Abstract
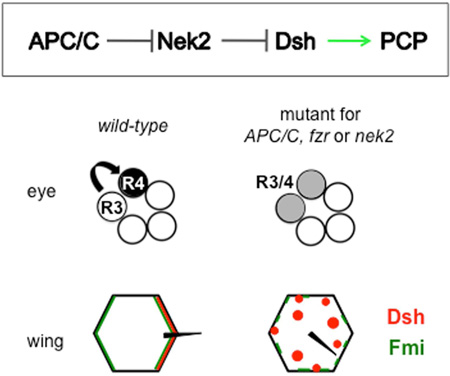
The Anaphase Promoting Complex/Cyclosome (APC/C) is an E3 ubiquitin ligase, well known for its role in cell cycle progression. However, it has been linked to additional functions, mainly in neuronal contexts, when using the co-activator Cdh1/Fzr. Here, our data indicate a post-mitotic requirement for the APC/CFzr/Cdh1 in epithelial cell patterning and planar cell polarity (PCP) in Drosophila. PCP signalling is critical for development by establishing cellular asymmetries and orientation within the plane of an epithelium, via differential localization of distinct complexes of core PCP factors. Loss of APC/C function leads to reduced Dishevelled (Dsh) levels, a core PCP factor. The effect of APC/C on Dsh is mediated by Nek2 kinase, which can phosphorylate Dsh and is a direct APC/CFzr/Cdh1 substrate. We have thus uncovered a pathway of regulation whereby APC/CFzr/Cdh1 negatively regulates Nek2, which negatively regulates Dsh, to ensure its proper stoichiometric requirement and localization during PCP establishment.
Keywords: Anaphase Promoting Complex, Post-mitotic function, Planar Cell Polarity, Drosophila, Asymmetric Protein localization
eTOC
Anaphase Promoting Complex (APC/C) is an E3 ubiquitin-ligase, known for its role in cell cycle progression. Weber and Mlodzik identify a post-mitotic APC/C function in Planar Cell Polarity establishment in Drosophila. The APC/C regulates levels of the Nek2 kinase, which regulates Dsh localization/levels via phosphorylation and targeting to the proteasome.
Introduction
Planar Cell Polarity (PCP) establishment is a process, which aligns single cells or groups of cells within the plane of an epithelium. When PCP genes are mutated this leads to the perturbation of otherwise almost crystalline-like arrays reflected by hairs on the Drosophila wing, inner ear sensory cells of the mammalian cochlea, or the trapezoidal arrangement of photoreceptors within ommatidia in the Drosophila eye. PCP establishment is well-conserved throughout the animal kingdom and associated gene lesions lead to severe developmental abnormalities and disease (Adler, 2002; Bayly and Axelrod, 2011; Goodrich and Strutt, 2011; Seifert and Mlodzik, 2007; Simons and Mlodzik, 2008; Singh and Mlodzik, 2012; Wallingford, 2012; Wang and Nathans, 2007).
In order for PCP to be set up the core PCP factors need to be asymmetrically localized. A key molecular cassette in this process is the Frizzled (Fz)/PCP core group, consisting of two complexes: Van Gogh (Vang, Strabismus/Stbm) and Prickle (Pk) localize proximally in wing cells, or to the R4 photoreceptor precursors in developing eyes; and Fz itself, Dishevelled (Dsh, Dvl), and Diego (Dgo, Diversin and Inversin in vertebrates) localize distally in wing cells or polar in photoreceptor R3 and R4 precursors in ommatidial preclusters. Flamingo (Fmi, Starry night/Stan or Celsr1–3 in vertebrates) localizes to both complexes and stabilizes them across cell membranes (Chen et al., 2008; Klein and Mlodzik, 2005; Lawrence et al., 2004; Strutt and Strutt, 2008, 2009). This asymmetric protein localization into specific complexes on opposing sides of the respective cells is delicately controlled and stoichiometric imbalances lead to PCP defects, which look similar in loss or gain-of-function scenarios.
As part of the interactions that generate stable PCP complexes, the cytoplasmic factors Dgo and Pk have been shown to antagonize each other in the context of Dsh (Jenny et al., 2005; Tree et al., 2002). We used these core PCP genes, dgo and pk, in a genome wide genetic screen and identified a subunit of the Anaphase Promoting Complex/Cyclosome (APC/C) as modifier of the dgo and pk induced phenotypes (Weber et al., 2012). The APC/C functions as an E3 ubiquitin ligase, which targets substrates for proteasomal degradation to ensure precise cell cycle progression (Foe and Toczyski, 2011; Peters, 2002; Pines, 2011). There is, however, accumulating evidence that it is expressed and also active in differentiating cells (reviewed by Pines, 2011; Primorac and Musacchio, 2013; also: Silies and Klambt, 2010; van Roessel et al., 2004). APC/C co-activators Cdc20/fizzy (fzy) and Cdh1/rap/fizzy-related (fzr) are sequentially associated with the APC/C complex during mitosis and G1 and are thought to convey substrate specificity (Jacobs et al., 2002; rev in Pines, 2011; Primorac and Musacchio, 2013). For example, Cdh1/Fzr has been shown to be the co-activator for Nek2 kinase degradation in Xenopus (Kimata et al., 2008; Pfleger and Kirschner, 2000).
The Nek2 serine/threonine kinase is a APC/C substrate, recruited by the subunit APC8, leading to its ubiquitination and consequently degradation (Fry, 2002; Hayes et al., 2006; Min et al., 2013; Pfleger and Kirschner, 2000; Sedgwick et al., 2013). Interestingly, in Drosophila it has been shown that Dsh is bound and phosphorylated by Nek2 (Schertel et al., 2013). Dsh is the core PCP protein, which links PCP signalling to actin polymerization and is also shared between the Wnt/β–catenin and Wnt-Fz/PCP signaling pathways (Boutros and Mlodzik, 1999; Wallingford and Habas, 2005). While, Nek2 has been suggested to promote canonical Wnt/Wg signaling in this context (Schertel et al., 2013), no role has been described in the modulation of PCP signaling.
Here we demonstrate that the APC/CFzr/Cdh1 regulates post-mitotic epithelial patterning and PCP establishment in Drosophila. We confirm this by showing that its negative regulator Rca1/emi interferes with this function. APC/C loss of function phenotypes in post-mitotic cells are caused by a reduction in Dsh levels, which is mediated by the Nek2 kinase acting on Dsh. Accordingly, changing Nek2 levels relieves or aggravates APC2 and APC8 induced PCP defects and, in a converse manner, dsh copy reduction or extra dsh copies do the same. Our in vivo data, together with previous observations (see above), are consistent with a model where the APC/C regulates Nek2 ubiquitination and proteasome degradation and Nek2 regulates Dsh via phosphorylation and targeting to the proteasome. Importantly, we observe that Nek2 behaves like a core PCP factor with too much or too little of it causing comparable PCP defects, suggesting that Nek2 acts directly on Dsh/Dvl (consistent with biochemical data; Cervenka et al., 2016). In summary, our data indicate that APC/C regulation of Nek2 is required for PCP establishment in post-mitotic cells and that it exerts its function via controlling Dsh levels.
Results
APC/CFzr/Cdh1 affects PCP independent of cell cycle regulation
PCP aligns cells along specific body axes; for example, ommatidia in the compound eye along the dorso-ventral axis, and wing cells with respect to the proximal-distal axis. In the eye, PCP signaling is required in post-mitotic cells leading to the consecutive establishment of cell fates of photoreceptors R3 and R4 and associated ommatidial rotation, which occurs in a clock- or counter-clockwise direction in the dorsal and ventral halves of the eye, respectively (Cooper and Bray, 1999; Fanto and Mlodzik, 1999; Mlodzik, 1999; Strutt and Strutt, 1999). Cell cycle activity in developing eyes is confined to undifferentiated cells anterior to the morphogenetic furrow (MF) and to a few yet uncommited cells between assembling ommatidial clusters of the 5-cell stage posterior to the MF. These cells of the second mitotic wave give rise only to photoreceptors R1, R6 and R7 and accessory cells. Importantly, they do not contribute to photoreceptor precursors R8, R2, R3, R4 and R5, which are post-mitotic and committed at this stage (Wolff and Ready, 1991), with photoreceptors R3 and R4 required for PCP establishment (Cooper and Bray, 1999; Fanto and Mlodzik, 1999; Mlodzik, 1999; Strutt and Strutt, 1999).
In a genome-wide screen, we previously identified APC8 as an enhancer of pk and dgo induced eye and wing PCP defects (Weber et al., 2012). APC/C components are consistently expressed in post-mitotic, differentiating cells in Drosophila: for example, Fzr/Cdh1 and APC1/Shtd are expressed at higher levels after cell cycle exit in developing eye discs posterior to the MF (Pimentel and Venkatesh, 2005; Tanaka-Matakatsu et al., 2007). In addition, Fzr/Cdh1 is also expressed in wing tissue of 24 and 38hrs old pupae (Buttitta et al., 2010; and Fly-FISH: Lecuyer et al., 2007; Wilk et al., 2016). Therefore, we first wanted to confirm with several APC/C subunits and its co-activators whether the entire complex and its enzymatic activity affected PCP in post-mitotic cells.
