Abstract
Adenosine A2a receptor (A2aR) signaling acts as a barrier to autoimmunity by promoting anergy, inducing regulatory T cells (Tregs), and inhibiting effector T cells. However, in vivo effects of A2aR signaling on polyclonal CD4 T cells during a primary response to foreign antigen has yet to be determined. To address this problem, we immunized mice with peptide antigen 2W1S coupled to PE in CFA and treated with the selective A2aR agonist CGS-21680 (CGS). 2W1S:I-Ab-specific tetramer-binding CD4 T cells did not become anergic or differentiate into Foxp3+ Tregs. Additionally, CGS treatment did not inhibit Th1 or Th17 differentiation. However, CGS did abrogate germinal center (GC) T follicular helper (Tfh) cells, and blunted PE-specific GC B cell responses. The use of A2aR-deficientCD4 T cells established that this CGS effect was T cell-intrinsic. Therefore, this study has identified a unique role for A2aRs in regulating CD4 T cell differentiation during vaccination.
Introduction
Adenosine is a purine nucleoside that regulates a broad spectrum of physiological responses via four different G coupled-protein receptors (A1, A2a, A2b, and A3). Adenosine A2a receptors (A2aRs) are expressed primarily on cells of hematopoietic origin, particularly on activated cytotoxic CD8 and helper CD4 T cells (1,2,3). Studies using mouse models of T cell-mediated autoimmune disorders (4,5) as well as graft-versus-host disease (6) have shown that A2aR signaling can restore immune homeostasis by promoting the induction of T cell anergy and regulatory T cell (Treg) differentiation. Loss of A2aR signaling in Adora2a-deficient mice also leads to enhanced control of tumor growth by CD8 T cells (7). In vitro polarizing assays suggest that A2aR signaling alters CD4 T cell differentiation by inhibiting the induction of Th1 (Tbet+), Th2 (GATA3+), and Th17 (RORγt+) effector T cell phenotypes (8,9). In vivo models of asthma (10), autoimmunity (4,5), and infection (11) also suggest that A2aR signaling can reduce Th1 and Th17 responses. However, CD4 T cell differentiation during a primary response to foreign antigen in the context of vaccination has not been assessed.
Most investigations of A2aRs to date have correlated changes in disease outcome in vivo with the in vitro modulation of signaling in cloned T cells, TCR-transgenic T cells, or bulk primary T cells. None the less, Zarek et al. (4) took advantage of hemagglutin in and pigeon cytochrome c peptide-specific TCR-transgenic CD4 T cells made deficient for A2aRs (Adora2a–/–) to show in vivo that both endogenous adenosine as well as an A2aR agonist can act to inhibit dangerous effector responses to an experimental self antigen and promote the development of anergy and Tregs. More recently, Shehade et al.(11) found that activation of A2aRs during acute infection with Toxoplasma gondii reduced the number of endogenous bulk IFN-γ producing CD4 T cells from secondary lymphoid organs. In cancer, an investigation of tumor antigen-specific CD8 polyclonal T cells in Adora2a-deficient mice has also indicated that endogenous adenosine limits their clonal expansion in response to tumor antigen (7). However, A2aR-mediated regulation of endogenous polyclonal antigen-specific CD4 T cells remains unknown.
To investigate the in vivo effects of A2aR signaling on naïve polyclonal CD4 T cells during a primary response to foreign antigen we utilized a vaccination approach that allowed us to track antigen-specific CD4 T cells that differentiate into various T cell lineages such as Th1, Th17, Tregs, and follicular helper T cells (Tfh). No reports to date have indicated a role for A2aR signaling in Tfh differentiation despite the role of other purinergic receptors such as P2X7 in regulating Tfh homeostasis (12). It is possible that A2aR mediated effects on Tfh cells may have been overlooked due a requirement of antigen specificity between T cells and B cells during Tfh differentiation (13). To address this we utilized a vaccine that consists of 2W1S peptide covalently coupled to phycoerythrin (PE) (14). This allowed us to look at the interplay between endogenous antigen specific 2W1S:I-Ab-specific T cells and PE-specific B cells (14). We initially sought to characterize polyclonal antigen-specific CD4 T cell anergy induction and Treg generation during A2aR signaling by tracking of 2W1S:I-Ab tetramer-binding T cells in mice treated with the selective A2aR agonist CGS-21680 (CGS). We discovered that CGS has no impact on the antigen-induced clonal expansion of polyclonal 2W1S-specific CD4 T cells, nor does it promote the induction of anergy or Treg differentiation in our vaccination system. CGS did not appear to reduce Th1 or Th17 differentiation; instead, A2aR signaling directly inhibited 2W1S:I-Ab-driven CD4 T cell differentiation toward the germinal center (GC)-Tfh fate, and reduced cognate GC B cell responses. Therefore, this work identifies a novel A2aR-mediated control mechanism during vaccination that regulates CD4 T differentiation and function.
