Abstract
Standard volumetric neuroimaging studies have demonstrated preferential atrophy of subcortical structures among individuals with HIV. However, to our knowledge, no study has investigated subcortical shape alterations secondary to HIV and whether advancing age impacts that relationship. This study employed 3D morphometry to examine the independent and interactive effects of HIV and age on shape differences in nucleus accumbens, amygdala, caudate, hippocampus, pallidum, putamen, and thalamus in 81 participants ranging in age from 24 to 76 including 59 HIV+ individuals and 22 HIV‐seronegative controls. T1‐weighted MRI underwent a preprocessing pipeline followed by automated subcortical segmentation. Parametric statistical analyses were used to determine independent effects of HIV infection and age on volume and shape in each region of interest (ROI) and the interaction between age and HIV serostatus in predicting volume/shape in each ROI. Significant main effects for HIV were found in the shape of right caudate and nucleus accumbens, left pallidum, and hippocampus. Age was associated with differences in shape in left pallidum, right nucleus accumbens and putamen, and bilateral caudate, hippocampus, and thalamus. Of greatest interest, an age × HIV interaction effect was found in the shape of bilateral nucleus accumbens, amygdala, caudate, and thalamus as well as right pallidum and putamen such that increasing age in HIV participants was associated with greater shape alterations. Traditional volumemetric analyses revealed main effects for both HIV and age but no age × HIV interaction. These findings may suggest that age and HIV infection conferred additional deleterious effects on subcortical shape abnormalities beyond the independent effects of these factors. Hum Brain Mapp 38:1025–1037, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: neuroimaging, HIV, age, subcortical, morphometry, volumetry
Abbreviations
- ANOVA
analysis of variance
- cART
combination antiretroviral therapy
- Cho/Cr
choline/creatine
- FDR
false discovery rate
- FSL
FMRIB Software Library
- HIV
human immunodeficiency virus
- MRI
magnetic resonance imaging
- MRS
magnetic resonance spectroscopy
- MI/Cr
myoinositol/creatine
- ROI
region of interest
- SCID
Structured Clinical Interview for DSM‐IV
- UCLA
University of California, Los Angeles
- VBM
voxel‐based morphometry
INTRODUCTION
As the HIV‐infected population continues to age, questions regarding the synergistic effects of HIV and normal aging on neurofunction are becoming of ever‐increasing importance. The neuroAIDS literature regarding the impact of aging is mixed. While a number of studies have found that older adults with HIV infection are more vulnerable to cognitive problems compared to healthy elderly controls as well as younger HIV+ cohorts [Becker et al., 2004; Gonzalez & Cherner, 2008; Rodriguez‐Penney et al., 2013; Hinkin et al., 2004; Sacktor et al., 2011; Valcour et al., 2004], few studies have reported an interactive effect of advancing age and HIV infection [Sacktor et al., 2010; Chang et al., 2011; Morgan et al., 2011; Vance, 2011], or have found evidence to support that advancing age is an additive risk factor for neurocognitive dysfunction among HIV+ individuals [Valcour et al., 2011; Cysique et al., 2011; Kissel et al., 2014].
On the other hand, studies using magnetic resonance spectroscopy (MRS) have reported significant age × HIV interactive effects, particularly within neurometabolites in frontal white matter [Ernst & Chang, 2004; Harezlak et al., 2011; Cysique et al., 2013]. Although a significant interaction effect of HIV and age was not reported, a study using task‐based functional MRI [Ances et al., 2010] demonstrated that the task‐related blood‐oxygen dependent level signal for HIV+ participants was equivalent to that of HIV‐ control participants who were 15 years older. Finally, a study using diffusion tensor imaging found significant interaction effects of HIV and age in subcortical white matter and frontal regions [Chen et al., 2009].
Together, these studies support the contention that HIV may accelerate brain aging. Furthermore, significant effects of advancing age and HIV infection on brain integrity have been reported in cortical regions and specific white mater tracts. To our knowledge, interactive effects of HIV infection and advancing age on brain structure have not been reported in subcortical regions. We suggest here that the previous inability to find significant age × HIV interactive effects in subcortical structures may be due to the methodologies employed rather than an absence of an age × HIV interaction.
Studies that have examined subcortical structures have used volumetrics as the primary method of investigation. While volumetric methods have provided critical insights into adverse effects of HIV and aging on brain structure, this method is limited by its poor spatial resolution, which makes it difficult to detect changes which may be specific to particular regions or nuclei of brain structures. Considering that pattern and rate of disease‐related neuronal loss does not occur in a uniform linear fashion, computing total volume for a particular subcortical structure may be suboptimal for capturing early changes. Rather, there is often an insidious progression of neurodegeneration, at first without overt clinical sequelae, that progresses until cognitive and/or motor symptoms become apparent. This pattern of insidious neural insult is seen in disorder such as Parkinson's disease [Braak et al., 2004], Alzheimer's disease [Hynd et al., 2004; Ballatore, Lee & Trojanowski, 2007], and other forms of progressive neurodegenerative disorders. Similarly, there is reason to believe that HIV associated neurologic damage unfolds well before overt cognitive manifestations emerge [Masliah et al., 1995; Zhang et al., 2003; Jones et al., 2007; Thames et al., 2011; Fields et al., 2014; Bryant et al., 2015]. Therefore, an analytic method with greater spatial resolution may provide the sensitivity needed to capture neural changes that occur due to deleterious interaction effects of advancing age and HIV infection
Shape analysis, a method of Bayesian surface based analysis, is one such methodology that may be more sensitive to regional (e.g., specific nuclei) changes not detectable by standard volumetric methods [Patenaude et al., 2011]. Indeed, shape analysis provides more direct measurements than voxel based morphometry (VBM), including identification of boundaries, as well as location and direction of shape changes [Fernández‐Espejo et al., 2015; Fallon et al., 2013; Kim et al., 2013]. For example, studies using shape analysis to examine normal aging have shown that age‐related atrophy of the thalamus is greatest in anterior regions [Hughes et al., 2012]. This is consistent with other investigations that have documented normal aging effects in the anterior nuclei of the thalamus (such as the ventral anterior and the ventral lateral nucleus) that project to cortical regions [Alexander et al., 1986; Raz et al., 1997; Salat et al., 2009]. Age‐related changes in hippocampal shape have been found to be more sensitive for detecting cognitive impairment, relative to hippocampal subfield volumes [Voineskos et al., 2015]. Studies of HIV+ populations suggest that shape analysis provides increased sensitivity beyond that of volumetrics for detecting deformations in subcortical structures between HIV+ and HIV‐seronegative groups [Wade et al., 2015; Thompson et al., 2006]. Similarly, studies have supported increased sensitivity for shape analysis, for detecting structural deformations in Parkinson's disease (PD) and Alzheimer's disease (AD) [McKeown et al., 2008; Csernansky et al., 2000]. Additionally, shape analysis, but not volumetrics were shown to relate to meaningful outcomes in these studies, such as severity of motor symptoms in PD and progression from mild cognitive impairment to AD [Nemmi et al., 2015; Costafreda et al., 2011]. These studies suggest that degeneration is not uniform and that shape analysis may detect subtle variations in specific regions of structures that may be undetected/lost when the volume of the entire structure is measured.
