Abstract
Objectives
Proton pump inhibitors (PPIs) have been used for treatment of Barrett's esophagus (BE) for many years. However, the connection between PPIs and esophageal adenocarcinoma (EAC) in patients with BE has still been controversial. The current systematic review and meta-analysis was designed to evaluate the association between PPIs and the risk of EAC or high-grade dysplasia (HGD) in patients with BE.
Methods
A systematic literature search of studies reporting the association between PPIs and the risk of EAC and/or HGD in patients with BE was conducted in PubMed, Embase, Web of Science and the Cochrane Library. Next, literature was screened using previously established criteria and relevant data were extracted from included studies. Finally, the software program Review Manage 5.2 was applied to aggregate data and analyze the results.
Results
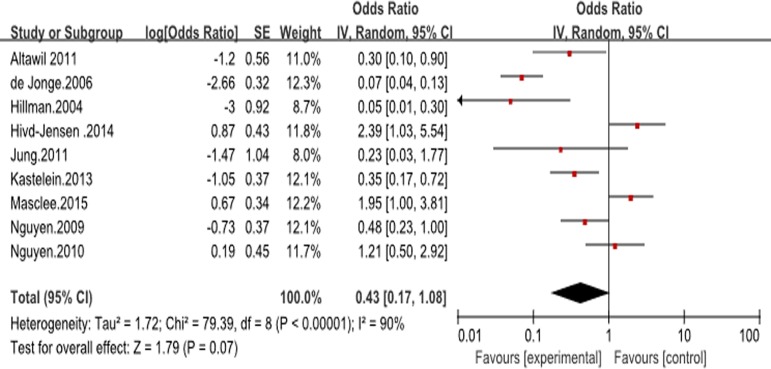
Nine observational studies, comprising five cohort and four case-control studies (including a total of 5712 patients with BE), were identified. Upon meta-analysis, PPIs were found to have no association with the risk of EAC and/or HGD in patients with BE (unadjusted OR 0.43, 95% CI 0.17–1.08). Analysis for duration response relationship revealed no significant trend toward protection against EAC or HGD with PPIs usage for >2~3 years (one study using 7-year cutoff) when compared to usage for shorter time periods (PPIs usage >2~3 years vs. <2~3 years: OR 0.91 (95% CI 0.25–3.31) vs. 0.91 (0.40–2.07)).There also was considerable heterogeneity between studies.
Conclusion
No dysplasia- or cancer-protective effects of PPIs usage in patients with BE were identified by our analysis. Therefore, we conclude that clinicians who discuss the potential chemopreventive effects of PPIs with their patients, should be aware that such an effect, if exists, has not been proven with statistical significance.
Introduction
Barrett’s esophagus (BE) is a condition in which the stratified squamous epithelium (SSE) of the distal esophagus under goes intestinal metaplasia (transformation to specialized columnar epithelium) [1]. Approximately 10% of patients with several longstanding chronic gastroesophageal reflux disease (GERD) will eventually develop BE as a complication of GERD [2]. In the general population, the prevalence of BE is estimated at 1–2%, with white males over 60 years of age predominantly affected [3]. As BE is the single most important risk factor for the development of esophageal adenocarcinoma (EAC), and since the incidence of EAC has increased exponentially over the past 3 decades, increased attention has been focused on preventing the progression from BE to EAC or its immediate precursor lesion, high-grade dysplasia (HGD) [4–7].
Proton pump inhibitors (PPIs) are the most commonly prescribed class of medications that are used for treating GERD. PPIs are quite effective and remarkably safe [8]. There have been limited studies and debate regarding the effect of such treatment on the risk of progression to dysplasia cancer. On the one hand, reduction of esophageal acid exposure by PPIs decreases inflammation and proliferation. Additionally, proposed beneficial effects of PPIs include anti-oxidant properties [9], effects on neutrophils, endothelial cells, epithelial cells [10], and anti-apoptotic cell modulation [11]. Moreover, PPIs are thought to inhibit binding to adhesion molecules in malignant cells and to suppress metastasis[12].On the other hand, PPIs therapy interferes with esophageal exposure to secondary bile acids, and increases circulating gastrin levels, which may induce proliferation, COX-2 upregulation, and perhaps expansion of metaplasia. It has been suggested that PPIs may promote the development of Barrett’s metaplasia and its progression to dysplasia or cancer [13]. Epidemiological studies of the association between PPIs and EAC risk have also yielded conflicting conclusions. Some studies have suggested that PPIs exert a protective effect against progression from BE to EAC. Singh et al carried out a previous meta-analysis whose results also suggested a protective effect of PPIs [14]. However, since an accumulating number of increasingly inconsistent results have now been reported, we felt the need to again carefully analyze existing data to formulate an objective overview of this topic.