Materials and Methods
Mice
B6 (WT) mice were purchased from Charles River Breeding Laboratories under a contract from the National Cancer Institute (Frederick, MD). Adora2af/f mice containing loxP sites on either side of exon 2 of the Adora2a gene (a gift from Joel Linden, La Jolla Institute for Allergy and Immunology, La Jolla, CA) (3) were crossed with CD4-Cre mice (a gift from Michael Farrar, University of Minnesota, Minneapolis, MN) to generate conditional A2aR T cell knockout (KO) mice. Non-Cre littermates were used as WT controls. Mice were bred and housed in specific-pathogen free conditions in animal facilities at the University of Minnesota, Twin Cities. All experimental protocols were performed in accordance with guidelines of the University of Minnesota Institutional Animal Care and Use Committee and the National Institutes of Health.
Immunization and selective A2aR agonist treatment
Mice were given an intraperitoneal (i.p.) vaccine containing 200 μl of 0.6 μg of 2W1S peptide conjugated to 25 μg of PE emulsified in Complete Freund's Adjuvant (CFA) (Sigma-Aldrich) as previously described (14). Mice were then given a 7d course of twice daily i.p. injection with the selective A2aR agonist, CGS-21680 (CGS; Tocris) 2.5 mg/kg or with vehicle alone (PBS) as previously described (4).
Cell enrichment and flow cytometry
Lymph nodes (LN) and spleens were collected and divided for separate enrichments of 2W1S:I-Ab tetramer-specific CD4 T cells and PE-specific B cells. 2W1S:I-Ab APC-labeled tetramers were used to stain and enrich for 2W1S-specific CD4 T cells (14). PE B cell enrichment was performed by mixing cell suspensions with 1 μg of PE (ProZyme) (14). Isolation of PE-specific B cells and for 2W1S:I-Ab-specific CD4 T cells was done using magnetic beads (STEM cell Technologies) (14). Enriched 2W1S T cells were first surface stained with CXCR5 (2G8), PD-1 (J43), CD4 (RM4-5), CD44 (IM7), as well as with the irrelevant cell exclusion antibodies CD11c (N418), B220 (RA3-6B2), CD8 (53-6.7), and F4/80 (BM8), and then fixed/permeabilized using a fixation/permeabilization kit (eBioscience) followed by intracellular staining with Foxp3 (FJK-16s), Tbet (4B10), Bcl6 (K112-91), RORγt (Q31-378), and Ki67 (SoA15). Enriched PE-specific B cells were surface stained with B220 (RA3-6B2), GL7 (GL-7), CD38 (90), IgM (RMM-1), and IgD (11-26c.2a), as well as with the irrelevant cell exclusion antibodies CD11c (N418), CD4 (GK1.5), CD8 (53-6.7), and F4/80 (BM8), and then they were fixed/permeabilized using a fixation/permeabilization kit (eBioscience) and intracellular stained with goat anti-mouse Ig (H+L) (A11068). Anergy in 2W1S:I-Ab-specific CD4 T cells T cells was assessed by staining with CD73 (TY11.8) and folate receptor 4 (FR4, 12A5) as previously outlined (15, 16). Cells were analyzed on a Fortessa (Becton Dickinson) flow cytometer and analyzed using FlowJo (TreeStar).
Statistical analysis
Statistical tests were performed using Prism (GraphPad) software, and p values were obtained using an unpaired one-tailed Student's t-test with a 95% confidence interval.
Results
Activation of adenosine A2a receptors during the primary response to vaccination fails to induce anergy or promote the differentiation of Foxp3+ Tregs
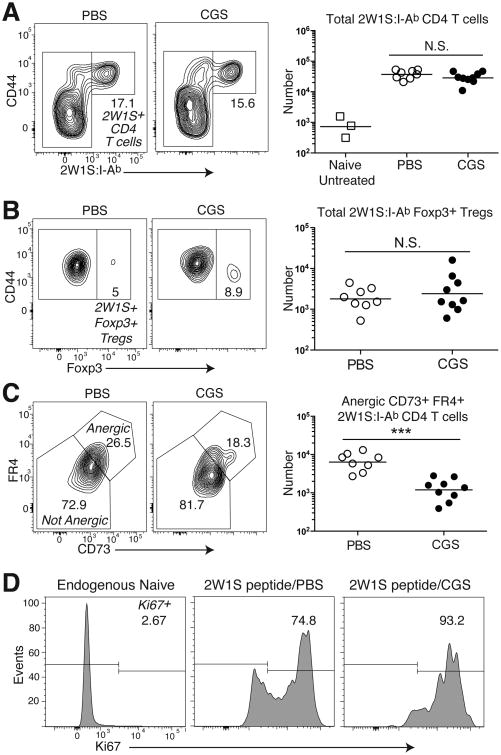
