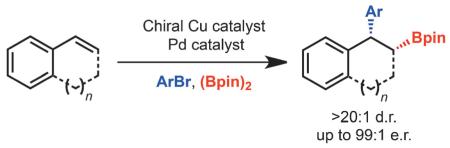
Abstract
A method for the catalytic enantioselective arylboration of alkenylarenes is disclosed. The reaction leads to the formation of 1,1-diarylalkanes that also incorporate an additional pinacol boronic ester which can be easily transformed to a variety of groups. The products are formed with excellent diastereoselectivities and enantioselectivities.
Keywords: alkenes, copper, cross-coupling, enantioselective catalysis, palladium
Graphical abstract
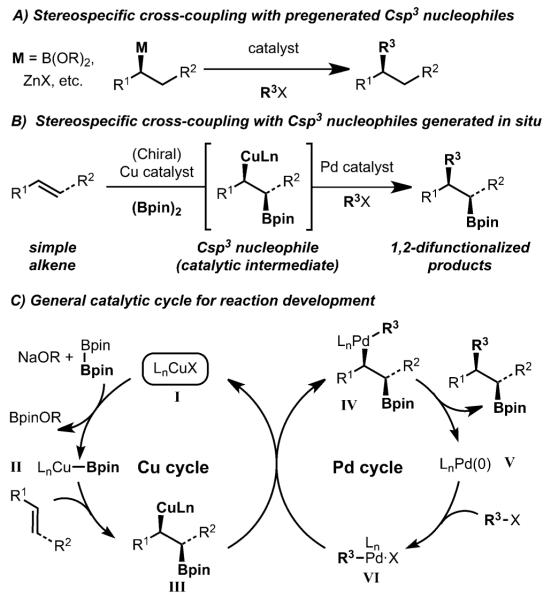
Metal-catalyzed cross-coupling reactions are a valuable method for the construction of Csp2–Csp2 bonds. The translation of such methods to the formation of Csp2–Csp3 bonds has met with significant challenges; however, in recent years notable progress has been made.[1] Particularly difficult cross-coupling reactions are those that involve secondary Csp3 nucleophiles. Moreover, instances in which stereodefined secondary nucleophiles are employed to achieve stereospecific cross-coupling are even more rare (Scheme 1A). It should be noted that recent and significant progress has been made in this area with Pd-catalyzed Suzuki–Miyaura,[2] Negishi,[3] and Stille[4] cross-coupling reactions.[1]
Scheme 1.
Approaches towards stereospecific cross-coupling.
In recent years, our laboratory has taken an interest in developing a complementary approach towards Pd-catalyzed stereospecific cross-coupling of stereodefined Csp3 nucleophiles. In particular, we envisioned a one-step process that would involve generation of a stereodefined Csp3 nucleophile (III) as a catalytic intermediate by addition of CuBpin complexes (Bpin: pinacol boronic ester) across a simple alkene, followed by Pd-catalyzed stereospecific cross-coupling (III to IV to V) to formally constitute a carboboration reaction (Schemes 1B,C).[5] This method is an attractive approach for chemical synthesis because simple alkenes are used as the starting material and two new bonds and potentially two new stereocenters are generated in a single operation. Our laboratory, and that of Semba and Nakao,[6] independently developed arylboration reactions of styrene derivatives that function according to this model. While diastereoselective variants of these dual catalyst reactions has been achieved, enantioselective processes have been slow to emerge.[7] However, recent progress has been made by Liao et al. with Pd/Cu-catalyzed enantioselective allylboration of alkenes (Scheme 2A);[8] one example of arylboration was also presented. Buchwald and coworkers recently disclosed an enantioselective hydroarylation that operates by Pd/Cu dual catalysis.[9]
Scheme 2.
