Abstract
Objectives
The tracheoesophageal puncture (TEP) restores verbal communication after total laryngectomy using a one-way valved voice prosthesis (VP). Microbial colonization can shorten VP device life. Our aims were to investigate patterns of prosthetic and oral colonization, and record changes in VP device life after targeted decontamination.
Materials and Methods
We conducted a retrospective review of TEP clinic patients who underwent microbial analysis of the VP between 01/2003 and 07/2013. Two subgroups were analyzed: 1) patients with microbial analysis of the VP and the mouth were analyzed to identify patterns of common contamination, and 2) patients who were prescribed targeted oral decontamination on the basis of the microbial analysis of the VP were analyzed to evaluate effects on device life.
Results
Among 42 patients, 3 patients had only fungal, 5 only bacterial, and 33 had polyspecies fungal and bacterial colonization. In the TEP-oral microflora subgroup (n=15), 7 had common microorganisms in the mouth and on the VP. Among the decontamination subgroup (n=23), 6 patients received broad spectrum rinse, 16 antifungal agents and 13 antibiotics, or a combination thereof. After targeted decontamination, the median device life of prostheses improved from 7.89 to 10.82 weeks (p=0.260).
Conclusion
The majority of patients with a suboptimal VP device life in this pilot had polyspecies bacterial and fungal colonization. VPs rarely had fungal contamination alone (3%), and non-albicans fungal species were more common than expected. For these reasons, we are exploring the use of targeted decontamination regimens that were associated with 1.4-fold improvement in VP duration.
Keywords: tracheoesophageal puncture, voice prosthesis, microbiological culture, culture and sensitivity, decontamination, device life, antifungal therapy, antibiotics
Introduction
For 15 to 40% of patients with locoregionally advanced or recurrent cancer of the larynx or hypopharynx, the definitive treatment modality is a total laryngectomy or laryngopharyngectomy, either as primary or salvage treatment. The consequences of surgical resection include loss of the natural laryngeal speaking voice and nasal function, along with changes in airway control and possible dysphagia that substantially impact quality of life among survivors [1]. A major emphasis of postsurgical rehabilitation is to restore voice after removal of the larynx.
Tracheoesophageal puncture (TEP) is a surgical procedure to reestablish speech after laryngectomy. In North America, tracheoesophageal (TE) voice restoration has been widely adopted since the TEP method was first published in 1979, with a success rate of >90% typically cited in cohorts of appropriately selected patients [2,3]. TEP necessitates lifelong use of a valved, silicone voice prosthesis (VP). The most commonly used TE VPs in the United States, Provox™ (Atos Medical AB, Hörby, Sweden) and Blom-Singer (InHealth Technologies, Carpinteria, CA, USA,) are indwelling type, silicone rubber devices with a low-resistance one-way valve mechanism. Indwelling style VPs are worn continuously and exchanged by a clinician when valve functions begin to fail. Average life of these devices is reported between 3–6 months, with a significant variability of several days to several years, based on patient characteristics and device features such as silicone material and valve strength [4–8]. Reasons for device replacement include leakage and various problems with the TEP site, such as enlargement, infection or granulation tissue formation [9]. Among the most prominent complications that decrease VP device life is the formation of microbial biofilm plaque and invasive growth into the silicone on the prosthesis valve that ultimately causes aspiration through the device or increased airflow resistance during speech [5,10].
The esophagus is a non-sterile environment, contiguous with the oral cavity and its residing microflora, the latter providing a constant source for biofilm and plaque formation on the VPs. Biofilms are highly organized bacterial or fungal colonies with a self-produced extracellular matrix and antimicrobial defense mechanisms. Biofilms can transfer resistance to microbials, act as a diffusion barrier and a shield against host defenses [11]. Biofilm-forming microbiological contaminants for VPs described in the literature have not changed significantly over the last three decades. Since the early 1980s, the majority of studies have implicated Candida species, typically albicans strains, as the most important pathogen causing device deterioration [4, 6, 10, 12–19]. Reported bacterial contaminants receive far less attention, and those reported mostly include commensal oral microflora, predominantly S. aureus, Pseudomonas sp., Enterobacter sp., Klebsiella sp., R. dentocariosa, and Proteus sp. [4, 5, 13–17, 20, 21]. However, a potential adhesive interaction between those and the colonizing Candida species, especially S. aureus, has been proposed [14, 16].
Nystatin remains the most frequently used pharmacologic strategy for early VP failures [22]. This practice is supported by evidence suggesting maximal efficacy of nystatin relative to other antifungal agents. For instance, a comparative study found that minimal inhibitory concentration values were narrowly distributed at lower concentration levels for nystatin, as opposed to broader ranges at higher concentration levels for miconazole and fluconazole [4]. More recent studies explore alternate decontamination therapies that include nutritional and other antifungal therapies, such as amphotericin B [12] and miconazole nitrate. Ultimately, frequent replacement of the deteriorated device is required when these therapies fail [6]. Many questions remain regarding the relationship between microbiota of the oral cavity and VP, and effects of decontamination regimens beyond use of antifungals. Our aims were to: 1) investigate patterns of prosthetic and oral colonization, 2) describe oral decontamination regimens recommended on the basis of microbial assessment, and 3) record changes in VP device life after targeted decontamination among patients with suboptimal VP device life.
