Abstract
The endocannabinoid system shifts energy balance toward storage and fat accumulation, especially in the context of diet-induced obesity. Relatively little is known about factors outside the central nervous system that may mediate the effect of high fat diet (HFD) on brain endocannabinoid levels. One candidate is the liver fatty acid binding protein (FABP1), a cytosolic protein highly prevalent in liver, but not detected in brain, which facilitates hepatic clearance of fatty acids. The impact of Fabp1 gene ablation (LKO) on the effect of high-fat diet (HFD) on brain and plasma endocannabinoid levels was examined and data expressed for each parameter as the ratio of high fat diet/control diet. In male wild-type mice, HFD markedly increased brain N-acylethanolamides, but not 2-monoacylglycerols. LKO blocked these effects of HFD in male mice. In female wild-type mice, HFD slightly decreased or did not alter these endocannabinoids as compared with male wild-type. LKO did not block the HFD effects in female mice. The HFD-induced increase in brain arachidonic acid -derived arachidonoylethanolamide in males correlated with increased brain free and total arachidonic acid. The ability of LKO to block the HFD-induced increase in brain arachidonoylethanolamide correlated with reduced ability of HFD to increase brain free and total arachidonic acid in males. In females, brain free and total arachidonic acid levels were much less affected by either HFD or LKO in the context of HFD. These data showed that LKO markedly diminished the impact of HFD on brain endocannabinoid levels, especially in male mice.
Keywords: mouse, FABP1, gene ablation, brain, endocannabinoid, sexual dimorphism
Graphical abstract
Fatty acid binding protein-1 (FABP-1) is not detectable in brain but is highly expressed in liver. FABP-1 null (LKO) male mice on control, but not high-fat, diet had increased brain levels of arachidonic acid-containing endocannabinoids (AEA, 2-AG), correlating with increased free and total arachidonic acid in brain and serum; however, this dietary phenomenon was not observed in female LKO.
INTRODUCTION
A major function of the endocannabinoid system (ECS) is in the control of appetite (Osei-Hyiaman et al. 2005;Watkins and Kim 2015;Naughton et al. 2013). Fasting increases endocannabinoid (EC) levels in brain hypothalamus and nucleus accumbens, while refeeding normalizes EC levels therein without altering EC levels in other brain regions not involved with feeding (Silvestri and DiMarzo 2013;Lillo 2007). Pharmacologic inhibition as well as genetic ablation of cannabinoid receptor-1 (CB1) decreases appetite and results in hypophagia (Silvestri and DiMarzo 2013). However, the impact of ECs on appetite is highly dependent on both the profile of individual EC produced as well as the presence of CB1 receptor (Osei-Hyiaman et al. 2005;Watkins and Kim 2015;Naughton et al. 2013). For example, in brain the arachidonic acid (ARA, 20:4n-6)-derived EC [arachidonoylethanolamide (anandamide, AEA), 2-arachidonoylglycerol (2-AG)] selectively act on CB1 (but not CB2) to stimulate food intake and hyperphagia (Naughton et al. 2013;Guzman et al. 2004). Upon conversion to ARA, linoleic acid (LNA, 18:2n-6) also increases food intake (Naughton et al. 2013;Alvheim et al. 2012;Alvheim et al. 2014). In general, other EC decrease food intake and weight gain by non-CB pathways—oleoylethanolamide (OEA) and stearoylethanolamide (SEA) by activating PPARα (Guzman et al. 2004;Naughton et al. 2013), eicosapentaenoylethanolamide (EPEA) and docosahexaenoylethanolamide (DHEA) indirectly by displacing ARA from cell membrane phospholipids to thereby reduce AEA and 2-AG production (Naughton et al. 2013). In contrast, palmitoylethanolamide (PEA) has no effect (Naughton et al. 2013).
The EC also function in shifting energy balance toward energy storage and fat accumulation, especially in the context of high fat diet (HFD) induced obesity (DIO) (Naughton et al. 2013;Silvestri and DiMarzo 2013). It is thought that HFD fed ad libitum induces DIO at least in part by increasing EC levels (AEA, 2-AG), diacyglycerol lipase (DAGL, a key enzyme in 2-AG production) (Naughton et al. 2013;Silvestri and DiMarzo 2013), and CB1 receptors in brain (Engeli 2008). CB1 activation not only stimulates appetite as indicated above, but also activates SREBP1c to induce transcription of lipogenic enzymes (ACC, FASN) and de novo lipogenesis—effects exacerbated by HFD (Osei-Hyiaman et al. 2005;Silvestri and DiMarzo 2013). In contrast, HFD does not elicit weight gain and obesity in cannabinoid receptor-1 (CB1) gene ablated mice even though caloric intake is similar (Osei-Hyiaman et al. 2005;Trillou et al. 2004). Obesity in human subjects and fat accumulation in rodents are also associated with increased plasma AEA and 2-AG, altered pattern of CB receptor expression, and increased de novo lipogenesis (Naughton et al. 2013;Engeli 2008;Silvestri and DiMarzo 2013). Conversely, weight loss in intra-abdominally obese men is accompanied by decreased plasma 2-AG, decreased visceral obesity, and normalization of plasma lipid parameters (Silvestri and DiMarzo 2013). It is important to note, however, that ad libitum feeding HFD does not discriminate effects of total food intake from those of increased fat content of the diet on serum endocannabinoids or the brain endocannabinoid system. In this regard, HFD also induced weight gain and obesity in wild-type (WT) mice pair-fed HFD where caloric intake of HFD and control diet did not differ (Atshaves et al. 2010b;McIntosh et al. 2013). However, nothing is known about the impact of pair-fed HFD on serum endocannabinoids or the brain endocannabinoid system.
Liver fatty acid binding protein-1 (FABP1), a cytosolic protein that enhances hepatic uptake and metabolism of fatty acids, significantly impacts body weight gain and obesity (Atshaves et al. 2010b;Atshaves et al. 2010a;McIntosh et al. 2013;Gajda et al. 2013). While hepatic FABP1 is elevated in obesity (Morrow et al. 1979), FABP1 is not detectable in brain (Owada et al. 2006;Myers-Payne et al. 1996a;Avdulov et al. 1998). However, FABP1 has high affinity for ARA (20:4, n-6) (Frolov et al. 1997;Murphy et al. 1999) and enhances ARA uptake in FABP1 overexpressing cells (McIntosh et al. 2010;Murphy et al. 1996b;Murphy et al. 1996a;Schroeder et al. 1993). FABP1 gene ablation (LKO) increases plasma ARA availability for brain uptake and concomitantly increases brain levels of ARA and ARA-derived EC (AEA, 2-AG) in male mice (Martin et al. 2016a;Schroeder et al. 2016). In contrast, female mice are resistant to the LKO-induced increase brain AEA and 2-AG (Martin et al. 2016b).
Taken together, these findings suggested that FABP1 may significantly impact the effect of HFD on brain EC levels and the EC system in a sexually dimorphic manner. This possibility was tested in male and female WT and LKO mice fed HFD versus control chow diet and data expressed for each parameter as the ratio of high fat diet/control diet. The results demonstrated that LKO inhibited the high fat diet-induced increase in brain EC levels, especially in male mice.
METHODS
Animal Care
Animal protocols were approved by the Institutional Animal Care and Use Committee at Texas A&M University. Wild-type (WT) male and female inbred C57BL/6NCr mice were purchased from the National Cancer Institute (Frederick Cancer Research and Development Center, Frederick, MD). Fabp1 gene ablated (LKO) mice were generated on the same C57BL/6NCr background as described (Martin et al. 2003) and backcrossed to WT C57BL/6NCr mice to the N10 generation. For colony maintenance, mice were fed a standard rodent chow mix [5% calories from fat; D8604 Teklad Rodent Diet, Teklad Diets (Madison, WI)] for 7 weeks, maintained in barrier cages on ventilated racks at 12-hr light/dark cycle in a temperature controlled facility (25°C), and allowed ad libitum food and water until study initiation. Mice were sentinel monitored quarterly and confirmed free of all known rodent pathogens.
Study Design
Sample size calculations and power analysis were performed utilizing the G*Power analysis tool available at http://www.gpower.hhu.de/en.html based on (Charan and Kantharia 2013;Faul et al. 2009;Faul et al. 2007). Prior to beginning the high-fat feeding study, mice from each genotype (16 male WT, 16 female WT, 16 male LKO, 16 female LKO) were randomized to generate the respective control diet or high-fat diet feeding group. Randomization was accomplished utilizing the RAND function in Microsoft Excel 2010. At the end of the dietary feeding study, all mice were randomized utilizing Microsoft Excel 2010 in order to minimize bias with respect to tissue collection and processing. All experimenters were blind with respect to group assignment and outcome assessment.
Experimental Diets
To avoid potential complications of phytoestrogens (Thigpen et al. 1999a;Thigpen et al. 1999b) and phytol (Ellinghaus et al. 1999;Hanhoff et al. 2005;Fuchs et al. 2001;Hanhoff et al. 2001;Wolfrum et al. 1999;Seedorf et al. 1998) present in standard lab chow on sex differences and FABP1 expression, four groups of mice (16 WT male, 16 WT female, 16 LKO male, 16 LKO female) were housed individually in Tecniplast Sealsafe individually ventilated cages (external water bottles, wire lid holders for food pellets) and acclimated for 1 week on a defined phytol-free, phytoestrogen-free, 10 kcal% fat control chow (#D12450B from Research Diets, New Brunswick, NJ, http://www.researchdiets.com/opensource-diets/stock-diets/dio-series-diets). Each group of mice was then split into two groups of 8 mice, all housed individually and fed for an additional 12 weeks either the same phytol-free, phytoestrogen-free, 10 kcal% fat control chow (#D12450B from Research Diets, New Brunswick, NJ) or pair fed isocaloric high fat diet (# D12451 from Research Diets, New Brunswick, NJ, http://www.researchdiets.com/opensource-diets/stock-diets/dio-series-diets) as described earlier (Atshaves et al. 2010b). The isocaloric high fat diet (HFD) was the phytol-free, phytoestrogen free diet modified to increase dietary fat from 10 kcal % to 45 kcal% high fat at the expense of carbohydrate which was reduced from 70 kcal % to 35 kcal % while protein was maintained constant (# D12451 from Research Diets, New Brunswick, NJ). Detailed analysis of the fatty acid composition of these diets (DIO FA Profile 11-11.xls, Research Diets, New Brunswick, NJ, http://www.researchdiets.com/opensource-diets/stock-diets/dio-series-diets) showed that in control chow the n-6 PUFA 20:4n-6 and 18:2n-6 comprised 18.3 and 0.1 g/kg, respectively, while n-3 PUFA 18:3n-3, 20:5n-3 and 22:6n-3 comprised 2.2, 0 and 0 g/kg, respectively. In the high fat diet the contents of C18:2n-6 and C20:4n-6 were increased over 3-fold to 56.7 and 0.5 g/kg, respectively, while those of the n-3PUFA18:3n-3, 20:5n-3 and 22:6n-3 were increased only slightly to 4.3, 0 and 0.2 g/kg, respectively.
Tissue Collection
At the end of the dietary study, mice were fasted overnight, brain and liver collected for flash freezing and storage at −80°C for subsequent analysis of arachidonic acid (ARA) and endocannabinoid levels, western blotting, and/or QrtPCR as described below and in Supplementary Methods. Serum was collected similarly, flash frozen, and stored at −80°C for subsequent determination of ARA and endocannabinoid levels also as described below.
Liquid Chromatography/Mass Spectrometry (LC/MS) Analysis of Tissue Fatty Acyl Ethanolamides (NAE), 2-Monoacylglycerols (2-MG), and Arachidonic Acid (ARA)
For LC/MS analysis of endocannabinoids in brain and serum, internal standards of deuterated AEA-d4, OEA-d2, PEA-d4, DHEA-d4, EPEA-d4, 2-AG-d8, and ARA-d8 were obtained from Cayman Chemical (Ann Arbor, MI). Cayman Chemical (Ann Arbor, MI) was the source of unlabeled N-acylethanolamides (NAE), unlabeled 2-monoacylglycerols (2-MG), and the following external standards: n-6 arachidonoylethanolamide (AEA), oleoylethanolamide (OEA), palmitoylethanolamide (PEA), n-3 docosahexaenoylethanolamide (DHEA), n-3 eicosapentaenoylethanolamide (EPEA), 2-arachidonoylglycerol (2-AG), 2-oleoylglycerol (2-OG), and 2-palmitoylglycerol (2-PG). All solvents and reagents were of highest commercially available grade.
