Abstract
Background
There is interest in using atherosclerotic cardiovascular disease (ASCVD) risk to personalize systolic blood pressure (SBP) treatment goals. Therefore, we studied whether Coronary Artery Calcium (CAC) can further guide the allocation of anti-hypertensive treatment intensity.
Methods
We included 3,733 participants from the Multi-Ethnic Study of Atherosclerosis with SBP between 120-179mmHg. Within subgroups categorized by both SBP (120-139, 140-159, 160-179mmHg) and estimated 10-year ASCVD risk (using the ACC/AHA pooled-cohort equations), we compared multivariable-adjusted hazard ratios (HRs) for the composite outcome of incident ASCVD or heart failure, after further stratifying by CAC (0, 1-100, or >100). We estimated 10-year number-needed-to-treat (NNT10) for an intensive SBP goal of 120mmHg by applying the treatment benefit recorded in meta-analyses to event rates within CAC strata.
Results
Mean age was 65 years. There were 642 composite events over a median of 10.2 years. In persons with SBP <160mmHg, CAC stratified risk for events. For example, among those with ASCVD risk <15% and who had SBP of either 120-139 or 140-159mmHg, respectively, we found increasing HRs for events with CAC 1-100 (1.7 [95% CI, 1.0-2.6] or 2.0 [1.1-3.8]) and CAC >100 (3.0 [1.8-5.0] or 5.7 [2.9-11.0]), all relative to CAC=0. There appeared to be no statistical association between CAC and events when SBP was 160-179mmHg, irrespective of ASCVD risk level. Estimated NNT10 for a SBP goal of 120mmHg varied substantially according to CAC levels when predicted ASCVD risk <15% and SBP <160mmHg (e.g. NNT10 of 99 for CAC=0 and 24 for CAC>100, when SBP 120-139mmHg). However, few participants with ASCVD risk <5% had elevated CAC. Furthermore, NNT10 estimates were consistently low and varied less among CAC strata when SBP was 160-179mmHg or when ASCVD risk was ≥15% at any SBP level.
Conclusion
Combined CAC-imaging and assessment of global ASCVD risk has potential to guide personalized SBP goals (e.g., choosing a traditional goal of 140 or a more intensive goal of 120 mmHg), particularly among adults with estimated ASCVD risk 5-15% and pre-hypertension or mild hypertension.
Keywords: Systolic BP, Antihypertensive therapy, CVD risk, Coronary Artery Calcium
Introduction
Elevated blood pressure (BP) is a major cause of heart disease, stroke, and heart failure, with over 972 million adults worldwide and approximately one in three U.S. adults diagnosed with hypertension.1 While effective antihypertensive pharmacotherapies are widely available,2 there has been recent controversy regarding the optimal systolic BP (SBP) threshold to initiate or intensify treatment. For example, relying on data from randomized trials (and excluding observational results), a 2014 report by the eighth panel appointed to the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC-8) found no trial evidence to support initiating therapy until a SBP of 150 mmHg or higher in adults older than 60 who do not have diabetes or chronic kidney disease.3 This recommendation was controversial4 and differs from other guidelines and advisories, the majority of which recommend a lower threshold of 140 mmHg.
Furthermore, after JNC-8 was released, the landmark Systolic Blood Pressure Intervention Trial (SPRINT)5 reported significant improvements in outcomes, notably ASCVD and heart failure, among 9,361 high-risk non-diabetic hypertensive patients, older than 50, treated to a SBP target of 120 mm Hg or less versus the standard target of 140 mmHg or less. Thus, questions remain about whom to treat and with what treatment intensity, particularly among individuals with pre-hypertension or mild hypertension.
In this context, there has been heightened interest in the use of global ASCVD risk estimates - in conjunction with SBP – to guide initiation and titration treatment decisions for hypertension.6-9 This strategy may allow providers to balance the tension between avoiding overtreatment among low risk persons who are unlikely to benefit and intensifying treatment to achieve lower SBP in higher-risk adults. Prior reports of risk-based allocation of BP therapy have focused exclusively on risk estimates derived from traditional clinical risk factors such as those included in the ACC/AHA 2013 ASCVD risk score. 6, 8, 10, 11
Coronary Artery Calcium (CAC), measured by non-contrast cardiac CT, is a powerful subclinical marker of absolute and relative ASCVD risk and has been demonstrated to add incremental prognostic information to risk estimates derived from traditional risk factors.12-14 In addition, prior analyses have suggested that CAC testing has potential to personalize allocation of other preventive therapies (e.g., aspirin or statin) by identifying individuals who are unlikely to obtain net benefit (e.g., those with zero CAC generally have very low absolute ten-year risk and, hence, high estimated number-needed-to-treat [NNT]), as well as those who may be more likely to benefit due to high absolute risk (e.g., CAC>100).15, 16
Therefore, in this study we sought to determine whether CAC might inform the identification of primary prevention candidates who are more likely to benefit from initiation or titration of antihypertensive therapy to a more intensive SBP goal of 120 mmHg (compared to the current standard of 140 mmHg).
Methods
Study Participants
MESA is a multi-center, multi-ethnic, prospective observational cohort study.17 Between July 2000 and August 2002, MESA recruited 6,814 men and women, aged 45 to 84 years, from four ethnic groups (Caucasian, African-American, Chinese-American, and Hispanic). Participants were enrolled from six geographically distinct U.S. communities. Exclusion criteria included clinical cardiovascular disease at baseline. All participants provided informed consent and the study was approved by the institutional review boards at all field centers.
The primary sample for this analysis excluded MESA participants with baseline systolic BP levels below 120 mmHg (n=2,939) and equal to or higher than 180 mmHg (n=136). We excluded persons with SBP <120 mmHg a priori because we determined that CAC screening among these adults for the purposes of BP management would be inappropriate due to the fact that treating adults with SBP <120 to even lower BPs (irrespective of CAC) is difficult to justify. We also excluded those with SBP ≥180 mmHg because, 1) this was an outlier SBP phenotype in the sample (just 1.9%), 2) SBP at this level is consistent with hypertensive urgency, is high risk, and requires rapid therapy- not CAC testing to target specific goals, and 3) we did not want to include individuals with possible secondary hypertension in the analysis. In addition, we excluded six persons with missing information on baseline systolic BP or BP medication use, leaving 3,733 participants in total. We also conducted secondary analyses using a subsample of MESA participants who fulfilled SPRINT criteria. 5 This subsample included only non-diabetics older than 50 with systolic BP ≥130 mmHg and who had any one of the following; Framingham CVD ten-year Risk ≥15% or left ventricular hypertrophy by EKG or ankle-brachial index <0.9 or estimated glomerular filtration rate between 20-59 mL/min/1.73 m2. After exclusions, this subsample included 1,394 participants.
Cardiovascular Risk Factors
Race, family history of myocardial infarction, and smoking status were collected by self-report. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Diabetes was defined as a fasting blood glucose concentration of ≥126 mg/dL, self-report, or the use of insulin or oral hypoglycemic medications. Seated blood pressure was recorded after a minimum of 5 minutes rest as the mean of the last two of three seated measurements using a Dinamap Pro-100 automated oscillometric sphygmomanometer. 18 Participants were asked to bring their medications to the clinic and antihypertensive and statin drug use was assessed with a medication inventory. Total cholesterol, high-density lipoprotein cholesterol (HDL-C), and triglyceride measurements were performed in blood samples obtained after a twelve-hour fast. Low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald equation. The ten-year risk of hard ASCVD events for MESA participants was estimated using the ACC/AHA Pooled Cohort Equations (with Hispanics/Chinese calculated as White). 10
Cardiac CT Protocol
The MESA scanning protocol has been published. 19 Cardiac CT was performed at baseline at three MESA sites using a cardiac-gated electron-beam CT scanner (Imatron C-150XL, GE-Imatron, San Francisco, CA) and at three sites using a four-slice multi-detector CT scanner. Both scanner-types produce near-identical results. 19 Intra-observer and inter-observer agreements were excellent (κ = 0.93 and κ = 0.90, respectively). While no action was required based on CAC results, participants were told they had no CAC or that the amount was less than average, average, or greater than average for their age and sex, and to discuss the results with their physicians.