The activation of A2aRs in many biological systems is associated with the production of intracellular cAMP, a second-messenger known for its anti-proliferative function (17). To investigate the effects of A2aR signaling during the primary CD4 T cell response to antigen, we used a tetramer of the major his to compatibility complex II I-Ab molecule containing the 2W1S peptide to study the in vivo proliferation and differentiation of polyclonal 2W1S:I-Ab-specific CD4 T cells following immunization with a 2W1S peptide coupled to PE in CFA. This vaccination approach induces 2W1S:I-Ab-specific CD4 T cells to undergo clonal expansion and differentiation to Th1, Th17, Tregs, and Tfh lineages (14). Coupling 2W1S and PE together also allows for the interplay between 2W1S-specific GC-Tfh cells and PE-reactive GC B cell. This occurs when PE-specific B cells internalize 2W1S-PE through BCR recognition of PE resulting in the display 2W1S:I-Ab complexes, and allows them to exchange helper signals with 2W1S:I-Ab–specific CD4 T cells (14). The effects of A2aR activation were tested by treating immunized mice twice daily with the selective A2aR agonist CGS (2.5 mg/kgi.p.) or vehicle alone as a control (PBS) (4). As shown in figure 1A, the clonal expansion of the 2W1S:I-Ab-specific polyclonal CD4 T cell population as a whole was no different in CGS-or PBS-treated mice. Consistent with preserved clonal expansion, we also observed no increase in the number of 2W1S:I-Ab-specific Foxp3+ Tregs during this immunization in the presence of CGS (Fig. 1B). Therefore, A2aR signaling did not inhibit clonal expansion nor did it enhance Treg cell differentiation after vaccination with antigen in CFA.
Figure 1. A2aR signaling using the selective agonist CGS-21680 does not promote anergy or Treg induction during primary immunization.
B6 mice were immunized with 2W1S-PE and subsequently given a 7d treatment course of the selective A2aR agonist CGS 2.5 mg/kg or vehicle alone (PBS). (A) The frequency and number of spleen and LN 2W1S:I-Ab-specific CD4 T cells (with naïve untreated mice shown for reference). (B) Foxp3+ Tregs within the 2W1S:I-Ab-specific CD4 T cell compartment. (C) CD73+ FR4+ anergic-phenotype cells within the conventional Foxp3– 2W1S:I-Ab-specific CD4 T cell population. (D) Ki67 expression in conventional Foxp3– 2W1S:I-Ab-specific CD4 T cells 7d after 2W1S-PE immunization in the presence of CGS (2W1S peptide/CGS) or vehicle alone (2W1S peptide/PBS) (with endogenous naive polyclonal CD44lo CD4 T cells shown as a control). Data are representative from three independent experiments (n=8-9 mice per group). *P < 0.05, **P < 0.01, and ***P < 0.001
We next examined the capacity of A2aR signaling to promote anergy in 2W1S:I-Ab-specific polyclonal CD4 T cells during vaccination. To test the effects of A2aR signaling on the development of anergy, we examined two surface molecules whose high gene expression marks functionally unresponsive anergic T cells: CD73 (Nt5e) and FR4 (Izumo1r) (15, 16). Remarkably, CGS treatment led to a reduction rather than rise in the number of 2W1S-specific CD4 T cells that expressed high levels of these two anergy markers (Fig. 1C). Also consistent with an inability to promote tolerance in this system, CGS treatment led to an increase in the fraction of 2W1S:I-Ab-specific CD4 T cells that continued to express high levels of the proliferative marker Ki67at day 7 (Fig. 1D). Taken together, these data indicated that A2aR signaling cannot inhibit the proliferation of polyclonal antigen-specific CD4 T cells during antigen priming in the presence of a strong adjuvant, and does not promote Treg generation or anergy induction.