We are unaware of any existing studies that have examined the independent and interactive effects of aging and HIV on subcortical structural abnormalities using methods of shape analysis. Based on findings derived using other imaging approaches, we identified the following candidate regions for shape analyses: nucleus accumbens, amygdala, caudate, hippocampus, pallidum, putamen, and thalamus. We examined (1) the effects of HIV infection on volume and shape in each region of interest (ROI); (2) the association between age and volume/shape in each ROI; and (3) the interaction between age and HIV in predicting volume/shape in each ROI.
METHODS
Participants
Participants included 59 HIV+ (confirmed by serologic testing) and 22 HIV‐negative participants who were enrolled as part of a larger study (K23 MH095661; PI: A.D.T.). All procedures were in accordance with the Declaration of Helsinki, reviewed and approved by the University of California, Los Angeles (UCLA) Institutional Review Board prior to enrollment and all participants provided written informed consent. The Structured Clinical Interview (SCID) for DSM‐IV [First et al., 1995], and questionnaires about neurological and medical history were used to screen for neurological, psychiatric, and medical confounds including: history of seizure disorder or other neurologic disorder; history of concussion or traumatic brain injury sufficient to warrant medical attention; history of Axis I psychiatric disorder or current substance use disorder (SCID‐IV diagnostic criteria); current prescription for psychotropic medication, except for anxiolytics and antidepressants; current substance dependence or methamphetamine use, comorbid CNS infection (e.g., Hepatitis C), HIV‐associated CNS opportunistic infection (e.g., toxoplasmosis) or neoplasm. Participants were also screened for MRI contraindications.
Group Demographic Comparison
Demographic factors (e.g., age, education) and clinical factors (e.g., past drug use) between HIV+ and HIV− groups were compared using one‐way analysis of variance (ANOVA). Group differences in dichotomous factors (e.g., gender, ethnicity, and urinalysis results) were assessed using chi‐square analyses. We used p < 0.05 as our cutoff for statistical significance for these demographic analyses (Table 1).
Table 1.
Demographic comparison between HIV+ & HIV− groups
| N = 59 | N = 22 | ||
|---|---|---|---|
| Demographic variable | HIV+ | HIV− | Cohen's d |
| Male sex (%) | 46 (79%) | 13 (59%) | 0.392 |
| Education | 13.54 ± 1.89 | 14.00 ± 2.56 | 0.205 |
| Age | 50.32 ± 12.05 | 53.25 ± 10.28 | 0.173 |
| Age range | 24–76 | 24–66 | |
| Ethnicitya | 35% C, 65% AA | 50% C, 50% AA | 0.315 |
| Nadir CD4 (#/mm3) | 219.16 ± 167.25 | ||
| Current CD4 (#/mm3) | 586.00 (296.26) | ||
| # (%) with detectable viral load | 13 (22%) | ||
| Peak viral load (IU/mL) | 362,318.87 (756358.02) | ||
| # (%) currently prescribed Truvada | 20 (34%) | ||
| # (%) previously prescribed Truvada | 10 (17%) | ||
| CNS penetrance efficiency of current ARV regimen | Median: 8.00 | Range: 2–16 | |
| (%) with major depression (SCID) | 4 (7%) | 1 (5%) | 0.064 |
|
# (%) current barbiturate use (urine screen) # (%) current cocaine use (urine screen) # (%) current amphetamine use (urine screen |
0 0 0 |
0 0 0 |
|
| # current MDMA use (urine screen) | 0 | 0 | |
| # (%) current phencyclidine use (urine screen) | 0 | 0 | |
| % with current substance abuse (SCID) | 14% | 4% | 0.412 |
| % with current alcohol abuse (SCID) | 8.8% | 4% | 0.316 |
| % with past substance dependence (SCID) | 42% | 27% | 0.248 |
| % with past alcohol dependence (SCID) | 22% | 23% | 0.045 |
| % with past substance abuse (SCID) | 42% | 20% | 0.424 |
| % with past alcohol abuse (SCID) | 23% | 12% | 0.309 |
Note. No significant (i.e., p < 0.05) differences across groups on these demographic variables.
C = Caucasian; AA = African American.
MRI Acquisition
T1‐weighted data was collected using a 12‐channel head coil on Siemens Tim Trio 3T scanner (Siemens Medical Solution, Erlangen, Germany) at the Center for Cognitive Neuroscience (Los Angeles, California) at the UCLA Semel Institute for Neuroscience and Human Behavior. Structural MP‐RAGE T1‐weighted scans were acquired with 120–1.0 mm sagittal slices, FOV = 256 mm (A‐P) × 192 mm (FH), matrix = 256–192, TR = 450.0 ms, TE = 10.0 ms, flip angle = 8, voxel size = 1.0m × 0.94 × 0.94 mm.
Shape Processing and Analysis
3D volumetric T1‐weighted anatomical scans were acquired from 59 HIV+ and 22 HIV− individuals. The following preprocessing steps were completed using the University of Southern California, Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) processing software (http://enigma.ini.usc.edu/protocols/). Anatomical scans subsequently underwent intensity inhomogeneity normalization using the MNI “nu_correct” tool (http://www.bic.mni.mcgill.ca/software/). Each T1‐weighted anatomical image was linearly aligned to a standard brain template (the Colin27) [Holmes et al., 1998] using FSL's FLIRT [Jenkinson et al., 2002] with 9 degrees of freedom to allow translations, rotations and scaling in 3D. Next, using FMRIB Software Library (FSL) [Smith et al., 2004], the preprocessed data was run through an automated, model‐based subcortical segmentation protocol (FSL FIRST) [Patenaude et al., 2011] using a boundary correction method. The vertex and bvar files of automatically segmented subcortical structures (which included the amygdala, nucleus accumbens, caudate, hippocampus, pallidum, putamen, and thalamus) in the left and right hemisphere were then visually inspected for quality and the bvar files for the segmented subcortical structures were concatenated both between‐group and within‐group.
All automatically segmented ROIs for each participant are aligned to the same standard space. Each vertex provides surface ROI data in the same location and space for each participant. To perform group‐level analyses, each participant's data must be registered to a standard space so that each vertex is aligned within a given space for group comparisons. The mean surface of the sample is used as the reference point.