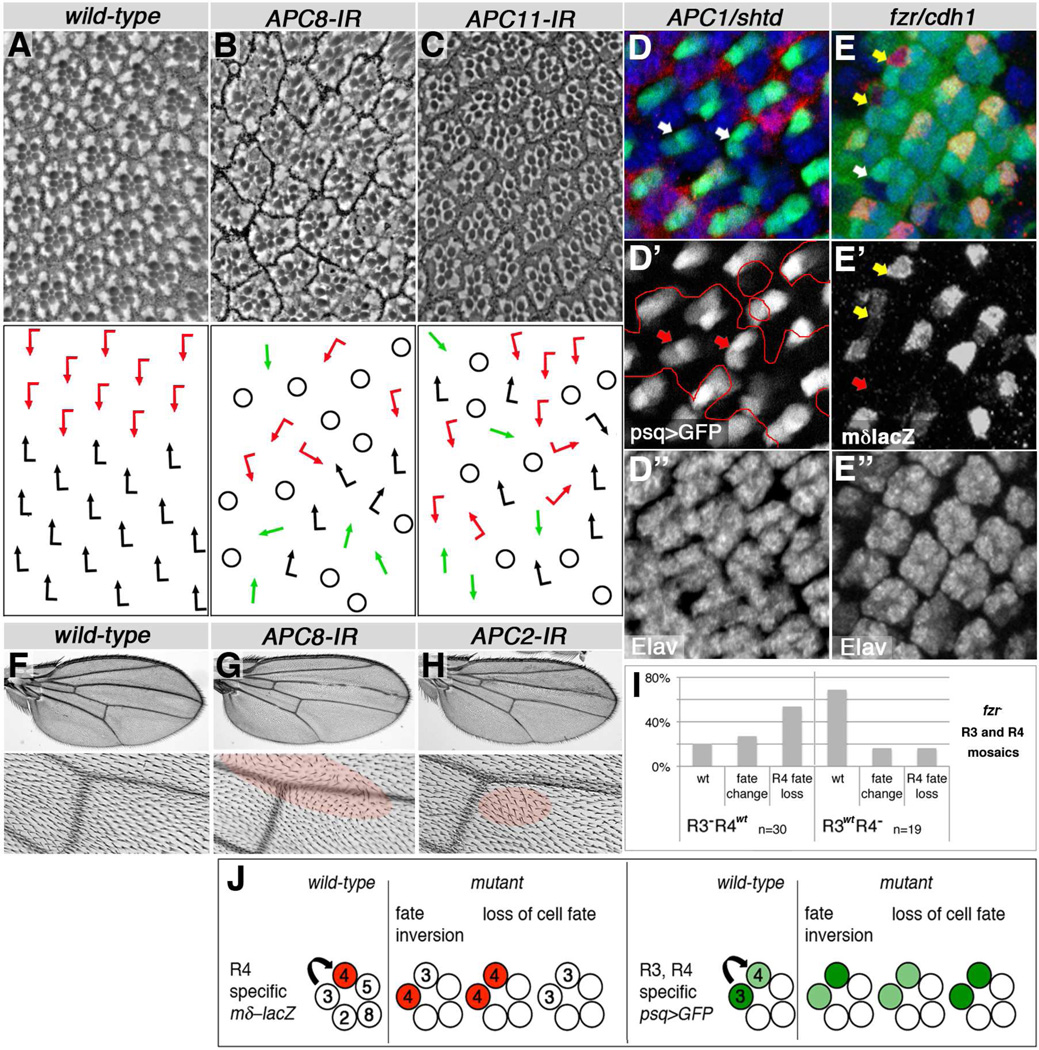
Strikingly, knockdown of the subunits APC2 (morula/mr), APC8 (cdc23) (Fig. 1A–B, and Suppl Fig. S1A, S1C), and APC11 (lemming/lmg) (Fig. 1C) with photoreceptor specific post-mitotic tools (Exp. Procedures) induced PCP defects as detected in adult eyes. Importantly, APC2/mr and APC11/lmg are the minimal catalytic subunits of the APC/C (Tang et al., 2001). Analyses of mosaic R3/R4 precursor pairs serve as functional indication of whether a PCP gene acts on the R3 or R4 side of the process. For example: fz, dsh and dgo display an R3 requirement, whereas Vang and pk function in R4 (Jenny et al., 2003; Jenny et al., 2005; Tomlinson et al., 1997; Wolff and Rubin, 1998; Zheng et al., 1995). Detailed examination of APC1/shattered (shtd) and fzr/cdh1 mutant eye tissue, using molecular markers for R3 and R4 cell fates (see schematic, Fig. 1J), confirmed PCP defects, reflected by frequent R3/R4 fate inversions or loss of R4 fate (Fig. 1D–E,I–J; Suppl. Fig. S1E). Furthermore, mosaic R3/R4 pairs in fzr/cdh1 mutant clones revealed a PCP requirement in R3 (Fig. 1E, quantif. in 1I), suggesting a function affecting the Fz-Dsh-Dgo complex. Together, these data indicate that the entire APC/C is required for PCP establishment in the eye, most likely acting in R3 during the process.
Figure 1. APC/C and Cdh1/Fzr affect PCP establishment in eyes and wings.
Adult eye and wing phenotypes, and photoreceptor R3/R4 fate changes in developing eyes are shown in APC/C and fzr/cdh1 knockdown or mutant tissue. Anterior is left and dorsal up for eye tissue, proximal is left and anterior up for wings. (A–C) Top panels show eye sections, bottom panels respective schematics (red and black arrows: chiral ommatidia, green arrows: symmetrical ommatidia, circles: loss of R-cells/unscorable ommatidia). (A) Wild-type eye (control). (B) APC8 knockdown: loss of chirality, misrotation, and R-cell loss (genotype: GMR-GAL4/+, UAS-APC8-IR52280/+ at 27°C). (C) APC11 knockdown: similar phenotypes as in (B) (genotype: UAS-APC11-IR103649, HMS02024/+, sev-GAL4 females shifted to 31°C for 3 days at early 3rd larval instar). (D,E) APC1/shtd and cdh1/fzr mutant eye disc tissue stained for R3 and R4 cell fate markers and photoreceptors by Elav (blue, monochrome in D",E"). (D) APC1/shtd1 clone (marked by loss of red, lacZ) displays R-cell fate defects. Ommatidial preclusters, marked with high GFP levels in R3 and low GFP in R4 fate via psq-GAL4>GFP/+ (green, monochrome D'), often show inverted cell fate (indicated by arrows). (E) cdh1/fzr clone (fzrrapA allele) marked by loss of GFP. Mosaic ommatidia with single fzr mutant R3/R4 cells are indicated by arrows; yellow arrows mark R3wt clusters and white/red arrows R3mut clusters. In 53.3% of R3 mutant ommatidia R4 fate is lost, via loss of mδ–lacZ R4-marker (red, monochrome E'), compared to 15.8% when R4 is mutant, indicating a requirement in R3; Quantified in (I). (F–H) Adult wings and central detail around posterior crossvein (bottom panels). (F) Control wing, (G) APC8 knockdown, and (H) APC2 knockdown: PCP defects evident by wing hair whorls and multiple cellular hairs (highlighted by red shading). Genotypes: (F) fng-GAL4/+, UAS-IR28548/+ (27°C), (G) fng-GAL4/+, UAS-APC8-IR52280/+ (18°C), and (H) fng-GAL4/+, UAS-APC2-IR2993/+ (27°C). Quantified in Suppl. Figure 1. (I) Quantification of R4 fate in mosaic cdh1/fzr R3/R4 pairs for alleles fzr8F3, fzrrapA and fzrrapB. (J) Schematic of photoreceptor R3/R4 specific marker expression and cell fate changes observed in PCP mutant ommatidia (mδ0.5-lacZ, Cooper and Bray, 1999, psq-GAL4 UAS-GFP, Weber et al., 2008). Arrow represents sequential PCP and Notch signaling setting up R3 and R4 fates.
In wings, the core PCP factors become segregated into a proximal complex comprised of Vang, Pk, Fmi and a distal complex containing Fz, Dsh, Dgo and Fmi. The Fz-Dsh-Dgo complex serves as an “organizing center” for actin polymerization, focusing actin-polymerization to give rise to one cellular hair, or trichome (Adler, 2002; Bayly and Axelrod, 2011; Goodrich and Strutt, 2011; Klein and Mlodzik, 2005; Seifert and Mlodzik, 2007; Singh and Mlodzik, 2012). Knockdown of APC2 and APC8 in wings induced PCP-type hair orientation defects and multiple hairs forming in individual cells (Fig. 1G–H). Examining pupal wing tissue during PCP establishment confirmed the presence of multiple trichomes per cell and their misorientation, as well as a delay in trichome formation (compared to surrounding wild-type tissue; Suppl. Fig. S3A–D).
To confirm that the effects of APC/C on PCP were independent of cell cycle control, we tested for a requirement of cell cycle specific effectors in eye and wing development. CyclinA (CycA) and String (Stg)/Cdc25 are targeted by the APC/C for proteasomal degradation during cell cycle progression (Donzelli et al., 2002; Geley et al., 2001). Knockdown or overexpression of CycA or Stg during PCP establishment in eyes and wings did not cause PCP defects (e.g. inversions or loss of chirality in eyes and wing hair whorls; Suppl. Fig. S2A–I). Cell cycle regulators affected wing hair spacing and grouping (Suppl. Fig S2E–I) but not cellular hair polarity. Furthermore, we tested Fizzy (fzy)/cdc20, the mitotic regulator of APC/C (Dawson et al., 1995; Izawa and Pines, 2011), in wings for PCP effects. fzy6/7 transheterozygotes were described to affect cell cycle control when animals were shifted to 29°C (Dawson et al., 1995; Swan and Schupbach, 2005). Temperature shifts applied to such animals during PCP establishment did not affect wing hair orientation (Suppl. Fig. 2J–L). We therefore conclude that APC/CFzr/Cdh1 is generally required for PCP establishment via a post-mitotic effect, independent of its function in cell cycle regulation.
Increased APC/CFzr/Cdh1 is sufficient to interfere with PCP in the eye
Next we tested gain-of-function scenarios for APC/C components, as in PCP establishment both loss and gain of a core component cause similar defects due to disturbances in the balance of the core factors. Post-mitotic overexpression of subunits APC8 and APC11 is possible specifically in R3/R4 cells in the eye, and this was sufficient to induce PCP defects (Fig. 2A, B). APC11 over-expression analyzed in developing eye tissue revealed R3/R4 cell fate defects, monitored with the R4 fate marker mδ–lacZ with frequent misexpression in R3 or loss in R4 precursors (see below; Fig. 4G).
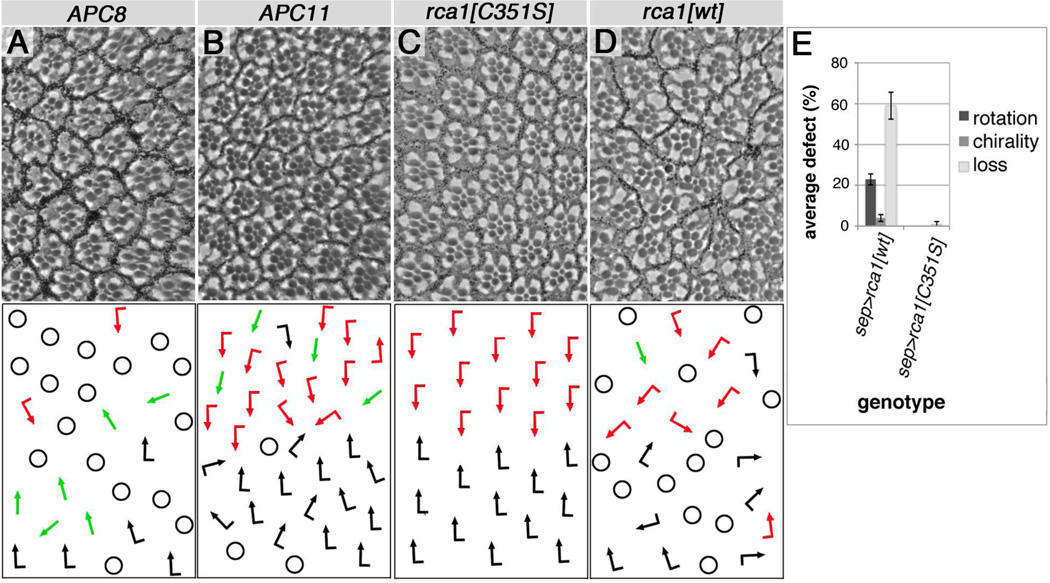
Figure 2. APC/C and rca1/emi (negative regulator of APC/C) overexpression is sufficient to induce PCP.
(A–B) APC8 and APC11/Lmg expression interferes with PCP in eyes. (A) GMR-GAL4/+, UAS-APC82508HA/+ at 29°C, (B) sev-GAL4/+, lmgEY11317/+, UAS-LmgA/+ (shifted to 31°C for 2 days in early 3rd instar), both genotypes induce PCP defects (green arrows or intermixed red and black arrows), and loss of R-cells (marked by circles), compare to Fig. 1A for wt. (C) Overexpression of Rca1 point mutant C351S, which inactivates Rca1: sep-GAL4>UAS-Rca1[C351S]/+ (25°C) did not induce defects, whereas (D) sep-GAL4>UAS-Rca1 (wt Rca1)/+ (25°C) induced PCP defects. (E) Quantification of Rca1 induced eye PCP defects. Average PCP defects (rotation and chirality) and loss of R-cells, 3 eyes each, n=272–400 ommatidia. All Rca1 truncation constructs (Zielke et al., 2006) were tested, showing no PCP effects.