Methods
Study identification
To identify relevant studies, we used the Preferred Reporting Items for Systematic reviews and Meta-Analyses for Protocols 2009 (PRISMA- 2009) for this systematic review and meta-analysis (S1 Table). We conducted a systematic literature search of Medline, Embase, Web of Science and the Cochrane Library, with retrieval until January 2016. By combining the use of medical subject heading terms and keywords, including ‘proton pump inhibitor*’, ‘PPI’, ‘acid suppress*’, ‘omeprazole’,‘pantoprazole’, ‘esomeprazole’, ‘lansoprazole’, ‘rabeprazole’, ‘dexlansoprazole’ AND ‘barrett’s’ OR‘oesophageal’ AND ‘neoplasia’, ‘high-grade dysplasia’, ‘oesophageal adenocarcinoma’, we searched randomized controlled trials (RCTs) or observational studies (cohort and case–control design) in the above databases. This computer search was also supplemented by manual searches of the reference lists of all retrieved studies, review articles and conference abstracts. Following these searches, based on specified inclusion criteria (research object being patients with BE, study compared differences in incidence of HGD or EAC after taking PPIs vs. not) and exclusion criteria (non-observational studies, studies without knowledge of BO status, studies without sufficient information on progression to OAC or BO-HGD, and studies comparing medical and surgical therapy for GERD and BO), two authors (Q.H and TT.S) independently screened the retrieved documents by browsing the title and abstract. Inclusion was not otherwise restricted by study size, language or publication type. We excluded letters to the editor, review articles, case reports and animal experimental studies. When multiple reports describing the same population cohort were published, the most recent or complete report was used. Differences were resolved by discussion or consultation with a third researcher (H.X) as needed. Since our study was a review of previous published studies, ethical approval or patient consent was not required.
Data extraction and quality assessment
After study identification, data from the included studies were extracted and summarized independently by two of the authors (Q.H and TT.S). We collected clinical information including patient characteristics, time and dosage of PPIs, potential confounding variables, and estimates of association. By using non-PPI users as a reference, we measured the association between patients exposed to PPIs for a specified time (<2~3 years or >2~3 years) vs. non-use to estimate duration–response relationship. Any disagreement was resolved by senior authors (H.X). We used the Newcastle–Ottawa scale to evaluate the methodological quality of case–control and cohort studies [15]. The Newcastle-Ottawa scale consists of three factors: patient selection, comparability of study groups, and assessment of outcome. A score of 0–9 (allocated as stars) was allocated to each study. Due to the theme of some included studies is not at all the same with this meta-analysis, we combine NOS and the level of theme similarity in these studies with this meta-analysis for study quality assessment. Any discrepancies were addressed by a joint re-evaluation of the original article.
Outcomes assessed
Not only did we assess the risk of progression to EAC and/or HGD in patients with BE between PPIs users and non-users, but we also analyzed effects of time and dosage of PPIs on progression to EAC and/or HGD. Based on the time of PPI usage (>2~3 years vs. <2~3 years), study design (cohort vs. case–control), number of outcomes (>60vs. <60) and mean follow-up time (>5years vs. <5years or not recorded), we performed pre-planned subgroup analysis. To assess the presence of a reflux-independent association between PPI usage and risk of progression to EAC and/or HGD, we performed an analysis restricted to studies which adjusted for the presence of erosive esophagitis or reflux symptoms; Similarly, we restricted our analysis to studies which adjusted for concomitant use of non-steroidal anti-inflammatory drugs (NSAIDs)/aspirin or statins to assess the presence of any independent chemopreventive association. Due to time-related biases have affected several observational studies reporting impressive results on the effectiveness of certain medications in reducing the incidence of major disease outcomes, we made a detailed analysis regarding the risk of time-related biases in the individual studies according to the methods provided by literatures [16, 17].
Statistical analysis
All analyses were performed using Review Manager 5.2(Cochrane Collaboration, Oxford, UK). Because two studies contributing to the estimate reported only the odds ratio (OR) and their 95% confidence intervals (CIs) [18, 19], we used the generic inverse variance method to include data in this meta-analysis. Since outcomes (i.e., progression to HGD/EAC) were relatively rare, Odds ratios (ORs) were considered approximations of RRs or HRs, and ORs were used to compare dichotomous variables. All results were reported with 95% CI. Statistical heterogeneity between studies was assessed using the chi-square test with significance set at p < 0.10, and heterogeneity was quantified using the I2 statistic. The random-effects model was used if the value of I2 was >70% between studies; otherwise, the fixed-effects model was used. Given the small number of studies identified in our analysis, statistical tests for assessing publication bias were not performed [20].