Next, scalar vertex values were then analyzed using parametric statistical analyses to investigate the relationship between age, HIV status and the size and shape of the aforementioned subcortical structures. These analyses were performed using a Monte Carlo simulation in FSL randomize script [Hayasaka and Nichols, 2003; Nichols and Holmes, 2002]. Regressions assessed the relationship of radial distance at each vertex to variables of interest (i.e., age, HIV status, and the HIV × Age interaction) after regressing out covariates. A multistep, quality‐controlled processing pipeline was used to correct for multiple sources of artifact; nonetheless, we chose to employ a conservative p‐value threshold (p < 0.0001). When results indicated that a variable of interest was associated with a deformed vertex, the angle of vector depicting the direction of this effect was used to determine the type of shape alteration this represented. Significant vertices where the direction of the effect indicated that the change was inward toward the center of the subcortical structure were classified as “shrinking” vertices, a finding indicative of atrophy. Significant vertices where the direction of effect indicated changes that were outward away from the structure but with an angle of <45° from the surface of the structure, were classified as “thinning” vertices. This distinction was made to clarify that some (<45°) positive vectors signify elongation of the subcortical surface whereas others (>45°) indicate enlargement, or hypertrophy of the structure subregion (Fig. 1).
Figure 1.
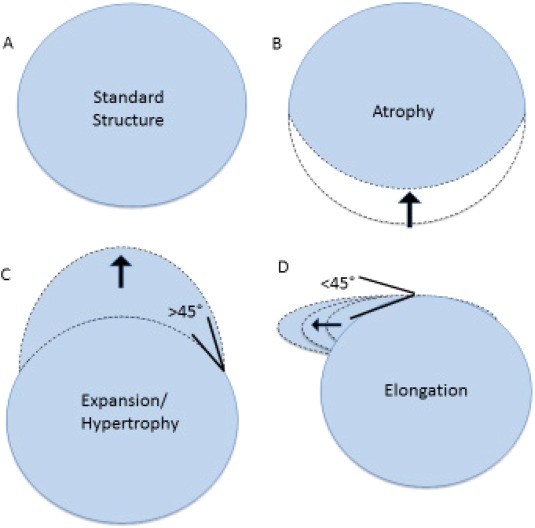
Illustration of types of subcortical shape deformation. [Color figure can be viewed at http://wileyonlinelibrary.com]
Illustration of shape changes as defined by the angle of deformation from the original position of a surface vertex (A). Any vertex with significant decrease in position, regardless of angle, denotes atrophy (B). Vertices with significant increase in position >45° denotes hypertrophy (C). A significant increase in position <45° denotes deformation of the vertex in such a way as to result in elongation or thinning of the structure (D).
Volumetric Analysis
Following quality inspection of automatic segmentation, mean volumes were extracted for each participant's nucleus accumbens, amygdala, caudate, hippocampus, pallidum, putamen, and thalamus. Age was centered and then used to create a centered age × HIV interaction term. Separate ordinary least square regressions were performed to investigate the relationship between HIV status, age, and the interactive effect of HIV and age on subcortical structure volume. False discovery rate (FDR) correction was applied to all regression results.
RESULTS
Demographic Group Comparison
The HIV+ and HIV‐seronegative groups did not significantly differ on age, years of education, ethnicity, or gender. None of the participants tested positive for barbiturates, cocaine, methamphetamine, phencyclidine, or MDMA. The HIV serostatus groups also did not differ on current alcohol or substance abuse, past (i.e., self‐reported lifetime) substance dependence, past substance abuse, past alcohol dependence, or past alcohol abuse. Participants were not included in the study if they reported previous methamphetamine abuse or dependence.
Bivariate correlations showed that years of education was significantly positively associated with volume of the left putamen (r = 0.25, P = 0.02), left amygdala (r = 0.23, P = 0.03), and right pallidum (r = 0.25, P = 0.02). We also found a significant positive relationship between years of education and enlarged shape of regions on all ROIs. There were no significant associations between sex or race/ethnicity and volume or shape of any ROI. Given that education did not differ between HIV groups, education was not included as a covariate in the regression models reported below.
Subcortical Shape Analysis
A multistep, quality‐controlled processing pipeline was used to correct for multiple sources of artifact; nonetheless, we chose to employ a conservative p‐value threshold (p < 0.001). All results reported below were significant (p < 0.001) and are displayed in empirically derived figures showing the location (i.e., subregion) of significant results on each subcortical structure.
A significant effect of HIV serostatus on subcortical structure shape was found in regions of the left putamen, right caudate, right nucleus accumbens and bilateral hippocampi (F > 4, p < 0.001; see Fig. 2 and Table 2 for specific subregion details]. HIV infection was associated with shrinking in regions of the right nucleus accumbens, left putamen, and bilateral hippocampus, and thinning in the right caudate. No significant effect of HIV was found in the shape of bilateral amygdala, pallidum, or thalamus.
Figure 2.
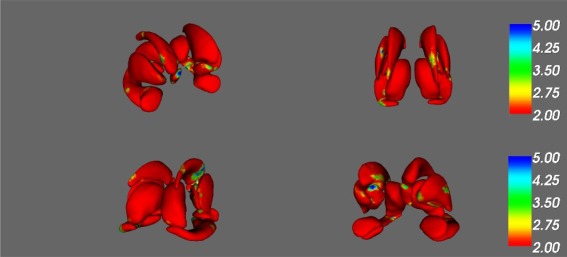
Effect of HIV on subcortical shape. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Differences in subcortical shape between HIV+ and HIV‐seronegative groups
| Hemisphere | Structurea | HIV+ group vertices | Subregion |
|---|---|---|---|
| Right | Nucleus accumbens | Reduction | Dorsal, ventral, lateral |
| Bilateral | Hippocampus | Reduction | Medial tail |
| Left | Putamen | Reduction | Dorsal |
| Right | Caudate | Elongation | Lateral body |
p < 0.001.
F‐statistic maps of the main effect of HIV on (a) right anterior, (b) posterior, (c) right posterior, and (d) left anterior nucleus accumbens, amygdala, caudate nucleus, hippocampus, putamen, pallidum, and thalamus. Significant results reported for F‐values ≤ 4, as indicated by regions in turquoise and blue.
Age was significantly correlated with shape changes in regions of the left pallidum, right nucleus accumbens, and right putamen, as well as bilateral caudate, hippocampus and thalamus (F > 4, p < 0.001; see Fig. 3 and Table 3 for specific subregion details). Age was associated with shrinking of regions of the right nucleus accumbens and bilateral hippocampus, in addition to thinning in regions of the bilateral caudate and bilateral thalamus. Furthermore, age was associated with enlargement in regions of the left pallidum, right putamen, and bilateral caudate. No significant associations were found between age and the left nucleus accumbens, left putamen, right pallidum, or bilateral amygdala.
