Summary
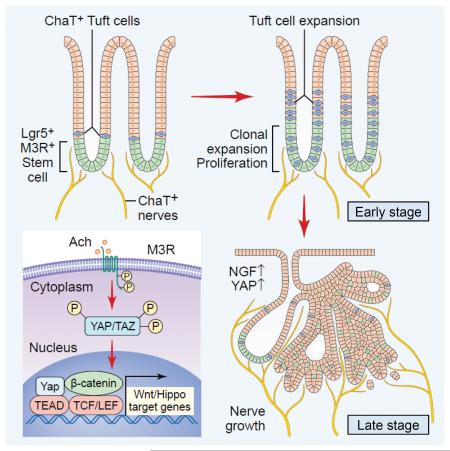
Within the gastrointestinal stem cell niche, nerves help to regulate both normal and neoplastic stem cell dynamics. Here, we reveal the mechanisms underlying the cancer-nerve partnership. We find that Dclk1+ tuft cells and nerves are the main sources of acetylcholine (ACh) within the gastric mucosa. Cholinergic stimulation of the gastric epithelium induced nerve growth factor (NGF) expression, and in turn NGF overexpression within gastric epithelium expanded enteric nerves and promoted carcinogenesis. Ablation of Dclk1+ cells or blockade of NGF/Trk signaling inhibited epithelial proliferation and tumorigenesis in a muscarinic acetylcholine receptor-3 (M3R)-dependent manner, in part through suppression of Yes-Associated Protein (YAP) function. This feed-forward ACh-NGF axis activates the gastric cancer niche and offers a compelling target for tumor treatment and prevention.
Keywords: NGF, gastric cancer, acetylcholine, Lgr5, Wnt, YAP, Dclk1, tuft cell, stem cell, muscarinic acetylcholine recepter type 3
Graphical abstract
Introduction
There has been considerable interest in the biologic and therapeutic implications of neural regulation of normal stem cells and cancer growth (Brownell et al., 2011; Hanoun et al., 2014; Katayama et al., 2006; Magnon et al., 2013; Mendez-Ferrer et al., 2010; Peterson et al., 2015; Stopczynski et al., 2014; Venkatesh et al., 2015; Zhao et al., 2014). In the gastrointestinal tract, acetylcholine (ACh) regulates epithelial stem cells, proliferation, and tumorigenesis via the muscarinic receptor-3 (M3R), in part through modulation of Wnt signaling (Lundgren et al., 2011; Raufman et al., 2008; Zhao et al., 2014). Canonical Wnt activation is characterized by nuclear translocation of β-catenin leading to activation of the transcriptional factor T-cell factor (TCF) family and target gene expression. However, to achieve TCF activation, multiple transcriptional co-activators are required, including the Yes-associated protein (YAP) (Rosenbluh et al., 2012), which appears to form an important part of the ACh-M3R axis.
Although many cancers, including stomach, pancreas, and colon, show increased nerve density (Albo et al., 2011; Ceyhan et al., 2010; Zhao et al., 2014), the overall significance of tumor-associated neural plasticity remains uncertain. The neurotrophin family molecules signal through Trk receptors to support neuron survival and axonal growth. In cancer, there is often an upregulation of neurotrophins or Trk receptors to activate cancer cell proliferation in an autocrine manner (Dolle et al., 2004; Weeraratna et al., 2001). Trk inhibitors that suppress neurotrophin signaling have been used in the treatment of cancers characterized by an activated Trk fusion protein (Vaishnavi et al., 2015). Given the relevance of neurotrophin/Trk signaling in neural development, and the possible importance of this pathway in cancer signaling, we hypothesized that neurotrophin/Trk signaling might represent a potent driver of peritumoral innervation and tumor growth.
The enteric nervous system (ENS) has an ability to regulate gastrointestinal homeostasis through direct innervations to gastrointestinal crypts (Gross et al., 2012; Neal and Bornstein, 2007), which appears to be linked to epithelial homeostasis. For example, sympathetic nerves accelerate crypt cell proliferation through norepinephrine (Tutton and Helme, 1973), and serotonin from ENS components promotes growth and turnover of the mucosal epithelium, by regulating muscarinic cholinergic innervation to epithelial effectors (Gross et al., 2012; Tutton and Barkla, 1986). Although the role of cholinergic signaling in gut proliferation and cancer has been suggested, the precise molecular mechanism in the ENS-cancer interaction remains uncertain.
Nerves also promote mucosal regeneration indirectly via Dclk1+ tuft cells. Dclk1+ tuft cells act, in part, as intermediary niche cells coordinating neural input to help regulate subsequent stem cell activity (Chandrakesan et al., 2015; Westphalen et al., 2014). Tuft cells express choline-acetyltransferase (ChAT), the enzyme responsible for ACh production, and they have a neuron-like gene expression signature (Schutz et al., 2015), and also express cytokines such as IL-25 and cyclooxygenase-2 (Cox2), and help to mediate inflammatory responses within gastrointestinal mucosa (Bezencon et al., 2008; von Moltke et al., 2016). Given their unique nature, we hypothesized that tuft cells are well placed to help coordinate the crosstalk between nerves and cancer. Accordingly, we conducted this study to reveal the whole picture of nerve-cancer interaction during tumorigenesis with multiple mouse models.
Results
ChAT+ tuft cells and nerves expand within gastric mucosa during tumorigenesis
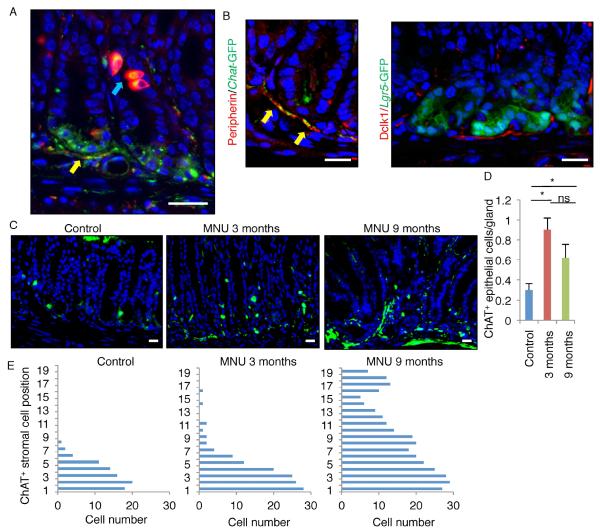
We first explored the source of ACh within the alimentary tract using Chat-GFP transgenic mice, in which all ACh-producing cells are GFP+ (Tallini et al., 2006). Chat-GFP is expressed in nerve fibers within the lamina propria and the submucosal and myenteric ganglia (Figure 1A, S1A). GFP+ nerve fibers surround the base of glands, where stem cells such as Lgr5+ cells reside, supporting the notion that cholinergic nerves contribute to the gastrointestinal stem cell niche through close physiological contact (Figure 1B, S1B-C). As previously shown (Schutz et al., 2015), Chat-GFP is also expressed in epithelial tuft cells that are positive for Dclk1 (Figure 1A, S1A). Immunostaining revealed that Dclk1 is strongly expressed in tuft cells, but also detected mild to moderate Dclk1 expression in Chat-GFP+ cholinergic nerve fibers and ganglia (Figure 1A-1B, S1A). Our Dclk1-CreERT mice (Westphalen et al., 2014) confirmed Dclk1 expression in a subset of ENS as well as epithelial tuft cells, and both of which showed immunopositivity for ACh (Figure S1D-E). However, Dclk1 is not expressed in other stromal lineages, such as α-smooth muscle actin (SMA)+ myofibroblasts, CD31+ endothelial cells, CD45+ hematopoietic cells, or NG2+ pericytes (Figure S1F). Taken together, these results suggest that expression of Dclk1 identifies most, if not all, cholinergic signaling cells within the gut, including both epithelial tuft cells and stromal neurons.
Figure 1. ChAT+ tuft cells and nerves expand during carcinogenesis.
(A) Dclk1 staining (red) in Chat-GFP (green) mice antrum. Blue arrow indicates tuft cells, and yellow arrow indicates nerves. (B) (Left) Peripherin staining (red) in Chat-GFP mice antrum. Arrows indicate GFP+ nerves. (Right) Dclk1 staining (red) in Lgr5-GFP mice antrum. (C) Chat-GFP expression with or without MNU treatment (3 months and 9 months after the beginning of MNU). (D) The number of ChAT+ epithelial cells per gland in MNU-treated or untreated stomachs. Total 100 glands per group are analyzed. (E) Cell position of ChAT+ stromal cells in MNU-treated or untreated stomachs. Total 50 glands per group are analyzed. Means ± SEM. *p < 0.05 (ANOVA). DAPI = blue. Bars = 20 m. See also Figure S1.