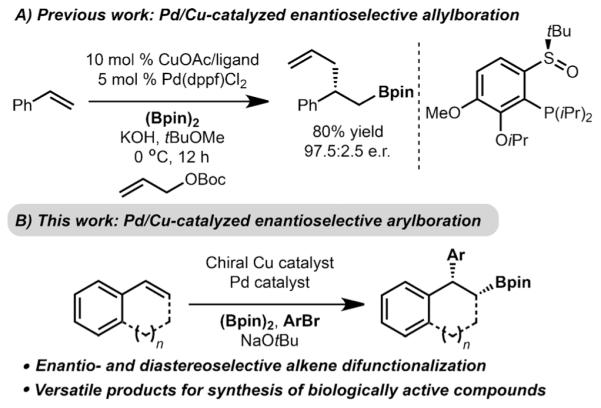
Pd/Cu-catalyzed enantioselective carboboration.
Herein, we describe an enantioselective arylboration of alkenylarenes that leads to the formation of 1,1-diarylalkanes (Scheme 2B). This method is significant and different from prior efforts in Pd/Cu-catalyzed carboboration because highly enantio- and diastereoselective difunctionalization can be achieved while establishing two stereocenters. It is also important to note that while other methods exist for the enantioselective synthesis of 1,1-diarylalkanes, such as hydrogenation,[10] conjugate addition,[11] and cross-coupling,[1a,12] one main feature of the process described herein is that, in addition to generating the 1,1-diarylalkane motif, a stereogenic carbon bearing a synthetically versatile Bpin group is also incorporated. With respect to the latter point, the utility of this method is highlighted in the synthesis of biologically relevant small molecules.
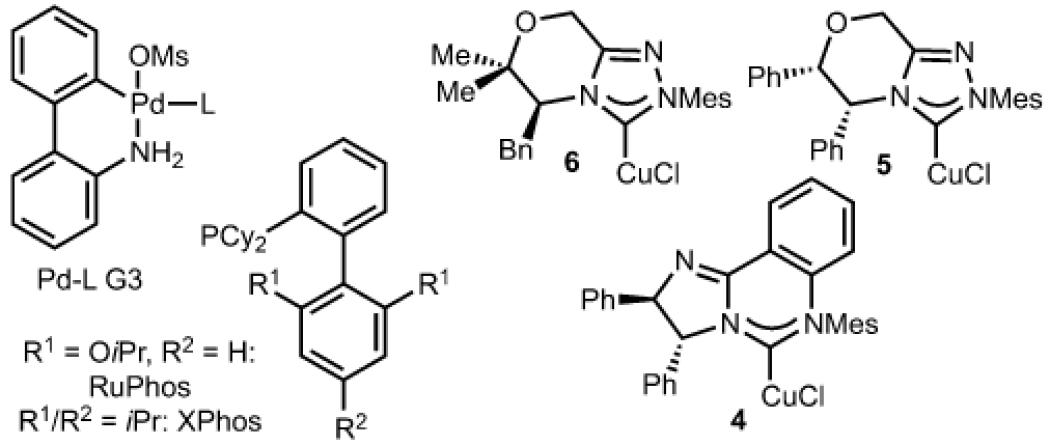
Based on prior efforts from our laboratory, N-heterocyclic carbene(NHC) copper complexes (vs. the analogous phosphine complexes) were uniquely effective for the Pd/Cu-arylboration process.[6b,c] To achieve highly enantioselective arylboration, a chiral copper catalyst that is stereodiscriminating at (or near) room temperature was required (ambient temperature is necessary for operation of the palladium catalysis described here).[13] This initiative led to evaluation of chiral complexes (4–6) that project substituents towards the metal center so as to create a defined chiral environment.[14,15]
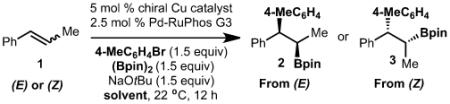
As illustrated in Table 1, entries 1–3, we evaluated the effectiveness of copper complexes 4–6 for arylboration of trans-β-methyl styrene ((E)-1) and observed formation of 2 with modest enantioselectivity. It is important to note that Pd-RuPhos G3[16] was used because prior efforts from our laboratory demonstrated its effectiveness at facilitating a stereoretentive transmetalation of Csp3 copper complexes.[6c] Further evaluation of reaction conditions and substrates revealed that reaction with cis-β-methyl styrene ((Z)-1) was significantly more enantioselective with the copper complex 4, generating the product in 91:9 e.r. (11:1 d.r.; Table 1, entry 4). Based on our prior reports, use of solvent mixtures can lead to improved results. As such, use of toluene:MeCN (10:1) allowed for product formation in 50:1 d.r. and 96:4 e.r. (Table 1, entry 11).