Results
Study selection and characteristics
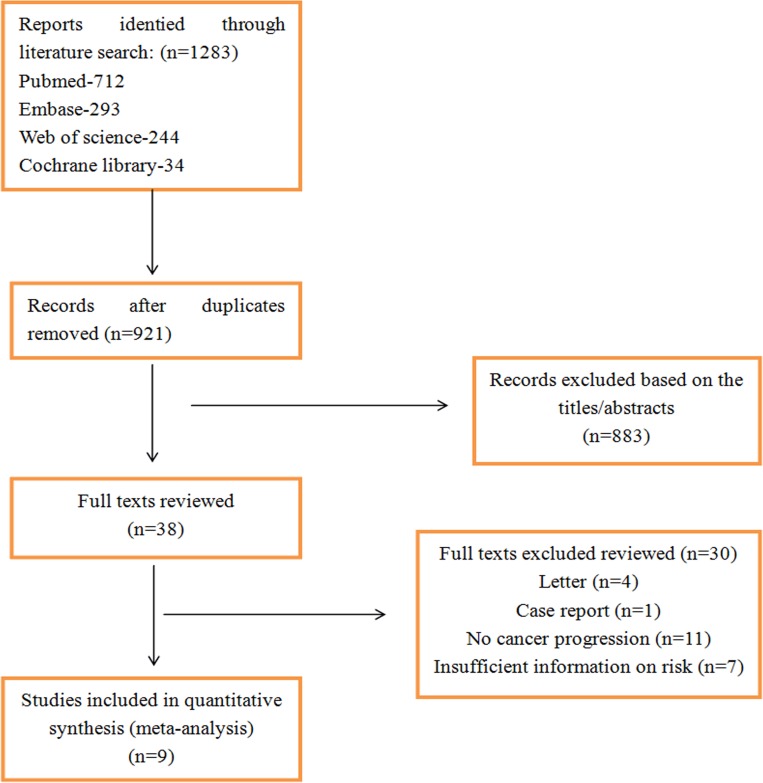
In our initial search, we identified 1,283 article records in databases. After removing duplicate documents, 921 articles were identified. We browsed titles and abstracts to further exclude irrelevant literature. After this browsing, there were 38 potentially eligible studies assessed for inclusion. Finally, nine studies (five cohort [18, 19, 21–23] and four case-control [24–27] studies) satisfied our stringent inclusion criteria. The study flow is shown in Fig 1. The principal characteristics of the 9 included studies are presented in Table 1. Among them, four were conducted in the USA, three in the Netherlands, one in Australia, one in the UK, and one in Denmark. Sample sizes ranged from 77 to 1437(total 5712), of whom 501 progressed to EAC and/or HGD. The mean age of patients at the time of BE diagnosis ranged from 58 to 65 years, and approximately 73.1% of patients with BE were men. Only three studies interpreted study population race [21, 24, 25]. In addition to the use of PPIs, a proportion of patients also used non-steroidal anti-inflammatory drugs (NSAIDs)/aspirin and statins.
Fig 1. Flow diagram showing results of search and reasons for exclusion of studies.
Table 1. Characteristics of individual studies included in the search.
| Study | Location | Time period; follow up | Age at BE diagnosis | Sex (%men) | Race (%Caucasian) | Obesity (%with BMI>30kg/m2) | Smoking (% smokers) | Length of BE (% with LSBE) |
|---|---|---|---|---|---|---|---|---|
| Cohort studies | ||||||||
| Hillman.2004 | Canberra,Australia; | 1981–2001; median4.7years | 58 (12) | 71 | NR | NR | NR | 45.0 |
| Nguyen.2009 | Arizona, USA; | 1982–2004; mean 7.6years | 61 (12) | 94 | 90 | NR | NR | 29.1 |
| Altawil.2011 | Michigan, USA; | 2004–2010; NR | 60 | 96 | 75 | 28.9 | NR | NR |
| Jung.2011 | Minnesota, USA; | 1976–2006; median 5.9years | 63 (14) | 69 | NR | 29 | 13 (current) | 59 |
| Kastelein .2013 | Rotterdam, The Netherlans; | 2003–2009; mean 5.2years | 61 (53–68) | 71 | NR | 19 | 19 (current) | 100 |
| Case-control studies | ||||||||
| de Jonge .2006 | Rotterdam, The Netherlans; | 2003–2005; NA | 62 (11) | 74 | 100 | 27 | 20 | NR |
| Nguyen .2010 | Nationwide VA,USA; | 2000–2002; NA | 65 (10) | 97 | 74 | NR | NR | NR |
| Hivd-Jensen .2014 | Nationwide Denmark | 1995to2009 median 10.2 years | 62.6 52.4–72.9 | 66.5 | NR | NR | NR | NR |
| Masclee.2015 | UK | 1996to2011 | 64.8 (SD13.8) | 63 | NR | 18.4 | 11.4 (current) | NR |
| The Netherlans | 1996to2012 | 61.2 (SD13.4) | 62 | NR | 11.4 | 49.5 (current) | NR |
Quality assessment
Due to the fact that our included studies were either cohort or case-control, we applied the Newcastle-Ottawa scale to assess their methodological quality. In overall quality score (maximum = 9), according to the scores of specific tables, the methodological quality of the included studies was moderate to high, ranging from 6 to 9. After combining with the level of theme similarity in these studies with this meta-analysis, we divided these included studies into high quality [21, 22, 25–27] and moderate quality [18, 19, 23, 24]. Only four studies assessed obesity in BE patients [19, 22, 24, 26]. Three studies accounted for reflux symptoms [19, 22, 24] and four for erosive esophagitis [19, 22, 24, 27].
PPI use and the risk of advanced neoplasia
With the incidence of EAC and/or HGD as an endpoint, no significant difference was identified between PPI users and non-users (unadjusted OR 0.43, 95% CI 0.17–1.08) (Fig 2). In six studies, which reported the time to progression to EAC or HGD in a cohort of patients with BE, PPI users were also not significantly different from nonusers (HR 0.61, 95% CI 0.28–1.34) [17, 19, 21, 22, 27, 28]. Only three studies assessed the association of PPI dosage with the risk of progression to EAC and/or HGD (defined daily dose [DDD] as evaluation reference), however, all 3 of these studies showed no statistical significance [22, 26, 27]. There was insufficient information in these studies to allow estimation of PPIs’ effect on the risk of progression to EAC alone or to HGD alone. Considerable heterogeneity was observed in the overall analysis (I2 = 90%), although this was primarily due to the different sample sizes: when the sample size was less than 600, the use of PPIs was strongly associated with a lower risk of dysplasia in patients with BE (OR 0.20, 95% CI 0.09–0.46; I2 = 77%) [18, 19, 21–24]. In contrast, three studies in which the sample sizes were more than 600, showed an inconsistent result (OR 1.83, 95% CI 1.16–2.86; I2 = 0%)[25, 27, 28]. The use of PPIs and other medication and the incidence of EAC and/or HGD in included studies are particularly showed in Table 2.
