Abstract
Toll-like receptors (TLRs) play a central role in the initiation of adaptive immune responses with several TLR agonists acting as known B-cell mitogens. Despite thousands of publications on TLRs, the function of TLR10 remains unknown. We have found that antibody mediated engagement of TLR10 on primary human B-cells suppresses B-cell proliferation, cytokine production and signal transduction. When challenged with either a T-independent or T-dependent antigen, TLR10 transgenic mice exhibit diminished antibody responses. Adoptive transfer of splenic B-cells into B-cell deficient mice revealed that the suppressive effects on antigen-specific humoral immune responses are entirely B-cell intrinsic. Our results demonstrate that TLR10 has a functional role within the B-cell lineage that is distinct from that of other TLR family members and may provide a potential therapeutic target for diseases characterized by dysregulated B-cell activity.
Introduction
As central elements of the innate immune system TLRs provide a first line of immune defense against infectious agents. Through direct sensing of bacterial, fungal or viral components TLRs activate intracellular signaling events that drive the cellular expression and release of immune mediators. These activation events not only drive inflammatory processes, but also initiate and orchestrate the longer term protective responses of the adaptive immune system (1).
Humans possess 10 TLR family members, numbered 1 through 10, which are differentially expressed in leukocytes and the epithelial cells of mucosal surfaces (2, 3, 4). Subsets of TLRs that are expressed on the plasma membrane stimulate the production of classic proinflammatory molecules while other TLRs expressed in endosomal compartments are best known for their ability to stimulate the production of type I IFNs (5, 6). All TLRs are type 1 transmembrane receptors comprised of extracellular leucine rich repeat domains and an intracellular TIR (Toll-Interleukin-1 Receptor homology) signaling domain. TLRs signal via ligand-induced receptor dimerization in which two juxtaposed TIR domains act as a scaffold for the recruitment of proximal adaptor molecules. With the exception of TLR3, which solely utilizes TRIF (TIR-domain-containing adaptor-inducing interferon-β), TLRs utilize the proximal adaptor MyD88 which is required for transducing signals that ultimately culminate in proinflammatory gene expression (7, 8).
TLR activation not only induces classic inflammatory mediators but also provides a critical link between the innate and adaptive arms of the immune response (9, 10). The ability of TLRs to induce adaptive responses is best understood through their actions on dendritic cells; however TLR subsets are also expressed on B-cells where they have direct stimulatory activity. For example, certain TLR agonists are well known T-independent (TI) antigens for B-cells. In addition, B-cell intrinsic TLR activation has been shown to promote antibody production and class-switching responses to both TI and T-dependent (TD) antigens (11, 12, 13). Importantly, TLR-mediated B-cell activation has been shown to be a major driver of disease progression in various mouse models of autoimmune disease. In addition to studies in mice, genome wide association studies, as well as in vitro studies with patient cells, have identified a significant role for TLRs in promoting both the progression and severity of autoimmune diseases, particularly systemic lupus erythematosus (SLE) (14, 15, 16).
TLRs have been the subject of intense research over the last decade providing a fairly clear picture of the ligand recognition, signaling and biologic functions of TLRs 1 through 9, but not TLR10. To date, TLR10 remains an orphan receptor with no agreed upon function in part due to the murine TLR10 gene being disrupted by several retroviral insertions making classical knockout studies impossible. Human TLR10, which was initially cloned and sequenced in 2001 (17), is most homologous to TLRs 1 and 6, and intact orthologues of the TLR10 gene have been found in every other sequenced mammal to date including several rodent species (18,19).
We have previously shown that similar to TLR1, TLR10 cooperates with TLR2 in the sensing of triacylated lipopeptide agonists. However, TLR10, either alone or in cooperation with TLR2, fails to induce typical TLR-associated signaling events including activation of NF-κB, IL-8 or IFN-β driven reporters (20). More recently, we and others have reported that TLR10 is able to suppress both MyD88-dependent and –independent signaling in mononuclear cell preparations ultimately inhibiting the production of inflammatory mediators including IL-6 and IFN-β (21, 22). We report here that TLR10 is functionally expressed on the surface of primary human B-cells and is able to suppress responses mediated by a variety of B-cell co-stimulatory signals. Furthermore, we show that in a TLR10 knock-in mouse model, TLR10 is able to suppress both TI and TD antibody production showing that human TLR10 is a functional receptor with a novel anti-inflammatory function in B-cells.
Material & Methods
Reagents
All cells were grown in RPMI 1640 supplemented with 10% FBS, 2mM glutamine and 1X non-essential amino acids. Anti-IgM and anti-mouse IgG antibodies were purchased from Jackson Laboratories. Anti-CD40 was purchased from R&D Systems. R848 and Class C CpG were purchased from InvivoGen. Phospho-specific antibodies p38 (clone D3F9), JNK (clone 81E11), Syk Y525/526 (C87C1), Akt S473 (D9E), β-actin (clone 13E5) were purchased from Cell Signaling Technologies. The isotype control antibody (clone MOPC-21) was purchased from BioLegend.
Two αTLR10 antibodies, 3C10C5 and 5C2C5, were generated with the assistance of the University of Illinois Immunological Resource Center. A soluble TLR10 fragment consisting of the extracellular domain of TLR10 (AA’s 20–474) was purified following the protocol we previously used for other TLRs (23). Female Balb/c mice were injected every three weeks with the TLR10 antigen and checked for serum antibody activity after the third immunization. Splenic cells were fused with SP2/0 tumor cells using polyethylene glycol and selected under HAT media. All hybridoma clones were counter screened against plate-bound soluble TLR1 to remove cross-reactive clones. Lastly, a subset of clones were chosen to determine their ability to stain HEK cell transfected with TLR10 or a CMV empty vector. From this screen, two clones, 3C10C5 and 5C2C5, were selected for future studies (Supplementary Figure 1). The αTLR10 clone 3C10C5 was chosen for licensing and is currently commercially available from several different biotechnology companies.
Flow Cytometry
Approximately 2–4 × 106 cells/test were blocked in flow buffer (1X PBS, 10% Rabbit Serum) for 30 min. Cells were centrifuged and re-suspended in 100 μL of flow buffer containing primary antibody at 10 μg/mL and incubated for 30 min. After washing, the cells were suspended in 100 μL of flow buffer containing αMouse IgG1-Biotin Conjugate (Jackson Laboratories) for 30 minutes. Cells were washed again and suspended in 100 μL of flow buffer containing a Streptavidin-APC conjugate (Jackson Laboratories). After washing, cells were fixed in 300 μL of 4% paraformaldehyde for 15 min after which they were washed and re-suspended in 100 μL of flow buffer. All labeling steps were performed on ice. Cells were analyzed on a BD FACS Canto II Flow Cytometer operated by the Roy J. Carver Biotechnology Center at the University of Illinois.