Definition of Cardiovascular events
The primary endpoint of all-cause CVD or hospitalized heart failure was pre-specified to match the composite outcome used in SPRINT.5 Secondary individual endpoints included all-cause CVD, heart failure, all-cause coronary heart disease (CHD), and stroke. At intervals of 9–12 months, an interviewer contacted each subject or a family member about outpatient diagnoses of CHD or CVD, interim hospitalizations, and deaths. Two physicians from the MESA mortality and morbidity review committee independently classified events; in the event of disagreement, the full committee adjudicated. With follow-up through 2012, MESA was successful in obtaining information on 98% of reported hospitalized CVD and 95% of reported outpatient CVD encounters.
All-cause CHD events were defined as: myocardial infarction, death from CHD, probable angina resulting in revascularization, or resuscitated cardiac arrest. All-cause CVD events were defined as: all-cause CHD events plus cerebrovascular accident (CVA, transient ischemic attack, or ischemic or hemorrhagic stroke), CVA death, or other CVD death. MESA reviewers classified incident heart failure as definite, probable, or absent. Probable or definite hospitalized heart failure both required symptoms, such as shortness of breath or edema as a baseline criteria. Probable hospitalized heart failure further required heart failure diagnosed by a physician and patient receiving medical treatment for heart failure. To meet criteria for definite hospitalized heart failure, one or more additional factors, such as pulmonary edema by X-ray, poor left ventricular systolic function, or diastolic dysfunction, were also required. Participants who suffered both CVD and heart failure were censored from this analysis after the first event. More details of the MESA follow-up methods are available at the MESA website (http://www.mesa-nhlbi.org).
Statistical Analysis
In order to examine the potential implications of CAC testing for both intensification (e.g. titration) and initiation of BP therapy to a more intensive SBP goal, we included persons with and without baseline anti-hypertensive medication use (Table 1). We calculated proportions for categorical variables and either mean ± standard deviation or median ± interquartile range for continuous variables with normal and non-normal distributions, respectively. Groups were compared using 2-sample t-test, Mann-Whitney, or Chi-square testing, as appropriate.
TABLE 1.
Baseline characteristics of the MESA sample (N=3,733) and the SPRINT-eligible subsample (N= 1,394), according to baseline blood pressure (BP) therapy
| MESA Study Sample | P-value* | Sprint-Eligible Subsample | P-value* | |||
|---|---|---|---|---|---|---|
| No BP Therapy (N= 1964) | BP Therapy (N= 1769) | No BP Therapy (N= 613) | BP Therapy (N= 781) | |||
| Age, years | 63.4 (±9.9) | 66.1 (±9.3) | <0.001 | 69.0 (±7.9) | 69.4 (±7.8) | 0.51 |
| Male | 1004 (51) | 796 (45) | <0.001 | 412 (67) | 401 (51) | <0.001 |
| Race | <0.001 | <0.001 | ||||
| White | 757 (38) | 548 (31) | 253 (41) | 280 (36) | ||
| Black | 505 (26) | 700 (40) | 148 (24) | 278 (36) | ||
| Hispanic | 464 (24) | 349 (20) | 139 (23) | 146 (19) | ||
| Chinese | 238 (12) | 172 (10) | 73 (12) | 77 (10) | ||
| Body Mass Index, kg/m2 | 28.3 (±5.3) | 29.8 (±5.6) | <0.001 | 27.7 (±4.6) | 28.8 (±5.32) | <0.001 |
| Systolic BP, mmHg | 137.2 (±13.4) | 141.2 (±14.1) | <0.001 | 147.2 (±12.3) | 148.4 (±12.2) | 0.035 |
| Diastolic BP, mmHg | 76.2 (±8.8) | 75.6 (±9.5) | 0.02 | 78.5 (±9.0) | 77.6 (±9.6) | 0.17 |
| Fasting Glucose, mg/dL | 97.0 (±30.7) | 103.8 (±33.0) | <0.001 | 92.1 (±10.5) | 93.7 (±11.4) | 0.01 |
| Diabetes | 174 (9) | 385 (22) | <0.001 | - | - | |
| Smoking Status | <0.001 | <0.001 | ||||
| Current Smoker | 269 (14) | 167 (9) | 88 (14) | 80 (10) | ||
| Never Smoker | 942 (48) | 932 (53) | 249 (40) | 391 (50) | ||
| Former Smoker | 747 (38) | 661 (38) | 273 (45) | 306 (40) | ||
| LDL-C, mg/dL | 120.8 (±30.9) | 113.4 (±31.1) | <0.001 | 125.1 (±30.8) | 116.7 (±30.9) | <0.001 |
| HDL-C, mg/dL | 51.2 (±14.9) | 50.4 (±14.1) | 0.18 | 48.8 (±14.2) | 50.7 (±14.3) | <0.005 |
| Triglycerides, mg/dL | 116 (79-164) | 113 (81-166) | 0.92 | 122 (87-166) | 113 (83-165) | 0.12 |
| Creatinine, mg/dL | 0.94 (±0.21) | 1.0 (±0.29) | <0.001 | 1.01 (±0.22) | 1.04 (±0.28) | 0.07 |
| Family History of MI | 771 (42) | 801 (49) | <0.001 | 238 (43) | 359 (50) | 0.001 |
| 10 year ASCVD Risk, % | 14 (±11) | 22 (±0.15) | <0.001 | 21 (±22) | 24 (±12) | <0.001 |
| ASCVD Risk Score Categories | <0.001 | <0.001 | ||||
| <7.5% | 683 (35) | 276 (16) | 23 (4) | 29 (4) | ||
| 7.5-15% | 552 (28) | 402 (23) | 192 (32) | 160 (21) | ||
| >15% | 716 (37) | 1072 (61) | 393 (65) | 584 (75) | ||
Values are for number (%), median (IQR) or mean (±SD)
P values are for differences between groups using 1-way ANOVA, Kruskal Wallis testing, or χ2, as appropriate.
ASCVD indicates atherosclerotic cardiovascular disease; BP, blood pressure; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; MESA, multi-ethnic study of atherosclerosis; and MI, myocardial infarction.
In survival analyses, participants were categorized into the following systolic BP categories, <140 mmHg (i.e. either 120-139 mmHg for the MESA study sample or 130-139 mmHg for the SPRINT-eligible subsample), 140-159 mmHg, and 160-179 mmHg. Then, to evaluate whether CAC can personalize risk assessment among subgroups of varying SBP and ASCVD risk estimates, these BP categories were further stratified on the basis of, first, ten-year ASCVD risk (<15% or ≥15%5, 6,20 which was the median level of risk in our primary sample) and, second, CAC group (0, 1-100, >100). We compared crude event (incidence) rates, as well as Cox multivariable-adjusted hazard ratios, within each of these CAC strata.
Models were adjusted for age, sex, race/ethnicity category, BMI, fasting-glucose, diabetes status (yes/no), creatinine, smoking category, LDL-C, HDL-C, triglycerides, statin use, and family history of myocardial infarction (yes/no). In models where the sample was stratified by ASCVD (<15% or ≥15%), we adjusted for core demographics and variables not included in the ASCVD equation (BMI, creatinine, triglycerides, statin use, and family history of myocardial infarction). We conducted sensitivity analyses with more parsimonious models adjusted just for, 1) demographics alone (age, sex, and race/ethnicity), and 2) using the 13 variables included in the primary model, we constructed a propensity score for the composite outcome within each of the CAC subgroups and adjusted the model for this score as a single variable.
We estimated a 10-year number needed to treat to prevent the primary outcome of all-cause CVD or HF (NNT10) with treatment initiation or intensification to a systolic goal of 120 mmHg. This was calculated by applying the expected relative risk reduction derived either from meta-analysis (22% reduction in CHD, 41% reduction in stroke and 24% reduction in heart failure for each 10 mmHg lowering of systolic BP21) in the primary sample, or directly from SPRINT (25% relative reduction for a target of 120 mmHg versus a target of 140 mmHg5) in the secondary analysis of the SPRINT-eligible subsample. The NNT10 was calculated directly as the reciprocal of the absolute risk difference at the median follow-up of the cohort on the basis of Kaplan-Meier estimates and was subsequently adjusted to a NNT10 according to the Altman-Anderson method.22 In a sensitivity analysis, using the same statistical techniques, we modeled NNT10 for a systolic goal of 130 mmHg. We also conducted a sensitivity analysis of NNT10 for a goal of 120mmHg that included lower cut-points of 10-year estimated ASCVD risk (<5% or <10%). Finally, we estimated NNT10 for a goal of 120mmHg to prevent each of the individual endpoints included in the main composite (CHD, stroke, and heart failure) and we also conducted analyses in the diabetic-subgroup of our primary MESA sample.