We previously reported that Lgr5+ gastric stem cells express high levels of M3R and expand in response to cholinergic signaling during carcinogenesis (Zhao et al., 2014). Interestingly, in a N-nitroso-N-methylurea (MNU) carcinogen mouse model of gastric cancer, we found a dynamic relationship between epithelium and stroma in terms of cholinergic cell distribution during tumor development. Early in the model (first 3 months after MNU treatment), there was a significant increase in Chat-GFP+ tuft cells in the epithelium. But after 9 months, there was a gradual loss of epithelial Chat-GFP+ tuft cells, accompanied by axonogenesis of cholinergic nerve fibers (Figure 1C-E). Thus, the early expansion of Chat+ tuft cells during carcinogenesis, followed by the later increase in cholinergic innervation with progression to dysplasia, suggests a requirement for ACh production in tumorigenesis from different sources, depending on tumor stage.
ACh signaling stimulates NGF production in gastric epithelial cells
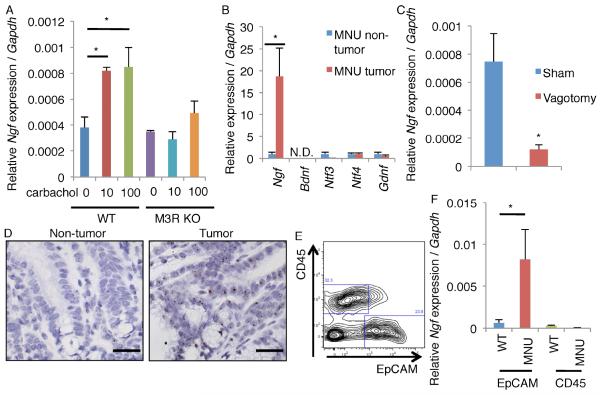
Cholinergic stimulation has been shown to induce the expression of certain neurotrophin family molecules (da Penha Berzaghi et al., 1993; Lapchak et al., 1993; Mahmoud et al., 2015). In support of these observations, treatment of gastric organoids with the cholinergic agonist carbachol upregulated the expression of Ngf in an M3R-dependent fashion (Figure 2A). Of all the major neurotrophins (NGF, brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT3), neurotrophin 4 (NT4), and glial-cell line derived neurotrophic factor (GDNF)), NGF was most highly and specifically upregulated by carbachol (Figure 2A, S2A). In mouse gastric tumors, there was also a specific upregulation of Ngf (almost 20 times higher expression than the normal stomach), and such upregulation was not observed in any other neurotrophins (Figure 2B). Similar upregulation of Ngf was found in mouse colorectal tumors induced by azoxymethane (AOM) and dextran sodium sulfate (DSS) (Figure S2B). Interestingly, abrogation of cholinergic signaling in the stomach by surgical vagotomy was able to inhibit Ngf upregulation in gastric tumors (Figure 2C). Immunostaining and in situ hybridization confirmed that NGF was expressed within the neoplastic epithelium rather than the stromal compartment (Figure 2D, S2C), a finding confirmed by cell sorting and subsequent quantitative PCR (Figure 2E-F). MNU tumor-derived organoids that are cultured in isolation, separated from their native microenvironment, lost Ngf expression within 7 days. However, treatment with the ACh mimetic carbachol partly reestablished Ngf upregulation (Figure S2D). Therefore, ACh production, possibly from tuft cells initially but later from innervated nerves with axonogenesis, is at least partly responsible for the neoplastic upregulation of NGF.
Figure 2. ACh signaling stimulates NGF production.
(A) Ngf expression in cultured gastric orgnoids from WT and Chrm3 knockout (M3R KO) mice. Organoids were treated with carbachol for 7 days. n = 4/group. (B) Relative expression per Gapdh of neurotrophin family in MNU-treated mouse non-tumor and tumor tissues. The average expression of each gene in non-tumor tissues is set as 1.0. n = 4/group. N.D. means “not detected”. (C) Relative Ngf expression per Gapdh in MNU tumors isolated from mice which have taken vagotomy or sham treatment. n = 3/group. (D) In situ hybridyzation of Ngf in MNU-treated non-tumor and tumor area. (E) FACS plot of EpCAM and CD45 from MNU tumors. (F) Relative Ngf expression per Gapdh in EpCAM+ cells and CD45+ cells isolated from WT and MNU-treated mice. n = 3/group. Means ± SEM. *p < 0.05 (ANOVA in A, t-test in B, C and F). Bars = 20 m. See also Figure S2.
NGF/Trk signaling regulates mucosal innervation
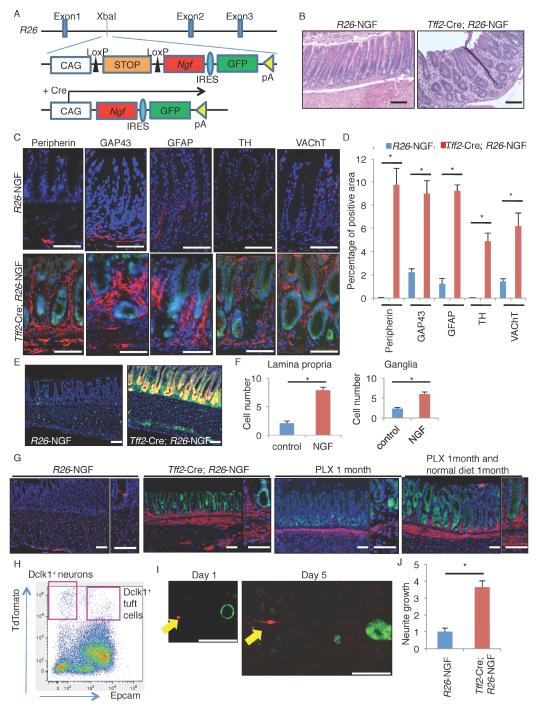
Based on our hypothesis that NGF plays a role in tumor-associated innervation, we generated knockin mice which conditionally express mouse Ngf gene to test this in vivo. To create this line, a Lox-STOP-Lox-Ngf-IRES-GFP construct was inserted into the Rosa26 (referred to as R26) gene locus (Figure 3A). To target NGF expression to the gastric epithelium, we used a Tff2-BAC-Cre line (Dubeykovskaya et al., 2016), which targeted Cre recombinase expression to the entire gastric epithelium (Figure S3A-B). Tff2-Cre; R26-NGF mice expressed a high level of Ngf in the gastric epithelium (Figure S3C), leading to disturbed glandular architecture and increased stromal cells within the lamina propria (Figure 3B). Immunostaining revealed that these stromal cells were nerves and glial cells, including both adrenergic (TH+) and cholinergic (vesicular acetylcholine transporter (VAChT)+) neurons that expressed Dclk1 (Figure 3C-D, S3D). We also found that submucosal ganglia were larger in NGF-overexpressing mice and indeed the number of HuC/D+ cells in ganglia was significantly increased (Figure S3E-F). In addition, Nestin+ cells were expanded within the lamina propria and submucosal ganglia in NGF-overexpressing mice (Figure 3E-F). As reported previously (Belkind-Gerson et al., 2013; Grundmann et al., 2016), the majority of these Nestin+ cells are positive for a glial marker S100B, thus including glial progenitors and mature glia, but negative for neuronal markers such as HuC/D, PGP9.5, and Dclk1, or other stromal markers including CD31 and α-SMA (Figure S3G-I). Targeting NGF overexpression to the small and large intestine using crosses to Vil1-Cre transgenic mice increased nerve density in these organs (Figure S3J-K). The expansion of stromal nerves in NGF-overexpressing mice was suppressed by the treatment with the Trk inhibitor PLX-7486 (PLX), although the nerves rapidly re-expanded after the discontinuation of PLX treatment (Figure 3G). In contrast, the numbers of Dclk1+ neuronal cells in ganglia did not change during this time course, and these cells did not show any uptake of BrdU even after 2 months continuous administration (Figure S3L-M), suggesting that the expansion of nerve fibers in NGF-overexpressing mice during adulthood occurs primarily through axonogenesis, rather than neurogenesis.
Figure 3. NGF/Trk signaling regulates mucosal innervation.