Reverse Transcriptase PCR
RNA was isolated using a RNeasy Mini Kit (Qiagen) following the manufacturer’s instructions. The cDNA was prepared with a 1:1 mixture of oligo-dT and random primers (Invitrogen), 1 μg of RNA and the Superscript III enzyme (Invitrogen). Reverse transcriptase negative samples were generated by withholding Superscript III from the reaction mixture. PCR was performed using AmpliTaq Gold 360 Master Mix (Applied Biosystems). Primers used for TLR10 (F -CAGTGGAACACTTTCAGATCC, R - CCAAGGGTGTGTTGTTAGC); HPRT1 (F - TGGGCTTACCTCACTGCTTT, R - CTAATCACGACGCTGGGACT).
Human B-cell Isolation
Peripheral blood B-cells were isolated by centrifuging whole blood over a Ficoll-Paque solution (GE Healthcare) at 1100xg for 10 min with 0 brake to obtain mononuclear cells (MNCs). B-cells were isolated from the MNCs using a MACS B-cell Isolation Kit II (Milteny Biotech) according to the manufacturer’s instructions. Tonsillar tissue, obtained from surgical discards at Carle Foundation hospital, was homogenized and passed through a 40μM filter. MNCs were obtained by centrifuging the filtered homogenate over a Ficoll-Paque solution as described above. MNCs were mixed at a 1:50 ratio with previously isolated human RBC’s and a Human B-cell Enrichment RossetteSep (STEMCELL Technologies) antibody cocktail prior to centrifuging over a Ficoll-Paque solution with the resulting buffy coat consisting of >95% B-cells.
Human B-cell Stimulation Assays
Isolated human B-cells were pre-incubated with either an isotype control (20μg/mL) or αTLR10 (3C10C5 at 20μg/mL) antibody with the addition of a secondary αMouse IgG1 F(ab)’2 fragment (20μg/mL) for 30 min. The secondary antibody is used to further cross-link the primary antibody and to prevent IgG FcR-mediated inhibition. After pre-incubation, cells are stimulated with a subset of the following: αIgM (20μg/mL), αCD40 (0.1μg/mL), the TLR7/8 agonist R848 (100ng/mL) or the TLR9 agonist CpG (2μg/mL). For the PCR Superarray, total RNA was isolated after 24 hours of stimulation and sampled using a T-cell and B-cell Activation PCR Array (SA Biosciences). Also after 24 hours, cell-free supernatants were assayed using a MIP-1β antibody pair (Life Technologies) using a 1:20 dilution. Proliferation was assayed using an ELISA based BrdU detection kit (Roche). In short, a 1X solution of BrdU was added to the cells. After 24 hours, the supernatant was removed and the cells fixed to the plate. Cells were then incubated with a HRP-conjugated-anti-BrdU antibody for 2 hours prior to washing and the addition of TMB for color detection at 450nm.
Human B-cell Signaling
Tonsillar B-cells were pre-incubated with an isotype control (20μg/mL) or αTLR10 antibody (20μg/mL) for 30 min prior to stimulation. After 15 min, cell lysates were generated in RIPA lysis buffer (150mM NaCl, 50mM Tris-HCl pH 8.0, 1% NP-40 and 1mM EDTA) supplemented with Halt protease and phosphatase inhibitor (Thermo Scientific). Equivalent amounts of protein lysate were separated by SDS-PAGE, transferred to PVDF membranes and blotted against specific phospho-antibodies (Cell Signaling Technologies).
Generation of TLR10 Transgenic Mice
A constitutive human TLR10 knock-in mouse expressing the full-length human TLR10 gene was generated by the UIUC Transgenic Facility. The BAC clone #1148D18 containing the full coding region of TLR10 with its native promoter was purchased from Empire Genomics. BAC DNA was isolated with Qiagen’s Large Construct Kit with genomic DNA removed by endonuclease digestion. Approximately 80μg of plasmid DNA was digested with 100 units of BsrB1 and HF-Not1 to release a 16,584bp fragment containing the full TLR10 transcript along with ~4kb of the upstream and ~3kb of the downstream sequence. Excised DNA was recovered with Qiagen’s Large Fragment Extraction Kit before continuing with the transgenic process. Fifty four transgenic pups from the C57BL/6 genetic background were received and screened for TLR10 insertion by PCR. Southern blot analysis revealed that seven of the transgenic pups carried the transgene and that 6 founders possessed at least 10 copies of the transgene. Three lines were selected for further characterization of TLR10 expression in various tissues by RT-PCR and immunoprecipitation. Line 5-1 was chosen for further experimental characterization. Genotyping of the mice was performed using For- CAGTGGAACACTTTCAGATCC and Rev-CCAAGGGTGTGTTGTTAGC primers for TLR10. All mice used for experiments were between the ages of 4 – 12 weeks. For consistency, only males were used in our experiments.
Splenic B-cell Isolation
Murine splenocytes were isolated by manual disruption of the spleen in sterile PBS followed by passage through a 40μM filter. MNCs were furthered purified by centrifuging through a Ficoll-Paque gradient. B-cells were isolated using STEMCELL Technologies EasySep Mouse B-cell Isolation Kit (Cat #19854A).
Murine B-cell Stimulations
Murine splenic B-cells are isolated as described above and prepared at a concentration of 2–3x106 B-cells/100μL of media in BD Falcon tubes. Prior to stimulation, cells were incubated in 10nM CFSE (Life Technologies) in PBS for 20 min at RT in the dark. Cells were then washed and re-suspended in media followed by stimulation with αIgM (20μg/mL), αCD40 (1μg/mL) or CpG (4μg/mL). After 72 hrs cell pellets were prepared for flow and run on a BD FACS Canto II flow cytometer to measure CFSE fluorescence in the FITC channel. Additionally, cell-free supernatants were collected and IL-6 was measured by paired antibody ELISA (Life Technologies).
Immunization & NP-Specific Antibody ELISA
Age and sex matched mice were immunized with either NP(27)-CGG or NP(7)-LPS (BioSearch Technologies) mixed in a 1:1 ratio with Imject Alum (ThermoScientific). Each mouse received a 200 μL intra-peritoneal injection of NP(27)-CGG or NP(7)-LPS equal to 100 ug and 20 ug of antigen respectively. Prior to immunization along with 7 and 14 days after immunization, blood was collected from the submandibular vein and the resulting serum stored. NP-specific ELISA plates were prepared by coating NP(5)-BSA (BioSearch Technologies) onto ThermoScientific Nunc plates at concentration of 10ug/mL in Coating Buffer B (4.3g NaHCO3, 5.3g Na2CO3, 1L H2O) and incubated at 4°C overnight. Plates were then blocked in Assay Buffer (1X PBS, 2% BSA, 0.05% Tween 20) for 1 hr. Samples used a 1:10,000 dilution for IgM and IgG1 and a 1:100 dilution for IgG2b, IgG2c, IgG3 and IgA. An 8-point standard curve was created by taking pooled undiluted serum from previous NP injections and creating serial dilutions starting at 1:50 or 1:5000 for each respective isotype. Samples and standards were allowed to incubate for 1 hr. Biotin conjugated goat anti-mouse detection antibodies (Southern Biotech) specific to each isotype were added at 50 ng/mL and allowed to incubate for 1 hr. Streptavidin-HRP (Jackson ImmunoResearch) was added at 0.1 ug/mL and incubated for 30 min before developing in an OPD solution. Wells were washed in ELISA Wash Buffer (1X PBS, 0.05% Tween 20) between each step and measured at 490 nm.