Activation of adenosine A2a receptors interferes with the differentiation of Tfh and GC-Tfh cells during antigen priming
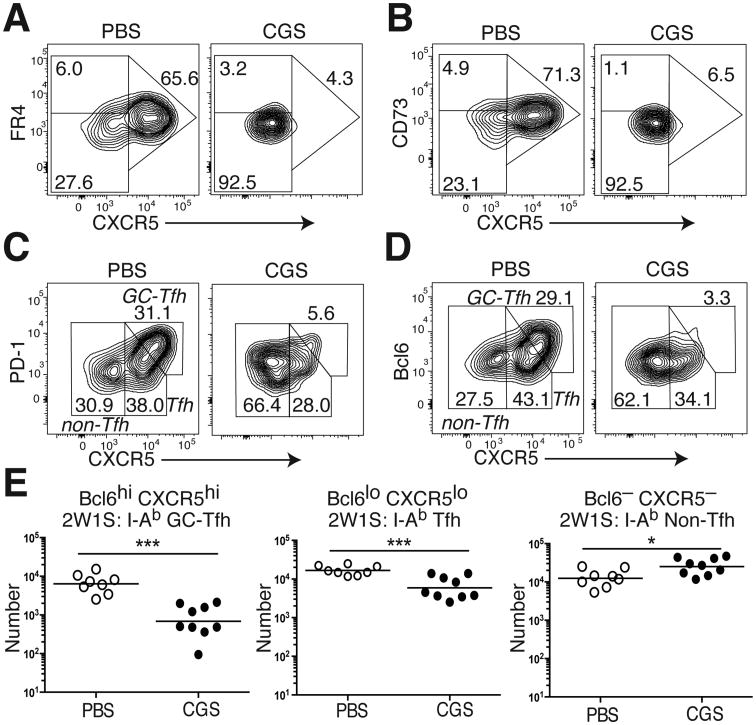
Paradoxically, primed CD4 T cells in CGS-treated mice expressed even lower levels of the anergy markers CD73 and FR4 than mice exposed to vehicle alone (Fig. 1C). Previous studies have demonstrated moderate levels of FR4 and CD73 expressed on Tfh cells (18). Although the role of these two molecules in Tfh generation and function remains unknown, this observation led us to the hypothesis that A2aR signaling interferes with Tfh differentiation. Mice primed with 2W1S in CFA did develop a large population of CXCR5+ 2W1S:I-Ab-specific CD4 T cells that expressed moderate levels of FR4 and CD73 (Fig. 2A,B). In contrast, treatment of primed mice with CGS led to a loss in the generation of this FR4int CD73int CXCR5+ 2W1S:I-Ab-specific CD4 T cell subset.
Figure 2. A2aR activation reduces Tfh and GC-Tfh differentiation.
2W1S:I-Ab tetramer-binding T cells were recovered from the spleen and LNs of 2W1S-PE immunized WT B6 mice after 7d of treatment with either CGS or vehicle alone (PBS). (A) FR4 and CXCR5, (B) CD73 and CXCR5, (C) PD-1 and CXCR5, and (D) Bcl6 and CXCR5 staining in 2W1S:I-Ab-specific CD4 T cells from CGS- or PBS-treated immunized mice. (E) Aggregate numbers of Bcl-6hi CXCR5hi GC-Tfh, Bcl-6lo CXCR5lo Tfh, and Bcl-6– CXCR5– non-Tfh cells that bind the 2W1S:I-Ab tetramer. Data are representative of three independent experiments (n=8-9 mice). *P < 0.05, **P < 0.01, and ***P < 0.001
To more formally assess A2aR-regulated Tfh differentiation, we investigated the expression of the cell surface marker PD-1 and the transcription factor Bcl6 in primed 2W1S:I-Ab-specific CD4 T cells (19,20). CD4T cells that express the highest levels of CXCR5, Bcl6, and PD-1 have been characterized as GC-Tfh cells (Bcl6hi CXCR5hi) and are known to provide cognate help to antigen-specific B cells within germinal centers, whereas CD4 T cells expressing lower levels are characterized as Tfh cells (Bcl6lo CXCR5lo) and reside at the T cell/B cell border (21). During primary immunization, CGS treatment reduced both the frequency and number of 2W1S:I-Ab-specific Tfh and GC-Tfh cells (Fig. 2C-E). Consistent with a shift toward alternate differentiation fates, CGS treatment also elicited a small but significant increase in the number of 2W1S:I-Ab-specific non-Tfh cells. Therefore, our experiments revealed a novel role for A2aR pathway activation in the inhibition of Tfh and GC-Tfh cell differentiation.
A2aR inhibition of Tfh differentiation is T cell intrinsic
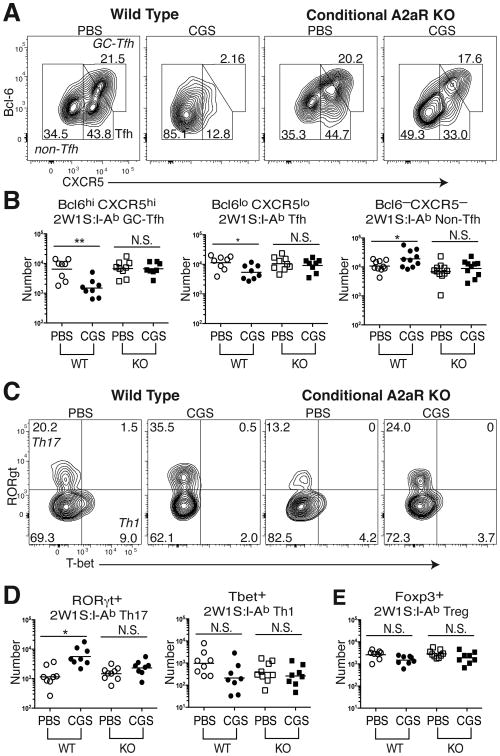
Although A2aR expression is known to be highly induced on CD4 T cells following TCR ligation (1-3), the expression of this adenosine receptor might also be expected on other cells of hematopoietic origin. Additionally, Tfh cell differentiation is a multifactorial process whose regulation likely involves multiple additional cell types, particularly dendritic cells and B cells (19-21). Therefore, it was important to determine whether the effects of CGS were the result of direct A2aR engagement on the 2W1S:I-Ab-specific CD4 T cells. To address this question, CD4-Cre Adora2af/f conditional knock-out (KO) mice lacking A2aRs only on their T cells were immunized with 2W1S-PE and compared to wild type (WT) A2aR-expressing littermates following a 7d course of CGS treatment. In the absence of T cell-expressed A2a receptors, CGS treatment lost its capacity to reduce Bcl6 