Summary
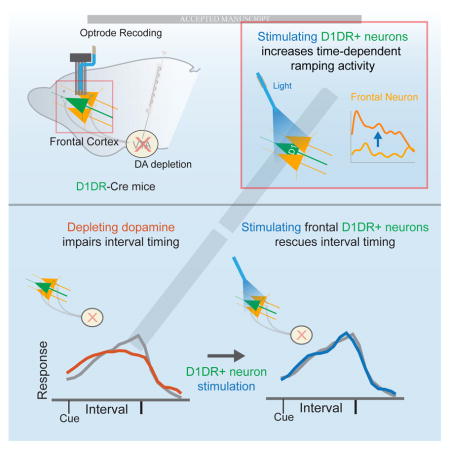
Disrupted mesocortical dopamine contributes to cognitive symptoms of Parkinson’s disease (PD). Past work has implicated medial frontal neurons expressing D1 dopamine receptors (D1DRs) in temporal processing. Here, we investigate if these neurons can compensate for behavioral deficits resulting from midbrain dopamine dysfunction. We report three main results. First, both PD patients and mice with ventral tegmental area (VTA) dopamine depletion had attenuated delta activity (1–4 Hz) in the medial frontal cortex (MFC) during interval timing. Second, we found that optogenetically stimulating MFC D1DR neurons could increase ramping activity among MFC neurons. Finally, stimulating MFC D1DR neurons specifically at delta frequencies (2 Hz) compensated for deficits in temporal control of action caused by VTA dopamine depletion. Our results suggest that cortical networks can be targeted by frequency-specific brain stimulation to improve dopamine-dependent cognitive processing.
Keywords: prefrontal cortex, interval timing, dopamine receptors
eTOC Blurb
Kim et al., study neuronal networks in medial frontal cortex during elementary cognitive processing. They find that frontal delta rhythms depend on dopamine in both humans and rodents. Stimulating frontal neurons expressing D1-type dopamine receptors improves temporal processing and stimulating frontal neurons at 2 Hz can improve the temporal control of action.
Introduction
Patients with Parkinson’s disease (PD) not only suffer from motor symptoms but also from debilitating cognitive symptoms[1–3]. There are few effective treatments for the cognitive symptoms of PD, leading to considerable morbidity and mortality[4]. We study an elementary cognitive process impaired in PD patients: interval timing[5]. This task requires that participants estimate an interval of several seconds as instructed by a cue, and requires executive resources such as working memory and attention to time[5,6]. Interval timing is particularly well-suited to study cognitive function in PD because it is consistently impaired in patients with PD[7,8] and requires common neuronal features in humans and rodents[8–10], facilitating translational research in rodent disease models.
Recent work from our group and others has demonstrated that the medial frontal cortex (MFC) is necessary for the temporal control of action[11,12]. Blocking D1- but not D2-type dopamine receptors impairs temporal processing and neuronal activity correlated with temporal control in rodents[11,13,14], paralleling work in primates and humans implicating frontal D1-dopamine receptors (D1DRs) in cognitive processing[15,16]. During interval timing, MFC neurons that ‘ramp’ – or monotonically increase/decrease their activity in time – are involved in temporal processing[17]. Ramping neurons can be strongly functionally coupled with delta rhythms (1–4 Hz), which are influenced by focal MFC D1DR agonists and antagonists[13,18]. These data lead to the hypothesis that D1DR-dependent MFC delta rhythms critically regulate temporal processing.
Here, we explore this idea by recording from MFC neurons in mice with disrupted midbrain dopamine function. We find that stimulating MFC neurons expressing D1DRs increased MFC ramping activity and could improve interval-timing deficits caused by depleting VTA dopamine. These results provide evidence that stimulation of MFC D1DR neurons can improve cognitive deficits in VTA dopamine-depleted mice, which could inform the development of therapies targeting D1DRs or cortical brain stimulation for human diseases that impair cognition.
Results
Medial frontal delta rhythms are attenuated in humans and rodents with disrupted midbrain dopamine
To examine how dopamine influences cognitive processing in mid-frontal brain regions, we collected EEG data during performance of an interval-timing task from 12 PD patients without dementia performing an fixed interval-timing task with a 12 s interval (Table S1; Figure 1A)[10]. In this task, a numerical cue stimulus appeared on the center of the screen indicating the 12 s interval the participants were instructed to estimate, and participants made responses by pressing the space bar on a keyboard using their dominant hand when they estimated the 12 s interval had elapsed. Participants received feedback about their response time at the end of each trial, and there was a uniformly varying, randomly chosen 3- to 6-s interval between response and feedback, after which participants moved to the next trial by pressing the space bar again. During fixed-interval timing tasks, temporal control of action can be quantified via two measures. First, we calculated the efficiency of responses by calculating the fraction of responses between 11–12s divided by the overall number of responses. As efficiency is closer to 1, the fraction of responses at 12 seconds is greater, indicating that participants guide their actions in time more accurately[18,19]. Secondly, we calculated the ‘curvature’ of time-response histograms by measuring the deviation from the cumulative distribution of a straight line[10,19–21]. This metric is 0 with a flat time-response curve during interval and is closer 1 when more responses are at 12 s and time-response histograms are more curved. In line with prior work by our group and others, PD patients had impaired efficiency and curvature compared to controls (Figure 1B–C; efficiency: t(22)=4.0, p<0.0008; curvature: t(22)=2.8, p<0.01; Fig S1)[7,10]. Mid-frontal scalp EEG cue-aligned delta band activity measured at electrode Cz was also attenuated in PD patients relative to demographically matched controls (Figure 1D–I; delta power at 1–4 Hz over the entire 12 s interval from lead Cz: t(22)=4.3, p<0.005)[9,10,22]. No consistent differences were found for cue-aligned theta (5–8 Hz), alpha (9–12 Hz), beta (13–30 Hz) activity or other frequency bands or around responses (Fig S2). Given the consistent differences in cue-aligned mid-frontal delta MFC activity in control vs. PD patients, we focused on delta frequencies for the remainder of this manuscript.
