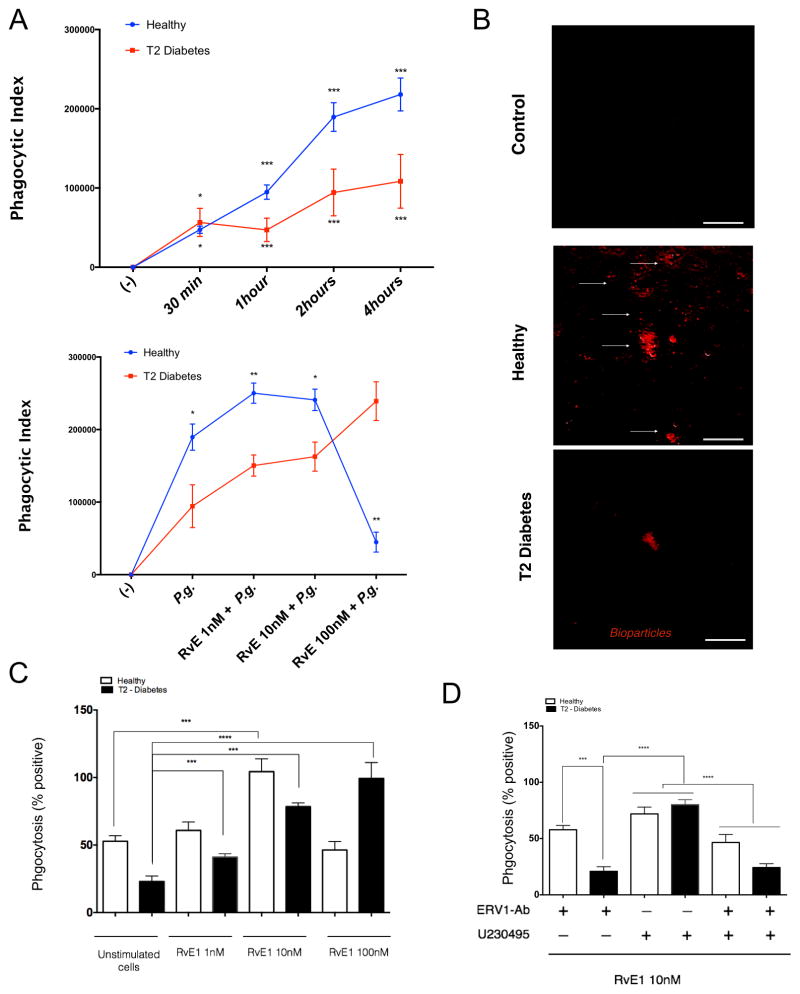
SPMs, like RvE1, have the ability to influence cell behavior and act as biochemical agonists, altering functional responses in infection and inflammation (47–51). Phagocytosis is a key biological process for neutrophils and macrophages in initial host response, promoting clearance and return to homeostasis. We have previously reported a deficiency of phagocytosis of the pathogen Porphyromonas gingivalis in type 2 diabetes (39, 41). To understand functional actions of neutrophils in T2D, we incubated cells with RvE1 and assayed phagocytosis in vitro with both bacterial pathogen and zymosan fluorescent bioparticles. In a kinetic assay, neutrophils from T2D exhibited decreased phagocytosis after 1–4 hours (Figure 6A, upper panel). Neutrophils were pretreated for 15 minutes with 1, 10, and 100 nM RvE1, and activation of ERV1 and phagocytosis were assessed (Figures 6A, lower panel). When compared to controls, diabetic neutrophils responded differently to RvE1 pretreatment in a dose-dependent manner (Figure 6A). The results were consistent when zymosan bioparticles were used (Figure 6B–D). For neutrophils from healthy subjects, low dose (1, 10 nM) RvE1 enhanced phagocytosis over baseline in a dose-response manner. Consistent with signaling results, phagocytosis is mediated through ERV-1 receptor activation in diabetes and BLT-1 in healthy neutrophils (Figure 6D). The 100nM RvE1 dose had no additive impact on normal neutrophil phagocytosis, suggesting an optimal dose range. For diabetic subjects, higher doses were required to achieve a similar response (Figure 6).
Figure 6. Neutrophil phagocytosis is mediated by ERV-1 activation.