Material and methods
Patients
The protocol for this retrospective pilot study was approved by the Institutional Review Board at the MD Anderson Cancer Center (DR08-0709). A waiver of informed consent was obtained. An unselected cohort of patients who underwent total laryngectomy and TEP at the University of Texas MD Anderson Cancer Center between 01/01/2003 and 07/01/2013 was identified. Patients who had valve problems precipitating microbial analysis of the voice prosthesis were included in the final analysis. Valve problems included suboptimal or declining prosthesis life (typically leakage through the prosthetic valve ≤8 weeks after fitting) or significant observable biofilm on the valve detectable on non-microscopic gross visual inspection of the prosthesis upon removal in clinic. All included patients had microbial analysis of the VP; specimens from the oral cavity and stoma were also examined when available.
Medical records were reviewed retrospectively for cancer treatment (demographics, the site and stage of underlying malignancy, disease status, type of surgical procedure, reconstruction and radiation), timing of TEP, reason of VP replacement, device life, and microbiological data related to the voice prosthesis, oral cavity or stoma and decontamination regimens. In addition, TEP VP device life was calculated. Two subgroups were analyzed: 1) patients with both VP and oral samples submitted for microbial analysis were examined to identify patterns of common contamination, and 2) patients who were prescribed targeted oral and systemic decontamination after microbial analysis of the VP were examined to evaluate effects on device life. The average device life was calculated for the three last prostheses pre-dating decontamination and the three first prostheses post-dating decontamination to minimize potential outliers but to focus on the prostheses immediately preceding and following decontamination.
All samples were transported at room temperature to the M D Anderson Microbiology laboratory for analysis. Specimens were typically received within 4 hours and incubating within six. Samples set up beyond these times were marked as delayed. All oral or stoma swabs were treated as wound culture specimens in terms of microbiology processing and the procedures used for the analysis. In our center these procedures place emphasis on recovery of respiratory tract pathogens and microorganisms commonly seen in opportunistic infections of cancer patients; including Streptococcus pneumoniae, Beta hemolytic streptococci, Gram negative rods, Staphylococcus aureus, Yeast, Nocardia, and molds. Further, if seen in high quantity, or as a predominant organism in a background of reduced normal species, then alpha hemolytic streptococci, enterococci, Corynebacterium spp, would be identified. The microbial ‘wound procedure’ set up also included a thioglycolate broth tube to facilitate the identification of anaerobes. Voice prosthesis devices were received dry, in sterile specimen cups and set up in a similar time frame as the above swab samples. VP were processed in a similar manner to the swabs above using semi-quantitate wound culture criteria, however, prior to media inoculation the device was suspended in 1 ml of Trypticase Soy Broth (BBL) and were either ‘Vortexed’ on high or sonicated in a Branson 2200 ultrasonic bath for 1 min before planting the eluted material on media. Organisms were given a preliminary identification using Gram stain morphology, colony morphology, rapid biochemicals (P disk, A disk, string test, oxidase, PYR disk, staph/aurex, odor, pigment, wet mount motility, spot indole, catalase, Van/Colistin disks, growth on differential and selective media, and standard biochemical protocols [25]. Detailed biochemical for species level identification was performed by on the Vitek II instrument GNI, GPI, and Yeast ID cards (bioMerieux, Box 15969 Durham, NC), and correlated with rapid biochemical and morphology. In unidentified cases or when discrepant findings were seen, identifications were made using 16s sequencing [23]. Yeast identifications were confirmed by correlated with cornmeal/Tween-80 agar (BBL, Becton Dickinson, 7 Loveton Circle, Sparks MD).
Decontamination
After the microbiological analysis of the biofilms, the patients were seen by the Oral Oncology Service at the MD Anderson Cancer Center. Based on the culture and sensitivity results, the patients were prescribed antibiotic, antifungal or broad spectrum decontaminants. This process was termed “targeted decontamination”. The patients were followed and if the symptoms and the VP device life did not improve, a new sample was taken and re-cultured.
Statistical analysis
Descriptive statistics were calculated. Patterns of microbial colonization were examined among all 42 patients included. Differences in device life-time before and after decontamination regimens were compared among the subgroup of 23 patients who underwent targeted decontamination using the Wilcoxon sign rank test, and further stratified by pre-decontamination device life (<6 weeks or ≥6 weeks). Statistical analysis was performed by the STATA version 10.0 (College Station, TX, USA). P-values of less than 0.05 were considered statistically significant.