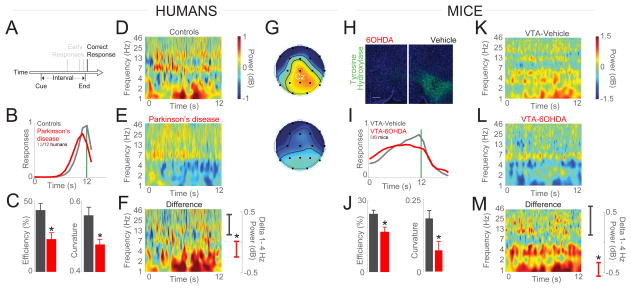
Figure 1. Interval timing and medial frontal delta rhythms in humans and rodents are attenuated with disrupted midbrain dopamine.
(A) Interval timing requires participants to estimate an interval of several seconds (12 s) by making a motor response as instructed by a cue; early responses are unreinforced. This task requires working memory for temporal rules and attention to time. (B) Humans with PD have broader and earlier time-response histograms compared to demographically-matched controls (12 controls and 12 PD patients). (C) Interval-timing behavior can be operationalized by using the efficiency of responses or the curvature of time-response histograms. PD patients are less efficient and have flatter time-response histograms compared to demographically-matched controls. (D–E) Time-frequency analysis of EEG electrode Cz during interval-timing tasks revealed that delta rhythms between 1–4 Hz are (F) attenuated in PD patients compared to controls. (G) Topographic distribution of delta power over the MFC in controls and PD patients; power same as in (D). White dot in (G) corresponds to electrode Cz. (H) In mice, mesocortical dopamine can be depleted using 6OHDA injections; we targeted bilateral mesocortical projections in the VTA. Representative images from animal with VTA-6OHDA (left) vs a different animal with vehicle (right) stained for tyrosine hydroxylase (green). (I) In mice, interval timing is impaired in VTA-6OHDA compared to VTA-Vehicle mice. (J) VTA-6OHDA mice were less efficient in interval timing and had flatter time-response histograms compared to VTA-Vehicle. (K–M) VTA-6OHDA mice have less delta power in MFC LFPs compared to control mice. EEG from 12 PD patients/12 controls, 6 VTA-Vehicle/6 VTA mice; *= p < 0.05. See also Figure S1, S2, S3 and Table S1.
Patients with PD can have degeneration of dopaminergic neurons including a group of neurons in the medial midbrain that constitute the major dopaminergic projection to frontal and limbic cortices[23,24]. To explore mesocortical dopamine circuits in detail, we depleted dopamine in medial midbrain by targeting the ventral tegmental area (VTA) with injection of the neurotoxin 6-hydroxydopamine (6OHDA; Figure 1H). As in humans, mice with VTA-6OHDA had impaired interval-timing performance (Figure 1I–J; efficiency: t(12)=3.4, p<0.01; curvature: t(12)=3.3, p<0.01; data from 6 VTA-Vehicle and 6-VTA-6OHDA mice). Similar to PD patients, local field potentials (LFPs) from VTA-6OHDA mouse MFC had attenuated delta activity during interval timing (Figure 1L–M; delta power: t(16)=2.9, p<0.01; average MFC LFPs in 6 VTA-Vehicle mice and 6 VTA-6OHDA mice). No consistent differences were found for response-related delta activity (Fig S3). These data indicate that MFC delta rhythms are decreased in both humans and rodents with disrupted dopaminergic signaling[10,21].
MFC D1DR-expressing neurons are modulated during interval timing
The data above indicate that VTA-6OHDA can influence MFC delta rhythms. To examine how dopamine influences temporal processing by MFC neuronal ensembles, we used optogenetics to study the activity of MFC neurons expressing dopamine receptors during interval-timing performance. In the MFC, there are two broad classes of dopamine receptors, D1-type and D2-type. Prior lines of research have demonstrated that MFC D1DRs—but not D2-dopamine receptors—are involved in cognitive processing[15,25], and our own work has implicated MFC D1DRs specifically in temporal processing[13,14,18,26].
We tested this idea using D1DR-Cre+ mice to record from MFC D1DR neurons (Figure 2A–B). In these mice, we can ‘tag’ MFC D1DR neurons by combining optogenetics and neuronal ensemble recording. MFC D1DR neurons virally expressing channelrhodopsin-2 (ChR2) will fire action potentials with a short latency (<5 ms) in response to blue light with consistent waveforms (Figure 2C–D). Of 206 MFC neurons in 6 VTA-Vehicle D1DR-Cre+ mice expressing ChR2, 30 neurons were optogenetically tagged as putative MFC D1DR neurons (14.6%; average spike latency = 1.84ms; average correlation between stim/non-stim waveforms = 0.993). Consistent with work in primates and rats, both putative MFC D1DR neurons and untagged MFC neurons could be modulated during interval-timing tasks (Figure 3A–C).
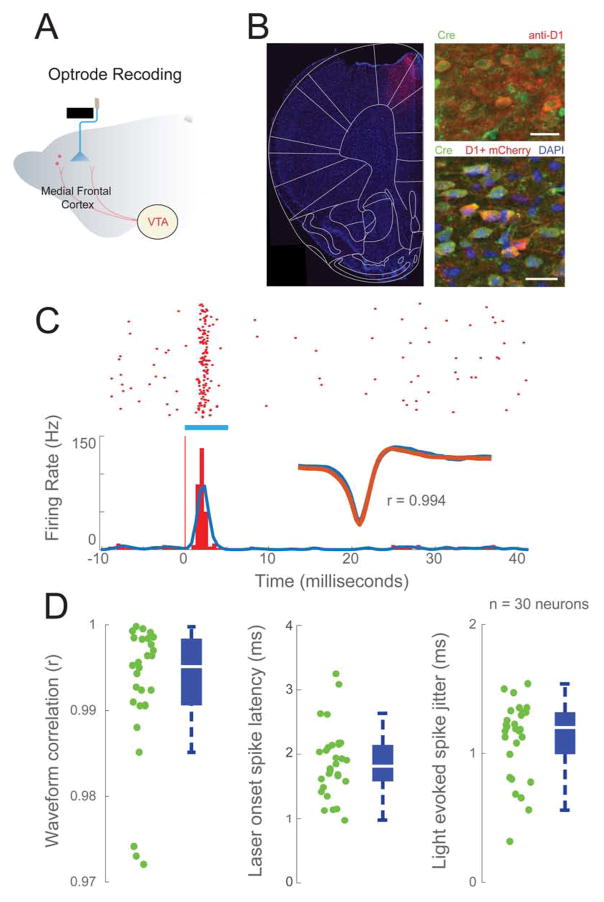
Figure 2. Optogenetic tagging of putative MFC D1DR-expressing neurons.