Phagocytosis rate analysis of Porphyromonas gingivalis (Pg) and zymosan particles by peripheral blood neutrophils obtained from healthy and type 2 diabetic adult volunteers. Pg was labeled with BacLight and opsonized in heat inactivated normal serum 30 minutes at room temperature and incubated with neutrophils from both groups (MOI = 20:1). (A) The rate of phagocytosis of positive labeled bacteria was evaluated by flow cytometry. (B) Deep red labeled zymosan particles were incubated with neutrophils (1 hour) and evaluated by immunofluorescence microscopy. (C) The actions of RVE1 treatment (1–10 nM) on phagocytosis were quantified. (D) Labeled bioparticles were readily visible within neutrophils and expressed as mean ± SD mean fluorescence intensity. Phagocytic index was measured after blocking ERV1 and BLT1 receptors. Cells were treated with RvE1 (10nM) alone, or in combination with BLT1 receptor antagonist (U-230495) or ERV1 receptor antagonist (ERV1/Ab) or both. Phagocytosis experiments are expressed as phagocytic index (% positive x MFI). Statistical significance was evaluated by Wilcoxon test (n=24; healthy n=12, T2D, n=12, * p<0.05, ** p<0.01, *** p<0.001, ns = non-significant).
DISCUSSION
Neutrophils are essential cells for responding to inflammation under normal health and T2D conditions. Dysregulated immune cell functions are clearly seen in T2D, but the molecular basis remains ill-defined. We demonstrate that uncontrolled T2D impacts the phenotypic expression of the RvE1 receptor, ERV1, on peripheral blood neutrophils. ERV1 expression was originally reported as an active, signaling receptor on macrophages and dendritic cells (52). However, the expression and function of ERV1 on neutrophils in T2D and other chronic inflammatory diseases has not been previously investigated. Our findings show that ERV1 expression is low on healthy human neutrophils and BLT1 mediated signaling is dominant. Neutrophils isolated from people with poorly controlled T2D express markedly elevated ERV1 on their surface that is functionally transducing signals, while expression and signaling through BLT1 is significantly decreased (Figure 1G and 2A). T2D neutrophils exhibit a markedly reduced capacity for phagocytosis of bioparticles and pathogenic bacteria, which can be rescued with RvE1 treatment, but the concentrations of RvE1 necessary for activity far exceed those required for normal neutrophils from healthy subjects. Lipid mediator metabololipidomics of serum lipid mediators revealed a marked increase in LTB4 and decreased RvE1, as well as other specialized pro-resolving lipid mediators (Figure 5). Exogenous LTB4 did not significantly change the baseline levels of ERV1 on neutrophils in vitro.
Development of metabolic syndrome prior to the onset of T2D is characterized by changes in the metabolism of glucose and fatty acids, activating innate immune responses that give rise to systemic insulin resistance and establishing a state of chronic systemic inflammation (2, 4, 38). As a result, T2D is associated with deficiencies in clearing microbial infections, impaired phagocytosis and chronic inflammation. In this study cohort, individuals with T2D presented marginally increased neutrophil counts, high cholesterol, and high glucose levels (Figure 1). Increased inflammation in T2D is associated with prolonged activation of neutrophils, as demonstrated by gene expression profile (Figure 1G), consistent with an inefficient clearance of bacteria, consequently increasing susceptibility to infection. Additionally, inflammation has been implicated in other complications of T2D, including cardiovascular disease, retinopathy and a high incidence of oral manifestations such as periodontal diseases and multiple abscesses (39).