Lipids were extracted from mouse brain or serum for determination of NAE composition and mass as well as 2-MG composition and mass by liquid chromatography/mass spectrometry (LC/MS) as described earlier by our lab (Martin et al. 2016a;Martin et al. 2016b). Likewise, separate aliquots of serum and brain for protein quantitation as well as for lipid extraction to determine free ARA and total ARA were performed as described earlier (Martin et al. 2016a).
Western Blotting
For western blotting, antibodies were obtained from the following commercial sources: Polyclonal anti-rabbit antibody to caveolin-1 (CAV1; 610060) was from BD Transduction Laboratories (Lexington, KY). Goat polyclonal anti-fatty acid amide hydrolase (FAAH, sc-26427), anti-N-acylphosphatidylethanolamide phospholipase-D (NAPE-PLD; sc-163117), anti- fatty acid transport protein 4 (FATP-4; sc-5834), and anti-cannabinoid receptor-1 (CB1; sc-10066) were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-rabbit fatty acid transport protein 1 (FATP-1; sc-25541), monoclonal anti-mouse fatty acid binding protein-3 (FABP3; sc-58275), as well as rabbit polyclonal anti-rabbit fatty acid binding protein-7 (FABP7; sc-30088), monoclonal anti-mouse N-acylethanolamide hydrolyzing acid amidase (NAAA; sc-100470), monoclonal anti-mouse β-Actin (sc-47778), rabbit polyclonal anti-monoacylglycerol lipase (MGL, sc-134789) and anti- diacylglycerol lipase α (DAGLα; sc-133307) were from Santa Cruz Biotech (Santa Cruz, CA). Mouse polyclonal anti-fatty acid transport protein 5 (FATP5, ab89008), rabbit polyclonal anti fatty acid transport protein 2 (FATP2, ab83763), anti-cytochrome C oxidase 4 (COX4, ab16056), and specific monoclonal anti-mouse heat shock protein-70 (HSP70; ab2787) were from Abcam (Cambridge, MA). Mouse monoclonal glyceraldehyde 3-phosphate dehydrogenase (GAPDH, MAB374) was from Millipore, Inc (Billerica, MA). Rabbit polyclonal anti-sterol carrier protein-2 (recognizing 58 kDa SCP-x, 15 kDa pro-SCP-2, and 13.2 kDa SCP-2) was prepared as described earlier (25). Monoclonal anti-mouse antibody to fatty acid translocase/cluster of differentiation 36/thrombospondin receptor (FAT/CD36; RDI-M1537db) was from Research Diagnostics (Flanders, NJ). Western blotting following sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed on brain homogenates using the above antibodies similarly as described in (Klipsic et al. 2015;Martin et al. 2016a;Martin et al. 2016b). Antibodies to GAPDH or β-Actin were used as internal gel loading controls. Selection of internal gel loading control protein was made on the basis of molecular size of the protein of interest to ensure adequate separation from the control protein. Images were obtained and quantified by densitometric analysis using ImageJ software (National Institutes of Health, Bethesda, MD). Values were normalized to internal housekeeper control.
Statistical Analysis
All values are expressed as means ± SEM. Comparison of the means was done using one-way ANOVA followed by the Newman-Keuls post hoc analysis. Differences of P < 0.05 were considered statistically significant; *, P < 0.05 for LKO vs WT; #, P < 0.05 for female vs male.
RESULTS
High Fat Diet (HFD) Differentially Alters Brain Levels of N-acylethanolamides (NAE) and 2-Monoacylglycerols (2-MG) in Male vs Female Mice
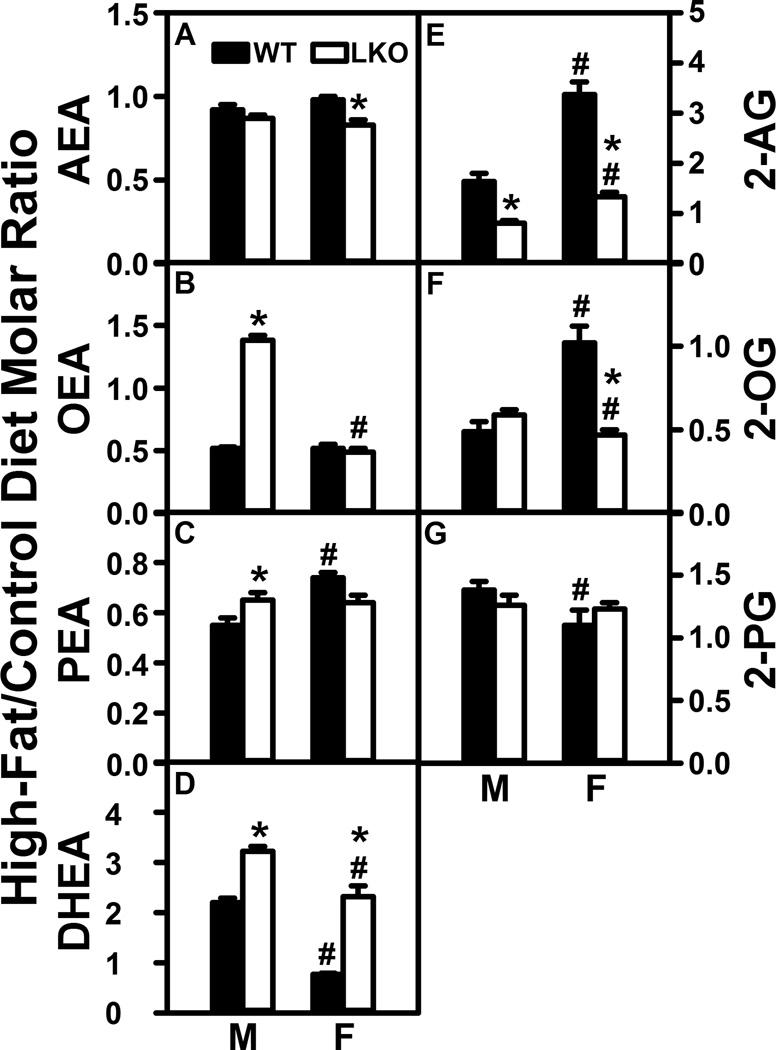
Although HFD has been reported to increase brain levels of arachidonic acid (ARA)-containing arachidonoylethanolamide (AEA) and 2-arachidonoylglycerol (2-AG), most rodent studies have been performed only with males and have also not reported levels of the non-ARA containing NAE and 2-MG (Naughton et al. 2013;Silvestri and DiMarzo 2013). Therefore, the impact of high fat diet (HFD) on brain NAE and 2-MG levels was determined in male and female C57BL/6N mice fed HFD or control diet. Brain NAE and 2-MG data were expressed as the molar ratio of each NAE in brain from mice fed HFD to that in mice fed control diet, expressed in the ordinate of Figure 1 as High-Fat/Control Diet Molar Ratio.
FIGURE 1.
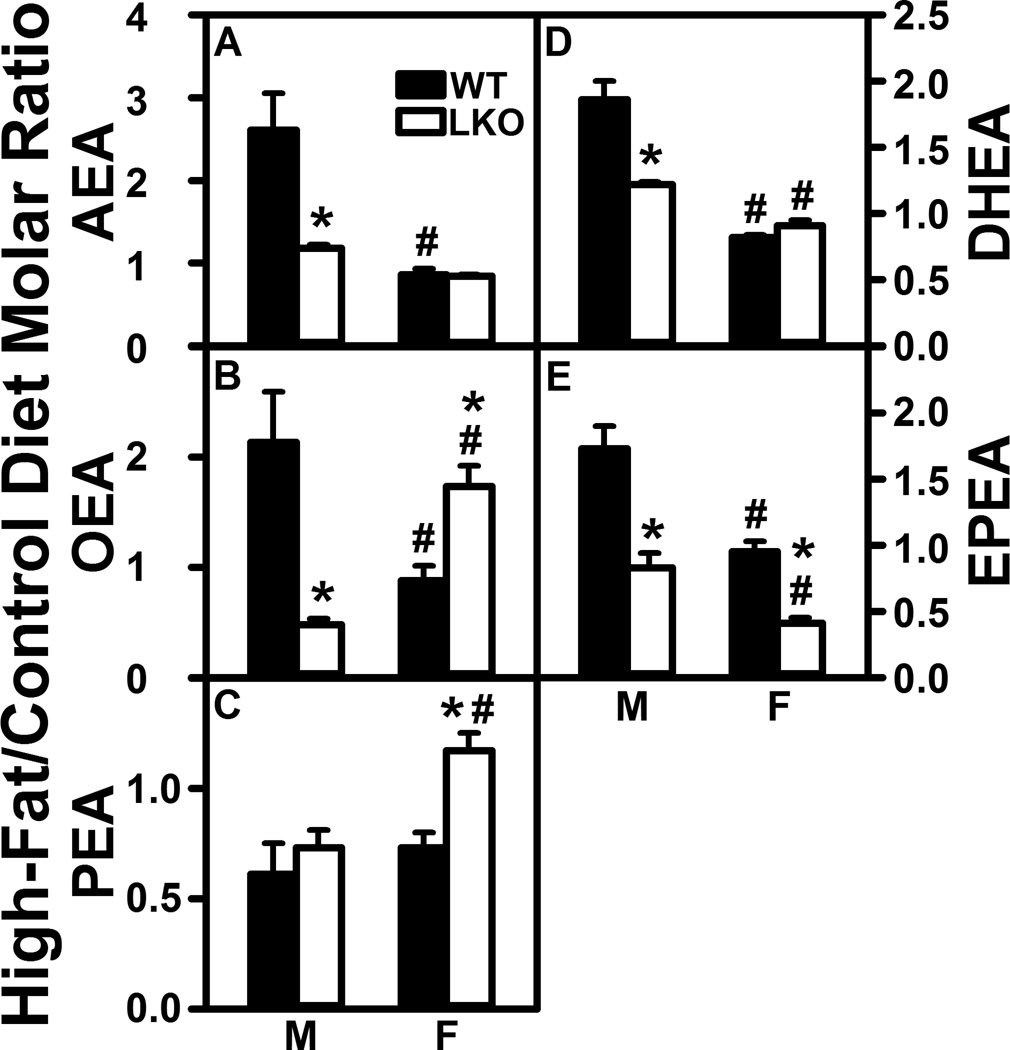
Fabp1 gene ablation (LKO) diminishes the impact of high fat diet (HFD) on brain N-acylethanolamide (NAE) levels. C57BL/6N male and female WT and FABP1 gene ablated mice were fed HFD or control diet, fasted overnight, and brains removed/flash frozen and stored at −80°C. LC-MS analysis to quantify N-acylethanolamides using deuterated internal standards (Cayman Chemical) was performed as described in Materials and Methods to quantify (A) arachidonoylethanolamide (AEA), (B) oleoylethanolamide (OEA), (C) palmitoylethanolamide (PEA), (D) docosahexaenoylethanolamide (DHEA), and (E) eicosapentaenoylethanolamide (EPEA). Results are expressed as the molar ratio of each NAE in High-Fat/Control diet and presented as mean ± SEM (n = 8); *, P < 0.05 for LKO vs WT; #, P < 0.05 for female (F) vs male (M).
