Abstract
Background
Human papillomavirus (HPV) testing allows women to self-collect cervico-vaginal cells at home (i.e., self-sampling). Using primary data from a randomized pilot study, we evaluated the long-term consequences and cost-effectiveness of using self-sampling to improve participation to routine cervical cancer screening in Norway.
Methods
We compared a strategy reflecting screening participation (using reminder letters) to strategies that involved mailing self-sampling device kits to women non-compliant to screening within a 5-year or 10-year period under two scenarios: A) self-sampling respondents had moderate under-screening histories, or B) respondents to self-sampling had moderate and severe under-screening histories. Model outcomes included quality-adjusted life-years (QALY) and lifetime costs. The ‘most cost-effective’ strategy was identified as the strategy just below $100,000 per QALY gained.
Results
Mailing self-sampling device kits to all women non-compliant to screening within a 5-year or 10-year period can be more effective and less costly than the current reminder letter policy; however, the optimal self-sampling strategy was dependent on the profile of self-sampling respondents. For example, ‘10-yearly self-sampling’ is preferred ($95,500 per QALY gained) if ‘5-yearly self-sampling’ could only attract moderate under-screeners; however, ‘5-yearly self-sampling’ is preferred if this strategy could additionally attract severe under-screeners.
Conclusions
Targeted self-sampling of non-compliers likely represents good value-for-money; however, the preferred strategy is contingent on the screening histories and compliance of respondents.
Impact
The magnitude of the health benefit and optimal self-sampling strategy is dependent on the profile and behavior of respondents. Health authorities should understand these factors prior to selecting and implementing a self-sampling policy.
Keywords: Mass screening, compliance, cost-effectiveness, cervical cancer, Pap smear, human papillomavirus, self-sampling
Introduction
Cervical cancer screening, aimed at detecting and removing cervical abnormalities before they have an opportunity to progress to invasive cancer, has contributed to significant reductions in cervical cancer burden worldwide (1). However, screening programs continue to be challenged with participation and follow-up compliance rates that are typically well below 100 percent. For example, in Norway, approximately 50% of eligible women consistently attend routine cytology-based (i.e., Pap smear) screening every three years according to guidelines-based recommendations, while the remaining never attend (~10%) or attend less frequently than recommended (~40%). These missed opportunities to prevent cervical cancer result in negative health and economic consequences.
The advent of new technologies that can detect human papillomavirus (HPV), the necessary cause of cervical cancer, has opened new opportunities to increase screening participation by allowing women to self-collect cervico-vaginal cells at home and return the sample in the mail for HPV testing. Meta-analyses have demonstrated comparable detection rates of high-risk HPV infections between self- and physician-collected tests (2, 3), In addition, studies assessing the impact of self-sampling on screening utilization have found that mailing a self-sampling device kit to non-compliant women may improve attendance rates beyond conventional approaches (4). For example, a recent randomized pilot study (SESAM-1 (SElf-SAMpling-1)) (5), tasked with demonstrating the feasibility and effectiveness of self-sampling in Norway, found that offering self-sampling as an alternative to mailing repeated reminder letters improved overall attendance to the screening program. However, the majority of empirical studies, including the Norwegian pilot study, were limited to reporting outcomes in terms of percent increase in coverage (4) and have not evaluated the long-term consequences (e.g., changes in cervical cancer risk and mortality) associated with improvements in screening attendance. Assessing the long-term health outcomes as well as monetary costs allows decision makers to evaluate whether the gains in coverage justify any additional costs associated with increasing adherence.
While self-sampling has the potential to overcome practical and personal barriers of traditional office-based screening, health authorities need to consider important country-specific factors prior to implementing a national policy regarding self-sampling. For example, a recent study in neighboring Denmark found that response rates to self-sampling were highest among women with more frequent screening history compared with women that infrequently or never attended screening (6). Failing to attract the most under-screened women may reduce the potential value of introducing self-sampling. In addition, the Norwegian pilot study indicated nearly half of self-sampling respondents substituted an office-based cytology exam with returning the self-sampling device. While substitution behavior may help reduce societal costs associated with a screening program, follow-up compliance among test-positive women advised to undergo office-based cytology must remain high in order to retain overall program effectiveness (7), Lastly, the Norwegian pilot study identified a proportion of self-sampling respondents that tested negative for an HPV infection, yet still attended an office-based exam. Excess screening generates costs without adding benefit, and should be considered when evaluating the efficiency of a self-sampling policy.
Using primary data from the randomized self-sampling pilot study in Norway, our objective was to use mathematical modeling to evaluate the long-term health consequences and cost-effectiveness of self-sampling as an approach to improve participation to routine cervical cancer screening. We considered the impact of the long-term screening participation history of self-sampling respondents (i.e., moderate vs. severe under-screeners), substitution behavior of current screeners, compliance to follow-up for women advised to undergo additional testing, and excess screening behavior.
