Abstract
Reactive oxygen species damage various cell components including DNA, proteins, and lipids, and these impairments could be a reason for severe human diseases including atherosclerosis. Forkhead box O1 (FOXO1), an important metabolic transcription factor, upregulates antioxidant and proapoptotic genes during oxidative stress. Apolipoprotein A-I (ApoA-I) forms high density lipoprotein (HDL) particles that are responsible for cholesterol transfer from peripheral tissues to liver for removal in bile in vertebrates. The main sources for plasma ApoA-I in mammals are liver and jejunum. Hepatic apoA-I transcription depends on a multitude of metabolic transcription factors. We demonstrate that ApoA-I synthesis and secretion are decreased during H2O2-induced oxidative stress in human hepatoma cell line HepG2. Here, we first show that FOXO1 binds to site B of apoA-I hepatic enhancer and downregulates apoA-I gene activity in HepG2 cells. Moreover, FOXO1 and LXRα transcription factors participate in H2O2-triggered downregulation of apoA-I gene together with Src, JNK, p38, and AMPK kinase cascades. Mutations of sites B or C as well as the administration of siRNAs against FOXO1 or LXRα to HepG2 cells abolished the hydrogen peroxide-mediated suppression of apoA-I gene.
Keywords: Apolipoprotein A-I, Oxidative stress, Hydrogen peroxide, FOXO1, LXRα
Introduction
Oxidative stress is a state in which cells are not capable to inactivate reactive oxygen and nitrogen species (ROS and NOS) formed by oxidative metabolism. ROS damage various cell components including DNA, proteins, and lipids, and these impairments could be a reason for severe human diseases including atherosclerosis. Moreover, some ROS can be involved in redox signaling as second messengers, so oxidative stress disturbs normal signal transduction in the cells (Rani et al. 2016). Hydrogen peroxide, hydroxyl radical, and superoxide anion are the important members of ROS family. H2O2 assists inflammatory response by inactivating phosphatases as well as activating specific signaling cascades (Kyriakis and Avruch 2012; Schieber and Chandel 2014). Hydrogen peroxide is produced by NADPH-oxidases in hepatocytes as a response to proinflammatory or hormone stimuli (Bedard and Krause, 2007; Schieber and Chandel, 2014). Although the release of reactive oxygen species was recognized as a critical step in combating invaders, excessive production of ROS can trigger oxidative stress that causes significant harm to cells (Schieber and Chandel 2014). Additionally, inflammation is now considered to be one of the main causes of metabolic syndrome, and hyperactivation of proinflammatory signaling pathways might be responsible for this process (Donath and Shoelson 2011).
Apolipoprotein A-I (ApoA-I) forms high density lipoprotein (HDL) particles that are responsible for the cholesterol transfer from peripheral tissues to liver for removal in bile in vertebrates (Lewis and Rader 2005). HDLs protect peripheral tissues from accumulation of excess cholesterol and subsequent development of atherosclerosis. ApoA-I also manifests anti-inflammatory and anti-thrombotic activities that contribute to its key role in preventing atherosclerosis development (Hopkins 2013; Kontush and Chapman 2006).
The main sources for plasma ApoA-I in mammals are liver and jejunum (Eggerman et al. 1991; Ginsburg et al. 1995). Recently, synthesis of ApoA-I was found in human macrophages where endogenous ApoA-I restricts macrophage response to proinflammatory stimuli and stabilizes cassette transporter ABCA1 (Mogilenko et al. 2012b). Treatment of hepatic cells with TNFα, IL-1β, or a bacterial lipopolysaccharide decreases the rates of ApoA-I expression and secretion (Haas et al. 2003; Mogilenko et al. 2009; Morishima et al. 2003; Orlov et al. 2010; Song et al. 1998).
Hepatic enhancer (HE) was shown to be the main element controlling apoA-I transcription in liver cells. HE is located in coordinates−222…−110 versus the canonical site of transcription initiation. There are three main regions within HE: sites A (−214…−192), B (−169…−146), and C (−134…−119) (Higuchi et al. 1988; Sastry et al. 1988; Widom et al. 1991). Metabolic regulators from nuclear receptor superfamily LXRα and LXRβ (Huuskonen et al. 2006) decrease apoA-I expression through binding to site C, PPARα (Martin et al. 2001) activates apoA-I transcription by interacting with site A, while HNF4α (Chan et al. 1993; Malik and Karathanasis 1996) upregulates, and PPARγ inhibits (Shavva et al. 2016b) apoA-I expression through binding to both sites A and C. Proinflammatory agents repress apoA-I in HepG2 cells by employing nuclear receptors that interact with sites A and C of HE (Mogilenko et al. 2009; Morishima et al. 2003; Orlov et al. 2010; Shavva et al. 2016b). Activators of apoA-I expression FOXA2 (Harnish et al. 1994; Harnish et al. 1996) and Sp1 (Oleaga et al. 2013) were shown to bind site B.
Little is known about apoA-I gene expression under oxidative stress. Administration of an oxidative stress inducer, gramoxone, to human hepatoma cells results in the simultaneous activation of apoA-I transcription and ApoA-I mRNA degradation (Cuthbert et al. 1997). Interestingly, a putative “antioxidant response element” (ARE) within the HE (−145...−130) may be involved in gramoxone-mediated induction of apoA-I transcription (Cuthbert et al. 1997). However, gramoxone is not an endogenous compound and is not found in vivo. Moreover, there is no data confirming the relevance of the gramoxone model to oxidative stress in vivo.
FOXO1 was implicated in protecting cells from excessive ROS production (Choi et al. 2009; Klotz et al. 2015) and mediating the apoptosis induced by oxidative stress (Shen et al. 2012). FOXO1 was shown to upregulate several survival genes, responsible for defense against excessive ROS accumulation, namely HO-1 (Cheng et al. 2009; Liu et al. 2013), catalase (Awad et al. 2014), and MnSOD (Sengupta et al. 2011). Despite the clear importance of FOXO1 in maintenance of hepatic cholesterol and glucose homeostasis, its role in the modulation of apoA-I gene expression is still unknown. Some of key FOXA2 target genes are also regulated by FOXO1 including GK, PEPK, and G6Pase (Hirota et al. 2008; Schmoll et al. 2000; Yeagley et al. 2001). FOXA2 and FOXO1 compete for the common binding site in Pdx2 promoter in pancreatic β-cells (Kitamura and Ido Kitamura 2007).
Here, we show that treatment of human hepatoma cells (HepG2 cell line) with hydrogen peroxide leads to the repression of ApoA-I synthesis and secretion. This effect depends on FOXO1 and LXRα transcription factors. Moreover, we have first found that FOXO1 binds to site B of apoA-I HE and inhibits apoA-I gene transcription. Mutations of sites B or C as well as transfection of HepG2 cells by siRNAs against FOXO1 or LXRα abolished the suppression of apoA-I gene by hydrogen peroxide.
Materials and methods
Chemical inhibitors
MAP-kinase inhibitors were purchased from Biomol, USA: SP600125 (JNK1/2/3 inhibitor)—cat. number EI-305; SB203580 (p38 inhibitor) – cat. number EI-286; U0126 (MEK1/2 inhibitor)—cat. number EI-282; PP2 (Src inhibitor)—cat. number SIH-470.
Antibodies
Mouse monoclonal antibodies against human β-actin (cat. number ab3280) were supplied by Abcam. Rabbit polyclonal antibodies against human FOXO1 (cat. number sc-11350) were purchased from Santa Cruz Biotechnology.
Plasmids
The pCMVLacZ plasmid (Dizhe et al. 2006) and pA1(−256/+72) (Lapikov et al. 2008) were described elsewhere. The plasmids pA1(−256/+72) with disrupted A or C sites (Shavva et al. 2016b) and B site (Shavva et al. 2016a) were described in the previous works.
Cell culture maintenance and electroporation
Human hepatoma cell line HepG2 was purchased from the Cell Culture Bank of the Institute of Cytology, Russian Academy of Sciences, Saint-Petersburg, Russia. The details of HepG2 cultivation and electroporation as well as a luciferase test were described earlier (Shavva et al. 2016b). The following concentrations of kinase inhibitors were used: SP600125 (10 μM), U0126 (10 μM), SB203580 (25 μM), and PP2 (10 μM). The inhibitors were added to cells 1 h before hydrogen peroxide administration.