expression and block 2W1S:I-Ab-specific GC-Tfh differentiation, and its inhibitory effects on Tfh cells appeared greatly blunted (Fig. 3A,B). Likewise, treatment of CD4-Cre Adora2af/f mice with CGS failed to induce an increase in non-Tfh cells during 2W1S antigen priming. Given that Bcl6 promotes differentiation to the Tfh and GC-Tfh fates in part by repressing other lineage-specific transcription factors such as Tbet and RORγt (19, 20), we further assessed these non-Tfh cells. WT mice significantly increased the frequency and number of 2W1S:I-Ab-specific RORγt+ Th17 cells when their A2aRs were directly bound by the adenosine agonist, whereas Tbet+ Th1 and Foxp3+ Treg differentiation appeared not to be regulated by A2aR signaling (Fig. 3C-E). A small, but statistically insignificant increase in RORγt+ Th17 cells was also observed in KO mice treated with CGS (Fig. 3C-E). Thus, direct CD4 T cell A2aR signaling shifts the balance of differentiation away from the GC-Tfh fate toward Th17 effector cell generation during the primary response to antigen.
Figure 3. A2aR inhibition of GC-Tfh differentiation is T cell intrinsic.
2W1S:I-Ab tetramer-bound CD4 T cells were enriched from spleen and LNs of CD4-Cre Adora2af/f conditional knock-out (KO) mice as well as non-Cre expressing WT littermates after 2W1S-PE immunization and a 7d course of either CGS or PBS treatment. (A, B) Frequency (A) and number (B) of 2W1S-specific CD44hi Foxp3– CD4 T cell subsets:Bcl6hi CXCR5hi GC-Tfh, Bcl6lo CXCR5lo Tfh, and Bcl-6– CXCR5– non-Tfh cells. (C, D) Frequency (C) and number (D) of Th17 (RORγt+ Tbet–) and Th1 (RORγt– Tbet+) lineage cells within the non-Tfh fraction of 2W1S:I-Ab tetramer-binding CD4 T cells. (E) 2W1S-specific Foxp3+ Treg numbers. Data are representative of three independent experiments (n=8-9). *P < 0.05, **P < 0.01, and ***P < 0.001
T cell-intrinsic A2aR activation reduces T-dependent B cell immunity
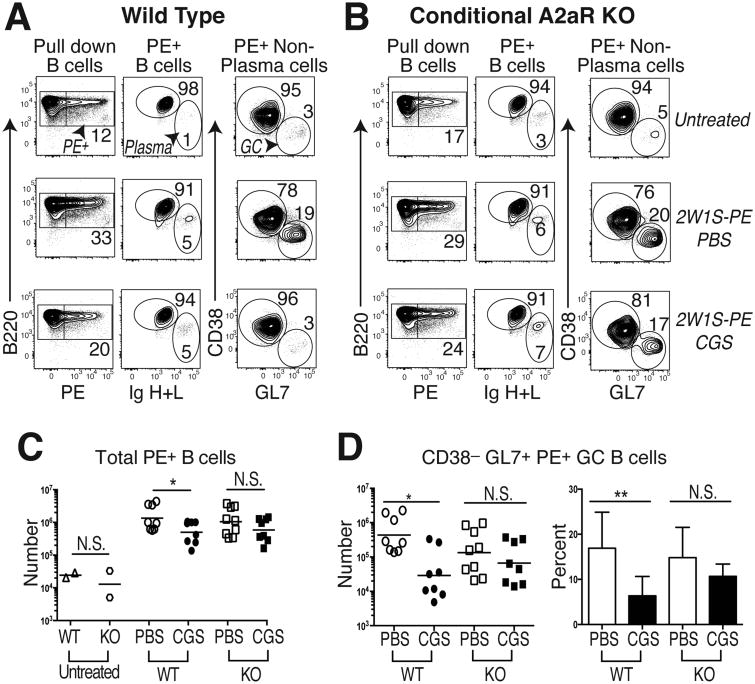
GC-Tfh cells promote the survival, differentiation, isotype class switch, and affinity maturation of antigen-specific B cells in germinal centers (21). Therefore, we hypothesized that A2aR signaling in T cells during primary immunization would interfere with the T-dependent B cell response to vaccination. To test this, WT and CD4-Cre Adora2af/f mice (KO) mice were immunized with a protein complex containing 2W1S coupled to PE (2W1S-PE) either with or without CGS treatment, and then PE-specific B cells were enriched using magnetic beads and characterized by flow cytometry. An ∼50-fold expansion of PE-specific B cells was seen in WT and KO PBS-treated mice following immunization, as compared to naïve mice (Fig. 4A-C). Consistent with the hypothesis, the number of PE-specific B cells found after vaccination was significantly reduced in CGS-treated WT hosts, but not in mice whose CD4 T cells lacked A2aRs. CGS treatment appeared to have its greatest inhibitory effect on the frequency and number of GC-phenotype CD38– GL7+ PE-binding B cells in the WT mice, although all B cell subpopulations were affected (Fig. 4A-D). Importantly, these inhibitory effects of CGS on PE-specific B cells during immunization were blunted in mice that lacked Adora2a gene expression specifically within the T cell compartment. Therefore, these data suggest that the loss of antigen-specific GC-Tfh differentiation that occurs during strong A2aR signaling on CD4 T cells is sufficient to abrogate the provision of cognate T cell help to GC B cells during their primary response to antigen.