Materials and methods
Analytic approach
We adapted a previously published decision-analytic model (8) to simulate HPV-induced cervical cancer burden in the context of Norway. In our analysis, we compared a baseline scenario representing status quo cytology-based screening and participation that is observed under the current “2-reminder letter policy” to strategies that involved mailing cervico-vaginal self-sampling device kits to women not compliant with routine screening. Model inputs were based on findings from a recent self-sampling pilot study that randomized Norwegian women non-compliant after the 1st reminder letter to receive either a 2nd reminder letter or a self-sampling device kit. We used an intention-to-treat approach to estimate the percentage-increase in screening as the study participants in the self-sampling arm, but not the control arm, were given an opportunity to opt-out of the trial prior to mailing sampling devices (5.9% of eligible women opted-out). Model outcomes included lifetime risk of cervical cancer, quality-adjusted life expectancy, lifetime costs, and resource use (e.g., office-based cytology exam, colposcopy referrals). We identified efficient strategies by calculating the incremental cost-effectiveness ratios (ICER), calculated as the incremental costs divided by the incremental benefit of one strategy compared with the next least costly strategy. Strategies that were more costly and less effective (dominated) or less costly but also less efficient (weakly dominated) were removed from calculations. The ‘most cost-effective’ strategy was identified as the strategy with the ICER just below the amount Norwegian society is willingness-to-pay for health improvements. Although no single willingness-to-pay threshold value exists in Norway, we used the commonly-cited threshold value of 500,000 Norwegian Kroner (~$80,000 in 2005-values (5)) per quality-adjusted life year (QALY) gained, and adjusted to 2014-values using changes in real income wage in Norway between 2005 and 2014 (4). Accordingly, we considered a strategy just below $100,000 per QALY gained as cost-effective, although explored the optimal strategy under alternative values. For all analyses, we selected 50 parameter sets that fit well to the empirical data to reflect uncertainty in the natural history model input values, and reported results as the mean of outcomes across the parameter sets. To capture uncertainty in the optimal strategy, we conducted a probabilistic sensitivity analysis and reported the minimum and maximum ICERs across the 50 good-fitting parameter sets for the base case analysis. We adopted a societal perspective to include direct health-related costs, direct non-health-related costs, and patient time costs (e.g., time spent by the patient attending screening or performing a self-collected sample), but evaluated a scenario with direct health-related costs only in sensitivity analysis. Costs and benefits were discounted by 4% per year, as recommended in Norway (9). Parameter uncertainty and alternative scenarios were evaluated in sensitivity analysis.
Model overview and input parameters
Individual girls enter the model at age 9, prior to sexual initiation and without HPV infection or cervical disease. Each month individuals may acquire type-specific HPV infection(s) categorized as HPV-16, -18, -31, -33, -45, -52 and -58, pooled other high-risk HPV, and pooled low-risk HPV. Individuals with a prevalent infection may transition to and from HPV-induced health states (i.e., cervical intraepithelial neoplasia grades 2 (CIN2) or 3 (CIN3), and invasive cancer) (8). The model was adapted to the Norwegian context using a likelihood-based calibration approach to identify unique parameter sets that maximized correspondence between model outputs and empirical outcomes observed in Norway, including age-specific prevalence of HPV, and type-specific distribution of HPV in CIN3 and invasive squamous cell carcinoma. Additional details of the model structure and calibration to Norway, based on a previous Norwegian analysis (10), are available at author’s website (11)).
Direct medical and non-medical costs associated with office-based screening, laboratory analysis, and treatment of precancer and cancer were based on guidelines and valued using a combination of Norwegian fee schedules and micro-costing. The costs associated with self-sampling reflected empirical data from the Norwegian self-sampling pilot study (Table 1). The cost of the self-sampling kit included the self-collection device (dry brush), information leaflet, resealable zipper bag, identification sheet, return envelope and postage. For the proportion of women that returned their device, we assumed 45 minutes to read, administer and return the sample to a mailbox, as well as the cost associated with analyzing the HPV test. All costs were measured in 2014 NOK and converted to US dollars (US $) using the average annual 2014 exchange rate (US $1=NOK6.30) (12). The office-based cost calculation approach has been reported previously (10); see author’s website for additional details (11).
Table 1.
Selected model inputs and assumptions
| Variable | Base value | Setting |
|---|---|---|
| Costs (2014 US$) | ||
| Reminder letter | $1.4 | Norway(5) |
| Primary office visita | $241 | Norwayf |
| Analysing Pap smear | $45 | Norwayf |
| Analysing hrHPV test | $40 | Norwayf |
| Self-collection kitb | $54 | Norway(5) |
| Colposcopy with biopsy | $532 | Norwayf |
| Treatment of precancer | $1,681 | Norwayf |
| Treatment of local cancer | $26,929 | Norwayf |
| Treatment of regional cancer | $56,573 | Norwayf |
| Treatment of distant cancer | $41,348 | Norwayf |
| % screening at different frequencies with current 2-reminder letter policy | ||
| 3-yearly | 47% | Norway (16) |
| 4-yearly | 10% | Norway (16) |
| 5-yearly | 22% | Norway (16) |
| 8-yearly | 5% | Assumption |
| 10-yearly | 5% | Norway (16) |
| 20-yearly | 5% | Assumption |
| Never | 6% | Norway (16) |
| ‘5-yearly self-sampling’ policy | ||
| % returning SS device | 20% | Norway (5) |
| % increase in absolute participation with SS | 10% | Norway (5) |
| % switch from physician-collected to SSc | 10% | Norway (5) |
| ‘10-yearly self-sampling’ policy | ||
| % returning SS device | 11% | Assumption |
| % increase in absolute participation with SS | 6% | Assumption |
| % switch from physician-collected to SSc | 5% | Assumption |
| Additional base case self-sampling parameters | ||
| % hrHPV-positive attending office-based cytology | 88% | Norway (5) |
| % hrHPV-negative attending office-based cytologyd | 18% | Norway (5) |
| % requesting SS device (opt-in policy) | 30% | Sweden (14) and Denmark (6) |
| Test characteristicse | ||
| Relative sensitivity of self-collected hrHPV test | 100% | Meta-analysis (2, 3) |
| Relative specificity of self-collected hrHPV test | 95% | Meta-analysis (2, 3) |
hrHPV: high-risk human papillomavirus.
Includes patient time and transport, and sending sample to laboratory;
Includes self-collection device (dry brush), leaflet, resealable zipper bag, identification sheet, return envelope and postage. For the proportion of women that returned their device, we included individual's time (i.e. 45 minutes) to read the instructions, take the sample and return the sample to a mailbox;
Proportion of women that would have attended an office-based exam within the same year if self-sample device was not mailed;
Proportion of women that were hrHPV-negative on their self-collected sample, but attended an office-based cytology exam;
Self-collected cervico-vaginal sample relative to physician-collected cervical sample. HPV test sensitivity is defined as the probability of detecting high-risk HPV given presence of high-risk HPV in the cervical canal. HPV test specificity is defined as the probability of not detecting hrHPV given hrHPV is not present in the cervix;
Based on previous Norwegian analyses (10) and updated to 2014 values.