Results
Patients
605 potentially eligible TEP patients were screened and 42 patients (33 male and 9 female) who had valve problems precipitating microbial analysis of the voice prosthesis were included in the final analysis (Table 1). Median patient age was 66 years, with a range of 37–86 years. The location of the treated tumors was glottic, subglottic or supraglottic in 78% of patients, mostly classified T3-4 or recurrent cancer. Eighty-three percent of patients underwent total laryngectomy with primary closure of the pharynx, and the remaining 17% percent required free flap reconstruction. Most (88%) had a history of radiotherapy, two patients had re-irradiation of the neck. The median time from TEP procedure to analysis of the 1st VP was 19.5 months. Five patients were wearing commercially available specialty valves when sent for analysis, 3 silver oxide impregnated silicone, 1 fluoroplastic coated silicone, and 1 customized by adding silicone glue to increase the weight of the valve seat.
Table 1.
Demographic and clinical characteristics in the series of 42 patients
| No. (%) | ||
|---|---|---|
| Sex | Male | 33 (78%) |
| Female | 9 (21%) | |
| Age | Median | 66 |
| Range | 37–86 | |
| Tumor site | Glottic/subglottic | 17 (40%) |
| Supraglottic | 16 (38%) | |
| Hypopharyngeal | 5 (12%) | |
| Unknown primary (function) | 2 (5%) | |
| Thyroid | 2 (5%) | |
| T classification | 0–2 | 5 (12%) |
| 3–4 | 20 (48%) | |
| Recurrent | 15 (36%) | |
| Unknown or N/A | 2 (5%) | |
| N classification | N0 | 10 (24%) |
| N+ | 15 (36%) | |
| Recurrent | 15 (36%) | |
| Unknown or N/A | 2 (5%) | |
| Surgical procedure | TL | 35 (81%) |
| TL + PP | 4 (10%) | |
| TLP | 4 (10%) | |
| Reconstruction | None | 32 (76%) |
| Pectoralis | 2 (5%) | |
| ALT (patch) | 3 (7%) | |
| ALT (tubed) | 2 (5%) | |
| Jejunal | 2 (5%) | |
| Radiation | None | 5 (12%) |
| Pre-operative RT | 11 (26%) | |
| Pre-operative chemoRT | 6 (14%) | |
| Post-operative RT | 12 (29%) | |
| Post-operative chemoRT | 6 (14%) | |
| Timing of TEP | Primary | 22 (53%) |
| Secondary | 17 (40%) | |
| Both | 3 (7%) | |
| TOTAL | 42 |
Microbiological findings
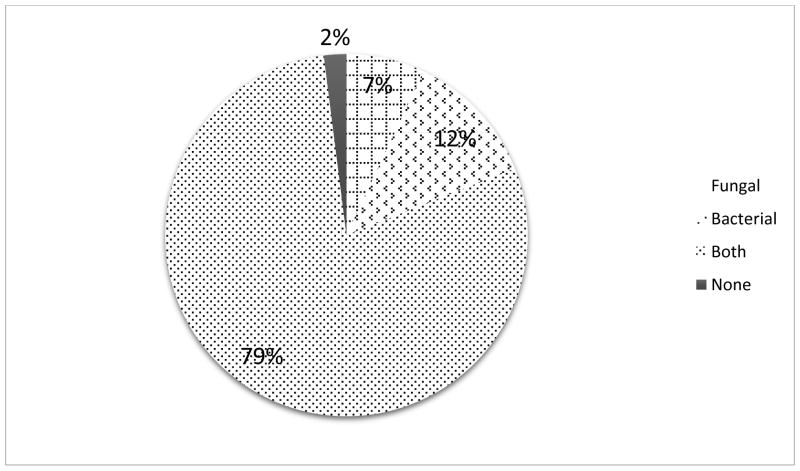
Of the 42 patients whose failed voice prostheses were analyzed, the initial culture results showed that 3 patients had only fungal, 5 only bacterial, 33 had polyspecies fungal and bacterial colonization and 1 had no detectable colonization (Figure 1).
Figure 1.
Distribution of microbiological flora of TEP voice prostheses in the analyzed 42 patients
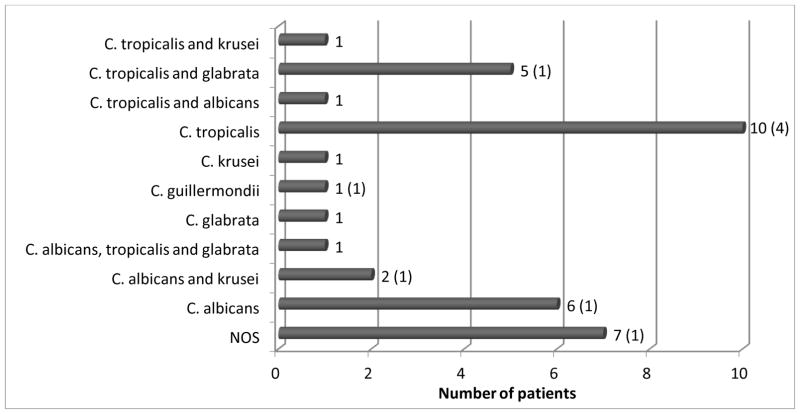
Thirty-six of 42 patients had some type of fungal colonization, single or polyspecies, which consisted of both C. albicans and non-albicans colonization, including C. tropicalis, C. glabrata and other multiple yeast species. C. tropicalis was the most common fungal strain (n=18), present almost twice as often as C. albicans (n=10). Nine patients presented with persisting Candida colonization despite nystatin therapy at the time of analysis (Figure 2).