Figure 4. T cell A2aR activation reduces GC B cell immunity.
PE-specific B cells were enriched from the spleen and LNs of 2W1S-PE primed WT or CD4-Cre Adora2af/f conditional knock-out (KO) mice given a 7d course of CGS or the PBS vehicle alone. (A) Gating strategy to identify PE-specific B220+ total B cells (left column), B220intermediate intracellular Ig (H+L)hi plasma cells (middle column), as well as intracellular Ig (H+L)intermediate CD38– GL7+ GC B cells (right column) in control untreated (upper row), 2W1S-PE immunized and PBS-treated (middle row), and 2W1S-PE immunized and CGS-treated (lower row) WT mice. (B) Representative KO mice treated as in (A). (C) Absolute numbers of total PE-specific B cells in WT and KO mice treated as in (A) and (B), with untreated mice shown as a control. (D) Absolute numbers and frequency of PE-specific polyclonal CD38– GL7+ GC B cells in immunized WT and KO mice treated as in (A) and (B). Data are representative of three independent experiments (n=8-9 mice). *P < 0.05, **P < 0.01, and ***P < 0.001
Discussion
The identification of novel signaling elements that fine-tune CD4 T cell lineage differentiation during primary immunization offers new opportunities to improve the efficacy of vaccines and targeted immunotherapies (22, 23, 24). Our data suggest that in vivo A2aR signaling during the primary response to antigen plus a strong adjuvant diverts CD4 T helper cells away from the Tfh and GC-Tfh lineages. Given the key role of alternative T differentiation fates such as Th17 in protection against mucosal barrier infections (22), it is conceivable that selective A2aR agonists could be useful during vaccination to shape an optimal T differentiation response against pathogens.
A2aRs also appear to be a particularly attractive therapeutic target for the treatment of B cell-dependent autoimmune disorders such as systemic lupus erythematosus (25) and rheumatoid arthritis (RA) (26). Indeed, previous studies have shown a positive correlation between the number of Tfh cells and disease burden in patients with RA (26). Perhaps consistent with this, A2aR agonists effectively suppress animal models of inflammatory arthritis (27). The fact that the first-line anti-rheumatic drugs methotrexate and sulfasalazine act, in part, through the generation of extracellular adenosine and A2aR signaling (28, 29), lends further support to the notion that A2aR signaling can ameliorate T-dependent B cell autoreactivity.
It should be noted that the ablation of A2aRs in these vaccination experiments using conditional knock-out mice failed to significantly enhance antigen-specific Tfh or GC-Tfh differentiation. Similarly, use of a selective antagonist of A2aRs during immunization did not reliably alter CD4 T cell differentiation (data not shown). Although strong A2aR signaling with an agonist can be inhibitory for Tfh and GC-Tfh differentiation in normal CD4 T cells, under normal circumstances endogenous extracellular adenosine may play no role in CD4 T cell fate selection within secondary lymphoid organs following i.p. immunization in adjuvant. Alternatively, unspecified factors (e.g., increased A2bRs, decreased adenosine kinase) may compensate for the loss of A2aRs in KO mice. Activated T cells are known to upregulate adenosine deaminase (ADA), an enzyme capable of metabolizing adenosine to inosine (30). Therefore, strong continuous A2aR signaling from endogenous adenosine sources may only occur under special circumstances such as hypoxia where CD73is up-regulated to facilitate increased extracellular adenosine production in close proximity to A2a receptors (31-33). Going forward, it will be important to identify the immunological context (spatial and temporal) whereby extracellular adenosine counter-regulates Tfh and GC-Tfh differentiation.
Acknowledgments
We thank Dr. Joel Linden from the La Jolla Institute for Allergy and Immunology for the Adora2af/f mice as well as Dr. Michael Farrar from the University of Minnesota for the CD4-Cre mice. We would also like to thank members of the Mueller and Jenkin's lab.