Figure 3.
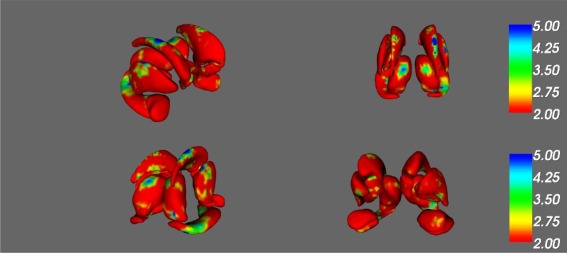
Effect of age on subcortical shape. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 3.
Effect of age on subcortical shape after controlling for HIV status
| Hemisphere | Structurea | Direction of effect | Subregion |
|---|---|---|---|
| Right | Nucleus accumbens | Reduction | Ventral, lateral |
| Left | Hippocampus | Reduction | Medial body |
| Right | Hippocampus | Reduction | Head, body, tail |
| Bilateral | Caudate | Elongation | Medial body, tail |
| Left | Thalamus | Elongation | Dorsomedial, ventroposterolateral |
| Right | Thalamus | Elongation | Dorsomedial, pulvinar |
| Left | Pallidum | Enlargement | Inferior, posterolateral |
| Right | Putamen | Enlargement | Dorsal |
| Bilateral | Caudate | Enlargement | Dorsal |
p < 0.001.
F‐statistic maps of the main effect of age on (a) right anterior, (b) posterior, (c) right posterior, and (d) left anterior nucleus accumbens, amygdala, caudate nucleus, hippocampus, putamen, pallidum, and thalamus. Significant results reported for F‐values ≥ 4, as indicated by regions in turquoise and blue.
An age × HIV interaction was found in regions of bilateral nucleus accumbens, amygdala, caudate, and thalamus as well as right pallidum and right putamen (F > 4, p < 0.001; see Fig. 4 and Table 4 for specific subregion details) such that with increased age the HIV+ participants demonstrated greater shape change. Specifically, this age × HIV interaction was seen with shrinking of regions of bilateral nucleus accumbens and the right lateral caudate, thinning in regions of the bilateral amygdala and bilateral caudate, as well as enlargement in regions of right pallidum, right putamen, and bilateral thalami.
Figure 4.
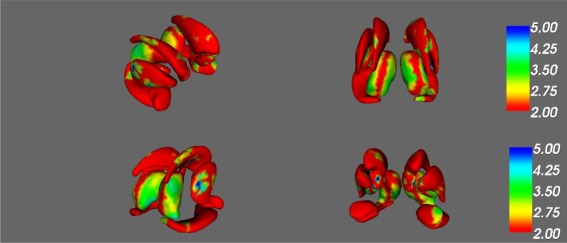
Interactive effect of HIV infection and age on subcortical shape. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 4.
Age × HIV interactive effect on subcortical shape
| Hemisphere | Structurea | Direction of effect | Subregion |
|---|---|---|---|
| Left | Nucleus accumbens | Reduction | Ventral |
| Right | Nucleus accumbens | Reduction | Dorsal |
| Right | Caudate | Reduction | Lateral body |
| Left | Amygdala | Elongation | Anterolateral, dorsomedial |
| Right | Amygdala | Elongation | Dosomedial |
| Left | Caudate | Elongation | Dorsal body |
| Right | Caudate | Elongation | Ventral & dorsal body |
| Right | Pallidum | Enlarged | Ventral, medial, lateral |
| Right | Putamen | Enlarged | Ventral, medial, lateral |
| Bilateral | Thalamus | Enlarged | Ventroanterior, ventrolateral, dorsomedial, ventroposterolateral, pulvinar |
p < 0.001.
F‐statistic maps of the interaction effect of HIV and aging on (a) right anterior, (b) posterior, (c) right posterior, and (d) left anterior nucleus accumbens, amygdala, caudate nucleus, hippocampus, putamen, pallidum, and thalamus. Significant results reported for F‐values ≥ 4, as indicated by regions in turquoise and blue.
Volumetric Analysis
Standard volumetric analyses were then conducted to determine whether the interactive effect of age and HIV infection was detectable in the average volume of these subcortical structures. To assess the effects of HIV status, age, and age × HIV interaction on subcortical volume, separate ordinary least squares regression analyses were conducted using the entire volume of each unilateral ROI as the outcome and HIV status, age, and age × HIV interaction term as predictors. While the overall regression models were significant for all ROIs except the left and right amygdala, the age × HIV interaction was not a significant predictor in any of these regressions.
DISCUSSION
This study examined the independent and interactive effects of age and HIV serostatus on subcortical brain structure by using shape analysis, a neuroimaging analysis method that provides a more precise measurement of morphometry than analyses conducted using a global metric of mean structure volume. We found significant age × HIV interactive effects suggesting that with older age, HIV+ participants demonstrated greater shape changes in several subcortical structures than younger HIV+ participants and both older and younger controls. In bilateral nucleus accumbens, amygdala, and caudate, results showed that increased age in HIV+ participants conferred regional elongation or thinning. Somewhat counterintuitively, the age × HIV interactive effect related to enlargement of regions in ventral and pulvinar regions of bilateral thalamus as well as right ventral, medial and lateral pallidum and right ventral, medial and lateral putamen. In the right pallidum, age × HIV associated hypertrophy was found in different subregions (ventral, medial, and lateral areas) than those where we found age‐related hypertrophy (dorsal areas).
Pathologic enlargement (i.e., hypertrophy) of subcortical structures has been found in studies of other HIV+ populations [e.g., Castelo et al., 2007; Wade et al., 2015] and may represent HIV‐related basal ganglia hypermetabolism [Hinkin et al., 1995; Rottenberg et al., 1996; von Giesen et al., 2000]. Meyerhoff et al. [1996] reported decreased NAA/Cho specifically in the putamen, pallidum, and thalamus in HIV+ participants compared to controls. However, these authors did not find neuronal loss related to decreased NAA/Cho. Therefore, these results were taken to suggest metabolic abnormalities that occurred without secondary atrophy in the putamen, pallidum, and thalamus. While HIV creates a neurotoxic environment within the CNS leading to cell death and regional atrophy, there are also several consequences of HIV infection which can lead to hypertrophy, including abnormal proliferation of astrocytes (i.e., astrocytosis) [Buffo et al., 2008; Ton & Xiong, 2013; Tavazzi et al., 2014], normal proliferation of CNS cells attempting to maintain homeostasis in the presence of a viral infection [Cassol et al., 2014; Vartak‐Sharma et al., 2016], aquaporin dysregulation [Aoki‐Yoshino et al., 2005; Benga and Huber, 2012; Xing et al., 2016], neurotropic factor dysregulation [Gage et al., 1989; Fields et al., 2014] and expansion of neuronal somas during infection prior to apoptosis [Kaul et al., 2001; Mbita et al., 2014]. It is possible that one or more of these cellular mechanisms drive the pattern of atrophy/hypertrophy found in this study and that the co‐occurrence of these events is obscured when assessing the entire structure as a single volume.