Figure 2.
Distribution of single and multi-organism fungal colonization
The numbers in parenthesis show the number of patients infected with the microorganisms while using nystatin.
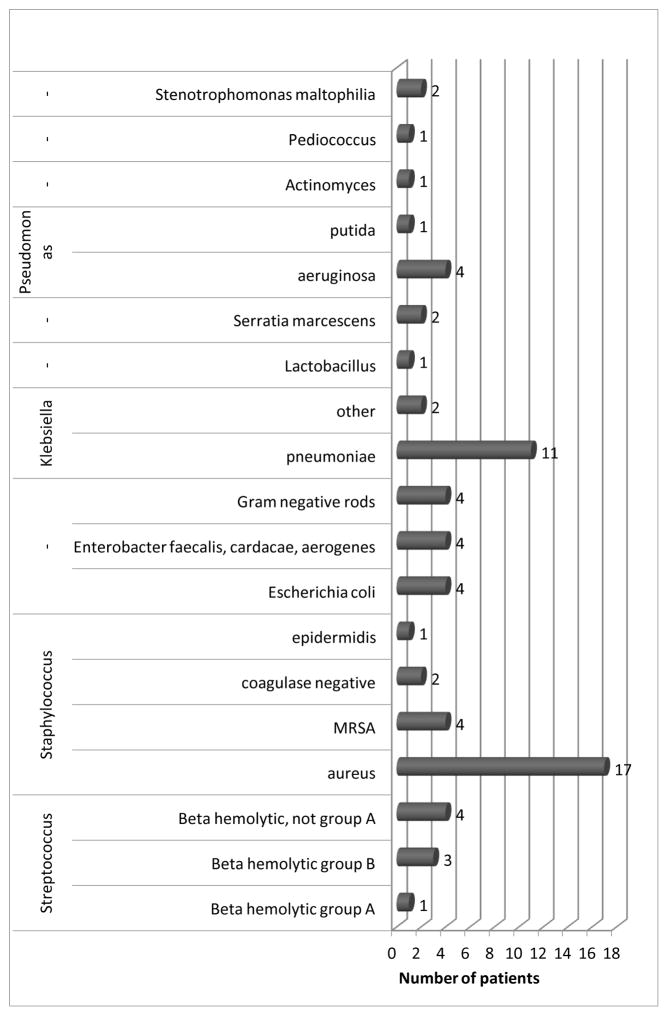
Forty-one of 42 had single or multi-bacterial colonization on the VP, mainly Staphylococci, such as S. aureus and methicillin resistant S. aureus (MRSA). Further microbial contaminants included Streptococci, Klebsiella and Pseudomonas (Figure 3).
Figure 3.
Distribution of species in bacterial colonization
Common microbiological findings in oral and VP specimens
Fifteen of the 42 patients had specimens for both VP and oral microflora. In this subgroup, 7 patients had common species in the mouth and on the VP.
Targeted decontamination
Twenty-three patients were prescribed targeted decontamination regimens based on microbial analysis of their VP. Among these 23, 12 had single and 11 had multimodality therapy: 6 initially had a broad spectrum rinse and 16 had an antifungal agent prescribed (Table 2). For bacterial colonization, patients received antibiotic therapies are listed in Table 3. In case of a polyspecies bacterial-fungal contamination, both antifungal and antibacterial agents were prescribed simultaneously.
Table 2.
Division of initial decontamination regimens prescribed among the 23 patients
| Chlorhexidine | Nystatin | Fluconazole | Clotrimazole | |
|---|---|---|---|---|
| Patients (%) | 6 (26%) | 3 (13%) | 11 (48%) | 2 (9%) |
Table 3.
Choice of antibiotics for bacterial infections
| Moxifloxacin | Levofloxacin | Ciprofloxacin | Clindamycin | Amoxicillin | |
|---|---|---|---|---|---|
| Patients (%) | 3 (13%) | 3 (13%) | 2 (9%) | 4 (17%) | 1 (4%) |
Device life analysis
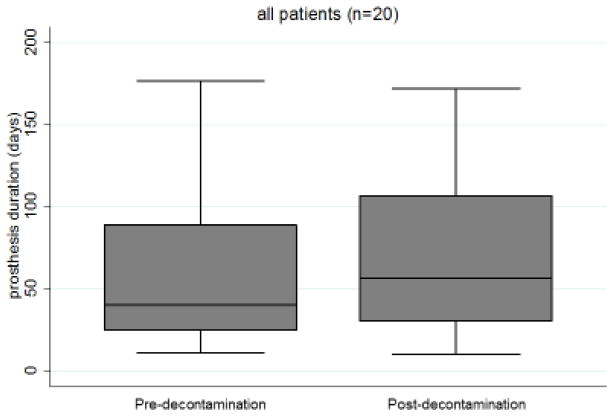
VP device life was examined in 23 patients who had oral or systemic decontamination therapy prescribed. Three were excluded because they did not have sufficient follow up data after decontamination. Among the 20 evaluable patients, the average device life before decontamination improved from 7.89 to 10.82 weeks after decontamination, but this was not statistically significant (p=0.260, Figure 4).