Table 1.
Reaction optimization.
| Entry | (E)/(Z) | Copper catalyst |
Solvent | Yield [%][a] | d.r.[b] | e.r.[c] |
|---|---|---|---|---|---|---|
| 1 | (E) | 4 | THF | 45 | 15:1 | 70:30 |
| 2 | (E) | 5 | THF | 32 | 5:1 | 82:18 |
| 3 | (E) | 6 | THF | 32 | 9:1 | 43:57 |
| 4 | (Z) | 4 | THF | 46 | 11:1 | 91:9 |
| 5 | (Z) | 5 | THF | <5 | – | – |
| 6 | (Z) | 6 | THF | <5 | – | – |
| 7 | (Z) | 4 | toluene | 68 | 8:1 | 90:10 |
| 8 | (Z) | 4 | toluene:THF (10:1) |
78 | 15:1 | 94:6 |
| 9 | (Z) | 4 | toluene:Et20 (10:1) |
26 | 6:1 | 86:14 |
| 10 | (Z) | 4 | toluene:DMF (10:1) |
58 | 16:1 | 95:5 |
| 11 | (Z) | 4 | toluene:MeCN (10:1) |
83 | 50:1 | 96:4 |
| ||||||
Yield was determined by gas chromatography (GC) analysis with a calibrated internal standard.
Diastereomeric ratio (d.r.) was determined by GC analysis of the unpurified reaction mixture.
Determined by HPLC (with a chiral column) of the purified products. Solvent key: tetrahydrofuran (THF); diethyl ether (Et2O); N,N,-dimethylformamide (DMF); acetonitrile (MeCN).
With an optimized set of conditions in hand, the scope of this process was explored (Table 2). With respect to the alkene component, electron-poor (product 7; Table 2) and electron-rich (product 18; Scheme 3) alkenylarenes could be used. Increased alkyl substitution was tolerated, provided that the β-substituent was not too sterically demanding (compare products 8 and 10; Table 2). Reaction with styrene led to formation of 11 in good yield, albeit with slightly reduced enantioselectivity.
Table 2.
Arylboration of various alkenes.

|
Yield reported as the average of at least two experiments. Diastereomeric ratio (d.r.) was determined by 1H NMR spectroscopic analysis of the unpurified reaction mixture. Enantiomeric ratio (e.r.) was determined by high performance liquid chromatography (HPLC) analysis (with a chiral column) of the purified products.
Yield determined by 1H NMR analysis with an internal standard.
5 mol% copper catalyst (4) was added in two portions (see the Supporting Information for details).
KOSiMe3 was used instead of NaOtBu.
Scheme 3.
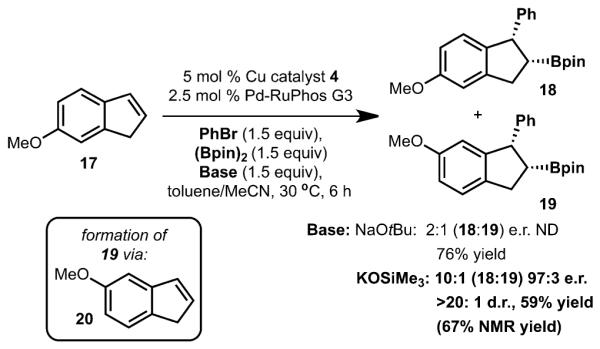
Base effects for arylboration of 6-methoxyindene (15).