Results
Baseline characteristics of the primary sample and of the SPRINT-eligible subsample, stratified by anti-hypertensive medication use, are shown in Table 1. Except for a lower proportion of males and being less likely to smoke, persons receiving BP therapy at baseline were older and had a higher burden of ASCVD risk factors than those who were not on BP therapy at baseline. Those receiving BP therapy also had higher SBP than those not on therapy. Diastolic BP levels, while clinically similar (75.6 vs. 76.2 mmHg), were statistically lower among those on BP therapy. The distribution of CAC also differed according to baseline BP treatment status (Figure 1).
Figure 1. CAC distribution by anti-hypertension treatment status in the primary MESA sample overall (3,733 U.S. adults aged 45 to 84 years with Systolic BP 120-180 mmHg) and the SPRINT-eligible subsample (N=1,394).
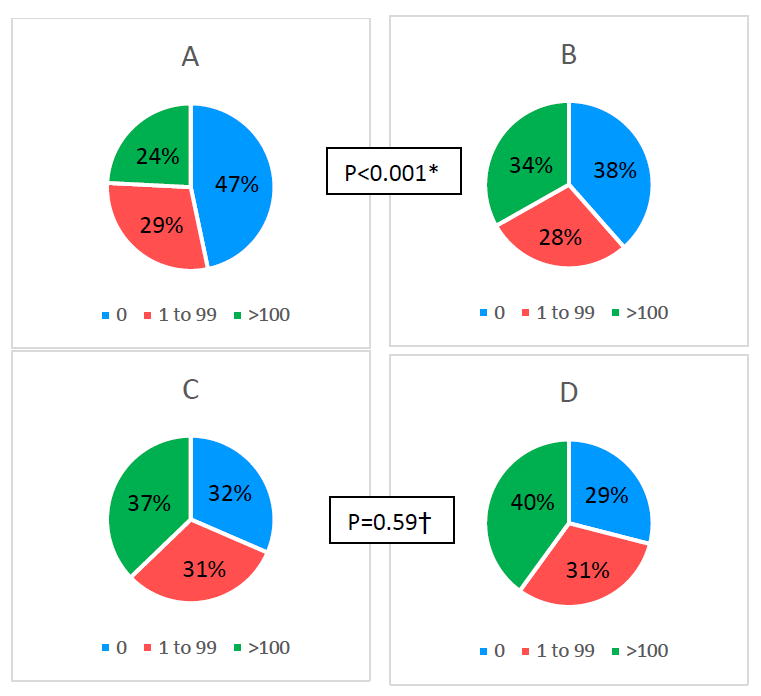
A) Primary Sample Untreated
B) Primary Sample on BP treatment
C) Sprint subsample Untreated
D) Sprint subsample on BP treatment
* p value comparing CAC level among treated to untreated in the primary sample
† p value comparing CAC level among treated to untreated in SPRINT sub-sample
Over a median (interquartile range) follow-up of 10.2 (9.7–10.7) years, 642 primary composite outcome events (all-cause CVD or heart failure) occurred in the sample overall. Figure 2 demonstrates that cumulative event-free survival was significantly lower, in both the primary sample and SPRINT-eligible subsample, among individuals with CAC 1-100 and >100, compared to those with zero CAC. Similar trends were demonstrated after stratification by baseline systolic BP category (eFigure 1). These trends were also qualitatively similar for the individual outcomes of CHD, stroke, and heart failure (eFigure 2).
Figure 2. Kaplan-Meier curves for survival free from the primary outcome of all-cause CVD or heart failure in the primary MESA study sample and the SPRINT-eligible subsample, according to categories of CAC.
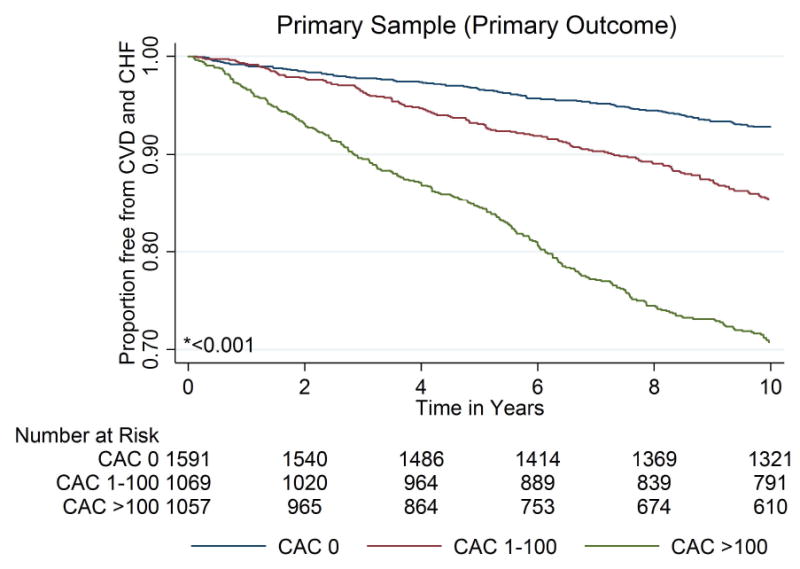
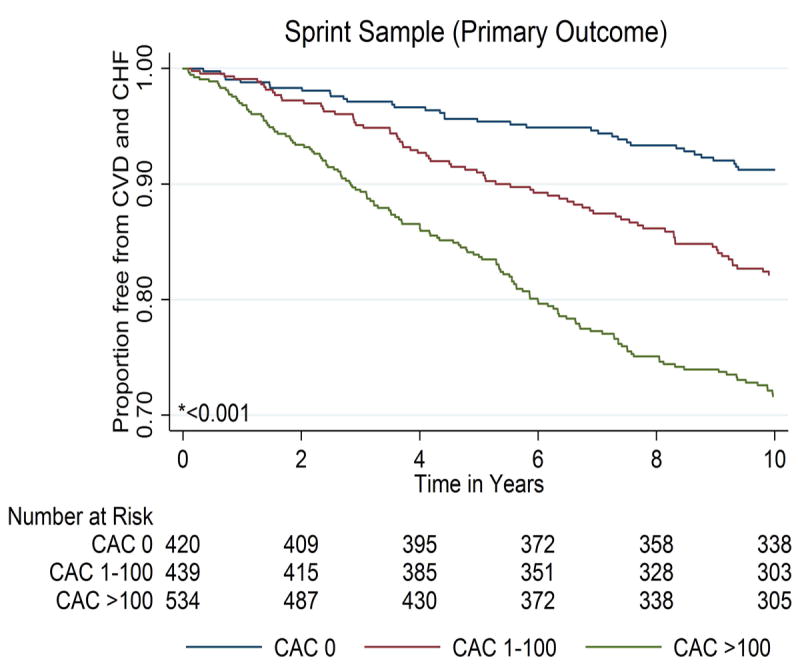
* P value by Log-Rank testing
Among persons in the primary sample who were not on baseline BP therapy, event rates were low for those with zero CAC and either SBP between 120-139 mmHg (5.6 per 1,000 person-years) or SBP between 140-159 mmHg (7.4 per 1,000 person-years). However, event rates appeared to be high, irrespective of CAC level, in persons with untreated SBP between 160-179 mmHg (ranging from approximately 20 to 40 per 1,000 person-years, Table 2). In general, event rates were also consistently higher among those on baseline BP therapy compared to untreated individuals, within each of the BP and CAC strata. Of note, however, persons not on BP therapy with SBP between 120-139 mmHg and CAC >100 had a similar event rate (24.3 per 1,000 person-years) as individuals on therapy with both poorly controlled hypertension (SBP 160-179 mmHg) and CAC=0 (20.2 per 1,000 person-years).
TABLE 2.