B-cell Transfer
Splenic B-cells were isolated as described previously from between 5–9 male mice per group. Splenocytes were pooled for subsequent B-cell isolation and then re-suspended in sterile PBS at a concentration of 1E8 B-cells/mL. A total of 200μL, equaling 2E7 B-cells was injected via intra-venous injection into the caudal tail vein of male μMT mice and allowed to colonize for 24 hrs before immunization. To further boost the immune reaction, LPS was added to the NP(27)-CGG immunizations equivalent to 5 ug of LPS per mouse.
Statistics
All statistics were performed using the GraphPad Prism software. Significance was determined by a t-test using the Holm-Sidak method.
Results
TLR10 Expression in Primary Lymphocytes
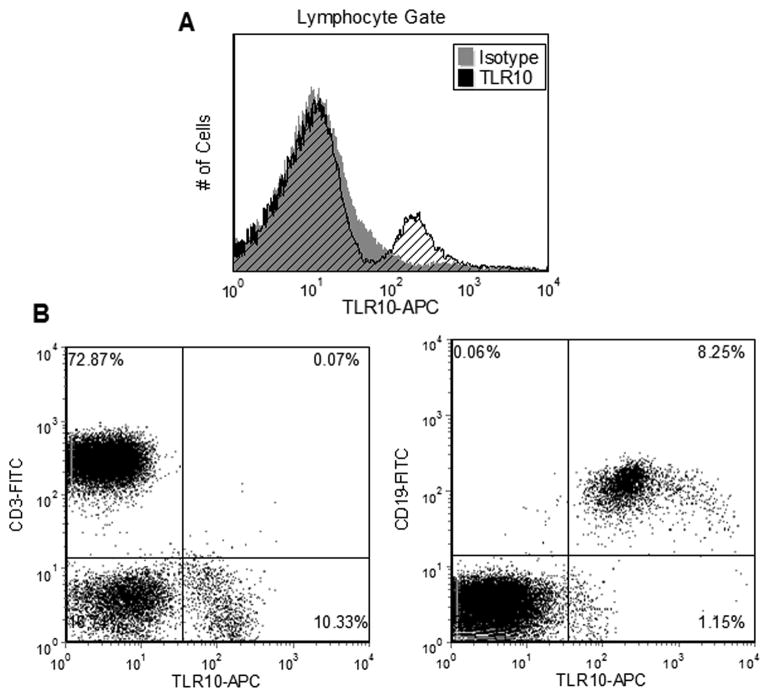
To assess human TLR10 expression, lymphocytes from whole blood of healthy individuals were labelled with an anti-TLR10 antibody developed in our lab. In order to detect weakly expressed TLR10, a biotin-streptavidin labeling procedure was employed prior to flow cytometric analysis (see Materials and Methods). Compared to an isotype control antibody, a shift in TLR10 fluorescence was detected in a subpopulation of the gated lymphocytes (Fig. 1A). Co-staining revealed that all lymphocytes expressing TLR10 also expressed the B-cell marker CD19, but not the T-cell marker CD3 (Fig. 1B). The presence of cell surface TLR10 on B-cells is consistent with high levels of TLR10 RNA message observed in lymphoid tissues believed to originate from B-cells. Our findings prompted us to investigate the function of this highly uncharacterized TLR in this immune cell.
Figure 1. TLR10 is expressed on the plasma membrane of primary human B-cells.
(a) Flow cytometry of lymphocytes gated from whole blood. Cells were triple labeled for TLR10 expression (see Materials & Methods) using either an isotype control or TLR10 antibody. (B) Lymphocytes were stained as in (A) for TLR10 with the addition of either the T-cell marker CD3 or the B-cell marker CD19 to differentiate the lymphocyte population into its constituent components.
TLR10 Antibody-Mediated Suppression of Primary Human B-cells
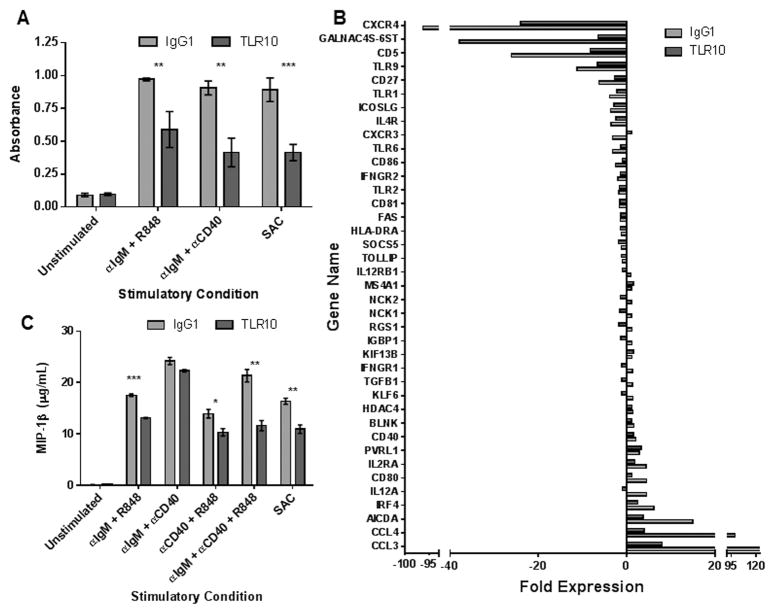
Upon appropriate co-stimulation, clonal B-cells undergo proliferation and differentiation to antibody producing plasma cells. To determine if TLR10 can suppress human B-cell proliferation, we assessed the effect of an αTLR10 monoclonal antibody on B-cell proliferation induced by co-stimulation through pairwise engagement of the BCR, TLR7 and the CD40 receptor. Compared to an isotype control antibody, the αTLR10 antibody suppressed the proliferation of B-cells, isolated from six different donors, irrespective of the stimulus (Fig. 2A). TLR10 engagement also suppressed B-cell proliferation induced by Staphylococcus aureus Cowan strain (SAC), a well-established B-cell mitogen. Together, these results suggest that TLR10 serves to dampen B-cell activation mediated by either T cell-independent or T cell-dependent mechanisms.
Figure 2. Primary human B-cell activation is suppressed by an αTLR10 antibody.