For more detailed information on cost calculations, please visit the author’s website at: http://www.med.uio.no/helsam/english/research/projects/preventive-strategies-hpv/harvardmodel-norway-technicalappendix.pdf. (US $1=NOK6.30)
Analysis and assumptions
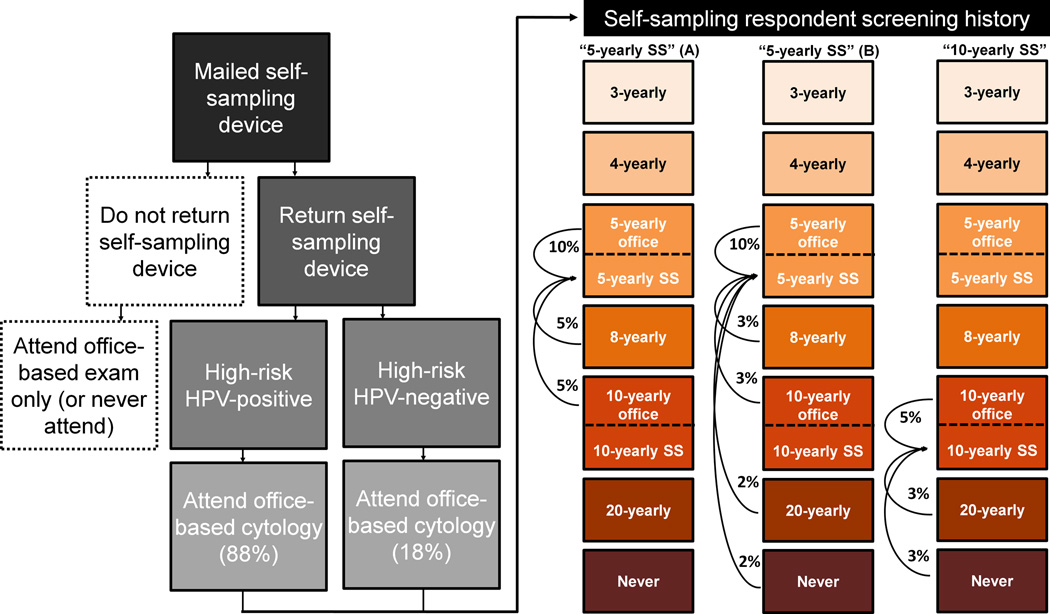
Primary data from the Cancer Registry of Norway were used to estimate a distribution of screening frequency (i.e., every 3-, 5-, 8-, 10-, 20-years, and never-screeners) associated with the current screening policy that involves mailing a 1st reminder letter to women who have not attended screening within a 3-year period and a 2nd reminder to those who did not comply within an additional year (Table 1). In the primary analysis, we compared this baseline status quo strategy to the self-sampling strategy that was evaluated in the pilot study (5). This strategy, herein referred to as ‘5-yearly self-sampling’, involves mailing a self-sampling kit to women non-compliant after the 1st reminder letter. Based on findings from the pilot study, we assumed that 20% of individuals returned their self-sampling device, among whom half (i.e., 10%) received the screening at 5 years and the other half (i.e., 10%) substituted attending an office-based exam with mailing a self-sampling device (at the same screening frequency) (Figure 1). Respondents that were positive for a high-risk HPV infection on their self-collected sample were referred to an office-based follow-up exam involving cytology. Consistent with the pilot trial (5), we assumed that compliance to the office-based exam for women positive for high-risk HPV was 88%, and that 18% of the women elected to attend an office-based cytology exam, despite a negative HPV test. Following guidelines-based screening in Norway at the time of pilot trial, women suspected of high-grade abnormalities on cytology were referred directly to colposcopy, while women with equivocal or low-grade results were recommended repeat cytology in combination with HPV testing 6–12 months later (13).
Figure 1. Self-sampling (SS) strategy flow chart and the alternative assumptions for participation and compliance (i.e., Scenario A and Scenario B).
Scenario A assumed that respondents to self-sampling had moderate under-screening histories (i.e., screened every 8-, or 10-years in the absence of self-sampling), while Scenario B assumed that respondents to self-sampling included women with moderate and severe under-screening histories (i.e., screen every 8-, 10-, 20-years or never-screened). For this scenario, we assumed there was a gradient of response related to screening history, i.e., women with more severe screening histories were less likely to respond compared with women with moderate screening histories. Flow chart reflects primary data from the SESAM-1 (SElf-SAMpling-1) pilot trial conducted in Norway (5).
To evaluate the impact of screening history on increased participation among the women who returned a self-sampling device, we projected the model outcomes for ‘5-yearly self-sampling’ under two scenarios (Figure 1). Scenario A assumed that respondents to self-sampling had moderate under-screening histories (i.e., screened every 8-, or 10-years in the absence of self-sampling), while Scenario B assumed that respondents to self-sampling included women with moderate and severe under-screening histories (i.e., screen every 8-, 10-, 20-years or never-screened). For this scenario, we assumed there was a gradient of response related to screening history, i.e., women with more severe screening histories were less likely to respond compared with women with moderate screening histories (Figure 1) (6).