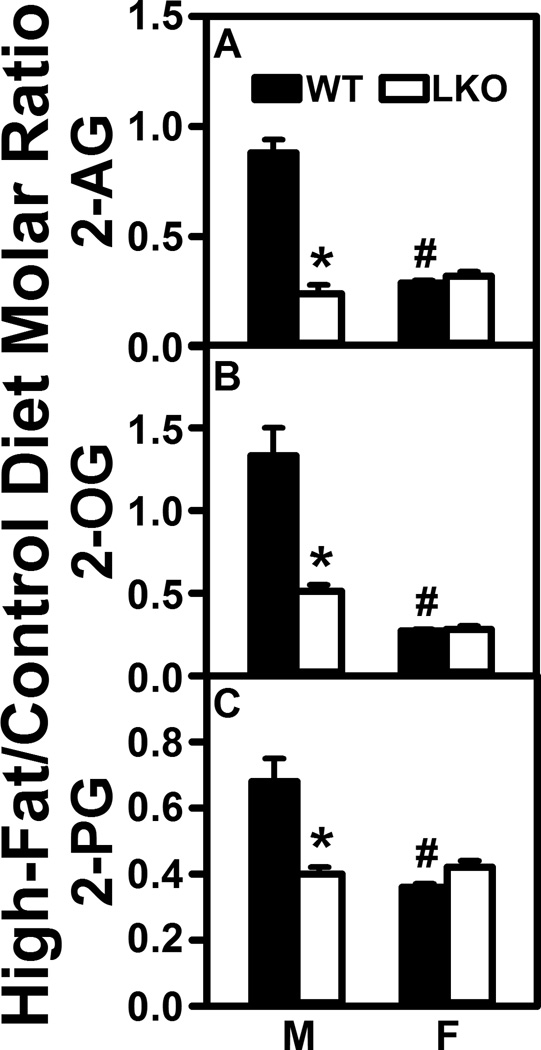
HFD selectively increased brain AEA nearly 3-fold in WT males but not WT females (Fig. 1A). In contrast, HFD did not alter brain level of 2-AG in males and actually decreased that in females by nearly 70% (Fig. 2A). Likewise, HFD also selectively impacted brain levels of non-ARA containing NAE and 2-MG—increasing that of oleoylethanolamide (Fig. 1B, OEA), docosahexaenoylethanolamide (Fig. 1D, DHEA), and eicosahexaenoylethanolamide (Fig. 1E, EPEA) in males, but not females. In contrast, HFD decreased brain levels of 2-OG and 2-PG in females while having little net effect on those in males (Fig. 2B,C).
FIGURE 2.
Fabp1 gene ablation (LKO) confers on high fat diet (HFD) the ability to decrease brain 2-monoacylglycerol levels. All conditions were as in Figure 1 except that LC-MS analysis of 2-monoacylglyerols was performed using deuterated internal standards (Cayman Chemical) as described in Materials and Methods to quantify (A) 2-arachidonoylglycerol (2-AG), (B) 2-oleoylglycerol (2-OG), and (C) 2-palmitoylglycerol (2-PG). Results are expressed as the molar ratio of each NAE in High-Fat/Control diet and presented as mean ± SEM (n = 8); *, P < 0.05 for LKO vs WT; #, P < 0.05 for female (F) vs male (M).
Taken together, these data indicated that NAE (AEA, OEA, DHEA, EPEA), but not 2-MG, levels in brains of male mice were dramatically elevated (2–3 fold) by HFD. In contrast, HFD did not increase NAE or 2-MG levels in female brain, but instead actually decreased 2-MG in female brains.
Fabp1 Gene Ablation (LKO) Markedly Diminishes the Impact of High Fat Diet (HFD) on Brain N-acylethanolamides (NAE) in Males and Selectively Alters the Effect of High Fat Diet on Female Brain NAE
LKO essentially abolished the ability of HFD to increase brain levels of AEA, OEA, DHEA, and EPEA in male mouse brain (Fig. 1A,B,DE). LKO also decreased the brain high-fat/control diet ratios of 2-MG in males (Fig. 2A–C). In contrast, LKO actually increased ratios for OEA and PEA (Fig. 1B,C) while decreasing that of EPEA (Fig. 1E) in females. However, LKO had no impact on brain ratios of 2-MG in females (Fig. 2A–C).
Impact of High Fat Diet (HFD) and Fabp1 Gene Ablation (LKO) on Brain and/or Serum Levels of Free and Total Arachidonic Acid (ARA)
Diets rich in linoleic acid (LNA, 18:2n-6; precursor of ARA) or ARA increase the level of ARA, AEA, and 2-AG (Artmann et al. 2008;Naughton et al. 2013;Alvheim et al. 2012;Alvheim et al. 2014). The HFD used herein contained 3-fold more LNA and ARA than did the control diet (see Methods). Therefore, the impact of LKO on serum and brain high-fat/control diet ratios of free ARA as well as total ARA was examined in mice fed HFD.
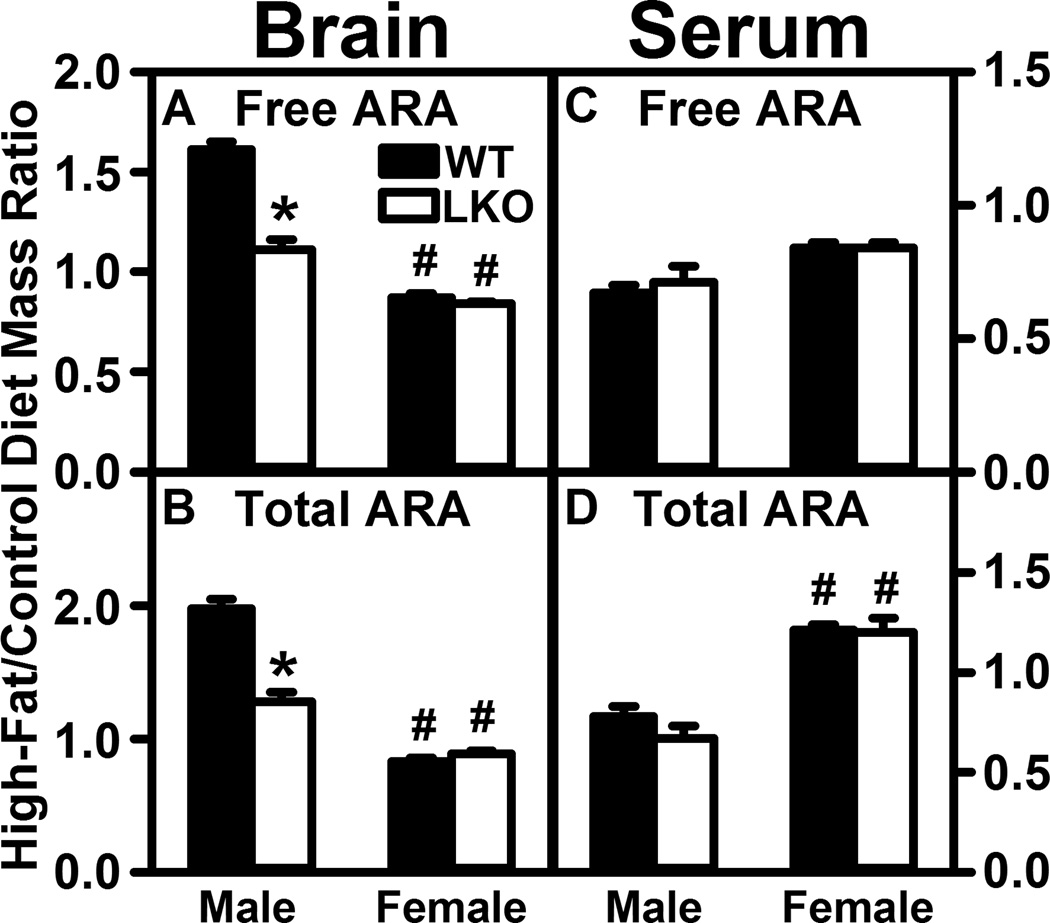
In WT male mice the HFD increased the high-fat/control diet ratios of free ARA and total ARA in brain homogenate by 60% and 100%, respectively (Fig. 3A,B), while not altering the ratios in serum (Fig. 3C,D). In contrast, in WT female mice the HFD did not significantly increase this ratio for free ARA and total ARA in brain homogenate (Fig. 3A,B), did not impact the ratio for free ARA in serum (Fig. 3C), and increased that for serum total ARA by 23% (Fig. 3D).
FIGURE 3.
Fabp1 gene ablation (LKO) diminishes the ability of high fat diet (HFD) to increase brain levels of unesterified and total arachidonic acid (ARA). All conditions were as in Figure 1 except that unesterified and total ARA levels were determined in brain and serum by LC-MS using deuterated internal standard (Cayman Chemical) as described in Materials and Methods. Panels refer to: (A) brain free ARA; (B) brain total ARA; (C) serum free ARA; (D) serum total ARA. Results are expressed as the mass ratio of each free ARA or total ARA in High-Fat/Control diet and presented as mean ± SEM (n = 4–8); *, P < 0.05 for LKO vs WT; #, P < 0.05 for female (F) vs male (M).
LKO essentially abolished the impact of HFD on high-fat/control diet fed ratios of free ARA and total ARA in brain homogenates of male mice (Fig. 3A,B) and did not alter those in serum (Fig. 3C,D). However, in female mice LKO did not alter the lack of effect of HFD on these ratios in brain (Fig. 3A,B) or further exacerbate the impact of HFD on increasing serum total ARA (Fig. 3D).
Thus, the HFD induced increase in brain ARA-derived AEA and 2-AG in males (Fig. 1A, 2A) correlated with increased brain free ARA and total ARA, while serum ARA levels were not altered. Conversely, the ability of LKO to block the HFD induced increase in brain AEA and 2-AG in males was associated with abolishing the ability of HFD to increase brain free and total ARA in males. In contrast, female brain and serum free and total ARA levels were much less affected by either HFD or LKO.
High Fat Diet (HFD) Differentially Alters Serum Levels of N-acylethanolamides (NAE) and 2-Monoacylglycerols (2-MG) in Male vs Female Mice
While HFD did not significantly alter serum AEA in either male or female WT mice (Fig. 4A), levels of 2-AG were markedly increased 1.6- and 3.4-fold, respectively (Fig. 4E). In contrast, HFD decreased serum levels of the non-ARA containing ECs such as OEA, PEA, and 2-OG (Fig. 4B,C,F) while increasing that of DHEA and 2-PG (Fig. 4D, G) in male WT mice. In contrast, HFD decreased serum levels of OEA and PEA (Fig. 4B,C) without altering DHEA, 2-OG, or 2-PG levels (Fig. 4D,F,G) in female WT mice.
FIGURE 4.
Fabp1 gene ablation (LKO) differentially impacts the ability of high fat diet (HFD) to alter serum N-acylethanolamide (NAE) and 2-monoacylglycerol (2-MG) levels. All conditions were as in Figures 1 and 2 except that NAE and 2-MG were determined in serum by LC/MS as described in Materials and Methods. Panels refer to: (A) arachidonoylethanolamide (AEA), (B) oleoylethanolamide (OEA), (C) palmitoylethanolamide (PEA), (D) docosahexaenoylethanolamide (DHEA), (E) 2-arachidonoylglycerol (2-AG), (F) 2-oleoylglycerol (2-OG), and (G) 2-palmitoylglycerol (2-PG). Results are expressed as the molar ratio of each NAE or 2-MG in High-Fat/Control diet and presented as mean ± SEM (n = 8); *, P < 0.05 for LKO vs WT; #, P < 0.05 for female (F) vs male (M).
LKO decreased the impact of HFD on serum AEA in females but not males (Fig. 4A) and more markedly diminished the impact of HFD on serum 2-AG in both males and females (Fig. 4E). LKO conferred on HFD the ability to increase OEA, PEA, and DHEA in serum of males (Fig. 4B,C,D), while enhancing the ability of HFD to increase DHEA (Fig. 4D) but decreasing 2-OG in females (Fig. 4F).
Thus, the overall pattern of brain levels of AEA and 2-AG as well as other NAE and 2-MG in general did not reflect that in the serum.