Fig 2. Forest plot of assessing the effects of PPIs on the patients with Barrett’s esophagus (BE) and the risk of esophageal adenocarcinoma (EAC) and/or high-grade dysplasia (HGD) in all included studies.
Table 2. The use of PPIs and other medication and the incidence of EAC and/or HGD in included studies.
| Study | Total no. Of patients with BE with baseline dysplasia status | Incident EAC and/or HGD | Patients on PPI | Patients not on PPI | Reflux symptoms; endoscopic esophagitis | Other medication use | |||
|---|---|---|---|---|---|---|---|---|---|
| Incident EAC and/or HGD | Total no. Of patients on PPI | Incident EAC and/or HGD | Total no. Of patients not on PPI | NSAIDs/aspirin | Statins | ||||
| Cohort studies | |||||||||
| Hillman.2004 | 350 NDBE—85.4% LGD—14.6% | HGD—9 EAC—7 Combined—11 | NR | NR | NR | NR | NR; 88% | 78 (22.0%) | NR |
| Nguyen.2009 | 344 NDBE—100% LGD—0 | HGD—20 EAC—13 Combined—33 | 17 | 231 (67.2%) | 16 | 113 (32.8%) | NR | 169 (49.1%) | 87 (25.3%) |
| Altawil.2011 | 77 NDBO—100% LGD—0 | 17 | 7 | 49 | 10 | 28 | NR | 20 (26.0%) | 27 (35.1%) |
| Jung.2011 | 355 NDBE—83% LGD—17% | HGD—12 EAC—7 Combined—19 | NR | NR | NR | NR | 77%; 31% | NR | NR |
| Kastelein.2013 | 540 NDB—86% LGD—14% | HGD—28 EAC—12 Combined—40 | 28 | 462 (85.6%) | 12 | 78 (14.4%) | 29%; 9% | 110 (20.4%) | 102 (18.9%) |
| Case-control studies | |||||||||
| de Jonge.2006 | 335 | EAC—91 | 43 | 270 (81.6%) | 44 | 61 (18.4%) | 72.5%; NR | 134 (40.0%) | NR |
| Nguyen.2010 | 812 | EAC—116 | 110 | 763 (94.0%) | 6 | 49 (6.0%) | NR | 468 (57.6%) | 377 (46.4%) |
| Hivd-Jensen .2014 | 1437 NDBE—89.8% LGD—10.2% | HGD—80 EAC—60 Combined—140 | 134 | 1306 | 6 | 131 | NR | 966(67.2%);439(30.5%) | 250 (17.4%) |
| Masclee.2015 | 1466 | 57 | 46 | 1005 | 11 | 461 | NR;4% | 128(22.8%);183(26.3%) | 248 (35.6%) |
| NR;30% | 104(13.5%);48(6.2%) | 126 (16.4%) | |||||||
Subgroup and sensitivity analysis
When exposure time intervals were dichotomized as either short term (<2~3 years) or long term (>2~3 years), the association between PPI usage and risk of EAC or HGD was not statistically significant (Table 3). When classified by study design, case-control studies showed a result (OR 0.78, 95% CI 0.13–4.7) similar to all studies, however, cohort studies, though accounting for 29% of the total sample size, suggested a protective association between PPIs and the risk of EAC and/or HGD (OR 0.31, 95% CI 0.18–0.54). Since most of patients in the entire BE cohort were taking PPIs, statistically significant risk estimates were not noted. Regarding assessment of time-related biases, we had not found any of them in these four studies [22, 25–27]. One study existed immortal time bias, but we could not use a proper person-time approach to eliminate this bias from its provided data [21]. The data from other four studies were such unspecific that we could not evaluate whether or not they existed time-related biases [18, 19, 23, 24]. We also systematically excluded each study from the main summary estimate to assess whether any single study had a dominant effect on the summary OR. Result revealed that no single study markedly affected the summary estimate or p value for heterogeneity among the other summary estimates, and the pooled point estimate remained statistically insignificant (range 0.16–1.51), with the corresponding 95% CI bounds including 1.