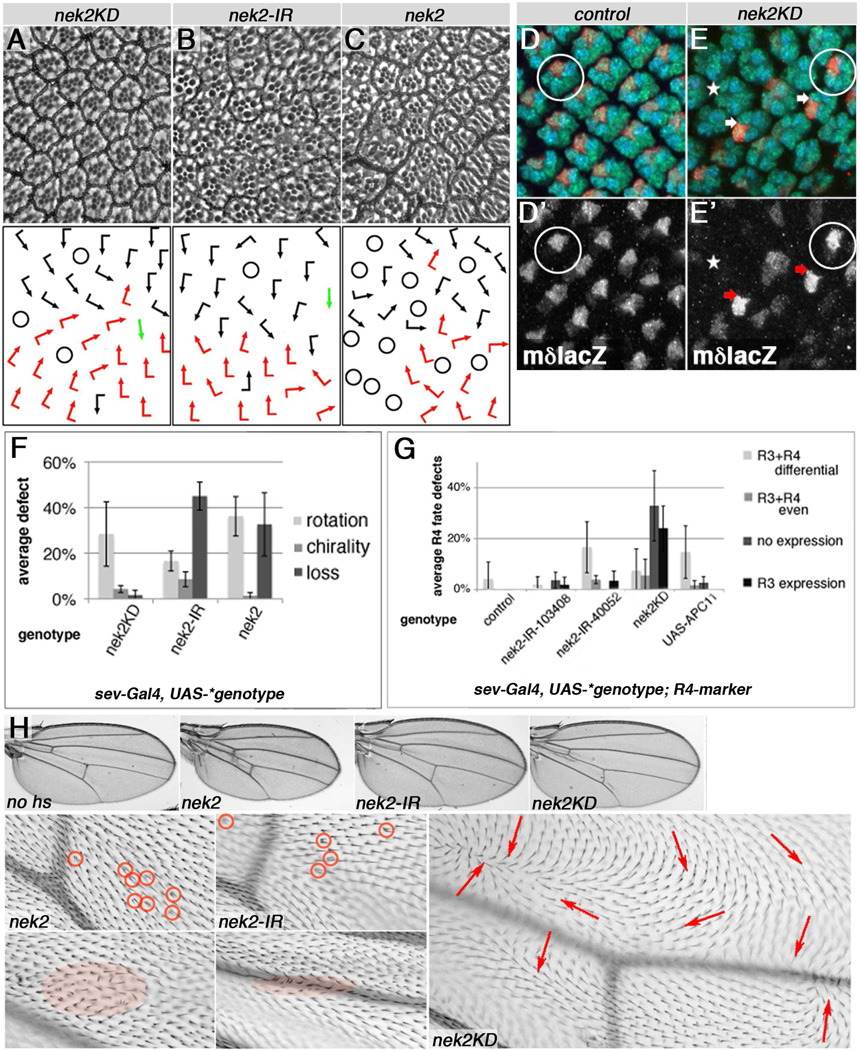
Figure 4. Nek2 kinase is required for PCP establishment in eyes and wings.
(A–C) Top panels show adult eye sections and bottom panels schematic presentations of PCP defects. (D,E) Developing eye discs stained for R4 fate (red, mδ–lacZ, monochrome D', E'), Elav (all R-cells, green), and Rough (blue). (F–G) Quantif. of eye phenotypes. (A) sev-GAL4/+; nek2KD/+ (25°C), (B) sev-GAL4/+, UAS-Dcr2/Y, nek2-IR40052/+ (following a 18°C to 31°C shift for 48 hrs at early 3rd instar), and (C) sev-GAL4; UAS-Nek2/+ (19°C) display PCP defects: evident by rotation, chirality, and R-cell loss defects (quantified in F). (D) mδ–lacZ/+, sev-GAL4/+, UAS-Dcr2/+ control, and (E) sev-GAL4/+, UAS-Dcr2/+, nek2KD/+, mδ–lacZ/+ (following a 18°C to 31°C temperature shift 50–75hrs prior to dissections). Wild-type example with normal R4 fate represented by mδ–lacZ expression is circled in (D,E). Arrows point to clusters with inverted R3/R4 fates (E), and asterisk indicates cluster where R4 fate failed to be established. (F) Quantification of rotation, chirality and loss of photoreceptor defects in adult eyes as shown in (A–C), average from 3–4 eyes, n=322–446 ommadia. (G) Quantification of R4 fate/mδ–lacZ expression in R3/R4 pairs based on 3–6 eye discs, n=73–185 preclusters each. nek2 knockdown (2 independent IRs), Nek2 kinase dead (nek2KD), and APC11/Lmg over-expression all induce R3 cells to express mδ–lacZ (R3+R4 differential, R3+R4 even, R3 expression) or loss of mδ–lacZ expression (no expression) compared to control. See Fig. 1J for schematic. (H) Adult wing phenotypes induced by temperature shifts to 30°C starting in early prepupae for 20–40hrs of en-GAL4, GAL80ts/+ UAS-construct/+ (respective constructs indicated in each panel). Multiple trichomes and orientation defects are circled, highlighted, or marked with arrows. Nek2 expression and knockdown induce multiple cellular hairs and occasional whorls, whereas Nek2KD induces such defects throughout entire posterior compartment (marked by red arrows). No defects are observed in wings kept at 18°C (no hs).
We confirmed APC/CFzr/Cdh1 requirements in PCP establishment by overexpressing Regulator of cyclinA (Rca1)/emi, a known negative APC/C regulator (Grosskortenhaus and Sprenger, 2002; Hsu et al., 2002; Zielke et al., 2006), which has been shown to affect post-mitotic cell fate establishment in the embryonic CNS (Lear et al., 1999) and to maintain cell cycle exit (Buttitta et al., 2010). Whereas over-expression of Rca1 does not affect cell cycle progression or disrupt mitosis (Grosskortenhaus and Sprenger, 2002), we detected PCP defects with wild-type Rca1 (Fig. 2D,E). An inactive Rca1C351S point mutant (Zielke et al., 2006) was not able to do so, serving as a specific control (Fig. 2C,E). As Rca1 antagonizes APC/CFzr/Cdh function (Grosskortenhaus and Sprenger, 2002; Hsu et al., 2002; Zielke et al., 2006), we tested if it could relieve APC8 induced phenotypes. Accordingly, combined knockdown of APC8 and Rca1 showed no hair inversions/whorls (compared to APC8 alone; Suppl. Fig. S1F–G). Taken together, our data on individual APC/C subunits (Fig. 1, 2 and Suppl. Fig. S1) and the observed Rca1 effects, indicate that the entire APC/CFzr/Cdh1 is required for PCP establishment.
APC/CFzr/Cdh1 specifically affects Dsh levels
As APC/CFzr/Cdh1 targets substrates for proteasomal degradation, we wished to determine which of the core PCP proteins might be affected via Western blot analyses and/or in vivo protein localization. Due to the functional requirement of fzr/cdh1 in R3 cells (Fig. 1E,I), we examined developing eye tissue with an APC8 knockdown in post mitotic cells with endogenously driven, GFP-tagged Dsh, dsh-DshGFP (Axelrod, 2001). We observed that Dsh levels were markedly reduced in APC8 knockdown eye discs (Fig. 3A–B). Furthermore, CyclinB, an APC/C target in cell cycle regulation, was not affected confirming specificity and post-mitotic effect of APC8 in these assays. The levels of other core PCP proteins, e.g. Pk, Dgo and Fmi, were not grossly affected in pupal wing tissue, albeit mispolarized in cells where APC8 was knocked down (Suppl. Fig. S3A–D; note that Fmi staining represents misoriented polarity at the cellular level).
Figure 3. Dsh levels are reduced in APC8 knockdown.
(A) Western blots of GMR-GAL4/+, UAS-APC8-IR/+, dshDshGFP/+ (expressed from endogenous dsh control sequences, Axelrod, 2001) or control eye discs (white-IR) at 27°C, probed for GFP. Left side shows 4 disc pairs, right side 8 disc pairs. APC8-IR52280 and APC8-IR52279 show reduced DshGFP levels as compared to control (white-IR). Loading controls: γ–Tub and CyclinB. (B) Quantification of 3 independent Western blots. DshGFP levels in red, controls grey, *P=0.07, **P=0.0027. Statistical analyses here and in subsequent figures were student t-test, unless indicated. Error bars are Standard Deviation in all figures.
These data suggest that Dsh is the core PCP factor affected by APC/CFzr/Cdh1. However, as Dsh levels are reduced upon APC8 knockdown, Dsh cannot be a direct target of APC/C mediated proteasomal degradation. We therefore tested Nek2 kinase, a known target of APC/CFzr/Cdh1 (Kimata et al., 2008; Pfleger and Kirschner, 2000) which has been shown to bind and phosphorylate Dsh in the context of Wnt/β–catenin signaling in Drosophila and mammalian cell culture (Schertel et al., 2013; Cervenka et al., 2016). Furthermore, Nek2A recruitment to the APC/C depends on APC8 in mammalian cell culture studies (Sedgwick et al., 2013).
Nek2 kinase is a APC/CFzr/Cdh1 target in PCP and expressed in tissue undergoing PCP
We observed that Nek2 knockdown in vivo in developing wing discs lead to a marked reduction in wing size (Suppl. Fig. S4A–B) consistent with a role in cell cycle progression. Moreover, regional RNAi-based Nek2 knockdown, e.g. in the posterior compartment via enGal4, was largely compensated by wild-type cells (Herrera et al., 2013), as evidenced by loss of marked enGal4, UAS-Nek2-IR cells (Suppl. Fig. S4C–E). Thus, Nek2 is required for cell cycle progression in vivo, as suggested by previous studies (Fry et al., 2012; Nigg, 2001; Prigent et al., 2005; also Discussion).
Little is known about post mitotic Nek2 functions except for a role in microtubule acetylation (Chang et al., 2009) and suggested promotion of Wnt/β–catenin signaling in Drosophila (Schertel et al., 2013). We wanted to confirm that Nek2 was expressed in tissues undergoing PCP establishment, using a Nek2 antibody (Prigent et al., 2005). Nek2 was indeed detected in photoreceptor precursors in punctae adjacent to the core PCP factor Fmi in developing eye discs (Suppl. Fig. S4F–J). In a nek2 knockdown domain, we detected markedly reduced punctate staining as compared to adjacent control tissue, indicating specific Nek2 staining (Suppl. Fig. S4K, L). To address Nek2 function in PCP, we used the post-mitotic assay (in analogy to the APC/C subunits) during eye development and asked whether Nek2 could affect R3/R4 fate decisions. Nek2 knockdown and expression of the kinase dead (KD) or wild-type form all caused PCP phenotypes in adult eyes (Fig. 4A–C, F). This was confirmed by assessing R3/R4 fate with molecular markers (Fig. 4D–E, G). For example, Nek2KD expression induced over 30% of ommatidia with loss of R4 fate and approximately 20% with switched R3/R4 fates, both clear manifestations of PCP defects (Fig. 4G; schematic in Fig. 1J). We thus conclude that precise levels of Nek2 are required for eye PCP establishment.
Due to cell cycle progression/growth defects associated with Nek2 (Suppl. Fig. S4 and above), we employed tight temperature shift experiments to be able to assess PCP defects in developing wings. Both Nek2 knockdown, Nek2KD expression, and over-expression of Nek2 induced PCP defects reflected in hair orientation and multiple cellular hairs, when specifically expressed during PCP establishment (Fig. 4H). The kinase dead version, Nek2KD, was particularly potent in inducing PCP defects (Fig. 4H, right panels), affecting the entire expression domain and causing whorls of cellular orientation. Together with published observations, these data suggest that Nek2 is a good candidate to convey the APC/CFzr/Cdh1 effects on PCP via Dsh.