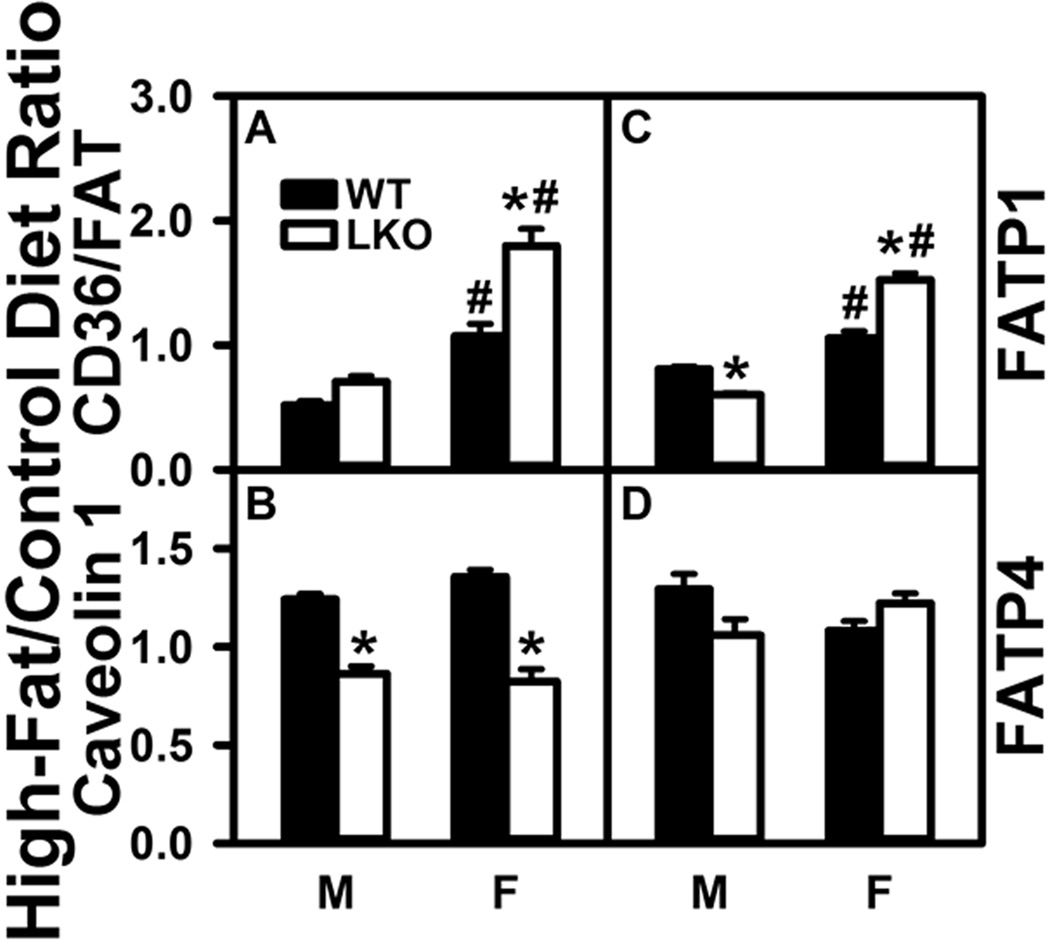
Fabp1 Gene Ablation (LKO) Has Little Overall Effect on the Impact of High-Fat Diet (HFD) on Membrane Fatty Acid Transport/Translocase Proteins In Brain
Brain expresses four membrane associated proteins (CD36/FAT, caveolin-1, FATP1, FATP4) that facilitate uptake/translocation of fatty acids such as ARA or LNA into the cytosol (Mitchell and Hatch 2011). As shown by western blotting, in WT males the HFD decreased CD36/FAT (Fig. 5A) and less so FATP1 (Fig. 5C) while increasing that of caveolin-1 and FATP4 (Fig. 5B,D). In contrast, in WT females the HFD increased CD36/FAT, caveolin-1, and FATP1 but not FATP4 (Fig. 5A–D).
FIGURE 5.
Impact of Fabp1 gene ablation (LKO) differentially alters the ability of high fat diet (HFD) to impact brain protein levels of membrane proteins involved in fatty acid uptake. All conditions were as described in Figure 1 except that SDS-PAGE followed by western blotting on aliquots of brain homogenate as described in Materials and Methods to determine levels of: (A) CD36/FAT (β-actin), (B) CAV1 (β-actin), (C) FATP1 (GAPDH), and (D) FATP4 (β-actin). Relative protein levels were normalized to gel-loading control protein (β-actin or GAPDH), values compared to male WT set to 1, results expressed as the relative ratio of each protein in High-Fat/Control diet, and data shown as mean ± SEM (n = 8); *, P < 0.05 for LKO vs WT; #, P < 0.05 for female (F) vs male (M).
LKO did not significantly impact the impact the ability of HFD to reduce CD36/FAT (Fig. 5A), but decreased HFD’s effects on caveolin-1 and FATP1 (Fig. 5A,B) in males. In females, LKO exacerbated the HFD-induced increase in CD36/FAT and FATP1 (Fig. 5A,C) while decreasing that on caveolin-1 (Fig. 5B).
These data suggested that the HFD-induced increase in brain free and total ARA as well as AEA in WT male mice may be attributable in part to an increase in protein levels of two membrane fatty acid transporter/translocase proteins (Caveolin-1, FATP4), although another (CD36/FAT) was decreased. Conversely, the ability of LKO to block the HFD-induced increase in the free ARA and total ARA levels in brain of males was associated in part with reduced level of caveolin-1 and FATP1 in males.
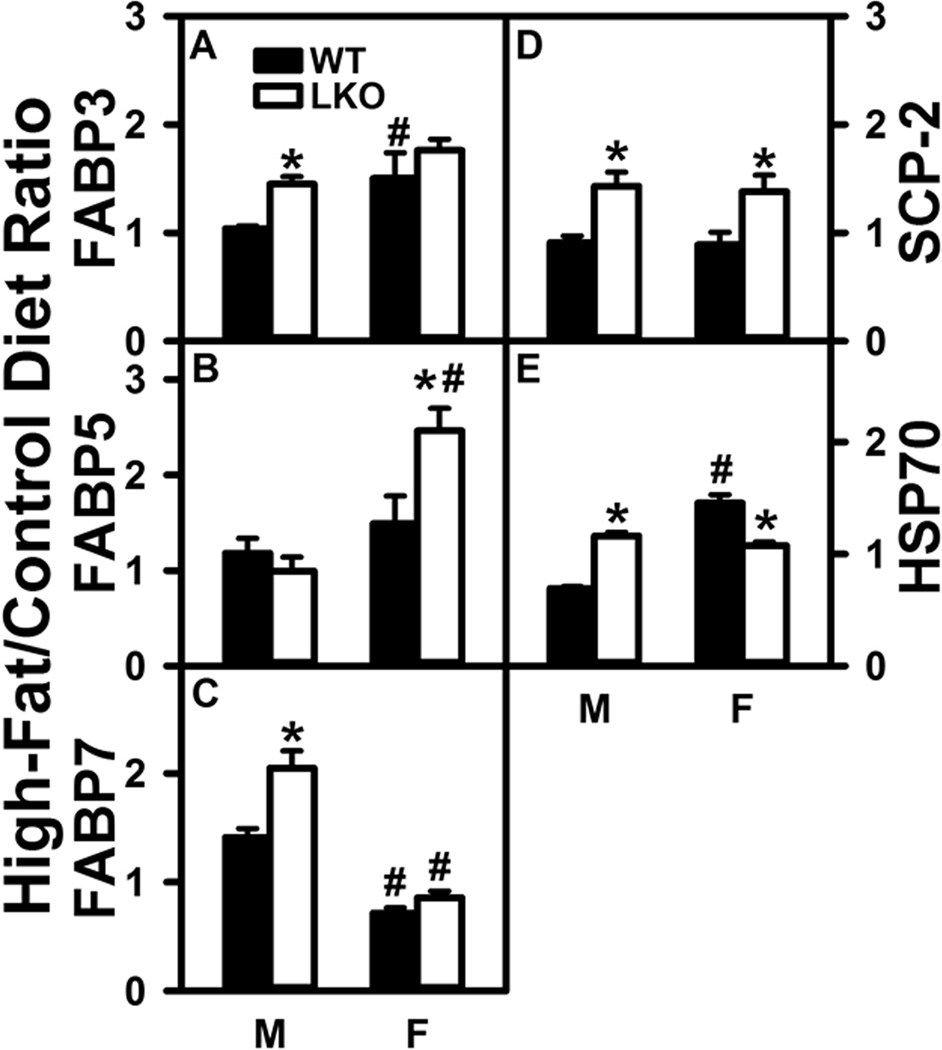
Effect of Fabp1 Gene Ablation (LKO) on Impact of High Fat Diet (HFD) on Cytosolic Fatty Acid Transport Proteins in Brain
Brain expresses two families of intracellular cytosolic proteins that facilitate cytosolic transport of poorly aqueous-soluble ARA and targeting to sites of ARA incorporation into phospholipids for AEA and 2-AG synthesis (endoplasmic reticulum) or ARA degradation (mitochondria) (Mitchell and Hatch 2011;Owada 2008;Moulle et al. 2012;Murphy et al. 2005): i) FABPs 3, 5, and 7 (Pu et al. 1999b;Pu et al. 1999a;Myers-Payne et al. 1996b;Murphy et al. 2005;Moulle et al. 2012), ii) sterol carrier protein-2 (SCP-2) (Frolov et al. 1996;Pu et al. 1998;Avdulov et al. 1998;Myers-Payne et al. 1996a), and iii) HSP70 (Oddi et al. 2009).
Western blotting showed that in male WT mice HFD did not significantly alter brain protein levels of FABP3 (Fig. 6A), FABP5 (Fig. 6B), SCP-2 (Fig. 6D), or HSP70 (Fig. 6E), but increased that of FABP7 (Fig. 6C). In female WT mice the HFD increased protein levels of FABP3 (Fig. 6A) and HSP70 (Fig. 6E) while decreasing that of FABP7 (Fig. 6C).
FIGURE 6.
Effect of Fabp1 gene ablation (LKO) on ability of high fat diet (HFD) to impact brain protein levels of cytosolic proteins that bind/chaperone ARA and endocannabinoids. All conditions were as in Figure 5 except that western blot analysis was performed as described in Materials and Methods to determine protein levels of: (A) FABP3 (GAPDH), (B) FABP5 (GAPDH), (C) FABP7 (β-actin), (D) SCP-2 (GAPDH), and (E) HSP70 (β-actin). Relative protein levels were normalized to gel-loading control protein (β-actin or GAPDH), values compared to male WT set to 1, results expressed as the relative ratio of each protein in High-Fat/Control diet, and data shown as mean ± SEM (n = 8); *, P < 0.05 for LKO vs WT; #, P < 0.05 for female (F) vs male (M).
In contrast, in male mice LKO conferred on HFD the ability of HFD to increase brain protein levels of FABP3 (Fig. 6A), FABP7 (Fig. 6C), SCP-2 (Fig. 6D), and HSP70 (Fig. 6E) in males. With regards to female mice, LKO conferred on HFD the ability to increase brain levels of FABP5 (Fig. 6B) and SCP-2 (Fig. 6D), but eliminated the HFD-induced increase in HSP70 (Fig. 6E).
QrtPCR was performed to determine if the effects of HFD and LKO on protein levels of brain cytosolic fatty acid transport proteins were associated with transcriptional regulation. Overall, there was very little correlation between the effects of HFD and/or LKO on mRNA vs protein levels of the cytosolic fatty acid transport proteins in brain of either males or females (Supplementary Fig. 1A–D).
Taken together, these findings indicated that the HFD-induced increase in brain free ARA and total ARA was not associated with overall upregulation of cytosolic transport proteins that facilitate uptake/cytosolic trafficking of fatty acids such as ARA and LNA. Likewise, the ability of LKO to block HFD-induced increases in brain free and total ARA levels in males was also not associated with any overall decrease in protein levels of cytosolic ARA transporters. Most of the observed changes in protein levels were not associated with concomitant altered mRNA levels.