(A) Schematic of multichannel electrode recording of MFC D1DR neurons with optical fiber. (B) Expression of D1DR-Cre dependent ChR2-mCherry from dorsal MFC (left). Immunohistochemistry with anti-Cre and anti-D1DR demonstrated expression of Cre recombinase in D1DR neurons (top right). Immunohistochemistry with anti-Cre confirmed Cre dependent expression of ChR2-mCherry (bottom right). Scale bar = 20μm. (C) A raster plot of a neuron with spikes evoked blue light (blue bar). Representative example of average spike waveforms compared between light-evoked and spontaneous activity. Pearson correlation coefficient > 0.95 indicated that light-evoked and non-light evoked spikes were the same; note that during optogenetic tagging stimulation, animals were not engaged in the task, and typically not moving. The timescale is shown in milliseconds to show evoked activity. (D) Properties of light-evoked waveforms. Light-evoked spike latencies and jitter of 30 putative MFC D1DR neurons (waveform correlation: 0.998±0.0015. spike latency: 1.84±0.098ms. spike jitter: 1.1±0.05ms; green dots from each putative MFC D1DR neuron). Box plot central bar represents the median, and edges represent the 25th and 75th percentiles of the data set.
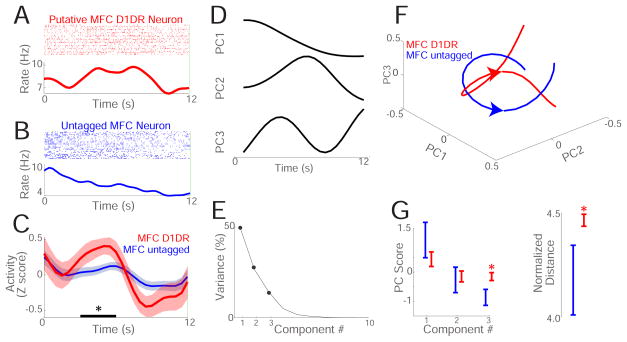
Figure 3. MFC D1DR neurons are involved in temporal processing.
(A) Peri-event raster and histogram of a putative MFC D1DR neuron during the 12 s interval during interval timing (red). (B) Peri-event raster and histogram of an untagged MFC neuron during the 12 s interval (blue). (C) Normalized average activity (Z score) of 30 optogenetically tagged putative MFC D1DR neurons (red) and 176 untagged MFC (blue) neurons during the 12 s interval; putative MFC D1DR neurons had stronger modulations in the middle of the interval from 3–6 s (black bar) compared to untagged MFC neurons. (D) Across all 206 neurons in 6 VTA-vehicle mice, we used data-driven PCA to find patterns of neuronal activity. PCA identified 3 major components among MFC neuronal ensembles. (E) Scree plot of variance explained by each component; only PC1-3 were analyzed. (F) 30 putative MFC D1DR neurons (red) and 176 untagged MFC neurons (blue) had distinct population trajectories in PC space. G) 30 putative MFC D1DR neurons (red) and 176 untagged MFC neurons (blue) had significantly different PC3 scores and had significantly different normalized distance in principal component space. Neuronal recordings from 6 VTA-Vehicle mice. *= p < 0.05.
We used principal component analysis (PCA) to explore MFC population activity using unbiased, data-driven approaches. This technique identifies a series of orthogonal basis functions that minimize variance across multivariate data and has been extensively used to analyze neuronal data[13,27–29]. In the MFC, PCA identified three prominent patterns of neuronal activity. As in previous studies, the first principal component (PC1) was a ‘ramp’—a monotonic linear change in neuronal activity over time (Figure 3D)[13,28–30]. Ramping activity, or PC1, explained 51% of neuronal variance. Of note, our past work indicates that ramping activity can be coherent with delta oscillations and can predict when animals respond during the interval-timing task[9,10,13,17]. PC2 showed modulation during the interval and explained 27% of variance. The third PC had biphasic modulation during the interval and explained 14% of variance. All other components explained <10% of variance and were not analyzed (Figure 3E). We projected the neuronal activity of the putative MFC D1DR population and the population of untagged MFC neurons in the same PC space using the first 3 components (Figure 3F), and only PC3 loaded differently on putative MFC D1DR neurons compared to untagged MFC neurons (t(204)=2.1, p<0.04). To test if the populations were different as a whole, we compared the normalized Euclidian distance of each population from the center of PC space. If two populations are different in PC space, then they will have different distances in PC space[31]. We found that PC distance was different for MFC D1DR populations vs untagged MFC populations (4.2±0.17 vs 4.5±0.03; t(204)=2.9, p<0.004; statistical power 0.84). These data provide evidence that putative MFC D1DRs are modulated during the interval, and this pattern of modulation can be identified by PCA.
Delta stimulation of MFC D1DR neurons can compensate for VTA-6OHDA
Our past work has shown that disrupting VTA dopamine or MFC D1DRs impairs interval timing and MFC delta rhythms[10,13,14,26], raising the question of how delta activity influences MFC neuronal ensembles responsible for the temporal control of action[12,13,29]. We focused on VTA 6-OHDA animals, as these animals had attenuated delta rhythms, and explored how delta stimulation of MFC D1DR neurons influenced temporal processing by MFC neurons and interval timing performance.