As Nek2/Nek2A is a known substrate of the APC/C and targeted for proteasomal degradation (Kimata et al., 2008; Pfleger and Kirschner, 2000; Sedgwick et al., 2013, Martins et al., 2017), we tested in vivo if reduced proteasomal function affected nek2 induced PCP defects. To this end we coexpressed dominant negative Prosβ2 and 6 subunits (Smyth and Belote, 1999; Schweisguth, 1999) with Nek2, which caused a marked increase in PCP defects in wings (Suppl. Fig. S5A–D), confirming a proteasomal involvement of APC/C – Nek2 during PCP establishment. We thus hypothesized that nek2 reduction might relieve the APC knockdown induced PCP defects. Consistently, in wings where APC/C subunits and Nek2 were simultaneously knocked down, PCP defects were markedly reduced, as compared to either APC8 or APC2 knockdown alone (Fig. 5A–N). Conversely, when Nek2 was expressed at levels where it alone did not cause phenotypic defects, both APC8 and APC2 knockdown PCP defects were enhanced (Fig. 5I–P). Here, Nek2KD fully suppressed the PCP defects (Fig. 5O), suggesting that the kinase dead version acts as a dominant negative. As Nek2 reduction was fully effective at suppressing APC/C knockdown induced defects, we conclude that Nek2 links the APC/C to PCP requirements. This model also predicts that changing Dsh levels might have an effect on APC/C caused PCP defects. We thus genetically decreased Dsh levels, and observed that dsh1/+ dominantly enhanced APC8 or APC2 knockdown induced PCP defects (Fig. 6A–J) and, conversely, increased Dsh levels suppressed APC8 or APC2 defects (Suppl. Fig. S6A–D), both effects consistent with the model. As Dsh also acts in canonical Wnt-signaling, we occasionally observed size reduction and perturbed vein and margin patterning within the expression domain (Fig. 5E–F and 6A,C,D,F,I and Suppl. Fig. S5B–C’ and S6A), defects described for loss of Wg/β–catenin signaling (Sharma and Chopra, 1976; Couso et al., 1994; Bejsovec, 2006). Together, these data are consistent with the notion that the role of APC/CFzr/Cdh1 in PCP is to regulate Dsh levels and this is mediated by Nek2 action on Dsh.
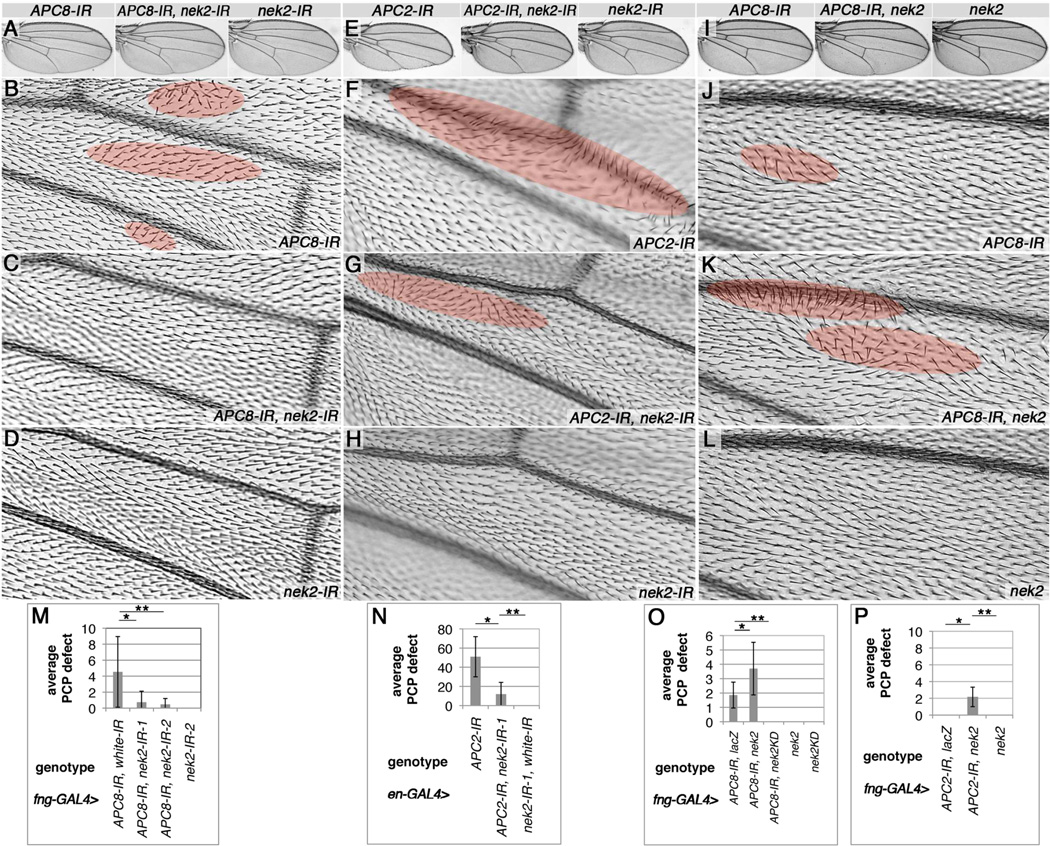
Figure 5. Reduction of nek2 suppresses and Nek2 over-expression enhances APC/C knockdown induced wing PCP whorls.
(A,E,I) Adult wing over-views, and high-mag. details in lower panels. PCP wing defects are highlighted by red shading. (A–D) APC8-IR, nek2-IR interactions, quantified in (M). (E–H) APC2-IR, nek2-IR interactions, quantified in (N). (I–L) APC8-IR, nek2 interactions, quantified in (O). (A,B,I,J,M,O) fng-GAL4/+ UAS-APC8-IR52280/+ (16°C) shows PCP orientation phenotypes (highlighted in red) in (B) and (J). (A,C,M) fng-GAL4/+, UAS-APC8-IR52280/+, UAS-nek2-IR103408 (16°C); and (A,M) fng-GAL4/+, UAS-APC8-IR52280/+, UAS-nek2-IR40052 (16°C): note reduced PCP orientation defects, compare to (B), with two independent nek2-IRs. (M) Quantification of PCP defects: 360° orientation whorls per wing were quantified for 14–16 wings each, *P=0.0038 and **P=0.0016. (E, F, N) en-GAL4/+, UAS-APC2-IR2993/+ (25°C), with trichome orientation defects (highlighted in red) and reduced size in knockdown domain. (G,N) en-GAL4/+, UAS-APC2-IR2993/+, UAS-nek2-IR103408/+ (25°C): note reduced defects. (H,N) en-GAL4/+, UAS-nek2-IR103408/+ (25°C) control wings with no effect on hair orientation and domain size. (N) Quantification of hair whorls (as in M) in posterior compartment, 12–13 wings each, *P<0.0001, **P=0.0018 (t-test). (I–P) Nek2 overexpression enhances APC8 and APC2 knockdown defects. (J–L) Distal area between L3 and L4 is shown. (J) Rare and mild defects are detected in fng-GAL4/+ UAS-APC8-IR52280/+ (16°C). (K,O) fng-GAL4/+ UAS-APC8-IR52280/+, UAS-nek2/+ (16°C), note more and larger patches with hair orientation defects. (L,O) fng-GAL4/+ UAS-nek2/+ (16°C): no effects. (O) Quantification of wing hair orientation defects of entire wing for APC8 knockdown. Note: nek2 enhances and nek2-KD suppresses APC8-IR induced whorls. *P=0.027, **P<0.0001, n=7–21 wings. (P) Quantification of APC2 knockdown and Nek2 over expression at 18°C. Individually they cause no phenotype, but together PCP trichome whorls are induced. Genotypes: fng-GAL4>UAS-APC2-IR2993/+ UAS-lacZ/+, fng-GAL4>UAS-APC2-IR2993/+ UAS-nek2/+ and fng-GAL4>UAS-nek2/+, *, **P<0.0001, n=6–15 wings.
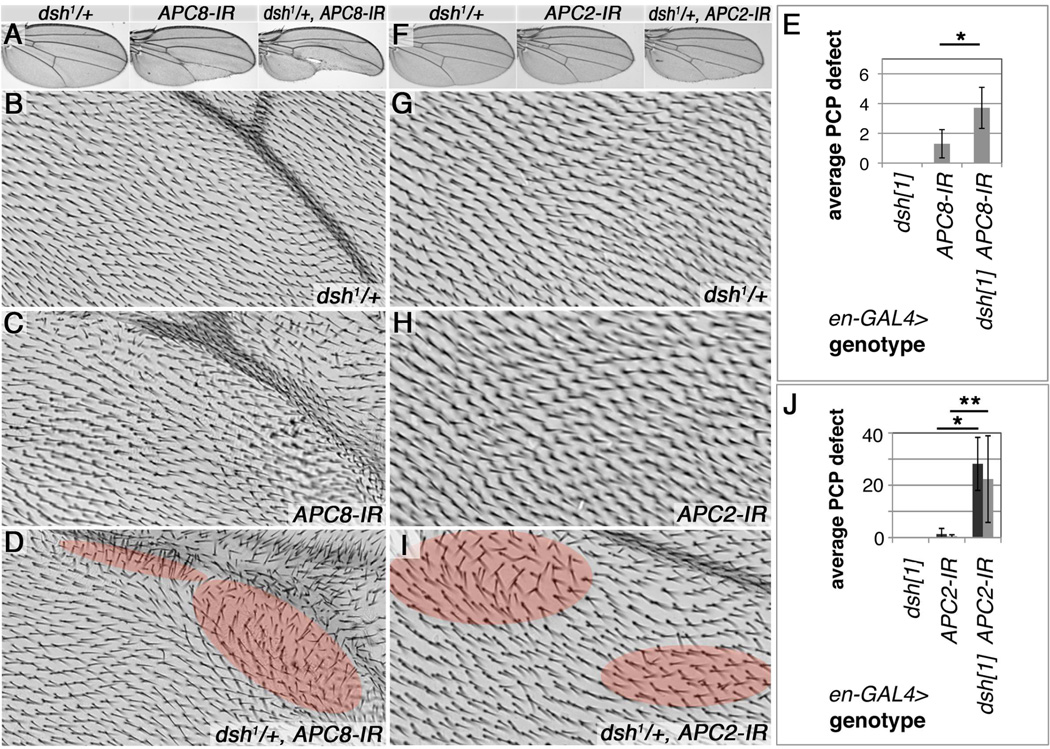
Figure 6. Reduction of dsh dominantly enhances APC/C induced PCP defects.
(A,F) Adult wing overviews and (B–D, G–I) high magnification images of wing area proximal to L5: (A–D') dsh1/+ enhances APC8 knockdown induced phenotypes. (A) Wing overviews of indicated genotypes. (A,B) Control dsh1/+, en-GAL4/+ (18°C), with normal trichome pattern. (A,C) en-GAL4/+ UAS-APC8-IR52280/+ (18°C), note mild trichome orientation defects proximal to L5, partial margin loss and reduced posterior compartment size. (D) dsh1/+; en-GAL4/+, UAS-APC8-IR52280/+ (18°C), note more severe orientation defects (highlighted by red shading) and posterior compartment size reduction. (E) Quantification of trichome orientation defects in APC8 knockdowns. 7–10 wings/genotype evaluated in wing area proximal to L5, 1=20 misoriented and/or multiple trichomes, *P= 0006.