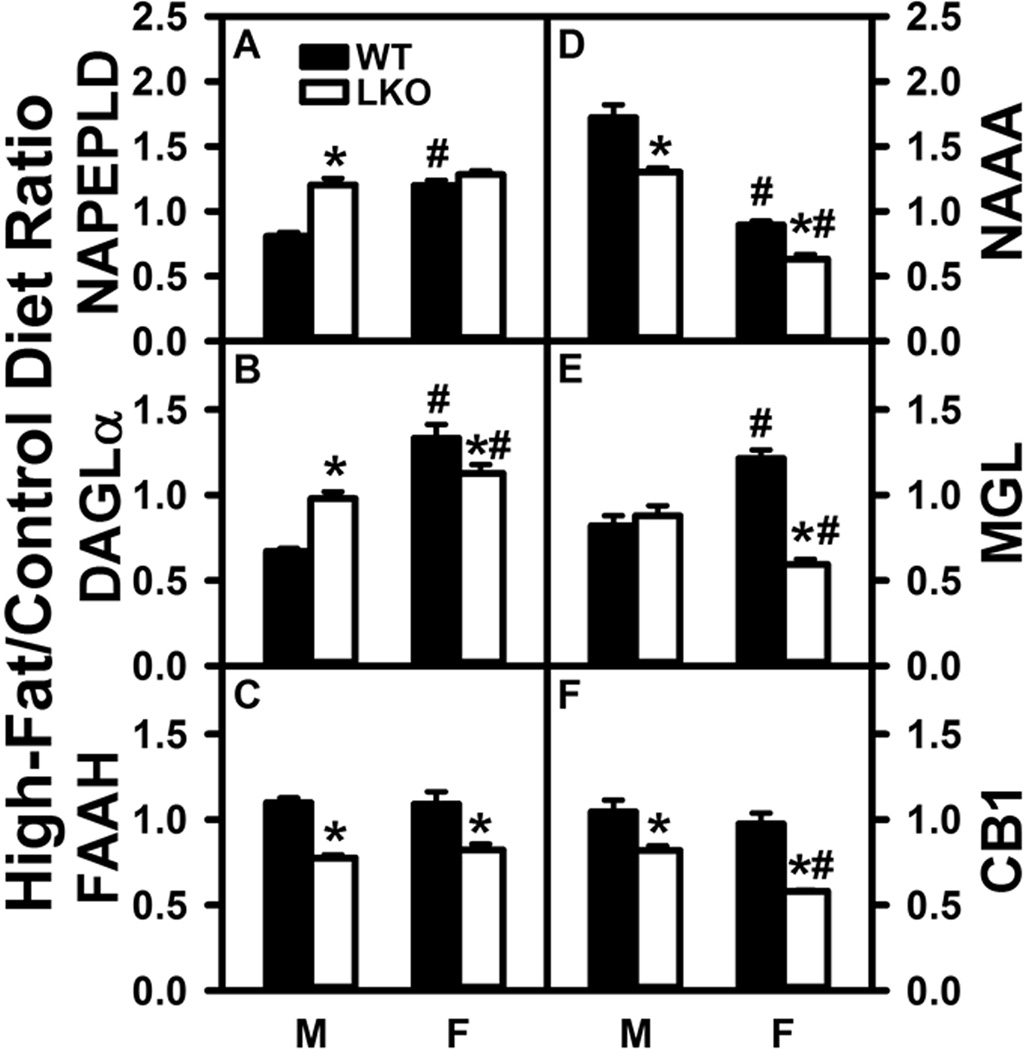
Overall Effect of Fabp1 Gene Ablation (LKO) on the Impact of High Fat Diet (HFD) on Levels of Brain Proteins for AEA and 2-AG Synthesis and Degradation
Brain AEA and 2-AG are synthesized by NAPEPLD and DAGLα, respectively, and degraded by FAAH, NAAA and MGL, respectively (Goparaju et al. 1989;Di Marzo et al. 1998;Blankman et al. 2007). While high-fat diet increases brain AEA and 2-AG in WT mice, but not in LKO mice (Fig. 1A,2A), it is not known whether these effects are attributable to alterations in protein levels of AEA and 2-AG synthetic or degradative enzymes.
Western blotting showed that HFD slightly decreased brain NAPEPLD in male WT mice, but increased that in females (Fig. 7A). Similarly, HFD decreased brain DAGLα in males but increased that in females (Fig. 7B). LKO conferred on HFD the ability to increase brain protein level of NAPEPLD and DAGLα in males, but not in females (Fig. 7A,B). The HFD-induced increase in NAPELD and DAGLα proteins did not correlate with increased Napeld mRNA (Supplementary Fig. 2A) or Dagla and Daglb mRNAs (Supplementary Fig. 2B,C).
FIGURE 7.
Impact of Fabp1 gene ablation on ability of high fat diet to modify brain protein levels of membrane proteins involved in endocannabinoid synthesis and degradation. All conditions were as in Figure 5 except that western blot analysis was performed as described in Materials and Methods to determine protein levels of: (A) NAPEPLD (GAPDH), (B) DAGLα (GAPDH), (C) FAAH (GAPDH), (D) NAAA (β-actin), (E) MGL (GAPDH), and (F) CB1 (GAPDH). Relative protein levels were normalized to gel-loading control protein (β-actin or GAPDH), values compared to male WT set to 1, results expressed as the relative ratio of each protein in High-Fat/Control diet, and results presented as mean ± SEM (n = 8); *, P < 0.05 for LKO vs WT; #, P < 0.05 for female (F) vs male (M).
With regards to degradative enzymes, HFD did not alter brain protein levels of FAAH (Fig. 7C) or MGL (Fig. 7E) in males, but increased that of NAAA in males (Fig. 7D). In contrast, LKO decreased the HFD ratio of FAAH (Fig. 7C) and NAAA (Fig. 7D) in both males and females while decreasing that of MGL only in females (Fig. 7E). The small, but significant, alteration in FAAH protein levels was not associated with markedly altered Faah mRNA level (Supplementary Fig. 2D). However, HFD-induced decrease in NAAA protein level in females, as well as the additional impact of LKO did correlate with the respective mRNA alterations (Supplementary Fig. 2E). Protein changes in MGL poorly correlated with mRNA levels in males; however, there was better correlation in females (Supplementary Fig. 2F).
These findings indicated that the HFD-induced increase in male brain AEA was not attributable to increased protein levels of the respective synthetic enzymes or decreased levels of degradative enzymes. Likewise, the ability of LKO to block the HFD-induced increases in male brain AEA and decrease 2-AG in male brains was not attributable to decreased protein levels of NAPELD and DAGLα or increased levels of degradative enzymes.
Effect of Fabp1 Gene Ablation on the Impact of High Fat Diet (HFD) On the Brain AEA and 2-AG Receptor System
CB1 is the most common cannabinoid receptor-1 in brain (Maccarrone et al. 2010). HFD had no effect on brain protein level of CB1 in either male or female WT mice (Fig. 7F). In contrast, LKO conferred on HFD the ability to decrease CB1 protein in both males and females (Fig. 7F). This lack of effect of HFD on CB1 protein level did not correlate with marked increase in brain Cnr1 mRNA, but decreased CB1 protein did correlate with decreased Cnr1 mRNA in HFD-fed LKO males and females (Supplementary Fig. 2G). A similar pattern was found for Cnr2 mRNA (Supplementary Fig. 2H).
Thus, the HFD-induced increases in brain AEA in male mice did not correlate with any increase in brain CB1 receptor level. However, the ability of LKO to impair the HFD effect on AEA in male, but not female, mice was correlated with decreased CB1 receptor level.
DISCUSSION
Fatty acid components of high-fat diet (HFD) have a major impact on components of the brain EC system involved in food intake and energy distribution (Naughton et al. 2013;Osei-Hyiaman et al. 2005;Trillou et al. 2004;Engeli 2008;Silvestri and DiMarzo 2013). For example, fatty acids such as arachidonic acid (ARA) or its precursor LNA increase ARA-derived AEA and 2-AG (Naughton et al. 2013;Alvheim et al. 2012;Alvheim et al. 2014;Artmann et al. 2008). Although HFD concomitantly decreases CB1 in several brain regions (Engeli 2008), the HFD-induced increase in AEA and 2-AG acts on the cannabinoid receptor-1 (CB1) to stimulate food intake, hyperphagia, and adiposity (Naughton et al. 2013;Alvheim et al. 2012;Alvheim et al. 2014;Osei-Hyiaman et al. 2005;Watkins and Kim 2015;Guzman et al. 2004). However, most studies examining the impact of HFD on the brain EC system: i) were performed under ad libitum feeding conditions and do not differentiate effects of increased food consumption from those of higher proportion of dietary fat; ii) examined only males, but not females; iii) did not consider the high hepatic clearance of dietary ARA (Mashek 2013;Havel et al. 1962;Kohout et al. 1971). The work presented herein presents the following new insights into these questions:
First, the HFD-induced increase in brain AEA levels of wild-type (WT) male mice is independent of hyperphagia. Under pair-feeding conditions, food intake did not significantly differ between groups (Atshaves et al. 2010b;McIntosh et al. 2013). Yet male mice pair-fed HFD exhibited markedly increased brain levels of free and total ARA as well as the AEA derived therefrom by nearly 2 and 3-fold, respectively. This increase was consistent with the 3-fold higher dietary level of ARA and its precursor LNA in the HFD as indicated in Results. The HFD-induced increase in AEA was not prevented by concomitant smaller increases in DHEA or EPEA, which are known to displace ARA from cell membranes to thereby decrease AEA (Naughton et al. 2013;Watkins and Kim 2015). However, HFD did concomitantly increase brain levels of OEA which decreases the action of AEA by another mechanism (Naughton et al. 2013). Although brain PEA was decreased by HFD, PEA has no effect on food intake (Naughton et al. 2013). Finally, while the pair-fed HFD increased brain AEA level, it did not alter brain 2-AG or CB1 receptor level. The net result of these actions of pair-fed HFD on male mouse whole body phenotype was to increase body weight gain, primarily as increased fat tissue mass (Atshaves et al. 2010b;McIntosh et al. 2013). These findings differed significantly from those of ad libitum fed HFD which increases both AEA and 2-AG (Naughton et al. 2013;Silvestri and DiMarzo 2013), but decreases CB1 (Engeli 2008) to result in binge eating and whole body weight gain (Naughton et al. 2013;Silvestri and DiMarzo 2013). Taken together, these findings indicated that HFD’s impact on individual components of the brain EC system was highly dependent on whether the HFD was fed ad libitum or pair fed. Furthermore, HFD’s impact on the brain EC system to shift energy balance toward energy storage and fat accumulation did not require increased food consumption.
Second, pair-fed WT female mice were resistant to HFD-induced changes in brain EC (Figure 8). HFD did not significantly increase brain levels of N-acylethanolamides (AEA, OEA, PEA, or DHEA) and even decreased those of the 2-monoacylglycerols (2-AG, 2-OG, 2-PG). This was in marked contrast to WT males where most of these EC were increased in response to HFD. HFD-fed females’ lack of change in brain AEA or 2-AG was associated with unaltered or only slightly decreased brain free ARA or total ARA. Although few papers have focused on sex differences in the EC system (Craft et al. 2013), what is known indicates that WT female brains have higher brain AEA and 2-AG levels than their male counterparts (Martin et al. 2016a;Martin et al. 2016b). This was attributed to sexual dimorphism in both ARA synthesis and FABP1 level as evidenced by females having: i) higher serum ARA level (Decsi and Kennedy 2011;Lohner et al. 2013;Kitson et al. 2012); ii) higher liver level of Δ6-desaturase, the key enzyme in initiating conversion of LNA to ARA (Kitson et al. 2012); and iii) reduced hepatic expression of FABP1 (Atshaves et al. 2005;Atshaves et al. 2007;Atshaves et al. 2009;Atshaves et al. 2004), especially in the context of HFD as shown herein. While FABP1 is not expressed in brain (Owada et al. 2006;Myers-Payne et al. 1996a;Avdulov et al. 1998), FABP1 is thought to be a major cytosolic ARA-binding/transfer protein contributing to the high ARA first-pass hepatic clearance rate to thereby diminish plasma availability of ARA for brain uptake and EC formation (Mashek 2013;Havel et al. 1962;Kohout et al. 1971;Martin et al. 2016a). Indeed, females’ nearly 50% lower hepatic FABP1 level (Atshaves et al. 2005;Atshaves et al. 2007;Atshaves et al. 2009;Atshaves et al. 2004) correlates with higher plasma ARA availability for brain uptake and AEA formation as compared to males (Martin et al. 2016a;Martin et al. 2016b).