The exact mechanisms by which neutrophils fail to activate resolution cascades in T2D remain unknown. Chronic inflammation in diabetes and/or obesity influences cell phenotype. In this study, subgroup analysis of BMI revealed no association with ERV-1 or BLT-1 receptor expression. We have previously demonstrated that exogenous application of RvE1 in diabetic mice increased cellular functions, but the reason for failure of resolution remained unclear. Neutrophils are central to both the initiation and resolution phases of the acute inflammatory response, and both phases are tightly regulated by pro-resolution mediators, but receptor expression and function in chronic diseases yet to be explored. We previously reported that human neutrophils express ERV-1 (53) and, consistent with our findings (54), this has been confirmed. In this study we demonstrate that the RvE1 receptor ERV1 is upregulated by neutrophils from people with poorly controlled T2D (Figures 1G and 2A). This observation was specific to neutrophils, as monocytes did not show similar increases. The signaling pathways activated in neutrophils in response to RvE1 were inferred from work with monocyte/macrophages (36), but had not been directly demonstrated. We confirm that normal peripheral blood neutrophils also signal with rS6/mTOR pathways primarily through the BLT1 receptor. T2D neutrophils signal via rS6 as well, however the main receptor is ERV1. Interestingly, another ERV1 ligand, chemerin 15, actively signals in chronic coronary lesions, but chemerin did not regulate the expression patterns of the receptor (49). There is precedent for gain of function of ERV1 dependent on macrophage phenotype. As has been recently reported, ERV1 expression was found on functional M1, but not M2, macrophages (55). Thus, a proinflammatory phenotype favors high expression of ERV1 on neutrophils. We also considered the possibility that a significant difference found in age among our subjects (Figure 1E) was a cofounder in ERV-1 receptor profile. In previous studies aging has shown to influence resolution lipids, bit no information was found on resolution receptors (56). Here, pearson’s correlation analysis demonstrated that no positive association between age and ERV-1 and BLT-1 receptors, BMI and serum glucose (p>0.05). Age showed a positive correlation with cholesterol levels (p=0.006).
The hypothesis that increased inflammation was the main driver of ERV1 expression was tested. LPS and TNFα activation of neutrophils showed increases of ERV1 expression (Figure 4A and B) similar to what was seen at baseline for neutrophils from T2D; the increase was additive to the upregulation of neutrophils when compared to healthy controls. This conforms to prior publications, which showed neutrophils treated with TNFα, the formyl-methionyl peptide, FMLP and IL-8 rapidly upregulated ERV1 (54). Exogenous addition of RvE1 downregulates overexpression by both elevated baseline in diabetes and induced overexpression with LPS and TNFα. We also found that only neutrophil ERV1 was upregulated, not monocyte or lymphocyte (54). While previously published results demonstrated that anti-inflammatory ligands such as chemerin, annexin A1 and α-melanocyte stimulating hormone did not influence ERV1 surface expression, RvE1 was able to regulate receptor expression in a dose-response manner (Figure 3). The key question, whether ERV1 expression was dependent on RvE1 acting directly on the receptor, was addressed by expressing this receptor in CHO cells. When CHO ERV1 + cells were incubated with RVE1 (1–100nM), the mRNA expression decreased (Figure 4E). This indicates the dose-dependent influence of RvE1 lipid mediator ligand on ERV1 receptor levels.
The activation of ERV1 signaling is partially known, and upon ligand binding, rS6 phosphorylation signals cellular biological functions, including increased phagocytosis and reduced NFκB induced cytokine reduction. Upon exogenous RvE1 activation at therapeutic doses considerably higher than those found in serum, neutrophils responded with rS6 phosphorylation (Figure 3B). LPS and TNFα treatment influenced ERV1 expression and rS6 signaling (Figure 3C). Upon exogenous RvE1 activation, both overexpression of the receptor and increased phosphorylation were rescued. Understanding the molecular mechanisms that regulate expression and function of the resolution receptors ERV1 and BLT1 is relevant to exogenous RvE1 biological functions.
It is plausible that RvE1 increases the rate of phagocytosis of healthy neutrophils, but, because ERV1 expression levels were differentially regulated, the concentration needed to activate T2D neutrophils was unknown. The deficient phagocytosis observed in T2D is a clear hallmark and formation of ulcers, and periodontal abscesses are a major consequence of this deficiency. In this study, RvE1/ERV1 binding activated phagocytosis in a dose-response manner, with higher responses when 100nM was added to T2D neutrophils. In healthy individuals, the effective dose (1–10nM) is logarithmically lower than in T2D (Figure 6A, 6B). Interestingly, at the highest dosage in healthy neutrophils, the effect of RvE1 was attenuated. At the highest dosage in, T2D neutrophils, phagocytosis reached the peak response of normal neutrophils at a ten-fold lower dosage (Figure 6).