In a secondary analysis, we considered an alternative self-sampling strategy that restricted mailing self-sampling device kits to women with more severe under-screening histories. This strategy targeted women non-compliant with routine screening within 10 years, herein referred to as ’10-yearly self-sampling’. For this strategy, we used empirical data from a neighboring Nordic country to inform response rates to self-sampling (6). The Danish study demonstrated that response to self-sampling was ~50% lower among women that infrequently or never-attended screening compared with women that more frequently attended screening. Therefore, we assumed that the overall increase in absolute participation to ’10-yearly self-sampling’ was 6%, with an additional 5% of women who substituted their office-based exam with a home-based self-sampling test; we considered this single behavioral assumption for all ‘10-yearly self-sampling’ analyses. In sensitivity analysis, we considered an opt-in “on-demand” self-sampling approach that involved mailing non-compliant individuals an invitation letter to request a self-sampling device kit. For this strategy, we assumed that 30% of the women requested a self-sampling device kit. Although a meta-analysis (4) has demonstrated that an “opt-in” approach does not provide statistically significant improvement in participation compared with reminder letters, we do not have Norwegian-specific data; therefore, we performed a threshold analysis to identify the maximum decrease in coverage the “opt-in” approach could permit in order to remain preferred over an “opt-out” self-sampling approach. For this scenario, we included the costs for reminders mailed to women who do not request the self-sampling device kit as well as for those who fail to return the device.
Results
5-yearly self-sampling
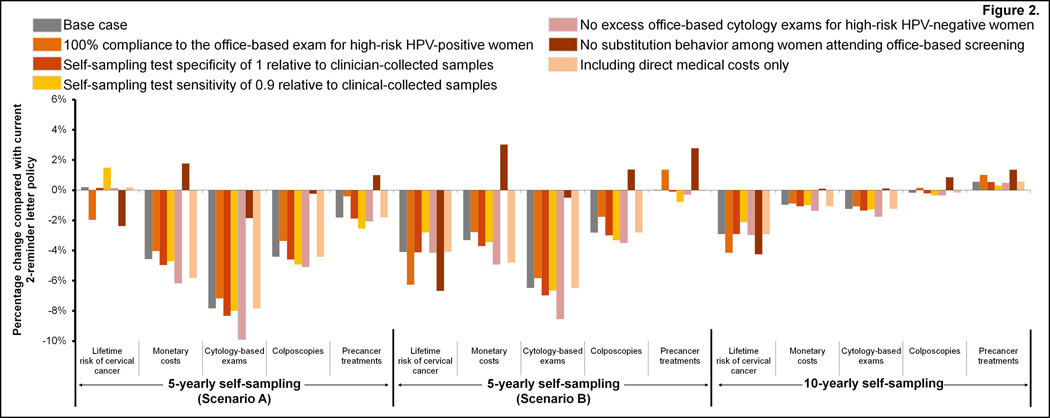
In the primary analysis and irrespective of self-sampling respondent screening history, mailing self-sampling devices to all women non-compliant after the 1st reminder letter is expected to be less costly and provide equal or greater health benefits (i.e., quality-adjusted life-years) compared with the current ‘2-reminder letter policy’ (Table 2). Importantly, if ‘5-yearly self-sampling’ could reach both moderate and severe under-screeners (i.e., Scenario B), self-sampling could further reduce the lifetime risk of developing cervical cancer, resulting in a more attractive cost-effectiveness profile (i.e., from $29,630 (range: $24,160–37,930) to $29,420 (range: $23,990–37,680) per QALY gained compared to no screening). Under both respondent screening history assumptions, ‘5-yearly self-sampling’ was generally associated with reductions in the total number of cytology exams, colposcopy referrals, and precancer treatments compared with current levels (Figure 2).
Table 2.
Primary cost-effectiveness analysis evaluating ‘5-yearly self-sampling’ compared with the current 2-reminder letter policy
| Respondent screening historya |
Strategy | Lifetime CC risk |
Discounted lifetime costs ($)b |
Discounted QALEc |
ICER ($)d |
|---|---|---|---|---|---|
|
Scenario A: Self-sampling respondents are moderate under-screeners |
No Screening | 2.17% | 328 | 19.7429 | -- |
| ‘5-yearly self-sampling’ | 0.75% | 1,719 | 19.7899 | 29,627 (24,159-37,926) | |
| ‘2-reminder letter policy’ | 0.75% | 1,801 | 19.7898 | Dominated | |
|
Scenario B: Self-sampling respondents are moderate and severe under- screeners |
No Screening | 2.17% | 328 | 19.7429 | -- |
| ‘5-yearly self-sampling’ | 0.72% | 1,741 | 19.7910 | 29,423 (23,990-37,683) | |
| ‘2-reminder letter policy’ | 0.75% | 1,801 | 19.7898 | Dominated | |
CC: Cervical cancer; ICER: Incremental cost-effectiveness ratio; QALE: Quality-adjusted life expectancy.
Moderate under-screening histories assumed women screen every 8-, or 10-years in the absence of self-sampling, and moderate and severe under-screening histories assumed women screen every 8-, 10-, 20-years or never-screened in the absence of self-sampling;
Discounting started at screening initiation (i.e., age 25 years);
Quality of life adjustment range from a health state utility weight of 0 (death) to 1 (perfect health). Weights for cervical cancer varied according to stage (local: 0.76 for five years; regional: 0.67 for five years; distant: 0.48 five years). Disease specific utility weights were multiplied to baseline age-specific utility weights from Denmark(17) to estimate overall utility;
Incremental cost-effectiveness ratios were calculated as the incremental mean costs divided by the incremental mean effects of two strategies. The range in ICER values reflects the minimum and maximum ICERs across the 50 good-fitting parameter sets. (US $1=NOK6.30)
Figure 2. Changes in health benefits and resources use for the baseline and influential parameters assumptions.
The figure displays the tradeoffs in reductions in lifetime cervical cancer risk, monetary costs, number of cytology exams, colposcopy referral rates, and precancer treatments for 5-yearly self-sampling (under Scenarios A and B) or 10-yearly self-sampling strategies compared with the current 2-reminder letter policy. Scenario A assumed that respondents to self-sampling had moderate under-screening histories (i.e., screened every 8-, or 10-years in the absence of self-sampling), while Scenario B assumed that respondents to self-sampling included women with moderate and severe under-screening histories (i.e., screen every 8-, 10-, 20-years or never-screened). For this scenario, we assumed there was a gradient of response related to screening history, i.e., women with more severe screening histories were less likely to respond compared with women with moderate screening histories.