(A) Gene construct of R26-LSL-Ngf-IRES-EGFP mice. (B) H&E staining of R26-NGF and Tff2-Cre; R26-NGF mouse stomach. (C-D) Neuron and glial marker staining (C, red) and quantification (D) in R26-NGF and Tff2-Cre; R26-NGF (green) mice. The percentages of positive area per total mucosal area are quantified in 4 images per group. (E-F) Nes-GFP expression (E) and the cell number of GFP+ cells (F) in lamina propria (per stromal gap between 2 glands) and ganglia (per ganglia) in 6-week-old R26-NGF; Nes-GFP and Tff2-Cre; R26-NGF; R26-TdTomato; Nes-GFP mice. Total 20 glands and 20 ganglia are analyzed. Both NGF+ Tff2-Cre lineage cells and Nes-GFP+ cells are colored by green. (G) Dclk1 staining (red) of R26-NGF mice, Tff2-Cre; R26-NGF (green) mice, Tff2-Cre; R26-NGF mice treated with PLX for 1 month, and Tff2-Cre; R26-NGF mice treated with PLX for 1 month and subsequently treated with normal diet for another 1 month. (H-J) Co-culture experiment of sorted Dclk1+ stromal cells (red) and Tff2-Cre; R26-NGF gastric organoids (green). (H) FACS plot of Dclk1-CreERT; R26-TdTomato mouse stomach with EpCAM staining 1 day after tamoxifen induction. (I) Day 1 and 5 neurite growth image in NGF+ organoid (green) co-culture. Arrows indicate Dclk1+ neurons (red). (J) Quantification of length of neurite growth. The length with WT organoid co-culture is set as 1.0. n = 20/group. Means ± SEM. *p < 0.05 (t-test). DAPI = blue. Bars = 100 m. See also Figure S3.
In adult Dclk1-CreERT; R26-mTmG mice treated with tamoxifen, the recombined GFP+ nerves were initially found near the gastric gland base. However, after 1 year, these recombined cells gradually expanded towards the top of glands (Figure S3N-O), suggesting time-dependent axonal growth and/or nerve turnover under normal homeostasis, as shown in other studies (Kabouridis et al., 2015). PLX treatment suppressed this stromal lineage tracing in Dclk1-CreERT; R26-mTmG mice (Figure S3P-Q), suggesting a role for NGF in the normal remodeling of the stroma. Consistent with this notion, stromal tracing in Dclk1-CreERT; R26-mTmG mice was enhanced in MNU-induced tumors that expressed high levels of NGF (Figure S3R). To further explore this relationship, we co-cultured sorted Epcam−Dclk1+ neurons with WT or Tff2-Cre; R26-NGF gastric organoids (Figure 3H). While cultured neurons rarely showed much neurite outgrowth when co-cultured with WT organoids, NGF-overexpressing organoids induced significant neurite growth (Figure 3I-J), mimicking the in vivo Dclk1+ axonal growth that was dependent on NGF.
ACh/M3R signaling regulates mucosal proliferation and clonal stem cell expansion
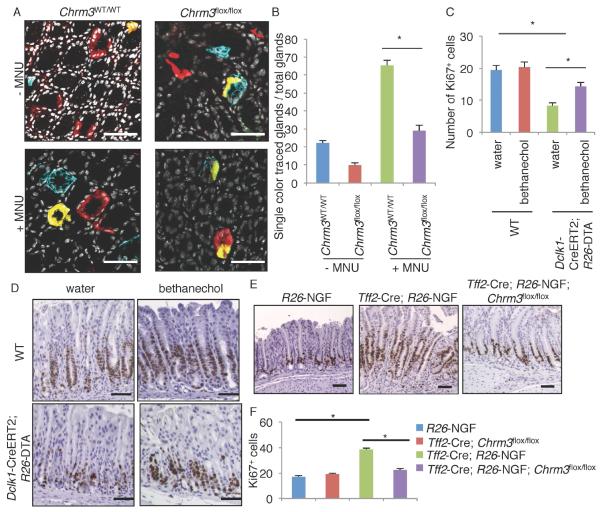
To test the role of muscarinic signaling in this process, Tff2-Cre; Chrm3flox/flox mice were used to conditionally delete Chrm3 gene in the gastric epithelium. Loss of epithelial M3R expression resulted in decreased proliferation following MNU-induced injury, while there were no significant changes in proliferation under normal conditions (Figure S4A-B). To examine the effect of M3R signaling in the gastric Lgr5+ population, we generated Lgr5-CreERT; R26-Confetti mice with or without the Chrm3flox/flox transgene, and traced the clonal expansion of Lgr5+ stem cells during MNU treatment. As reported previously (Leushacke et al., 2013), multiple Lgr5+ cells began to lineage trace each gland, with some glands showing all 4 of the different fluorescent reporters, but within 2 months most glands consolidated to a single color in a “neutral drift” manner. This single-color conversion in a gland was more frequently observed in MNU-treated mice than in non-treated mice, suggesting more rapid loss of progenitors or faster emergence of a dominant clone after MNU treatment (Figure 4A-B). However, knockout of Chrm3 in the Lgr5+ population suppressed single-color conversion, with the majority of glands in these mice demonstrating multi-color tracing or incomplete tracing even 2 months after tamoxifen/MNU treatment. Thus, M3R signaling may regulate gastric epithelial proliferation and stem cell division during mucosal regeneration.
Figure 4. ACh/M3R signaling regulates mucosal proliferation and stem cell expansion.
(A-B) Cross sectional images (A) of Lgr5-CreERT2; R26-Confetti mice (Chrm3WT/WT) and Lgr5-CreERT; Chrm3flox/flox; R26-Confetti mice. Mice were treated with tamoxifen, following with or without 5 cycles of MNU. DAPI = white. (B) Percentages of the glands that were fully traced by single color per total glands where recombination occurs. Total 80 glands from 4 mice per group are analyzed. (C-D) The number of Ki67+ cells per gland (C) and Ki67 staining (D) in WT and Dclk1-CreERT; R26-DTA mice. Mice were treated with tamoxifen on day 1, and given bethanechol for 5 days. Total 90 glands from 3 mice per group are analyzed. (E-F) Ki67 staining (E) and the number of Ki67+ cells per gland (F) in R26-NGF, Tff2-Cre; Chrm3flox/flox, Tff2-Cre; R26-NGF, and Tff2-Cre; R26-NGF; Chrm3flox/flox mice. Total 90 glands from 3 mice per group are analyzed. Means ± SEM. *p < 0.05 (ANOVA). Bars = 100 m. See also Figure S4.
We previously reported that ablation of Dclk1+ cells leads to a decrease in epithelial proliferation in the intestine and colon (Westphalen et al., 2014). We confirmed a similar decrease in gastric proliferation following Dclk1+ cell ablation in Dclk1-CreERT; R26-Diphtheria toxin A (DTA) mice (Figure 4C-D). In the Dclk1-CreERT; R26-DTA model, tuft cells were efficiently ablated after tamoxifen administration through cell specific expression of DTA. However, the majority of labeled Dclk1+ nerves were not ablated and remained within the stroma, probably because of the requirement of high levels of Dclk1 expression for DTA-driven ablation. This provided us with the opportunity to examine the effect of specific ablation of tuft cells, as opposed to neurons in these mice. We tested the effects of a cholinergic agonist bethanechol following ablation of Dclk1+ tuft cells, and administration of bethanechol significantly rescued the loss of proliferation by Dclk1+ tuft cell ablation, suggesting that the effect by tuft cell ablation in proliferation depends, at least in part, on the loss of their cholinergic signaling.
Dclk1+ cholinergic signaling also contributed to intestinal regeneration following DSS-colitis. While ablation of Dclk1+ cells worsened DSS-colitis (Westphalen et al., 2014), restoration of cholinergic signaling by bethanechol restored mucosal regeneration (Figure S4C-D). Furthermore, consistent with previous reports using systemic Chrm3 knockout mice (Hirota and McKay, 2006), conditional knockout of M3R in the colonic epithelium exacerbated the severity of DSS-colitis (Figure S4E-F). Intestinal overexpression of NGF in Vil1-Cre;R26-NGF mice with marked cholinergic innervation improved regeneration following DSS-induced injury, whereas simultaneous knockout of M3R blocked the NGF-mediated regenerative effects (Figure S4E-F). Taken together, these findings suggest that cholinergic signaling from tuft cells and nerves is critical for mucosal homeostasis and regeneration.
Compared to control mice, Tff2-Cre; R26-NGF mice showed increased proliferation within the stomach (Figure 4E-F). The increased proliferation was abrogated by the concomitant knockout of Chrm3, but unaffected by the deletion of β-adrenergic receptor 2 (Adrb2), indicating that it was the cholinergic, rather than adrenergic, neurons that were the major drivers of epithelial proliferation in this setting (Figure 4E-F, S4G-H). Treatment with the Trk inhibitor PLX for 1 month dramatically reduced the nerve density within the gastric mucosa, as well as the level of epithelial proliferation (Figure S4I). However, the gastric epithelium returned to its baseline hyperproliferation following removal of the Trk inhibitor. This demonstrates that gastric ACh/NGF/Trk signaling is important for promoting both mucosal nerve fiber growth and epithelial proliferation.