(F–I’) dsh1/+ enhances all aspects of APC2 knockdown induced phenotypes. (F) Wing overviews of indicated genotypes. (F,G) Control dsh1/+; en-GAL4/+ (22°C) wing. (F,H) en-GAL4/+, UAS-APC2-IR2993/+ (22°C), note rare PCP hair orientation defects. (F,I) dsh1/+; en-GAL4/+, UAS-APC2-IR2993/+ (22°C): note enhanced multiple trichome and PCP hair orientation defects (highlighted by red shading). (J) Quantification of multiple trichome (dark column) and orientation defects (90–180°, light column) between veins L4 and L5; 6 wings/genotype, * P<0.0001, ** P=0.0091.
Nek2 controls Dsh levels in vivo
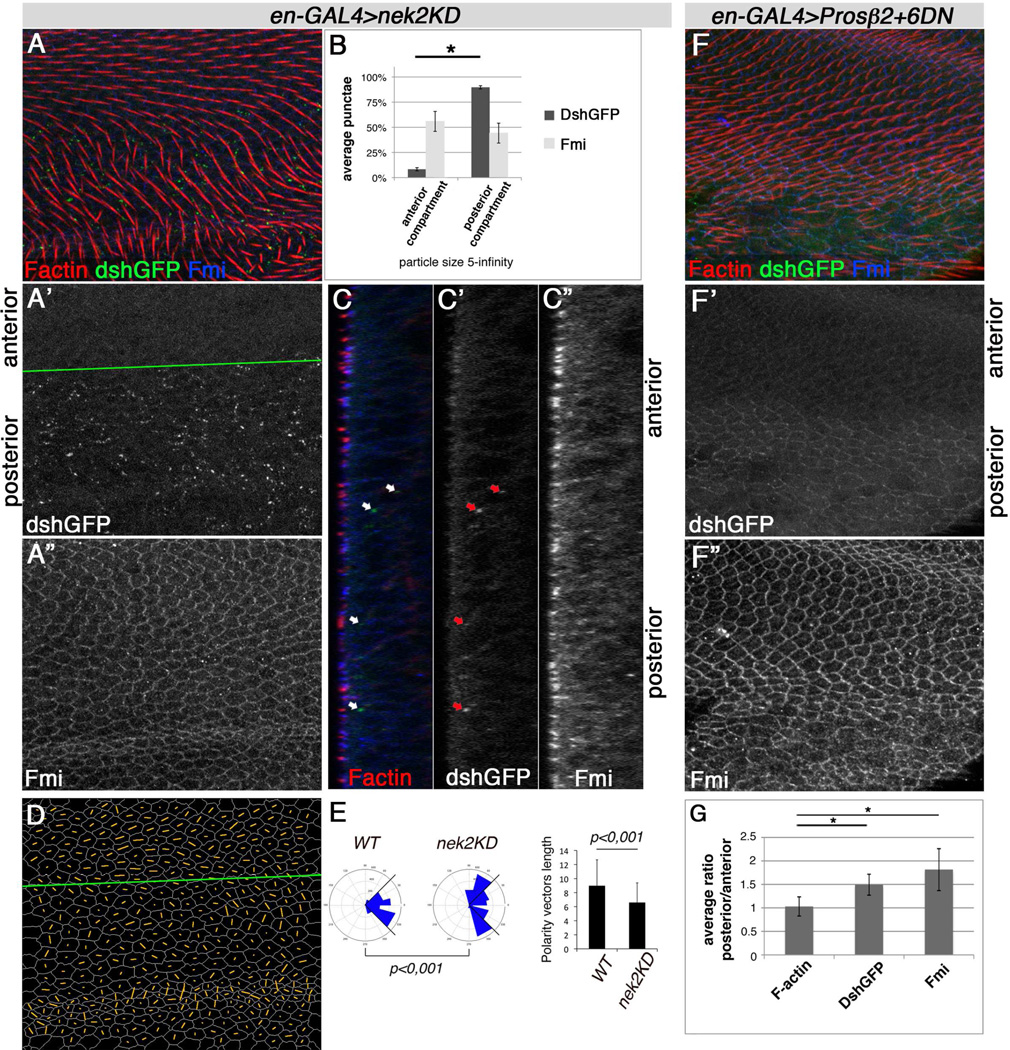
Based on the genetic experiments, we wanted to address the type of effect Nek2 exerts on Dsh protein during PCP establishment. We thus monitored Dsh-GFP levels (endogenous expression via dsh-DshGFP, Axelrod, 2001) in pupal wings during PCP establishment prior to and during actin hair formation with Nek2 and Nek2KD expression (see Fig. 4H for adult PCP effects). Whereas Nek2 expression caused reduced Dsh levels (Suppl. Fig. S7G,H), Nek2KD induced Dsh level increase (compared to adjacent control tissue) with the wild-type asymmetric, membrane associated DshGFP pattern barely visible (Fig. 7A-A', B). Fmi levels were largely unaffected (Fig. 7A,A",B, and C"). The Nek2 and Nek2KD expression regions displayed PCP defects as detected by randomized polarity vector angles and reduced polarity strength (nematic order), assimilated from Fmi localization (Fig. 7A”,D–E, and Suppl. Fig. S7G). This was consistent with the PCP defects seen in adult wings (Fig. 4H). Blocking proteasomal degradation with the dominant negative Prosβ2 and 6 subunits in vivo (see above) confirmed that Dsh levels are controlled via degradation in the PCP context (Fig. 7F–G), which has also been observed in cell culture assays (Schertel et al., 2013).
Figure 7. Nek2KD expression causes Dsh protein increase in vivo and affects PCP similar to interference with the proteasome.
Pupal wing stained for F-actin (Phalloidin, red), Fmi (blue), and direct Dsh levels (dsh-DshGFP, green, no antibody applied) in XY projections (A) or XZ single sections (C). Anterior/posterior compartments are indicated. (A) Trichomes are misoriented in posterior compartment (genotype enGAL4/+, UAS-nek2KD/+ dshGFP/+, temperature shift from 18°C to 28°C for 29hrs prior to fixation). (A',B,C') Dsh localizes in a faint zig-zag pattern on membranes in anterior compartment and ectopic punctae in posterior compartment (compared to anterior compartment). (A",B,C") Fmi punctae size and number are not affected, but apical asymmetric Fmi localization is perturbed by Nek2KD, reflecting PCP defects. (B) Quantification of DshGFP and Fmi punctae in posterior vs. anterior compartments of XY projections (as in A). Average relative count of large cytoplasmic punctae of 5 ROIs in 4 wings, *P<0.001, n=71–298. (D) Nematic order representing polarity strength and angle for each cell stained for Fmi in A" indicated by orange lines (via Packing_analyzer). Green line marks compartment border (A',D). (E) Quantification of Fmi polarity in Nek2KD domain. Blue sectors in rosettes represent average polarity angle distribution in anterior compartment (wt) and posterior compartment (Nek2KD), graph on right shows polarity length. Fmi polarity angles are random and polarization strength is reduced in Nek2KD as compared to control tissue. Calculations are based on 3 independent wings, n=1760 wt/anterior compartment, n=2425 Nek2KD/posterior compartment cells; P value for rosette is from χ2-test.
(F,G) Proteasomal interference in posterior compartment of pupal wings stained as in (A) of en-GAL4/+, GAL80ts/+, dshGFP/+, UAS-Prosb2+6DN/+, temperature shift from 18°C to 29°C for 29hrs prior to dissection. (F) Trichomes are lost or form in bundles in posterior compartment. (F') Dsh levels on the membrane and in cytoplasm are increased and (F") Fmi levels are also increased. (G) Quantification of protein levels with increase in DshGFP and Fmi upon interfering with proteasomal degradation. Measurements from 3 wings in 4 Z sections each perpendicular to antero-posterior compartment boundary. *P<0.0001.
Within the Nek2KD expression domain, Dsh-GFP levels were not only generally increased in wing cells (Fig. 7A–A’ and Suppl. Fig. S7A–B), but the majority of Dsh was localized to large intracellular punctae near apical juntional plasma membrane regions (Fig. 7A’,B,C-C’, and Suppl. Fig. S7A–B). Interestingly, these resemble Dsh punctae that have been observed in Dsh overexpression studies in cell culture and shown to be Dsh aggregates mediated by dimerizaton/multimerization of the Dsh DIX domain (Schwarz-Romond et al., 2005; 2007). This suggests that Dsh levels in Nek2KD expressing cells are increased beyond the cellular capacity to recruit Dsh to the membrane (and hence such aggregates might form), or by a sequestration mechanism that is caused by a Nek2-Dsh physical interaction (as suggested by Cervenka et al., 2016).
We thus examined the net outcome of Dsh PCP signalling in this context, and analyzed tissue with combined Dsh knockdown and Nek2KD overexpression (Suppl. Fig. S7C–F). At levels where the individual expression had no effect, the combination induced PCP wing hair whorls (Suppl. Fig. S7D, D'), suggesting that Dsh present in a Nek2KD background is largely inactive, likely due to the trapping in the large aggregates. Thus Nek2 binding to Dsh is likely to serve a second role beyond controlling protein levels.
In summary, these data indicate that Nek2 acts specifically on Dsh levels and localization and thus causes PCP defects.
Discussion
We demonstrate a post-mitotic role of the APC/CFzr/Cdh1 in epithelial cell patterning and PCP. Loss and gain-of-function studies of several APC/C subunits indicate that the entire APC/C, its regulator rca1/emi, and co-activator Cdh1/Fzr are affecting PCP establishment via an effect on Dsh. As Dsh levels are reduced in APC8 knockdowns, the APC/CFzr/Cdh1 proteasomal targeting function cannot directly act on Dsh. We identified Nek2 kinase, a known APC/CFzr/Cdh1 substrate (Kimata et al., 2008; Min et al., 2013; Pfleger and Kirschner, 2000; Sedgwick et al., 2013; Martins et al., 2017), as mediator of the effect on Dsh. Nek2 binds and phosphorylates Dsh, and has been suggested to promote its function in canonical Wg/Wnt-signaling (Schertel et al., 2013). Dsh levels are indeed increased in Nek2 loss-of-function scenarios. Importantly, the increase in Dsh levels is observed before PCP signaling is set, suggesting that it is the primary cause of the PCP defects associated with APC/C. Accordingly, APC/C knockdown mediated PCP defects depend on and are modified by both Nek2 levels and dsh. Thus APC/CFzr/Cdh1 affects the levels of Dsh positively by inhibiting Nek2, and Nek2 in turn affects the stability and subcellular localization of Dsh.
Post-mitotic APC/C function in epithelial cells
The APC/C is an E3 ubiquitin ligase, tightly regulated by its co-activators. During meiosis, its co-activator is Cortex (Kronja et al., 2014; Pesin and Orr-Weaver, 2007), and in mitotic cell cycle progression Cdc20 is the activating subunit (rev. in Pines, 2011). More recently, post-mitotic APC/C functions have been described and often linked to the Cdh1/Fzr co-activator family (rev. in Pines, 2011; Primorac and Musacchio, 2013), which are differentially expressed in vertebrates (Wan and Kirschner, 2001). Post-mitotic APC/C effects have largely been linked to neuronal cells (rev. in Peters, 2002; Pines, 2011; Primorac and Musacchio, 2013), based on expression studies in mice (Gieffers et al., 1999) or functional studies in Drosophila, which again link it to fzr/cdh1 (Silies and Klambt, 2010; van Roessel et al., 2004). Nonetheless, only few neuronal functions of the APC/CCdh1/Fzr have been established, including effects on neuromuscular synapses (van Roessel et al., 2004) and axonal path-finding in the Drosophila CNS (Silies and Klambt, 2010). We describe a post-mitotic APC/CCdh1/Fzr function in epithelial cell polarity and patterning, to our knowledge the first of its kind. Interestingly, its effect on Dsh levels and associated cellular polarity and PCP patterning is indirect, and mediated by the Nek2 kinase. Nek2 kinases, most closely related to “never-in-mitosis-again” (NIMA) kinases, are best known for their role as regulators of cell cycle and centrosome integrity from vertebrate cell culture experiments (Fry et al., 2012; Nigg, 2001). In Drosophila cultured cells, Nek2 is required for centrosome maturation and upon over-expression causes centrosome fragmentation (Prigent et al., 2005). Nek2/Nek2A is a direct APC/C substrate during cell cycle progression (Kimata et al., 2008; Min et al., 2013; Pfleger and Kirschner, 2000; Sedgwick et al., 2013). Our data, together with the above publications, are consistent with the model where the APC/C regulates Nek2 levels via ubiquitination and proteasome degradation and Nek2 regulates Dsh levels via its phosphorylation and subsequent targeting to the proteasome. As such, in PCP signaling it is not only the APC/CCdh1/Fzr, which is used post-mitotically in epithelial patterning, but also Nek2, one of its cell cycle associated substrates. A similar observation has been made by Martins et al (2017) in the context of early stages of eye development and MF progression.