MTT assay
HepG2 cells were seeded into 96-well plates at a density of 1 × 104 cells/cm2 in DMEM with 10% fetal calf serum (FCS) in 5% CO2 at 37 °C. After 24 h incubation, culture medium was changed to fresh DMEM without FCS and cells were cultured for 24 h. Culture medium was changed to fresh DMEM without FCS again; different concentrations of hydrogen peroxide were added to cells, and the cell viability was accessed by MTT assay after 24 h incubation as described (Mosmann 1983). Briefly, one half of the culture medium (100 μl) was removed from wells and 20 μl of MTT (3-(4,5-dimethylthiazol-2-yl)-diphenyltetrazolium bromide) (Sigma) solution (5 mg/ml on phosphate saline buffer, pH 8.0) was added. Cells were incubated at 37 °C for 4 h and the medium with dye was removed. Cells were lysed in 200 μl of DMSO and absorbance at 570 nm was measured by Synergy 2 plate reader (Bio-Tek Instruments). The cell death (in %) was calculated by following formula:
where D 570 is the absorbance at 570 nm in a sample well, D 570 DMEM is the absorbance at 570 nm in a well without cells (fresh DMEM), and D 570 K is the absorbance at 570 nm in a well with control untreated HepG2 cells.
siRNA-mediated knockdown
Scrambled control RNA oligonucleotides (sc-37007) and siRNA against FOXO1 (sc-35382) were supplied by Santa Cruz Biotechnology. siRNA against AMPKα1 and AMPKα2 (Tangeman et al. 2012) was purchased from DNA-Synthesis (Moscow, Russia). The Lipofectamine RNAiMAX (Invitrogen) was used for delivery of siRNAs into cells in accordance to manufacturer’s instructions. The cells were harvested 72 h after transfection.
Reverse transcription and real time PCR
RNA isolation, reverse transcription, and real-time PCR including multiplexing details were performed as described earlier (Mogilenko et al. 2013; Shavva et al. 2016b). Primers for human β-actin (Mogilenko et al. 2012a), human RPLP0, human Cyclophilin A (Shavva et al. 2016b), and human ApoA-I (Mogilenko et al. 2009) were described earlier. Primers for human FOXO1: 5′-AACCAAAGCTTCCCACACAG-3′, 5′-GTAACCTGCTCACTAACCCTCAG-3′, 5′-ROX-GACAACGACACATAGCTGGGTGTCAGG-RTQ2–3′; AMPKα1: 5′-TGGATTTCCGTAGTATTGATGATG-3′, 5′-CCAGGTCTTGGAGTTAGGTCA-3′; AMPKα2: 5′-TGGACTTTAAAAGCATTGATGATG-3′, 5′-AGGCGAGGTGAAACTGAAGA-3′; and MnSOD2: 5′-TGCAAGGAACAACAGGCC-3′, 5′-CGTGGTTTACTTTTTGCAAGC-3′ were selected by Primer3 software (http://primer3.sourceforge.net).
ChIP assay
Chromatin immunoprecipitation (ChIP) was performed as described (Mogilenko et al. 2009). Primers for apoA-I HE were published elsewhere (Mogilenko et al. 2009); for human G6Pase promoter region containing FOXO1 RE: 5′-CTGTGTCTCTGGCCTGGTTT-3′ and 5′-CAACCCAGCCCTGATCTTT-3′. Non-specific binding was assessed with the use of antibodies against human β-actin and by primers to GAPDH pseudogene.
Nuclear extract preparation and DNA affinity precipitation
Nuclear extracts were obtained as described elsewhere (Andrews and Faller, 1991). DNA affinity precipitation was performed as described earlier (Orlov et al. 2007). The following double-stranded synthetic oligonucleotides were used (sequences of sense strands only are shown): HRE-B 5′-TTTGCCCACTCTATTTGCCCAGCCCCATG-3′ (matches to site B of HE) or HRE-B-mut, 5′-TTTGCTGTGAGTCCACTCTATTTGCCCAG-3′ (mutated site B; altered nucleotides are underlined). All oligonucleotides were obtained from Syntol. Western blotting was used for the analysis of the samples.
Enzyme-linked immunosorbent assay (ELISA)
Determination of ApoA-I concentration in the cultural medium or cell lysates was performed by ELISA as described earlier (Mogilenko et al. 2009). Polyclonal antibodies to human apoA-I were obtained as described previously (McVicar et al. 1984).
Statistical analysis
Results are shown as means ± standard error of mean. The group comparisons were performed by an unpaired t test or Dunnett’s criterion (for multiple comparisons). The values of p < 0.05 were accepted for statistically significant differences. Statistical analysis was carried out by Statistica 5.0 software (StatSoft).
Results and discussion
Hydrogen peroxide downregulates apoA-I expression in concentration-dependent manner in HepG2 cells
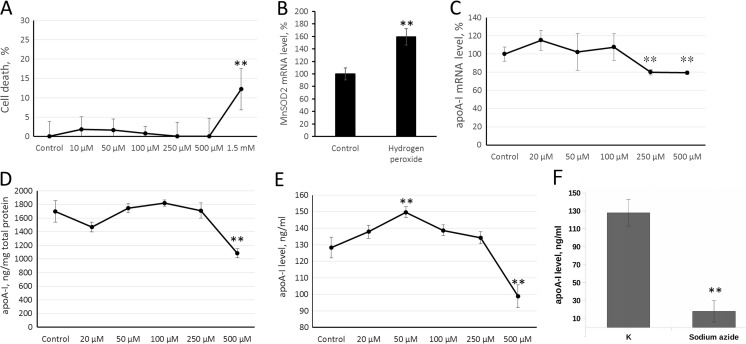
Gramoxone, an inducer of oxidative stress, activates apoA-I gene but destabilized its mRNA, resulting in overall decrease in the amount of ApoA-I mRNA in HepG2 cells (Cuthbert et al. 1997). To investigate the effect of ROS on apoA-I gene activity we treated HepG2 cells with H2O2 for 24 h. This term was chosen because of rather high stability of ApoA-I mRNA (ApoA-I mRNA half life is about 23 h (Cuthbert et al. 1997)). Toxicity of H2O2 was assessed using MTT test (Fig. 1a). Only a high (1.5 mM) concentration of hydrogen peroxide appeared to be toxic (Fig. 1a). Treatment of HepG2 cells with 250 μM of hydrogen peroxide resulted in an increase of MnSOD2 mRNA levels, indicating that this amount of H2O2 provoked the oxidative stress response (Fig. 1b). Administration of 20–100 μM of hydrogen peroxide failed to affect apoA-I gene expression, while 250 μM triggered a decrease in gene activity. There were no significant differences between 250 and 500 μM concentrations of hydrogen peroxide and this fact is in a good agreement with threshold model of oxidative stress induction (Fig. 1c).
Fig. 1.
Hydrogen peroxide downregulates apoA-I gene expression in HepG2 cells. a MTT test. HepG2 cells were treated with various concentrations of H2O2 for 24 h and subjected to MTT test. b Real-time RT-PCR. HepG2 cells were treated with 250 μM of H2O2 for 24 h. The Y-axis values correspond to the relative level of MnSOD2 mRNA (100% in control cells). c Real-time RT-PCR. HepG2 cells were treated with various concentrations of H2O2 for 24 h. The Y-axis values correspond to the relative level of apoA-I mRNA (100% in control cells). d–f ELISA. HepG2 cells were treated with various concentrations of H2O2 (d, e) or 10 mM sodium azide (f) for 24 h. Analysis of ApoA-I protein in whole-cell extracts (d) or culture medium (e, f). The Y-axis values correspond to the ApoA-I protein level. Values are presented as means ± the standard error of the mean of three independent experiments. The statistical analyses of differences between compared groups were performed using a non-paired Student’s t test (**p < 0.05)
To see if mRNA level alteration was in parallel with decrease in protein content and secretion, we measured the amount of secreted and intracellular ApoA-I protein by ELISA. The concentration of ApoA-I protein in cellular extracts and cultivation medium was decreased after treatment with 500 μM of H2O2 (Fig. 1d, e). Interestingly, treatment of HepG2 cells with 50 μM of hydrogen peroxide resulted in a modest increase in ApoA-I secretion (Fig. 1e). This effect might be a result of an independent regulation of ApoA-I secretion as it had been shown earlier for different stimuli (Mogilenko et al. 2009; Shavva et al. 2016a; Shavva et al. 2016b).