5-yearly or 10-yearly self-sampling
When we expanded the analysis to include a strategy that restricts mailing self-sampling device kits to women who have not screened within 10 or more years, the optimal (i.e., most cost-effective) self-sampling policy was contingent on the screening histories of self-sampling respondents. For example, ‘10-yearly self-sampling’, which improves absolute participation by 6%, was considered the preferred strategy ($95,490 (range: $76,450–133,110) per QALY gained) if ‘5-yearly self-sampling’, which improves absolute participation by 10%, was only able to attract moderate under-screeners (Table 3). Provided willingness-to-pay thresholds of $50,000 and $100,000 per QALY gained, ‘10-yearly self-sampling’ was optimal in 0% and 70% of the simulations, respectively. Conversely, if ‘5-yearly self-sampling’ was able to attract both moderate and severe under-screeners, the health benefits associated with sending self-sampling devices more frequently were greater than the health benefits gained from the ‘10-yearly self-sampling’ strategy. Compared with the current 2-reminder letter policy, ‘10-yearly self-sampling’ reduced lifetime costs, the number of cytology exams, but resulted in equal or greater number of colposcopy referrals and precancer treatments (Figure 2).
Table 3.
Secondary cost-effectiveness analysis evaluating ‘5-yearly self-sampling’ or ‘10-yearly self-sampling’ compared with the current 2-reminder letter policy
| Respondent screening historya |
Strategy | Lifetime CC risk |
Discounted lifetime costs ($)b |
Discounted QALEcb |
ICER ($)d |
|---|---|---|---|---|---|
|
Scenario A: 5-yearly self-sampling respondents are moderate under-screeners (Figure 1) |
No Screening | 2.17% | 328 | 19.7429 | -- |
| ‘5-yearly self-sampling’ | 0.75% | 1,719 | 19.7899 | 29,627 (24,159-37,926) | |
| ‘10-yearly self-sampling’ | 0.73% | 1,783 | 19.7905 | 95,486 (76,454-133,108) | |
| ‘2-reminder letter policy’ | 0.75% | 1,801 | 19.7898 | Dominated | |
|
Scenario B: 5-yearly self-sampling respondents are moderate and severe under-screeners (Figure 1) |
No Screening | 2.17% | 328 | 19.7429 | -- |
| ‘5-yearly self-sampling’ | 0.72% | 1,741 | 19.7910 | $29,423 (23,990-37,683) | |
| ‘10-yearly self-sampling’ | 0.73% | 1,783 | 19.7905 | Dominated | |
| ‘2-reminder letter policy’ | 0.75% | 1,801 | 19.7898 | Dominated | |
CC: Cervical cancer; ICER: Incremental cost-effectiveness ratio; QALE: Quality-adjusted life expectancy.
Refers only to ‘5-yearly self-sampling’. Moderate under-screening histories assumed women screen every 8-, or 10-years in the absence of self-sampling, and moderate and severe under-screening histories assumed women screen every 8-, 10-, 20-years or never-screened in the absence of self-sampling. A single self-sampling scenario was considered for the ’10-yearly self-sampling’ strategy (see Figure 1);
Discounting started at screening initiation (i.e., age 25 years);
Quality of life adjustments ranged from a health state utility weight of 0 (death) to 1 (perfect health). Weights for cervical cancer varied according to stage (local: 0.76 for five years; regional: 0.67 for five years; distant: 0.48 five years). Disease specific utility weights were multiplied to baseline age-specific utility weights from Denmark (17) to estimate overall utility;
Incremental cost-effectiveness ratios were calculated as the incremental mean costs divided by the incremental mean effects of two strategies. The range in ICER values reflects the minimum and maximum ICERs across the 50 good-fitting parameter sets. (US $1=NOK6.30)
Sensitivity analysis
For all parameter explorations, the current 2-reminder letter policy remained less beneficial (i.e., quality-adjusted life expectancy) and more costly than 5- or 10-yearly self-sampling (Table 4). Similarly, assuming self-sampling could reach both moderate and severe under-screeners (i.e., Scenario B) always yielded more attractive incremental cost-effectiveness ratios compared with the scenarios that assumed self-sampling reached only moderate under-screeners (i.e., Scenario A). We found that cancer reductions improved considerably when we assumed that all high-risk HPV-positive women complied with the recommended office-based cytology exam, or when we assumed there was no substitution behavior. However, these two scenarios had differing effects on monetary costs, resource use and harms (Figure 2). For example, eliminating substitution behavior increased the average cost per woman compared with current levels, but assuming 100% compliance to follow-up was still less costly than current levels (Figure 2). Removing substitution behavior did not change the preferred strategy in the primary or secondary analyses. When we allowed for substitution behavior to continue, but assumed a lower relative self-sampling test sensitivity (0.9 rather than 1), the lifetime risk of cancer increased by 1.5% compared with current levels, if self-sampling reached only moderate under-screeners (i.e., Scenario A). However, improvements in cancer prevention continued to occur either when ‘5-yearly self-sampling’ reached severe under-screeners or with a ’10-yearly self-sampling’ policy.
Table 4.