Optogenetic stimulation of MFC D1DR neurons at delta frequencies (2 Hz) increased the firing rate of MFC D1DR neurons (Figure 4A–B) and delta power in local field potentials (6 VTA-6OHDA mice: unstimulated: −0.1±0.3dB vs stimulated: 2.6±0.9dB: paired t(10)=2.6, p<0.03). MFC neuronal ensembles have dense recurrent connectivity; thus MFC D1DR neurons are likely to strongly influence the activity of other medial frontal neurons[32–34]. We noticed that stimulation of MFC D1DR neurons in the same MFC network could increase ramping activity of untagged MFC neurons (Figure 4C–F). As above, ramping activity can be represented by PC1 in PCA[13,17,18]; and we found that MFC D1DR delta stimulation markedly affected PCA resulting in significant differences for PC1 among untagged MFC neurons (Figure 4G–H; PC1: paired t(233)=2.1, p<0.04). PC3 also increased with MFC D1DR delta stimulation (paired t(233)=3.9, PC3: p<0.0001; no difference was found for PC2). The population distance in PC space was also significantly different for stimulated vs. unstimulated trials (Fig 4H; 4.1±0.06 vs. 4.3±0.04; paired t(233)=2.9, p<0.005). A complementary analysis that captures ramping activity is linear regression, which finds that ramping neurons have a significant linear regression fit[17,18]. Consistent with the PCA above, more ramping neurons were found during MFC D1DR delta stimulation trials (89 vs. 67; X2=4.7,p<0.03; Fig 4I). Furthermore, these ramping neurons had a significantly steeper slope on stimulation trials (|1.1|±0.1 vs.|2.0|±0.25 spikes/sec2; t(154)=3.1, p<0.003; Fig 4I). Finally, to quantify timing information in untagged MFC neuronal ensembles, we used a naïve Bayesian classifier to predict time from MFC neuronal firing rate during stimulated and unstimulated trials (Fig 4J–K). During unstimulated trials, MFC neurons predicted time poorly (R2 of 0.13), albeit better than shuffled data (R2=0.003; z(2,40) =16.3, p<0.000). However, during trials with optogenetic delta stimulation, the R2 increased to 0.53, significantly higher than unstimulated trial classification (z(2,40) = 6.64, p<0.003). These data indicate that delta stimulation of MFC D1DR neurons could change MFC networks by increasing ramping patterns of activity in mice with disrupted mesocortical dopamine.
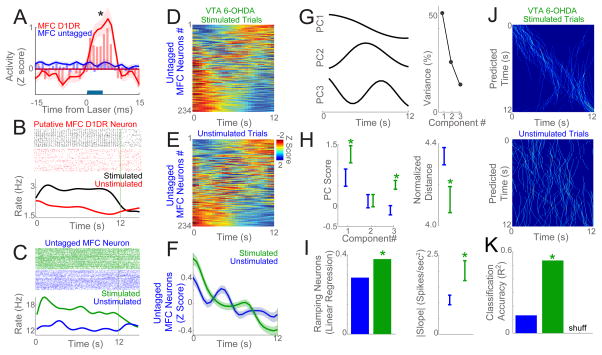
Figure 4. Delta optogenetic stimulation of MFC D1DR neurons modulates temporal processing.
We compared the activity of MFC neurons with and without optogenetic stimulation of MFC D1DR neurons at 2 Hz in VTA-6OHDA mice. (A) Laser stimulation (blue bar) of MFC D1DRs only triggered increased activity in 41 putative MFC D1DR neurons (red) but not 234 untagged MFC neurons (blue). (B) This putative MFC D1DR neuron had an increased firing rate with optogenetic stimulation during interval timing (stimulation trials – black; unstimulated trials – red). (C) Stimulation of MFC D1DR neurons could change the activity of untagged MFC neurons by increasing its activity early in the trial (stimulation trials – green; unstimulated trials – blue). (D–E) Heat maps of peri-event activity from 234 untagged MFC neurons in 6 VTA-6OHDA mice were different for stimulated vs unstimulated trials early in the interval (white boxes). F) On average, stimulation increased activity early in the interval in 234 untagged MFC neurons (0–3 s; black bar; stimulation trials –green; unstimulated trials - blue). G–H) To quantify this effect, we turned to PCA which revealed stronger PC1 and PC3 scores on stimulated trials compared to unstimulated trials, resulting in significant differences in normalized distance among 234 untagged MFC neurons (stimulation trials – green; unstimulated trials – blue). I) More untagged MFC neurons had a significant linear regression fit on stimulated vs unstimulated trials. These neurons had a steeper regression slope on stimulation trials. J) Bayesian classification of predicted time from neuronal ensemble activity on stimulated vs unstimulated trials in VTA-6OHDA mice. The predicted time on each trial at each bin shown in in white; 50 trials are shown. K) On stimulated trials, classifiers appeared to predict time better than on unstimulated trials, and had significantly higher classification accuracy as quantified by R2 of predicted vs. actual time (stimulation trials – green; unstimulated trials – blue). Data from 6 VTA-6OHDA mice; *= p< 0.05.
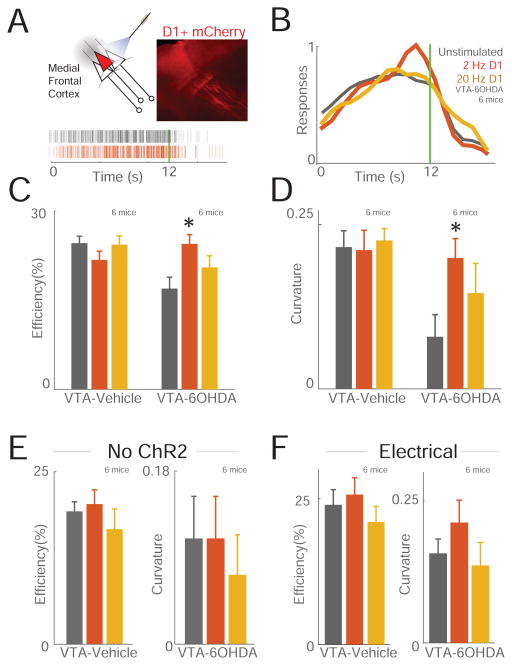
These results imply that stimulation of MFC D1DR neurons in delta frequencies may be able to compensate for cognitive deficits caused by VTA-6OHDA. We tested this idea by training D1DR-Cre+ mice to perform an interval-timing task, depleting VTA dopamine, and then expressing channelrhodopsin (ChR2) in MFC D1DR neurons as above. We used delta frequencies (2 Hz) because they were specifically attenuated in PD patients and in VTA-6OHDA mice (Fig 1)[10], and we also used beta frequencies (20 Hz), as we have used this frequency previously and because cortical beta frequencies have been implicated in PD[11,35]. We stimulated MFC D1DR neurons with 5 ms pulses at 8mW laser power at 2 Hz, 20 Hz, or withheld stimulation (unstimulated trials) on randomly selected trials. Strikingly, stimulating MFC D1DR neurons at 2 Hz but not 20 Hz compensated for behavioral deficits in interval-timing performance VTA 6-OHDA mice (Fig 5C–D; efficiency post-hoc ANOVA; F(2,12)=5.8, p<0.05; curvature F(2,12)=3.17, p<0.05; 6 VTA-Vehicle/6 VTA-6OHDA mice). No effects were observed in mice expressing control virus with mCherry; that is, these D1DR-Cre+ mice had a ‘blank’ virus without ChR2 and laser light stimulation (Figure 5E; 6 VTA-Vehicle/6 VTA-6OHDA mice). Electrical stimulation using bipolar stimulating electrodes at 2 or 20 Hz of medial frontal networks was ineffective (Figure 5F; 6 VTA-Vehicle/6 VTA-6OHDA mice). Finally, in mice with intact dopamine circuits, stimulation of prefrontal D1 neurons had no observable effect on interval-timing performance at any frequency (Figure 5A–D; 6 VTA-Vehicle/6 VTA-6OHDA mice). VTA-6OHDA did not exhibit observable differences in patterns of movement as measured by open-field activity, and did not change the number of responses, rewards, or nosepoke durations (Fig S4). Furthermore, optogenetic stimulation of MFC D1DR neurons did not change responding, reward acquisition, or movements (Fig S4). Taken together, these data suggest that frequency-specific stimulation of MFC D1DR neurons at delta frequencies can potentially compensate for interval timing deficits caused depleting dopamine in mesocortical circuits.