Initiating the ACh-NGF axis is sufficient to cause gastric cancer
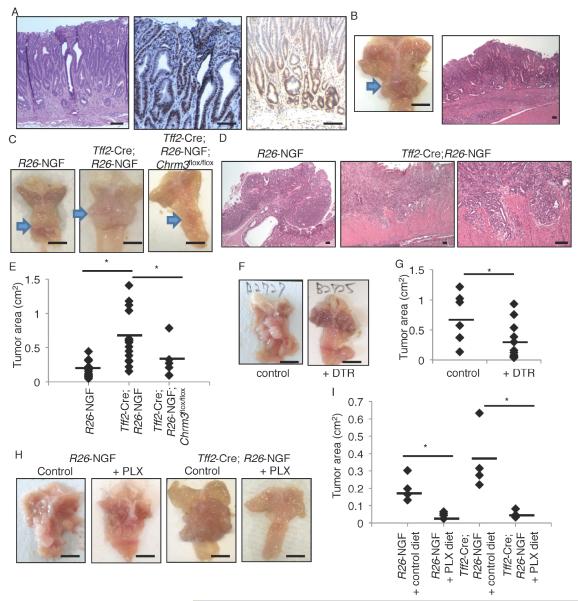
By 8 months of age, Tff2-Cre; R26-NGF mice developed spontaneous metaplasia and dysplasia in the stomach, with an expansion of dysplastic CD44+/Ki67+ epithelial cells (Figure 5A and S5A). By 18 months, the mice developed large gastric tumors with intramucosal adenocarcinoma (Figure 5B). Overexpression of NGF in colonic epithelium also induced dysplastic tumors in the rectum (Figure S5B). These NGF-mediated effects in the stomach were attenuated by knockout of Chrm3 in gastric epithelium (Figure S5A, C). Overexpression of NGF in the Tff2-Cre; R26-NGF mice significantly accelerated tumor growth and invasion in response to MNU (Figure 5C-E). The tumor-promoting effect by NGF was also evident in the AOM-DSS colon cancer model (Figure S5D-F). Knockout of Chrm3 in the gastric epithelium blocked MNU-dependent tumor development in the setting of NGF overexpression (Figure 5C, E). By contrast, knockout of Adrb2 did not suppress MNU-dependent tumor development (Figure S5G-H), again establishing the role of cholinergic rather than adrenergic nerves in gastric carcinogenesis. Ablation of ACh-producing Dclk1+ tuft cells in the MNU model by treatment of Dclk1-CreERT; R26-DTR mice with DT significantly inhibited tumor development, with reduction of NGF expression and innervation (Figure 5F-G, S5I-K). Treatment with the Trk-inhibitor PLX prevented MNU tumor growth both in WT and NGF-overexpressing mice, and reduced peritumoral nerve density, CD44+ cancer stem cell expansion, and β-catenin nuclear translocation (Figure 5H-I, S5L-N).
Figure 5. Initiating the ACh-NGF axis is sufficient to cause gastric cancer.
(A) H&E (left), Ki67 (middle), and CD44 (right) staining of 8-month-old Tff2-Cre; R26-NGF mice. (B) Gross picture and H&E staining of 18-month-old Tff2-Cre; R26-NGF mice stomach. Arrow indicates tumor. (C-E) MNU-induced tumors in R26-NGF (n = 19), Tff2-Cre; R26-NGF (n = 16), and Tff2-Cre; R26-NGF; Chrm3flox/flox (n = 6) mice. Mice were sacrificed at 48 weeks after the beginning of MNU. Gross picture (C), H&E image (D), and tumor area (cm2) (E) are shown. Arrows indicate tumors. (F-G) MNU-treated Dclk1-CreERT; R26-DTR mice were treated with vehicle (n = 7) or DT (10 g/kg, n = 13). Vehicle or DT and tamoxifen were given once a week from 28 weeks to 52 weeks after the beginning of MNU. Representative tumor image (F) and tumor area (G) are shown. (H-I) Gross images (H) and tumor area (I) of MNU-treated R26-NGF and Tff2-Cre; R26-NGF mice with or without PLX treatment. PLX was given from 24 weeks to 36 weeks after the beginning of MNU. n = 4/group. Average tumor area is indicated by black bars. Means ± SEM. *p < 0.05 (ANOVA in E, t-test in G and I). Bars = 100 m (A, B right), 5 mm (B left, C, F, H). See also Figure S5.
We tested the effect of NGF inhibition in an allograft tumor model (Li et al., 2014). Kras, Apc, and Tp53-mutated gastric glands were isolated from tamoxifen-treated Lgr5-CreERT; LSL-KrasG12D/+; LSL-p53R172H/+; Apcflox/flox mice and tumor organoids cultured. These organoids were then implanted into immunodeficient NOD-SCID mice, and the mice were treated with control or PLX7486-containing diet. After 3 weeks, tumor size was significantly reduced by Trk inhibition, and the expansion of nerves in the peritumoral site was inhibited (Figure S5O-Q). Taken together, these experiments suggest a central role for NGF/Trk signaling, mediated through a cholinergic niche, in the initiation and progression of stomach cancer.
M3R signaling regulates Apc-dependent tumor growth through YAP activation
We recently reported that Mist1+ stem cells in the oxyntic glands of the proximal stomach that express Bhlha15 gene (also known as Mist1) can give rise to gastric cancer (Hayakawa et al., 2015a). In Mist1-CreERT; R26-Tomato mice, we also found robust lineage tracing in the distal stomach (Figure S6A), suggesting the fair and broad expression of Mist1 in the gastric antrum. Interestingly, loss of Apc gene in the Mist1 lineage was not sufficient to create tumors in the proximal corpus, as reported previously (Hayakawa et al., 2015a; Hayakawa et al., 2016); however, Apc deletion in the Mist1 lineage did induce rapid macroscopic tumors in the gastric antrum (Figure S6B).
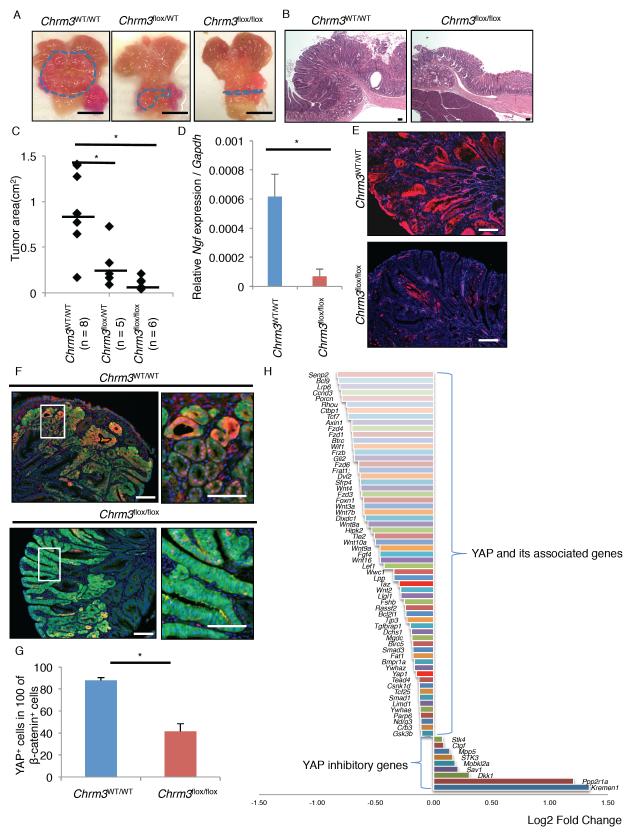
Using this mouse model of antral gastric tumor, we generated Mist1-CreERT; Apcflox/flox; Chrm3flox/flox mice to explore the role of M3R signaling in Apc/β-catenin-dependent tumor growth. In keeping with the role of cholinergic signaling described above, we also found a significant reduction in tumor burden with loss of just one copy of Chrm3 gene, and hemizygous Chrm3 floxed mice showed an almost complete absence of tumor development (Figure 6A-C). NGF expression and nerve density were significantly decreased in Apc and Chrm3 double knockout mice, supporting the role of cholinergic signaling on NGF-mediated innervation (Figure 6D-E, S6C-D). Nevertheless, immunostaining showed strong β-catenin nuclear accumulation both in Chrm3-WT and Chrm3-null stomachs (Figure S6E), suggesting that knockout of M3R suppresses tumor growth downstream of β-catenin nuclear translocation, possibly by inhibiting TCF transcriptional activity.