Crude Event Rates* and Adjusted† Hazard Ratios (95% Cls) for Incident ASCVD or Heart Failure in the MESA study sample and the SPRINT-eligible subsample, according to baseline systolic BP (with or without therapy) and stratified by CAC
| MESA Study Sample | Sprint-Eligible Subsample | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) | n (%) | Crude event rates (95% CI) * | Adjusted HR (95% CI) † | N (%) | n (%) | Crude event rates (95% CI) * | Adjusted HR (95% CI) † | ||
| SBP <140‡ | |||||||||
| No BP therapy | CAC=0 | 635 (50) | 40 (6) | 5.6 ( 4.1, 7.6) | 1.0 | 61(29) | 5 (8) | 7.3 ( 3.0, 17.6) | 1.0 |
| CAC 1-100 | 363 (29) | 47 (13) | 12.1 ( 9.1, 16.1) | 1.6 ( 1.01, 2.6) | 69(33) | 8 (12) | 11.3 ( 5.6, 22.5) | 1.4 ( 0.4, 4.6) | |
| CAC>100 | 263 (21) | 61 (23) | 24.3 ( 18.9, 31.3) | 3.0 ( 1.8, 5.0) | 14(18) | 14 (18) | 19.0 ( 11.3, 32.1) | 1.4 ( 0.4, 4.9) | |
| BP Therapy | CAC=0 | 369 (41) | 28 (8) | 7.0 ( 4.8, 10.1) | 1.0 | 47 (22) | 3 (6) | 6.0 ( 1.9, 18.7) | 1.0 |
| CAC 1-100 | 258 (29) | 35 (14) | 12.8 ( 9.2, 17.8) | 1.3 ( 0.7, 2.2) | 79(37) | 13 (16) | 15.6 ( 9.1, 28.9) | 3.1 ( 0.8, 11.6) | |
| CAC>100 | 274 (30) | 86 (31) | 35.5 ( 28.7, 43.8) | 2.6 ( 1.6, 4.3) | 90 (42) | 21 (23) | 26.0 ( 17.0, 40.0) | 4.8 ( 1.3, 17.5) | |
| SBP 140-159 | |||||||||
| No BP therapy | CAC=0 | 225 (41) | 19 (8) | 7.4 ( 4.7, 11.7) | 1.0 | 94(32) | 11 (12) | 10.2 ( 5.6, 18.4) | 1.0 |
| CAC 1-100 | 162 (29) | 27 (17) | 16.5 (11.3, 24.1) | 1.9 (1.01, 3.7) | 89(30) | 15 (17) | 17.2 ( 10.4, 28.6) | 1.9 ( 0.8, 4.6) | |
| CAC>100 | 162 (29) | 54 (33) | 36.9 ( 29.0, 49.5) | 5.2 (2.7, 10.2) | 112 (38) | 37 (33) | 36.5 ( 26.4, 50.4) | 3.8 ( 1.7, 8.7) | |
| BP Therapy | CAC=0 | 243 (36) | 31 (13) | 11.9 ( 8.4, 17.0) | 1.0 | 125(30) | 13 (10) | 9.6 ( 5.6, 16.5) | 1.0 |
| CAC 1-100 | 183 (27) | 40 (22) | 21.8 ( 16.0, 29.7) | 1.7 ( 1.01, 2.8) | 126(30) | 28 (22) | 21.3 ( 15.4, 32.3) | 2.1 ( 1.01, 4.4) | |
| CAC>100 | 240 (36) | 80 (33) | 38.8 ( 29.0, 49.5) | 2.3 ( 1.4, 3.7) | 164(40) | 55 (33) | 39.2 ( 30.1, 51.1) | 2.9 ( 1.5, 5.9) | |
| SBP 160-179 | |||||||||
| No BP therapy | CAC=0 | 57 (37) | 9 (16) | 16.7 ( 8.7, 32.1) | 1.0 | 38 (35) | 6 (16) | 15.5 ( 6.9, 34.4) | 1.0 |
| CAC 1-100 | 47 (30) | 15 (32) | 34.4 ( 20.7, 57.1) | 1.1 ( 0.2, 5.5) | 34 (31) | 11 (32) | 38.3 ( 20.3, 66.2) | 2.3 ( 0.8, 6.9) | |
| CAC>100 | 50 (32) | 16 (32) | 37.1 ( 22.7, 60.6) | 1.9 (0.1, 26.0) | 37 (34) | 12 (32) | 36.4 ( 20.7, 64.1) | 1.1 ( 0.3, 3.9) | |
| BP Therapy | CAC=0 | 70 (35) | 14 (20) | 20.2 ( 12.0, 34.1) | 1.0 | 55 (37) | 9 (16) | 15.8 ( 8.2, 30.4) | 1.0 |
| CAC 1-100 | 57 (30) | 14 (26) | 26.6 ( 15.7, 44.9) | 1.0 ( 0.5, 2.0) | 43 (29) | 10 (23) | 25.1 ( 13.5, 46.5) | 2.3 ( 0.7, 6.9) | |
| CAC>100 | 70 (35) | 26 (37) | 44.6 ( 30.4, 65.5) | 1.2 ( 0.6, 2.3) | 52 (35) | 17 (33) | 36.3 ( 23.8, 61.6) | 2.9 ( 0.9, 9.3) | |
Event rates are per 1,000 person years.
Adjusted for age, sex, race, BMI, fasting glucose, diabetes status, creatinine, smoking category, LDL-C, HDL-C, triglycerides, statin use and family history of MI. Significant Hazard Ratios are in bold (p<0.05). N (%) and n (%) represent numbers of persons and events in each category.
<140 SBP is 120-139 for the MESA study sample and 130-139 for the SPRINT-eligible subsample SBP indicates systolic blood pressure in mmHg; CAC, coronary artery calcium; all other abbreviations as per Table 1
Adjusted Cox models confirmed that, relative to CAC=0, CAC 1-100 and CAC >100 carried incremental excess in hazard for events among persons with SBP in the 120-139 mmHg and 140-159 mmHg ranges, irrespective of baseline treatment status (Table 2). However, associations between CAC and hazard for events among those with SBP 160-179 mmHg were not statistically significant, either with or without baseline therapy. All of these trends were qualitatively similar in the SPRINT-eligible subsample.
Table 3 demonstrates findings after individuals within each SBP category, both untreated and treated combined, were stratified by estimated ten-year ASCVD risk (above or below the sample median of 15%). Those with CAC=0 had low event rates in both the 120-139 mmHg (4.6 per 1,000 person-years) and 140-159 mmHg BP categories (6.9 per 1,000 person-years), as long as ASCVD risk was <15%. Event rates were comparably higher (>7.5 per 1,000 person-years) in all persons with SBP between 160-179 mmHg. Furthermore, persons with baseline ASCVD risk ≥15% at all levels of baseline SBP also had higher event rates, again irrespective of CAC level (ranging from approximately 13 to 46 per 1,000 person-years).
TABLE 3.