This work was supported by National Institutes of Health Grants: P01AI035296 (to D.L.M. and M.K.J.), and NIH Institutional Pre-Doctoral Training Grant: T32AI007313 (to S.S.), and by a research grant from the Lupus Foundation of Minnesota (to D.L.M.).
Abbreviations
- B6
C57BL/6
- A2aR
adenosine 2a receptors
- FR4
folate receptor 4
- GC
germinal center
- CGS
CGS-21680
- LN
lymph node
- Tfh
T follicular helper
- Treg
regulatory T cell
References
- 1.Streitová D, Sefc L, Savvulidi F, Pospísil M, Holá J, Hofer M. Adenosine A(1), A(2a), A(2b), and A(3) receptors in hematopoiesis. 1. Expression of receptor mRNA in four mouse hematopoietic precursor cells. Physiol Res. 2010;59:133–137. doi: 10.33549/physiolres.931723. [DOI] [PubMed] [Google Scholar]
- 2.Koshiba M, Rosin DL, Hayashi N, Linden J, Sitkovsky MV. Patterns of A2A extracellular adenosine receptor expression in different functional subsets of human peripheral T cells. Flow cytometry studies with anti-A2A receptor monoclonal antibodies. Mol Pharmacol. 1999;55:614–624. [PubMed] [Google Scholar]
- 3.Cekic C, Sag D, Day Y, Linden J. Extracellular adenosine regulates naive T cell development and peripheral maintenance. J Exp Med. 2013;210:2693–706. doi: 10.1084/jem.20130249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, Drake CG, Powell JD. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;11:251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB. Cutting edge: Critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol. 2006;177:2765–2769. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 6.Sevigny CP, Li L, Awad AS, Huang L, McDuffie M, Linden J, Lobo PI, Okusa MD. Activation of adenosine 2A receptors attenuates allograft rejection and alloantigen recognition. J Immunol. 2007;178:4240–4249. doi: 10.4049/jimmunol.178.7.4240. [DOI] [PubMed] [Google Scholar]
- 7.Cekic C, Linden J. Adenosine A2A receptors intrinsically regulate CD8+ T cells in the tumor microenvironment. Cancer Res. 2014;74:7239–7249. doi: 10.1158/0008-5472.CAN-13-3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 9.Liang D, Zuo A, Shao H, Chen M, Kaplan HJ, Sun D. Anti-inflammatory or proinflammatory effect of an adenosine receptor agonist on the Th17 autoimmune response is inflammatory environment-dependent. J Immunol. 2014;193:5498–5505. doi: 10.4049/jimmunol.1401959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Wan H, Tang W, Ni Y, Hou X, Pan L, Song Y, Shi G. Critical roles of adenosine A2A receptor in regulating the balance of Treg/Th17 cells in allergic asthma. Clin Respir J. 2016 doi: 10.1111/crj.12503. [DOI] [PubMed] [Google Scholar]
- 11.Francois V, Shehade H, Acolty V, Preyat N, Delrée P, Moser M, Oldenhove G. Intestinal immunopathology is associated with decreased CD73-generated adenosine during lethal infection. Mucosal Immunol. 2015;8:773–784. doi: 10.1038/mi.2014.108. [DOI] [PubMed] [Google Scholar]
- 12.Proietti M, Cornacchione V, Rezzonico Jost T, Romagnani A, Faliti CE, Perruzza L, Rigoni R, Radaelli E, Caprioli F, Preziuso S, Brannetti B, Thelen M, Mc Coy KD, Slack E, Traggiai E, Grassi F. ATP-gated ionotropic P2X7 receptor controls follicular T helper cell numbers in peyer's patches to promote host-microbiota mutualism. Immunity. 2014;41:789–801. doi: 10.1016/j.immuni.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang JA, Tubo NJ, Gearhart MD, Bardwell VJ, Jenkins MK. Cutting Edge: Bcl6-interacting corepressor contributes to germinal center T follicular helper cell formation and B cell helper function. J Immunol. 2015;194:5604–5608. doi: 10.4049/jimmunol.1500201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalekar LA, Schmiel SE, Nandiwada SL, Lam WY, Barsness LO, Zhang N, Stritesky GL, Malhotra D, Pauken KE, Linehan JL, O'Sullivan MG, Fife BT, Hogquist KA, Jenkins MK, Mueller DL. CD4(+) T cell anergy prevents autoimmunity and generates regulatory T cell precursors. Nature Immunol. 2016;17:304–314. doi: 10.1038/ni.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez R, Zhang N, Thomas S, Nandiwada SL, Jenkins MK, Binstadt BA, Mueller DL. Arthritogenic self-reactive CD4+ T cells acquire an FR4hi CD73hi anergic state in the presence of Foxp3+ regulatory T cells. J Immunol. 2012;188:170–181. doi: 10.4049/jimmunol.1101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erdmann AA, Gao Z, Jung U, Foley J, Borenstein T, Jacobson KA, Fowler DH. Activation of Th1 and Tc1 cell adenosine A2A receptors directly inhibits IL-2 secretion in vitro and IL-2-driven expansion in vivo. Blood. 