Figure 6. M3R signaling regulates Apc-dependent tumor growth through YAP activation.
(A-C) Gross pictures (A) and H&E images (B) of Mist1-CreERT; Apcflox/flox, Mist1-CreERT; Apcflox/flox; Chrm3flox/WT, and Mist1-CreERT; Apcflox/flox; Chrm3flox/flox mouse tumors. Lines indicate tumor area. Tumor area (cm2) is quantified in (C). Average tumor area is indicated by black bars. (D-G) Ngf gene expression per Gapdh (D, n = 3) and immunofluorescence of NGF (E, red) and doublestaining of YAP (F, red) and β-catenin (green) in Mist1-CreERT; Apcflox/flox and Mist1-CreERT; Apcflox/flox; Chrm3flox/flox mouse tumors. Bottom panels in F are enlarged images of white box area in top panels. Numbers of YAP+ cells in 100 nuclear β-catenin+ cells are shown in (G). Total 500 nuclear β-catenin+ cells per group are analyzed. (H) Fold changes of YAP-related gene expression in mouse gastric tumor on the vagotomized side compared to tumor on the non-vagotomized side. Means ± SEM. *p < 0.05 (ANOVA in C, t-test in D and G). DAPI = blue. Bars = 100 m (B, E, F), 5 mm (A). See also Figure S6.
YAP modulates Wnt/β-catenin signaling as a transcriptional coactivator, and is required for intestinal tumor growth following the loss of Apc (Azzolin et al., 2014; Rosenbluh et al., 2012). While YAP is minimally expressed in normal gastric epithelium or cultured organoids, strong YAP upregulation is observed in dysplastic tissues or organoids after Apc deletion (Figure 6F, S6F-G). However, YAP was rarely upregulated within the Chrm3-null gastric tumors, even though they showed strong β-catenin expression (Figure 6F-G). A gene downstream of YAP, BCL2L1, was also downregulated in the Chrm3-null gastric tumors compared to the Chrm3-WT tumors (Figure S6H). The Wnt target genes, Sox9 and CD44, were prominently expressed in Chrm3-WT, nuclear β-catenin+ dysplastic cells, but not in the Chrm3-null, β-catenin+ cells (Figure S6I-J). Furthermore, we revisited our previous microarray data (GEO accession no. GSE30295) which compared gene expression between gastric tumors isolated from the vagotomized anterior stomach and the non-vagotomized posterior stomach in a hypergastrinemic mouse model (Zhao et al., 2014). We found downregulation of YAP-associated genes along with upregulation of YAP inhibitory genes in the vagotomized anterior stomach, suggesting that the inhibition of ACh signaling by vagotomy can block YAP activity in this model (Figure 6H). Thus, M3R signaling may regulate tumor growth in mice by controlling YAP activity.
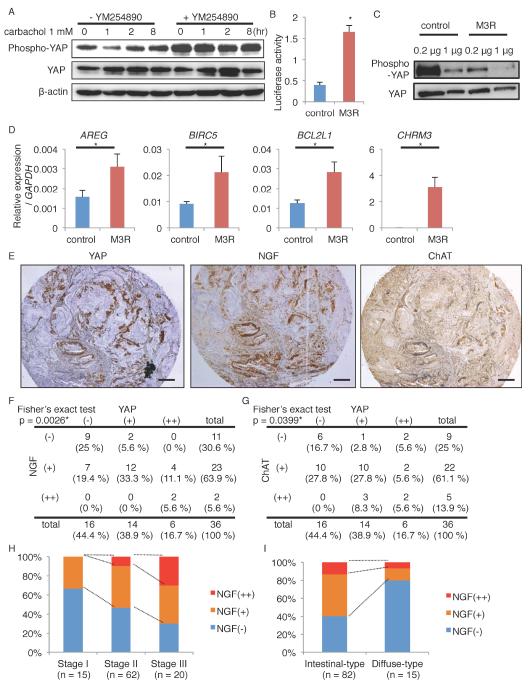
The M3R, a G-protein coupled receptor (GPCR), selectively couples to G-proteins of the Gq/11 family, and it has been suggested that GPCRs regulate YAP activation by controlling large tumor suppressor (LATS) kinase activity (Feng et al., 2014; Yu et al., 2014; Yu et al., 2012). In the TMK1 gastric cancer cell line which expresses the M3R (Kodaira et al., 1999), treatment with carbachol reduced the level of phosphorylated YAP, with no significant changes in the levels of total YAP (Figure 7A). Similarly, carbachol treatment in Apc-deleted gastric organoids reduced the level of phosphorylated YAP (Figure S6G). However, treatment with a Gq/11 specific inhibitor YM254890 blocked the changes of YAP phosphorylation in TMK1 cells after carbachol stimulation, suggesting that cholinergic stimuli dephosphorylates YAP indeed through a Gq/11 family protein. We next transfected a human M3R gene expressing construct into the M3R-negative AGS cell line. Overexpression of M3R decreased the level of phosphorylated YAP in a Gq-dependent manner, and activated the transcriptional activity of YAP in a luciferase assay (Dupont et al., 2011) (Figure 7B-C, S7A). YAP target genes including AREG, BIRC5, and BCL2L1 were significantly upregulated by M3R overexpression (Figure 7D). Consistent with the findings in our mouse models, carbachol treatment or M3R overexpression significantly upregulated NGF expression in human cancer cells, although NGF treatment did not cause YAP activation contrary to carbachol (Figure S7B-C).
Figure 7. M3R activates YAP signaling in human gastric cancer cells.
(A) Immunoblotting of TMK-1 cells treated with 1 mM carbachol for the indicated times. Cells were pretreated with vehicle or 10 m YM254890. β-actin was used as a loading control. (B-D) Relative YAP luciferase activity (B, n = 3/group), immunoblotting (C), and relative gene expression (D, n = 3/group) in AGS cells transfected with control or M3R-expressing vectors. Samples are collected 24 hours after transfection. (E) Representative images of YAP, NGF, ChAT staining in human gastric cancers. (F-G) Correlation between the expression levels of YAP and NGF (F), and of YAP and ChAT (G) in 36 gastric cancer cases. (H-I) NGF positivity in different cancer stages (H) and histological forms (I) in 97 gastric cancer cases. Means ± SEM. *p < 0.05 (t-test in B and D, Fisher in F and G). Bars = 200 m. See also Figure S7, Table S1, and Table S2.
We investigated NGF expression levels in 16 gastric cancer cell lines (Figure S7D-E), and confirmed NGF mRNA expression in 10 lines (62.5 %), and NGF protein expression in 9 lines (56.3 %). Staining of 36 human gastric cancer samples with NGF antibody demonstrated that NGF was moderately expressed in 63.9 % of cancer patients and strongly expressed in 5.6 % patients, while non-dysplastic stomach does not express NGF or YAP (Figure 7E-G, S7F-G). Importantly, there was a significant correlation between YAP immunoreactivity and NGF or ChAT immunoreactivity. In addition, we evaluated NGF and YAP expression in an additional set of 97 human gastric cancer samples, and correlated expression with clinical and histopathological data. In this additional cohort, NGF was expressed in 53.6 % of cancer cases. NGF expression was significantly associated with a higher cancer stage (Adjusted odds ratio (AOR) 4.57), and expression was more evident in intestinal-type cancers than in diffuse-type cancers (Figure 7H-I and Table S1). YAP expression was also significantly associated with cancer stage (AOR 5.71), as well as an increased risk of lymphoid node metastasis (AOR 6.55) (Table S2). Taken together, these results support the significance of the NGF-ACh-YAP axis in human gastric cancers, particularly in advanced, intestinal-type cancers.
Discussion
We found that Dclk1 and ChAT are co-expressed in both tuft cells and nerves within the stomach and intestine, and that both cholinergic sources expand at discrete times during carcinogenesis. Tuft cells are dramatically expanded in early carcinogenesis, particularly in inflammation-associated cancer models. Previous studies suggested that tuft cells could potentially influence carcinogenesis through production of inflammatory mediators (Bailey et al., 2014; Okumura et al., 2010; Quante et al., 2012), but here we have shown that tuft cells are also a local source of ACh that contributes to early cancer growth and remodeling of the peritumoral neural microenvironment.