These findings demonstrate that loss of regulation of inflammation is a biological event common to human chronic diseases, including T2D. Understanding deficient pathways in the resolution of inflammation is critical for designing new therapeutics for treating individuals with T2D. The traditional focus when managing T2D has been the control of hyperglycemia and insulin, not the resolution of inflammation. Receptors of inflammation resolution should be included as potential markers for cell phenotype and possible therapeutic targets. The results of this study demonstrate that ERV1 is responsive to inflammatory stimuli and that the lipid mediator ligand RvE1 improves neutrophil-mediated inflammatory responses in uncontrolled T2D. Importantly, these results suggest that it is possible to correct deficient responses to RvE1 with added exogenous RvE1. Further studies are necessary to characterize ERV1 receptor dysfunction in other inflammatory disease models. Understanding these pathways and their regulation of disease is important for personalized medicine approaches for the treatment of inflammatory diseases.
Supplementary Material
Acknowledgments
The authors thank Paul Chung for his excellent technical assistance, and Laurie K. McCauley for helpful discussions.
FUNDING
This work was supported by USPHS grants K99/R00 DE 0235804 (to Freire, M), R01 DE025020 and DE025383 (to Van Dyke, T.E.) from the National Institutes of Dental and Craniofacial Research and P01GM095467 (to Serhan, C.N.). This work was performed at the Forsyth Institute and at the Lipid Mediator Metalolipidomics Core, Brigham and Women’s Hospital, Harvard Medical School.
Footnotes
AUTHOR CONTRIBUTIONS
MOF designed the studies and wrote the manuscript. MOF, JD, CNS, TVD performed experiments, acquired and/or analyzed data, and assisted in manuscript preparation.
CONFLICT OF INTEREST
The authors have declared no conflicts of interest.
References
- 1.Maertens JA, Raad II, Marr KA, Patterson TF, Kontoyiannis DP, Cornely OA, Bow EJ, Rahav G, Neofytos D, Aoun M, Baddley JW, Giladi M, Heinz WJ, Herbrecht R, Hope W, Karthaus M, Lee DG, Lortholary O, Morrison VA, Oren I, Selleslag D, Shoham S, Thompson GR, 3rd, Lee M, Maher RM, Schmitt-Hoffmann AH, Zeiher B, Ullmann AJ. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387:760–769. doi: 10.1016/S0140-6736(15)01159-9. [DOI] [PubMed] [Google Scholar]
- 2.Guariguata L, Whiting D, Weil C, Unwin N. The International Diabetes Federation diabetes atlas methodology for estimating global and national prevalence of diabetes in adults. Diabetes research and clinical practice. 2011;94:322–332. doi: 10.1016/j.diabres.2011.10.040. [DOI] [PubMed] [Google Scholar]
- 3.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 4.Li P, Hotamisligil GS. Metabolism: Host and microbes in a pickle. Nature. 2010;464:1287–1288. doi: 10.1038/4641287a. [DOI] [PubMed] [Google Scholar]
- 5.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nature reviews Immunology. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 7.Serhan CN, Drazen JM. Antiinflammatory potential of lipoxygenase-derived eicosanoids: a molecular switch at 5 and 15 positions? J Clin Invest. 1997;99:1147–1148. doi: 10.1172/JCI119268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fierro IM, Serhan CN. Mechanisms in anti-inflammation and resolution: the role of lipoxins and aspirin-triggered lipoxins. Braz J Med Biol Res. 2001;34:555–566. doi: 10.1590/s0100-879x2001000500002. [DOI] [PubMed] [Google Scholar]
- 9.Serhan CN, Chiang N. Lipid-derived mediators in endogenous anti-inflammation and resolution: lipoxins and aspirin-triggered 15-epi-lipoxins. Scientific World Journal. 2002;2:169–204. doi: 10.1100/tsw.2002.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. The Journal of experimental medicine. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Dyke TE, Kornman KS. Inflammation and factors that may regulate inflammatory response. Journal of periodontology. 2008;79:1503–1507. doi: 10.1902/jop.2008.080239. [DOI] [PubMed] [Google Scholar]