Table 3. Subgroup analyses and duration–response relationship on the association of PPIs use and risk of EAC and/or HGD in patients with BE.
| Groups | Categories | No. of studies | Adjusted OR | 95% CI | Heterogeneity within groups (I2) | P interaction |
|---|---|---|---|---|---|---|
| Study design | Cohort | 5 | 0.31 | [0.18, 0.54] | 26 | 0.34 |
| Case–control | 4 | 0.78 | [0.13, 4.70] | 96 | ||
| Number of outcomes | <60 | 6 | 0.39 | [0.16, 0.95] | 79 | 0.07 |
| >60 | 3 | 0.58 | [0.06, 5.81] | 96 | ||
| mean follow-up time | <5years or no record | 5 | 0.20 | [0.04, 0.94] | 90 | 0.13 |
| >5years | 4 | 0.80 | [0.33, 1.94] | 81 | ||
| Duration–response | <2~3 years | 5 | 0.91 | [0.40, 2.07] | 80 | 1.00 |
| >2~3years | 5 | 0.91 | [0.25, 3.31] | 92 | ||
| Study quality | High | 5 | 0.98 | [0.46, 2.10] | 80 | <0.001 |
| Moderate | 4 | 0.12 | [0.05, 0.29] | 53 | ||
| Time related bias | No | 4 | 1.18 | [0.49, 2.85] | 81 | 0.005 |
| Yes or unclear | 5 | 0.17 | [0.06, 0.48] | 78 |
Discussion
This systematic review and meta-analysis of nine observational studies was performed to evaluate the effect of PPIs on progressing to HGD and/or EAC in patients with BE. In this meta-analysis, published studies included a total of 5712 patients with non-dysplastic BE (or LGD), of whom 501 progressed to EAC and/or HGD. Our results conflict with results of previous studies, most of which reported an inverse relationship between PPI use and the risk of neoplastic progression, as well as a decreased risk of neoplastic progression with prolonged PPI use. A previous meta-analysis on this topic was performed by Singh et al and published in 2014[14]. This previous article included four cohort and two case-control studies for analysis, as partly described here, involving a total of 2813 patients, and showed that PPI use was associated with a 71% risk reduction in progression to EAC and/or HGD in a duration-dependent manner. We included two new studies (NOS = 7, 8) [26, 27]. Although these new included studies were case-control, they presented several advantages in study quality. Firstly, they featured a large cohort, including BE patients nationwide, the use of registries with validated high data coverage, and a complete prescription and hospitalization history. Secondly, nested case–control design in a well-defined population represents the general population minimized selection bias. In addition, because all prescription medications were recorded prospectively, there was no recall bias, and the use of the unique civil registration numbers allowed population-based design, complete follow-up, and linkage across registries. But most of all, both the two newer studies found no evidence of a protective effect from PPIs on the development of OAC or HGD, which results were in contrary with the conclusion of the previous meta-analysis. Hvid-Jensen et al identified the RR of OAC or HGD was 2.2 (95% CI: 0.7–6.7) and 3.4 (95% CI: 1.1–10.5) in long-term low- and high-adherence PPI users respectively. Masclee et al found PPIs used at highest dose showed an OR for HGD–OAC of 0.9 (95% CI 0.3 to 2.3). Therefore, because of these conflicting results, we seriously considered the relationship between these two studies and the previous meta-analysis.
When considering PPIs as potential chemopreventive agents, the protective mechanism likely involves decreasing intra-esophageal acid and bile exposure, thus promoting esophageal mucosal healing. The up-regulated production of cyclooxygenase-2 (COX-2) has been implicated in the progression of BE to EAC [29]. Because acid and bile exposure have been shown to increase COX-2 expression, PPIs should in theory counter this effect. Indeed, in experimental studies, COX-2 inhibitors suppressed the growth of BE cells, potentially through suppression of basic fibroblast growth factor [30]. Another study confirmed that prostaglandin E2, the product of COX-2 conversion, is reduced in patients with BE taking esomeprazole combined with high doses (325mg/day) of aspirin [31]. It is likely that a large proportion of registered PPI usage is symptom-driven, and reflux symptoms have also been associated with the risk of EAC even in persons without known BE [32]. The observation that PPIs may increase the risk of EAC is explained by the treatment indication being a risk factor for EAC, i.e., reverse causation and the phenomenon of ‘channeling’, where in high-risk patients are being prescribed high-dose PPIs whereas low-risk patients are being prescribed lower doses or not at all [25, 33, 34]. Therefore, it may be the severity of reflux that predisposes to cancer, rather than the usage of PPIs per se. However, in contravention with this hypothesis, the increased use of PPIs (introduced in the late 1980s [35]), is associated with the increasing incidence of EAC [36, 37]. Concerns that PPI-induced hypergastrinaemia may increase the risk of adenocarcinoma development have also been expressed [38]. In vitro studies have revealed that gastrin has a pro-proliferative effect on Barrett’s epithelium [39]. A potential causal effect of gastrin on neoplastic progression in human BE has been supported by one study showing that serum gastrin levels were significantly correlated with cellular proliferation in nondysplastic BE patients on PPI therapy [40]. Moreover, it is well-known that reflux symptoms correlate poorly with the actual amount of refluxate in patients with GERD, and that BE may even make patients hyposensitive to acid refluxate [41]. PPI usage and severity of reflux are therefore not necessarily linearly corrected. Hence, the risk correlation between PPIs and incidence of EAC reflects the therapeutic picture–not measurable reflux [42, 43]. This is in line with national guidelines, which recommend PPIs for symptom control alone, and not for the prevention of EAC [44, 45].