Crude Event Rates* and Adjusted† Hazard Ratios (95% Cls) for Incident ASCVD or Heart Failure in the MESA study sample and the SPRINT-eligible subsample, according to baseline systolic BP, stratified by ASCVD risk and sub-stratified by CAC
| MESA Study Sample | Sprint-Eligible Subsample | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) | n (%) | Crude event rates (95% CI) * | Adjusted HR (95% CI) † | N (%) | n (%) | Crude event rates (95% CI) * | Adjusted HR (95% CI) † | ||
| SBP <140‡ | |||||||||
| ASCVD risk<15% | CAC=0 | 796 (59) | 42 (5) | 4.6 ( 3.4, 6.2) | 1.0 | 54 (36) | 4 (7) | 6.8 (2.5 , 18.1) | 1.0 |
| CAC 1-100 | 387 (29) | 41 (11) | 9.5 ( 7.0, 12.9) | 1.7 ( 1.01, 2.6) | 58(39) | 7 (12) | 11.1 ( 5.3, 23.3) | 2.3 ( 0.6, 8.9) | |
| CAC>100 | 164 (12) | 33 (20) | 19.7 ( 14.0, 27.7) | 3.0 ( 1.8, 5.0) | 38 (25) | 10 (26) | 26.7 (14.4, 49.6) | 2.9 ( 0.8, 10.3) | |
| ASCVD risk≥15% | CAC=0 | 198 (25) | 25 (13) | 12.7 ( 8.6, 18.8) | 1.0 | 53 (20) | 4 (8) | 6.9 ( 2.6, 18.5) | 1.0 |
| CAC 1-100 | 230 (29) | 41 (18) | 18.2 ( 13.4, 24.7) | 1.3 ( 0.7, 2.2) | 88 (33) | 14 (16) | 15.7 ( 9.3, 26.6) | 2.1 ( 0.7, 6.8) | |
| CAC>100 | 371 (46) | 33 (20) | 35.0 ( 29.1, 42.1) | 2.6 ( 1.6, 4.3) | 130 (48) | 25 (19) | 21.7 ( 14.6, 32.1) | 2.4 ( 0.8, 7.6) | |
| SBP 140-159 | |||||||||
| ASCVD risk<15% | CAC=0 | 264 (56) | 21 (8) | 6.9 ( 4.5, 10.6) | 1.0 | 93 (47) | 9 (10) | 8.3 ( 4.3, 15.9) | 1.0 |
| CAC 1-100 | 131 (28) | 18 (14) | 12.8 ( 8.1, 20.3) | 2.0 ( 1.1, 3.8) | 59 (30) | 10 (17) | 16.8 ( 9.0, 31.2) | 2.3 ( 0.9, 6.0) | |
| CAC>100 | 80 (19) | 32 (40) | 43.4 ( 30.7, 61.4) | 5.7 ( 2.9,11.0) | 47 (24) | 20 (43) | 45.9 ( 29.6, 71.1) | 4.6 (1.8, 11.6) | |
| ASCVD risk≥15% | CAC=0 | 198 (27) | 28 (14) | 13.6 ( 9.4, 19.7) | 1.0 | 124 (25) | 15 (12) | 11.3 ( 6.8, 18.8) | 1.0 |
| CAC 1-100 | 213 (29) | 49 (23) | 23.7 ( 17.9, 31.4) | 1.7 ( 1.1, 2.8) | 156 (31) | 33 (21) | 21.5 ( 15.3, 30.3) | 1.9 ( 1.01, 3.6) | |
| CAC>100 | 316 (43) | 101(32) | 37.6 ( 30.9, 45.6) | 2.3 ( 1.5, 3.8) | 225 (45) | 71 (32) | 36.6 ( 29.0, 46.2) | 2.6 ( 1.4, 5.00) | |
| SBP 160-179 | |||||||||
| ASCVD risk<15% | CAC=0 | 48 (53) | 4 (8) | 7.9 ( 3.0, 21.2) | 1.0 | 27 (49) | 2 (7) | 6.4 ( 1.6, 25.7) | 1.0 |
| CAC 1-100 | 29 (32) | 7 (24) | 21.8 ( 10.4, 45.7) | 1.0 ( 0.2, 5.8) | 18 (33) | 4 (22) | 20.1 ( 7.5, 53.5) | 38.5 ( 0.3, 526.7) | |
| CAC>100 | 14 (15) | 3 (21) | 19.8 ( 6.4, 61.3) | 4.0 ( 0.4,40.2) | 10(18) | 3 (30) | 29.2 ( 9.4, 90.5) | 3.9 ( 0.1, 839.4) | |
| ASCVD risk≥15% | CAC=0 | 77 (29) | 19 (25) | 26.7 ( 17.0, 41.9) | 1.0 | 64 (32) | 13 (20) | 20.7 (12.0, 35.6) | 1.0 |
| CAC 1-100 | 78 (30) | 22 (28) | 34.3 ( 22.6, 52.1) | 1.0 ( 0.5, 2.0) | 59 (29) | 17 (30) | 34.0 ( 21.1, 54.7) | 1.5 ( 0.7, 3.5) | |
| CAC>100 | 106 (41) | 39 (37) | 45.9 ( 33.6, 62.9) | 1.1 ( 0.6, 2.1) | 78 (39) | 26 (33) | 39.5 ( 26.9, 58.0) | 1.3 ( 0.6, 2.9) | |
Event rates are per 1,000 person years.
Adjusted for age, sex, race, BMI, creatinine, triglycerides, statin use and family history of MI. Significant Hazard Ratios are in bold (p<0.05). N (%) and n (%) represent numbers of persons and events in each category.
<140 SBP is 120-139 for the MESA study sample and 130-139 for the SPRINT-eligible subsample SBP indicates systolic blood pressure in mmHg; CAC, coronary artery calcium; all other abbreviations as per Table 1
Adjusted Cox models demonstrated increased hazard for events with CAC 1-100 and CAC >100 (versus CAC=0) among those who had SBP levels in the range of 120-139 mmHg and 140-159 mmHg, but, no statistical association of CAC with CVD among those with SBP 160-179 mmHg (Table 3). Excess relative hazard with increasing CAC strata was most pronounced in those with estimated ASCVD risk <15%. Parsimonious demographic-adjusted and propensity score-adjusted models produced similar results (eTables 1-4). As in Table 2, all of these trends were qualitatively similar in the SPRINT-eligible subsample. None of the hazard ratios presented in Table 3 demonstrated any interaction by race/ethnicity.
The absolute differences in event rates according to baseline CAC translated into substantial variation in estimated NNT10 to prevent all-cause CVD or heart failure with BP lowering to a SBP goal of 120 mmHg. For example, a low NNT10 (between 4 and 8), was estimated for persons with CAC >100 in both the SBP 140-159 and 160-179 mmHg categories, irrespective of baseline estimated ASCVD risk (Table 4). In contrast, participants with CAC=0 had higher estimated NNT10 at all levels of baseline SBP and ASCVD risk. Persons with SBP <140 mmHg, ASCVD risk <15% and zero CAC had the highest NNT10 estimates (NNT10= 99). Likely due to the higher baseline SBP and ASCVD risk in those who were SPRINT eligible, with higher consequent event rates, all NNT10 levels were relatively low in this sub-sample. The NNT10 results were qualitatively similar when the sample overall was stratified by baseline treatment status (as such, NNT10 for a goal SBP of 120 mmHg was similar for both initiation of BP therapy and intensification of prior therapy, eTable 5). Because CAC stratifies absolute risk for CHD, stroke, and heart failure, the NNT10 trends seen for the composite outcome are mirrored in each of the individual outcomes (eTable 6).
TABLE 4.
Estimated 10-year NNT for the prevention of ASCVD or heart failure with blood pressure (BP) therapy to a target systolic BP of 120 mmHg, stratified by ASCVD risk and sub-stratified by CAC
| MESA Study Sample | Sprint-Eligible Subsample | ||||||
|---|---|---|---|---|---|---|---|
| Mean (SD) SBP | 10 year cumulative incidence (95% CI) | NNT * | Mean (SD) SBP | 10 year cumulative incidence (95% CI) | NNT † | ||
| SBP <140‡ | |||||||
| ASCVD risk<15% | CAC=0 | 129 (6) | 0.04 ( 0.03, 0.06) | 99 | 135 (2) | 0.08 ( 0.03, 0.19) | 39 |
| CAC 1-100 | 129 (6) | 0.09 ( 0.06, 0.12) | 52 | 135 (3) | 0.09 ( 0.04, 0.21) | 32 | |
| CAC>100 | 129 (6) | 0.19 ( 0.13, 0.26) | 24 | 134 (3) | 0.2 ( 0.12, 0.39) | 14 | |
| ASCVD risk ≥15% | CAC=0 | 130 (6) | 0.12 ( 0.08, 0.17) | 29 | 135 (3) | 0.08 ( 0.03, 0.20) | 35 |
| CAC 1-100 | 131 (6) | 0.18 ( 0.13, 0.24) | 21 | 136 (3) | 0.15 ( 0.09, 0.25) | 19 | |
| CAC>100 | 130 (6) | 0.27 ( 0.25, 0.35) | 15 | 135 (3) | 0.21 (0.15, 0.30) | 13 | |
| SBP 140-159 | |||||||
| ASCVD risk<15% | CAC=0 | 147 (5) | 0.05 ( 0.03, 0.09) | 36 | 147 (6) | 0.06 ( 0.02, 0.13) | 31 |
| CAC 1-100 | 147 (6) | 0.12 ( 0.08, 0.20) | 15 | 148 (6) | 0.19 ( 0.11, 0.33) | 9 | |
| CAC>100 | 147 (6) | 0.38 ( 0.28, 0.50) | 5 | 147 (6) | 0.39 ( 0.27, 0.55) | 5 | |
| ASCVD risk ≥15% | CAC=0 | 150 (6) | 0.10 ( 0.07, 0.16) | 15 | 150 (6) | 0.09 ( 0.05, 0.16) | 20 |
| CAC 1-100 | 148 (5) | 0.19 ( 0.15, 0.26) | 9 | 148 (5) | 0.18 ( 0.13, 0.25) | 10 | |
| CAC>100 | 148 (6) | 0.32 ( 0.27, 0.38) | 5 | 148 (6) | 0.31 ( 0.25, 0.38) | 6 | |
| SBP 160-179 | |||||||
| ASCVD risk<15% | CAC=0 | 167 (5) | 0.07 ( 0.02, 0.20) | 20 | 166 (5) | 0.04 ( 0.01, 0.26) | 33 |
| CAC 1-100 | 168 (6) | 0.18 ( 0.08, 0.37) | 18 | 168 (7) | 0.22 ( 0.09, 0.49) | 6 | |
| CAC>100 | 166 (4) | 0.14 ( 0.04, 0.46) | 8 | 166 (4) | 0.20 (0.05, 0.59) | 7 | |
| ASCVD risk≥15% | CAC=0 | 168 (6) | 0.24 ( 0.15, 0.35) | 5 | 168 (6) | 0.18 ( 0.11, 0.31) | 7 |
| CAC 1-100 | 168 (6) | 0.28 ( 0.19, 0.41) | 4 | 168 (6) | 0.29 ( 0.19, 0.44) | 5 | |
| CAC>100 | 168 (6) | 0.34 ( 0.25, 0.44) | 4 | 169 (7) | 0.32 ( 0.22, 0.44) | 4 | |
NNT for the MESA study sample is calculated as follows; for each SBP category, we took the mean SBP in this category and subtracted 120 to get the target BP reduction. (e.g. if mean is 130 mmHg in the SBP <140 mmHg category then, to achieve 120 mmHg, the target reduction would be 10 mmHg). For each 10 mmHg reduction we estimate a 22% reduction in CHD, 41% reduction in stroke and 24% reduction in HF.