Human primary B-cells were isolated from peripheral blood of healthy donors by negative selection and pre-incubated with either an isotype control or TLR10 mAb for 30 minutes prior to stimulation. (A) B-cells were stimulated with the indicated agonists for 96 hours with BrdU added after 72 hours of stimulation. BrdU incorporation was assayed by ELISA on six different donors. (B) B-cells were stimulated with αIgM and CpG for 24 hours after which RNA was isolated and assayed for activation using a B-cell PCR superarray. Bars represent the fold expression of stimulated cells compared to control cells which were left unstimulated in the presence of the isotype control antibody for 30 min. (C) B-cells were stimulated with the indicated agonists. Cell-free supernatants were collected after 24 hours and assayed for MIP-1β by ELISA.* p < 0.1; ** p < 0.05; *** p < 0.01
B-cell stimulation results in changes in expression of a wide range of genes which function to drive functional B-cell responses. To more broadly examine the effect of TLR10 engagement on B-cell activation, we co-stimulated B-cells through the BCR and TLR9 for 24 hours in the presence of either αTLR10 or an isotype control antibody. After stimulation, RNA was collected and the expression of an array of lymphocyte specific activation genes was measured by real-time PCR. Of the 84 genes tested, 39 exhibited statistically significant changes as either an increase or decrease in expression following B-cell stimulation. Compared to an isotype control antibody, αTLR10 affected 33 of the 39 genes by consistently dampening the increase or decrease in individual gene expression normally induced by B-cell stimulation (Fig. 2A, Supplementary Table 1). The three genes that exhibited the greatest degree of suppression were Chemokine Ligand 3 (CCL3), Chemokine Ligand 4 (CCL4) and Activation Induced Cytosine Deaminase (AICDA). The chemokine CCL3 and CCL4, also known as MIP-1α and MIP-1β respectively, are each potent chemoattractants secreted by lymphocytes that function to drive lymphocyte migration in the germinal center. AICDA is necessary for both somatic hypermutation and class switch recombination during clonal expansion in the germinal center.
To confirm that the suppression observed in the RNA array translates to functional effects, secretion of MIP-1 β (CCL4) was measured from B-cells stimulated in the presence of either isotype control or the αTLR10 antibody. TLR10 antibody engagement suppressed the production of MIP-1 β induced by pairwise co-stimulation of either the BCR and TLR7 or the BCR and CD40 which serve to mimic T-independent and T-dependent activation, respectively (Fig. 2C). TLR10 engagement also suppressed MIP-1 β production induced by Staphylococcus aureus Cowan strain. These results suggest that the TLR10 not only suppresses TLR-mediated activation events but also broadly regulates B-cell activation induced by a variety of stimuli.
Suppression of BCR Signaling through TLR10
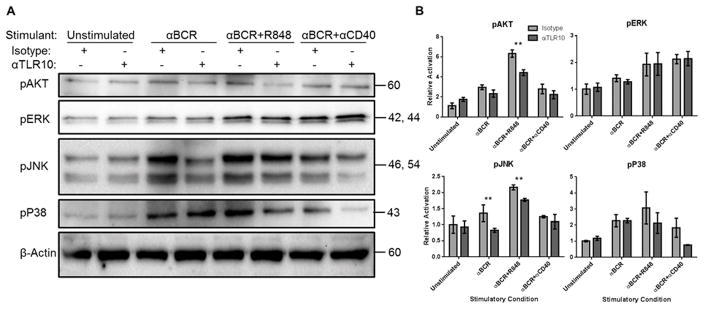
Given the broad suppressive activity of TLR10, we next assessed the effect of the αTLR10 antibody on a number of early signaling events which drive B-cell activation. To this end, primary human B-cells were stimulated through the BCR either alone or with TLR7 or CD40 as a co-stimulus. Compared to isotype control, we observed that the αTLR10 antibody was able to suppress the phosphorylation of the MAP kinase JNK but not P38 or ERK. Additionally, the αTLR10 antibody was able to suppress Akt signaling when stimulated with αBCR and R848, suggesting TLR10 may also be able to target the PI3 kinase pathway (Fig. 3).
Figure 3. BCR signaling is suppressed by a TLR10 mAb on primary human B-cells.
Primary human B-cells were isolated from tonsillar tissue by homogenization, density gradient centrifugation and a negative selection antibody cocktail. B-cells were pre-incubated with an isotype control or TLR10 mAb for 30 min prior to stimulation with the indicated agonist for 15 minutes after which the cells were lysed and frozen at −20°C. (A) Lysates were probed for phosphorylated targets of TLR signaling. Data is representative of three independent experiments (two for pP38). (B) Blots were normalized against β-Actin and densities shown relative to the unstimulated isotype control cells. Error bars represent the standard error of the mean. * p < 0.1; ** p < 0.05
Generation of TLR10 Transgenic Mice
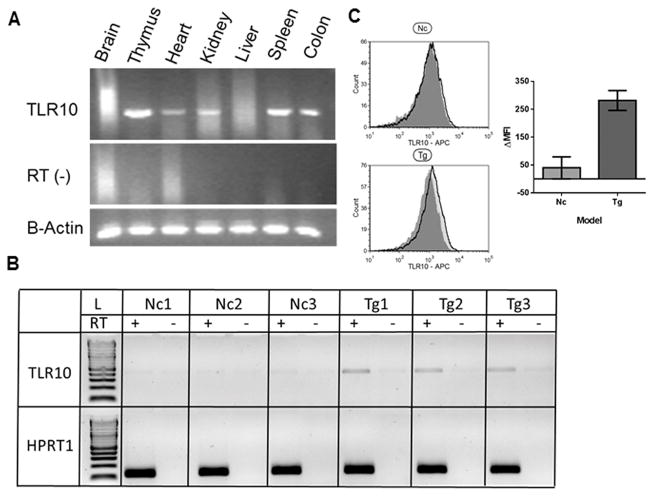
To better study TLR10, we sought to develop a suitable animal model. Since TLR10 is a pseudogene in mice, and orthologues of the TLR10 gene are present in all other rodents and mammals examined thus far, it seemed reasonable to believe that humanTLR10 would function in mice. Therefore, the full-length human TLR10 gene, including several kilobases of the 5′ and 3′ UTR, was stably integrated in the mouse genome (see Materials and Methods). A total of three TLR10 transgenic founder lines were established that both expressed detectable levels of TLR10 and successfully passed the transgene to offspring. Of the three founder lines, line 5-1 was selected for further functional assessment based on intermediate levels of TLR10 expression and sizable litters. Line 5-1 exhibits RT-dependent expression of TLR10 in a variety of different tissues (Fig. 4A). TLR10 transgenic mice are viable, breed well and do not exhibit any overt physical abnormities.
Figure 4. Human TLR10 transgenic mice stably express TLR10 on B-cells.