To ensure the energy-dependent rather than passive (induced by the loss of plasma membrane integrity during hydrogen peroxide exposure) mechanism of ApoA-I secretion, cells were treated with sodium azide (10 mM) (blocks oxidative phosphorylation). Treatment of HepG2 cells with sodium azide led to the almost complete absence of secreted ApoA-I in culture medium, that suggests an energy-dependent mechanism of ApoA-I secretion (Fig. 1f).
Hydrogen peroxide activates Src, p38, JNK, and AMPK to downregulate apoA-I gene expression
Oxidative stress is known to employ several different signaling pathways to regulate gene expression. Stress-regulated MAP-kinases of JNK and p38 families are activated by ASK1/2/MKK4/7 and ASK1/2/MKK3/6 pathways respectively (Kyriakis and Avruch 2012). Another MAPK Erk1/2 are phosphorylated in the response to ROS by Src/Ras signaling (Aikawa et al. 1997). Moreover, p38 and JNK MAPK can also be activated by Src under oxidative stress (Kim et al. 2008; Yoshizumi et al. 2000). Both Ask1/2 and Src are activated by similar mechanisms, where ROS oxidize cysteine residues on Trx (in the case of Ask1/2) to release kinases from Trx-dependant inhibition or on Src itself to allow for autophosphorylation and activation (Giannoni et al. 2005; Kyriakis and Avruch 2012). We treated HepG2 cells with inhibitors of Src (PP2), JNK1/2/3 (SP600125), MEK1/2 (U0126), and p38 (SB203580) to evaluate their possible involvement in the apoA-I gene repression by hydrogen peroxide (Fig. 2a, b). Pretreatment of HepG2 cells with JNK, p38, or Src inhibitors weakened the hydrogen peroxide-mediated decrease in apoA-I gene activity (Fig. 2a, b). In contrast, MEK1/2 inhibitor failed to interfere with apoA-I expression downregulation under hydrogen peroxide (Fig. 2a).
Fig. 2.
JNK and AMPK are employed in the inhibition of apoA-I gene activity by H2O2 in HepG2 cells. a, b Real-time RT-PCR. HepG2 cells were pretreated with SP600125 (10 μM), SB203580 (25 μM), PP2 (10 μM), and/or U0126 (10 μM) for 1 h before administration of 250 μM of H2O2 for 24 h. The Y-axis values correspond to the relative level of apoA-I mRNA (100% in control cells). c Real-time RT-PCR. HepG2 cells were transfected with 2.4 pmoles per well (96 well plate) of scrambled siRNA or siRNA against AMPKα1 and AMPKα2 for 72 h before RNA isolation and treated with 250 μM of H2O2 for 24 h before RNA isolation. The Y-axis values correspond to the relative level of apoA-I mRNA (100% in control cells). d, e Real-time RT-PCR. HepG2 cells were transfected with 2.4 pmoles per well (96 well plate) of scrambled siRNA or siRNA against AMPKα1 and AMPKα2 for 72 h before RNA isolation. The Y-axis values correspond to the relative level of AMPKα1 (d) or AMPKα2 (e) mRNA mRNA (100% in control cells). f Western assay. HepG2 cells (24 well plate) were transfected with 15 pmoles per well of scrambled siRNA or siRNA against AMPKα1 and AMPKα2 for 72 h before protein isolation. The results were normalized by detection of β-actin. Values are presented as means ± the standard error of the mean of three independent experiments. The statistical analyses of differences between compared groups were performed using a non-paired Student’s t -test (**p < 0.05; #p < 0.01; N.S. non-significant (p > 0.05))
The other potential target of oxidative stress is AMP-activated protein kinase (AMPK) (Han et al. 2010). The possible role of AMPK in apoA-I gene expression decrease during oxidative stress was assessed by transfecting HepG2 cells with siRNA against two isoforms of catalytic subunit of AMPK—α1 and α2 (Fig. 2d–f). siRNA decreased the amounts of AMPKα mRNAs as well as AMPKα protein (Fig. 2d–f) and canceled hydrogen peroxide-induced downregulation of apoA-I expression (Fig. 2c), indicating that AMPK is involved in this process.
Transcription factors FOXO1 and LXRα mediate repression of apoA-I gene by H2O2
Transcription factor FOXO1 protects cells during oxidative stress by increasing production of Catalase, HO-1, and MnSOD (Awad et al. 2014; Cheng et al. 2009; Liu et al. 2013; Sengupta et al. 2011; Klotz et al. 2015). The nucleo-cytoplasmic translocation plays an important role in the FOXO1-dependent transcriptional regulation. The most important signal cascade controlling FOXO1 nucleo-cytoplasmic translocation is phosphatidylinositol 3-kinase/protein kinase B (PI3K/PKB) pathway that results in the phosphorylation of FOXO1 at Thr24, Ser256, and Ser319 and the exclusion of phosphorylated FOXO1 from nucleus to cytoplasm with its following proteasomal degradation (Rena et al. 1999). However, while this signal pathway plays a central role in the FOXO1 inactivation under cell administration of insulin or other growth factors, it appears not to be involved in the FOXO1 regulation under oxidative stress. Moreover, phosphorylation of FOXO1 by MST1 (Lehtinen et al. 2006) or methylation by PRMNT1 (Yamagata et al. 2008) protect FOXO1 from the protein kinase B-mediated phosphorylation at Ser256 in the response to oxidative stress and prevent FOXO1 nuclear exclusion.
P38, JNK and AMPK may be implicated in FOXO1-dependent signaling during oxidative stress. MAP-kinases, Erk1/2, and p38, but not JNK, phosphorylate and activate FOXO1 (Asada et al. 2007). Nevertheless, FOXO1 and JNK appear to cooperate during a stress response in multiple cell types (Martinez et al. 2008; Yinghua et al. 2014). JNK was shown to phosphorylate FOXO4 (Essers et al. 2004), but not FOXO1 (Asada et al. 2007). JNK-mediated activation of FOXO1 might depend on its ability to phosphorylate 14-3-3ζ, a protein known to induce FOXO1 nuclear-cytoplasmic translocation and inactivation (Van Der Heide et al. 2004). Phosphorylation of 14-3-3ζ by JNK was shown to trigger the release of a related forkhead factor FOXO3a (Sunayama et al. 2005), suggesting that similar mechanism might be possible for FOXO1. AMPK was shown to activate FOXO1 in pulmonary arterial myocytes in response to hypoxia-generated hydrogen peroxide (Awad et al. 2014). Whether AMPK directly phosphorylates FOXO1 or activates it through any other mechanism is unknown. However, inhibition of AMPK resulted in a decrease in FOXO1 activity (Awad et al. 2014). Taking into account our data about the role of AMPK and MAP kinases p38, and JNK in the downregulation of apoA-I expression under oxidative stress (Fig. 2), it is plausible to suggest that FOXO1 is responsible for this effect.