Figure 4.
Device life before and after decontamination
Average device life increased 1.4-fold after targeted antimicrobial therapy.
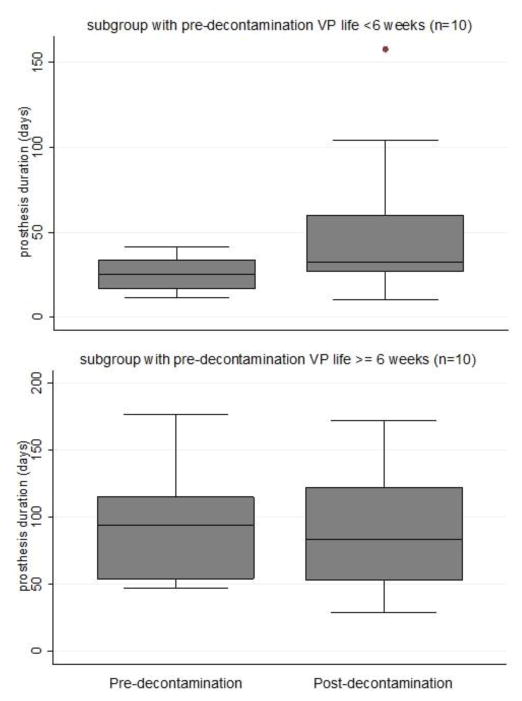
Patients were then stratified by pre-decontamination VP life. The subgroup with particularly poor device life (<6 weeks) prior to decontamination appeared to have more benefit from targeted decontamination, but this was not statistically compared due to non-significant main effect of decontamination therapy (Figure 5). Among the 10 patients whose pre-therapy device life averaged <6 weeks, device life improved by 191% (mean: 26.8 days pre- vs 51.4 days post-decontamination) Further examining 9 patients whose device life did not improve after decontamination, 1 reported non-compliance to the prescribed antifungal agent and 4 had co-existing anatomical/mechanical abnormality such as stricture or anterior diverticulum on their modified barium swallow test that could contribute to early device failure. Among non-responding patients there was initially a relatively higher device life, which did not improve much after decontamination, whereas the responding group initially had a much lower device life that improved substantially.
Figure 5.
Stratified device life before and after decontamination
Average device life almost doubled among those with mean duration <6 weeks prior to decontamination (top), whereas average device life was unchanged after decontamination among those with mean duration ≥6 weeks prior to decontamination (bottom).
Discussion
The highest quality of voice rehabilitation after total laryngectomy can be accomplished with tracheoesophageal VPs. These devices have a reported average life of 3 to 6 months, which can significantly decrease with microbial colonization by bacteria and fungi, posing a major limiting factor [9]. Pathophysiology of biofilm maturation is well characterized. Formation of a biofilm is a complex and dynamic process involving numerous anatomic, physiologic, and biologic stages: 1) initial attachment, 2) irreversible attachment, 3) maturation I, 4) maturation II, and 5) dispersion. Topical antimicrobials have reduced impact in stages 4 and 5 as biofilm matures [24]. Multifactorial processes related to the esophagus, mouth, and biomaterial of the VP influence biofilm development. Examples are salivary composition, variations in normal microbiota, rough surfaces of silicone VP material, and high humidity that promote biofilm formation and eventual valve failure [8,25]. Microbial plaque deposits on the silicone ultimately lead to leakage and aspiration, and the device must be replaced.
The oral cavity has a plethora of microorganisms that provide a constant supply of colonizers to the aerodigestive tract, but reports on VP colonization to date have mostly focused on Candida. Our study confirms a high proportion (79%) of Candida contamination on malfunctioning VPs, with a dominance of non-albicans strains, such as C. tropicalis or C. glabrata. In our setting as a major cancer center with current trends of more aggressive cancer treatment protocols and a shift towards faster immunosuppression, these data are not surprising. In our center 27% of C. tropicalis and 57% of C. glabrata are fluconazole resistant. Also, the generalized use of fluconazole and or echinocandin prophylaxis and the presence of a more mature biofilm might have shifted Candida contamination to the non-albicans strains. The latter is concerning since the non-albicans strains are less susceptible to antifungals (e.g. C. glabrata to fluconazole), have intrinsic resistance to some agents (e.g. C. krusei to fluconazole), or show resistance to echinocandins (e.g. C. tropicalis) [26].
