Abstract
Drug-induced hypersensitivity reactions can significantly impact drug development and use. Studies to understand risk factors for drug-induced hypersensitivity reactions have identified genetic association with specific human leukocyte antigen (HLA) alleles. Interestingly, drug-induced hypersensitivity reactions can occur in non-human primates; however association between drug-induced hypersensitivity reactions and major histocompatibility complex (MHC) alleles has not been described. In this study, tissue samples were collected from 62 cynomolgus monkeys from preclinical studies in which 9 animals had evidence of drug-induced hypersensitivity reactions. Microsatellite analysis was used to determine MHC haplotypes for each animal. A total of 7 haplotypes and recombinant MHC haplotypes were observed, with distribution frequency comparable to known MHC I allele frequency in cynomolgus monkeys. Genetic association analysis identified alleles from the M3 haplotype of the MHC I B region (B*011:01, B*075:01, B*079:01, B*070:02, B*098:05, and B*165:01) to be significantly associated (χ2 test for trend, P < 0.05) with occurrence of drug-induced hypersensitivity reactions. Sequence similarity from alignment of alleles in the M3 haplotype B region and HLA alleles associated with drug-induced hypersensitivity reactions in humans was 86 to 93%. These data demonstrate that MHC alleles in cynomolgus monkeys are associated with drug-induced hypersensitivity reactions, similar to HLA alleles in humans.
Keywords: MHC, haplotype, drug-induced hypersensitivity reaction, cynomolgus monkey
Introduction
The major histocompatibility complex (MHC) gene loci encode proteins that play a critical role in immune response. Human MHC genes are divided into three classes: class I and II which encode complexes that present endogenous and exogenous peptide sequences, respectively (Vyas et al., 2008), and class III which encode specialized immune function genes (Milner and Campbell, 2001). The genes encoded by MHC Class I and II are involved in antigen presentation such as HLA-A, HLA-B (class I), and HLA-DR, HLA-DP, HLA-DQ (class II) genes (Traherne, 2008). These genes are highly polymorphic and experience genomic recombination (HLA-DRβ duplication) to introduce additional sequence variation which impacts peptide binding specificity and receptor interaction (Parham, 1988, Rollini et al., 1985). In the cynomolgus monkey (Macaca fascicularis), MHC genes have been extensively studied based on the diverse geographic locations that harbor cynomolgus monkeys (Campbell et al., 2009, Pendley et al., 2008, Creager et al., 2011, Krebs et al., 2005). MHC class I genes are defined as either Mafa-A or Mafa-B (HLA homologues). Interestingly, the MHC genes in these animals have undergone extensive genetic rearrangement and duplication when compared to human MHC gene loci (O’Connor et al., 2007, Doxiadis et al., 2006, Blancher et al., 2006). The result is thousands of non-human primate MHC haplotypes, with cynomolgus monkey and the closely related rhesus monkey sharing MHC class I and II haplotypes across species (Doxiadis et al., 2006, de Groot et al., 2012, Robinson et al., 2013). Despite this high degree of variation, similarities to human MHC genes persist, with human and cynomolgus monkeys sharing MHC epitopes across species (Mothe et al., 2015).
Specific groups of linked genetic variants in HLA genes have been defined as HLA* allele haplotypes and are associated with variable immune responses (Price et al., 1999, Marsh et al., 2010). HLA* alleles have also been associated with drug-induced hypersensitivity reactions for patients receiving therapies such as abacavir, flucloxacillin, carbamazepine, and allopurinol (Daly et al., 2009, Mallal et al., 2002, Martin et al., 2004, Hung et al., 2006, Hung et al., 2005). Skins reactions associated with drug administration are one of the most common adverse drug reactions (ADRs) observed in humans (Bigby et al., 1986, Pichler, 2003, Roujeau, 2005). ADRs can be predictable in humans when due to the drug’s pharmacologic action (type A reactions) or unpredictable when not related to the drug’s pharmacologic action (type B reactions) (Rawlins and Thompson, 1977). It has been reported that the vast majority of drug-induced skin reactions in humans tend to be type B reactions and are idiosyncratic in nature (Uetrecht and Naisbitt, 2013).