Impact of model assumptions on the discounted incremental cost-effectiveness ratio (ICER) evaluating ‘5-yearly self-sampling’ and ‘10-yearly self-sampling’ compared with the current 2-reminder letter policy in Norway
| Respondent screening historya |
Sensitivity analysis scenario | ‘5-yearly self- sampling’ ICERb |
‘10-yearly self- sampling’ ICERb |
Current 2- reminder letter policy |
|---|---|---|---|---|
|
Scenario A: 5-yearly Self- sampling respondents are moderate under-screeners (Figure 1) |
Base case | $29,627 | $95,486 | Dominated |
| hrHPV+ compliance 100% | $32,408 | $144,002 | Dominated | |
| Relative test specificity of 1 | $29,475 | $103,772 | Dominated | |
| Relative SS sensitivity of 0.9 | $29,800 | $77,719 | Dominated | |
| No excess office-based exams | $29,004 | $126,030 | Dominated | |
| No substitution behaviorc | Dominated | $30,768 | Dominated | |
| Direct medical costs only | $16,209 | $76,536 | Dominated | |
| ’5-yearly SS’ participation 8%d | $29,910 | $67,745 | Dominated | |
| ’5-yearly SS’ participation 12%d | $29,268 | $509, 890 | Dominated | |
| ’10-yearly SS’ participation 4%d | $29,627 | $209,944 | Dominated | |
| ’10-yearly SS’ participation 8%d | $29,627 | $61,325 | Dominated | |
|
Scenario B: 5-yearly self- sampling respondents are moderate and severe under-screeners (Figure 1) |
Base case | $29,423 | Dominated | Dominated |
| hrHPV+ compliance 100% | $32,116 | Dominated | Dominated | |
| Relative test specificity of 1 | $29,274 | Dominated | Dominated | |
| Relative SS sensitivity of 0.9 | $29,590 | Dominated | Dominated | |
| No excess office-based exams | $28,814 | Dominated | Dominated | |
| No substitution behaviorc | $72,362 | $30,768 | Dominated | |
| Direct medical costs only | $16,067 | Dominated | Dominated | |
| ’5-yearly SS’ participation 8%d | $29,802 | $161,611 | Dominated | |
| ’5-yearly SS’ participation 12%d | $29,054 | Dominated | Dominated | |
| ’10-yearly SS’ participation 4%d | $29,423 | Dominated | Dominated | |
| ’10-yearly SS’ participation 8%d | $29,423 | Dominated | Dominated | |
hrHPV+: high-risk HPV-positive.
Refers only to ‘5-yearly self-sampling’. Moderate under-screening histories assumed women screen every 8-, or 10-years in the absence of self-sampling, and moderate and severe under-screening histories assumed women screen every 8-, 10-, 20-years or never-screened in the absence of self-sampling. A single self-sampling scenario was considered for the ’10-yearly self-sampling’ strategy (see Figure 1);
Cost per quality-adjusted life year gained. Quality of life adjustments ranged from a health state utility weight of 0 (death) to 1 (perfect health). Weights for cervical cancer varied according to stage (local: 0.76 for five years; regional: 0.67 for five years; distant: 0.48 five years). Disease specific utility weights were multiplied to baseline age-specific utility weights from Denmark (17) to estimate overall utility;
Rank order of strategies changed. ‘5-yearly self-sampling’ was more costly but less effective than 10-yearly self-sampling,
Assumes substitution behavior continues;
For more detailed information on cost calculations, please visit the author’s website at: http://www.med.uio.no/helsam/english/research/projects/preventive-strategies-hpv/harvardmodel-norway-technicalappendix.pdf. (US $1=NOK6.30)
The largest reductions in monetary costs compared with current levels were projected when we assumed that a self-sampling program could eliminate all excess office-based exams for women testing HPV-negative. Importantly, reducing excess office-based exams led to further reductions in unnecessary colposcopy referrals as well as unnecessary precancer treatments without compromising health benefits. For this scenario, ‘10-yearly self-sampling’ exceeded $100,000 per QALY gained compared with ‘5-yearly self-sampling’ for Scenario A only. When we varied the effectiveness of self-sampling to increase participation, we found that if ‘5-yearly self-sampling’ increased participation by 12% (instead of by 10%), or if ’10-yearly self-sampling’ was less effective (increasing participation by 4% instead of 6%), offering self-sampling at the more frequent 5-year interval was preferred (Table 4).
When we directly compared a self-sampling policy that required women to request a self-sampling device kit (i.e., opt-in) against our base case strategy involving mailing a self-sampling device kit to all women (i.e., opt-out), overall monetary costs were reduced by <2%. Subsequently, an opt-in self-sampling approach was preferred over an opt-out approach, provided they yielded the same increase in screening attendance (results not shown). However, this finding only remained true until screening participation for the opt-in strategy did not decrease by more than 1% (i.e., from 10% to 9%) compared with the opt-out approach.
Discussion
Our results suggest that self-sampling in Norway may be a more effective and cost-effective intervention to improve participation to screening than existing efforts that involve mailing repeated reminder letters to women non-compliant with screening guidelines. However, the magnitude of the health benefit and optimal self-sampling strategy is dependent on the profile and behavior of self-sampling respondents. Our findings demonstrate that the assumptions around the screening histories of self-sampling respondents and compliance to recommended follow-up drive the projected benefits and costs of self-sampling, highlighting the importance of understanding these factors prior to selecting and implementing a self-sampling strategy.
We also demonstrated the importance of accounting for substitution behavior when evaluating self-sampling strategies. Substitution of office-based screening with self-sampling is not currently the target use of a self-sampling policy but may be an unintended consequence of offering self-sampling. When we removed substitution behavior in the analysis, the absolute costs of screening changed but the preferred strategy in the primary and secondary analyses were unaffected. Using data from a Norwegian self-sampling pilot trial, our base case assumed that 50% of the respondents to self-sampling were among women who substituted an office-based exam for self-sampling. When we coupled substitution behavior with less than perfect compliance for women advised to undergo follow-up testing, we found that this effectively reduced overall participation to routine screening for this group of women. Although compliance to follow-up testing among women who substituted an office-based exam with self-sampling is unknown, self-sampling itself can introduce an added step to the screening algorithm, and therefore, increase loss to follow-up. In addition, self-sampling may not sufficiently reassure women who test HPV-negative. In the Norwegian pilot study, 18% of women who did not have a high-risk HPV infection attended an office-based exam, despite being explicitly advised they did not need further screening. In this analysis, we showed that eliminating potential over-screening practices was one of the most important cost-saving practices that a self-sampling screening program could undertake.