Figure 5. Delta stimulation of MFC D1DR neurons can compensate for impaired temporal control of action caused by VTA-6OHDA.
(A) Optogenetic stimulation of MFC D1DR neurons and histology (upper right) of ChR2-mCherry expression driven by D1-Cre+. All stimulation was unilateral. Bottom, a raster plot of responses from one mouse during interval timing compared in MFC D1DR stimulated (red) and unstimulated (grey) trials. (B) Normalized time-response histograms demonstrate that 2 Hz but not 20Hz stimulation of putative MFC D1DR neurons improved interval-timing deficits in VTA-6OHDA mice. (C) Efficiency and (D) curvature analysis demonstrated that interval-timing performance significantly changed in VTA-6OHDA mice with 2 Hz stimulation. No effects were seen in VTA-Vehicle mice with intact dopamine, in control mice, or with mice with electrical stimulation at 2 or 20 Hz. (E) Optogenetic stimulation in D1-Cre+ mice with AAV-mCherry without ChR2 did not significantly change interval timing efficiency or curvature. (F) MFC electrical stimulation also did not significantly affect interval timing. Data from 6 VTA-Vehicle and 6 VTA-6OHDA mice with ChR2, 6 VTA-Vehicle and 6 VTA-6OHDA with virus without ChR2, and 6 VTA-Vehicle and 6 VTA-6OHDA mice for electrical stimulation. *= p<0.05 via mixed-effects ANOVA. See also Figure S5.
Discussion
We tested the hypothesis that D1DR-dependent delta rhythms in the MFC can critically regulate temporal processing. We used an interval timing task that is impaired in PD patients to study MFC networks in detail. We report novel evidence that 1) MFC delta oscillations are decreased in both humans with PD and rodents with VTA-6OHDA, 2) that stimulating MFC D1DR neurons at delta frequencies (2Hz) can increase MFC ramping activity, and 3) that stimulating MFC D1DRs can compensate for deficits in temporal control of action caused by VTA-6OHDA. Although MFC delta activity has been shown previously to be attenuated in PD and in rodents with VTA 6-OHDA, our data describing the effects of MFC D1DR stimulation are, to our knowledge, novel. These results provide new insight into how MFC D1DRs influence processing and performance during cognitive tasks. Our findings are particularly significant because there are no clinically-approved drugs that specifically target D1DRs. Current clinical brain stimulation approaches target basal ganglia circuits explicitly for movement disorders. Our work implies that cortical brain stimulation can influence cognitive processing and might provide useful guidance for future therapies targeting cognition in PD.
This work extends a large body of research implicating frontal D1DR signaling in the intricacies of cognitive processing[10,13,15,16,26,36]. Our results were presaged by work demonstrating that frontal D1DR blockade markedly attenuated performance of and neuronal encoding of working memory, that frontal D1DRs are impaired in human patients with deficits in executive function, and finally, that MFC D1DRs are involved in temporal processing[13,15,25,37]. Our study is the first to optogenetically ‘tag’ cortical D1DR-expressing neurons and record from these neurons during a cognitive task. We found that stimulating MFC D1DR neurons can modulate MFC neuronal activity and improve interval timing performance in animals with disrupted MFC dopamine.
Consistent with extensive past work by our group and others, PD patients and VTA-6OHDA rodents had impairments in interval timing[5,7,10,13,26,38]. We found that PD patients and VTA-6OHDA rodents had ‘earlier’ response times, but this is likely an artifact of greater variance in time-response histograms. Feedback given immediately following the first response after 12 s would quickly terminate responding and thus abbreviate time-estimation. Interval-timing tasks using ‘peak’ trials, in which feedback is withheld, can more appropriately capture time estimation. PD patients and dopamine-depleted rodents have marked changes during peak interval-timing[7,39]. However, without peak trials it is difficult to draw firm conclusions about temporal shifts in response times vs. increased variance of time estimation[40]. Interval timing deficits in PD have been interpreted as impaired memory for temporal rules[5,7,8], and our study provides some insight into how MFC neurons expressing D1DRs are involved in temporal processing.
MFC D1DR neurons have diverse synaptic targets in the cortex, striatum, amygdala, hypothalamus, and other brain regions[34,41]. Several of these projections might influence MFC activity and exert powerful control over goal-directed behaviors[41]. While the MFC exerts top-down control over other brain regions such as the motor cortex, the exact role of MFC neurons expressing D1DRs in this network is unclear[9,42]. Defining the exact network will require systematic circuit-level exploration but could be highly relevant to understanding cognitive processing.