Consistent with previous literature, we found that HIV was associated with subcortical shape abnormalities that were specific to the right caudate, right nucleus accumbens, left pallidum, and left hippocampal tail. These shape abnormalities were exclusively shrinking and/or thinning which is consistent with our knowledge of HIV effects on subcortical degeneration [e.g., Becker et al., 2011; Wade et al., 2015]. Additionally, significant age effects were found among a number of subcortical regions. Specifically, increased age was related to shrinking of the right nucleus accumbens and bilateral hippocampi. Increased age was also related to thinning of bilateral caudate and bilateral thalami. The shrinking and thinning findings are in line with prior research demonstrating age‐related volumetric loss in subcortical structures [e.g., Goodro et al., 2012; Walhovd et al., 2005] and provide further insights into specific ROI subregions that may be most susceptible to age‐related morphology changes.
Interestingly, age was also related to enlargement of the dorsal caudate body, as well as regions of the left lateral pallidum and right dorsal putamen. To the best of our knowledge, there have been no studies using shape analysis to investigate effect of aging on the ROIs we investigated here. However, age‐related enlargement has been report by authors using shape analysis to interrogate other ROIs. One such study employing shape analysis found age‐related enlargement of the substantia nigra and the medial portion of the subthalamic nucleus [Visser et al., 2016]. Regarding age‐based trajectories of subcortical volume, the literature is inconsistent [for a review, see Walhovd et al., 2011]. For example, several studies report age‐related decline in caudate volume [Greenberg et al., 2008; Gunning‐Dixon et al., 1998; Jernigan et al., 2001; Raz et al. 2005] while a more recent study found volumetric increase in the caudate in advanced aging [Fjell et al., 2014]. Walhovd et al. [2005] report age‐related volume loss across all cortical and subcortical regions investigated. However, these authors also found age‐related deceleration of atrophy in the caudate, putamen, and pallidum, suggesting unique age‐related anatomical changes in these structures. One possible mechanism underlying these corpus striatum findings may be increased neuron soma size as a compensatory mechanism modulating against age‐related atrophy [Cabello et al., 2002] and against progression of neurodegeneration into dementia [Iacono et al., 2008]. Further studies using shape analysis and histology may be needed to further elucidate these findings.
We are intrigued by our findings related to aging and enlargement of subcortical regions, as it not only provides more detail into neuropathological changes, but may also account for why previous studies using volume‐based methods have failed to find interactive effects. Averaging volume across a region with structural enlargement in certain subregions and degeneration within others (as was the case for the right pallidum) may not fully capture the complexity of structural changes. We also did not find age‐related volumetric increases in any ROI, further suggesting that the spatial sensitivity of shape analysis provides additional information about subcortical morphology than volumetric findings. Further, recent literature suggests that HIV‐associated accelerated brain aging likely occurs via similar cellular mechanisms as normal aging [Rickabaugh et al., 2015]. Thus, it is possible that some of the underlying neuropathology driving these aging‐related hypertrophies are also involved in the hypertrophy found in older HIV+ participants. Such age‐related mechanisms may include increased microglia proliferation with age [Hua et al., 2012], morphologic hypertrophy of activated microglia [Streit et al., 2004; Flanary, 2005] leading to improper removal of damaged or senescent microglia [Mosher & Wyss‐Coray, 2014], increased trafficking of peripheral leukocytes into the CNS [Ritzel et al., 2016], altered neuroprotective and inflammatory functions of microglia [Nakanishi & Wu, 2009; von Bernhardi et al., 2010] and inflammation‐related (e.g., CD4+ T lymphocyte) neurogenesis within healthy tissue [Kipnis et al., 2004; Ziv et al., 2006; Wolf et al., 2009; for a detailed review, please see Schwartz et al., 2013].
Results of volumetric analyses investigating the independent effects of HIV status and age on subcortical volume were largely in agreement with the results of shape analysis. Significant independent effects of age and HIV status were found on subcortical volume. However, we did not find an interactive effect on subcortical volume despite finding interactive effects on subcortical shape, further supporting the notion that shape analysis is a more appropriate method for investigating the interaction effects of advancing age and HIV infection.
Together, these findings both support and expand upon previous literature demonstrating deleterious effects of HIV and aging on subcortical volume [Cohen et al., 2010; Becker et al., 2011; Ances et al., 2012] and subcortical shape. Importantly, these findings support the contention that the lack of age × HIV interactive findings to date does not necessarily translate to the absence of effects. Rather, it is possible that the presence of both enlargement and thinning/reduction within a particular region obscures the results of volumetric analyses by reducing the sensitivity for detecting regionally specific change. Volumetric measurements only allow for inference about atrophy or hypertrophy of the entire structure. Shape analyses allow for inferences about different mechanisms of atrophy, including shrinking and elongation, that are highly spatially specific and which suggest different forms of pathologic neuronal changes, and possibly explains the heterogeneity of cognitive impairments seen in HIV‐associated neurocognitive disorder (HAND).
There are limitations of this study worth noting. First, even for an imaging study, we had a relatively small sample of participants that consisted of primarily African American and Caucasian males, which may not generalize to other ethnic/racial subgroups or women. Considering that this was a cross‐sectional study, we cannot make inferences about long‐term outcomes or the rates of neuroanatomic change in our HIV+ cohort. HIV+ average age was 50, which could be considered young for aging studies. However, our age range included HIV+ participants from 24 to 76 years old. Additionally, this does represent a relatively older HIV+ population and, given that recent studies have demonstrated that HIV infection is associated with accelerated aging [Horvath et al., 2015], it is not surprising that we were able to find age effects in this sample. Further, the results of shape analyses must be interpreted with some caution, as changes on the shape of a structures surface do not give direct information about changes occurring at the cellular level.
Despite these limitations, the findings of the current study provide a unique contribution to the existing literature on HIV and aging and have important potential clinical implications for neurobehavioral outcomes. Given that this study found that age and HIV infection conferred additional deleterious effects on subcortical shape beyond the independent effects of these factors, it is possible that older HIV+ individuals are at risk for cognitive problems in functions associated with these structures as well as an accelerated trajectory of cognitive decline.