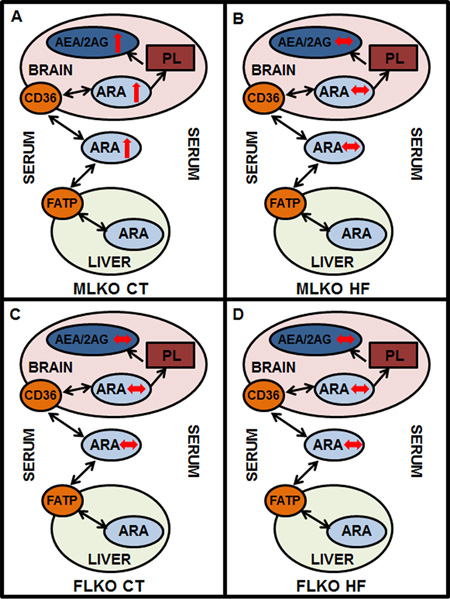
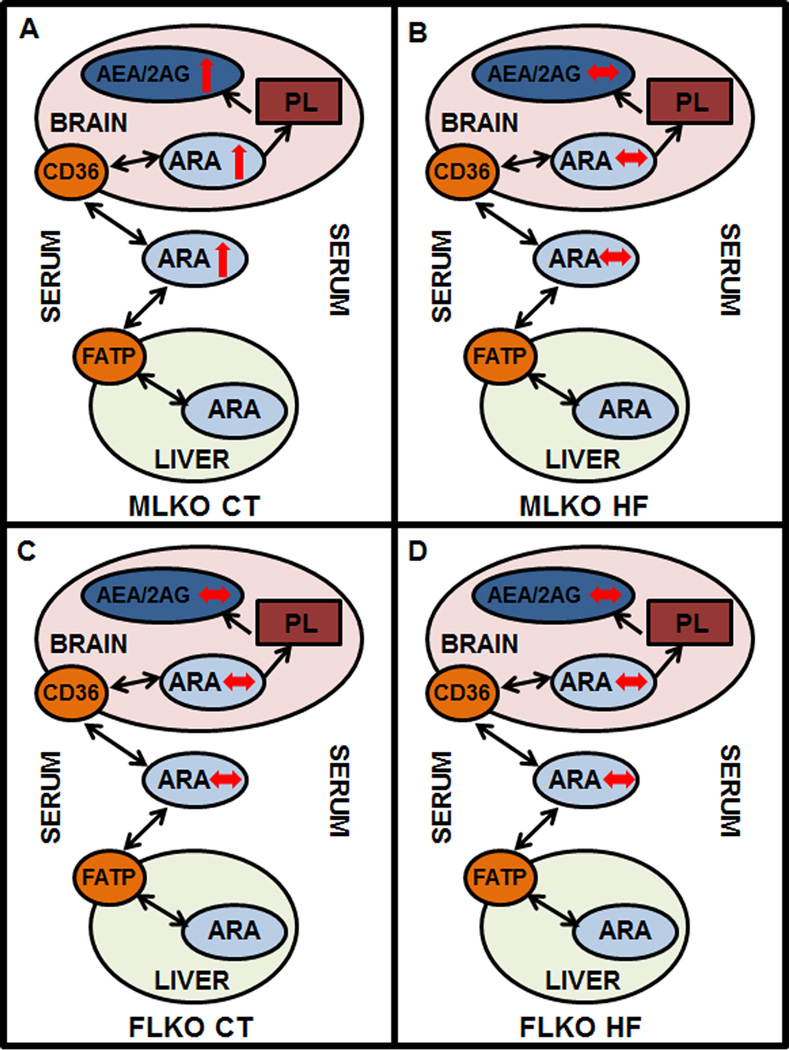
FIGURE 8.
Schematic representation of phenotypic changes in brain and serum endocannabinoid (EC) levels. Panel A: Brain/serum arachidonic acid (ARA) and ARA-containing EC (AEA, 2-AG) levels are increased (↑) in male Fabp1 gene-ablated (LKO) mice fed control (CT) diet vs male wild-type (WT) mice fed CT diet. Panel B: Brain/serum ARA and AEA/2-AG levels are unchanged (↔) in male LKO mice fed high-fat (HF) diet vs male WT mice fed HF diet. Panel C: Brain/serum ARA and AEA/2-AG levels are unchanged (↔) in female LKO mice fed CT diet vs female WT mice fed CT diet. Panel D: Brain/serum ARA and AEA/2-AG levels are unchanged (↔) female LKO mice fed HF diet vs female WT mice fed HF diet.
Third, FABP1 gene ablation (LKO) abolished the HFD-induced increase in brain AEA in pair fed male mice (Figure 8). This was associated primarily with concomitantly increased levels of brain cytosolic proteins (FABP3, FABP7, SCP2, HSP70) that bind/chaperone AEA to endoplasmic reticulum for degradation. Conversely, knockdown of FABP5 or FABP7 (Kaczocha et al. 2012;Kaczocha et al. 2015) or chemical inhibition of all brain FABPs (Elmes et al. 2015;Kaczocha et al. 2009;Kaczocha et al. 2012) is known to elevate AEA levels due to decreased targeting to degradative enzymes in the endoplasmic reticulum. Concomitantly, LKO increased weight gain as lean tissue mass in males and fat tissue mass in both males and females pair fed HFD (Atshaves et al. 2010b). This LKO-induced shift in the impact of HFD on whole body phenotype was attributed at least in part to: i) increased brain levels of cytosolic proteins in males (FABP3, FABP5, SCP-2, and possibly HSP70) and females (FABP5, SCP-2). Many of these proteins directly target fatty acids towards oxidative organelles (Atshaves et al. 2010a;Gallegos et al. 2001) and/or target both fatty acids and non-ARA containing ECs to the nucleus for activating PPARs (Schroeder et al. 2016;Schroeder et al. 2008); ii) marked downregulation of hepatic fatty acid oxidation as reflected in decreased serum β-hydroxybutyrate levels (Atshaves et al. 2010b). Finally, it is important to note that the increased body weight, % weight gain, fat tissue mass, and at least in females also lean tissue mass in HFD-fed LKO mice (Atshaves et al. 2010b) was not attributable to any increase in brain CB1 receptor levels—which was instead decreased in LKO HFD-fed mice regardless of sex. This was surprising since complete loss of CB1 (i.e. CB1 gene ablation) elicits resistance to HFD-induced obesity even in the context of similar caloric intake (Osei-Hyiaman et al. 2005;Trillou et al. 2004). Although reasons for this discrepancy remain to be resolved, one possibility is that CB1 ablation may impact FABP1 level or non-CB pathways may be operative. Thus, LKO blocked the ability of HFD to increase brain AEA levels not only through regulating levels of serum and brain ARA (from which brain AEA is primarily derived) but potentially also by additional non-CB pathways.
Taken together, these findings suggested that the impact of HFD on the brain endocannabinoid system, appetite, and energy metabolism depended significantly on the feeding regimen (pair-fed vs ad libitum), sex, and expression of FABP1. It was shown for the first time that FABP1 significantly impacts the effect of HFD on brain EC levels (Figure 8), brain CB1 receptor, and weight gain independent of differences in food intake, but in a sexually dimorphic manner. HFD dramatically increased brain levels of AEA in males, but not females, while concomitantly decreasing 2-AG in females but not males. LKO inhibited the high fat-induced increase in brain EC levels, especially in male mice. These effects in turn contributed to FABP1’s impact on whole body phenotype. Significantly, this work underscores the importance for considering FABP1 level in development of FABP1-targeted therapeutics, since hepatic FABP1 levels are pharmacologically inducible (Brandes and Arad 1983;Sanderson et al. 2010;Richert et al. 2003;Rakhshandehroo et al. 2009;Rajaraman et al. 2007;Hashimoto et al. 1999;Martin et al. 2013;Madsen et al. 1999;Huang et al. 2014), increased or decreased depending on expression highly prevalent SNP-variant genotypes (Peng et al. 2015;McIntosh et al. 2014), and upregulated in human liver pathologies such as obesity (Morrow et al. 1979), non-alcoholic fatty liver disease (NAFLD) (Yang et al. 1987;Higuchi et al. 2011;Charlton et al. 2009;Baumgardner et al. 2007), and alcoholic fatty liver disease (AFLD) (Pignon et al. 1987;Gyamfi et al. 2008).
Supplementary Material
Summary Statement.
FABP1 ablation inhibits high fat-induced increase in brain endocannabinoids in males.
Acknowledgments
This work was supported in part by the US Public Health Service/National Institutes of Health Grant R25 OD016574 (S.C., A.B.K.), and Merial Veterinary Scholars Program, CVM (S.C., A.B.K.).
Abbreviations
- AEA
n-6 arachidonoylethanolamide (anandamide)
- 2-AG
2-arachidonoylglycerol
- ARA
arachidonic acid
- Cnr1
cannabinoid receptor-1 (CB1)
- Cnr2
cannabinoid receptor-2 (CB2)
- Dagla
diacylglycerol lipase A (DAGL-A)
- Daglb
diacylglycerol lipase B (DAGL-B)
- DHEA
n-3 docosahexaenoylethanolamide
- DIO
high fat diet induced obesity
- EC
endocannabinoid
- EPEA
n-3 eicosapentaenoylethanolamide
- Faah
fatty acid amide hydrolase (FAAH)
- Fabp1
liver fatty acid binding protein-1 (FABP1, L-FABP)
- Fabp3
fatty acid binding protein-3 (FABP-3)
- Fabp5
fatty acid binding protein-5 (FABP-5)
- Fabp7
fatty acid binding protein-7 (FABP-7)
- FAT/CD36
fatty acid translocase/thrombospondin receptor
- FATP-1
fatty acid transport protein-1
- FATP-4
fatty acid transport protein-4
- LKO
FABP1 gene ablated mouse on C57BL/6NCr background
- HFD
high fat diet
- LCFA
long chain fatty acid
- LCFA-CoA
long chain fatty acyl CoA
- LNA
linoleic acid (18:2n-6)
- 2-MG
2-monoacylglycerol
- Mgll
2-monoacylglycerol lipase (MGL)
- Naaa
N-acylethanolamine-hydrolyzing acid amidase (NAAA)
- NAE
N-acylethanolamide
- NAPE
N-acylphosphatidylethanolamide
- Nape-pld
N-acylphosphatidylethanolamide phospholipase-D (NAPE-PLD)
- OEA
oleoylethanolamide
- 2-OG
2-oleoylglycerol
- PEA
palmitoylethanolamide
- 2-PG
2-palmitoylglycerol
- Scp2
sterol carrier protein-2 (SCP-2)
- WT
wild-type C57BL/6NCr mouse.
Footnotes
Conflict of interest: The authors declare that they have no conflicts of interest with the contents of this article.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author contributions: GGM and LJD designed, performed, and analyzed the experiments shown in Figures 1, 2, and 4. DL designed, performed, and analyzed the experiments shown in Figures 5, 6, and 7. SC designed, performed, and analyzed the experiments shown in Supplementary Figures 1 and 2. DRS, EJM, and MYG designed, performed, and analyzed the experiment shown in Figure 3. EJM, ABK, and FS conceived and coordinated the study and wrote the paper. All authors reviewed the results and approved the final version of the manuscript.