(A) Mouse organs were harvested and homogenized in Trizol with a Dounce homogenizer prior to RNA isolation from each indicated tissue. RNA was reverse transcribed in the presence and absence of reverse transcriptase (RT-) and checked for TLR10 expression by PCR. (B) Isolated murine B-cell RNA was isolated from 3 non-transgenic and 3 transgenic mice before preparing cDNA and assaying for TLR10 as in (A). (C) Murine spleenocytes from 3 non-transgenic and 3 transgenic mice were triple labeled for TLR10 and the B-cell markers B220 and IgM. Histogram shows the TLR10 fluorescence compared to an isotype control antibody from a representative non-transgenic and transgenic mouse. Bar graph shows the ΔMFI from the 3 transgenic and non-transgenic mice.
Suppression of ex vivo Transgenic B-cell Activation
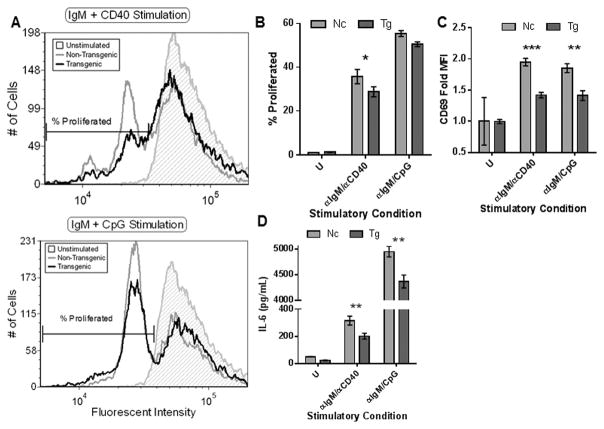
Given that TLR10 is expressed by human B-cells we examined isolated splenic B-cells from TLR10 transgenic mice and found that the receptor was expressed at both the mRNA and protein level (Fig. 4B,C). To assess the effects of TLR10 expression, we compared the proliferative responses of splenic B-cells as a result of ex vivo stimulation. Compared to non-transgenic control mice, splenocytes from TLR10 transgenic mice exhibited less proliferation when stimulated with αIgM/αCD40 according to the CFSE profile of viable cells as determined by their forward and side scatter characteristics. Although αIgM/CpG stimulation showed similar trends, the data did not reach statistical significance (p = 0.116). Since we did not measure absolute numbers of dividing cells, the data do not address potential differences in activation-induced cell death (Fig 5A, B). Transgenic B-cells, irrespective of either of the two stimulatory conditions we tested, exhibited significantly decreased CD69 expression and IL-6 secretion compared to non-transgenic control mice (Fig. 5C, D). These data demonstrate that human TLR10 is capable of suppressing murine B-cell activation ex vivo and that TLR10-mediated suppression of B-cells is conserved across different mammalian species.
Figure 5. Primary TLR10 transgenic murine B-cell activation is suppressed compared to non-transgenic control mice.
Murine spleens from 3 transgenic and 3 non-transgenic mice were collected, pooled and a single-cell suspension was obtained. Murine B-cells were further purified by a negative selection antibody cocktail. (A, B, C) B-cells were pre-incubated with CFSE prior to stimulation with the indicated agonists for 72 hours. After stimulation, live cells were gated by forward and side scatter characteristics. (A) Representative example of a fluorescent CFSE B-cell cycling profile. The percentage of divided B-cells (B) and CD69 expression (C) was analyzed from three different replicates based upon the marker present in (A). (D) Pooled B-cells were stimulated for 24 hours with the indicated agonists and cell-free supernatant collected to measure mIL-6 production. Data is representative of three independent experiments. * p < 0.1; ** p < 0.05; *** p < 0.01
The ex vivo experiments on splenic B-cells were conducted without the addition of an αTLR10 antibody suggesting that the observed suppression is the direct result of heterologous expression of human TLR10. This prompted us to assess whether TLR10 transgenic mice had deficiencies in either B-cell development or antibody production. To assess the former, we examined the ratio and abundance of the major B-cell subpopulations in the bone marrow, blood, spleen and lymph nodes but no differences were observed between TLR10 transgenic and non-transgenic littermate control mice (Supplementary Table 2). Additionally, there was no difference in the steady state serum concentrations between the TLR10 transgenic mice compared to the non-transgenic control mice for any of the immunoglobulin isotypes (Supplementary Figure 2). Together, these results demonstrate that human TLR10 transgene expression in mice does not grossly effect B-cell development and antibody production.
Transgenic B-cells are Suppressed in vivo
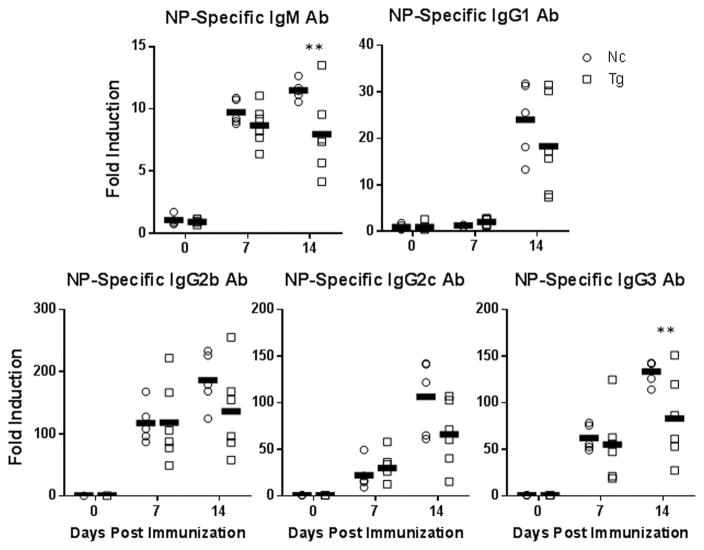
To assess the effect of TLR10 expression on B-cell responses in vivo, we immunized transgenic or non-transgenic control mice with the TI antigen, and B-cell mitogen, LPS which was conjugated to the hapten nitrophenol (NP). One and two weeks after immunization, serum levels of NP-specific antibodies in TLR10 transgenic mice were significantly decreased in both the IgM and IgG3 subclasses compared to serum levels in immunized non-transgenic control mice (Fig. 6). NP-specific antibody levels for other immunoglobulin subclasses also trended lower in TLR10 transgenic mice but did not reach significance in our studies. These results demonstrate that TLR10 transgenic mice are deficient in TI antibody responses to LPS however, since TLR10 is expressed throughout various cells and tissues of the mice the in vivo effects observed may be extrinsic to B-cells.
Figure 6. TLR10 transgenic mice exhibit suppressed type 1 T-independent antibody responses.