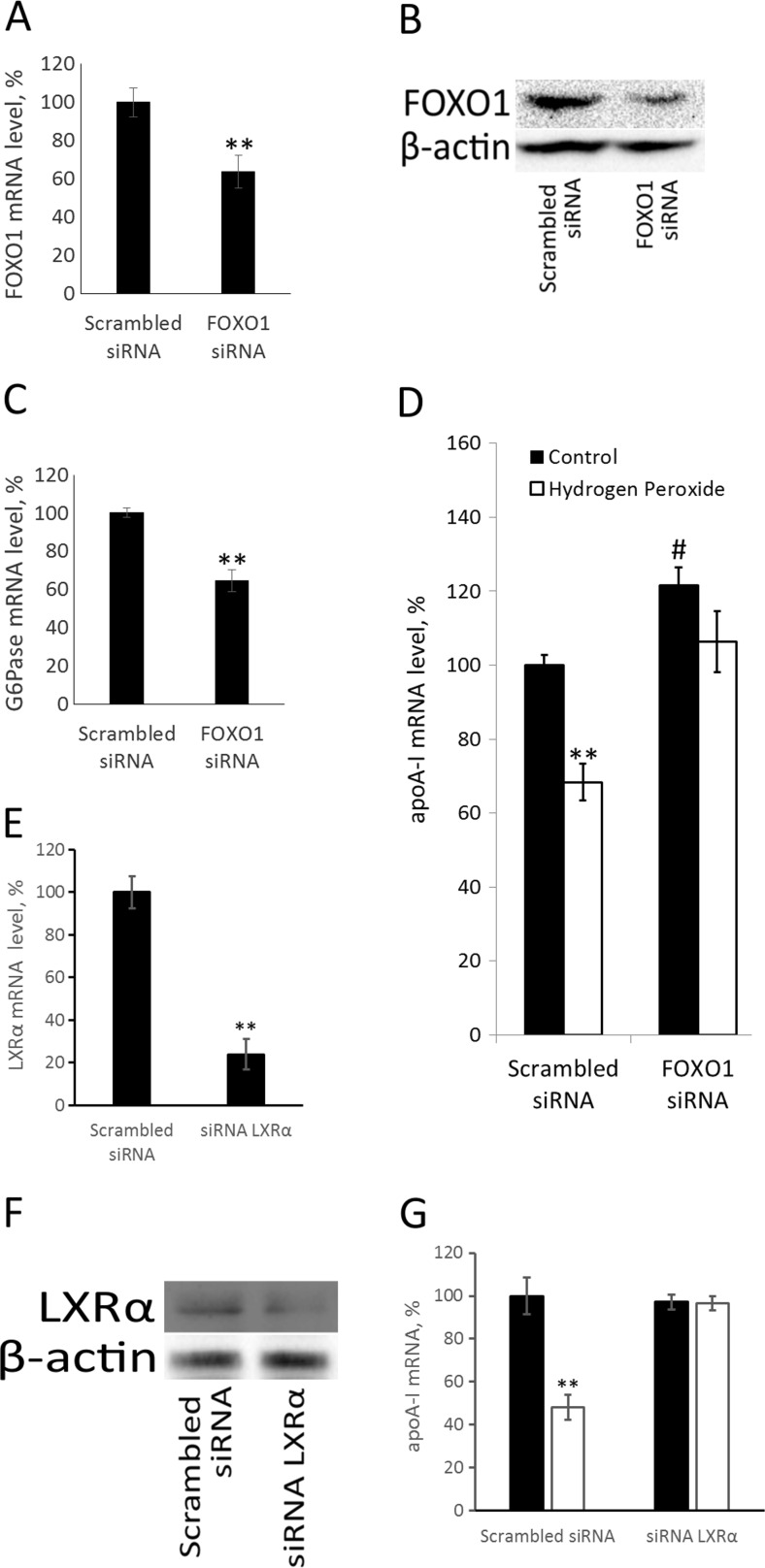
To study the role of FOXO1 in apoA-I transcription inhibition during oxidative stress, we employed siRNA-mediated knockdown of FOXO1. siRNA against FOXO1 decreased both FOXO1 mRNA and protein levels (Fig. 3a, b). Additionally, siRNA against FOXO1 downregulated the canonical FOXO1 target gene G6Pase (Fig. 3c). siRNA against FOXO1 led to ~20% elevation in ApoA-I mRNA suggesting that FOXO1 might be a repressor of apoA-I gene (Fig. 3d). Administration of hydrogen peroxide to HepG2 cells repressed apoA-I gene while siRNA against FOXO1 abrogated this effect (Fig. 3d). These data indicate that the hydrogen peroxide-mediated downregulation of apoA-I gene expression is FOXO1-dependent.
Fig. 3.
LXRα and FOXO1 play a key role in hydrogen peroxide-dependent downregulation of apoA-I gene expression. a Real-time RT-PCR. HepG2 cells (96 well plate) were transfected with 2.4 pmoles per well of scrambled siRNA or siRNA against FOXO1 for 72 h before RNA isolation. The Y-axis values correspond to the relative level of FOXO1 mRNA (100% in control cells). b Western assay. HepG2 cells (24 well plate) were transfected with 15 pmoles per well of scrambled siRNA or siRNA against FOXO1 for 72 h before protein isolation. c Real-time RT-PCR. HepG2 cells (96 well plate) were transfected with 2.4 pmoles per well of scrambled siRNA or siRNA against FOXO1 for 72 h before RNA isolation. The Y-axis values correspond to the relative level of G6Pase mRNA mRNA (100% in control cells). d Real-time RT-PCR. The Y-axis values correspond to the relative level of apoA-I mRNA (100% in control cells). HepG2 cells (96 well plate) were transfected with mock 2.4 pmoles per well of siRNA or siRNA against FOXO1 for 72 h as described in Materials and methods and treated with 250 μM of hydrogen peroxide for 24 h. e Real-time RT-PCR. HepG2 cells (96 well plate) were transfected with 2.4 pmoles per well of scrambled siRNA or siRNA against LXRα for 72 h. The Y-axis values correspond to the relative level of LXRα mRNA (100% in control cells). f Western blotting. HepG2 cells (24 well plate) were transfected with 15 pmoles per well of scrambled siRNA or siRNA against LXRα for 72 h before protein isolation. g Real-time RT-PCR. HepG2 cells (96 well plate) were transfected with 2.4 pmoles per well of scrambled siRNA or siRNA against LXRα for 72 h before RNA isolation and treated with 250 μM of H2O2 for 24 h before RNA isolation. The Y-axis values correspond to the relative level of apoA-I mRNA (100% in control cells). Values are presented as means ± the standard error of the mean of three independent experiments. The statistical analyses of differences between compared groups were performed using a non-paired Student’s t test (**p < 0.05; #p < 0.01)
The other putative target of AMPK under oxidative stress is transcription factor LXRα. AMPK was shown to phosphorylate and inactivate LXRα (Hwahng et al. 2009). To see if LXRα might be responsible for downregulation of apoA-I gene during oxidative stress, we transfected HepG2 cells with siRNA against LXRα or scrambled siRNA and treated them with hydrogen peroxide for 24 h (Fig. 3e–g). siRNA against LXRα significantly decreased the amount of LXRα mRNA (Fig. 3e) and protein (Fig. 3f) and completely abrogated hydrogen peroxide-induced decrease in apoA-I gene activity (Fig. 3g), showing that LXRα is indeed important for the repression of apoA-I gene under an oxidative stress.
FOXO1 binds to the site B of hepatic enhancer
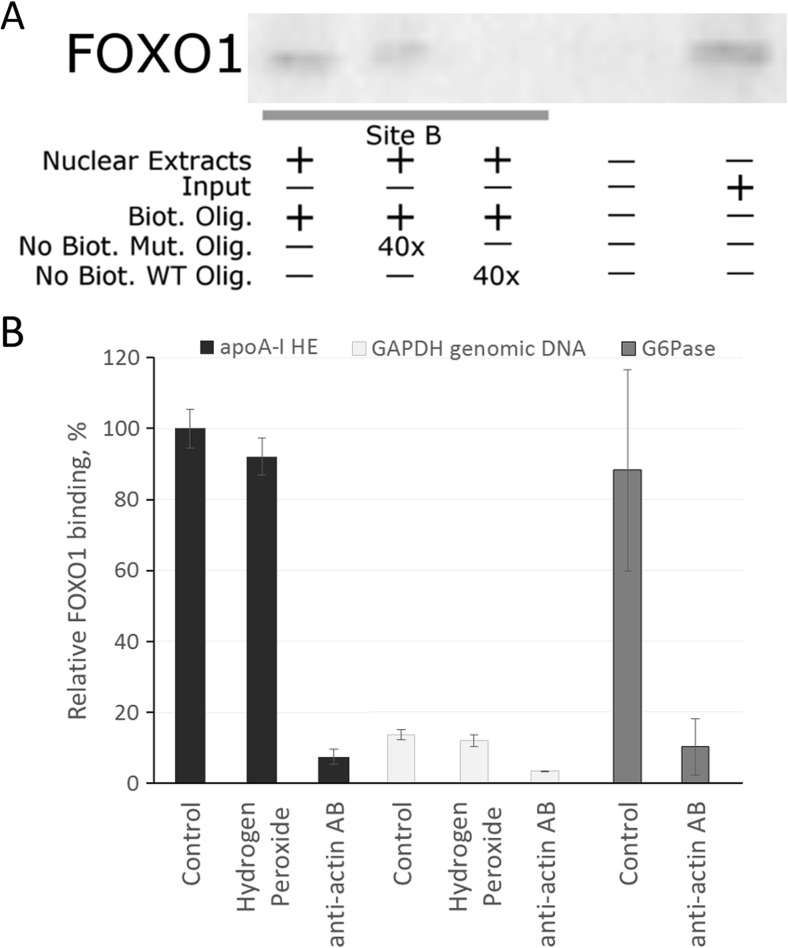
FOXO1-related transcription factor FOXA2 is capable of interacting with site B of HE (Harnish et al. 1994; Harnish et al. 1996). Given the close DNA-binding specificity of FOXO1 and FOXA2, site B of HE is likely to be a putative binding site for FOXO1. Moreover, we have shown the role of FOXO1 in the insulin-dependent regulation of apoA-I gene earlier (Shavva et al. 2016a). To test whether FOXO1 can bind to site B in vitro, we performed DNA-affinity precipitation. As shown on Fig. 4a, FOXO1 is capable of specifically binding site B-containing probes. While addition of the excess amount of unlabeled oligonucleotide corresponding to site B diminished the amount of bound FOXO1, mutated oligonucleotide failed to compete with the biotin-containing probe. To test the capacity of FOXO1 to bind HE in vivo, we employed chromatin immunoprecipitation assay (ChIP) (Fig. 4b). The G6Pase promoter that contains sites for FOXO1 (Vander Kooi et al. 2003) was selected as a positive control. ChiP confirmed that FOXO1 interacts with the HE of apoA-I and the G6Pase promoter in vivo. Hydrogen peroxide failed to affect FOXO1 binding to the HE (Fig. 4b). Based on the above mentioned results, we can conclude that FOXO1 binds to the HE of apoA-I gene in HepG2 cells in vivo.