Our analysis also has several limitations that must be taken into account when interpreting our results. Firstly, our meta-analysis included only observational studies, which lacked the experimental random allocation of intervention necessary to test exposure–outcome hypotheses optimally. No RCTs have been performed to explore this association. We also did not have complete information about body mass index, tobacco and alcohol consumption, or H. pylori status, which may be important factors in neoplastic progression. Thirdly, several studies lacked detailed pathologic information on Barrett’ s segment length and grade of dysplasia, as is current practice for risk stratification of patients with BE. This deficiency may have resulted in misclassification of BE and EAC. In addition, we were unable to rule out publication bias. With such a limited number of studies, statistical testing for publication bias assessment is not recommended.
Conclusions
In summary, no definitive protective effects against the development of EAC and/or HGD were seen for patients with BE with long-term PPI usage. Until and unless results of future studies can confirm such an association, PPI usage should be restricted to symptom control according to current guidelines. These findings indicate that for an unselected group of patients with BE, chemoprevention by use of PPIs to reduce progression should not be considered directly as routine care.
Study Highlights
What is current knowledge
The connection between PPIs and esophageal adenocarcinoma (EAC) in patients with Barrett's esophagus (BE) has still been controversial.
What is new here
No definitive protective effects against the development of EAC and/or HGD were seen for patients with BE with long-term PPI usage.
PPIs were found to have no association with the risk of EAC and/or HGD in patients with BE (unadjusted OR 0.43, 95% CI 0.17–1.08).
Supporting Information
(DOC)
Abbreviations
- PPIs
Proton pump inhibitors
- BE
Barrett's esophagus
- EAC
esophageal adenocarcinoma
- HGD
high-grade dysplasia
- SSE
stratified squamous epithelium
- GERD
gastroesophageal reflux disease
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the National Natural Science Foundation of China (no. 81270480, no. 81570494). There was no additional external funding received for this study.
References
- 1.Alsalahi O, Dobrian AD. Proton Pump Inhibitors: The Culprit for Barrett's Esophagus? Frontiers in oncology. 2014;4:373 Epub 2015/01/27. PubMed Central PMCID: PMCPmc4288325. 10.3389/fonc.2014.00373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan X, Snyder N. Prevalence of Barrett's esophagus in patients with or without GERD symptoms: role of race, age, and gender. Digestive diseases and sciences. 2009;54(3):572–7. Epub 2008/07/26. 10.1007/s10620-008-0395-7 [DOI] [PubMed] [Google Scholar]
- 3.Kastelein F, Spaander MC, Biermann K, Vucelic B, Kuipers EJ, Bruno MJ. Role of acid suppression in the development and progression of dysplasia in patients with Barrett's esophagus. Digestive diseases. 2011;29(5):499–506. Epub 2011/11/19. 10.1159/000331513 [DOI] [PubMed] [Google Scholar]
- 4.Reid BJ, Li X, Galipeau PC, Vaughan TL. Barrett's oesophagus and oesophageal adenocarcinoma: time for a new synthesis. Nature reviews Cancer. 2010;10(2):87–101. PubMed Central PMCID: PMC2879265. 10.1038/nrc2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abrams JA, Sharaiha RZ, Gonsalves L, Lightdale CJ, Neugut AI. Dating the rise of esophageal adenocarcinoma: analysis of Connecticut Tumor Registry data, 1940–2007. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20(1):183–6. PubMed Central PMCID: PMC3018857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. Journal of the National Cancer Institute. 2008;100(16):1184–7. PubMed Central PMCID: PMC2518165. 10.1093/jnci/djn211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. Journal of the National Cancer Institute. 2005;97(2):142–6. 10.1093/jnci/dji024 [DOI] [PubMed] [Google Scholar]
- 8.Miyashita T, Shah FA, Harmon JW, Marti GP, Matsui D, Okamoto K, et al. Do proton pump inhibitors protect against cancer progression in GERD? Surgery today. 2013;43(8):831–7. Epub 2012/11/01. 10.1007/s00595-012-0395-2 [DOI] [PubMed] [Google Scholar]
- 9.Takagi T, Naito Y, Okada H, Ishii T, Mizushima K, Akagiri S, et al. Lansoprazole, a proton pump inhibitor, mediates anti-inflammatory effect in gastric mucosal cells through the induction of heme oxygenase-1 via activation of NF-E2-related factor 2 and oxidation of kelch-like ECH-associating protein 1. The Journal of pharmacology and experimental therapeutics. 2009;331(1):255–64. 10.1124/jpet.109.152702 [DOI] [PubMed] [Google Scholar]
- 10.Handa O, Yoshida N, Fujita N, Tanaka Y, Ueda M, Takagi T, et al. Molecular mechanisms involved in anti-inflammatory effects of proton pump inhibitors. Inflammation research: official journal of the European Histamine Research Society [et al]. 2006;55(11):476–80. [DOI] [PubMed] [Google Scholar]