Six transgenic and non-transgenic age-matched male mice were immunized with 10 μg of NP-LPS by i.p. injection. Serum was collected one day pre-immunization and seven and fourteen day’s post-immunization. Serum was assayed for the presence of NP-specific antibodies by ELISA. Arbitrary units were calculated by a 7-point serially diluted standard curve of previously immunized mouse serum and then normalized to day 0. * p < 0.1; ** p < 0.05; *** p < 0.01
Transgenic TLR10 Suppression is B-cell Intrinsic
To determine whether the in vivo effects of TLR10 on antibody responses are B-cell intrinsic we performed adoptive transfer experiments in which μMT mice, naturally deficient in B-cells, received pooled splenic B-cells from either TLR10 transgenic or non-transgenic control mice prior to immunization with NP-LPS. Initial adoptive transfer experiments showed no difference in the populations of transgenic or non-transgenic B220+ B-cells residing in the spleen of μMT mice 24 hrs after injection (unpublished data).
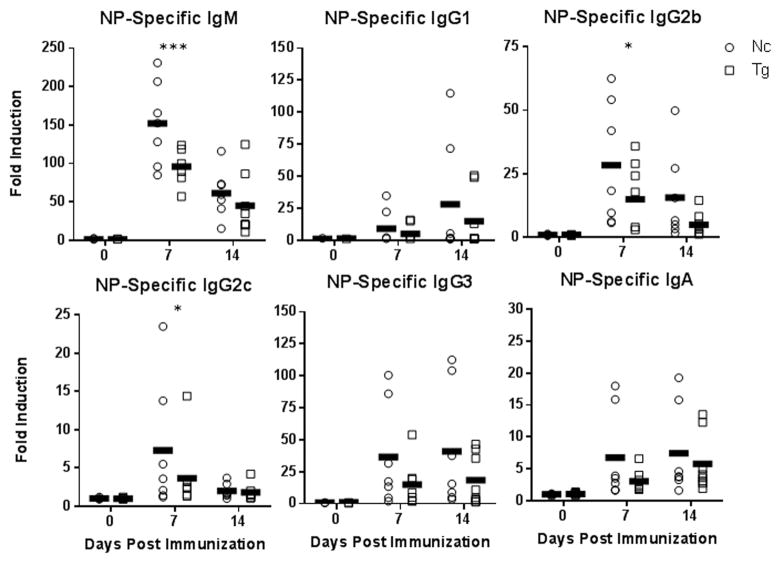
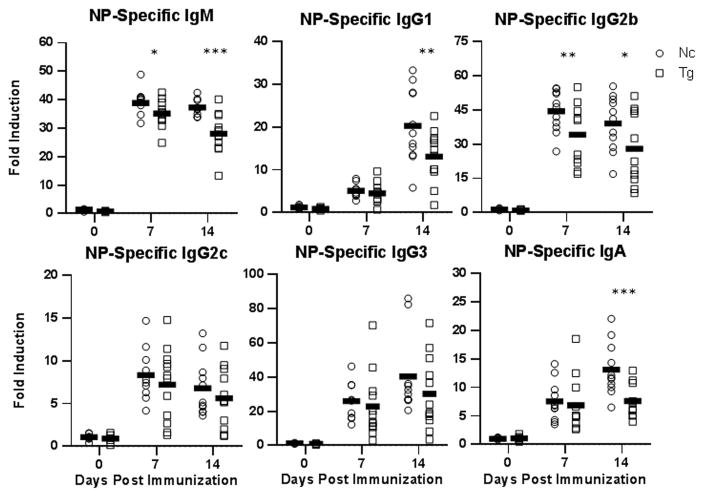
Similar to the above immunization findings, mice reconstituted with B-cells from TLR10 transgenic mice had significantly lower NP-specific IgM antibody responses to NP-LPS that those reconstituted with B-cells from non-transgenic control mice. Additionally, NP-specific antibody levels for all the other immunoglobulin isotypes trended lower in mice receiving B-cells from TLR10 transgenic mice (Fig. 7). Together, these findings show that the suppression of antibody responses by TLR10 to the TI antigen LPS is B-cell intrinsic. Our in vitro studies showed that TLR10 suppresses B-cell responses and signaling in response to CD40 co-stimulation suggesting that TLR10 may also regulate immune responses to TD antigens. To assess this, we immunized μMT mice, reconstituted with splenic B-cells from either TLR10 transgenic or non-transgenic mice, with the TD protein antigen NP-CGG (chicken-gamma globulin) along with an immune adjuvant. In addition to IgM, NP-specific serum antibody levels of IgG1, IgG2b and IgA were all significantly reduced in mice receiving TLR10 transgenic B-cells with other antibody isotypes also trending lower (Fig. 8). This shows that, even when presented with co-stimulatory signals from T-cells, TLR10 is able to suppress B-cell antibody responses. Taken together, we conclude that TLR10 is a B-cell intrinsic suppressor of both TI and TD B-cell activation.
Figure 7. TLR10 transgenic mice type-1 T-independent antibody response suppression is B-cell intrinsic.
B-cells from five male transgenic and five male non-transgenic mice were isolated and pooled. Approximately 2E7 B-cells were i.v. injected into eight age-matched μMT mice. One day post-transfer, mice were immunized with 20 μg of NP-LPS by i.p. injection. Serum was collected and assayed as described in figure 6. * p < 0.1; ** p < 0.05; *** p < 0.01
Figure 8. TLR10 transgenic mice T-dependent antibody response suppression is B-cell intrinsic.
B-cells were collected and transferred as described in figure 7. One day post-transfer mice were immunized with 100 μg of NP-CGG and 5 μg of LPS by i.p. injection. Serum was collected and assayed as described in figure 6. * p < 0.1; ** p < 0.05; *** p < 0.01
Discussion
Since its reported discovery in 2001 (17), the biological function of TLR10 has remained unknown. Recently, several groups have ascribed different biological functions for TLR10 of whose function is now controversial. We have previously shown that when overexpressed in a monocytic cell line or in a transgenic mouse, TLR10 is able to suppress TLR signaling irrespective of either MyD88 or TRIF recruitment (22). In this manuscript, we support the hypothesis that TLR10 is a novel suppressor of inflammatory responses and can suppress the adaptive immune responses of B-cells in vivo.
Since the discovery of TLRs, much of the focus has been on how TLRs positively regulate inflammation although more recently research has focused on the negative regulation of TLR signaling. Two proteins, SIGIRR and ST2, have been shown to negatively regulate TLR signaling by sequestering the proximal adaptor MyD88 (24, 25, 26). This regulation is most likely not how TLR10 functions though as TLR10 has the ability to suppress both TLR-dependent and TLR-independent signaling in B-cells (22).