An opt-in (“on demand”) self-sampling policy may be an attractive approach to reduce costs and waste; however, requiring women to request a self-sampling device kit may introduce an added screening barrier. A meta-analysis found there were no significant differences between the effects of opt-in self-sampling compared with reminder letters; however, no studies have been conducted within Norway. An intention-to-treat analysis based on a study performed in Sweden found that introducing an opt-in self-sampling approach may yield comparably high improvements in coverage as an opt-out approach (14). We found that the cost-saving generated by an opt-in approach were relatively small and were overshadowed by other larger screening-related costs (e.g., office-based exams, colposcopies). Furthermore, we found that as soon as improvements in screening participation decreased by more than 1%, requiring women to request a device would not be an attractive policy.
To our knowledge, this analysis represents the most comprehensive evaluation of the benefits and value of introducing self-sampling that accounts for variations in respondent screening history (i.e., stratifying non-compliant women by screening frequency) (7, 15). Rozemeijer and colleagues (7) conducted a model-based analysis in the Netherlands to evaluate a single self-sampling strategy for women non-compliant with routine screening. The Dutch study crudely categorized screening participation into two groups (i.e., regular attendees and non-attendees) and did not reflect more nuanced screening histories among the “non-attendee” group, potentially overestimating the benefits of self-sampling. Similar to our findings, the Dutch study demonstrated that substitution behavior may have an important impact on overall health benefits associated with self-sampling. Additionally, both studies found that follow-up compliance among women testing positive for high-risk HPV on their self-sample is important, particularly among women who substitute behavior.
Limitations
Several limitations due to data availability and model simplifications deserve consideration. First, we did not have empirical data from the Norwegian pilot trial to inform the distribution of respondent screening history for the ‘5-yearly self-sampling’ strategy. When we explored two possible scenarios (i.e., Scenario A and Scenario B; Figure 1), we found that the optimal self-sampling strategy was dependent on respondent screening history, and should be the focus of future studies. In addition, data were not available to inform the effectiveness of the ‘10-yearly self-sampling’ strategy. Based on preliminary data from a Danish implementation study, we assumed that ‘10-yearly self-sampling’ would not achieve the same improvements in participation as ‘5-yearly self-sampling’ (6). As expected, we found that varying the percent-increase in participation for ’10-yearly self-sampling’ was one of the most important parameters. Understanding the response rates to self-sampling when restricting mailing the device kits to the most under-screened women should be empirically investigated. We also assumed that self-sampling respondents would require a self-sampling device kit to prompt each screen for their remaining lifetime. To our knowledge, no empirical study has followed women over repeated intervals to observe future screening behavior after receiving and returning a self-sampling device, warranting future studies to collect these data. We did not include process-related utility decrements related to traveling or attending screening exams, and associated test results. Attending an office-based exam may impose greater disutility compared with a home-based exam; however, the magnitude of these potential differences is not known. Although we have accounted for monetary differences between home- and office-based screenings, accounting for process utility may yield a more attractive cost-effectiveness profile for a self-sampling policy.
We did not assume that moderate or severe under-screeners faced a higher background risk of developing cervical cancer. When we compared the prevalence of HPV in the Norwegian pilot study with calibrated model output, we found that uncertainty bounds generally overlapped (Supplementary Fig. S1), suggesting that HPV-induced disease burden among self-sampling respondents may be similar to the general population. A recent Danish self-sampling implementation study, found no significant differences in HPV prevalence between self-sampling respondents with moderate or severe under-screening histories compared with mild under-screening histories. Self-sampling would likely be more attractive if we accounted for a potentially higher background risk of developing cervical cancer among women who responded to self-sampling with a severe screening history; however, in our analysis, self-sampling yields a robust cost-effectiveness profile well-below commonly-cited willingness-to-pay thresholds in Norway and elsewhere. Lastly, we did not consider a scenario that assumed women compliant to 3- or 4-yearly screening would delay routine participation in order to receive a self-sampling device to avoid an office-based exam. We expect that societal costs would decrease substantially under this scenario; however, there is a potential for loss in health benefits associated with delaying screening from three to five years, especially if HPV-positive women advised to undergo an office-based cytology exam fail to attend.
The findings from the SESAM-1 pilot trial (5) performed in the Norwegian capital of Oslo are consistent with findings from a recent meta-analysis (4), but may not be generalizable to the rest of Norway or outside a trial setting. Routine surveillance of the screening program indicates that screening participation following receipt of the 2nd reminder letter is higher in other regions of Norway compared with participation in Oslo. Subsequently, the percent-increase in participation observed by the self-sampling arm of the study compared with control arm may be lower. Conversely, settings outside of Oslo (e.g., rural areas) may be more willing to respond to an offer for self-sampling. When we varied the assumptions around the benefits of self-sampling on screening participation, self-sampling remained more attractive than current approaches (Table 4). Ongoing research at the Cancer Registry of Norway will help inform important regional differences in willingness to respond to screening using self-sampling.
Policy implications
Studies aiming to evaluate the expected percent-increase in routine screening participation using self-sampling, while important, will not be sufficient to ascertain the long-term value of implementing a self-sampling policy. Our study underscores the importance of eliciting additional information, such as a respondent’s screening history over repeated routine intervals. Attracting fewer (e.g., 6%), but higher-risk women (due to rarely attending screening) to participate in screening (even every 10-years) provides more benefit compared with attracting a larger (e.g., 10%) number of women at moderate risk who attend screening, albeit less frequently. Furthermore, continued monitoring of screening behaviors, including both unnecessary office-based exams for HPV-negative women or missed follow-up exams for HPV-positive women, are important to reach the full potential of implementing self-sampling. Patient counselling and clinical interpretation of test results are also necessary programmatic services that should be offered to women before they have an opportunity to meet with health care professionals.