We have proposed that the ACh-NGF positive feedback loop is the basis for the abnormal innervation observed in the tumor microenvironment. Previous studies have reported that NGF can be induced by cholinergic stimuli (da Penha Berzaghi et al., 1993; Lapchak et al., 1993; Mahmoud et al., 2015), and in turn NGF can promote cholinergic nerve growth (Collins, 1984; Collins and Dawson, 1983; Kniewallner et al., 2014), consistent with our results. While the major receptor for NGF is TrkA, another neurotrophin receptor p75NTR can bind to NGF and pro-NGF (Howard et al., 2013). Given the significant effects by the Trk inhibitor in our study, it appears that Trk is the primary receptor within the ACh-NGF axis. Many clinical studies have investigated the efficacy of Trk inhibitors in cancers with mitogenic Trk-fused mutations (Vaishnavi et al., 2015). Furthermore, anti-NGF antibody has been used in several clinical studies for testing its effect on pain management in osteoarthritis patients (Bannwarth and Kostine, 2014). Our preclinical results suggest that Trk inhibitors and anti-NGF antibody may be effective for the therapy for stomach cancer by targeting the ACh-NGF axis.
The muscarinic acetylcholine receptors are classified into 5 distinct subtypes; gastric epithelial cells primarily express M3R, and also low level of muscarinic acetylcholine receptor-1 (M1R) and -5 (M5R) (Aihara et al., 2005; Zhao et al., 2014). The activation of M3R leads to a variety of biochemical and electrophysiological responses, and the resulting physiological effects may depend on the cell types. It has been suggested that M3R can activate mitogen-activated protein kinase (MAPK) (Kodaira et al., 1999), Akt (Song et al., 2007), or RhoA (Belo et al., 2011) and contribute to tumor growth in various cancers. We and others reported that M3R activates the Wnt pathway, and our current study suggests that M3R-mediated Wnt activation may be through YAP, a downstream target of M3R.
YAP, a downstream effector of the Hippo pathway, regulates various cellular functions such as proliferation, survival, stemness, or pluripotency. YAP is upregulated and activated by loss of Apc, and in turn YAP activation is required for β-catenin-dependent cancer growth (Azzolin et al., 2014; Cai et al., 2015; Rosenbluh et al., 2012). Accordingly, YAP controls tissue regeneration and tumorigenesis in various organs including stomach, by activating tissue stem cells (Gregorieff et al., 2015; Imajo et al., 2015; Jiao et al., 2014). GPCRs that activate G12/13, Gq/11, or Gi/o, can activate YAP by repressing YAP phosphorylation, while GPCRs that mainly activate Gs signaling such as Adrb2, are able to induce YAP phosphorylation and subsequent YAP inhibition (Yu et al., 2012). Our data suggests that the M3R can activate YAP signaling in a manner similar to other Gq/11 family receptors. Thus, destruction of the Apc complex is only able to induce full activation of Wnt targets and aggressive tumor development when there is sufficient, permissive cholinergic signaling through the M3R. Although it seems that M3R regulates YAP activity predominantly through its dephosphorylation, the involvement of M3R in YAP upregulation during initial malignant transformation remains uncertain, and thus needs to be elucidated in future studies.
In summary, we elucidated the machinery of nerve-epithelial interaction within the stomach, including the source of ACh, the cause of abnormal innervations in cancer, and the regulation of the Wnt and YAP pathways by M3R signaling, constituting what we term the ACh-NGF-YAP axis. Inhibition of ACh, M3R, or NGF may be important future therapeutic strategies to consider in the treatment of gastrointestinal cancers.
Experimental Procedures
Mice
Mist1-CreERT2 (Shi et al., 2009), Dclk1-CreERT (Westphalen et al., 2014), Tff2-Cre (Dubeykovskaya et al., 2016), Chrm3flox (Gautam et al., 2006), Chrm3 knockout (Aihara et al., 2003), Adrb2flox (Hinoi et al., 2008), and Nes-GFP (Mignone et al., 2004) mice were described previously. Apcflox mice were obtained from the National Cancer Institute. Lgr5-CreERT-IRES-EGFP, Vil1-Cre, Chat-GFP, NOD-SCID, Adrb2 knockout, R26-mTmG, R26-TdTomato, R26-Confetti, R26-DTR, and R26-DTA mice were purchased from the Jackson Laboratory. R26-LSL-Ngf-IRES-EGFP mice were generated as follows: the R26-LSL-Ngf-IRES-GFP alleles were generated by inserting a CAG–loxP-STOP-LoxP-Ngf-IRES-GFP-polyA cassettes into a Rosa-acceptor targeting plasmid (CTV plasmid, a gift from Dr. Klaus Rajewsky (Addgene #15912)). Mouse lines where generated by homologous recombination in KV1 (129S6/SvEvTac × C57BL/6J) embryonic stem cells followed by injection into C57BL/6J blastocysts. Chimeras were bred for germline transmission. Mice were backcrossed to C57BL/6J background for at least 6 generations. Cre recombinase was activated in CreERT mouse lines by oral administration of TAM (3 mg/0.2 mL corn oil). All animal studies and procedures were approved by the ethics committees at Columbia University and the University of Tokyo. All protocols using human materials were approved by the ethics committees at Columbia University and Gifu University, and written informed consent was obtained from all patients.
Treatment
MNU (Sigma) were prepared as previously described, and mice (8-week-old) were given drinking water containing 240 ppm MNU on alternate weeks for a total of 10 weeks (total exposure of 5 weeks)(Hayakawa et al., 2015b). For tumor analysis, mice were analyzed 36 – 52 weeks after the beginning of MNU, as indicated. For Dclk1+ cell ablation in Dclk1-CreERT; R26-DTR mice, tamoxifen and diphtheria toxin (DT) (10 g/kg) was administered via intraperitoneal injection once a week. PLX-7486, provided from Plexxicon, has been used in Phase I clinical study and detailed information is on NCI drug dictionary: http://www.cancer.gov/publications/dictionaries/cancer-drug?cdrid=747694. PLX-7486 was mixed into the AIN-76A mouse chow (100 mg/kg), and feeded for the indicated periods. Control group was given AIN-76A chow without PLX-7486. Bethanechol was dissolved in drinking water at a concentration of 800 mg/L. DSS was dissolved in drinking water at 3 % and given for 5 days. Vagotomy was performed as previously (Zhao et al., 2014).
Statistical Analysis
The differences between the means were compared using either the Student’s t-test (2-tailed). One-way ANOVA with post-hoc test was performed for multiple comparisons. Fisher’s exact test was used to determine if there are nonrandom associations between two categorical variables. In Table S1 and S2, a logistic regression analysis was conducted to evaluate the odds ratios as an estimate of whether NGF and YAP expression (“Positive” includes (+) and (++) cases) was associated with each parameters. p values < 0.05 were considered to indicate statistical significance. All statistical analyses were performed using the JMP (ver. 11) and the SAS (ver. 9.4) software.
Supplementary Material
Highlights.
NGF expression is induced in gastric cancer by ACh from nerves and tuft cells.
NGF promotes innervation and proliferation in gastric epithelium.
Blockade of NGF or ablation of cholinergic tuft cells inhibits tumor development.
Cholinergic signaling activates YAP signaling that is essential for Wnt activation.
Significance.
The factors driving nerve expansion during tumorigenesis and the downstream targets of nerve signaling are not well understood. This study investigates the extensive crosstalk that occurs during carcinogenesis between cancer cells and nerves, and identifies the ACh-NGF-M3R-YAP axis that is central to gastric cancer biology. This work proposes potential targets for cancer therapy, such as NGF and M3R, which can be clinically applied in the near future.
In brief.
Hayakawa et al. use a series of mouse models to show that acetylcholine from Dclk1+ tuft cells and nerves induces NGF in gastric epithelial cells, which promotes neuron proliferation and tumorigenesis. YAP is activated through the cholinergic signaling, and inhibition of this pathway can block NGF-driven tumors.
Acknowledgements
We thank Dr. Stephen Konieczny for providing the mice, Ms. Theresa Swayne for producing the 3D images, Dr. Rani Sellers for assisting with the in situ hybridization, Ms. Wendy Beth Jackelow (Medical & Scientific Illustration) for creating schematic images, and Plexxikon Inc. for providing PLX-7486.
Funding
This research was supported by the National Institute of Health grant U54CA126513, R01CA093405, R01CA120979, R01DK052778, the Clyde Wu Family Foundation (T.C.W.), the Nakayama Cancer Research Institute, the Okinaka Memorial Institute for Medical Research, and the Project for Cancer Research And Therapeutic Evolution (P-CREATE) from the Japan Agency of Medical Research and Development, AMED (Y.H.). Y.H. and K.S. were supported by Japan Society for the Promotion of Science, and Y.H. and T.T. were supported by Uehara Memorial Foundation.
Footnotes
Accession Number
Microarray data which compared gene expression between gastric tumors isolated from the vagotomized anterior stomach and the non-vagotomized posterior stomach in hypergastrinemic mice were deposited in GEO database (accession no. GSE30295).