2005;105:4707–4714. doi: 10.1182/blood-2004-04-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iyer SS, Latner DR, Zilliox MJ, McCausland M, Akondy RS, Penaloza-Macmaster P, Hale JS, Ye L, Mohammed AU, Yamaguchi T, Sakaguchi S, Amara RR, Ahmed R. Identification of novel markers for mouse CD4(+) T follicular helper cells. Eur J Immunol. 2013;43:3219–3232. doi: 10.1002/eji.201343469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatzi K, Nance JP, Kroenke MA, Bothwell M, Haddad EK, Melnick A, Crotty S. BCL6 orchestrates Tfh cell differentiation via multiple distinct mechanisms. J Exp Med. 2015;212:539–553. doi: 10.1084/jem.20141380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Nurieva R, Dong C. Transcriptional regulation of follicular T-helper (tfh) cells. Immunol Rev. 2013;252:139–145. doi: 10.1111/imr.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitano M, Moriyama S, Ando Y, Hikida M, Mori Y, Kurosaki T, Okada T. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity. 2011;34:961–972. doi: 10.1016/j.immuni.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 22.Tubo N, Pagán A, Taylor J, Nelson RW, Linehan JL, Ertelt JM, Huseby ES, Way SS, Jenkins MK. Single naive CD4+ T cells from a diverse repertoire produce different effector cell types during infection. Cell. 2013;153:785–796. doi: 10.1016/j.cell.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malhotra D, Linehan JL, Dileepan T, Lee YJ, Purtha WE, Lu JV, Nelson RW, Fife BT, Orr HT, Anderson MS, Hogquist KA, Jenkins MK. Tolerance is established in polyclonal CD4(+) T cells by distinct mechanisms, according to self-peptide expression patterns. Nature Immunol. 2016;17:187–195. doi: 10.1038/ni.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong C, Linderman JJ, Kirschner D. Harnessing the Heterogeneity of T Cell Differentiation Fate to Fine-Tune Generation of Effector and Memory T Cells. Front Immunol. 2014;5:57. doi: 10.3389/fimmu.2014.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terrier B, Costedoat-Chalumeau N, Garrido M, Geri G, Rosenzwajg M, Musset L, Klatzmann D, Saadoun D, Cacoub P. Interleukin 21 correlates with T cell and B cell subset alterations in systemic lupus erythematosus. J Rheum. 2012;39:1819–1828. doi: 10.3899/jrheum.120468. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Shan Y, Jiang Z, Feng J, Li C, Ma L, Jiang Y. High frequencies of activated B cells and T follicular helper cells are correlated with disease activity in patients with new-onset rheumatoid arthritis. Clin Exp Immunol. 2013;174:212–20. doi: 10.1111/cei.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vincenzi F, Padovan M, Targa M, Corciulo C, Giacuzzo S, Merighi S, Gessi S, Govoni M, Borea PA, Varani K. A(2A) adenosine receptors are differentially modulated by pharmacological treatments in rheumatoid arthritis patients and their stimulation ameliorates adjuvant-induced arthritis in rats. PLoS One. 2013;8:e54195. doi: 10.1371/journal.pone.0054195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morabito L, Montesinos MC, Schreibman DM, Balter L, Thompson LF, Resta R, Carlin G, Huie MA, Cronstein BN. Methotrexate and sulfasalazine promote adenosine release by a mechanism that requires ecto-5′-nucleotidase-mediated conversion of adenine nucleotides. J Clin Invest. 1998;101:295–300. doi: 10.1172/JCI1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riksen NP, Barrera P, van den Broek PHH, van Riel PLCM, Smits P, Rongen GA. Methotrexate modulates the kinetics of adenosine in humans in vivo. Ann Rheum Dis. 2006;65:465–470. doi: 10.1136/ard.2005.048637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martín M, Huguet J, Centelles JJ, Franco R. Expression of ecto-adenosine deaminase and CD26 in human T cells triggered by the TCR-CD3 complex possible role of adenosine deaminase as costimulatory molecule. J Immunol. 1995;155:4630–4643. [PubMed] [Google Scholar]
- 31.Choukèr A, Thiel M, Lukashev D, Ward JM, Kaufmann I, Apasov S, Sitkovsky MV, Ohta A. Critical role of hypoxia and A2A adenosine receptors in liver tissue-protecting physiological anti-inflammatory pathway. Mol Med. 2008;14:116–123. doi: 10.2119/2007-00075.Chouker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatfield SM, Sitkovsky MV. A2A adenosine receptor antagonists to weaken the hypoxia-HIF-1α driven immunosuppression and improve immunotherapies of cancer. Curr Opin Pharmacol. 2016;29:90–6. doi: 10.1016/j.coph.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]