Conclusions
Targeted self-sampling of non-compliers may improve the effectiveness of the Norwegian screening program and likely represents good value-for-money; however, the magnitude of the health benefit and optimal self-sampling strategy are dependent on the profile and behavior of self-sampling respondents. Health authorities should understand these factors prior to selecting and implementing a self-sampling program.
Supplementary Material
Acknowledgments
Funding source
EA Burger is partially supported by the Norwegian Research Council (grant number 238042). JJ Kim is partially supported by the U.S. National Cancer Institute of the National Institutes of Health (R01CA160744). All work by the authors was independent of the funders, and the funding sources had no involvement in the study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
We’d like to thank Maarit Leinonen for insightful discussions during manuscript development.
Footnotes
Conflicts of interest
All authors have no conflicts of interest to declare.
References
- 1.Arbyn M, Raifu AO, Weiderpass E, Bray F, Anttila A. Trends of cervical cancer mortality in the member states of the European Union. European journal of cancer. 2009;45:2640–2648. doi: 10.1016/j.ejca.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Snijders PJF, Verhoef VMJ, Arbyn M, Ogilvie G, Minozzi S, Banzi R, et al. High-risk HPV testing on self-sampled versus clinician-collected specimens: A review on the clinical accuracy and impact on population attendance in cervical cancer screening. International Journal of Cancer. 2013;132:2223–2236. doi: 10.1002/ijc.27790. [DOI] [PubMed] [Google Scholar]
- 3.Petignat P, Faltin DL, Bruchim I, Tramèr MR, Franco EL, Coutlée F. Are self-collected samples comparable to physician-collected cervical specimens for human papillomavirus DNA testing? A systematic review and meta-analysis. Gynecologic Oncology. 2007;105:530–535. doi: 10.1016/j.ygyno.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 4.Verdoodt F, Jentschke M, Hillemanns P, Racey CS, Snijders PJF, Arbyn M. Reaching women who do not participate in the regular cervical cancer screening programme by offering self-sampling kits: A systematic review and meta-analysis of randomised trials. European journal of cancer. 2015;51:2375–2385. doi: 10.1016/j.ejca.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Enerly E, Bonde J, Schee K, Pedersen H, Lonnberg S, Nygard M. Self-Sampling for Human Papillomavirus Testing among Non-Attenders Increases Attendance to the Norwegian Cervical Cancer Screening Programme. PLOS One. 2016:14. doi: 10.1371/journal.pone.0151978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lam J, Rebolj M, Ejegod DM, Rygaard C, Lynge E, Thomsen LT, et al. Non-attenders' screening history and participation rate in HPV self sampling: A pilot implementation. Proceedings of the 30th International Papillomavirus Conference & Clinical Workshop; 2015 September 17–21; Lisbon, Portugual. [Google Scholar]
- 7.Rozemeijer K, de Kok IMCM, Naber SK, van Kemenade FJ, Penning C, van Rosmalen J, et al. Offering Self-Sampling to Non-Attendees of Organized Primary HPV Screening: When Do Harms Outweigh the Benefits? Cancer Epidemiology Biomarkers & Prevention. 2015;24:773–782. doi: 10.1158/1055-9965.EPI-14-0998. [DOI] [PubMed] [Google Scholar]
- 8.Campos NG, Burger EA, Sy S, Sharma M, Schiffman M, Rodriguez AC, et al. An updated natural history model of cervical cancer: derivation of model parameters. American journal of epidemiology. 2014;180:545–555. doi: 10.1093/aje/kwu159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norwegian Directorate of Health. Økonomisk evaluering av helsetiltak. Norwegian: [cited 2014 Sept 15]. Available at: https://helsedirektoratet.no/retningslinjer/veileder-i-okonomisk-evaluering-av-helsetiltak. [Google Scholar]
- 10.Burger EA, Ortendahl JD, Sy S, Kristiansen IS, Kim JJ. Cost-effectiveness of cervical cancer screening with primary human papillomavirus testing in Norway. Br J Cancer. 2012;106:1571–1578. doi: 10.1038/bjc.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Technical Appendix: Harvard Cervical Cancer Natural History Model Calibration and Costing Approach for Norway. [cited 2016 Jun 01]; Available from http://www.med.uio.no/helsam/english/research/projects/preventive-strategies-hpv/harvardmodel-norway-technicalappendix.pdf. [Google Scholar]
- 12.Federal Reserve. Average rates of exchange 2014. [cited 2015 Nov 22]; Available from: http://www.federalreserve.gov/releases/G5A/current/
- 13.Cancer Registry of Norway. Cancer in Norway 2012: Cancer incidence, mortality, survival and prevalence in Norway. [cited 2014 Oct 12];2014 Available at: http://www.kreftregisteret.no/Global/Cancer%20in%20Norway/2012/CIN_2012-web.pdf. [Google Scholar]
- 14.Broberg G, Gyrd-Hansen D, Miao Jonasson J, Ryd M-L, Holtenman M, Milsom I, et al. Increasing participation in cervical cancer screening: Offering a HPV self-test to long-term non-attendees as part of RACOMIP, a Swedish randomized controlled trial. International Journal of Cancer. 2014;134:2223–2230. doi: 10.1002/ijc.28545. [DOI] [PubMed] [Google Scholar]
- 15.Mendes D, Bains I, Vanni T, Jit M. Systematic review of model-based cervical screening evaluations. BMC cancer. 2015;15:334. doi: 10.1186/s12885-015-1332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedersen K, Burger EA, Campbell S, Nygard M, Lonnberg S. Risk of Cervical Cancer by Screening Intensity: A Registry-based Analysis. Proceedings of the 30th International Papillomavirus Conference & Clinical Workshop; 2015 September 17–21; Lisbon, Portugual. [Google Scholar]
- 17.Olsen J, Jepsen MR. Human papillomavirus transmission and cost-effectiveness of introducing quadrivalent HPV vaccination in Denmark. International Journal of Technology Assessment in Health Care. 2010;26:183–191. doi: 10.1017/S0266462310000085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.