REFERENCES
- Alvheim AR, Malde MK, Osei-Hyiaman D, et al. Dietary linoleic acid elevates endogenous 2-AG and anandamide and induces obesity. Integrative Physiology. 2012;20:1984–1994. doi: 10.1038/oby.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvheim AR, Torstensen BE, Lin YH, et al. Dietary linoleic acid elevates teh endocannabinoids 2-AG and anandamide and promotes weight gain in mice fed a low fat diet. Lipids. 2014;49:59–69. doi: 10.1007/s11745-013-3842-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artmann A, Petersen G, Hellgren LI, et al. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochim. Biophys. Acta. 2008;1781:200–212. doi: 10.1016/j.bbalip.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Atshaves BP, Martin GG, Hostetler HA, McIntosh AL, Kier AB, Schroeder F. Liver fatty acid binding protein (L-FABP) and Dietary Obesity. Journal of Nutritional Biochemisty. 2010a;21:1015–1032. doi: 10.1016/j.jnutbio.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atshaves BP, McIntosh AL, Kier AB, Schroeder F. High dietary fat exacerbates weight gain and obesity in female liver fatty acid binding protein gene ablated mice. Lipids. 2010b;45:97–110. doi: 10.1007/s11745-009-3379-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atshaves BP, McIntosh AL, Martin GG, Landrock D, Payne HR, Bhuvanendran S, Landrock K, Lyuksyutova OI, Johnson JD, Macfarlane RD, Kier AB, Schroeder F. Overexpression of sterol carrier protein-2 differentially alters hepatic cholesterol accumulation in cholesterol-fed mice. J. Lipid Res. 2009;50:1429–1447. doi: 10.1194/jlr.M900020-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atshaves BP, McIntosh AL, Landrock D, Payne HR, Mackie J, Maeda N, Ball JM, Schroeder F, Kier AB. Effect of SCP-x gene ablation on branched-chain fatty acid metabolism. Am. J. Physiol. 2007;292:939–951. doi: 10.1152/ajpgi.00308.2006. [DOI] [PubMed] [Google Scholar]
- Atshaves BP, McIntosh AL, Payne HR, Mackie J, Kier AB, Schroeder F. Effect of branched-chain fatty acid on lipid dynamics in mice lacking liver fatty acid binding protein gene. Am. J. Physiol. 2005;288:C543–C558. doi: 10.1152/ajpcell.00359.2004. [DOI] [PubMed] [Google Scholar]
- Atshaves BP, Payne HR, McIntosh AL, Tichy SE, Russell D, Kier AB, Schroeder F. Sexually dimorphic metabolism of branched chain lipids in C57BL/6J mice. J. Lipid Res. 2004;45:812–830. doi: 10.1194/jlr.M300408-JLR200. [DOI] [PubMed] [Google Scholar]
- Avdulov NA, Chochina SV, Myers-Payne S, Hubbell T, Igbavboa U, Schroeder F, Wood WG. Expression and lipid binding of sterol carrier protein-2 and liver fatty acid binding proteins: differential effects of ethanol in vivo and in vitro. In: Riemersma RAARKRWaWR., editor. Essential Fatty Acids and Eicosanoids: Invited Papers from the Fourth International Congress. Champaign, IL: American Oil Chemists Society Press; 1998. pp. 324–327. [Google Scholar]
- Baumgardner JN, Shankar K, Hennings L, Badger TM, Ronis MJJ. A new model for nonalcoholic steatohepatitis in the rat utilizing total enteral nutrition to overfeed a high-polyunsaturated fat diet. Am. J. Physiol. Gastrointest. and Liver Phys. 2007;294:G27–G38. doi: 10.1152/ajpgi.00296.2007. [DOI] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF. Comprehensive profile of brain enzymes that hydrolyze endocannabinoid 2-arachidonoylglycerol. Chemistry and Biology. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes R, Arad R. Liver cytosolic fatty acid-binding proteins. Effect of diabetes and starvation. Biochim. Biophys. Acta. 1983;750:334–339. doi: 10.1016/0005-2760(83)90037-1. [DOI] [PubMed] [Google Scholar]
- Charan J, Kantharia ND. How to calculate sample size in animal studies. J. Pharmacology and Pharmacotherapeutics. 2013;4:303–306. doi: 10.4103/0976-500X.119726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton M, Viker K, Krishnan A, Sanderson S, Veldt B, Kaalsbeek AJ, Kendrick M, Thompson G, Que F, Swain J, Sarr M. Differential expression of lumican and fatty acid binding protein-1: new insights into the histologic spectrum of nonalcoholic fatty liver disease. Hepatology. 2009;49:1375–1384. doi: 10.1002/hep.22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, Marusich JA, Wiley JL. Sex differences in cannabinoid pharmacology: a reflection of differences in endocannabinoid system? Life Sci. 2013;92:476–481. doi: 10.1016/j.lfs.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decsi T, Kennedy K. Sex-specific differences in essential fatty acid metabolism. Am. J. Clin. Nutr. 2011;94:1914S–1919S. doi: 10.3945/ajcn.110.000893. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bisogno T, Sugiura T, et al. The novel endogenous cannabinoid 2-AG is inactivated by neuronal- and basophil-like cells: connections with anandamide. Biochem. J. 1998;331:15–19. doi: 10.1042/bj3310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinghaus P, Wolfrum C, Assmann G, Spener F, Seedorf U. Phytanic acid activates the peroxisome proliferator-activated receptor alpha (PPARalpha) in sterol carrier protein-2-/sterol carrier protein x-deficient mice. J. Biol. Chem. 1999;274:2766–2772. doi: 10.1074/jbc.274.5.2766. [DOI] [PubMed] [Google Scholar]
- Elmes MW, Kaczocha M, Berger WT, Leung KN, Ralph BP, Wang L, Sweeney JM, Miyauchi JT, Tsirka SE, Ojima I, Deutsch DG. Fatty acid binding proteins are intracellular carriers for delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) J. Biol. Chem. 2015;290:8711–8721. doi: 10.1074/jbc.M114.618447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeli S. Dysregulation of the endocannabinoid system in obesity. Journal of Neuroendocrinology. 2008;20:110–115. doi: 10.1111/j.1365-2826.2008.01683.x. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power3.1: Tests for correlation and regression analyses. Behavior Res. Meth. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power3: A lexible statistical power analysis program for the socia, behavioral, and biomedical sciences. Behavior Res. Meth. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Frolov A, Cho TH, Billheimer JT, Schroeder F. Sterol carrier protein-2, a new fatty acyl coenzyme A-binding protein. J. Biol. Chem. 1996;271:31878–31884. doi: 10.1074/jbc.271.50.31878. [DOI] [PubMed] [Google Scholar]
- Frolov A, Cho TH, Murphy EJ, Schroeder F. Isoforms of rat liver fatty acid binding protein differ in structure and affinity for fatty acids and fatty acyl CoAs. Biochemistry. 1997;36:6545–6555. doi: 10.1021/bi970205t. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Hafer A, Muench C, Kannenberg F, Teichmann S, Scheibner J, Stange EF, Seedorf U. Disruption of the sterol carrier protein 2 gene in mice impairs biliary lipid and hepatic cholesterol metabolism. J. Biol. Chem. 2001;276:48058–48065. doi: 10.1074/jbc.M106732200. [DOI] [PubMed] [Google Scholar]
- Gajda AM, Zhou YX, Agellon LB, Fried SK, Kodukula S, Fortson W, Patel K, Storch J. Direct comparison of mice null for liver- or intestinal fatty acid binding proteins reveals highly divergent phenotypic responses to high fat feeding. J. Biol. Chem. 2013;288:30330–30344. doi: 10.1074/jbc.M113.501676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos AM, Atshaves BP, Storey SM, Starodub O, Petrescu AD, Huang H, McIntosh A, Martin G, Chao H, Kier AB, Schroeder F. Gene structure, intracellular localization, and functional roles of sterol carrier protein-2. Prog. Lipid Res. 2001;40:498–563. doi: 10.1016/s0163-7827(01)00015-7. [DOI] [PubMed] [Google Scholar]
- Goparaju SK, Ueda N, Yamaguchi H, Yamamoto S. Anandamide amidohydrolase reacting with 2-AG, another cannabinoid receptor ligand. FEBS Lett. 1989;422:69–73. doi: 10.1016/s0014-5793(97)01603-7. [DOI] [PubMed] [Google Scholar]
- Guzman M, Lo Verme J, Fu J, Oveisi F, Blazquez C, Piomelli D. Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor PPARa. J. Biol. Chem. 2004;279:27849–27854. doi: 10.1074/jbc.M404087200. [DOI] [PubMed] [Google Scholar]
- Gyamfi MA, He L, French SW, Damjanov I, Wan Y-JY. Hepatocyte retinoid X receptor alpha-dependent regulation of lipid homeostasis and inflammatory cytokine expression contributes to alcohol-induced liver injury. J. Pharm. Exp. Ther. 2008;324:443–453. doi: 10.1124/jpet.107.132258. [DOI] [PubMed] [Google Scholar]
- Hanhoff T, Benjamin S, Borchers T, Spener F. Branched-chain fatty acids as activators of peroxisome proliferators. Eur. J. Lip. Sci. Technol. 2005;107:716–729. [Google Scholar]
- Hanhoff T, Wolfrum C, Ellinghaus P, Seedorf U, Spener F. Pristanic acid is activator of PPARalpha. Eur. J. Lipid Sci. 2001;103:75–80. [Google Scholar]
- Hashimoto T, Fujita T, Usuda N, Cook W, Qi C, Peters JM, Gonzalez FJ, Yeldandi AV, Rao MS, Reddy JK. Peroxisomal and mitochondrial fatty acid beta-oxidation in mice nullizygous for both PPARalpha and peroxisomal fatty acyl CoA oxidase. J. Biol. Chem. 1999;274:19228–19236. doi: 10.1074/jbc.274.27.19228. [DOI] [PubMed] [Google Scholar]
- Havel RJ, Felts JM, Van Duyne CM. Formation and fate of endogenous triglycerides in blood plasma of rabbits. J. Lipid Res. 1962;3:297–308. [Google Scholar]
- Higuchi N, Kato M, Tanaka M, et al. Effects of insulin resistance and hepatic lipid accumulation on hepatic mRNA expression levels of apoB, MTP, and L-FABP in non-alcoholic fatty liver disease. Exp. and Ther. Med. 2011;2:1077–1081. doi: 10.3892/etm.2011.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, McIntosh AL, Martin GG, Landrock K, Landrock D, Gupta S, Atshaves BP, Kier AB, Schroeder F. Structural and functional interaction of fatty acids with human liver fatty acid binding protein (L-FABP) T94A variant. FEBS J. 2014;281:2266–2283. doi: 10.1111/febs.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczocha LM, Glaser ST, Deutsch DG. Identification of intracellular carriers for the endocannabinoid anandamide. Proc. Natl. Acad. Sci. U. S. A. 2009;106:6375–6380. doi: 10.1073/pnas.0901515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczocha M, Glaser ST, Maher T, Clavin B, Hamilton J, O'Rourke J, Rebecchi M, Puopolo M, Owada Y, Thanos PK. Fatty acid binding protein deletion suppresses inflammatory pain through endocannabinoid/N-acylethanolamine-dependent mechanisms. Molecular Pain. 2015;11:52. doi: 10.1186/s12990-015-0056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczocha M, Vivieca S, Sun J, Glaser ST, Deutsch DG. Fatty acid binding proteins transport N-acylethanolamines to nuclear receptors and are targets of endocannabinoid transport inhibitors. J. Biol. Chem. 2012;287:3415–3424. doi: 10.1074/jbc.M111.304907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitson AP, Smith TL, Marks KA, Stark KD. Tissue specific sex differences in docosahexaenoic acid and delta-6 desaturase in rats fed a standard chow diet. Appl. Physiol. Nutr. Metab. 2012;37:1200–1211. doi: 10.1139/h2012-103. [DOI] [PubMed] [Google Scholar]
- Klipsic D, Landrock D, Martin GG, McIntosh AL, Landrock KK, Mackie JT, Schroeder F, Kier AB. Impact of SCP-2/SCP-x gene ablation and dietary cholesterol on hepatic lipid accumulation. Am. J. Physiol. Gastrointest. and Liver Phys. 2015;309:G387–G399. doi: 10.1152/ajpgi.00460.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohout M, Kohoutova B, Heimberg M. The regulaton of hepatic triglyceride metabolism by free fatty acids. J. Biol. Chem. 1971;246:5067–5074. [PubMed] [Google Scholar]
- Lillo JL. The endocannabinoid system as a novel approach for managing obesity. J. Am. Osteopath. Assoc. 2007;107(suppl. 2):512–520. [PubMed] [Google Scholar]
- Lohner S, Fekete K, Marosvolgyi T, Desci T. Gender differences in long chain PUFA status: systematic review of 51 publications. Ann. Nutr. Metab. 2013;62:98–112. doi: 10.1159/000345599. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Gasperi V, Catani MV, Diep TI, Dainese E, Hansen HS, Avigliano L. The endocannabinoid system and its relevance to nutrition. Annu. Rev. Nutr. 2010;30:423–440. doi: 10.1146/annurev.nutr.012809.104701. [DOI] [PubMed] [Google Scholar]