NNT for the SPRINT subsample assumes a 25% relative reduction in the main outcome
<140 SBP is 120-139 mmHg for primary sample and 130-139 mmHg for SPRINT subsample SBP indicates systolic blood pressure in mmHg; CAC, coronary artery calcium; NNT, number needed to treat, all other abbreviations as per Table 1
Figure 3 summarizes the range of NNT10 estimates after stratification by baseline CAC, with findings most widely dispersed among those with ASCVD risk <15% and who had either pre-hypertension or mild hypertension. In addition, sensitivity analyses evaluating lower ASCVD risk cut-points suggested that, among participants with SBP 120-139 mmHg, 32% of persons with ASCVD risk <7.5% had CAC>0 (with NNT10 estimates for a 120 mmHg SBP goal of 76 for CAC1-100 and 47 for CAC>100), whereas CAC>0 was less frequent and NNT10 estimates were higher among those with ASCVD risk <5% (e.g., NNT10 estimates for a 120 mmHg SBP goal of 180 for the 20% with CAC 1-100 and 37 for the 3% with CAC>100) (eTable 7).
Figure 3. CAC Stratifies a Range of Estimated Number Needed to Treat to a target systolic BP of 120 mmHg; among Categories of Baseline Systolic BP and ASCVD risk (primary MESA study sample, N=3,733).
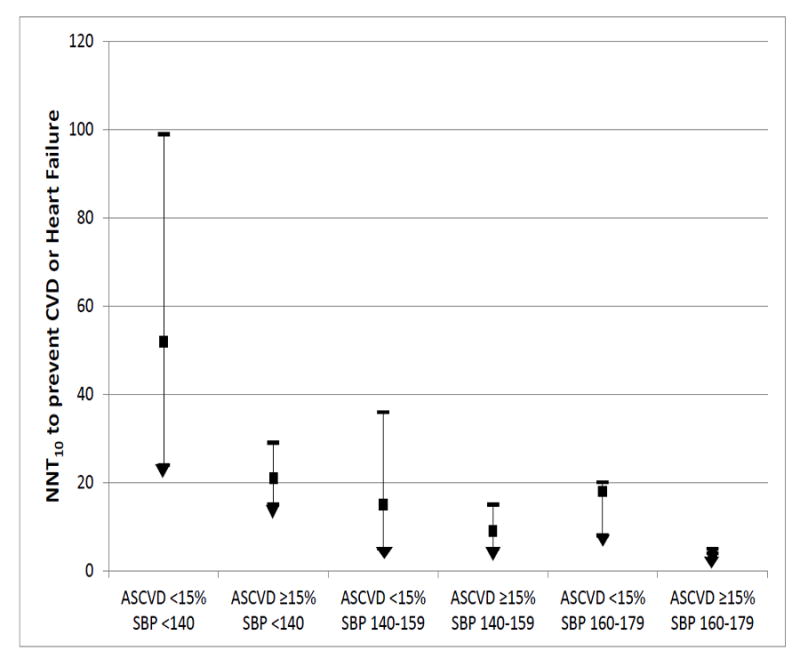
The NNT estimates within each category of ASCVD risk (calculated using traditional risk factors according to the 2013 ACC/AHA pooled cohort equations14) and Systolic BP consist of mean NNT10 for persons with CAC=0 (upper limit), mean NNT10 for persons with CAC 1-100 (solid square), and mean NNT10 for persons with CAC >100 (lower limit)
The exploratory analysis of diabetics in our sample suggested that NNT10 estimates were low, irrespective of CAC, among those with 10-year ASCVD risk ≥15%. Too few diabetics in our sample had ASCVD risk <15% to judge whether CAC has any role in guiding risk-based BP therapy in this setting (eTable 8). Finally, eTable 9 demonstrates our NNT10 estimates from the sensitivity analysis evaluating a SBP goal of 130mmHg. As expected, NNT10 estimates were higher (i.e., less benefit) when targeting 130 mmHg compared to 120 mmHg systolic, particularly among those at highest risk due to elevated baseline CAC.
Discussion
Our results add to an emerging body of literature suggesting that ASCVD risk may be useful in defining more personalized BP goals and could guide a precision medicine approach for both initiation and intensification of anti-hypertensive treatment. First, CAC was a powerful determinant of absolute risk for the composite of all-cause CVD or heart failure. Second, persons with zero CAC in both the prehypertension (120-139 mm Hg) and mild hypertension (140-159 mmHg) SBP categories had low ten-year event rates (e.g., <7.5 per 1,000 person-years). This was particularly true for those not already on BP therapy at baseline in whom the decision to initiate treatment may be under consideration, but also applied to persons on baseline therapy in whom intensification of treatment may be considered. All participants with SBP >160 mmHg had high event rates, irrespective of CAC levels.
Third, CAC may be most suitable for guiding therapeutic decisions (specifically, either initiation or intensification to a more intensive systolic goal of 120 mmHg) when both SBP is between 120-159 mmHg and ten-year ASCVD has been estimated as <15%. In these individuals, CAC=0 yielded a higher estimated NNT for persons with SBP 140-159 (NNT10= 36), and, above all, for those with SBP between 120 and 139 mmHg (NNT10= 99), suggesting lower likelihood for benefit. The latter group consists of those in whom the decision to treat to a more intensive goal of 120 mmHg (compared to the traditional goal of 140 mmHg) may be most challenging in the context of results from SPRINT. Given that 97% of MESA participants with estimated ASCVD risk <5% have CAC <100 and NNT10 estimates ranging from 180-273, our sensitivity analyses suggest that CAC may be most practical for this purpose when SBP is between 120 and 159 mmHg and estimated ASCVD risk is between 5 and 15%.
The above inferences are most appropriately applied to general community intermediate to low risk populations similar to MESA. Our secondary analysis results suggest that the relatively few adults fulfilling strict SPRINT eligibility criteria (just 7.6% of the overall U.S. population23) are, by definition, high risk for CVD or heart failure and the further use of CAC imaging in these individuals may be less helpful in deciding SBP goals.
The traditional paradigm of allocating BP therapy solely on BP values makes intuitive and physiological sense. However, data have consistently demonstrated that, while the relative risk reduction in events per unit of SBP lowering is the same, the absolute risk reduction, NNT, and, hence, clinical efficacy of BP treatment increases as baseline absolute ASCVD risk increases. 8 In fact, the idea of using baseline ASCVD risk to guide BP therapy is not new. 24, 25 Moreover, the concept of using risk to allocate ASCVD prevention therapies has taken center stage after the release of 2013 ACC/AHA guidelines for the treatment of cholesterol in adults, which recommend statins be considered based on an ASCVD risk of ≥7.5% and not solely on LDL-C values. 26
Indeed, recent data from the Heart Outcomes Prevention Evaluation (HOPE)-3 trial support the concept of risk-based allocation of BP therapy. In this study, 12,705 intermediate risk adults with baseline SBP of 138 mmHg were randomized to placebo or to a combination of 12.5mg hydrochlorothiazide and 16mg candesartan. Despite a relative SBP reduction of 6 mmHg (which was notably less than the 14.8 mmHg achieved in SPRINT), the intermediate risk adults enrolled in HOPE- 3 did not derive benefit. 27 Thus, SPRINT supports intensive BP control (SBP goal of 120 mmHg) in high risk patients, whereas HOPE-3 suggested that intermediate risk patients may be suitable for less stringent SBP goals. However, our findings introduce the potential value of CAC testing in this intermediate risk group in order to reclassify individual risk and inform more personalized intensive SBP goals in those with advanced subclinical atherosclerosis.