Our studies also reveal an in vivo function of the APC8 subunit in metazoa, which has previously been placed in the APC/C through studies in yeast (Matyskiela and Morgan, 2009). Interestingly, APC8 is known to recruit Nek2 (Sedgwick et al., 2013), which is the mediator of the APC/CCdh1/Fzr in PCP establishment, providing a functional link. The requirement of the Cdh1/Fzr co-activator (but not Cdc20/Fzy) and the inhibitory effect of Rca1/Emi on the APC/C indicate that the full APC/CCdh1/Fzr complex with its enzymatic activity is involved in PCP, again consistent with our in vivo data and the link of APC/CCdh1/Fzr to Nek2 (also Martins et al., 2017).
APC/CCdh1/Fzr-Nek2 mediated Dsh regulation
APC/CCdh1/Fzr affects PCP establishment via Dsh being its indirect “target” and this being mediated by Nek2, a Serine/Threonine (S/T)-kinase (Fry et al., 2012). Nek2 has been shown to phosphorylate Dsh/Dvl on multiple sites (Schertel et al., 2013; Cervenka et al., 2016). Schertel et al (2013) described increased Dsh protein stability in a Dsh mutant with reduced Nek2 phosphorylation in cell culture, which is supported by our observation that inhibition of proteasomal function increases Nek2 induced PCP and canonical Wnt-signaling defects in vivo. In contrast to Schertel et al (2013), we also detect clear in vivo requirements of Nek2 in cell growth through loss-of-function studies (Suppl. Fig. S4). Our data are consistent with the proposed Nek2 function derived from cell culture studies. Identifying specific Nek2 target residues on Dsh is difficult, as Dsh/Dvl proteins are very S/T rich (1/6 residues in Dsh/Dvl is an S/T with a total of over 100 potential phosphorylation sites). Nek2 phosphorylation of Dsh has been shown to affect all domains of the protein (Cervenka et al., 2016). Our previous functional data suggest that mutating individual clusters has no effect on Dsh function in vivo (Yanfeng et al., 2011). It is likely that that effect of Nek2 mediated phosphorylation is more subtle than just affecting Dsh levels, as in Nek2 interference scenarios we detect Dsh protein in large cytoplasmic punctae, likely to correspond to non-functional Dsh aggregates (Schwarz-Romond et al., 2007; 2005) with a strong dsh loss-of-function effect, as opposed to Nek2-wt (Suppl. Fig. S7G,C–F). While Dsh levels are increased in Nek2KD scenarios, Dsh is also mislocalized, and thus the resulting functional effect(s) can be pleiotropic and affect the canonical and PCP branches of Wnt-signaling and possibly other Dsh functions (Mlodzik, 2016). Accordingly, in some of our assays, the effects on Wnt/β–catenin and PCP signaling are not fully separated and wing size reduction and margin pattern defects (both Wg/β–catenin associated defects) are evident. Complex future experiments in vivo will be needed to dissect potential roles of the many Nek2-target sites on Dsh/Dvl (Cervenka et al., 2016).
The APC/CCdh1/Fzr effect on Dsh, albeit indirect, is more straightforward. Loss of APC/C function reduces Dsh levels to a point that is insufficient to support its PCP role. Consistently, the genetic requirements in the multi-cellular PCP-context in eye development are overlapping between the APC/CCdh1/Fzr and dsh, both are required in the R3 precursor for induction of the neighboring cell as R4. In developing wings, the phenotypic defects of APC-subunits analyzed at the cellular level are also reminiscent of dsh LOF alleles, with actin-hair formation being delayed and trichomes often forming in the center of a cell (our data; Strutt and Strutt, 2007). Additionally, core PCP factors are mislocalized, e.g. Fmi, reflecting aberrant cellular polarity. However, the strong effect on Dsh levels and the similarity in the cell biological and genetic requirements between dsh and APC/CCdh1/Fzr subunits define Dsh as the target of the APC/C during PCP establishment.
Dsh levels have to be tightly regulated, because of its several functions (Wallingford and Habas, 2005; Wynshaw-Boris, 2012; Mlodzik, 2016). It has thus been an enigmatic protein family for decades, with new functions still being discovered (Mlodzik, 2016; Wallingford and Habas, 2005; Wynshaw-Boris, 2012). It is the only core PCP factor displaying general lethality when overexpressed (Mlodzik, 2016), which is only in part explained by its role in canonical Wnt-signaling. Dsh behaviour is different from overactivation of canonical Wnt-signaling, as Wnt-pathway components generally cause overgrowth and cell fate defects but not cell lethal outcomes. Dsh might thus have still undiscovered functions in vivo, which are masked by strong maternal effects in Drosophila and redundancy among the three Dvl genes in mammals (Mlodzik, 2016; Wallingford and Habas, 2005; Wynshaw-Boris, 2012). However, Dsh levels need to be maintained within a specific range, a function that is at least in part provided by the APC/C and Nek2.
Experimental Procedures
Fly stocks
Crosses were set up at 25°C, unless otherwise indicated. The GAL4/UAS system (Brand and Perrimon, 1993) was used for expression of RNAi and constructs, in combination with GAL80ts (McGuire, 2003) or UAS-Dcr2 where indicated. GAL4 lines for post-mitotic expression in eye development were: sev-GAL4 (sev-enhancer with heatshock promoter) expressed in R3/R4, R1, R6 and R7 (Ye and Fortini, 1999), sep-GAL4 (sev-enhancer with sev promoter, same expression pattern as sevGal4; M. Fanto, (Djiane et al., 2005) and GMR-GAL4, expressed in photoreceptors posterior to MF (Ye and Fortini, 1999). Other GAL4 lines used: psq-GAL4 (Weber et al., 2008), fng-GAL4, en-GAL4, nub-GAL4, sd-GAL4, dpp-GAL4, act>y+>GAL4, mirr-GAL4 (Bloomington stock center). Following stocks were received from: fzr8F3 FRT19A, UAS-fzr-IR25550 (Ch. Klämbt), UAS-mr (T. Orr-Weaver; Kashevsky et al., 2002), UAS-nek2-IR103408, UAS-nek2-IR40052 (VDRC, 103408 and 40052 target independent sequences), sev-GAL4, UAS-nek2, UAS-nek2KD, en-GAL4 GAL80ts UAS-GFP (K. Basler), UAS-lmgA (P. Deak; Nagy et al., 2012), UAS-rca1, UAS-rca1C351S and deletions thereof (F. Sprenger; Zielke et al., 2006), mδlacZ (Cooper and Bray, 1999), fzy6, fzy7 (I. Dawson), UAS-Prosβ2 and UAS-Prosβ6 (H. Steller; Cho-Park et al, 2013). shtd1 was recombined on FRT19A. UAS-dshIR10361R2 was recombined onto an enGAL4, GAl80ts, UAS-GFP chromosome. Other stocks were from Bloomington, VDRC or NIG Japan stock centers. Temperature shift experiments for pupal wing: 3rd instar larvae collection, aging to prepupal stage at 18°C and shift to 28–31°C as indicated. For eye development temperature shifts were from 18°C at late 2nd or early 3rd instar to 29–31°C as indicated.
Transgenic flies and constructs
APC8/CG2508 open reading frame was amplified from LP08985 with primers containing cloning sites and tag: N-terminal EcoR1, C-terminal HA followed by Xba1. Forward primer: TCCGAATTCATGGCGATGCAGAAGTTCTTCAG, reverse-primer: TCCTCTAGATTAAGCGTAGTCTGGGACGTCGTATGGGTATCCATCGATGGATACGCTGGATATTTCCATGGAGCTGTTATCATCCGA. PCR product was sequenced and cloned into pUAST and transgenics established.
Immunostaining and histology
Larval and pupal tissue was dissected, fixed and stained and adult eyes and wings collected as described (Gault et al., 2012). Pupal aging at temperatures different from 25°C or with temperature shifts was calculated according to full developmental time at a given temperature and empirically determined. Confocal images were acquired on Zeiss LSM510 and LSM880 scopes. Super resolution microscopy was performed on a Leica TCS SP8 STED3X, at 647nm exitation (ext) with 775nm depletion (dep), 532nm ext with 660nm dep and 488nm ext with 592 dep laser lines. Gating was 0.2–4.85 ns for 532nm and 0.3–6.0 ns for 488nm laser lines.
Quantification
mδlacZ and psq>GFP count in mutant shtd and mosaic fzr tissue was taken on confocal projections of ommatidial 5 cell preclusters with all nuclei present based on Elav or Rough staining at clonal borders. Trichome orientation and number was recorded under light microscopy illumination. DshGFP and Fmi punctae in pupal wing confocal projections were counted with Fiji (threshold, particle count). Average protein levels determined in single Z sections via Fiji (mean grey value). For statistical analysis unpaired t-test was used to calculate P-values. Fmi polarity assessment for pupal wing cells was done with Packing_analyzer_V2 as described (Aigouy et al., 2010), edited, and strength of polarization (nematic order) and polarization angle/direction calculated for each cell. MATLAB script (Carvajal-Gonzalez et al., 2015; Wu et al. 2013) was applied to generate rosette diagrams and charts of individual compartments; χ2-Test was used for statistical analysis.
Immunoblotting
Third instar eye-antennal imaginal discs were dissected into PBS, 0.1% triton X-100, brought to 1× SDS loading buffer, boiled, run on 4–20% gradient SDS PAGE gels and transferred to nitrocellulose. Proteins were detected by the respective primary and secondary donkey-HRP antibodies (1:200, Jackson ImmunoResearch labs [IRL]) and ECL Plus (GE healthcare).
Antibodies and staining reagents
Chicken-anti-βgal (1:200, Immunology Consultants Laboratory), rabbit-anti-βgal (1:1000, Molecular Probes), mouse-anti-CycB (1:100, Developmental Studies Hybridoma Bank/DSHB), rat-anti-DEcad (1:50, DSHB), rabbit-anti-Dgo (1:100, Feiguin et al., 2001), rat-anti-Elav (1:50, DSHB), Mouse-anti-Fmi (1:10, DSHB), mouse-anti-GFP (1:1000, Roche), rabbit-anti-Nek2 (1:1000, Prigent et al., 2005), rat-anti-Pk (1:25; Strutt et al., 2013) mouse-anti-Ro (1:10, DSHB), mouse-anti-γTub (1:1000, Sigma), Rhodamine phalloidin (1:1000, Invitrogen), phalloidin-AF647 (1:1000, Jackson IRL), Hoechst 33342 (10mg/ml, 1:500). All fluorophore coupled secondaries were from Jackson IRL used at 1:200.