Prefrontal dopamine receptors involve complex dynamics[13,18,25,43,44]. Prefrontal dopamine is thought to adhere to a ‘U-shaped’ curve, with too high or too low amounts of dopamine degrading neuronal processing and cognitive performance. We optogenetically stimulated MFC neurons expressing D1DRs. This approach allowed us to study how spiking activity in these neurons influences interval timing behavior. However, we recorded comparatively few MFC D1DR neurons, limiting inferences about how distinct these neurons are during interval timing. Future studies might record from more MFC D1DR neurons in more animals to address this issue. Regardless, we found that frequency-specific 2 Hz stimulation of MFC D1DR neurons mimics the effects of optimal dopamine, while 20 Hz stimulation is too far from optimal to improve behavior. U-shaped models of dopaminergic function could also explain why optogenetic stimulation in mice without dopamine depletion was ineffective, as these mice would already have optimal levels of frontal dopamine. While our data implicate neurons expressing MFC D1DRs in cognitive control, drug design for clinical applications will likely need to carefully consider these dynamics to bring frontal dopaminergic signaling into its optimal range and maximize its effect on cognition.
Our results are consonant with existing literature postulating that mid-frontal low-frequency oscillations are a mechanism of cognitive control[22,45]. Low-frequency rhythms can synchronize diverse brain networks during a range of behaviors[45–47] and neurons involved in temporal and error processing can be coherent with low-frequency rhythms[9,13]. While we and others have reported that these rhythms appear to be modulated by salient environmental stimuli, such as the instructional cue during interval timing tasks, these are the first data indicating that increasing delta activity via optogenetic stimulation can improve temporal control of action, at least in animals with impaired mesocortical dopamine. Scalp EEG can detect delta oscillations at electrodes Cz or Fz, although the cortical source of these oscillations can be difficult to localize. In rodents, we can record directly from MFC neuronal networks, and we found common low-frequency rhythms in human EEG at electrode Cz and the mouse MFC in this study, consistent with two prior studies from our group[9,10]. This line of work indicates that MFC delta oscillations require intact mesocortical dopamine signaling, and could be a useful insight for neurophysiological biomarkers of human diseases that impair cognition.
Our data suggest that highly selective and specific stimulation of MFC D1DR neurons is required to influence cognitive performance. Indeed, only optogenetic delta-band stimulation at 2 Hz improved interval timing, and higher frequencies did not. However, translating this work to PD patients will prove challenging because PD is challenging to model in animals and PD patients have dysfunction in multiple circuits, including the basal ganglia and other brainstem and subcortical nuclei[48]. In the present study, we did not model PD, but rather studied mesocortical circuits by depleting VTA dopamine. We did not study substantia nigra pars compacta dopaminergic neurons, which are heavily involved in PD. Although neurons in both nuclei degenerate in PD, we focused on the VTA because this area projects heavily to the MFC, and nigrostriatal dopamine depletion can produce severe motor deficits[23,48]. Future studies will examine how the two ascending dopaminergic projections interact during interval timing.
Our proof-of-principle studies in mice performing an interval-timing task—which involves only elementary cognitive processing—also needs to be generalized to other cognitive paradigms. In addition, we used a highly limited fixed-interval timing task. Future efforts might employ detailed measurement of movement using force-controlled levers, task-specific distractors, perturbations in performance feedback, peak-trials, and multiple intervals. These modifications would allow more precise insight into how dopamine influences temporal processing. However, other executive tasks such as working memory tasks, trail-making, verbal fluency, choice-reaction time tasks, or Stroop tasks are not reliably disrupted in PD patients[48], likely involve the lateral frontal cortex (which does not exist in rodents), and can be difficult to train in animal models. Tracing the flow of cognitive information from MFC D1DR neurons could identify areas of functional convergence that can be targeted by next-generation brain-stimulation approaches for cognitive symptoms of PD as well as other diseases.
Experimental Procedures
Human interval timing
All procedures were approved by the Institutional Review Board at the University of Iowa Protocol #201301713. Informed consent was obtained after explaining the procedures in detail as well as the associated risks and benefits. Data was collected from 12 PD patients and 12 age- and education-matched control participants who performed an interval-timing task according to procedures described in detail elsewhere[10]. All participants had normal or corrected-to-normal vision and were not demented at the time of evaluation (Montreal Cognitive Assessment or MOCA score ≥ 26). Patients with PD did not have other confounding diseases, and control participants were free from brain disease. PD patients took medication as usual. The interval-timing task consisted of 160 trials with either a 3 or 12 s interval; only data from the 12 s interval was included in this manuscript, although detailed analyses of these data were described previously[10]. All trials began when a numerical cue stimulus appeared on the center of the screen indicating the temporal interval the participants were instructed to estimate (3 or 12 s). Participants made responses by pressing the space bar on a keyboard using their dominant hand when they estimated the temporal interval had elapsed. Participants received feedback about their response time at the end of each trial. There was a uniformly varying, randomly chosen 3- to 6-s interval between response and feedback. After feedback, participants moved to the next trial by pressing the space bar again. The task was self-paced, and the participants were asked not to count in their head during the task. For both human and rodent interval timing, we quantified behavioral performance using methods that we and others have used extensively[10,20,21,26]. Briefly, we measured the efficiency as the number of responses at 12 s divided by the total number of responses; this number is closer to 1 if most responses are at 12 s. Second, we measured the curvature of time-response histograms by calculating the deviation of the cumulative sum from a straight line; curvature has been used for over 50 years to quantify interval timing behavior[19,20]. Curvature indices are higher with more ‘curved’ time-response histograms. All behavioral data was tested for normality via the Lilliefors composite goodness of fit test (lillietest.m) prior to further analysis.
Transgenic Mice
This study used mice in which Cre-recombinase was driven by the D1 receptor promoter (Drd1a-cre+; derived from Gensat strain EY262; aged 3 months; 25–32 g), or littermate controls. Mice were bred and verified by genotyping using primers for D1-Cre recombinase transgene (D1-Cre-F: AGG GGC TGG GTG GTG AGT GAT TG, D1-Cre-R: CGC CGC ATA ACC AGT GAA ACA GC). Mice consumed 1.5–2 g of sucrose pellets during each behavioral session and additional food was provided 1–3 hours after each behavioral session in the home cage. Single housing and a 12 hour light/dark cycle were used; all experiments took place during the light cycle. Mice were maintained at ~85–90% of their free-access body weight during the course of these experiments for motivation. All procedures were approved by the Animal Care and Use Committee at the University of Iowa #4071105. A total of 12 mice were used for recording experiments (6 D1-Cre+ control mice for recording experiments, 6 D1-Cre+ mice with dopamine depletion) and a separate group of 36 mice for stimulation experiments: 6 control D1-Cre+ mice expressing ChR2 in the MFC with no dopamine depletion, 6 D1-Cre+ mice expressing ChR2 in the MFC with mesocortical depletion, 6 control D1-Cre+ mice expressing control virus in the MFC with no dopamine depletion, 6 control D1-Cre+ mice expressing control virus in the MFC with mesocortical depletion, and 6 wild-type mice with and 6 mice without mesocortical depletion for electrical stimulation.