Cyclic alkenylarenes, such as indene, 1,2-dihydronaphthalene, and 2H-chromene, also work well in these reactions (products 12, 13, and 14, respectively; Table 2). In the case of reactions with styrene, 1,2-dihydronaphthalene, and 2H-chromene, use of KOTMS was slightly superior to that of NaOtBu. Finally, it should be noted that in all cases the products were generated as a single diastereomer (> 20:1 d.r.). Additionally, strained alkenes could also be used to provide 15 and 16.[5h,17]
6-Methyoxyindene (17) was also evaluated under the standard reaction conditions and gave rise to a mixture of regioisomeric products 18 and 19 in a 2:1 ratio (Scheme 3). It was reasoned that the formation of 19 likely arises from a base-mediated isomerization of 6-methoxyindene (17) to 5-methoxyindene (20). To avoid the undesired isomerization reaction, the weaker base KOTMS (vs. NaOtBu) was used. Under the new reaction conditions, the formation of 19 was drastically suppressed such that 18 could be generated in 59% yield as a 10:1 mixture of regioisomers in 97:3 e.r. and > 20:1 d.r.
As illustrated in Table 3, a range of arylbromides were evaluated. Under the standard reaction conditions, electron-rich (products 22 and 29), electron-deficient (products 21, 23, 24, and 25) and various heterocyclic aryl bromides (products 27–29) all functioned well. The primary limitation with respect to aryl bromides is that sterically large groups were not tolerated (for example, 2-MeC6H4Br, product 26). The only ortho-substitution that was found to work was 2-ClC6H4Br (product 25). Finally, the absolute configuration of 30 was established by obtaining an X-ray crystal structure.
Table 3.
Reactions with various aryl bromides.
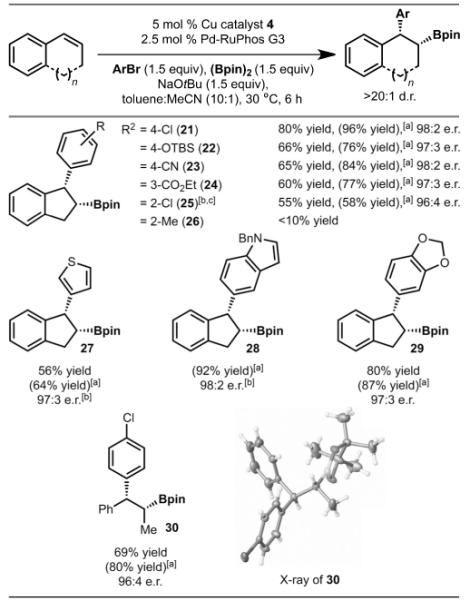
|
Yield reported as the average of at least two experiments. Diastereomeric ratio (d.r.) was determined by 1H NMR spectroscopic analysis of the unpurified reaction mixture. Enantiomeric ratio (e.r.) was determined by HPLC analysis (with a chiral column) of the purified reaction mixture.
Yield determined by 1H NMR spectroscopic analysis of the unpurified reaction mixture with an internal standard.
5 mol% copper catalyst (4) was added in two portions, (see the Supporting Information for details).
Generated in 18:1 d.r.
To demonstrate the utility of this method, glucocorticoid receptor partial agonist 34 was prepared by a short synthetic sequence (see Scheme 4).[18] The synthesis commenced with catalytic enantioselective arylboration of cis-β-methyl styrene ((Z)-1) and 5-bromoindazole derivative 31 to generate 32 with good selectivity. Homologation and oxidation of 32 led to formation of 33 in 46% yield. Finally, 34 could be prepared by the Mitsunobu reaction with BocNHMs followed by Boc deprotection in 50% yield over two steps.
Scheme 4.