- 11.Maity P, Bindu S, Choubey V, Alam A, Mitra K, Goyal M, et al. Lansoprazole protects and heals gastric mucosa from non-steroidal anti-inflammatory drug (NSAID)-induced gastropathy by inhibiting mitochondrial as well as Fas-mediated death pathways with concurrent induction of mucosal cell renewal. The Journal of biological chemistry. 2008;283(21):14391–401. 10.1074/jbc.M800414200 [DOI] [PubMed] [Google Scholar]
- 12.Kuipers EJ. Barrett's oesophagus, proton pump inhibitors and gastrin: the fog is clearing. Gut. 2010;59(2):148–9. Epub 2010/02/24. 10.1136/gut.2009.191403 [DOI] [PubMed] [Google Scholar]
- 13.Dall'Olmo L, Fassan M, Dassie E, Scarpa M, Realdon S, Cavallin F, et al. Role of proton pump inhibitor on esophageal carcinogenesis and pancreatic acinar cell metaplasia development: an experimental in vivo study. PloS one. 2014;9(11):e112862 Epub 2014/11/22. PubMed Central PMCID: PMCPmc4240576. 10.1371/journal.pone.0112862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh S, Garg SK, Singh PP, Iyer PG, El-Serag HB. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett's oesophagus: a systematic review and meta-analysis. Gut. 2014;63(8):1229–37. Epub 2013/11/14. PubMed Central PMCID: PMCPmc4199831. 10.1136/gutjnl-2013-305997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology. 2010;25(9):603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 16.Suissa S. Immortal time bias in observational studies of drug effects. Pharmacoepidemiology and drug safety. 2007;16(3):241–9. 10.1002/pds.1357 [DOI] [PubMed] [Google Scholar]
- 17.Suissa S, Dell'aniello S, Vahey S, Renoux C. Time-window bias in case-control studies: statins and lung cancer. Epidemiology. 2011;22(2):228–31. 10.1097/EDE.0b013e3182093a0f [DOI] [PubMed] [Google Scholar]
- 18.Hillman LC, Chiragakis L, Shadbolt B, Kaye GL, Clarke AC. Proton-pump inhibitor therapy and the development of dysplasia in patients with Barrett's oesophagus. The Medical journal of Australia. 2004;180(8):387–91. Epub 2004/04/20. [DOI] [PubMed] [Google Scholar]
- 19.Jung KW, Talley NJ, Romero Y, Katzka DA, Schleck CD, Zinsmeister AR, et al. Epidemiology and natural history of intestinal metaplasia of the gastroesophageal junction and Barrett's esophagus: a population-based study. The American journal of gastroenterology. 2011;106(8):1447–55; quiz 56. PubMed Central PMCID: PMC3150349. 10.1038/ajg.2011.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne JA1 EM, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323(7304):101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen DM, El-Serag HB, Henderson L, Stein D, Bhattacharyya A, Sampliner RE. Medication usage and the risk of neoplasia in patients with Barrett's esophagus. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2009;7(12):1299–304. Epub 2009/06/16. PubMed Central PMCID: PMCPmc2789910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kastelein F, Spaander MC, Steyerberg EW, Biermann K, Valkhoff VE, Kuipers EJ, et al. Proton pump inhibitors reduce the risk of neoplastic progression in patients with Barrett's esophagus. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2013;11(4):382–8. Epub 2012/12/04. [DOI] [PubMed] [Google Scholar]
- 23.Altawil JIB, Jinjuvadia R, Antaki F. Can progression to dysplasia in Barrett’s esophagus be prevented by proton pump inhibitors? Am J Gastroenterol. 2011; 106:S31. [Google Scholar]
- 24.de Jonge PJ, Steyerberg EW, Kuipers EJ, Honkoop P, Wolters LM, Kerkhof M, et al. Risk factors for the development of esophageal adenocarcinoma in Barrett's esophagus. The American journal of gastroenterology. 2006;101(7):1421–9. Epub 2006/07/26. 10.1111/j.1572-0241.2006.00626.x [DOI] [PubMed] [Google Scholar]
- 25.Nguyen DM, Richardson P, El-Serag HB. Medications (NSAIDs, statins, proton pump inhibitors) and the risk of esophageal adenocarcinoma in patients with Barrett's esophagus. Gastroenterology. 2010;138(7):2260–6. Epub 2010/03/02. PubMed Central PMCID: PMCPmc2883678. 10.1053/j.gastro.2010.02.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hvid-Jensen F, Pedersen L, Funch-Jensen P, Drewes AM. Proton pump inhibitor use may not prevent high-grade dysplasia and oesophageal adenocarcinoma in Barrett's oesophagus: a nationwide study of 9883 patients. Alimentary pharmacology & therapeutics. 2014;39(9):984–91. Epub 2014/03/13. [DOI] [PubMed] [Google Scholar]
- 27.Masclee GM1 CP, Spaander MC3, Kuipers EJ3, Sturkenboom MC4. NSAIDs, statins, low-dose aspirin and PPIs, and the risk of oesophageal adenocarcinoma among patients with Barrett's oesophagus: a population-based case-control study. BMJ Open. 2015;29(5):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hvid-Jensen F, Drewes AM, Funch-Jensen P. Letter: Proton pump inhibitor usage still seems to reduce the risk of high-grade dysplasia and/or oesophageal adenocarcinoma in Barrett's oesophagus—Authors' reply. Alimentary Pharmacology and Therapeutics. 2014;40(7):860–1. 10.1111/apt.12931 [DOI] [PubMed] [Google Scholar]