Standard preclinical toxicology testing has generally poorly predicted clinical drug-induced hypersensitivity reactions (Olson et al., 2000). However, it was recently reported that following treatment with metabotropic glutamate receptor 5 (mGluR5) negative allosteric modulators, clinical skin lesions were observed in Mauritius cynomolgus monkeys (Palanisamy et al., 2015). Further, similar skin lesions were observed during preclinical development of a second drug candidate (Pfizer internal data), and in total, 9 out of 62 cynomolgus monkeys exhibited clinical and histopathologic skin effects that were consistent with characteristics of drug-induced skin reactions observed in humans. Clinical effects in the monkeys appeared between 5 and 8 days of dosing and ranged from generalized to localized erythema often involving the genital region (scrotum) in males and/or the head or limbs in either sex. Localized skin reactions in the genital region were often edematous and occasionally associated with bulla formation. With one of the mGluR5 compounds, a dosing holiday followed by re-challenge resulted in more rapid clinical development of skin effects (Day 3) which is consistent with a hypersensitivity reaction. Microscopically, the lesions were characterized by lymphocytic inflammation at the dermal-epidermal interface (interface dermatitis) with additional perivascular lymphocytic inflammation in the dermis that was sometimes associated with edema. Interface inflammation was accompanied by single cell necrosis of keratinocytes, particularly along the basal epidermal cell layer (Fig 1); in some instances the degenerating keratinocytes were surrounded by lymphocytes. Bullae from the genital region were characterized by complete separation of the entire epidermis from the dermis (subepidermal bullae), sometimes with full thickness necrosis of the overlying epidermis. Bullae were filled with amphophilic fluid containing small numbers of inflammatory cells (predominantly lymphocytes). Immunohistochemical characterization of the skin lesions observed in the cynomolgus monkeys given mGluR5 inhibitors demonstrated a T lymphocyte-dominant immunophenotype. Collectively, the nature of the clinical effects (localized to generalized erythema with or without bullae formation), microscopic lesions (interface dermatitis with basal keratinocyte necrosis), the immunophenotype (T lymphocyte dominant), and the time of initial clinical onset with earlier onset upon re-challenge are consistent with an immune-mediated delayed type-IV hypersensitivity reaction (Palanisamy et al., 2015). These drug-induced clinical and microscopic skin effects in the monkeys have several overlapping features with human forms of drug-induced skin reactions, suggesting a similar underlying mechanism. While the mechanism may be similar, how drugs cause skin hypersensitivity reactions in humans and monkeys is not well understood and hypotheses are that the drug can form a hapten or superantigen-like structure that results in an immune response (Uetrecht, 2007).
Figure 1.
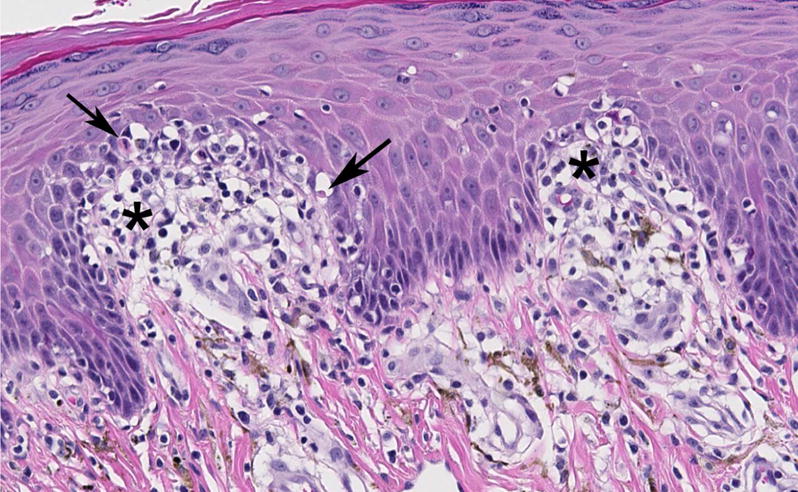
Representative microscopic image (original objective 20×) of a drug-induced skin lesion in a cynomolgus macaque treated with a small molecule mGluR5 negative allosteric modulator. The lesion is localized to the dermal-epidermal junction and characterized by lymphocytic interface inflammation (asterisks) accompanied by single cell necrosis of basal keratinocytes (arrows).
In this study, the association between MHC haplotypes and the skin hypersensitivity reactions observed in Mauritius cynomolgus monkeys was evaluated. The Mauritius cynomolgus monkey subpopulation provides a unique opportunity to study MHC haplotypes and their relationship to immune response in monkeys, as geographic isolation of a small founder population on the island of Mauritius has reduced MHC Class I and Class II sequence diversity to only 7 haplotypes (O’Connor et al., 2007, Wiseman et al., 2007, Krebs et al., 2005, Bonhomme et al., 2008). This study characterized these haplotypes in a study population of cynomolgus monkeys and identified that alleles in the M3 haplotype of the MHC I B region are associated with drug-induced hypersensitivity reactions in cynomolgus monkeys.