- 12.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 13.Bannenberg GL, Aliberti J, Hong S, Sher A, Serhan C. Exogenous pathogen and plant 15-lipoxygenase initiate endogenous lipoxin A4 biosynthesis. The Journal of experimental medicine. 2004;199:515–523. doi: 10.1084/jem.20031325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serhan CN, Chiang N. Novel endogenous small molecules as the checkpoint controllers in inflammation and resolution: entree for resoleomics. Rheum Dis Clin North Am. 2004;30:69–95. doi: 10.1016/S0889-857X(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 15.Serhan CN, Gotlinger K, Hong S, Arita M. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their aspirin-triggered endogenous epimers: an overview of their protective roles in catabasis. Prostaglandins Other Lipid Mediat. 2004;73:155–172. doi: 10.1016/j.prostaglandins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. The Journal of experimental medicine. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwab JM, Serhan CN. Lipoxins and new lipid mediators in the resolution of inflammation. Curr Opin Pharmacol. 2006;6:414–420. doi: 10.1016/j.coph.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Tjonahen E, Oh SF, Siegelman J, Elangovan S, Percarpio KB, Hong S, Arita M, Serhan CN. Resolvin E2: identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem Biol. 2006;13:1193–1202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, Hellstrom A, Kang JX, Chew EY, Salem N, Jr, Serhan CN, Smith LE. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, Serhan CN, Ji RR. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16:592–597. doi: 10.1038/nm.2123. 591p following 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim TH, Kim GD, Jin YH, Park YS, Park CS. Omega-3 fatty acid-derived mediator, Resolvin E1, ameliorates 2,4-dinitrofluorobenzene-induced atopic dermatitis in NC/Nga mice. Int Immunopharmacol. 2012;14:384–391. doi: 10.1016/j.intimp.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Mas E, Croft KD, Zahra P, Barden A, Mori TA. Resolvins D1, D2, and Other Mediators of Self-Limited Resolution of Inflammation in Human Blood following n-3 Fatty Acid Supplementation. Clin Chem. 2012;58:1476–1484. doi: 10.1373/clinchem.2012.190199. [DOI] [PubMed] [Google Scholar]
- 24.Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012;26:1755–1765. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serhan CN, Chiang N, Dalli J, Levy BD. Lipid mediators in the resolution of inflammation. Cold Spring Harbor perspectives in biology. 2015;7:a016311. doi: 10.1101/cshperspect.a016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brink C, Dahlen SE, Drazen J, Evans JF, Hay DW, Nicosia S, Serhan CN, Shimizu T, Yokomizo T. International Union of Pharmacology XXXVII. Nomenclature for leukotriene and lipoxin receptors. Pharmacol Rev. 2003;55:195–227. doi: 10.1124/pr.55.1.8. [DOI] [PubMed] [Google Scholar]
- 28.Aoki H, Hisada T, Ishizuka T, Utsugi M, Kawata T, Shimizu Y, Okajima F, Dobashi K, Mori M. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem Biophys Res Commun. 2008;367:509–515. doi: 10.1016/j.bbrc.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. Journal of immunology. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 30.Campbell EL, Louis NA, Tomassetti SE, Canny GO, Arita M, Serhan CN, Colgan SP. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. FASEB J. 2007;21:3162–3170. doi: 10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- 31.El Kebir D, Gjorstrup P, Filep JG. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:14983–14988. doi: 10.1073/pnas.1206641109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. Journal of immunology. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 33.Hasturk H, Kantarci A, Ohira T, Arita M, Ebrahimi N, Chiang N, Petasis NA, Levy BD, Serhan CN, Van Dyke TE. RvE1 protects from local inflammation and osteoclast-mediated bone destruction in periodontitis. FASEB J. 2006;20:401–403. doi: 10.1096/fj.05-4724fje. [DOI] [PubMed] [Google Scholar]
- 34.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong S, Porter TF, Lu Y, Oh SF, Pillai PS, Serhan CN. Resolvin E1 metabolome in local inactivation during inflammation-resolution. Journal of immunology. 2008;180:3512–3519. doi: 10.4049/jimmunol.180.5.3512. [DOI] [PubMed] [Google Scholar]