We also found that the majority of patients analyzed had either a polyspecies bacterial-fungal or bacterial colonization, only 3 patients had solely fungal contaminants on poorly functioning VPs. The most prominent bacteria found in our specimens were P. aeruginosa, K. pneumoniae and S. aureus, all three facultative anaerobes and part of the normal oral or skin flora. In accordance with our results, it has been reported previously that these bacteria are part of the microbial plaque on VPs, [6, 14, 15, 17, 21] including normal oral or periodontopathogenic flora, such as P. aeruginosa [19]. It has been hypothesized that the interaction with bacterial strains, such as S. aureus enhances the initial steps of plaque formation and ultimate device failure. Still, to date the most commonly accepted decontamination methods are antifungal, [14, 16, 18] based on early studies conducted to characterize the effects of antifungal therapy on device life of VPs. Historical studies suggested promising results using various agents, [4, 6, 20] but the available level of evidence from early studies is low [27] and biofilms are several magnitudes more resistant to antifungal decontamination efforts than planktonic cells [28]. Biofilm offers effective protection against antimicrobials to which planktonic organisms are usually susceptible. In addition, drawbacks of the prolonged general use of daily oral antifungal lozenges or rinses are poor patient compliance, possible cariogenic activity [29], drug-specific side effects and eventual drug resistance with generation of persister cells and colonies [26]. Therefore, based on the available evidence to date, clinical practice guidelines state that the most effective treatment of persistent Candida biofilms on VPs is removal and replacement of the medical device [28].
Our results show that in spite of a long-standing nystatin decontamination regimen, several patients had a Candida contamination, most often with a polyspecies flora. Among the investigated strains there were common pathogens between the oral and VP microflora, which induced us to attempt a systematic targeted antibiotic or antifungal therapy. That is, prospective decontamination based on the culture and sensitivity reports after removal of the colonized device. To our knowledge, this is the first attempt to apply targeted microbial therapy for failing VP due to contamination. While significant effects were not seen in the cohort at large, subgroup analyses suggested a potential efficacy of this strategy. In our patient population, device life increased almost 2-fold after our targeted therapy in those patients who started out with a particularly poor device life (<6 weeks). Those who started out with a relatively higher device life were less likely to improve VP duration after targeted decontamination therapy, which may be due to other persisting problems beyond biofilm that contribute to premature leakage, such as anatomical challenges of the neopharynx (e.g., stenosis) or gastroesophageal reflux disease. These important potential confounds were not consistently characterized in the medical charts of participants and could not be fully explored in this pilot study.
In practical terms, it is best to remove the device and start anew with a clean prosthesis at the time you begin decontamination therapy. An inherent limitation of the workflow in the clinical setting is the lag time between removal of the failed (contaminated) valve that is sent for microbial assessment and the results of the culture and sensitivity analysis required to initiate a personalized decontamination regimen. By the time therapy began in this series, the replacement valve had been indwelling for no less than one to two weeks which was sufficient time to have become colonized with the contaminating flora. This is particularly the case in the setting of mature biofilm conditions. This undesired lead time allowing for colonization of the replacement valve before starting individualized decontamination may have limited the efficacy of our therapy during this initial clinical roll-out. As we refine our algorithm and workflow, it is a priority to harmonize TEP and oral oncology clinical appointment schedules such that a fresh (i.e., uncolonized) VP is inserted at the onset of a new decontamination therapy.
Herein, we present evidence of polyspecies microbial colonization on failing VPs, alongside that of reduced efficacy of long term oral antifungal therapy, suggesting the development of drug resistance. These pilot data also support exploration of targeted decontamination to extend VP device life. However, the ability to examine efficacy of this strategy in this retrospective case series was limited by a small cohort of patients, as well as inconsistencies in processing microbial samples and implementation of individualized decontamination therapy in the clinical setting. We encountered temporal lapses between cultures of the oral cavity, voice prosthesis and stoma, and microbiological results were not consistently provided quantitatively in the medical records. We are also limited by the acquisition of culture and sensitivity data only in patients with valve problems; a controlled prospective study is required to understand the colonization patterns in a broad postlaryngectomy cohort that includes control patients with optimal device life. The standard clinical microbiological culture methodology (1 minute sonication in 1 mL broth) may not have been optimal to fully capture bacteria nested in glycoprotein-based glycocalyx produced by some biofilm bacteria (“slime”) resulting in over-representation of yeast and under-representation of slime-producing bacteria. To understand efficacy of targeted oral decontamination and the optimal therapeutic algorithm, further prospective studies are needed with a larger patient population, standardized quantitative laboratory methods for biofilm analysis on explanted devices, serial testing to screen for recurrent contamination, and multidimensional functional testing to exclude (or adjust for) competing anatomic or physiologic sources of early device failure.