Materials and Methods
Tissue Samples
Peripheral blood or formalin-fixed, paraffin-embedded (FFPE) spleen samples obtained from 62 cynomolgus monkeys of Mauritian origin (Charles River laboratory, Houston, TX) were selected from preclinical toxicology studies conducted for small molecule drug candidates from 2 independent and unrelated programs at Pfizer, Inc (5 studies over approximately 2 years). All procedures performed on animals were in accordance with regulations and guidelines reviewed and approved by the Pfizer Institutional Animal Care and Use Committee. In total, 9 out of the 62 samples collected were from monkeys that had clinical evidence of drug-induced skin reactions (localized or generalized erythema, edema and/or bullae formation), and 53 were from monkeys that had no observed clinical signs following administration of the same test article.
Genomic DNA isolation and microsatellite analysis
Genomic DNA was isolated from FFPE tissues using QIAamp DNA FFPE Tissue Kit (Qiagen, Valencia, CA), and from peripheral blood using DNeasy Blood and Tissue Kit (Qiagen), according to manufacturer instructions and was used as a template for microsatellite analysis. Microsatellite analysis was performed by the University of Wisconsin using published methods (Wiseman et al., 2007). Microsatellite profiles for each animal were used to infer the MHC class I and class II transcripts previously defined for each of the seven ancestral MHC haplotypes in the Mauritius cynomolgus monkey population (Wiseman et al., 2013).
Sequence alignment
Sequences for MHC M3 B region alleles and human HLA alleles were aligned using Clustal W (http://bio.lundberg.gu.se/edu/msf1.html) and sequence similarity scores were reported.
Statistical analysis
Due to the clinical and histological similarity of the drug-induced skin reactions (reviewed in Introduction) that occurred with small molecule drugs from two independent drug programs, the samples were combined for genetic association analysis in order to increase statistical power. Genetic association analysis was performed using the Chi-square trend test. Significance was considered at P < 0.05.
Results
MHC Haplotype Identification and Distribution
Using microsatellite analysis, there were 7 haplotypes and recombinant MHC class I haplotypes observed in the 62 monkeys. The distribution frequencies of these haplotypes in this population are shown in Table I. Overall, the MHC class I haplotype frequencies were very similar to what has been published for the general Mauritius cynomolgus monkey population (Budde et al., 2010). Haplotypes in the MHC class II region were also identified in the study population (Table I), and the distribution was similarly comparable to the general Mauritius monkey population (O’Connor et al., 2007), although to a lesser extent. Notably, the M4 haplotype was relatively enriched in the study population. Also notable was that no recombination was detected within the MHC A, B or class II regions in the 62 cynomolgus monkeys analyzed in this study (data not shown).
Table I.
Haplotype distribution of MHC I and II alleles in the study population and the general Mauritius cynomolgus monkey population.
| MHC I | MHC II | ||||
|---|---|---|---|---|---|
| Haplotypes | Study Population (N=62) | General Mauritius Population (N=425)a | Haplotypes | Study Population (N=62) | General Mauritius Population (N=117)b |
| M1 | 19% | 19% | H1 | 25% | 31% |
| M2 | 11% | 16% | H2 | 13% | 16% |
| M3 | 17% | 16% | H3 | 22% | 19% |
| M4 | 19% | 13% | H4 | 24% | 15% |
| M5 | 2% | 4% | H5 | 4% | 4% |
| M6 | 7% | 7% | H6 | 10% | 8% |
| M7 | 2% | 1% | Recombinant | 0% | 8% |
| Recombinant | 17% | 22% | Otherc | 2% | 0% |
| Otherc | 7% | 0.2% | |||
Data from Budde et al., 2010
Data from O’Connor et al., 2007
Haplotypes not determined by microsatellite analysis
Genetic Association Analysis
There were 4 MHC I haplotypes (M2, M3, M4, and M6) identified in the 9 animals that experienced drug-induced hypersensitivity reactions (Fig 2), suggesting that the alleles from these 4 haplotypes are more likely involved in drug-induced hypersensitivity reactions. Genetic analysis was conducted using the alleles from the 4 MHC haplotypes to identify possible association between these haplotypes and drug-induced hypersensitivity reactions in cynomolgus monkeys. Interestingly, the alleles from the M3 haplotype of the MHC class I B region (B*011:01, B*075:01, B*079:01, B*070:02, B*098:05, and B*165:01) were found to be significantly associated with drug-induced hypersensitivity reactions (Table II, χ2 test for trend, P < 0.05). In contrast, the remaining 11 MHC I haplotype regions (A, B, and class II regions in the M2, M4, and M6 haplotypes, and A and class II regions in the M3 haplotype) had no statistical association with drug-induced hypersensitivity reactions in the 62 cynomolgus monkeys analyzed.