Fig. 4.
FOXO1 binds to site B of apoA-I hepatic enhancer in HepG2 cells. a DNA-affinity precipitation. Assay was performed as described in Materials and methods. Biot. Olig. biotinilated oligonucleotide, No Biot. Mut. Olig. mutated oligonucleotide without biotin, No Biot. WT Olig. wild type oligonucleotide without biotin, Input reaction mixture before adding streptavidin-coated magnetic particles (positive control). b Real-time PCR calculation of ChIP. HepG2 cells were treated with hydrogen peroxide (250 μM) for 24 h. Histograms represent the relative level of FOXO1 binding (100% in control cells) to apoA-I hepatic enhancer (HE) or G6Pase promoter (positive control). Primers to GAPDH genomic DNA were used as non-specificity control. AB against β-actin were used as the control for non-specific binding. Values are presented as means ± the standard error of the mean of three independent experiments. The statistical analyses of differences between compared groups were performed using a non-paired Student’s t -test. There are no significant differences between levels of FOXO1 binding to apoA-I HE in the control cells and in the cells under oxidative stress
Hydrogen peroxide downregulates apoA-I gene expression through the sites B and C
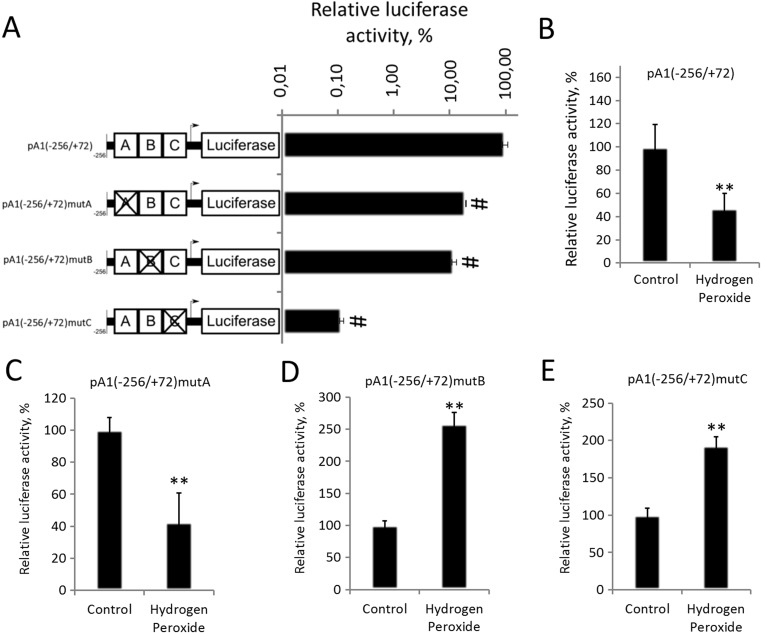
apoA-I gene expression in hepatocytes is mainly controlled by the hepatic enhancer. To test the possible role of the HE in the hydrogen peroxide-mediated repression of apoA-I gene, we transfected HepG2 cells with the plasmid bearing the reporter gene encoding luciferase driven by the intact HE (pA1(−256/+72)) or by the HE with disrupted sites A (pA1(−256/+72) mutA), B (pA1(−256/+72) mutB), or C (pA1(−256/+72) mutC) (Fig. 5a). Expression of the reporter gene under the control of intact HE was significantly suppressed in cells treated with H2O2 compared to the control cells, indicating that HE is sufficient for hydrogen peroxide-mediated repression of the apoA-I gene (Fig. 5b). All three mutations significantly suppressed luciferase activity (Fig. 5a). Hydrogen peroxide downregulated apoA-I HE activity in cells transfected with pA1(−256/+72) mutA (Fig. 5c), yet increased luciferase expression in cells transfected with pA1(−256/+72) mutB (Fig. 5d) and pA1(−256/+72) mutC (Fig. 5e). The experiments with plasmid constructs suggest that sites B and C participate in H2O2-dependent downregulation of apoA-I expression.
Fig. 5.
Hydrogen peroxide decreases apoA-I gene activity through sites B and C of the hepatic enhancer. Luciferase test. (a) HepG2 cells were transfected with plasmids carrying intact or mutated HE of apoA-I. RLA is the relative luciferase activity (100% is the activity of pA1(−256/+72) in control HepG2 cells). b–e HepG2 cells were transfected with plasmids carrying luciferase under control of intact or mutated HE and treated with 250 μM of hydrogen peroxide for 24 h. RLA is the relative luciferase activity (100% is the activity of pA1(−256/+72) (B), pA1(−256/+72) mutA (c), pA1(−256/+72) mutB (d), pA1(−256/+72) mutC (e) in control HepG2 cells). Values are presented as means ± the standard error of the mean of four independent experiments. The statistical analyses of differences between compared groups were performed using a non-paired Student’s t test (**p < 0.05) and Dunnett’s criterion (#p < 0.05)
We have shown that FOXO1 binds site B of the HE in HepG2 cells (Fig. 4a, b), and LXRα was earlier shown to bind to site C (Huuskonen et al. 2006). Moreover, we have demonstrated that both FOXO1 (Fig. 3d) and LXRα (Fig. 3g) are necessary for H2O2-induced repression of apoA-I gene in HepG2 cells. These data are in agreement with the necessity of both sites B and C (Fig. 5d, e) for the decrease in apoA-I promoter activity during oxidative stress.
AMPK downregulates apoA-I expression through LXRα
AMPK was shown to regulate both LXRα (Hwahng et al. 2009) and FOXO1 (Awad et al. 2014). To determine the transcription factor through which hydrogen peroxide-induced AMPK downregulates apoA-I expression, we transfected cells with siRNA against FOXO1, LXRα, or AMPK and treated them with either hydrogen peroxide or a well-known AMPK activator metformin (Fig. 6a). Treatment of HepG2 cells with metformin for 24 h resulted in the significant decrease in apoA-I gene activity, confirming that treatment of HepG2 cells with AMPK activators leads to suppression of apoA-I expression. According to the previous experiments (Figs. 2 and 3), knockdown of all three genes resulted in abolishment of H2O2-driven decrease in apoA-I expression. However, only siRNAs against LXRα or AMPK canceled metformin-induced downregulation of apoA-I transcription. These experiments show that activation of AMPK by hydrogen peroxide or metformin results in the downregulation of apoA-I expression through LXRα activation. Interestingly, AMPK-induced LXRα phosphorylation prevented the induction of several LXRα target genes by its ligand (Hwahng et al. 2009). However, we have shown that LXRα binds to apoA-I HE in complex with FOXA2 and FOXA2 is necessary for the downregulation of apoA-I expression by LXRα ligand (Shavva et al. 2016a). LXRα bound to FOXA2 may behave differently from its other forms.