ACKNOWLEDGMENTS
The anatomic neuroimaging data was preprocessed by our collaborators (D.S.) at University of Southern California, Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) using their processing pipeline (http://enigma.ini.usc.edu/protocols/). The authors report no conflicts of interest.
REFERENCES
- Alexander GE, DeLong MR, Strick PL (1986): Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci 9:357–381. [DOI] [PubMed] [Google Scholar]
- Ances BM, Vaida F, Yeh MJ, Liang CL, Buxton RB, Letendre S, McCutchan JA, Ellis RJ (2010): HIV infection and aging independently affect brain function as measured by functional magnetic resonance imaging. J Infect Dis 201:336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Ortega M, Vaida F, Heaps J, Paul R (2012): Independent effects of HIV, aging, and HAART on brain volumetric measures. J AIDS 59:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki‐Yoshino K, Uchihara T, Duyckaerts C, Nakamura A, Hauw JJ, Wakayama Y (2005): Enhanced expression of aquaporin 4 in human brain with inflammatory diseases. Acta Neuropathologica 110:281–288. [DOI] [PubMed] [Google Scholar]
- Ballatore C, Lee VMY, Trojanowski JQ (2007): Tau‐mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci 8:663–672. [DOI] [PubMed] [Google Scholar]
- Becker JT, Lopez OL, Dew MA, Aizenstein HJ (2004): Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS 18:11–18. [PubMed] [Google Scholar]
- Becker JT, Sanders J, Madsen SK, Ragin A, Kingsley L, Maruca V, Cohen B, Goodkin K, Martin E, Miller EN, Sacktor N (2011): Subcortical brain atrophy persists even in HAART‐regulated HIV disease. Brain Imag Behav 5:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benga O, Huber VJ (2012): Brain water channel proteins in health and disease. Mol Asp Med 33:562–578. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K (2004): Stages in the development of Parkinson's disease‐related pathology. Cell Tissue Res 318:121–134. [DOI] [PubMed] [Google Scholar]
- Bryant AK, Ellis RJ, Umlauf A, Gouaux B, Soontornniyomkij V, Letendre SL, Achim CL, Masliah E, Grant I, Moore DJ (2015): Antiretroviral therapy reduces neurodegeneration in HIV infection. AIDS 29:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Götz M (2008): Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc Natl Acad Sci 105:3581–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello CR, Thune JJ, Pakkenberg H, Pakkenberg B (2002): Ageing of substantia nigra in humans: cell loss may be compensated by hypertrophy. Neuropathol Appl Neurobiol 28:283–291. [DOI] [PubMed] [Google Scholar]
- Cassol E, Misra V, Dutta A, Morgello S, Gabuzda D (2014): Cerebrospinal fluid metabolomics reveals altered waste clearance and accelerated aging in HIV patients with neurocognitive impairment. AIDS 28:1579–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo J, Courtney MG, Melrose RJ, Stern CE “ (2007): Putamen hypertrophy in nondemented patients with human immunodeficiency virus infection and cognitive compromise. Arch Neurol 64:1275–1280. ” [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Wang L, Joshi S, Miller JP, Gado M, Kido D, McKeel D, Morris JC, Miller MI (2000): Early DAT is distinguished from aging by high‐dimensional mapping of the hippocampus. Neurology 55:1636–1643. [DOI] [PubMed] [Google Scholar]
- Chang L, Andres M, Sadino J, Jiang CS, Nakama H, Miller E, Ernst T (2011): Impact of apolipoprotein E ε4 and HIV on cognition and brain atrophy: Antagonistic pleiotropy and premature brain aging. Neuroimage 58:1017–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Holt JL, Yakupov R, Jiang CS, Ernst T (2013): Lower cognitive reserve in the aging human immunodeficiency virus‐infected brain. Neurobiol Aging 34:1240–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Lee PL, Yiannoutsos CT, Ernst T, Marra CM, Richards T, Kolson D, Schifitto G, Jarvik JG, Miller EN, Lenkinski R (2004): A multicenter in vivo proton‐MRS study of HIV‐associated dementia and its relationship to age. Neuroimage 23:1336–1347. [DOI] [PubMed] [Google Scholar]
- Chen Y, An H, Zhu H, Stone T, Smith JK, Hall C, Bullitt E, Shen D, Lin W (2009): White matter abnormalities revealed by diffusion tensor imaging in non‐demented and demented HIV+ patients. Neuroimage 47:1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Harezlak J, Schifitto G, Hana G, Clark U, Gongvatana A, Paul R, Taylor M, Thompson P, Alger J, Brown M (2010): Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. J Neurovirol 16:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda SG, Dinov ID, Tu Z, Shi Y, Liu CY, Kloszewska I, Mecocci P, Soininen H, Tsolaki M, Vellas B, Wahlund LO (2011): Automated hippocampal shape analysis predicts the onset of dementia in mild cognitive impairment. Neuroimage 56:212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Maruff P, Bain MP, Wright E, Brew BJ (2011): HIV and age do not substantially interact in HIV‐associated neurocognitive impairment. J Neuropsych Clin Neurosci 23:83–89. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Moffat K, Moore DM, Lane TA, Davies NW, Carr A, Brew BJ, Rae C (2013): HIV, vascular and aging injuries in the brain of clinically stable HIV‐infected adults: A (1) H MRS study. PLoS One 8:e61738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Chang L (2004): Effect of aging on brain metabolism in antiretroviral‐naive HIV patients. AIDS 18:61–67. [PubMed] [Google Scholar]
- Ernst T, Jiang CS, Nakama H, Buchthal S, Chang L (2010): Lower brain glutamate is associated with cognitive deficits in HIV patients: A new mechanism for HIV‐associated neurocognitive disorder. J Magn Reson Imag 32:1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Yakupov R, Nakama H, Crocket G, Cole M, Watters M, Ricardo‐Dukelow ML, Chang L (2009): Declined neural efficiency in cognitively stable human immunodeficiency virus patients. Ann Neurol 65:316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon N, Alghamdi J, Chiu Y, Sluming V, Nurmikko T, Stancak A (2013): Structural alterations in brainstem of fibromyalgia syndrome patients correlate with sensitivity to mechanical pressure. NeuroImage Clin 3:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields J, Dumaop W, Langford TD, Rockenstein E, Masliah E (2014): Role of neurotrophic factor alterations in the neurodegenerative process in HIV associated neurocognitive disorders. J Neuroimmune Pharmacol 9:102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB (1995): Structured clinical interview for the DSM (SCID). John Wiley & Sons, Inc. [Google Scholar]
- Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB; Alzheimer's Disease Neuroimaging Initiative (2014): What is normal in normal aging? Effects of aging, amyloid and Alzheimer's disease on the cerebral cortex and the hippocampus Prog Neurobiol 117:20–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanary B (2005): The role of microglial cellular senescence in the aging and Alzheimer diseased brain. Rejuven Res 8:82–85. [DOI] [PubMed] [Google Scholar]
- Gage FH, Batchelor P, Chen KS, Chin D, Higgins GA, Koh S, Deputy S, Rosenberg MB, Fischer W, Bjorklund A (1989): NGF receptor reexpression and NGF‐mediated cholinergic neuronal hypertrophy in the damaged adult neostriatum. Neuron 2:1177–1184. [DOI] [PubMed] [Google Scholar]
- Goodro M, Sameti M, Patenaude B, Fein G (2012): Age effect on subcortical structures in healthy adults. Psychiatry Res 203:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Cherner M (2008): Co‐factors in HIV neurobehavioural disturbances: Substance abuse, hepatitis C and aging. Int Rev Psychiatry 20:49–60. [DOI] [PubMed] [Google Scholar]
- Greenberg DL, Messer DF, Payne ME, MacFall JR, Provenzale JM, Steffens DC, Krishnan RR (2008): Aging, gender, and the elderly adult brain: An examination of analytical strategies. Neurobiol Aging 29:290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning‐Dixon FM, Head D, McQuain J, Acker JD, Raz N (1998): Differential aging of the human striatum: A prospective MR imaging study. Am J Neuroradiol 19:1501–1507. [PMC free article] [PubMed] [Google Scholar]
- Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar ES, Alger J, Singer E, Campbell T, Yiannoutsos C, Cohen R (2011): HIV Neuroimaging Consortium: Persistence of HIV‐associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS 25:625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka S, Nichols TE (2003): Validating cluster size inference: random field and permutation methods. Neuroimage 20:2343–2356. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, Stefaniak M (2004): Medication adherence in HIV‐infected adults: Effect of patient age, cognitive status, and substance abuse. AIDS (Lond) 18:S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gorp WG, Mandelkern MA, Gee M, Satz P, Hoiston S, Marcotte TD, Evans MG, Paz DH, Ropchan JR, Quinones N, Khonsary A (1995): Cerebral metabolic change in patients with AIDS: Report of a six‐month follow‐up using positron‐emission tomography. Neurosciences 7:180–187. [DOI] [PubMed] [Google Scholar]
- Hua K, Schindler MK, McQuail JA, Forbes ME, Riddle DR (2012): Regionally distinct responses of microglia and glial progenitor cells to whole brain irradiation in adult and aging rats. PLoS One 7:e52728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EJ, Bond J, Svrckova P, Makropoulos A, Ball G, Sharp DJ, Edwards AD, Hajnal JV, Counsell SJ (2012): Regional changes in thalamic shape and volume with increasing age. Neuroimage 63:1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynd MR, Scott HL, Dodd PR (2004): Glutamate‐mediated excitotoxicity and neurodegeneration in Alzheimer's disease. Neurochem Int 45:583–595. [DOI] [PubMed] [Google Scholar]
- Iacono D, O'Brien R, Resnick SM, Zonderman AB, Pletnikova O, Rudow G, An Y, West MJ, Crain B, Troncoso JC (2008): Neuronal hypertrophy in asymptomatic Alzheimer disease. J Neuropathol Exp Neurol 67:578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema‐Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR (2001): Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging 22:581–594. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S (2001): A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S (2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841. [DOI] [PubMed] [Google Scholar]
- Jones GJ, Barsby NL, Cohen ÉA, Holden J, Harris K, Dickie P, Jhamandas J, Power C (2007): HIV‐1 Vpr causes neuronal apoptosis and in vivo neurodegeneration. J Neurosci 27:3703–3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim JB, Seo WK, Suh SI, Koh SB (2013): Volumetric and shape analysis of thalamus in idiopathic generalized epilepsy. J Neurol 260:1846–1854. [DOI] [PubMed] [Google Scholar]
- Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M (2004): T cell deficiency leads to cognitive dysfunction: Implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci USA 101:8180–8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissel EC, Pukay‐Martin ND, Bornstein RA (2014): The relationship between age and cognitive function in HIV‐infected men. J Neuropsych Clin Neurosci. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA (2001): Pathways to neuronal injury and apoptosis in HIV‐associated dementia. Nature 410:988–994. [DOI] [PubMed] [Google Scholar]
- Masliah E, Ge N, Achim CL, DeTeresa R, Wiley CA (1995): Patterns of neurodegeneration in HIV encephalitis. J Neuro‐AIDS 1:161–173. [DOI] [PubMed] [Google Scholar]
- Mbita Z, Hull R, Dlamini Z (2014): Human immunodeficiency virus‐1 (HIV‐1)‐mediated apoptosis: New therapeutic targets. Viruses 6:3181–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown MJ, Uthama A, Abugharbieh R, Palmer S, Lewis M, Huang X (2008): Shape (but not volume) changes in the thalami in Parkinson disease. BMC Neurol 8:(1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhoff DJ, Weiner MW, Fein G (1996): Deep gray matter structures in HIV infection: A proton MR spectroscopic study. Am J Neuroradiol 17:973–978. [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Delano‐Wood L, Bondi MW, Grant I (2011): HIV Neurobehavioral Research Program (HNRP) Group Intraindividual variability in HIV infection: Evidence for greater neurocognitive dispersion in older HIV seropositive adults. Neuropsychology 25:645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher KI, Wyss‐Coray T (2014): Microglial dysfunction in brain aging and Alzheimer's disease. Biochem Pharmacol 88:594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi H, Wu Z (2009): Microglia‐aging: Roles of microglial lysosome‐and mitochondria‐derived reactive oxygen species in brain aging. Behav Brain Res 201:1–7. [DOI] [PubMed] [Google Scholar]
- Nemmi F, Sabatini U, Rascol O, Péran P (2015): Parkinson's disease and local atrophy in subcortical nuclei: Insight from shape analysis. Neurobiol Aging 36:424–433. [DOI] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy D, Jenkinson M (2011): A Bayesian model of shape and appearance for subcortical brain. NeuroImage 56:907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD (1997): Selective aging of the human cerebral cortex observed in vivo: Differential vulnerability of the prefrontal gray matter. Cereb Cortex 7:268–282. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD (2005): Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex 15:1676–1689. [DOI] [PubMed] [Google Scholar]