Figure 2.
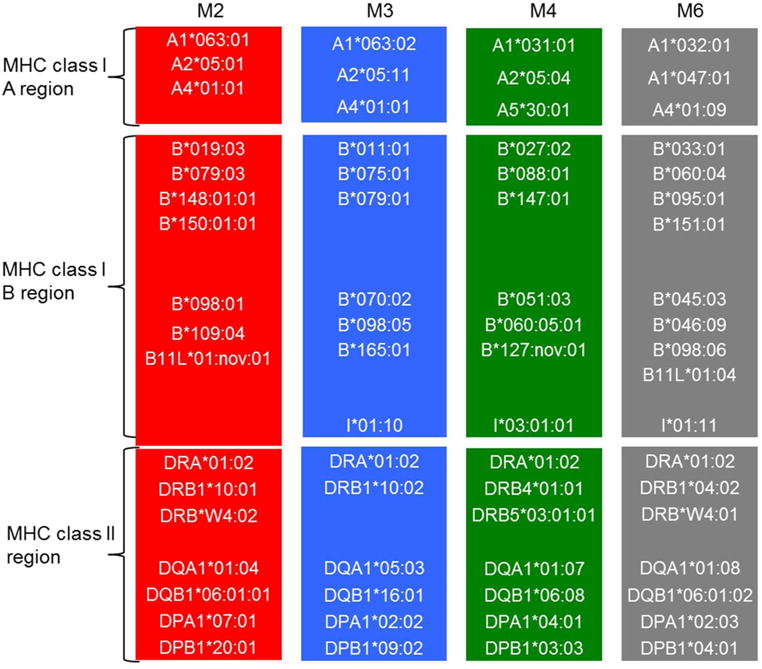
Alleles of the 4 haplotypes identified in cynomolgus monkeys experiencing drug-induced hypersensitivity reactions. Microsatellite analysis was used to determine the MHC haplotypes in 62 cynomolgus monkeys, and 4 haplotypes were identified in the 9 monkeys with drug-induced hypersensitivity reactions: M2 (red); M3 (blue); M4 (green); and M6 (gray).
Table II.
Results of genetic association analysis with alleles from MHC haplotypes and drug-induced hypersensitivity reactions in cynomolgus monkeys.
| MHC region | Number of Samples Analyzed | P value |
|---|---|---|
| M3 haplotype of MHC B region | 62 | 0.0065 |
| 11 MHC haplotype regions (A, B and class II regions in M2, M4, and M6 haplotype; A and class II regions in M3 haplotype) | 62 | >0.05 |
Homology Between HLA Alleles Associated With Drug-Induced Hypersensitivity Reactions and MHC I M3 B Alleles
The DNA sequences for HLA alleles (HLA-B*57:01, B*15:02, B*58:01 and B*35:05) that have been reported to be associated with drug-induced hypersensitivity reactions (Pompeu et al., 2012) were compared with the DNA sequences of the cynomolgus monkey MHC I M3 B region alleles that were genetically associated with the skin reactions observed in the monkeys. Sequence similarity ranged from 86 to 93% (Table III).
Table III.
Sequence similarity scores from alignments between alleles in MHC I M3 haplotype B region (Mafa-B) and HLA alleles.
| Sequence Similarity Score (%)a | ||||
|---|---|---|---|---|
| Alleles | HLA-B*57:01:01b | HLA-B*15:02:01b | HLA-B*58:01:01b | HLA-B*35:05:01b |
| Mafa-B*011:01 | 91% | 92% | 92% | 92% |
| Mafa-B*075:01 | 92% | 93% | 92% | 92% |
| Mafa-B*079:01 | 90% | 92% | 91% | 91% |
| Mafa-B*070:02:01 | 91% | 92% | 92% | 92% |
| Mafa-B*098:05 | 91% | 92% | 92% | 92% |
| Mafa-B*165:01 | 86% | 87% | 86% | 86% |
Homology between cynomolgus monkey MHC class I M3 B region alleles (Mafa-B* alleles) and human HLA alleles were aligned using Clustal W (http://bio.lundberg.gu.se/edu/msf1.html).
HLA allele known to be associated with drug-induced hypersensitivity reaction (Pompeu et al., 2012); only subtype: 01 was selected for each HLA allele.