- 36.Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, Serhan CN. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J Biol Chem. 2010;285:3451–3461. doi: 10.1074/jbc.M109.044131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schif-Zuck S, Gross N, Assi S, Rostoker R, Serhan CN, Ariel A. Saturated-efferocytosis generates pro-resolving CD11b low macrophages: modulation by resolvins and glucocorticoids. European journal of immunology. 2011;41:366–379. doi: 10.1002/eji.201040801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spranger J, Kroke A, Mohlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 39.Herrera BS, Hasturk H, Kantarci A, Freire MO, Nguyen O, Kansal S, Van Dyke TE. Impact of resolvin E1 on murine neutrophil phagocytosis in type 2 diabetes. Infection and immunity. 2015;83:792–801. doi: 10.1128/IAI.02444-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Micro. 2012;10:717–725. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Dyke T, Herrera B, Hasturk H, Kantarci A. RvE1 and phagocytosis of Porphyromonas gingivalis by PMN in type 2 diabetes (P4227) The Journal of Immunology. 2013;190:130.113. [Google Scholar]
- 42.American Diabetes, A. 2. Classification and Diagnosis of Diabetes. Diabetes care. 2016;39(Suppl 1):S13–22. doi: 10.2337/dc16-S005. [DOI] [PubMed] [Google Scholar]
- 43.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. The Journal of experimental medicine. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120:e60–72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishida T, Yoshida M, Arita M, Nishitani Y, Nishiumi S, Masuda A, Mizuno S, Takagawa T, Morita Y, Kutsumi H, Inokuchi H, Serhan CN, Blumberg RS, Azuma T. Resolvin E1, an endogenous lipid mediator derived from eicosapentaenoic acid, prevents dextran sulfate sodium-induced colitis. Inflamm Bowel Dis. 2010;16:87–95. doi: 10.1002/ibd.21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishizuka T, Hisada T, Aoki H, Mori M. Resolvin E1: a novel lipid mediator in the resolution of allergic airway inflammation. Expert Rev Clin Immunol. 2008;4:669–672. doi: 10.1586/1744666X.4.6.669. [DOI] [PubMed] [Google Scholar]
- 47.Cotran RS. Endothelial Phagocytosis: An Electron-Microspopic Study. Exp Mol Pathol. 1965;28:217–231. doi: 10.1016/0014-4800(65)90034-1. [DOI] [PubMed] [Google Scholar]
- 48.Ramon S, Bancos S, Serhan CN, Phipps RP. Lipoxin A(4) modulates adaptive immunity by decreasing memory B-cell responses via an ALX/FPR2-dependent mechanism. European journal of immunology. 2014;44:357–369. doi: 10.1002/eji.201343316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Widmann JJ, Cotran RS, Fahimi HD. Mononuclear phagocytes (Kupffer cells) and endothelial cells. Identification of two functional cell types in rat liver sinusoids by endogenous peroxidase activity. J Cell Biol. 1972;52:159–170. doi: 10.1083/jcb.52.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samson M, Edinger AL, Stordeur P, Rucker J, Verhasselt V, Sharron M, Govaerts C, Mollereau C, Vassart G, Doms RW, Parmentier M. ChemR23, a putative chemoattractant receptor, is expressed in monocyte-derived dendritic cells and macrophages and is a coreceptor for SIV and some primary HIV-1 strains. European journal of immunology. 1998;28:1689–1700. doi: 10.1002/(SICI)1521-4141(199805)28:05<1689::AID-IMMU1689>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 53.Freire MO, ND, Van Dyke TE. IADR/AADR. Journal of Dental Research; Seattle, Washington: 2013. ChemR23 Expression and Function in Chronic Inflammation. [Google Scholar]
- 54.Cash JL, Bena S, Headland SE, McArthur S, Brancaleone V, Perretti M. Chemerin15 inhibits neutrophil-mediated vascular inflammation and myocardial ischemia-reperfusion injury through ChemR23. EMBO reports. 2013;14:999–1007. doi: 10.1038/embor.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herova M, Schmid M, Gemperle C, Hersberger M. ChemR23, the receptor for chemerin and resolvin E1, is expressed and functional on M1 but not on M2 macrophages. Journal of immunology. 2015;194:2330–2337. doi: 10.4049/jimmunol.1402166. [DOI] [PubMed] [Google Scholar]
- 56.Arnardottir HH, Dalli J, Colas RA, Shinohara M, Serhan CN. Aging Delays Resolution of Acute Inflammation in Mice: Reprogramming the Host Response with Novel Nano-Proresolving Medicines. The Journal of Immunology. 2014;193:4235–4244. doi: 10.4049/jimmunol.1401313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.