Fig. 6.
The relationships between AMPK cascade and FOXO1 and LXRα transcription factors in the hydrogen peroxide-mediated downregulation of apoA-I gene. a Real-time RT-PCR. HepG2 cells (96 well plate) were transfected with 2.4 pmoles per well of scrambled siRNA (black columns), siRNA against FOXO1 (white columns), siRNA against LXRα (light gray columns or siRNA against AMPKa (dark gray columns) for 72 h before RNA isolation and treated with 250 μM of H2O2 for 24 h before RNA isolation. The activator of AMPK metformin (2 mM) was added 24 h before RNA isolation. Values are presented as means ± the standard error of the mean of four independent experiments. The statistical analyses of differences between compared groups were performed using a non-paired Student’s t test (**p < 0.05) and Dunnett’s criterion (#p < 0.05). b Western blotting analysis of subcellular localization of FOXO1 transcription factor. HepG2 cells were exposed to H2O2 (250 μM) for 2 h. JNK inhibitor SP600125 (10 μM) had been added 1 h before hydrogen peroxide. Crude cytoplasmic and nuclear fractions were isolated as described in Materials and methods and analyzed by Western assay. β-actin in cytosolic fractions was used as a normalization control. Diagram shows the quantification of Western blotting by densitometry. The Y-axis values correspond to the ratio (%) of nuclear pool of the FOXO1 transcription factor to its total amount. The total amounts of the transcription factor were normalized by β-actin. Values are presented as means ± the standard error of the mean of three independent blots. The statistical analyses of differences between compared groups were performed using a non-paired Student’s t test (**p < 0.05). c, d Real-time PCR calculation of ChIP. HepG2 cells were treated with hydrogen peroxide (250 μM) for 24 h. JNK inhibitor SP600125 (10 μM) had been added to cells 1 h before H2O2 administration. Histograms represent the relative level of FOXO1 (c) or LXRα (d) binding (100% in control cells) to apoA-I hepatic enhancer (HE) (black columns). Primers to GAPDH genomic DNA were used as non-specificity control (gray columns). AB against β-actin were used as the control for non-specific binding. Values are presented as means ± the standard error of the mean of three independent experiments. The statistical analyses of differences between compared groups were performed using a non-paired Student’s t test. There are no significant differences between levels of FOXO1 and LXRα binding to apoA-I HE in the control cells and in the cells under an oxidative stress
JNK induces FOXO1 nuclear translocation but fails to affect its binding to apoA-I HE
JNK may participate in the hydrogen peroxide-mediated downregulation of apoA-I gene expression by promoting FOXO1 nuclear localization (see above). To check this assumption, cells were pretreated for 1 hour with JNK inhibitor SP600125 before addition of H2O2 and harvested 2 hours later. Hydrogen peroxide treatment triggered FOXO1 translocation from cytoplasm to the nucleus. Pretreatment with JNK inhibitor prevented this effect (Fig. 6b). These data confirm the involvement of JNK in nuclear accumulation of FOXO1 during oxidative stress possibly by phosphorylation of 14-3-3ζ. To assess the role of JNK in promoting FOXO1 binding to apoA-I HE, chromatin immunoprecipitation was performed. In agreement with earlier experiments (Fig. 4b), hydrogen peroxide treatment had no significant effect on FOXO1 binding to HE, and pretreatment of HepG2 cells with SP600125 had no effect either (Fig. 6c). Similar results were obtained with LXRα (Fig. 6d). The possible reason for this discrepancy (the decrease of nuclear FOXO1 caused by JNK inhibitor under oxidative stress does not lead to departure of FOXO1 from apoA-I HE) is the fact that FOXO1 interacts with HE of apoA-I as part of a complex with LXRβ that can attach FOXO1 to the HE of apoA-I (Shavva et al. 2016a). However, knockdown of LXRβ does not prevent the hydrogen peroxide-mediated downregulation of apoA-I gene expression (data not shown). Alternatively, direct or indirect effects of JNK on FOXO1 under oxidative stress may be based on the change of FOXO1 trans-activation potential rather than the change of FOXO1 DNA-binding properties. Therefore, further investigations should be carried out to clarify the exact role of FOXO1 in the JNK-mediated downregulation of apoA-I gene expression under oxidative stress.
CONCLUSIONS
Here, we have first shown that apoA-I gene expression, protein synthesis, and secretion are decreased during H2O2-induced oxidative stress. This decline depends on the FOXO1 and LXRα transcription factors and on JNK, p38, and/or AMPK kinase cascades. We have first demonstrated that FOXO1 interacts with apoA-I hepatic enhancer and represses apoA-I gene activity. The similarities between inflammatory response, hypoxia, and oxidative stress are well known (Görlach et al. 2015). ApoA-I protein is a negative marker of acute response; its synthesis is strongly downregulated under inflammation in hepatocytes, so the negative regulation of apoA-I gene expression under oxidative stress demonstrated in this work is not surprising. Interestingly, both transcription factors responsible for H2O2-mediated repression of apoA-I gene—FOXO1 and LXRα take part in the TNFα-driven downregulation of apoA-I gene (Mogilenko et al. 2009; Orlov et al. 2010; Shavva et al. 2016a). Reduced levels of plasma HDL are observed in cigarette smokers (Assmann et al. 1984). Taking into account the known facts about high levels of generation of reactive oxygen species such as superoxides and hydrogen peroxide in cigarette smokers’ plasma, our work suggests a molecular mechanism explaining a decline of HDL in cigarette smokers.
Acknowledgements
The work has been supported by the Russian Foundation for Basic Research (Grants 15-04-07512, 15-04-08186, 15-04-07918 and 16-04-01312).
Contributor Information
Vladimir S. Shavva, Phone: +7 (812) 346-0644, Email: shavva@iem.sp.ru
Sergey V. Orlov, Phone: +7 (812) 346-0644, Email: serge@iem.sp.ru
References
- Aikawa R, Komuro I, Yamazaki T, Zou Y, Kudoh S, Tanaka M, Shiojima I, Hiro Y, Yazaki Y. Oxidative stress activates extracellular signal-regulated kinases through Src and Ras in cultured cardiac myocytes of neonatal rats. J Clin Invest. 1997;100:1813–1821. doi: 10.1172/JCI119709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews NC, Faller DV. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991;19:2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada S, Daitoku H, Matsuzaki H, Saito T, Sudo T, Mukai H, Iwashita S, Kako K, Kishi T, Kasuya Y, Fukamizu A. Mitogen-activated protein kinases, Erk and p38, phosphorylate and regulate Foxo1. Cell Signal. 2007;19:519–527. doi: 10.1016/j.cellsig.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Assmann G, Schulte H, Schriewer H. The effects of cigarette smoking on serum levels of HDL cholesterol and HDL apolipoprotein A-I. Findings of a prospective epidemiological study on employees of several companies in Westphalia, West Germany. J Clin Chem Clin Biochem. 1984;22:397–402. doi: 10.1515/cclm.1984.22.6.397. [DOI] [PubMed] [Google Scholar]