Supplementary Material
Highlights.
-
-
Post-mitotic Anaphase Promoting Complex (APC) function in planar cell polarity
-
-
APC regulates the function of Nek2 during PCP establishment.
-
-
Dishevelled (Dsh) is the core PCP target of the APC/C during PCP signaling
-
-
APC regulates Nek2 levels and Nek2 regulates Dsh via phosphorylation
Acknowledgments
We are grateful to Jose Maria Carvajal-Gonzalez and Angel Roman for help with quantitative analyses of nematic order in pupal wings. We thank Y. Kimata for sharing unpublished results, C. Schertel, K. Basler, T. Orr-Weaver, A. Brand, F. Sprenger, C. Prigent, R. Giet, P. Deak, C. Klämbt, I. Dawson, C. Lehner, H. Steller and D. Strutt for flies and reagents, and the stock centers in Bloomington, Vienna, Hungary and Japan for fly strains, G. Joseph, M.J. Smith and A. Eustache for technical help, G. Collu, A. Humphries, C. Iomini, and L. Vuong for helpful comments on the manuscript, and all Mlodzik lab and department members for critical comments and helpful discussions. Confocal microscopy was performed at the Tisch Cancer Institute Microscopy Core, supported by grant P30 CA196521 from the NCI. We thank P. Carman from Leica together with Mount Sinai’s Microscopy Core for access to the Leica TCS SP8 STED3X microscope and acquisition of STED images. This work was supported by NIH grants GM102811 and GM104241 to MM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
UW and MM designed the experiments and wrote the manuscript, UW performed the experiments.
References
- Adler PN. Planar signaling and morphogenesis in Drosophila. Dev Cell. 2002;2:525–535. doi: 10.1016/s1534-5807(02)00176-4. [DOI] [PubMed] [Google Scholar]
- Aigouy B, Farhadifar R, Staple DB, Sagner A, Roper JC, Julicher F, Eaton S. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell. 2010;142:773–786. doi: 10.1016/j.cell.2010.07.042. [DOI] [PubMed] [Google Scholar]
- Axelrod JD. Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes Dev. 2001;15:1182–1187. doi: 10.1101/gad.890501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayly R, Axelrod JD. Pointing in the right direction: new developments in the field of planar cell polarity. Nat Rev Genet. 2011;12:385–391. doi: 10.1038/nrg2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejsovec A. Flying at the head of the pack: Wnt biology in Drosophila. Oncogene. 2006;25:7442–7449. doi: 10.1038/sj.onc.1210051. [DOI] [PubMed] [Google Scholar]
- Boutros M, Mlodzik M. Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech Dev. 1999;83:27–37. doi: 10.1016/s0925-4773(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Buttitta LA, Katzaroff AJ, Edgar BA. A robust cell cycle control mechanism limits E2F-induced proliferation of terminally differentiated cells in vivo. J Cell Biol. 2010;189:981–996. doi: 10.1083/jcb.200910006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal-Gonzalez JM, S B, Mendoza M, Dussert A, Collu G, Roman AC, Weber U, Ciruna B, Mlodzik M. The Clathrin Adaptor AP-1 complex and Arf1 regulate Planar Cell Polarity in vivo. Natur Communications. 2015 doi: 10.1038/ncomms7751. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervenka I, Valnohova J, Bernatik O, Harnos J, Radsetoulal M, Sedova K, Hanakova K, Potesil D, Sedlackova M, Salasova A, et al. Dishevelled is a NEK2 kinase substrate controlling dynamics of centrosomal linker proteins. Proc Natl Acad Sci U S A. 2016;113:9304–9309. doi: 10.1073/pnas.1608783113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Baloh RH, Milbrandt J. The NIMA-family kinase Nek3 regulates microtubule acetylation in neurons. J Cell Sci. 2009;122:2274–2282. doi: 10.1242/jcs.048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WS, Antic D, Matis M, Logan CY, Povelones M, Anderson GA, Nusse R, Axelrod JD. Asymmetric homotypic interactions of the atypical cadherin flamingo mediate intercellular polarity signaling. Cell. 2008;133:1093–1105. doi: 10.1016/j.cell.2008.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho-Park PF, Steller H. Proteasome regulation by ADP-ribosylation. Cell. 2013;153:614–627. doi: 10.1016/j.cell.2013.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MT, Bray SJ. Frizzled regulation of Notch signalling polarizes cell fate in the Drosophila eye. Nature. 1999;397:526–530. doi: 10.1038/17395. [DOI] [PubMed] [Google Scholar]
- Couso JP, Bishop SA, Martinez Arias A. The wingless signalling pathway and the patterning of the wing margin in Drosophila. Development. 1994;120:621–636. doi: 10.1242/dev.120.3.621. [DOI] [PubMed] [Google Scholar]
- Dawson IA, Roth S, Artavanis-Tsakonas S. The Drosophila cell cycle gene fizzy is required for normal degradation of cyclins A and B during mitosis and has homology to the CDC20 gene of Saccharomyces cerevisiae. J Cell Biol. 1995;129:725–737. doi: 10.1083/jcb.129.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djiane A, Yogev S, Mlodzik M. The apical determinants aPKC and dPatj regulate Frizzled-dependent planar cell polarity in the Drosophila eye. Cell. 2005;121:621–631. doi: 10.1016/j.cell.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Donzelli M, Squatrito M, Ganoth D, Hershko A, Pagano M, Draetta GF. Dual mode of degradation of Cdc25 A phosphatase. EMBO J. 2002;21:4875–4884. doi: 10.1093/emboj/cdf491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanto M, Mlodzik M. Asymmetric Notch activation specifies photoreceptors R3 and R4 and planar polarity in the Drosophila eye. Nature. 1999;397:523–526. doi: 10.1038/17389. [DOI] [PubMed] [Google Scholar]
- Feiguin F, Hannus M, Mlodzik M, Eaton S. The Ankyrin repeat protein Diego mediates Frizzled-dependent planar polarization. Developmental Cell. 2001;1:93–101. doi: 10.1016/s1534-5807(01)00010-7. [DOI] [PubMed] [Google Scholar]
- Foe I, Toczyski D. Structural biology: a new look for the APC. Nature. 2011;470:182–183. doi: 10.1038/470182a. [DOI] [PubMed] [Google Scholar]
- Fry AM. The Nek2 protein kinase: a novel regulator of centrosome structure. Oncogene. 2002;21:6184–6194. doi: 10.1038/sj.onc.1205711. [DOI] [PubMed] [Google Scholar]
- Fry AM, O'Regan L, Sabir SR, Bayliss R. Cell cycle regulation by the NEK family of protein kinases. J Cell Sci. 2012;125:4423–4433. doi: 10.1242/jcs.111195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gault WJ, Olguin P, Weber U, Mlodzik M. Drosophila CK1γ, gilgamesh, Controls PCP-mediated Morphogenesis Through Regulation of Vesicle Trafficking. J Cell Biol. 2012 doi: 10.1083/jcb.201107137. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geley S, Kramer E, Gieffers C, Gannon J, Peters JM, Hunt T. Anaphase-promoting complex/cyclosome-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J Cell Biol. 2001;153:137–148. doi: 10.1083/jcb.153.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieffers C, Peters BH, Kramer ER, Dotti CG, Peters JM. Expression of the CDH1-associated form of the anaphase-promoting complex in postmitotic neurons. Proc Natl Acad Sci U S A. 1999;96:11317–11322. doi: 10.1073/pnas.96.20.11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosskortenhaus R, Sprenger F. Rca1 inhibits APC-Cdh1(Fzr) and is required to prevent cyclin degradation in G2. Dev Cell. 2002;2:29–40. doi: 10.1016/s1534-5807(01)00104-6. [DOI] [PubMed] [Google Scholar]
- Hayes MJ, Kimata Y, Wattam SL, Lindon C, Mao G, Yamano H, Fry AM. Early mitotic degradation of Nek2A depends on Cdc20-independent interaction with the APC/C. Nat Cell Biol. 2006;8:607–614. doi: 10.1038/ncb1410. [DOI] [PubMed] [Google Scholar]
- Herrera SC, Martin R, Morata G. Tissue homeostasis in the wing disc of Drosophila melanogaster: immediate response to massive damage during development. PLoS Genet. 2013;9:e1003446. doi: 10.1371/journal.pgen.1003446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu JY, Reimann JD, Sorensen CS, Lukas J, Jackson PK. E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APC(Cdh1) Nat Cell Biol. 2002;4:358–366. doi: 10.1038/ncb785. [DOI] [PubMed] [Google Scholar]
- Izawa D, Pines J. How APC/C-Cdc20 changes its substrate specificity in mitosis. Nat Cell Biol. 2011;13:223–233. doi: 10.1038/ncb2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs H, Richter D, Venkatesh T, Lehner C. Completion of mitosis requires neither fzr/rap nor fzr2, a male germline-specific Drosophila Cdh1 homolog. Curr Biol. 2002;12:1435–1441. doi: 10.1016/s0960-9822(02)01074-6. [DOI] [PubMed] [Google Scholar]
- Jenny A, Darken RS, Wilson PA, Mlodzik M. Prickle and Strabismus form a functional complex to generate a correct axis during planar cell polarity signaling. EMBO J. 2003;22:4409–4420. doi: 10.1093/emboj/cdg424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny A, Reynolds-Kenneally J, Das G, Burnett M, Mlodzik M. Diego and Prickle regulate Frizzled planar cell polarity signalling by competing for Dishevelled binding. Nat Cell Biol. 2005;7:691–697. doi: 10.1038/ncb1271. [DOI] [PubMed] [Google Scholar]
- Kashevsky H, Wallace JA, Reed BH, Lai C, Hayashi-Hagihara A, Orr-Weaver TL. The anaphase promoting complex/cyclosome is required during development for modified cell cycles. Proc Natl Acad Sci U S A. 2002;99:11217–11222. doi: 10.1073/pnas.172391099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata Y, Baxter JE, Fry AM, Yamano H. A role for the Fizzy/Cdc20 family of proteins in activation of the APC/C distinct from substrate recruitment. Mol Cell. 2008;32:576–583. doi: 10.1016/j.molcel.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Klein TJ, Mlodzik M. Planar cell polarization: an emerging model points in the right direction. Annu Rev Cell Dev Biol. 2005;21:155–176. doi: 10.1146/annurev.cellbio.21.012704.132806. [DOI] [PubMed] [Google Scholar]
- Kronja I, Whitfield ZJ, Yuan B, Dzeyk K, Kirkpatrick J, Krijgsveld J, Orr-Weaver TL. Quantitative proteomics reveals the dynamics of protein changes during Drosophila oocyte maturation and the oocyte-to-embryo transition. Proc Natl Acad Sci U S A. 2014;111:16023–16028. doi: 10.1073/pnas.1418657111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence PA, Casal J, Struhl G. Cell interactions and planar polarity in the abdominal epidermis of Drosophila. Development. 2004;131:4651–4664. doi: 10.1242/dev.01351. [DOI] [PubMed] [Google Scholar]
- Lear BC, Skeath JB, Patel NH. Neural cell fate in rca1 and cycA mutants: the roles of intrinsic and extrinsic factors in asymmetric division in the Drosophila central nervous system. Mech Dev. 1999;88:207–219. doi: 10.1016/s0925-4773(99)00190-2. [DOI] [PubMed] [Google Scholar]
- Lecuyer E, Yoshida H, Parthasarathy N, Alm C, Babak T, Cerovina T, Hughes TR, Tomancak P, Krause HM. Global analysis of mRNA localization reveals a prominent role in organizing cellular architecture and function. Cell. 2007;131:174–187. doi: 10.1016/j.cell.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Matyskiela ME, Morgan DO. Analysis of activator-binding sites on the APC/C supports a cooperative substrate-binding mechanism. Mol Cell. 2009;34:68–80. doi: 10.1016/j.molcel.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Temporal and Regional Gene Expression Targeting with the Conventional GAL4/UAS System in Drosophila. A. Dros. Res. Conf. 2003;44:153. [Google Scholar]