Additionally, the observation that TLR10 is not under convergent evolution, has numerous nonsynonymous mutations in its TIR domain and is able to form a stable homodimer may allow TLR10 to recruit novel TIR-domain containing proteins (18, 19, 27). TLR10 contains several mutations within the BB loop of its TIR domain which has been shown to be important in TLR signaling. Furthermore, those mutations are conserved across different mammalian TLR10 proteins suggesting it has important implications in TLR10 signaling (18). Much of TLR biology focuses on the five well-studied TIR-domain containing proteins; MyD88, MAL (TIRAP), TRIF, TRAM and SARM while there exists the possibility that there are additional TIR-domain containing proteins either understudied or undiscovered (5). Two such examples are the proteins B-cell adaptor for PI3K (BCAP) (28) and B-cell scaffold with ankyrin repeats 1 (BANK1) (29) which are novel TIR-domain containing protein that has been linked to TLR signaling. More importantly, a BCAP knockout mouse was shown to have elevated levels of inflammation in response to a S. typhimurium infection (28). This highlights the possibility that TLR10 could be recruiting a novel TIR-domain protein to mediate the suppression.
There is also a growing body of evidence that there is a large synergy between the BCR, TLRs and TNF receptors in B-cells. Several key mediators of each pathway have been shown to be important in the regulation of the complementary pathway such as the proteins Protein Kinase D (PKD) (30), Bruton’s Tyrosine Kinase (BTK) (31), TRAF5 (32) and Syk (33). It is possible that by suppressing one of these pathways, TLR10 is able to alter B-cell signaling that allows it to suppress several pathways simultaneously.
In support of our study, another manuscript from our lab has shown that when TLR10 is overexpressed in cell lines or in a mouse model, it is able to suppress proinflammatory signaling and cytokine production. We also show that TLR10-engagment with a monoclonal antibody is able to suppress IL-6 production in primary mononuclear cells (22). In a different study, another group showed that TLR2 responses to either PAM3CSK4 or whole Borrelia bugdorferi are suppressed after TLR10-engagment with a monoclonal antibody. They also use a transgenic mouse model to confirm that TLR10 is able to suppress TLR2-mediated inflammatory signaling (21). Our study expands upon this work by showing that TLR10-mediated suppression is not limited to mononuclear cells and can also suppress B-cell adaptive immune responses.
Two other studies have presented an opposing hypothesis that TLR10 is a pro-inflammatory receptor. They use either THP-1 or HT-29 cell lines to knockdown TLR10 followed by challenge with either H1N1 flu virus or live Listeria monocytogenes. In each case, they reported increased expression of proinflammatory cytokines only upon infection with live pathogen (34, 35). This suggests a possible alternative function for TLR10, perhaps in the context of live intracellular pathogens that will require further investigation.
Nonetheless, our study has vast implications for many B-cell malignancies that are driven by dysregulated TLR activation. It is now well established that unwanted or over activation of TLR 7, 8 and 9 in B-cells are drivers of autoimmune diseases. Studies have shown that mice deficient in TLRs 7–9 have attenuated autoimmune diseases characterized by autoantibody production as in rheumatoid arthritis and lupus (16, 36). Additionally, genetic polymorphisms in several TLR signaling components such as IRF5, IRAK1 and TNFAIP3 have been shown to predispose individuals to lupus (37). This manuscript highlights the ability of TLR10-engagment to suppress B-cell responses after TLR activation, potentially offering a therapeutic opportunity for individuals with B-cell autoimmune diseases.
TLR10 remains a vastly understudied TLR that for many years was believed to have no biological function. In this manuscript, we have shown that TLR10 is expressed within human B-cells and through antibody-mediated crosslinking can suppress the activation of human B-cells. Furthermore, we have shown that the TLR10 machinery is conserved across mammals by creating a knock-in mouse model that expresses the full-length human TLR10 gene under its native human promoter which has the ability to suppress murine B-cell activation. Lastly, we have shown that the TLR10-mediated suppression is a B-cell intrinsic property and is able to suppress both T-cell independent and T-cell dependent antibody responses. This initial evidence that TLR10 is a B-cell suppressor may assist in the future development of novel immunotherapies for B-cell autoimmune diseases.
Supplementary Material
Acknowledgments
This work was supported by the NIAID of the NIH; grant 1R01-AI097639
The authors would like to the thank the Transgenic Mouse Facility at the Roy J. Carver Biotechnology Center at the University of Illinois Urbana-Champaign for their assistance in designing and creating the TLR10 transgenic mouse line used in this study.
Abbreviations
- TIR
Toll/Interleukin Receptor
- TRIF
TIR-domain-containing adaptor-inducing interferon-β
- TI
T-cell independent
- TD
T-cell dependent
- SLE
systemic lupus erythematosus
- SAC
staphylococcus aureus cowan strain
- NP
nitrophenol
Footnotes
Author Contributions
S.J., X.L., Y.G. and R.I.T. designed the study; N.J.H., S.J., X.L. performed the research; N.J.H. and S.J. analyzed the data; and N.J.H. and R.I.T. wrote the manuscript.
Competing Financial Interests
The authors declare no competing financial interests.