- Madsen L, Rustan AC, Vaagenes H, Berge K, Dyroy E, Berge RK. Eicosapentaenoic and docosahexaenoic acid affect mitochondrial and peroxisomal fatty acid oxidation in relation to substrate preference. Lipids. 1999;34:951–963. doi: 10.1007/s11745-999-0445-x. [DOI] [PubMed] [Google Scholar]
- Martin G, Chung S, Landrock D, Landrock KK, Huang H, Dangott LJ, Peng X, Kaczocha M, Seeger DR, Murphy EJ, Golovko MY, Kier AB, Schroeder F. FABP1 gene ablation impacts brain endocannabinoid system in male mice. J. Neurochem. 2016a;138:407–422. doi: 10.1111/jnc.13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GG, Chung S, Landrock D, Landrock K, Dangott LJ, Peng X, Kaczocha M, Murphy EJ, Kier AB, Schroeder F. Female mice are resistant to Fabp1 gene ablation induced alterations in brain endocannabinoid levels. Lipids. 2016b;5:1007–1020. doi: 10.1007/s11745-016-4175-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GG, Danneberg H, Kumar LS, Atshaves BP, Erol E, Bader M, Schroeder F, Binas B. Decreased liver fatty acid binding capacity and altered liver lipid distribution in mice lacking the liver fatty acid binding protein (L-FABP) gene. J. Biol. Chem. 2003;278:21429–21438. doi: 10.1074/jbc.M300287200. [DOI] [PubMed] [Google Scholar]
- Martin GG, McIntosh AL, Huang H, Gupta S, Atshaves BP, Kier AB, Schroeder F. Human liver fatty acid binding protein (L-FABP) T94A variant alters structure, stability, and interaction with fibrates. Biochemistry. 2013;52:9347–9357. doi: 10.1021/bi401014k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashek DG. Hepatic fatty acid trafficking: multiple forks in the road. Adv. Nutr. 2013;4:697–710. doi: 10.3945/an.113.004648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AL, Huang H, Atshaves BP, Wellburg E, Kuklev DV, Smith WL, Kier AB, Schroeder F. Fluorescent n-3 and n-6 very long chain polyunsaturated fatty acids: three photon imaging and metabolism in living cells overexpressing liver fatty acid binding protein. J. Biol. Chem. 2010;285:18693–18708. doi: 10.1074/jbc.M109.079897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AL, Atshaves BP, Landrock D, Landrock KK, Martin GG, Storey SM, Kier AB, Schroeder F. Liver fatty acid binding protein gene-ablation exacerbates weight gain in high fat fed female mice. Lipids. 2013;48:435–448. doi: 10.1007/s11745-013-3777-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AL, Huang H, Storey SM, Landrock K, Landrock D, Petrescu AD, Gupta S, Atshaves BP, Kier AB, Schroeder F. Human FABP1 T94A variant impacts fatty acid metabolism and PPARa activation in cultured human female hepatocytes. Am. J. Physiol. Gastrointest. and Liver Phys. 2014;307:G164–G176. doi: 10.1152/ajpgi.00369.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RW, Hatch GM. Fatty acid transport into the brain: of fatty acid fables and lipid tails. Prost. Leukot. Essen. Fatty Acids. 2011;85:293–302. doi: 10.1016/j.plefa.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Morrow FD, Allen CE, Martin RJ. Intracellular fatty acid-binding protein: Hepatic levels in lean and obese rats. Fed. Proc. 1979;38:280. [Google Scholar]
- Moulle VSF, Cansell C, Luquet S, Cruciani-Guglielmacci C. Multiple roles of fatty acid handling proteins in brain. Frontiers in Physiology. 2012;3:1–6. doi: 10.3389/fphys.2012.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy EJ, Edmondson RD, Russell DH, Colles SM, Schroeder F. Isolation and characterization of two distinct forms of liver fatty acid binding protein from the rat. Biochim. Biophys. Acta. 1999;1436:413–425. doi: 10.1016/s0005-2760(98)00150-7. [DOI] [PubMed] [Google Scholar]
- Murphy EJ, Owada Y, Kitanaka N, Kondo H, Glatz JFC. Brain arachidonic acid incorportion is decreased in heart FABP gene ablated mice. Biochem. 2005;44:6350–6360. doi: 10.1021/bi047292r. [DOI] [PubMed] [Google Scholar]
- Murphy EJ, Prows DR, Jefferson JR, Incerpi S, Hertelendy ZI, Heiliger CE, Schroeder F. Cis-parinaric acid uptake in L-cells. Arch. Biochem. Biophys. 1996a;335:267–272. doi: 10.1006/abbi.1996.0507. [DOI] [PubMed] [Google Scholar]
- Murphy EJ, Prows DR, Jefferson JR, Schroeder F. Liver fatty acid binding protein expression in transfected fibroblasts stimulates fatty acid uptake and metabolism. Biochim. Biophys. Acta. 1996b;1301:191–198. doi: 10.1016/0005-2760(96)00024-0. [DOI] [PubMed] [Google Scholar]
- Myers-Payne S, Fontaine RN, Loeffler AL, Pu L, Rao AM, Kier AB, Wood WG, Schroeder F. Effects of chronic ethanol consumption on sterol transfer protein in mouse brain. J. Neurochem. 1996a;66:313–320. doi: 10.1046/j.1471-4159.1996.66010313.x. [DOI] [PubMed] [Google Scholar]
- Myers-Payne SC, Hubbell T, Pu L, Schnutgen F, Borchers T, Wood WG, Spener F, Schroeder F. Isolation and characterization of two fatty acid binding proteins from mouse brain. J. Neurochem. 1996b;66:1648–1656. doi: 10.1046/j.1471-4159.1996.66041648.x. [DOI] [PubMed] [Google Scholar]
- Naughton SS, Mathai ML, Hryciw DH, McAinch AJ. Fatty acid modulation of the endocannabinoid system and the effect on food intake and metabolism. Int. J. Endocrinol. 2013;ID 361895:1–11. doi: 10.1155/2013/361895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddi S, Fezza F, Pasquoriello N, et al. Molecular identification of albumin and Hsp70 as cytosolic anandamide binding proteins. Chemistry and Biology. 2009;16:624–632. doi: 10.1016/j.chembiol.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Osei-Hyiaman D, DePetrillo M, Pacher P, Kunos G. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J. Clin. Inv. 2005;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owada Y. Fatty acid binding protein: localization and functional significance in brain. Tohoku J. Exp. Med. 2008;214:213–220. doi: 10.1620/tjem.214.213. [DOI] [PubMed] [Google Scholar]
- Owada Y, Abdelwahab S, et al. Altered emotional behavioral responses in mice lacking brain type FABP gene. Eur. J. Neurosci. 2006;24:175–187. doi: 10.1111/j.1460-9568.2006.04855.x. [DOI] [PubMed] [Google Scholar]
- Peng X-E, Wu Y-L, Zhu Y, Huang R-D, Lu Q-Q, Lin X. Association of a human FABP1 gene promoter region polymorphism with altered serum triglyceride levels. PLoS ONE. 2015:1–13. doi: 10.1371/journal.pone.0139417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignon J-P, Bailey NC, Baraona E, Lieber CS. Fatty acid-binding protein: a major contributor to the ethanol-induced increase in liver cytosolic proteins in the rat. Hepatology. 1987;7:865–871. doi: 10.1002/hep.1840070512. [DOI] [PubMed] [Google Scholar]
- Pu L, Annan RS, Carr SA, Frolov A, Wood WG, Spener F, Schroeder F. Isolation and identification of a native fatty acid binding protein form mouse brain. Lipids. 1999a;34:363–373. doi: 10.1007/s11745-999-0374-8. [DOI] [PubMed] [Google Scholar]
- Pu L, Foxworth WB, Kier AB, Annan RS, Carr SA, Edmondson R, Russell D, Wood WG, Schroeder F. Isolation and Characterization of 26- and 30-kDa Rat Liver Proteins Immunoreactive to Anti-Sterol Carrier Protein-2 Antibodies. Protein Expression And Purification. 1998;13:337–348. doi: 10.1006/prep.1998.0908. [DOI] [PubMed] [Google Scholar]
- Pu L, Igbavboa U, Wood WG, Roths JB, Kier AB, Spener F, Schroeder F. Expression of Fatty Acid Binding Proteins Is Altered in Aged Mouse Brain. Molecular and Cellular Biochemistry. 1999b;198:69–78. doi: 10.1023/a:1006946027619. [DOI] [PubMed] [Google Scholar]
- Rajaraman G, Wang GQ, Yan J, Jiang P, Gong Y, Burczynski FJ. Role of cytosolic liver fatty acid binding protein in hepatocellular oxidative stress: effect of dexamethasone and clofibrate treatment. Mol. Cell. Biochem. 2007;295:27–34. doi: 10.1007/s11010-006-9268-6. [DOI] [PubMed] [Google Scholar]
- Rakhshandehroo M, Hooiveld G, Muller M, Kersten S. Comparative analysis of gene regulation by the transcription factor PPARa between mouse and human. PLoS ONE. 2009;4:e6796. doi: 10.1371/journal.pone.0006796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richert L, Lamboley C, Viollon-Abadie C, Grass P, Hartmann N, Laurent S, Heyd B, Mantion G, Chibout S-D, Staedtler F. Effects of clofibric acid on mRNA expression profiles in primary cultures of rat, mouse, and human hepatocytes. Toxicol. Appl. Pharmacol. 2003;191:130–146. doi: 10.1016/s0041-008x(03)00231-x. [DOI] [PubMed] [Google Scholar]
- Sanderson LM, Boekschoten MV, Desvergne B, Muller M, Kersten S. Transcriptional profiling reeals divergent roles of PPARa and PPARb/d in regulatoin of gene expression in mouse liver. Physiol. Genomics. 2010;41:42–52. doi: 10.1152/physiolgenomics.00127.2009. [DOI] [PubMed] [Google Scholar]
- Schroeder F, Jefferson JR, Powell D, Incerpi S, Woodford JK, Colles SM, Myers-Payne S, Emge T, Hubbell T, Moncecchi D, Prows DR, Heyliger CE. Expression of rat L-FABP in mouse fibroblasts: role in fat absorption. Mol. Cell. Biochem. 1993;123:73–83. doi: 10.1007/BF01076477. [DOI] [PubMed] [Google Scholar]
- Schroeder F, McIntosh AL, Martin GG, Huang H, Landrock D, Chung S, Landrock KK, Dangott LJ, Li S, Kaczocha M, Murphy EJ, Atshaves BP, Kier AB. Fatty acid binding protein-1 (FABP1) and the human FABP1 T94A variant: Roles in the endocannabinoid system and dyslipidemias. Lipids. 2016;51:655–676. doi: 10.1007/s11745-016-4155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder F, Petrescu AD, Huang H, Atshaves BP, McIntosh AL, Martin GG, Hostetler HA, Vespa A, Landrock K, Landrock D, Payne HR, Kier AB. Role of fatty acid binding proteins and long chain fatty acids in modulating nuclear receptors and gene transcription. Lipids. 2008;43:1–17. doi: 10.1007/s11745-007-3111-z. [DOI] [PubMed] [Google Scholar]
- Seedorf U, Raabe M, Ellinghaus P, Kannenberg F, Fobker M, Engel T, Denis S, Wouters F, Wirtz KWA, Wanders RJA, Maeda N, Assmann G. Defective peroxisomal catabolism of branched fatty acyl coenzyme A in mice lacking the sterol carrier protein-2/sterol carrier protein-x gene function. Genes and Development. 1998;12:1189–1201. doi: 10.1101/gad.12.8.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri C, DiMarzo V. The endocannabinoid system in energy homeostasis and the etiophathology of metabolic disorders. Cell Metabolism. 2013;17:475–490. doi: 10.1016/j.cmet.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Thigpen JE, Setchell KD, Ahlmark KB, Kocklear J, Spahr T, Caviness GF, Goelz MF, Haseman JK, Newbold RR, Forsythe DB. Phytoestrogen content of purified, open- and closed-formula laboratory animal diets. Lab. An. Science. 1999a;49:530–536. [PubMed] [Google Scholar]
- Thigpen JE, Setchell KD, Goelz MF, Forsythe DB. The phytoestrogen content of rodent diets. Envron. Health Persp. 1999b;107:A182–A183. doi: 10.1289/ehp.107-1566530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trillou CR, Delgorge C, Menet C, Arnone M, Soubrie P. CB1 receptro knockout in mic eleads to leanness, resistanc eto diet-induced obesity and enhanced leptin sensitivity. Int. J. Obesity. 2004;28:640–648. doi: 10.1038/sj.ijo.0802583. [DOI] [PubMed] [Google Scholar]
- Watkins BA, Kim J. The endocannabinoid system: directing eating behavior and macronutrient metabolism. Frontiers in Psychology. 2015:1–10. doi: 10.3389/fpsyg.2014.01506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfrum C, Ellinghaus P, Fobker M, Seedorf U, Assmann G, Borchers T, Spener F. Phytanic acid is ligand and transcriptional activator of murine liver fatty acid binding protein. J. Lipid Res. 1999;40:708–714. [PubMed] [Google Scholar]
- Yang SY, He XY, Schulz H. Fatty acid oxidation in rat brain is limited by the low activity of 3-ketoacyl-coenzyme A thiolase. J. Biol. Chem. 1987;262:13027–13032. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.