Presumably BP values will always be important in allocating antihypertensive therapy and our data support this. Specifically, participants in our analysis with SBP >160 mmHg, had high event rates and low NNT, irrespective of baseline ASCVD risk or CAC. With the exception of those with ASCVD risk <15% and CAC=0, this was also true for persons with BP 140-159 mmHg. Nonetheless, adding ASCVD risk into BP treatment decisions could potentially allow consideration of therapy for large number of persons with SBP levels that, prior to SPRINT, were otherwise not typically considered to benefit from treatment initiation or intensification (e.g. those with SBP 120-139 mmHg). 6 For example, Karmali et al. found that most excess ASCVD events occur in persons with BP levels considered at goal by JNC-8 and that the vast majority of those who suffer these events have elevated ASCVD risk. 11
Estimating risk based on traditional risk factors alone can be misleading28 and CAC has been repeatedly shown to improve the accuracy of risk assessment. 29, 30 Furthermore, we have previously shown that CAC may inform NNT estimation for other ASCVD prevention therapies. 15, 16 In addition, CAC and intensive BP control such as that used in SPRINT both have supportive evidence for cost-effectiveness. 31, 32 As such, our data could extend the utility of CAC to guiding risk-based determination of more personalized systolic BP goals in persons with mild hypertension and pre-hypertension. This may be relevant for deciding whether to refer for CAC-imaging but is particularly meaningful for those who have already had CAC testing for other reasons.
Importantly, our analyses incorporate clinically relevant information on both baseline BP and estimated ASCVD risk into the calculation of CAC-based NNT estimates. This is crucial as we believe that CAC should not be used in isolation in this context. Specifically, as long as ASCVD risk is <15% and SBP is between 120-159 mmHg, our results suggest the potential for CAC=0 to allow more liberal BP treatment goals, like 140 mmHg for example, particularly if based on individual patient preferences. 33 Indeed, CAC may be most helpful in cases where physicians are considering intensifying treatment to a SPRINT-based SBP goal of <120 mm Hg among persons with SBP between 120-139 mmHg (i.e. levels below the current traditional goal of 140 mmHg). In this setting, when ASCVD risk is <15%, a CAC=0 yields a NNT10 of approximately 100, information which could guide the clinical-patient treatment discussion. Of note, given the low burden of CAC and events among those with ASCVD estimates <5%, CAC-imaging to guide personalized SBP goals may be best suited to persons with estimated ASCVD risk 5-15%.
While we found that CAC-based NNT10 estimates were generally higher for a target of 130 mmHg (vs. 120 mmHg), the overall message was the same: NNT10 estimates for the prevention ASCVD or heart failure suggest that lower systolic targets (e.g. either 120 or 130 mmHg) may be superior to the traditional target of 140 mmHg when; 1) systolic BP is >160 mmHg, 2) estimated CVD risk using traditional risk factors is >15%, and, most importantly, 3) when CAC>100 among those individuals currently in the therapeutic ‘grey zone’ (i.e., those with SBP in the prehypertension and mild hypertension range and who are intermediate risk by CVD risk scores).
Our analysis has some limitations. While we believe that our findings may have important clinical implications and can guide future investigation, they are hypothesis-generating due to the observational nature of the data and the limited numbers of events among certain subgroups. The latter consideration is most relevant among those with SBP 160-179 mmHg and for our SPRINT-eligible subsample. Our NNT estimates are based on a number of assumptions (in particular that the relative risk reduction for BP therapy is similar among CAC strata), nonetheless, we feel they are informative. While some have argued that SPRINT SBP values cannot easily be translated into routine care34, we note that the MESA BP measurement protocol was nearly identical to SPRINT and that MESA also used automated oscillometric BP measurement devices. Because MESA was not designed to capture accurate time-to-event data on side effects of anti-hypertensive medication (e.g. electrolyte imbalance or injurious falls), we do not have absolute event rates for these outcomes among CAC strata and are unable to generate number-needed-to-harm estimates. For simplicity, we did not incorporate information on diastolic BP because the optimal goal for this parameter (80-89 mmHg) is more widely agreed upon, because diastolic BP does not typically add to ASCVD risk estimation over and above SBP, and because so few MESA participants had isolated diastolic hypertension (n=40, 0.6%).
Conclusion
Assessment of CAC may inform more personalized BP goals (e.g., choosing between a traditional SBP goal of 140 mmHg or a more intensive goal of 120 mmHg), particularly among persons with baseline ten-year ASCVD risk estimates between 5-15% and who have systolic BP levels between 120-159 mmHg. Specifically, among these individuals, CAC >100 appears to identify those who would likely benefit from an intensive systolic BP goal of 120 mmHg, whereas CAC=0 identifies individuals who may be suitable for more traditional SBP goals; thereby avoiding unnecessary intensification of medication and instead focusing on healthy lifestyle measures. A trial of risk-based allocation of BP treatment goals, preferably incorporating CAC, is needed.
Supplementary Material
CLINICAL PERSPECTIVE.
What is new?
A given magnitude of blood pressure lowering provides similar relative benefit at all levels of cardiovascular disease risk, but progressively greater absolute benefit (and therefore lower number needed to treat) as risk increases; suggesting that persons at high risk are more likely to derive net benefit from more intensive blood pressure goals (e.g. systolic ≤120 mmHg as supported by the SPRINT trial).
To our knowledge, this is the first study to incorporate information on individual coronary artery calcium burden in order to personalize the risk-based treatment of hypertension.
Added to both traditional risk-factor based estimation of cardiovascular disease risk and a discussion of patient treatment preferences, coronary artery calcium can help identify individuals who may benefit from more intensive treatment to a systolic BP goal of ≤120 mmHg versus a more traditional goal of ≤140 mmHg.
What are the clinical implications?
Information on CAC burden (particularly when CAC results have already been obtained for other reasons) may be considered when making personalized treatment decisions about blood pressure targets, particularly among persons with estimated cardiovascular disease risk between 5-15% and who have either pre-hypertension or mild hypertension.
A precision medicine clinical trial evaluating risk-based blood pressure treatment goals, preferably incorporating CAC and not just risk-factor based estimations, is now needed.
Acknowledgments
We thank the other investigators, staff, and participants of the MESA study for their contributions. Dr. McEvoy is supported by the P.J. Schafer Cardiovascular Research Fund and by the Johns Hopkins Magic That Matters Research Fund for Cardiovascular Research.
Funding sources: This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from NCRR.
Footnotes
Supplementary Material provided in an Online Appendix.