Author Contributions
S.A., M.K., B.W.R., H.T., M.M., Z.J., T.T., Z.A.D., W.K., A.U., M.M., Y.T., X.C., K.N., C.B.W., M.Q., A.H., C.M.Z., D.C., and D.L.W. performed various portions of the experiments. C.S.L. assisted making gene constructs and generated transgenic mice. R.N. analyzed the data of human samples. Y.H., K.S., D.L.W., and T.C.W. wrote the manuscript. Y.H., K.S., M.D.G., K.K., D.L.W., and T.C.W. contributed to the study supervision and coordination.
Conflict of interest:
The authors disclose no conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aihara T, Fujishita T, Kanatani K, Furutani K, Nakamura E, Taketo MM, Matsui M, Chen D, Okabe S. Impaired gastric secretion and lack of trophic responses to hypergastrinemia in M3 muscarinic receptor knockout mice. Gastroenterology. 2003;125:1774–1784. doi: 10.1053/j.gastro.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Aihara T, Nakamura Y, Taketo MM, Matsui M, Okabe S. Cholinergically stimulated gastric acid secretion is mediated by M(3) and M(5) but not M(1) muscarinic acetylcholine receptors in mice. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1199–1207. doi: 10.1152/ajpgi.00514.2004. [DOI] [PubMed] [Google Scholar]
- Albo D, Akay CL, Marshall CL, Wilks JA, Verstovsek G, Liu H, Agarwal N, Berger DH, Ayala GE. Neurogenesis in colorectal cancer is a marker of aggressive tumor behavior and poor outcomes. Cancer. 2011;117:4834–4845. doi: 10.1002/cncr.26117. [DOI] [PubMed] [Google Scholar]
- Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, et al. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Bailey JM, Alsina J, Rasheed ZA, McAllister FM, Fu YY, Plentz R, Zhang H, Pasricha PJ, Bardeesy N, Matsui W, et al. DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology. 2014;146:245–256. doi: 10.1053/j.gastro.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannwarth B, Kostine M. Targeting nerve growth factor (NGF) for pain management: what does the future hold for NGF antagonists? Drugs. 2014;74:619–626. doi: 10.1007/s40265-014-0208-6. [DOI] [PubMed] [Google Scholar]
- Belkind-Gerson J, Carreon-Rodriguez A, Benedict LA, Steiger C, Pieretti A, Nagy N, Dietrich J, Goldstein AM. Nestin-expressing cells in the gut give rise to enteric neurons and glial cells. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2013;25:61–69. e67. doi: 10.1111/nmo.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belo A, Cheng K, Chahdi A, Shant J, Xie G, Khurana S, Raufman JP. Muscarinic receptor agonists stimulate human colon cancer cell migration and invasion. Am J Physiol Gastrointest Liver Physiol. 2011;300:G749–760. doi: 10.1152/ajpgi.00306.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezencon C, Furholz A, Raymond F, Mansourian R, Metairon S, Le Coutre J, Damak S. Murine intestinal cells expressing Trpm5 are mostly brush cells and express markers of neuronal and inflammatory cells. J Comp Neurol. 2008;509:514–525. doi: 10.1002/cne.21768. [DOI] [PubMed] [Google Scholar]
- Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8:552–565. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Maitra A, Anders RA, Taketo MM, Pan D. beta-Catenin destruction complex-independent regulation of Hippo-YAP signaling by APC in intestinal tumorigenesis. Genes Dev. 2015;29:1493–1506. doi: 10.1101/gad.264515.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceyhan GO, Schafer KH, Kerscher AG, Rauch U, Demir IE, Kadihasanoglu M, Bohm C, Muller MW, Buchler MW, Giese NA, et al. Nerve growth factor and artemin are paracrine mediators of pancreatic neuropathy in pancreatic adenocarcinoma. Annals of surgery. 2010;251:923–931. doi: 10.1097/SLA.0b013e3181d974d4. [DOI] [PubMed] [Google Scholar]
- Chandrakesan P, May R, Qu D, Weygant N, Taylor VE, Li JD, Ali N, Sureban SM, Qante M, Wang TC, et al. Dclk1+ small intestinal epithelial tuft cells display the hallmarks of quiescence and self-renewal. Oncotarget. 2015;6:30876–30886. doi: 10.18632/oncotarget.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. An effect of nerve growth factor on the parasympathetic ciliary ganglion. J Neurosci. 1984;4:1281–1288. doi: 10.1523/JNEUROSCI.04-05-01281.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F, Dawson A. An effect of nerve growth factor on parasympathetic neurite outgrowth. Proc Natl Acad Sci U S A. 1983;80:2091–2094. doi: 10.1073/pnas.80.7.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Penha Berzaghi M, Cooper J, Castren E, Zafra F, Sofroniew M, Thoenen H, Lindholm D. Cholinergic regulation of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) but not neurotrophin-3 (NT-3) mRNA levels in the developing rat hippocampus. J Neurosci. 1993;13:3818–3826. doi: 10.1523/JNEUROSCI.13-09-03818.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolle L, Adriaenssens E, El Yazidi-Belkoura I, Le Bourhis X, Nurcombe V, Hondermarck H. Nerve growth factor receptors and signaling in breast cancer. Current cancer drug targets. 2004;4:463–470. doi: 10.2174/1568009043332853. [DOI] [PubMed] [Google Scholar]
- Dubeykovskaya Z, Si Y, Chen X, Worthley DL, Renz BW, Urbanska AM, Hayakawa Y, Xu T, Westphalen CB, Dubeykovskiy A, et al. Neural innervation stimulates splenic TFF2 to arrest myeloid cell expansion and cancer. Nature communications. 2016;7:10517. doi: 10.1038/ncomms10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Feng X, Degese MS, Iglesias-Bartolome R, Vaque JP, Molinolo AA, Rodrigues M, Zaidi MR, Ksander BR, Merlino G, Sodhi A, et al. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell. 2014;25:831–845. doi: 10.1016/j.ccr.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam D, Han SJ, Hamdan FF, Jeon J, Li B, Li JH, Cui Y, Mears D, Lu H, Deng C, et al. A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell metabolism. 2006;3:449–461. doi: 10.1016/j.cmet.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL. Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature. 2015;526:715–718. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
- Gross ER, Gershon MD, Margolis KG, Gertsberg ZV, Li Z, Cowles RA. Neuronal serotonin regulates growth of the intestinal mucosa in mice. Gastroenterology. 2012;143:408–417. e402. doi: 10.1053/j.gastro.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundmann D, Markwart F, Scheller A, Kirchhoff F, Schafer KH. Phenotype and distribution pattern of nestin-GFP-expressing cells in murine myenteric plexus. Cell Tissue Res. 2016 Aug 13; doi: 10.1007/s00441-016-2476-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Hanoun M, Zhang D, Mizoguchi T, Pinho S, Pierce H, Kunisaki Y, Lacombe J, Armstrong SA, Duhrsen U, Frenette PS. Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell Stem Cell. 2014;15:365–375. doi: 10.1016/j.stem.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y, Ariyama H, Stancikova J, Sakitani K, Asfaha S, Renz BW, Dubeykovskaya ZA, Shibata W, Wang H, Westphalen CB, et al. Mist1 Expressing Gastric Stem Cells Maintain the Normal and Neoplastic Gastric Epithelium and Are Supported by a Perivascular Stem Cell Niche. Cancer Cell. 2015a;28:800–814. doi: 10.1016/j.ccell.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y, Jin G, Wang H, Chen X, Westphalen CB, Asfaha S, Renz BW, Ariyama H, Dubeykovskaya ZA, Takemoto Y, et al. CCK2R identifies and regulates gastric antral stem cell states and carcinogenesis. Gut. 2015b;64:544–553. doi: 10.1136/gutjnl-2014-307190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y, Sethi N, Sepulveda AR, Bass AJ, Wang TC. Oesophageal adenocarcinoma and gastric cancer: should we mind the gap? Nat Rev Cancer. 2016;16:305–318. doi: 10.1038/nrc.2016.24. [DOI] [PubMed] [Google Scholar]
- Hinoi E, Gao N, Jung DY, Yadav V, Yoshizawa T, Myers MG, Jr., Chua SC, Jr., Kim JK, Kaestner KH, Karsenty G. The sympathetic tone mediates leptin's inhibition of insulin secretion by modulating osteocalcin bioactivity. J Cell Biol. 2008;183:1235–1242. doi: 10.1083/jcb.200809113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota CL, McKay DM. M3 muscarinic receptor-deficient mice retain bethanechol-mediated intestinal ion transport and are more sensitive to colitis. Canadian journal of physiology and pharmacology. 2006;84:1153–1161. doi: 10.1139/y06-068. [DOI] [PubMed] [Google Scholar]