- Awad H, Nolette N, Hinton M, Dakshinamurti S. AMPK and FoxO1 regulate catalase expression in hypoxic pulmonary arterial smooth muscle. Pediatr Pulmonol. 2014;49:885–897. doi: 10.1002/ppul.22919. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Chan J, Nakabayashi H, Wong NC. HNF-4 increases activity of the rat Apo A1 gene. Nucleic Acids Res. 1993;21:1205–1211. doi: 10.1093/nar/21.5.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Guo S, Copps K, Dong X, Kollipara R, Rodgers JT, Depinho RA, Puigserver P, White MF. Foxo1 integrates insulin signaling with mitochondrial function in the liver. Nat Med. 2009;15:1307–1311. doi: 10.1038/nm.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Oh S, Lee D, Oh HJ, Park JY, Lee SB, Lim DS. Mst1-FoxO signaling protects naive T lymphocytes from cellular oxidative stress in mice. PLoS One. 2009;4:1–10. doi: 10.1371/journal.pone.0005361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert C, Wang Z, Zhang X, Tam SP. Regulation of human apolipoprotein A-I gene expression by gramoxone. J Biol Chem. 1997;272:14954–14960. doi: 10.1074/jbc.272.23.14954. [DOI] [PubMed] [Google Scholar]
- Görlach A, Dimova EY, Petry A, Martínez-Ruiz A, Hernansanz-Agustín P, Rolo AP, Palmeira CM, Kietzmann T. Reactive oxygen species, nutrition, hypoxia and diseases: problems solved? Redox Biol. 2015;6:372–385. doi: 10.1016/j.redox.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizhe EB, Ignatovich IA, Burov SV, Pohvoscheva AV, Akifiev BN, Efremov AM, Perevozchikov AP, Orlov SV. Complexes of DNA with cationic peptides: conditions of formation and factors effecting internalization by mammalian cells. Biochem Mosc. 2006;71:1350–1356. doi: 10.1134/S0006297906120108. [DOI] [PubMed] [Google Scholar]
- Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- Eggerman TL, Hoeg JM, Meng MS, Tombragel A, Bojanovski D, Brewer JHB. Differential tissue-specific expression of human apoA-l and apoA-II. J Lipid Res. 1991;32:821–828. [PubMed] [Google Scholar]
- Essers MG, Weijzen S, de Vries-Smits AMM, Saarloos I, de Ruiter ND, Bos JL, Burgering BMT. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 2004;23:4802–4812. doi: 10.1038/sj.emboj.7600476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannoni E, Buricchi F, Raugei G, Ramponi G, Chiarugi P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol Cell Biol. 2005;25:6391–6403. doi: 10.1128/MCB.25.15.6391-6403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg GS, Ozer J, Karathanasis SK. Intestinal apolipoprotein AI gene transcription is regulated by multiple distinct DNA elements and is synergistically activated by the orphan nuclear receptor, hepatocyte nuclear factor 4. J Clin Invest. 1995;96:528–538. doi: 10.1172/JCI118065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas MJ, Horani M, Mreyoud A, Plummer B, Wong NCW, Mooradian AD. Suppression of apolipoprotein AI gene expression in HepG2 cells by TNFalpha and IL-1beta. Biochim Biophys Acta. 2003;1623:120–128. doi: 10.1016/j.bbagen.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Han Y, Wang Q, Song P, Zhu Y, Zou MH. Redox regulation of the AMP-activated protein kinase. PLoS One. 2010;5:e15420. doi: 10.1371/journal.pone.0015420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnish DC, Malik S, Karathanasis SK. Activation of apolipoprotein AI gene transcription by the liver-enriched factor HNF-3. J Biol Chem. 1994;269:28220–28226. [PubMed] [Google Scholar]
- Harnish DC, Malik S, Kilbourne E, Costa R, Karathanasis SK. Control of apolipoprotein AI gene expression through synergistic interactions between hepatocyte nuclear factors 3 and 4. J Biol Chem. 1996;271:13621–13628. doi: 10.1074/jbc.271.23.13621. [DOI] [PubMed] [Google Scholar]
- Higuchi K, Law SW, Hoeg JM, Schumacher UK, Meglin N, Brewer HB. Tissue-specific expression of apolipoprotein A-I (apoA-I) is regulated by the 5′-flanking region of the human apoA-I gene. J Biol Chem. 1988;263:18530–18536. [PubMed] [Google Scholar]
- Hirota K, Sakamaki JI, Ishida J, Shimamoto Y, Nishihara S, Kodama N, Ohta K, Yamamoto M, Tanimoto K, Fukamizu A. A combination of HNF-4 and Foxo1 is required for reciprocal transcriptional regulation of glucokinase and glucose-6-phosphatase genes in response to fasting and feeding. J Biol Chem. 2008;283:32432–32441. doi: 10.1074/jbc.M806179200. [DOI] [PubMed] [Google Scholar]
- Hopkins PN. Molecular biology of atherosclerosis. Physiol Rev. 2013;93:1317–1542. doi: 10.1152/physrev.00004.2012. [DOI] [PubMed] [Google Scholar]
- Huuskonen J, Vishnu M, Chau P, Fielding PE, Fielding CJ. Liver X receptor inhibits the synthesis and secretion of apolipoprotein A1 by human liver-derived cells. Biochemistry. 2006;45:15068–15074. doi: 10.1021/bi061378y. [DOI] [PubMed] [Google Scholar]
- Hwahng SH, Ki SH, Bae EJ, Kim HE, Kim SG. Role of adenosine monophosphate-activated protein kinase-p70 ribosomal S6 kinase-1 pathway in repression of liver X receptor-alpha-dependent lipogenic gene induction and hepatic steatosis by a novel class of dithiolethiones. Hepatology. 2009;49:1913–1925. doi: 10.1002/hep.22887. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Byun JY, Yun CH, Park IC, Lee KH, Lee SJ. c-Src-p38 mitogen-activated protein kinase signaling is required for Akt activation in response to ionizing radiation. Mol Cancer Res. 2008;6:1872–1880. doi: 10.1158/1541-7786.MCR-08-0084. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Ido Kitamura Y. Role of FoxO proteins in pancreatic beta cells. Endocr J. 2007;54:507–515. doi: 10.1507/endocrj.KR-109. [DOI] [PubMed] [Google Scholar]
- Klotz LO, Sánchez-Ramos C, Prieto-Arroyo I, Urbánek P, Steinbrenner H, Monsalve M. Redox regulation of FoxO transcription factors. Redox Biol. 2015;6:51–72. doi: 10.1016/j.redox.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontush A, Chapman MJ. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol Rev. 2006;58:342–374. doi: 10.1124/pr.58.3.1. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. 2012;92:689–737. doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]
- Lapikov IA, Mogilenko DA, Dizhe EB, Ignatovich IA, Orlov SV, Perevozchikov AP. Ap1-like cis-elements in 5′-regulatory region of human apolipoprotein A-I gene. Mol Biol (Mosk) 2008;42:295–305. doi: 10.1134/S002689330802012X. [DOI] [PubMed] [Google Scholar]
- Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villen J, Becker EB, DiBacco S, de la Iglesia N, Gygi S, Blackwell TK, Bonni A. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ Res. 2005;96:1221–1232. doi: 10.1161/01.RES.0000170946.56981.5c. [DOI] [PubMed] [Google Scholar]
- Liu X, Cui Y, Li M, Xu H, Zuo J, Fang F, Chang Y. Cobalt protoporphyrin induces HO-1 expression mediated partially by FOXO1 and reduces mitochondria-derived reactive oxygen species production. PLoS One. 2013;8:1–9. doi: 10.1371/journal.pone.0080521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S, Karathanasis SK. TFIIB-directed transcriptional activation by the orphan nuclear receptor hepatocyte nuclear factor 4. Mol Cell Biol. 1996;16:1824–1831. doi: 10.1128/MCB.16.4.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G, Duez H, Blanquart C, Berezowski V, Poulain P, Fruchart JC, Najib-Fruchart J, Glineur C, Staels B. Statin-induced inhibition of the rho-signaling pathway activates PPARα and induces HDL apoA-I. J Clin Invest. 2001;107:1423–1432. doi: 10.1172/JCI10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez SC, Tanabe K, Crasme C, Abumrad NA, Bernal-mizrachi E, Permutt MA. Inhibition of Foxo1 protects pancreatic islet beta-cells stress against fatty acid and endoplasmic reticulum stress–induced apoptosis. Diabetes. 2008;57:846–859. doi: 10.2337/db07-0595. [DOI] [PubMed] [Google Scholar]
- McVicar JP, Kunitake ST, Hamilton RL, Kane JP. Characteristics of human lipoproteins isolated by selected-affinity immunosorption of apolipoprotein A-I. Proc Natl Acad Sci U S A. 1984;81:1356–1360. doi: 10.1073/pnas.81.5.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogilenko DA, Dizhe EB, Shavva VS, Lapikov IA, Orlov SV, Perevozchikov AP. Role of the nuclear receptors HNF4 alpha, PPAR alpha, and LXRs in the TNF alpha-mediated inhibition of human apolipoprotein A-I gene expression in HepG2 cells. Biochemistry. 2009;48:11950–11960. doi: 10.1021/bi9015742. [DOI] [PubMed] [Google Scholar]