- 29.Wilson KT, Fu S, Ramanujam KS, Meltzer SJ. Increased expression of inducible nitric oxide synthase and cyclooxygenase-2 in Barrett's esophagus and associated adenocarcinomas. Cancer research. 1998;58(14):2929–34. Epub 1998/07/29. [PubMed] [Google Scholar]
- 30.Buttar NS, Wang KK, Anderson MA, Dierkhising RA, Pacifico RJ, Krishnadath KK, et al. The effect of selective cyclooxygenase-2 inhibition in Barrett's esophagus epithelium: an in vitro study. Journal of the National Cancer Institute. 2002;94(6):422–9. Epub 2002/03/21. [DOI] [PubMed] [Google Scholar]
- 31.Falk GW, Buttar NS, Foster NR, Ziegler KLA, DeMars CJ, Romero Y, et al. A Combination of Esomeprazole and Aspirin Reduces Tissue Concentrations of Prostaglandin E2 in Patients With Barrett's Esophagus. Gastroenterology. 2012;143(4):917–26.e1. 10.1053/j.gastro.2012.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lagergren J1 BR, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. The New England journal of medicine. 1999;340(11):825–31. 10.1056/NEJM199903183401101 [DOI] [PubMed] [Google Scholar]
- 33.Garcia Rodriguez LA, Lagergren J, Lindblad M. Gastric acid suppression and risk of oesophageal and gastric adenocarcinoma: a nested case control study in the UK. Gut. 2006;55(11):1538–44. Epub 2006/06/21. PubMed Central PMCID: PMCPmc1860118. 10.1136/gut.2005.086579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alexandre L, Clark AB, Bhutta HY, Holt S, Lewis MP, Hart AR. Statin use is associated with reduced risk of histologic subtypes of esophageal cancer: a nested case-control analysis. Gastroenterology. 2014;146(3):661–8. Epub 2013/12/10. 10.1053/j.gastro.2013.11.046 [DOI] [PubMed] [Google Scholar]
- 35.Garnett WR. History of acid suppression: focus on the hospital setting. Pharmacotherapy. 2003;23(10 Pt 2):56s–60s. Epub 2003/11/01. [DOI] [PubMed] [Google Scholar]
- 36.Nasr AO, Dillon MF, Conlon S, Downey P, Chen G, Ireland A, et al. Acid suppression increases rates of Barrett's esophagus and esophageal injury in the presence of duodenal reflux. Surgery. 2012;151(3):382–90. Epub 2011/10/25. 10.1016/j.surg.2011.08.021 [DOI] [PubMed] [Google Scholar]
- 37.Huo X, Juergens S, Zhang X, Rezaei D, Yu C, Strauch ED, et al. Deoxycholic acid causes DNA damage while inducing apoptotic resistance through NF-kappaB activation in benign Barrett's epithelial cells. American journal of physiology Gastrointestinal and liver physiology. 2011;301(2):G278–86. Epub 2011/06/04. PubMed Central PMCID: PMCPmc3154602. 10.1152/ajpgi.00092.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang JS, Varro A, Lightdale CJ, Lertkowit N, Slack KN, Fingerhood ML, et al. Elevated serum gastrin is associated with a history of advanced neoplasia in Barrett's esophagus. The American journal of gastroenterology. 2010;105(5):1039–45. Epub 2009/11/12. PubMed Central PMCID: PMCPmc3139948. 10.1038/ajg.2009.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haigh CR, Attwood SE, Thompson DG, Jankowski JA, Kirton CM, Pritchard DM, et al. Gastrin induces proliferation in Barrett's metaplasia through activation of the CCK2 receptor. Gastroenterology. 2003;124(3):615–25. Epub 2003/03/04. 10.1053/gast.2003.50091 [DOI] [PubMed] [Google Scholar]
- 40.Green DA, Mlynarczyk CM, Vaccaro BJ, Capiak KM, Quante M, Lightdale CJ, et al. Correlation between serum gastrin and cellular proliferation in Barrett's esophagus. Therapeutic advances in gastroenterology. 2011;4(2):89–94. Epub 2011/06/23. PubMed Central PMCID: PMCPmc3105623. 10.1177/1756283X10392444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krarup AL, Olesen SS, Funch-Jensen P, Gregersen H, Drewes AM. Proximal and distal esophageal sensitivity is decreased in patients with Barrett's esophagus. World journal of gastroenterology: WJG. 2011;17(4):514–21. PubMed Central PMCID: PMC3027019. 10.3748/wjg.v17.i4.514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moayyedi P, Talley NJ. Gastro-oesophageal reflux disease. The Lancet. 2006;367(9528):2086–100. [DOI] [PubMed] [Google Scholar]
- 43.Zagari RM, Law GR, Fuccio L, Pozzato P, Forman D, Bazzoli F. Dyspeptic Symptoms and Endoscopic Findings in the Community: The Loiano-Monghidoro Study. The American journal of gastroenterology. 2010;105(3):565–71. 10.1038/ajg.2009.706 [DOI] [PubMed] [Google Scholar]
- 44.Spechler SJ SP, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association Medical Position Statement on the Management of Barrett's Esophagus. Gastroenterology. 2011;140(3):1084–91. 10.1053/j.gastro.2011.01.030 [DOI] [PubMed] [Google Scholar]
- 45.Fitzgerald RC, di Pietro M, Ragunath K, Ang Y, Kang JY, Watson P, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut. 2014;63(1):7–42. 10.1136/gutjnl-2013-305372 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.