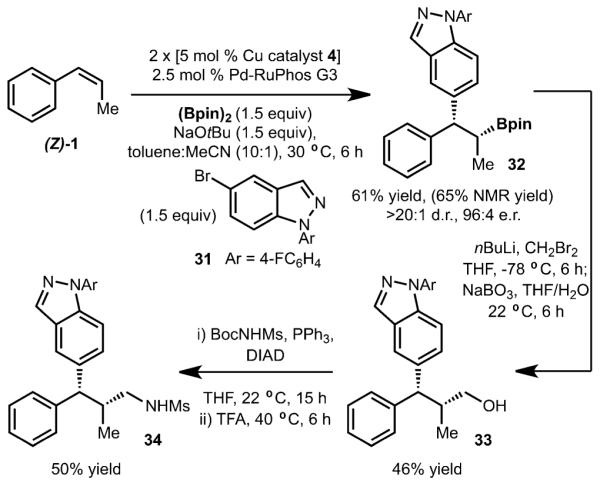
Synthesis of 34.
As noted in prior efforts from our laboratory, formation of diastereomeric products could be achieved by simply changing the palladium complex (Pd-RuPhos to -XPhos; see Table 1 for structures) and solvent (toluene/MeCN or tetrahydrofuran to toluene).[6c] Therefore, the possibility for synthesis of the anti-diastereomers was also investigated with chiral NHC copper complex 4. This was found to work well with 1,2-dihydronaphthalene to generate 36 in good yield, with diastereo- and enantioselectivity (Scheme 5).[19] Additionally, anti-selective arylboration of 37 with bromo-benzene led to formation of 38 in > 20:1 d.r. and > 99:1 e.r. in 83% yield. Borane 38 was converted to amine 39,[20] which is a known intermediate in the synthesis of dopamine receptor agonist, dihydrexidine (40).[21]
Scheme 5.

Anti-selective arylboration.
In conclusion, a method for the enantioselective arylboration of styrene derivatives has been developed. The reactions functioned well for a variety of alkenylarenes and provided rapid access to molecules of biological interest. This method represents an attractive strategy for the synthesis of 1,1-diarylalkanes from simple precursors.
Supplementary Material
Acknowledgements
We thank Indiana University and the National Institutes of Health (1R01GM114443) for generous financial support.
Footnotes
Supporting information for this article can be found under: http://dx.doi.org/10.1002/anie.201609844.
Conflict of interest
The authors declare no conflict of interest.
References
- [1] a).For reviews, see: Jana R, Pathak TP, Sigman MS. Chem. Rev. 2011;111:1417. doi: 10.1021/cr100327p.; Swift EC, Jarvo ER. Tetrahedron. 2013;69:5799. doi: 10.1016/j.tet.2013.05.001.; Wang C-Y, Derosa J, Biscoe MR. Chem. Sci. 2015;6:5105. doi: 10.1039/c5sc01710f.; Cherney AH, Kadunce NT, Reisman SE. Chem. Rev. 2015;115:9587. doi: 10.1021/acs.chemrev.5b00162.
- [2] a).For selected recent examples, see: Awano T, Ohmura T, Suginome M. J. Am. Chem. Soc. 2011;133:20738. doi: 10.1021/ja210025q.; Li L, Zhao S, Joshi-Pangu A, Diane M, Biscoe MR. J. Am. Chem. Soc. 2014;136:14027. doi: 10.1021/ja508815w.; Matthew SC, Glasspoole BW, Eisenberger P, Crudden CM. J. Am. Chem. Soc. 2014;136:5828. doi: 10.1021/ja412159g.
- [3].For a recent example, see: Campos KR, Klapars A, Waldman JH, Dormer PG, Chen C-Y. J. Am. Chem. Soc. 2006;128:3538. doi: 10.1021/ja0605265.
- [4].For a recent example, see: Li L, Wang C-Y, Huang R, Biscoe MR. Nat. Chem. 2013;5:607. doi: 10.1038/nchem.1652.