- Mogilenko DA, Kudriavtsev IV, Shavva VS, Dizhe EB, Vilenskaya EG, Efremov AM, Perevozchikov AP, Orlov SV. Peroxisome proliferator-activated receptor α positively regulates complement C3 expression but inhibits tumor necrosis factor α-mediated activation of C3 gene in mammalian hepatic-derived cells. J Biol Chem. 2013;288:1726–1738. doi: 10.1074/jbc.M112.437525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogilenko DA, Kudriavtsev IV, Trulioff AS, Shavva VS, Dizhe EB, Missyul BV, Zhakhov AV, Ischenko AM, Perevozchikov AP, Orlov SV. Modified low density lipoprotein stimulates complement C3 expression and secretion via liver X receptor and toll-like receptor 4 activation in human macrophages. J Biol Chem. 2012;287:5954–5968. doi: 10.1074/jbc.M111.289322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogilenko DA, Orlov SV, Trulioff AS, Ivanov AV, Nagumanov VK, Kudriavtsev IV, Shavva VS, Tanyanskiy DA, Perevozchikov AP. Endogenous apolipoprotein A-I stabilizes ATP-binding cassette transporter A1 and modulates toll-like receptor 4 signaling in human macrophages. FASEB J. 2012;26:2019–2030. doi: 10.1096/fj.11-193946. [DOI] [PubMed] [Google Scholar]
- Morishima A, Ohkubo N, Maeda N, Miki T, Mitsuda N. NFkappaB regulates plasma apolipoprotein A-I and high density lipoprotein cholesterol through inhibition of peroxisome proliferator-activated receptor alpha. J Biol Chem. 2003;278:38188–38193. doi: 10.1074/jbc.M306336200. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxic assays. J Immunol Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Oleaga C, Ciudad CJ, Izquierdo-Pulido M, Noé V. Cocoa flavanol metabolites activate HNF-3β, Sp1, and NFY-mediated transcription of apolipoprotein AI in human cells. Mol Nutr Food Res. 2013;57:986–995. doi: 10.1002/mnfr.201200507. [DOI] [PubMed] [Google Scholar]
- Orlov SV, Kuteykin-Teplyakov KB, Ignatovich IA, Dizhe EB, Mirgorodskaya OA, Grishin AV, Guzhova OB, Prokhortchouk EB, Guliy PV, Perevozchikov AP. Novel repressor of the human FMR1 gene—identification of p56 human (GCC)n-binding protein as a Kruppel-like transcription factor ZF5. FEBS J. 2007;274:4848–4862. doi: 10.1111/j.1742-4658.2007.06006.x. [DOI] [PubMed] [Google Scholar]
- Orlov SV, Mogilenko DA, Shavva VS, Dizhe EB, Ignatovich IA, Perevozchikov AP. Effect of TNFalpha on activities of different promoters of human apolipoprotein A-I gene. Biochem Biophys Res Commun. 2010;398:224–230. doi: 10.1016/j.bbrc.2010.06.064. [DOI] [PubMed] [Google Scholar]
- Rani V, Deep G, Singh RK, Palle K, Yadav UCS. Oxidative stress and metabolic disorders: pathogenesis and therapeutic strategies. Life Sci. 2016;148:183–193. doi: 10.1016/j.lfs.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Rena G, Guo S, Cichy SC, Unterman TG, Cohen P. Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J Biol Chem. 1999;274:17179–17183. doi: 10.1074/jbc.274.24.17179. [DOI] [PubMed] [Google Scholar]
- Sastry KN, Seedorf U, Karathanasis SK. Different cis-acting DNA elements control expression of the human apolipoprotein AI gene in different cell types. Mol Cell Biol. 1988;8:605–614. doi: 10.1128/MCB.8.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoll D, Walker KS, Alessi DR, Grempler R, Burchell A, Guo S, Walther R, Unterman TG. Regulation of glucose-6-phosphatase gene expression by protein kinase Balpha and the forkhead transcription factor FKHR. Evidence for insulin response unit-dependent and-independent effects of insulin on promoter activity. J Biol Chem. 2000;275:36324–36333. doi: 10.1074/jbc.M003616200. [DOI] [PubMed] [Google Scholar]
- Sengupta A, Molkentin JD, Paik JH, DePinho RA, Yutzey KE. FoxO transcription factors promote cardiomyocyte survival upon induction of oxidative stress. J Biol Chem. 2011;286:7468–7478. doi: 10.1074/jbc.M110.179242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavva VS, Bogomolova AM, Nikitin AA, Dizhe EB, Tanyanskiy DA, Efremov AM, Oleinikova GN, Perevozchikov AP, Orlov SV. Insulin-mediated downregulation of apolipoprotein A-I gene in human hepatoma cell line HepG2: the role of interaction between FOXO1 and LXRβ transcription factors. J Cell Biochem. 2016 doi: 10.1002/jcb.25651. [DOI] [PubMed] [Google Scholar]
- Shavva VS, Mogilenko DA, Bogomolova AM, Nikitin AA, Dizhe EB, Efremov AM, Oleinikova GN, Perevozchikov AP, Orlov SV. PPARγ represses apolipoprotein A-I gene but impedes TNFα-mediated apoA-I downregulation in HepG2 cells. J Cell Biochem. 2016;117:2010–2022. doi: 10.1002/jcb.25498. [DOI] [PubMed] [Google Scholar]
- Shen M, Lin F, Zhang J, Tang Y, Chen WK, Liu H. Involvement of the up-regulated FoxO1 expression in follicular granulosa cell apoptosis induced by oxidative stress. J Biol Chem. 2012;287:25727–25740. doi: 10.1074/jbc.M112.349902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Saito K, Fujigaki S, Noma A, Ishiguro H, Nagatsu T, Seishima M. IL-1 beta and TNF-alpha suppress apolipoprotein (apo) E secretion and apo A-I expression in HepG2 cells. Cytokine. 1998;10:275–280. doi: 10.1006/cyto.1997.0291. [DOI] [PubMed] [Google Scholar]
- Sunayama J, Tsuruta F, Masuyama N, Gotoh Y. JNK antagonizes Akt-mediated survival signals by phosphorylating 14-3-3. J Cell Biol. 2005;170:295–304. doi: 10.1083/jcb.200409117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangeman L, Wyatt CN, Brown TL. Knockdown of AMP-activated protein kinase alpha 1 and alpha 2 catalytic subunits. J RNAi Gene Silenc. 2012;8:470–478. [PMC free article] [PubMed] [Google Scholar]
- Van Der Heide LP, Hoekman MFM, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J. 2004;380:297–309. doi: 10.1042/bj20040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Kooi BT, Streeper RS, Svitek CA, Oeser JK, Powell DR, O’Brien RM. The three insulin response sequences in the glucose-6-phosphatase catalytic subunit gene promoter are functionally distinct. J Biol Chem. 2003;278:11782–11793. doi: 10.1074/jbc.M212570200. [DOI] [PubMed] [Google Scholar]
- Widom RL, Ladias J, Kouidou S, Karathanasis SK. Synergistic interactions between transcription factors control expression of the apolipoprotein AI gene in liver cells. Mol Cell Biol. 1991;11:677–687. doi: 10.1128/MCB.11.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K, Daitoku H, Takahashi Y, Namiki K, Hisatake K, Kako K, Mukai H, Kasuya Y, Fukamizu A. Arginine methylation of FOXO transcription factors inhibits their phosphorylation by Akt. Mol Cell. 2008;32:221–231. doi: 10.1016/j.molcel.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Yeagley D, Guo S, Unterman T, Quinn PG. Gene- and activation-specific mechanisms for insulin inhibition of basal and glucocorticoid-induced insulin-like growth factor binding protein-1 and phosphoenolpyruvate carboxykinase transcription. Roles of forkhead and insulin response sequences. J Biol Chem. 2001;276:33705–33710. doi: 10.1074/jbc.M101215200. [DOI] [PubMed] [Google Scholar]
- Yinghua JU, Taojun XU, Zhang H, Aiming YU. FOXO1-dependent DNA damage repair is regulated by JNK in lung cancer cells. Int J Oncol. 2014;44:1284–1292. doi: 10.3892/ijo.2014.2269. [DOI] [PubMed] [Google Scholar]
- Yoshizumi M, Abe J, Haendeler J, Huang Q, Berk BC. Src and Cas mediate JNK activation but not ERK1/2 and p38 kinases by reactive oxygen species. J Biol Chem. 2000;275:11706–11712. doi: 10.1074/jbc.275.16.11706. [DOI] [PubMed] [Google Scholar]