Discussion
Given the extensively studied and well known associations between certain HLA alleles and drug-induced hypersensitivity reactions in humans (Sukasem et al., 2014, Yip et al., 2015), it was hypothesized that MHC haplotypes in cynomolgus monkeys may also be associated with drug-induced hypersensitivity reactions in this species. Drug-induced hypersensitivity reactions were observed in cynomolgus monkeys with small molecule drug candidates from 2 independent and unrelated programs, and had similar clinical and histological characteristics suggesting that a common immune-mediated pathway (such as MHC signaling) downstream of or unrelated to either pharmacological mechanism of action was involved in the pathogenesis. Indeed, similar to the observations in humans, 6 alleles from the M3 haplotype of the MHC B region were identified to be significantly associated with the drug-induced hypersensitivity reactions observed in these monkeys.
Interestingly, unlike drug-induced hypersensitivity reactions in humans which typically manifest as idiosyncratic (low incidence), the skin reactions in the cynomolgus monkeys displayed a clear dose response relationship and high incidence (Palanisamy et al., 2015). Given that certain populations of cynomolgus monkeys have relatively limited MHC diversity (Aarnink et al., 2010), it was initially suspected that the M3 haplotype was over-represented in the population of Mauritian monkeys utilized in these preclinical studies, making what would typically appear as an idiosyncratic pattern in a genetically diverse human population appear more dose-related in the less diverse study population, which would in turn skew the genetic association analysis. However, very comparable distribution of MHC I haplotypes was observed between the study population and the general cynomolgus monkey population of Mauritian origin, including the M3 haplotype which had a distribution frequency of 17% in the study population and 16% in the general population (Budde et al., 2010), excluding the possibility that the observed significant association between the M3 haplotype and drug-induced hypersensitivity reactions resulted from a statistical sampling artifact. Furthermore, no recombination was detected within MHC A or B, or MHC class II regions in the study population, confirming the relatively limited genetic diversity of MHC molecules in cynomolgus monkeys of Mauritius origin. Of note, alleles from the MHC II region were not found to be associated with the drug-induced hypersensitivity reactions in cynomolgus monkeys, which is surprising given that CD4 positive T lymphocytes were predominant in the interface inflammation observed microscopically in the skin from these monkeys (Palanisamy et al., 2015). This might be explained by the less comparable distribution of MHC II haplotypes that were observed between the study population and the general Mauritian monkey population, which may have skewed the statistical analysis.
The mechanism of how small molecule drugs can cause skin hypersensitivity reactions is not well understood, but it is hypothesized that the parent drug or a reactive metabolite binds to a host protein forming a hapten and resulting in an autoimmune response against the drug-modified protein, or alternatively forming a superantigen-like crosslink between MHC and the T-cell receptor resulting in an autoimmune-like response (Uetrecht, 2007). Abacavir-induced skin hypersensitivity in HLA B*57:01 carriers is thought to involve the drug binding within the antigen-binding cleft of HLA B*57:01 in an allelic-specific manner and altering the HLA-bound peptide repertoire leading to subsequent T cell responses (Ostrov et al., 2012). It has been reported that there are specific structural features of the antigen-binding cleft that are common or shared with different HLA subtypes in humans (Pompeu et al., 2012). In the present study, it is undetermined which specific allele(s) or residues of the M3 haplotype in the MHC B region are causative for the observed skin reactions in the cynomolgus monkeys, but it is likely that the molecular mechanisms are similar to what is reported in humans given the high sequence conservation between alleles in the M3 haplotype and human HLA alleles.
In humans, immunological variations in T cell receptor repertoire and development of tolerance have been reported to contribute to the idiosyncratic nature of drug-induced hypersensitivity reactions (Uetrecht and Naisbitt, 2013). Although inter-animal variability in T cell receptor repertoire was not evaluated in the current study, CD4 positive T lymphocytes were predominate at the interface between the dermis and epidermis or within the epidermis itself (Palanisamy et al., 2015). T cells have previously been shown to contribute to skewed peripheral PBMC repertoire in rhesus monkeys (Currier et al., 1999), suggesting it is possible that variability in T cell receptor repertoire may be one of the factors that contributed to the observed drug-induced hypersensitivity reactions in the cynomolgus monkeys.
In conclusion, this is the first report showing that alleles from the M3 haplotype of the MHC I B region in cynomolgus monkeys are significantly associated with drug-induced hypersensitivity reactions in this species. These findings suggest that cynomolgus monkeys may be a good preclinical model for investigating the mechanisms underlying drug-induced hypersensitivity reactions. Moreover, because of the high degree of sequence similarity between these alleles in the M3 haplotype and HLA alleles associated with drug-induced hypersensitivity reactions in humans, identifying the specific alleles in the M3 haplotype associated with drug-induced hypersensitivity reactions in the monkeys may enable better human translation and will help in the identification of HLA alleles that may impart risk for patients administered these drugs.