Mice were trained to perform an interval-timing task with a 12 s interval according to methods described in detail previously (see Supplementary Methods) [26]. Time-response histograms were normalized to total responses to investigate timing independent of response rate. Temporally correct response and curvature index statistics were calculated from cumulative time-response histograms[20,26]. As above, all behavioral data was tested for normality. Response rates were compared between stimulation parameters via mixed-effects ANOVA; p values less than 0.05 were interpreted as statistically significant.
Mice trained in the 12 s interval-timing task were implanted with recording electrodes and an optical fiber (Microprobes) in the MFC [26]. Surgical procedures, neurophysiological recordings, neuronal analyses, and time-frequency analyses of mouse LFP and human EEG were conducted identical to methods described in detail previously [10,13,26,41,48]; see supplementary methods for details.
Optogenetics
We used an AAV construct with floxed inverted channelrhodopsin (AAV-ChR2) along with mCherry (UNC Viral Core; AAV5-EF1a-DIO-hChR2(H134R)-mCherry) [49]. When delivered to transgenic D1-Cre+ mice, Cre recombination leads to high expression driven by an EF-1a promoter selectively in neurons expressing D1DRs. Mice were injected with AAV-ChR2 into the prefrontal cortex (Mouse: AP: +1.8, ML −0.5, DV −1.5), with immediate placement of an optical fiber cannula (200 μm core, 0.22NA, Doric Lenses). The injection consisted of 0.5 μL of approximately 10 infectious particles per milliliter.
On testing days, D1-Cre+ mice with optical cannula were connected to the optical patch cable through Zirconia ferrule (Doric Lenses) without anesthesia. Light was generated from a 473 nm DPSS laser source (OEM Laser Systems) and an optical rotary joint (Doric Lenses) was used to facilitate animal rotation during performance of the interval-timing task. During testing, each mouse performed the fixed-interval timing task for 1 hr with light delivered with specific frequencies of stimulation. Specific frequencies of laser light were generated by TTL signal through microcontroller controlled by the operant behavior computer. In stimulation sessions, light was delivered from 0 to 12 s during the fixed-interval at 0, 2, and 20 Hz with pulse width 5 ms on randomly selected trials (33% for each condition; 0 Hz meant that the laser was off and no laser light was delivered). The power output of laser was adjusted to be 8 mW at the fiber tip before every experiment, power measurements verified that the laser reached 90% power within 0.74 ms of TTL triggers and maintained 8 mW with <5% error.
Histology
When experiments were complete, mice were anesthetized, sacrificed by injections of 100 mg/kg sodium pentobarbital. All mice were intracardially perfused with 4% paraformaldehyde. The brain was removed and post-fixed in paraformaldehyde overnight, and immersed in 30% sucrose until the brains sank. 50μm sections were made on cryostat (Leica) and store in PBS. Standard immunostaining procedures were performed in free-floating brain sections. Primary antibodies to Cre (mouse anti-Cre; Millipore-MAB 3120; 1:500), D1 receptor (rat anti-D1 dopamine receptor; Sigma-D2944; 1:200), tyrosine hydroxylase (rabbit anti-TH; Millpore-AB152; 1:500), Neurofilament (mouse anit-2H3; DSHB hybridoma-2H3; 1:100) were incubated overnight at 4 °C. Sections were visualized with Alexa Flour fluorescent secondary antibodies (goat anti-mouse IgG Alexa 633, goat anti-rat IgG Alexa 568, goat anti-rabbit IgG Alexa 488, and goat anti-mouse IgG Alexa 350; ThermoFisher; 1:1000) matched with the host primary by incubating for 2 hours at room temperature. Images were captured on Leica SP5 laser scanning confocal microscope or Zeiss Apotome.2 Axio Imager.
Supplementary Material
Highlights.
Humans and mice have dopamine-dependent delta rhythms in medial frontal cortex
Stimulating medial frontal neurons expressing D1DRs improves temporal processing
Delta stimulation of medial frontal neurons at 2 Hz can improve interval timing
Acknowledgments
This work was funded by The National Institute of Neurological Disorders and Stroke R01NS078100/K08 NS078100, The National Institute of Mental Health, NARSAD Young Investigator Grant from Brain & Behavior Foundation 22611, and grant #2014/22817-1, São Paulo Research Foundation (FAPESP).