Disclosures: Dr. Budoff serves on a speakers’ bureau for GE Healthcare. The remaining authors have no competing interests to declare.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB American Heart Association Statistics C, Stroke Statistics S. Executive summary: Heart disease and stroke statistics-2016 update: A report from the american heart association. Circulation. 2016;133:447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 2.Turnbull F Blood Pressure Lowering Treatment Trialists C. Effects of different blood-pressure-lowering regimens on major cardiovascular events: Results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 3.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the eighth joint national committee (jnc 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 4.Wright JT, Jr, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm hg in patients aged 60 years or older: The minority view. Ann Intern Med. 2014;160:499–503. doi: 10.7326/M13-2981. [DOI] [PubMed] [Google Scholar]
- 5.Group SR, Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC, Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Navar AM, Pencina MJ, Peterson ED. Assessing cardiovascular risk to guide hypertension diagnosis and treatment. JAMA Cardiol. 2016;1:864–871. doi: 10.1001/jamacardio.2016.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin SA, Boucher M, Wright JM, Saini V. Mild hypertension in people at low risk. BMJ. 2014;349:g5432. doi: 10.1136/bmj.g5432. [DOI] [PubMed] [Google Scholar]
- 8.Blood Pressure Lowering Treatment Trialists C. Sundstrom J, Arima H, Woodward M, Jackson R, Karmali K, Lloyd-Jones D, Baigent C, Emberson J, Rahimi K, MacMahon S, Patel A, Perkovic V, Turnbull F, Neal B. Blood pressure-lowering treatment based on cardiovascular risk: A meta-analysis of individual patient data. Lancet. 2014;384:591–598. doi: 10.1016/S0140-6736(14)61212-5. [DOI] [PubMed] [Google Scholar]
- 9.Xie X, Atkins E, Lv J, Bennett A, Neal B, Ninomiya T, Woodward M, MacMahon S, Turnbull F, Hillis GS, Chalmers J, Mant J, Salam A, Rahimi K, Perkovic V, Rodgers A. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: Updated systematic review and meta-analysis. Lancet. 2016;387:435–443. doi: 10.1016/S0140-6736(15)00805-3. [DOI] [PubMed] [Google Scholar]
- 10.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Sr, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW. 2013 acc/aha guideline on the assessment of cardiovascular risk: A report of the american college of cardiology/american heart association task force on practice guidelines. J AM Coll Cardiol. 2014;63:2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karmali KN, Ning H, Goff DC, Lloyd-Jones DM. Identifying individuals at risk for cardiovascular events across the spectrum of blood pressure levels. J Am Heart Assoc. 2015;4:e002126. doi: 10.1161/JAHA.115.002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silverman MG, Blaha MJ, Krumholz HM, Budoff MJ, Blankstein R, Sibley CT, Agatston A, Blumenthal RS, Nasir K. Impact of coronary artery calcium on coronary heart disease events in individuals at the extremes of traditional risk factor burden: The multi-ethnic study of atherosclerosis. Eur Heart Jl. 2014;35:2232–2241. doi: 10.1093/eurheartj/eht508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O’Leary D, Carr JJ, Goff DC, Greenland P, Herrington DM. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valenti V, OH B, Heo R, Schulman-Marcus J, Cho I, Kalra DK, Truong QA, Giambrone AE, Gransar H, Callister TQ, Shaw LJ, Lin FY, Chang HJ, Sciarretta S, Min JK. Long-term prognosis for individuals with hypertension undergoing coronary artery calcium scoring. Int J Cardiol. 2015;187:534–540. doi: 10.1016/j.ijcard.2015.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blaha MJ, Budoff MJ, DeFilippis AP, Blankstein R, Rivera JJ, Agatston A, O’Leary DH, Lima J, Blumenthal RS, Nasir K. Associations between c-reactive protein, coronary artery calcium, and cardiovascular events: Implications for the jupiter population from mesa, a population-based cohort study. Lancet. 2011;378:684–692. doi: 10.1016/S0140-6736(11)60784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miedema MD, Duprez DA, Misialek JR, Blaha MJ, Nasir K, Silverman MG, Blankstein R, Budoff MJ, Greenland P, Folsom AR. Use of coronary artery calcium testing to guide aspirin utilization for primary prevention: Estimates from the multi-ethnic study of atherosclerosis. Circ Cardiovasc Qual Outcomes. 2014;7:453–460. doi: 10.1161/CIRCOUTCOMES.113.000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: Objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 18.Chang JJ, Rabinowitz D, Shea S. Sources of variability in blood pressure measurement using the dinamap pro 100 automated oscillometric device. Am J Epidemiol. 2003;158:1218–1226. doi: 10.1093/aje/kwg274. [DOI] [PubMed] [Google Scholar]
- 19.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac ct in population-based studies: Standardized protocol of multi-ethnic study of atherosclerosis (mesa) and coronary artery risk development in young adults (cardia) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 20.Kovell LC, Ahmed HM, Misra S, Whelton SP, Prokopowicz GP, Blumenthal RS, McEvoy JW. Us hypertension management guidelines: A review of the recent past and recommendations for the future. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: Meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319:1492–1495. doi: 10.1136/bmj.319.7223.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bress AP, Tanner RM, Hess R, Colantonio LD, Shimbo D, Muntner P. Generalizability of sprint results to the u.S. Adult population. J Am Coll Cardiol. 2016;67:463–472. doi: 10.1016/j.jacc.2015.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alderman MH, Furberg CD, Kostis JB, Laragh JH, Psaty BM, Ruilope LM, Volpe M, Jackson R. Hypertension guidelines: Criteria that might make them more clinically useful. American J Hypertens. 2002;15:917–923. doi: 10.1016/s0895-7061(02)03001-7. [DOI] [PubMed] [Google Scholar]
- 25.Jackson R, Lawes CM, Bennett DA, Milne RJ, Rodgers A. Treatment with drugs to lower blood pressure and blood cholesterol based on an individual’s absolute cardiovascular risk. Lancet. 2005;365:434–441. doi: 10.1016/S0140-6736(05)17833-7. [DOI] [PubMed] [Google Scholar]
- 26.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW. 2013 acc/aha guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the american college of cardiology/american heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Lonn EM, Bosch J, Lopez-Jaramillo P, Zhu J, Liu L, Pais P, Diaz R, Xavier D, Sliwa K, Dans A, Avezum A, Piegas LS, Keltai K, Keltai M, Chazova I, Peters RJ, Held C, Yusoff K, Lewis BS, Jansky P, Parkhomenko A, Khunti K, Toff WD, Reid CM, Varigos J, Leiter LA, Molina DI, McKelvie R, Pogue J, Wilkinson J, Jung H, Dagenais G, Yusuf S Investigators H. Blood-pressure lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med. 2016;374:2009–2020. doi: 10.1056/NEJMoa1600175. [DOI] [PubMed] [Google Scholar]
- 28.DeFilippis AP, Young R, Carrubba CJ, McEvoy JW, Budoff MJ, Blumenthal RS, Kronmal RA, McClelland RL, Nasir K, Blaha MJ. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162:266–275. doi: 10.7326/M14-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeboah J, Young R, McClelland RL, Delaney JC, Polonsky TS, Dawood FZ, Blaha MJ, Miedema MD, Sibley CT, Carr JJ, Burke GL, Goff DC, Jr, Psaty BM, Greenland P, Herrington DM. Utility of nontraditional risk markers in atherosclerotic cardiovascular disease risk assessment. J Am Coll Cardiol. 2016;67:139–147. doi: 10.1016/j.jacc.2015.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erbel R, Lehmann N, Mohlenkamp S, Churzidse S, Bauer M, Kalsch H, Schmermund A, Moebus S, Stang A, Roggenbuck U, Brocker-Preuss M, Dragano N, Weimar C, Siegrist J, Jockel KH Heinz Nixdorf Recall Study I. Subclinical coronary atherosclerosis predicts cardiovascular risk in different stages of hypertension: Result of the heinz nixdorf recall study. Hypertension. 2012;59:44–53. doi: 10.1161/HYPERTENSIONAHA.111.180489. [DOI] [PubMed] [Google Scholar]
- 31.Roberts ET, Horne A, Martin SS, Blaha MJ, Blankstein R, Budoff MJ, Sibley C, Polak JF, Frick KD, Blumenthal RS, Nasir K. Cost-effectiveness of coronary artery calcium testing for coronary heart and cardiovascular disease risk prediction to guide statin allocation: The multi-ethnic study of atherosclerosis (mesa) PloS One. 2015;10:e0116377. doi: 10.1371/journal.pone.0116377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richman IB, Fairley M, Jorgensen ME, Schuler A, Owens DK, Goldhaber-Fiebert JD. Cost-effectiveness of intensive blood pressure management. JAMA Cardiol. 2016;1:872–879. doi: 10.1001/jamacardio.2016.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin SS, Sperling LS, Blaha MJ, Wilson PW, Gluckman TJ, Blumenthal RS, Stone NJ. Clinician-patient risk discussion for atherosclerotic cardiovascular disease prevention: Importance to implementation of the 2013 acc/aha guidelines. J Am Coll Cardiol. 2015;65:1361–1368. doi: 10.1016/j.jacc.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kjeldsen SE, Lund-Johansen P, Nilsson PM, Mancia G. Unattended blood pressure measurements in the systolic blood pressure intervention trial: Implications for entry and achieved blood pressure values compared with other trials. Hypertension. 2016;67:808–812. doi: 10.1161/HYPERTENSIONAHA.116.07257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