Conclusions
Based on our pilot data, the majority of patients with a suboptimal VP device life have bacterial or polyspecies bacterial and fungal contamination. VPs rarely had fungal contamination alone (3%). Nonetheless, the importance of fungi is not excluded by the fact they are found in a mixed culture. Furthermore, we found common pathogens between the oral and VP microflora. For these reasons, we are exploring broad-spectrum and targeted oral decontamination regimens that were associated in this preliminary study with 1.4-fold improvement in VP duration, and might be more beneficial for patients than long-standing nonspecific antifungal therapy. An unexpectedly high number of non-albicans colonizations (i.e., tropicalis) motivates exploration of next generation biomaterials for efficacy against polyspecies and mature biofilm.
Acknowledgments
The authors have received funding from the following:
(5P30CA016672) This study was funded by the National Institutes of Health Center Core Grant, The National Institute of Health
(R03 CA188162) This study was funded by National Cancer Institute
(1R56DE025248-01) This study was funded by the National Institutes of Dental and Craniofacial Research
(5R01CA160880-04) This study was funded by the National Institutes of Health for Complementary and Integrated Health
(1R01DE-25248-01A1) This study was funded by the National Institutes of Health/National Institutes of Dental and Craniofacial Research
Dr. Katherine Hutcheson received research grants from the National Cancer Institute and the National Institutes of Dental and Craniofacial Research. Dr. Jan Lewin, Dr. Katherine Hutcheson, Dr. Mark Chambers and Dr. Jeffrey Tarrand have received research grants from National Institutes of Health Center Core Grant. Dr. Mark Chambers received research grants from the National Institutes of Health/National Institutes of Dental and Craniofacial Research.
Footnotes
Conflict of Interest: The authors declared that they have no conflicts of interest to this work.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent:
A waiver of informed consent was obtained from all individual participants included in the study.
References
- 1.Tomeh C, Holsinger FC. Laryngeal cancer. Curr Opin Otolaryngol Head Neck Surg. 2014;22(2):147–153. doi: 10.1097/MOO.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 2.Kazi R, Sayed SI, Dwivedi RC. Post laryngectomy speech and voice rehabilitation: past, present and future. ANZ J Surg. 2010;80(11):770–771. doi: 10.1111/j.1445-2197.2010.05500.x. [DOI] [PubMed] [Google Scholar]
- 3.Singer MI, Blom ED. An endoscopic technique for restoration of voice after laryngectomy. Ann Otol Rhinol Laryngol. 1980;89(6 Pt 1):529–533. doi: 10.1177/000348948008900608. [DOI] [PubMed] [Google Scholar]
- 4.Bauters TGM, Moermann M, Vermeersch H, Nelis HJ. Colonization of voice prostheses by albicans and non-albicans Candida species. Laryngoscope. 2002;112:708–712. doi: 10.1097/00005537-200204000-00021. [DOI] [PubMed] [Google Scholar]
- 5.van Weissenbruch R, Albers FW, Bouckaert S, et al. Deterioration of the Provox silicone tracheoesophageal voice prosthesis: microbial aspects and structural changes. Acta Otolaryngol. 1997;117(3):452–458. doi: 10.3109/00016489709113420. [DOI] [PubMed] [Google Scholar]
- 6.van Weissenbruch R, Bouckaert S, Remon JP, Nelis HJ, Aerts R, Albers FW. Chemoprophylaxis of fungal deterioration of the Provox silicone tracheoesophageal prosthesis in postlaryngectomy patients. Ann Otol Rhinol Laryngol. 1997;106(4):329–337. doi: 10.1177/000348949710600413. [DOI] [PubMed] [Google Scholar]
- 7.de Carpentier JP, Ryder WD, Saeed SR, Woolford TJ. Survival times of Provox valves. J Laryngol Otol. 1996;110(1):37–42. doi: 10.1017/s0022215100132670. [DOI] [PubMed] [Google Scholar]
- 8.Kress P, Schäfer P, Schwerdtfeger FP, Rösler S. Are modern voice prostheses better? A lifetime comparison of 749 voice prostheses. Eur Arch Otorhinolaryngol. 2014;271(1):133–140. doi: 10.1007/s00405-013-2611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hancock KL, Lawson NR, Ward EC. Device life of the Provox Vega voice prosthesis. Eur Arch Otorhinolaryngol. 2013;270(4):1447–1453. doi: 10.1007/s00405-012-2154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leunisse C, van Weissenbruch R, Busscher HJ, van der Mei HC, Dijk F, Albers FW. Biofilm formation and design features of indwelling silicone rubber tracheoesophageal voice prostheses--an electron microscopical study. J Biomed Mater Res. 2001;58(5):556–563. doi: 10.1002/jbm.1054. [DOI] [PubMed] [Google Scholar]