Literature Cited
- 1.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 2.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdörfer B, Giese T, Endres S, Hartmann G. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 3.Dorner M, Brandt S, Tinguely M, Zucol F, Bourquin JP, Zauner L, Berger C, Bernasconi M, Speck RF, Nadal D. Plasma cell toll-like receptor (TLR) expression differs from that of B cells, and plasma cell TLR triggering enhances immunoglobulin production. Immunology. 2009;128:573–579. doi: 10.1111/j.1365-2567.2009.03143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourke E, Bosisio D, Golay J, Polentarutti N, Mantovani A. The toll-like receptor repertoire of human B lymphocytes: inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood. 2003;102:956–963. doi: 10.1182/blood-2002-11-3355. [DOI] [PubMed] [Google Scholar]
- 5.Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Neill LAJ. When signaling pathways collide: positive and negative regulation of toll-like receptor signal transduction. Immunity. 2008;29:12–20. doi: 10.1016/j.immuni.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Gay NJ, Symmons MF, Gangloff M, Bryant CE. Assembly and localization of Toll-like receptor signalling complexes. Nat Rev Immunol. 2014;14:546–558. doi: 10.1038/nri3713. [DOI] [PubMed] [Google Scholar]
- 8.Lin SC, Lo YC, Wu H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature. 2010;465:885–890. doi: 10.1038/nature09121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janeway CA. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Medzhitov R. Approaching the asymptote: 20 years later. Immunity. 2009;30:766–775. doi: 10.1016/j.immuni.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Rawlings DJ, Schwartz MA, Jackson SW, Meyer-Bahlburg A. Integration of B cell responses through Toll-like receptors and antigen receptors. Nat Rev Immunol. 2012;12:282–294. doi: 10.1038/nri3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 13.Browne EP. Regulation of B-cell responses by Toll-like receptors. Immunology. 2012;136:370–379. doi: 10.1111/j.1365-2567.2012.03587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koh YT, Scatizzi JC, Gahan JD, Lawson BR, Baccala R, Pollard KM, Beutler BA, Theofilopoulos AN, Kono DH. Role of nucleic acid-sensing TLRs in diverse autoantibody specificities and anti-nuclear antibody-producing B cells. J Immunol. 2013;190:4982–4990. doi: 10.4049/jimmunol.1202986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pelka K, Shibata T, Miyake K, Latz E. Nucleic acid-sensing TLRs and autoimmunity: novel insights from structural and cell biology. Immunol Rev. 2016;269:60–75. doi: 10.1111/imr.12375. [DOI] [PubMed] [Google Scholar]
- 16.Shlomchik MJ. Activating systemic autoimmunity: B’s, T’s, and tolls. Curr Opin Immunol. 2009;21:626–633. doi: 10.1016/j.coi.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chuang T, Ulevitch RJ. Identification of hTLR10: a novel human Toll-like receptor preferentially expressed in immune cells. Biochim Biophys Acta. 2001;1518:157–161. doi: 10.1016/s0167-4781(00)00289-x. [DOI] [PubMed] [Google Scholar]
- 18.Roach JC, Glusman G, Rowen L, Kaur A, Purcell MK, Smith KD, Hood LE, Aderem A. The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci USA. 2005;102:9577–9582. doi: 10.1073/pnas.0502272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mikami T, Miyashita H, Takatsuka S, Kuroki Y, Matsushima N. Molecular evolution of vertebrate Toll-like receptors: evolutionary rate difference between their leucine-rich repeats and their TIR domains. Gene. 2012;503:235–243. doi: 10.1016/j.gene.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Guan Y, Ranoa DRE, Jiang S, Mutha SK, Li X, Baudry J, Tapping RI. Human TLRs 10 and 1 share common mechanisms of innate immune sensing but not signaling. J Immunol. 2010;184:5094–5103. doi: 10.4049/jimmunol.0901888. [DOI] [PubMed] [Google Scholar]
- 21.Oosting M, Cheng SC, Bolscher JM, Vestering-Stenger R, Plantinga TS, Verschueren IC, Arts P, Garritsen A, van Eenennaam H, Sturm P, Kullberg BJ, Hoischen A, Adema GJ, van der Meer JWM, Netea MG, Joosten LAB. Human TLR10 is an anti-inflammatory pattern-recognition receptor. Proc Natl Acad Sci USA. 2014;111:E4478–4484. doi: 10.1073/pnas.1410293111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang S, Li X, Hess NJ, Guan Y, Tapping RI. TLR10 Is a Negative Regulator of Both MyD88-Dependent and -Independent TLR Signaling. J Immunol. 2016;196:3834–3841. doi: 10.4049/jimmunol.1502599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ranoa DRE, Kelley SL, Tapping RI. Human lipopolysaccharide-binding protein (LBP) and CD14 independently deliver triacylated lipoproteins to Toll-like receptor 1 (TLR1) and TLR2 and enhance formation of the ternary signaling complex. J Biol Chem. 2013;288:9729–9741. doi: 10.1074/jbc.M113.453266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kondo T, Kawai T, Akira S. Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol. 2012;33:449–458. doi: 10.1016/j.it.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Wald D, Qin J, Zhao Z, Qian Y, Naramura M, Tian L, Towne J, Sims JE, Stark GR, Li X. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2003;4:920–927. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- 26.Brint EK, Xu D, Liu H, Dunne A, McKenzie ANJ, O’Neill LAJ, Liew FY. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol. 2004;5:373–379. doi: 10.1038/ni1050. [DOI] [PubMed] [Google Scholar]
- 27.Nyman T, Stenmark P, Flodin S, Johansson I, Hammarström M, Nordlund P. The crystal structure of the human toll-like receptor 10 cytoplasmic domain reveals a putative signaling dimer. J Biol Chem. 2008;283:11861–11865. doi: 10.1074/jbc.C800001200. [DOI] [PubMed] [Google Scholar]
- 28.Troutman TD, Hu W, Fulenchek S, Yamazaki T, Kurosaki T, Bazan JF, Pasare C. Role for B-cell adapter for PI3K (BCAP) as a signaling adapter linking Toll-like receptors (TLRs) to serine/threonine kinases PI3K/Akt. Proc Natl Acad Sci USA. 2012;109:273–278. doi: 10.1073/pnas.1118579109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu YY, Kumar R, Iida R, Bagavant H, Alarcón-Riquelme ME. BANK1 Regulates IgG Production in a Lupus Model by Controlling TLR7-Dependent STAT1 Activation. PLoS ONE. 2016;11:e0156302. doi: 10.1371/journal.pone.0156302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haxhinasto SA, Bishop GA. A novel interaction between protein kinase D and TNF receptor-associated factor molecules regulates B cell receptor-CD40 synergy. J Immunol. 2003;171:4655–4662. doi: 10.4049/jimmunol.171.9.4655. [DOI] [PubMed] [Google Scholar]
- 31.Kenny EF, Quinn SR, Doyle SL, Vink PM, van Eenennaam H, O’Neill LAJ. Bruton’s tyrosine kinase mediates the synergistic signalling between TLR9 and the B cell receptor by regulating calcium and calmodulin. PLoS ONE. 2013;8:e74103. doi: 10.1371/journal.pone.0074103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buchta CM, Bishop GA. TRAF5 negatively regulates TLR signaling in B lymphocytes. J Immunol. 2014;192:145–150. doi: 10.4049/jimmunol.1301901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ying H, Li Z, Yang L, Zhang J. Syk mediates BCR- and CD40-signaling integration during B cell activation. Immunobiology. 2011;216:566–570. doi: 10.1016/j.imbio.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Regan T, Nally K, Carmody R, Houston A, Shanahan F, Macsharry J, Brint E. Identification of TLR10 as a key mediator of the inflammatory response to Listeria monocytogenes in intestinal epithelial cells and macrophages. J Immunol. 2013;191:6084–6092. doi: 10.4049/jimmunol.1203245. [DOI] [PubMed] [Google Scholar]
- 35.Lee SMY, Kok KH, Jaume M, Cheung TKW, Yip TF, Lai JCC, Guan Y, Webster RG, Jin DY, Peiris JSM. Toll-like receptor 10 is involved in induction of innate immune responses to influenza virus infection. Proc Natl Acad Sci USA. 2014;111:3793–3798. doi: 10.1073/pnas.1324266111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herlands RA, Christensen SR, Sweet RA, Hershberg U, Shlomchik MJ. T cell-independent and toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity. 2008;29:249–260. doi: 10.1016/j.immuni.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delgado-Vega A, Sánchez E, Löfgren S, Castillejo-López C, Alarcón-Riquelme ME. Recent findings on genetics of systemic autoimmune diseases. Curr Opin Immunol. 2010;22:698–705. doi: 10.1016/j.coi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.