- Rickabaugh TM, Baxter RM, Sehl M, Sinsheimer JS, Hultin PM, Hultin LE, Quach A, Martínez‐Maza O, Horvath S, Vilain E, Jamieson BD (2015): Acceleration of age‐associated methylation patterns in HIV‐1‐infected adults. PLoS One 10:e0119201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel RM, Crapser J, Patel AR, Verma R, Grenier JM, Chauhan A, Jellison ER, McCullough LD (2016): Age‐associated resident memory CD8 T cells in the central nervous system are primed to potentiate inflammation after ischemic brain injury. J Immunol 196:3318–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Penney AT, Iudicello JE, Riggs PK, Doyle K, Ellis RJ, Letendre SL, Woods SP, The HIV Neurobehavioral Research Program (HNRP) Group (2013): Co‐morbidities in persons infected with HIV: Increased burden with older age and negative effects on health‐related quality of life. AIDS Patient Care and STDs, 27(1), 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg DA, Sidtis JJ, Strother SC, Schaper KA, Anderson JR, Nelson MJ, Price RW (1996): Abnormal cerebral glucose metabolism in HIV‐1 seropositive subjects with and without dementia. J Nuclear Med 37:1133–1141. [PubMed] [Google Scholar]
- Sacktor N, Skolasky RL, Cox C, Selnes O, Becker JT, Cohen B, Martin E, Miller EN (2010): Longitudinal psychomotor speed performance in human immunodeficiency virus‐seropositive individuals: Impact of age and serostatus. J Neurovirol 16:335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N, Miyahara S, Deng L, Evans S, Schifitto G, Cohen BA, Paul R, Robertson K, Jarocki B, Scarsi K, Coombs RW (2011): Minocycline treatment for HIV‐associated cognitive impairment Results from a randomized trial. Neurol 77:1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Greve DN, Pacheco JL, Quinn BT, Helmer KG, Buckner RL, Fischl B (2009): Regional white matter volume differences in nondemented aging and Alzheimer's disease. Neuroimage 44:1247–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, Kipnis J, Rivest S, Prat A (2013): How do immune cells support and shape the brain in health, disease, and aging? J Neurosci 33:17587–17596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK (2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:S208–S219. [DOI] [PubMed] [Google Scholar]
- Streit WJ (2004): Microglia and Alzheimer's disease pathogenesis. J Neurosci Res 77:1–8. [DOI] [PubMed] [Google Scholar]
- Tavazzi E, Morrison D, Sullivan P, Morgello S, Fischer T (2014): Brain inflammation is a common feature of HIV‐infected patients without HIV encephalitis or productive brain infection. Curr HIV Res 12:97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, Kim MS, Becker BW, Foley JM, Hines LJ, Singer EJ, Heaton RK, Castellon SA, Hinkin CH (2011): Medication and finance management among HIV‐infected adults: The impact of age and cognition. J Clin Exp Neuropsychol 33:200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JB, Brier MR, Snyder AZ, Vaida FF, Ances BM (2013): Pathways to neurodegeneration: Effects of HIV and aging on resting‐state functional connectivity. Neurology 80:1186–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Lu A, Lee SE, Lee JY, Becker JT (2006): 3D mapping of ventricular and corpus callosum abnormalities in HIV/AIDS. Neuroimage 31:12–23. [DOI] [PubMed] [Google Scholar]
- Ton H, Xiong H (2013): Astrocyte dysfunctions and HIV‐1 neurotoxicity. J AIDS Clin Res 4:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Paul R, Neuhaus J, Shikuma C (2011): The effects of age and HIV on neuropsychological performance. J Int Neuropsychol Soc 17:190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour VG, Shikuma CM, Watters MR, Sacktor NC (2004): Cognitive impairment in older HIV‐1‐seropositive individuals: Prevalence and potential mechanisms. AIDS (Lond) 18:S79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance DE, Mugavero M, Willig J, Raper JL, Saag MS (2011): Aging with HIV: a cross‐sectional study of comorbidity prevalence and clinical characteristics across decades of life. J Assoc Nurses AIDS Care 22:17–25. [DOI] [PubMed] [Google Scholar]
- Vartak‐Sharma N, Nooka S, Ghorpade A (2016): Astrocyte elevated gene‐1 (AEG‐1) and the A (E) Ging HIV/AIDS‐HAND. Progr Neurobiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser E, Keuken MC, Forstmann BU, Jenkinson M (2016): Automated segmentation of the substantia nigra, subthalamic nucleus and red nucleus in 7T data at young and old age. NeuroImage 139:324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos AN, Winterburn JL, Felsky D, Pipitone J, Rajji TK, Mulsant BH, Chakravarty MM (2015): Hippocampal (subfield) volume and shape in relation to cognitive performance across the adult lifespan. Hum Brain Mapp 36:3020–3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Bernhardi R, Tichauer JE, Eugenín J (2010): Aging‐dependent changes of microglial cells and their relevance for neurodegenerative disorders. J Neurochem 112:1099–1114. [DOI] [PubMed] [Google Scholar]
- von Giesen HJ, Antke C, Hefter H, Wenserski F, Seitz RJ, Arendt G (2000): Potential time course of human immunodeficiency virus type 1–associated minor motor deficits: Electrophysiologic and positron emission tomography findings. Arch Neurol 57:1601–1607. [DOI] [PubMed] [Google Scholar]
- Wade BS, Valcour V, Busovaca E, Esmaeili‐Firidouni P, Joshi SH, Wang Y, Thompson PM (2015): Subcortical shape and volume abnormalities in an elderly HIV+ cohort In: SPIE Medical Imaging. International Society for Optics and Photonics; pp. 94171S–94171S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B (2005): Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging 26:1261–1270. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM (2011): Consistent neuroanatomical age‐related volume differences across multiple samples. Neurobiol Aging 32:916–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SA, Steiner B, Akpinarli A, Kammertoens T, Nassenstein C, Braun A, Blankenstein T, Kempermann G (2009): CD4‐positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. J Immunol 182:3979–3984. [DOI] [PubMed] [Google Scholar]
- Xing HQ, Zhang Y, Izumo K, Arishima S, Kubota R, Ye X, Xu Q, Mori K, Izumo S (2016): Decrease of aquaporin‐4 and excitatory amino acid transporter‐2 indicate astrocyte dysfunction for pathogenesis of cortical degeneration in HIV‐associated neurocognitive disorders. Neuropathology [DOI] [PubMed] [Google Scholar]
- Zhang K, McQuibban GA, Silva C, Butler GS, Johnston JB, Holden J, Clark‐Lewis I, Overall CM, Power C (2003): HIV‐induced metalloproteinase processing of the chemokine stromal cell derived factor‐1 causes neurodegeneration. Nat Neurosci 6:1064–1071. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M (2006): Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci 9:268–275. [DOI] [PubMed] [Google Scholar]