- 11.López D, Vlamakis H, Kolter R. Biofilms. Cold Spring Harb Perspect Biol. 2010;2(7):a000398. doi: 10.1101/cshperspect.a000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahieu HF, van Saene JJ, den Besten J, van Saene HK. Oropharynx decontamination preventing Candida vegetation on voice prostheses. Arch Otolaryngol Head Neck Surg. 1986;112:1090–1092. doi: 10.1001/archotol.1986.03780100078012. [DOI] [PubMed] [Google Scholar]
- 13.Mahieu HF, van Saene JJ, den Besten J, van Saene HK. Fungal colonization of tracheoesophageal voice prosthesis. Laryngoscope. 1987;97(5):594–597. doi: 10.1288/00005537-198705000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Palmer MD, Johnson AP, Elliott TS. Microbial colonization of Blom-Singer prostheses in postlaryngectomy patients. Laryngoscope. 1993;103(8):910–914. doi: 10.1288/00005537-199308000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Eerenstein SE, Grolman W, Schouwenburg PF. Microbial colonization of silicone voice prostheses used in laryngectomized patients. Clin Otolarygol. 1999;24:398–403. doi: 10.1046/j.1365-2273.1999.00245.x. [DOI] [PubMed] [Google Scholar]
- 16.Elving GJ, van der Mei HC, Busscher HJ, van Weissenbruch R, Albers FW. Comparison of the microbial composition of voice prosthesis biofilms from patients requiring frequent versus infrequent replacement. Ann Otol Rhinol Laryngol. 2002;111(3 Pt 1):200–203. doi: 10.1177/000348940211100302. [DOI] [PubMed] [Google Scholar]
- 17.Schuldt T, Dommerich S, Pau HW, Kramp B. Time course of microbial colonization of different voice prostheses. Laryngorhinootologie. 2010;89(10):606–611. doi: 10.1055/s-0030-1261969. [DOI] [PubMed] [Google Scholar]
- 18.Buijssen KJ, van der Laan BF, van der Mei HC, Atema-Smit J, van den Huijssen P, Busscher HJ. Composition and architecture of biofilms on used voice prostheses. Head Neck. 2012;34(6):863–871. doi: 10.1002/hed.21833. [DOI] [PubMed] [Google Scholar]
- 19.Bertl K, Zatorska B, Leonhard M, Rechenmacher-Strauss J, Roesner I, Schneider-Stickler B. Oral microbial colonization in laryngectomized patients as a possible cofactor of biofilm formation on their voice prostheses. J Clin Periodontol. 2013;40(9):833–840. doi: 10.1111/jcpe.12131. [DOI] [PubMed] [Google Scholar]
- 20.Mahieu HF, van Saene HK, Rosingh HJ, Schutte HK. Candida vegetations on silicone voice prostheses. Arch Otolaryngol Head Neck Surg. 1986;112(3):321–325. doi: 10.1001/archotol.1986.03780030085017. [DOI] [PubMed] [Google Scholar]
- 21.Sayed SI, Kazi R, Sengupta S, Chowdhari A, Jagade M. Microbial colonization of Blom-Singer indwelling voice prostheses in laryngectomized patients: A perspective from India. ENT Journal. 2012;91(4):E19–22. doi: 10.1177/014556131209100418. [DOI] [PubMed] [Google Scholar]
- 22.Blom ED, Hamaker RC. Tracheoesophageal voice restoration following total laryngectomy. In: Myers EN, Suen J, editors. Cancer of the head and neck. W. B. Saunders; Philadelphia: 1996. pp. 839–852. [Google Scholar]
- 23.Han XY, Tarrand JJ, Dickey BF, Han Esteva FJ. Helicobacter pylori bacteremia with sepsis syndrome. J Clin Microbiol. 2010;48(12):4661–4663. doi: 10.1128/JCM.01481-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monroe D. Looking for chinks in the armor of bacterial biofilms. PLoS Biol. 2007;5(11):e307. doi: 10.1371/journal.pbio.0050307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talpaert MJ, Balfour A, Stevens S, Baker M, Muhlschlegel FA, Gourlay CW. Candida biofilm formation on voice prostheses. J Med Microbiol. 2015;64(Pt 3):199–208. doi: 10.1099/jmm.0.078717-0. [DOI] [PubMed] [Google Scholar]
- 26.Sardi JC, Scorzoni L, Bernardi T, Fusco-Almeida AM, Mendes Giannini MJ. Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol. 2013;62(Pt 1):10–24. doi: 10.1099/jmm.0.045054-0. [DOI] [PubMed] [Google Scholar]
- 27.Worthington HV, Clarkson JE, Khalid T, Meyer S, McCabe M. Interventions for treating oral candidiasis for patients with cancer receiving treatment. Cochrane Database Syst Rev. 2010;7(7):CD001972. doi: 10.1002/14651858.CD001972.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr, Calandra TF, Edwards JE., Jr Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(5):503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips SM, Dellinger TM. Dental decay due to xerostomia and nystatin. Ann Pharmacother. 2005;39(10):1758. doi: 10.1345/aph.1G016. [DOI] [PubMed] [Google Scholar]