Acknowledgments
The authors would like to acknowledge Karrie Tartaro and Linda Nelms for assistance with DNA isolation. This study was made possible in part by Grant Number P51 RR000167 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), to the Wisconsin National Primate Research Center, University of Wisconsin-Madison. This research was conducted in part at a facility constructed with support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01. This publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Abbreviations
- MHC
major histocompatibility complex
- HLA
human leukocyte antigen
- ADRs
adverse drug reactions
- mGluR5
metabotropic glutamate receptor 5
- FFPE
Formalin-fixed, paraffin-embedded
References
- Aarnink A, Estrade L, Apoil PA, Kita YF, Saitou N, Shiina T, Blancher A. Study of cynomolgus monkey (Macaca fascicularis) DRA polymorphism in four populations. Immunogenetics. 2010;62:123–136. doi: 10.1007/s00251-009-0421-8. [DOI] [PubMed] [Google Scholar]
- Bigby M, Jick S, Jick H, Arndt K. Drug-induced cutaneous reactions. A report from the Boston Collaborative Drug Surveillance Program on 15,438 consecutive inpatients, 1975 to 1982. JAMA. 1986;256:3358–3363. doi: 10.1001/jama.256.24.3358. [DOI] [PubMed] [Google Scholar]
- Blancher A, Tisseyre P, Dutaur M, Apoil PA, Maurer C, Quesniaux V, Raulf F, Bigaud M, Abbal M. Study of Cynomolgus monkey (Macaca fascicularis) MhcDRB (Mafa-DRB) polymorphism in two populations. Immunogenetics. 2006;58:269–282. doi: 10.1007/s00251-006-0102-9. [DOI] [PubMed] [Google Scholar]
- Bonhomme M, Blancher A, Cuartero S, Chikhi L, Crouau-Roy B. Origin and number of founders in an introduced insular primate: estimation from nuclear genetic data. Mol Ecol. 2008;17:1009–1019. doi: 10.1111/j.1365-294X.2007.03645.x. [DOI] [PubMed] [Google Scholar]
- Budde ML, Wiseman RW, Karl JA, Hanczaruk B, Simen BB, O’Connor DH. Characterization of Mauritian cynomolgus macaque major histocompatibility complex class I haplotypes by high-resolution pyrosequencing. Immunogenetics. 2010;62:773–780. doi: 10.1007/s00251-010-0481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KJ, Detmer AM, Karl JA, Wiseman RW, Blasky AJ, Hughes AL, Bimber BN, O’Connor SL, O’Connor DH. Characterization of 47 MHC class I sequences in Filipino cynomolgus macaques. Immunogenetics. 2009;61:177–187. doi: 10.1007/s00251-008-0351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creager HM, Becker EA, Sandman KK, Karl JA, Lank SM, Bimber BN, Wiseman RW, Hughes AL, O’Connor SL, O’Connor DH. Characterization of full-length MHC class II sequences in Indonesian and Vietnamese cynomolgus macaques. Immunogenetics. 2011;63:611–618. doi: 10.1007/s00251-011-0537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier JR, Stevenson KS, Kehn PJ, Zheng K, Hirsch VM, Robinson MA. Contributions of CD4+, CD8+, and CD4+CD8+ T cells to skewing within the peripheral T cell receptor beta chain repertoire of healthy macaques. Hum Immunol. 1999;60:209–222. doi: 10.1016/s0198-8859(98)00109-8. [DOI] [PubMed] [Google Scholar]
- Daly AK, Donaldson PT, Bhatnagar P, Shen Y, Pe’er I, Floratos A, Daly MJ, Goldstein DB, John S, Nelson MR, Graham J, Park BK, Dillon JF, Bernal W, Cordell HJ, Pirmohamed M, Aithal GP, Day CP, Study, D. and International, S.A.E.C HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet. 2009;41:816–819. doi: 10.1038/ng.379. [DOI] [PubMed] [Google Scholar]
- de Groot NG, Otting N, Robinson J, Blancher A, Lafont BA, Marsh SG, O’Connor DH, Shiina T, Walter L, Watkins DI, Bontrop RE. Nomenclature report on the major histocompatibility complex genes and alleles of Great Ape, Old and New World monkey species. Immunogenetics. 2012;64:615–631. doi: 10.1007/s00251-012-0617-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxiadis GG, Rouweler AJ, de Groot NG, Louwerse A, Otting N, Verschoor EJ, Bontrop RE. Extensive sharing of MHC class II alleles between rhesus and cynomolgus macaques. Immunogenetics. 2006;58:259–268. doi: 10.1007/s00251-006-0083-8. [DOI] [PubMed] [Google Scholar]