- Min M, Mayor U, Lindon C. Ubiquitination site preferences in anaphase promoting complex/cyclosome (APC/C) substrates. Open Biol. 2013;3:130097. doi: 10.1098/rsob.130097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlodzik M. Planar polarity in the Drosophila eye: a multifaceted view of signaling specificity and cross-talk. EMBO J. 1999;18:6873–6879. doi: 10.1093/emboj/18.24.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlodzik M. The Dishevelled Protein Family: Still Rather a Mystery After Over 20 Years of Molecular Studies. Curr Top Dev Biol. 2016;117:75–91. doi: 10.1016/bs.ctdb.2015.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy O, Pal M, Udvardy A, Shirras CA, Boros I, Shirras AD, Deak P. lemmingA encodes the Apc11 subunit of the APC/C in Drosophila melanogaster that forms a ternary complex with the E2-C type ubiquitin conjugating enzyme, Vihar and Morula/Apc2. Cell Div. 2012;7:9. doi: 10.1186/1747-1028-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol. 2001;2:21–32. doi: 10.1038/35048096. [DOI] [PubMed] [Google Scholar]
- Pesin JA, Orr-Weaver TL. Developmental role and regulation of cortex, a meiosis-specific anaphase-promoting complex/cyclosome activator. PLoS Genet. 2007;3:e202. doi: 10.1371/journal.pgen.0030202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol Cell. 2002;9:931–943. doi: 10.1016/s1097-2765(02)00540-3. [DOI] [PubMed] [Google Scholar]
- Pfleger CM, Kirschner MW. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 2000;14:655–665. [PMC free article] [PubMed] [Google Scholar]
- Pimentel AC, Venkatesh TR. rap gene encodes Fizzy-related protein (Fzr) and regulates cell proliferation and pattern formation in the developing Drosophila eye-antennal disc. Dev Biol. 2005;285:436–446. doi: 10.1016/j.ydbio.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Pines J. Cubism and the cell cycle: the many faces of the APC/C. Nat Rev Mol Cell Biol. 2011;12:427–438. doi: 10.1038/nrm3132. [DOI] [PubMed] [Google Scholar]
- Prigent C, Glover DM, Giet R. Drosophila Nek2 protein kinase knockdown leads to centrosome maturation defects while overexpression causes centrosome fragmentation and cytokinesis failure. Exp Cell Res. 2005;303:1–13. doi: 10.1016/j.yexcr.2004.04.052. [DOI] [PubMed] [Google Scholar]
- Primorac I, Musacchio A. Panta rhei: the APC/C at steady state. J Cell Biol. 2013;201:177–189. doi: 10.1083/jcb.201301130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schertel C, Huang D, Bjorklund M, Bischof J, Yin D, Li R, Wu Y, Zeng R, Wu J, Taipale J, et al. Systematic screening of a Drosophila ORF library in vivo uncovers Wnt/Wg pathway components. Dev Cell. 2013;25:207–219. doi: 10.1016/j.devcel.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T, Fiedler M, Shibata N, Butler PJ, Kikuchi A, Higuchi Y, Bienz M. The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat Struct Mol Biol. 2007;14:484–492. doi: 10.1038/nsmb1247. [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T, Merrifield C, Nichols BJ, Bienz M. The Wnt signalling effector Dishevelled forms dynamic protein assemblies rather than stable associations with cytoplasmic vesicles. J Cell Sci. 2005;118:5269–5277. doi: 10.1242/jcs.02646. [DOI] [PubMed] [Google Scholar]
- Schweisguth F. Dominant-negative mutation in the beta2 and beta6 proteasome subunit genes affect alternative cell fate decisions in the Drosophila sense organ lineage. Proc. Natl. Acad. Sci. USA. 1999;96:11382–11386. doi: 10.1073/pnas.96.20.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick GG, Hayward DG, Di Fiore B, Pardo M, Yu L, Pines J, Nilsson J. Mechanisms controlling the temporal degradation of Nek2A and Kif18A by the APC/C-Cdc20 complex. EMBO J. 2013;32:303–314. doi: 10.1038/emboj.2012.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8:126–138. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- Sharma RP, Chopra VL. Effect of the Wingless (wg1) mutation on wing and haltere development in Drosophila melanogaster. Dev Biol. 1976;48:461–465. doi: 10.1016/0012-1606(76)90108-1. [DOI] [PubMed] [Google Scholar]
- Silies M, Klambt C. APC/C(Fzr/Cdh1)-dependent regulation of cell adhesion controls glial migration in the Drosophila PNS. Nat Neurosci. 2010;13:1357–1364. doi: 10.1038/nn.2656. [DOI] [PubMed] [Google Scholar]
- Simons M, Mlodzik M. Planar cell polarity signaling: from fly development to human disease. Annual review of genetics. 2008;42:517–540. doi: 10.1146/annurev.genet.42.110807.091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Mlodzik M. Planar cell polarity signaling: coordination of cellular orientation across tissues. Wiley Interdiscip Rev Dev Biol. 2012;1:479–499. doi: 10.1002/wdev.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth KA, Belote JM. The dominant temperature-sensitive lethal DTS7 of Drosophila melanogaster encodes an altered 20S proteasome beta-type subunit. Genetics. 1999;151:211–220. doi: 10.1093/genetics/151.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt D, Strutt H. Differential activities of the core planar polarity proteins during Drosophila wing patterning. Dev Biol. 2007;302:181–194. doi: 10.1016/j.ydbio.2006.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H, Searle E, Thomas-Macarthur V, Brookfield R, Strutt D. A Cul-3-BTB ubiquitylation pathway regulates junctional levels and asymmetry of core planar polarity proteins. Development. 2013;140:1693–1702. doi: 10.1242/dev.089656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H, Strutt D. Polarity determination in the Drosophila eye. Curr Opin Genet Dev. 1999;9:442–446. doi: 10.1016/S0959-437X(99)80067-7. [DOI] [PubMed] [Google Scholar]
- Strutt H, Strutt D. Differential stability of flamingo protein complexes underlies the establishment of planar polarity. Curr Biol. 2008;18:1555–1564. doi: 10.1016/j.cub.2008.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H, Strutt D. Asymmetric localisation of planar polarity proteins: Mechanisms and consequences. Semin Cell Dev Biol. 2009;20:957–963. doi: 10.1016/j.semcdb.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Swan A, Schupbach T. Drosophila female meiosis and embryonic syncytial mitosis use specialized Cks and CDC20 proteins for cyclin destruction. Cell Cycle. 2005;4:1332–1334. doi: 10.4161/cc.4.10.2088. [DOI] [PubMed] [Google Scholar]
- Tanaka-Matakatsu M, Thomas BJ, Du W. Mutation of the Apc1 homologue shattered disrupts normal eye development by disrupting G1 cell cycle arrest and progression through mitosis. Dev Biol. 2007;309:222–235. doi: 10.1016/j.ydbio.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Li B, Bharadwaj R, Zhu H, Ozkan E, Hakala K, Deisenhofer J, Yu H. APC2 Cullin protein and APC11 RING protein comprise the minimal ubiquitin ligase module of the anaphase-promoting complex. Mol Biol Cell. 2001;12:3839–3851. doi: 10.1091/mbc.12.12.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson A, Strapps WR, Heemskerk J. Linking Frizzled and Wnt signaling in Drosophila development. Development. 1997;124:4515–4521. doi: 10.1242/dev.124.22.4515. [DOI] [PubMed] [Google Scholar]
- Tree DR, Shulman JM, Rousset R, Scott MP, Gubb D, Axelrod JD. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell. 2002;109:371–381. doi: 10.1016/s0092-8674(02)00715-8. [DOI] [PubMed] [Google Scholar]
- van Roessel P, Elliott DA, Robinson IM, Prokop A, Brand AH. Independent regulation of synaptic size and activity by the anaphase-promoting complex. Cell. 2004;119:707–718. doi: 10.1016/j.cell.2004.11.028. [DOI] [PubMed] [Google Scholar]
- Wallingford JB. Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annu Rev Cell Dev Biol. 2012;28:627–653. doi: 10.1146/annurev-cellbio-092910-154208. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132:4421–4436. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- Wan Y, Kirschner MW. Identification of multiple CDH1 homologues in vertebrates conferring different substrate specificities. Proc Natl Acad Sci U S A. 2001;98:13066–13071. doi: 10.1073/pnas.231487598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–658. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- Weber U, Gault WJ, Olguin P, Serysheva E, Mlodzik M. Novel regulators of planar cell polarity: a genetic analysis in Drosophila. Genetics. 2012;191:145–162. doi: 10.1534/genetics.111.137190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber U, Pataki C, Mihaly J, Mlodzik M. Combinatorial signaling by the Frizzled/PCP and Egfr pathways during planar cell polarity establishment in the Drosophila eye. Dev Biol. 2008;316:110–123. doi: 10.1016/j.ydbio.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk R, Hu J, Blotsky D, Krause HM. Diverse and pervasive subcellular distributions for both coding and long noncoding RNAs. Genes Dev. 2016;30:594–609. doi: 10.1101/gad.276931.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff T, Ready DF. The beginning of pattern formation in the Drosophila compound eye: the morphogenetic furrow and the second mitotic wave. Development. 1991;113:841–850. doi: 10.1242/dev.113.3.841. [DOI] [PubMed] [Google Scholar]
- Wolff T, Rubin GM. Strabismus, a novel gene that regulates tissue polarity and cell fate decisions in Drosophila. Development. 1998;125:1149–1159. doi: 10.1242/dev.125.6.1149. [DOI] [PubMed] [Google Scholar]
- Wu J, Roman AC, Carvajal-Gonzalez JM, Mlodzik M. Wg and Wnt4 provide long-range directional input to planar cell polarity orientation in Drosophila. Nat Cell Biol. 2013;15:1045–1055. doi: 10.1038/ncb2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynshaw-Boris A. Dishevelled: in vivo roles of a multifunctional gene family during development. Curr Top Dev Biol. 2012;101:213–235. doi: 10.1016/B978-0-12-394592-1.00007-7. [DOI] [PubMed] [Google Scholar]
- Yanfeng WA, Berhane H, Mola M, Singh J, Jenny A, Mlodzik M. Functional dissection of phosphorylation of Disheveled in Drosophila. Dev Biol. 2011;360:132–142. doi: 10.1016/j.ydbio.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Fortini ME. Apoptotic activities of wild-type and Alzheimer's disease-related mutant presenilins in Drosophila melanogaster. J Cell Biol. 1999;146:1351–1364. doi: 10.1083/jcb.146.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Zhang J, Carthew RW. frizzled regulates mirror-symmetric pattern formation in the Drosophila eye. Development. 1995;121:3045–3055. doi: 10.1242/dev.121.9.3045. [DOI] [PubMed] [Google Scholar]
- Zielke N, Querings S, Grosskortenhaus R, Reis T, Sprenger F. Molecular dissection of the APC/C inhibitor Rca1 shows a novel F-box-dependent function. EMBO Rep. 2006;7:1266–1272. doi: 10.1038/sj.embor.7400851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.