- Howard L, Wyatt S, Nagappan G, Davies AM. ProNGF promotes neurite growth from a subset of NGF-dependent neurons by a p75NTR-dependent mechanism. Development. 2013;140:2108–2117. doi: 10.1242/dev.085266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imajo M, Ebisuya M, Nishida E. Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nat Cell Biol. 2015;17:7–19. doi: 10.1038/ncb3084. [DOI] [PubMed] [Google Scholar]
- Jiao S, Wang H, Shi Z, Dong A, Zhang W, Song X, He F, Wang Y, Zhang Z, Wang W, et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25:166–180. doi: 10.1016/j.ccr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Kabouridis PS, Lasrado R, McCallum S, Chng SH, Snippert HJ, Clevers H, Pettersson S, Pachnis V. Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron. 2015;85:289–295. doi: 10.1016/j.neuron.2014.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Kniewallner KM, Grimm N, Humpel C. Platelet-derived nerve growth factor supports the survival of cholinergic neurons in organotypic rat brain slices. Neuroscience letters. 2014;574:64–69. doi: 10.1016/j.neulet.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodaira M, Kajimura M, Takeuchi K, Lin S, Hanai H, Kaneko E. Functional muscarinic m3 receptor expressed in gastric cancer cells stimulates tyrosine phosphorylation and MAP kinase. J Gastroenterol. 1999;34:163–171. doi: 10.1007/s005350050238. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Araujo DM, Hefti F. Cholinergic regulation of hippocampal brain-derived neurotrophic factor mRNA expression: evidence from lesion and chronic cholinergic drug treatment studies. Neuroscience. 1993;52:575–585. doi: 10.1016/0306-4522(93)90407-7. [DOI] [PubMed] [Google Scholar]
- Leushacke M, Ng A, Galle J, Loeffler M, Barker N. Lgr5+ Gastric Stem Cells Divide Symmetrically to Effect Epithelial Homeostasis in the Pylorus. Cell Reports. 2013;5:349–356. doi: 10.1016/j.celrep.2013.09.025. [DOI] [PubMed] [Google Scholar]
- Li X, Nadauld L, Ootani A, Corney DC, Pai RK, Gevaert O, Cantrell MA, Rack PG, Neal JT, Chan CW, et al. Oncogenic transformation of diverse gastrointestinal tissues in primary organoid culture. Nat Med. 2014;20:769–777. doi: 10.1038/nm.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren O, Jodal M, Jansson M, Ryberg AT, Svensson L. Intestinal epithelial stem/progenitor cells are controlled by mucosal afferent nerves. PLoS One. 2011;6:e16295. doi: 10.1371/journal.pone.0016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, Frenette PS. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- Mahmoud AI, O'Meara CC, Gemberling M, Zhao L, Bryant DM, Zheng R, Gannon JB, Cai L, Choi WY, Egnaczyk GF, et al. Nerves Regulate Cardiomyocyte Proliferation and Heart Regeneration. Dev Cell. 2015;34:387–399. doi: 10.1016/j.devcel.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol. 2004;469:311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- Neal KB, Bornstein JC. Mapping 5-HT inputs to enteric neurons of the guinea-pig small intestine. Neuroscience. 2007;145:556–567. doi: 10.1016/j.neuroscience.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Okumura T, Ericksen RE, Takaishi S, Wang SS, Dubeykovskiy Z, Shibata W, Betz KS, Muthupalani S, Rogers AB, Fox JG, et al. K-ras mutation targeted to gastric tissue progenitor cells results in chronic inflammation, an altered microenvironment, and progression to intraepithelial neoplasia. Cancer Res. 2010;70:8435–8445. doi: 10.1158/0008-5472.CAN-10-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson SC, Eberl M, Vagnozzi AN, Belkadi A, Veniaminova NA, Verhaegen ME, Bichakjian CK, Ward NL, Dlugosz AA, Wong SY. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell. 2015;16:400–412. doi: 10.1016/j.stem.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quante M, Bhagat G, Abrams JA, Marache F, Good P, Lee MD, Lee Y, Friedman R, Asfaha S, Dubeykovskaya Z, et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell. 2012;21:36–51. doi: 10.1016/j.ccr.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raufman JP, Samimi R, Shah N, Khurana S, Shant J, Drachenberg C, Xie G, Wess J, Cheng K. Genetic ablation of M3 muscarinic receptors attenuates murine colon epithelial cell proliferation and neoplasia. Cancer Res. 2008;68:3573–3578. doi: 10.1158/0008-5472.CAN-07-6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, Zack TI, Wang X, Tsherniak A, Schinzel AC, et al. beta-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457–1473. doi: 10.1016/j.cell.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutz B, Jurastow I, Bader S, Ringer C, von Engelhardt J, Chubanov V, Gudermann T, Diener M, Kummer W, Krasteva-Christ G, et al. Chemical coding and chemosensory properties of cholinergic brush cells in the mouse gastrointestinal and biliary tract. Frontiers in physiology. 2015;6:87. doi: 10.3389/fphys.2015.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G, Zhu L, Sun Y, Bettencourt R, Damsz B, Hruban RH, Konieczny SF. Loss of the acinar-restricted transcription factor Mist1 accelerates Kras-induced pancreatic intraepithelial neoplasia. Gastroenterology. 2009;136:1368–1378. doi: 10.1053/j.gastro.2008.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P, Sekhon HS, Lu A, Arredondo J, Sauer D, Gravett C, Mark GP, Grando SA, Spindel ER. M3 muscarinic receptor antagonists inhibit small cell lung carcinoma growth and mitogen-activated protein kinase phosphorylation induced by acetylcholine secretion. Cancer Res. 2007;67:3936–3944. doi: 10.1158/0008-5472.CAN-06-2484. [DOI] [PubMed] [Google Scholar]
- Stopczynski RE, Normolle DP, Hartman DJ, Ying H, DeBerry JJ, Bielefeldt K, Rhim AD, DePinho RA, Albers KM, Davis BM. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res. 2014;74:1718–1727. doi: 10.1158/0008-5472.CAN-13-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallini YN, Shui B, Greene KS, Deng KY, Doran R, Fisher PJ, Zipfel W, Kotlikoff MI. BAC transgenic mice express enhanced green fluorescent protein in central and peripheral cholinergic neurons. Physiological genomics. 2006;27:391–397. doi: 10.1152/physiolgenomics.00092.2006. [DOI] [PubMed] [Google Scholar]
- Tutton PJ, Barkla DH. Serotonin receptors influencing cell proliferation in the jejunal crypt epithelium and in colonic adenocarcinomas. Anticancer Res. 1986;6:1123–1126. [PubMed] [Google Scholar]
- Tutton PJ, Helme RD. Proceedings: The role of catecholamines in the regulation of crypt cell proliferation. I. Adrenergic stimulation and blockade. Journal of anatomy. 1973;116:467–468. [PubMed] [Google Scholar]
- Vaishnavi A, Le AT, Doebele RC. TRKing down an old oncogene in a new era of targeted therapy. Cancer discovery. 2015;5:25–34. doi: 10.1158/2159-8290.CD-14-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh HS, Johung TB, Caretti V, Noll A, Tang Y, Nagaraja S, Gibson EM, Mount CW, Polepalli J, Mitra SS, et al. Neuronal Activity Promotes Glioma Growth through Neuroligin-3 Secretion. Cell. 2015;161:803–816. doi: 10.1016/j.cell.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016;529:221–225. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeraratna AT, Dalrymple SL, Lamb JC, Denmeade SR, Miknyoczki S, Dionne CA, Isaacs JT. Pan-trk inhibition decreases metastasis and enhances host survival in experimental models as a result of its selective induction of apoptosis of prostate cancer cells. Clin Cancer Res. 2001;7:2237–2245. [PubMed] [Google Scholar]
- Westphalen CB, Asfaha S, Hayakawa Y, Takemoto Y, Lukin DJ, Nuber AH, Brandtner A, Setlik W, Remotti H, Muley A, et al. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J Clin Invest. 2014;124:1283–1295. doi: 10.1172/JCI73434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Luo J, Mo JS, Liu G, Kim YC, Meng Z, Zhao L, Peyman G, Ouyang H, Jiang W, et al. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell. 2014;25:822–830. doi: 10.1016/j.ccr.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao CM, Hayakawa Y, Kodama Y, Muthupalani S, Westphalen CB, Andersen GT, Flatberg A, Johannessen H, Friedman RA, Renz BW, et al. Denervation suppresses gastric tumorigenesis. Science translational medicine. 2014;6:250ra115. doi: 10.1126/scitranslmed.3009569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.