- Hung SI, Chung WH, Jee SH, Chen WC, Chang YT, Lee WR, Hu SL, Wu MT, Chen GS, Wong TW, Hsiao PF, Chen WH, Shih HY, Fang WH, Wei CY, Lou YH, Huang YL, Lin JJ, Chen YT. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet Genomics. 2006;16:297–306. doi: 10.1097/01.fpc.0000199500.46842.4a. [DOI] [PubMed] [Google Scholar]
- Hung SI, Chung WH, Liou LB, Chu CC, Lin M, Huang HP, Lin YL, Lan JL, Yang LC, Hong HS, Chen MJ, Lai PC, Wu MS, Chu CY, Wang KH, Chen CH, Fann CS, Wu JY, Chen YT. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005;102:4134–4139. doi: 10.1073/pnas.0409500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs KC, Jin Z, Rudersdorf R, Hughes AL, O’Connor DH. Unusually high frequency MHC class I alleles in Mauritian origin cynomolgus macaques. J Immunol. 2005;175:5230–5239. doi: 10.4049/jimmunol.175.8.5230. [DOI] [PubMed] [Google Scholar]
- Mallal S, Nolan D, Witt C, Masel G, Martin AM, Moore C, Sayer D, Castley A, Mamotte C, Maxwell D, James I, Christiansen FT. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet. 2002;359:727–732. doi: 10.1016/s0140-6736(02)07873-x. [DOI] [PubMed] [Google Scholar]
- Marsh SG, Albert ED, Bodmer WF, Bontrop RE, Dupont B, Erlich HA, Fernandez-Vina M, Geraghty DE, Holdsworth R, Hurley CK, Lau M, Lee KW, Mach B, Maiers M, Mayr WR, Muller CR, Parham P, Petersdorf EW, Sasazuki T, Strominger JL, Svejgaard A, Terasaki PI, Tiercy JM, Trowsdale J. Nomenclature for factors of the HLA system, 2010. Tissue Antigens. 2010;75:291–455. doi: 10.1111/j.1399-0039.2010.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AM, Nolan D, Gaudieri S, Almeida CA, Nolan R, James I, Carvalho F, Phillips E, Christiansen FT, Purcell AW, McCluskey J, Mallal S. Predisposition to abacavir hypersensitivity conferred by HLA-B*5701 and a haplotypic Hsp70-Hom variant. Proc Natl Acad Sci U S A. 2004;101:4180–4185. doi: 10.1073/pnas.0307067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner CM, Campbell RD. Genetic organization of the human MHC class III region. Front Biosci. 2001;6:D914–926. doi: 10.2741/milner. [DOI] [PubMed] [Google Scholar]
- Mothe BR, Lindestam Arlehamn CS, Dow C, Dillon MB, Wiseman RW, Bohn P, Karl J, Golden NA, Gilpin T, Foreman TW, Rodgers MA, Mehra S, Scriba TJ, Flynn JL, Kaushal D, O’Connor DH, Sette A. The TB-specific CD4(+) T cell immune repertoire in both cynomolgus and rhesus macaques largely overlap with humans. Tuberculosis (Edinb) 2015;95:722–735. doi: 10.1016/j.tube.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor SL, Blasky AJ, Pendley CJ, Becker EA, Wiseman RW, Karl JA, Hughes AL, O’Connor DH. Comprehensive characterization of MHC class II haplotypes in Mauritian cynomolgus macaques. Immunogenetics. 2007;59:449–462. doi: 10.1007/s00251-007-0209-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson H, Betton G, Robinson D, Thomas K, Monro A, Kolaja G, Lilly P, Sanders J, Sipes G, Bracken W, Dorato M, Van Deun K, Smith P, Berger B, Heller A. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul Toxicol Pharmacol. 2000;32:56–67. doi: 10.1006/rtph.2000.1399. [DOI] [PubMed] [Google Scholar]
- Ostrov DA, Grant BJ, Pompeu YA, Sidney J, Harndahl M, Southwood S, Oseroff C, Lu S, Jakoncic J, de Oliveira CA, Yang L, Mei H, Shi L, Shabanowitz J, English AM, Wriston A, Lucas A, Phillips E, Mallal S, Grey HM, Sette A, Hunt DF, Buus S, Peters B. Drug hypersensitivity caused by alteration of the MHC-presented self-peptide repertoire. Proc Natl Acad Sci U S A. 2012;109:9959–9964. doi: 10.1073/pnas.1207934109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanisamy GS, Marcek JM, Cappon GD, Whritenour J, Shaffer CL, Brady JT, Houle C. Drug-induced Skin Lesions in Cynomolgus Macaques Treated with Metabotropic Glutamate Receptor 5 (mGluR5) Negative Allosteric Modulators. Toxicol Pathol. 2015;43:995–1003. doi: 10.1177/0192623315588114. [DOI] [PubMed] [Google Scholar]