- [5] a).For reviews, see: Shimizu Y, Kanai M. Tetrahedron Lett. 2014;55:3727.; Semba K, Fujihara T, Terao J, Tsuji Y. Tetrahedron. 2015;71:2183. Lazreg F, Nahra F, Cazin CSJ. Coord. Chem. Rev. 2015;293 – 294:48.; Neeve EC, Geier SJ, Mkhalid IAI, Westcott SA, Marder TB. Chem. Rev. 2016;116:9091. doi: 10.1021/acs.chemrev.6b00193.; For selected recent studies, see: Li X, Meng F, Torker S, Shi Y, Hoveyda AH. Angew. Chem. Int. Ed. 2016;55:9997. doi: 10.1002/anie.201605001.; Angew. Chem. 2016;128:10151.; Semba K, Ohtagaki Y, Nakao Y. Org. Lett. 2016;18:3956. doi: 10.1021/acs.orglett.6b01675.; Yeung K, Ruscoe RE, Rae J, Pulis AP, Procter DJ. Angew. Chem. Int. Ed. 2016;55:11912. doi: 10.1002/anie.201606710.; Angew. Chem. 2016;128:12091.; Seiple IB, Su S, Rodriguez RA, Gianatassio R, Fujiwara Y, Sobel AL, Baran PS. J. Am. Chem. Soc. 2010;132:13194. doi: 10.1021/ja1066459.
- [6] a).Semba K, Nakao Y. J. Am. Chem. Soc. 2014;136:7567. doi: 10.1021/ja5029556. [DOI] [PubMed] [Google Scholar]; b) Smith KB, Logan KM, You W, Brown MK. Chem. Eur. J. 2014;20:12032. doi: 10.1002/chem.201404310. [DOI] [PubMed] [Google Scholar]; c) Logan KM, Smith KB, Brown MK. Angew. Chem. Int. Ed. 2015;54:5228. doi: 10.1002/anie.201500396. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015;127:5317. [Google Scholar]
- [7] a).For examples of Cu-catalyzed enantioselective carboboration, see: Ito H, Kosaka Y, Nonoyama K, Sasaki Y, Sawamura M. Angew. Chem. Int. Ed. 2008;47:7424. doi: 10.1002/anie.200802342.; Angew. Chem. 2008;120:7534.; Zhong C, Kunii S, Kosaka Y, Sawamura M, Ito H. J. Am. Chem. Soc. 2010;132:11440. doi: 10.1021/ja103783p.; Burns AR, Solana Gonz lez J, Lam HW. Angew. Chem. Int. Ed. 2012;51:10827. doi: 10.1002/anie.201205899.; Angew. Chem. 2012;124:10985.; Meng F, Jang H, Jung B, Hoveyda AH. Angew. Chem. Int. Ed. 2013;52:5046. doi: 10.1002/anie.201301018.; Angew. Chem. 2013;125:5150.; Meng F, Haeffner F, Hoveyda AH. J. Am. Chem. Soc. 2014;136:11304. doi: 10.1021/ja5071202.; Meng F, McGrath KP, Hoveyda AH. Nature. 2014;513:367. doi: 10.1038/nature13735.; g) ref. [5d]; h) ref. [5f].
- [8].Jia T, Cao P, Wang B, Lou Y, Yin X, Wang M, Liao J. J. Am. Chem. Soc. 2015;137:13760. doi: 10.1021/jacs.5b09146. [DOI] [PubMed] [Google Scholar]
- [9] a).Friis SD, Pirnot MT, Buchwald SL. J. Am. Chem. Soc. 2016;138:8372. doi: 10.1021/jacs.6b04566.; b) For a related Pd/Cu-catalyzed hydroarylation, see: Semba K, Ariyama K, Zheng H, Kameyama R, Sakaki S, Nakao Y. Angew. Chem. Int. Ed. Angew. Chem. 2016;2016;55128:6275, 6383. doi: 10.1002/anie.201511975.