- Parham P. Function and polymorphism of human leukocyte antigen-A,B,C molecules. Am J Med. 1988;85:2–5. doi: 10.1016/0002-9343(88)90369-5. [DOI] [PubMed] [Google Scholar]
- Pendley CJ, Becker EA, Karl JA, Blasky AJ, Wiseman RW, Hughes AL, O’Connor SL, O’Connor DH. MHC class I characterization of Indonesian cynomolgus macaques. Immunogenetics. 2008;60:339–351. doi: 10.1007/s00251-008-0292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichler WJ. Delayed drug hypersensitivity reactions. Ann Intern Med. 2003;139:683–693. doi: 10.7326/0003-4819-139-8-200310210-00012. [DOI] [PubMed] [Google Scholar]
- Pompeu YA, Stewart JD, Mallal S, Phillips E, Peters B, Ostrov DA. The structural basis of HLA-associated drug hypersensitivity syndromes. Immunol Rev. 2012;250:158–166. doi: 10.1111/j.1600-065X.2012.01163.x. [DOI] [PubMed] [Google Scholar]
- Price P, Witt C, Allcock R, Sayer D, Garlepp M, Kok CC, French M, Mallal S, Christiansen F. The genetic basis for the association of the 8.1 ancestral haplotype (A1, B8, DR3) with multiple immunopathological diseases. Immunol Rev. 1999;167:257–274. doi: 10.1111/j.1600-065x.1999.tb01398.x. [DOI] [PubMed] [Google Scholar]
- Rawlins M, Thompson J. Pathogenesis of adverse drug reactions. In: Davies D, editor. Textbook of adverse drug reactions. Oxford University Press; Oxford: 1977. [Google Scholar]
- Robinson J, Halliwell JA, McWilliam H, Lopez R, Marsh SG. IPD–the Immuno Polymorphism Database. Nucleic Acids Res. 2013;41:D1234–1240. doi: 10.1093/nar/gks1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollini P, Mach B, Gorski J. Linkage map of three HLA-DR beta-chain genes: evidence for a recent duplication event. Proc Natl Acad Sci U S A. 1985;82:7197–7201. doi: 10.1073/pnas.82.21.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roujeau JC. Clinical heterogeneity of drug hypersensitivity. Toxicology. 2005;209:123–129. doi: 10.1016/j.tox.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Sukasem C, Puangpetch A, Medhasi S, Tassaneeyakul W. Pharmacogenomics of drug-induced hypersensitivity reactions: challenges, opportunities and clinical implementation. Asian Pac J Allergy Immunol. 2014;32:111–123. [PubMed] [Google Scholar]
- Traherne JA. Human MHC architecture and evolution: implications for disease association studies. Int J Immunogenet. 2008;35:179–192. doi: 10.1111/j.1744-313X.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetrecht J. Idiosyncratic drug reactions: current understanding. Annu Rev Pharmacol Toxicol. 2007;47:513–539. doi: 10.1146/annurev.pharmtox.47.120505.105150. [DOI] [PubMed] [Google Scholar]
- Uetrecht J, Naisbitt DJ. Idiosyncratic adverse drug reactions: current concepts. Pharmacol Rev. 2013;65:779–808. doi: 10.1124/pr.113.007450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas JM, Van der Veen AG, Ploegh HL. The known unknowns of antigen processing and presentation. Nat Rev Immunol. 2008;8:607–618. doi: 10.1038/nri2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman RW, Karl JA, Bohn PS, Nimityongskul FA, Starrett GJ, O’Connor DH. Haplessly hoping: macaque major histocompatibility complex made easy. ILAR J. 2013;54:196–210. doi: 10.1093/ilar/ilt036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman RW, Wojcechowskyj JA, Greene JM, Blasky AJ, Gopon T, Soma T, Friedrich TC, O’Connor SL, O’Connor DH. Simian immunodeficiency virus SIVmac239 infection of major histocompatibility complex-identical cynomolgus macaques from Mauritius. J Virol. 2007;81:349–361. doi: 10.1128/JVI.01841-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip VL, Alfirevic A, Pirmohamed M. Genetics of immune-mediated adverse drug reactions: a comprehensive and clinical review. Clin Rev Allergy Immunol. 2015;48:165–175. doi: 10.1007/s12016-014-8418-y. [DOI] [PubMed] [Google Scholar]