- [10] a).For representative examples, see: Tolstoy P, Engman M, Paptchikhine A, Bergquist J, Church TL, Leung AWM, Andersson PG. J. Am. Chem. Soc. 2009;131:8855. doi: 10.1021/ja9013375.; Bess EN, Sigman MS. Org. Lett. 2013;15:646. doi: 10.1021/ol303465c.
- [11].Paquin J-F, Defieber C, Stephenson CRJ, Carreira EM. J. Am. Chem. Soc. 2005;127:10850. doi: 10.1021/ja053270w. [DOI] [PubMed] [Google Scholar]
- [12] a).For selected recent studies of stereoselective/stereospecific cross-coupling with chiral electrophiles to access 1,1-diarylalkanes, see: Harris MR, Hanna LE, Greene MA, Moore CE, Jarvo ER. J. Am. Chem. Soc. 2013;135:3303. doi: 10.1021/ja311783k.; Do H-Q, Chandrashekar ERR, Fu GC. J. Am. Chem. Soc. 2013;135:16288. doi: 10.1021/ja408561b.; Zhou Q, Cobb KM, Tan T, Watson MP. J. Am. Chem. Soc. 2016;138:12057. doi: 10.1021/jacs.6b08075.
- [13].Lee Y, Hoveyda AH. J. Am. Chem. Soc. 2009;131:3160. doi: 10.1021/ja809382c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14] a).McQuade and coworkers have reported that this catalyst was effective for Cu-catalyzed allylic substitution and conjugate addition reactions. Park JK, Lackey HH, Rexford MD, Kovnir K, Shatruk M, McQuade DT. Org. Lett. 2010;12:5008. doi: 10.1021/ol1021756.; Park JK, McQuade DT. Angew. Chem. Int. Ed. 2012;51:2717. doi: 10.1002/anie.201107874.; Angew. Chem. 2012;124:2771.; Park J, McQuade DT. Synthesis. 2012;44:1485.; Delvos L, Hensel A, Oestreich M. Synthesis. 2014;46:2957.
- [15] a).Wadamoto M, Phillips EM, Reynolds TE, Scheidt KA. J. Am. Chem. Soc. 2007;129:10098. doi: 10.1021/ja073987e. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Maki BE, Chan A, Phillips E, Scheidt MKA. Org. Lett. 2007;9:371. doi: 10.1021/ol062940f. [DOI] [PubMed] [Google Scholar]
- [16].Bruno NC, Tudge MT, Buchwald SL. Chem. Sci. 2013;4:916. doi: 10.1039/C2SC20903A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Guisán-Ceinos M, Parra A, Martín-Heras V, Tortosa M. Angew. Chem. 2016;128:7083. doi: 10.1002/anie.201601976. [DOI] [PubMed] [Google Scholar]
- [18].Gilmore JL, Sheppeck JE, II, Wang J, Dhar TGM, Cavallaro C, Doweyko AM, Mckay L, Cunningham MD, Habte SF, Nadler SG, Dodd JH, Somerville JE, Barrish JC. Bioorg. Med. Chem. Lett. 2013;23:5448. doi: 10.1016/j.bmcl.2013.06.085. [DOI] [PubMed] [Google Scholar]
- [19].Reactions with indene or cis-β-methylstyrene generated the product with low levels of diastereoselectivity under the anti-selective conditions (refs. [6b,c]). Studies are ongoing to solve this problem.
- [20].Mlynarski SN, Karns AS, Morken JP. J. Am. Chem. Soc. 2012;134:16449. doi: 10.1021/ja305448w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21] a).Asano Y, Yamashita M, Nagai K, Kuriyama M, Yamada K, Tomioka K. Tetrahedron Lett. 2001;42:8493. [Google Scholar]; b) Hajra S, Maji B, Mal D. Adv. Synth. Catal. 2009;351:859. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.