Footnotes
Author Contributions Y.C.K and N.S.N designed the study. Y.C.K., S.W.H, R.R, S.L.A, and K.H.C conducted the experiments and analyzed the data; B.D.C analyzed some data; Y.C.K and N.S.N wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chaudhuri KR, Healy DG, Schapira AH. Non-motor symptoms of Parkinson’s disease: diagnosis and management. The Lancet Neurology. 2006;5:235–245. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- 2.Robbins TW, Cools R. Cognitive deficits in Parkinson’s disease: A cognitive neuroscience perspective. Mov Disord. 2014;29:597–607. doi: 10.1002/mds.25853. [DOI] [PubMed] [Google Scholar]
- 3.Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neurosci Biobehav Rev. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Forsaa EB, Larsen JP, Wentzel-Larsen T, Alves G. What predicts mortality in Parkinson disease? : a prospective population-based long-term study. Neurology. 2010;75:1270–1276. doi: 10.1212/WNL.0b013e3181f61311. [DOI] [PubMed] [Google Scholar]
- 5.Parker KL, Lamichhane D, Caetano MS, Narayanan NS. Executive dysfunction in Parkinson’s disease and timing deficits. Front Integr Neurosci. 2013;7:75. doi: 10.3389/fnint.2013.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown SW. Timing and executive function: Bidirectional interference between concurrent temporal production and randomization tasks. Memory & Cognition. 2006;34:1464–1471. doi: 10.3758/bf03195911. [DOI] [PubMed] [Google Scholar]
- 7.Malapani C, Rakitin B, Levy R, Meck WH, Deweer B, Dubois B, Gibbon J. Coupled temporal memories in Parkinson’s disease: a dopamine-related dysfunction. J Cogn Neurosci. 1998;10:316–331. doi: 10.1162/089892998562762. [DOI] [PubMed] [Google Scholar]
- 8.Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- 9.Narayanan NS, Cavanagh JF, Frank MJ, Laubach M. Common medial frontal mechanisms of adaptive control in humans and rodents. Nat Neurosci. 2013;16:1888–1897. doi: 10.1038/nn.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker KL, Chen K-H, Kingyon JR, Cavanagh JF, Naryanan NS. Medial frontal ~4 Hz activity in humans and rodents is attenuated in PD patients and in rodents with cortical dopamine depletion. J Neurophysiol. 2015 doi: 10.1152/jn.00412.2015. jn.00412.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narayanan NS, Land BB, Solder JE, Deisseroth K, DiLeone RJ. Prefrontal D1 dopamine signaling is required for temporal control. Proc Natl Acad Sci USA. 2012;109:20726–20731. doi: 10.1073/pnas.1211258109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J, Ghim JW, Lee JH, Jung MW. Neural correlates of interval timing in rodent prefrontal cortex. J Neurosci. 2013;33:13834–13847. doi: 10.1523/JNEUROSCI.1443-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker KL, Chen KH, Kingyon JR, Cavanagh JF, Narayanan NS. D1-Dependent 4 Hz Oscillations and Ramping Activity in Rodent Medial Frontal Cortex during Interval Timing. J Neurosci. 2014;34:16774–16783. doi: 10.1523/JNEUROSCI.2772-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker KL, Alberico SL, Miller AD, Narayanan NS. Prefrontal D1 dopamine signaling is necessary for temporal expectation during reaction time performance. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldman-Rakic PS, Muly EC, 3rd, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- 16.Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van Heertum R, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narayanan NS. Ramping activity is a cortical mechanism of temporal control of action. Curr Opin Behav Sci. 2016;8:226–230. doi: 10.1016/j.cobeha.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker KL, Ruggiero RN, Narayanan NS. Infusion of D1 Dopamine Receptor Agonist into Medial Frontal Cortex Disrupts Neural Correlates of Interval Timing. Front Behav Neurosci. 2015;9:294. doi: 10.3389/fnbeh.2015.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narayanan NS, Land BB, Solder JE, Deisseroth K, DiLeone RJ. Prefrontal D1 dopamine signaling is required for temporal control. PNAS. 2012;109:20726–20731. doi: 10.1073/pnas.1211258109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fry W, Kelleher RT, Cook L. A mathematical index of performance on fixed-interval schedules of reinforcement. J Exp Anal Behav. 1960;3:193–199. doi: 10.1901/jeab.1960.3-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker KL, Narayanan NS, Andreasen NC. The therapeutic potential of the cerebellum in schizophrenia. Front Syst Neurosci. 2014;8:163. doi: 10.3389/fnsys.2014.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavanagh JF, Zambrano-Vazquez L, Allen JJB. Theta lingua franca: A common mid-frontal substrate for action monitoring processes. Psychophysiol. 2012;49:220–238. doi: 10.1111/j.1469-8986.2011.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alberico SL, Cassell MD, Narayanan NS. The Vulnerable Ventral Tegmental Area in Parkinson’s Disease. Basal Ganglia. 2015;5:51–55. doi: 10.1016/j.baga.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cereb Cortex. 1998;8:321–345. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- 25.Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AFT. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 26.Narayanan NS, Land BB, Solder JE, Deisseroth K, DiLeone RJ. Prefrontal D1 dopamine signaling is required for temporal control. PNAS. 2012;109:20726–20731. doi: 10.1073/pnas.1211258109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chapin JK, Nicolelis MA. Principal component analysis of neuronal ensemble activity reveals multidimensional somatosensory representations. J Neurosci Methods. 1999;94:121–140. doi: 10.1016/s0165-0270(99)00130-2. [DOI] [PubMed] [Google Scholar]
- 28.Bekolay T, Laubach M, Eliasmith C. A spiking neural integrator model of the adaptive control of action by the medial prefrontal cortex. J Neurosci. 2014;34:1892–1902. doi: 10.1523/JNEUROSCI.2421-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narayanan NS, Laubach M. Delay activity in rodent frontal cortex during a simple reaction time task. J Neurophysiol. 2009;101:2859–2871. doi: 10.1152/jn.90615.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durstewitz D. Self-Organizing Neural Integrator Predicts Interval Times through Climbing Activity. J Neurosci. 2003;23:5342–5353. doi: 10.1523/JNEUROSCI.23-12-05342.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Witten I, Frank E. Data Mining. San Diego, CA: Academic Press; 2000. [Google Scholar]
- 32.Constantinidis C, Franowicz MN, Goldman-Rakic PS. Coding specificity in cortical microcircuits: a multiple-electrode analysis of primate prefrontal cortex. J Neurosci. 2001;21:3646–55. doi: 10.1523/JNEUROSCI.21-10-03646.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Markram H, Goodman PH, Berger TK, Ma J, Goldman-Rakic PS. Heterogeneity in the pyramidal network of the medial prefrontal cortex. Nat Neurosci. 2006;9:534–542. doi: 10.1038/nn1670. [DOI] [PubMed] [Google Scholar]
- 34.Gaspar P, Bloch B, Le Moine C. D1 and D2 receptor gene expression in the rat frontal cortex: cellular localization in different classes of efferent neurons. Eur J Neurosci. 1995;7:1050–1063. doi: 10.1111/j.1460-9568.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 35.Pogosyan A, Gaynor LD, Eusebio A, Brown P. Boosting cortical activity at Beta-band frequencies slows movement in humans. Curr Biol. 2009;19:1637–1641. doi: 10.1016/j.cub.2009.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci. 1998;18:1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 38.Malapani C, Deweer B, Gibbon J. Separating Storage from Retrieval Dysfunction of Temporal Memory in Parkinson’s Disease. Journal of Cognitive Neuroscience. 2002;14:311–322. doi: 10.1162/089892902317236920. [DOI] [PubMed] [Google Scholar]
- 39.Meck WH. Neuroanatomical localization of an internal clock: a functional link between mesolimbic, nigrostriatal, and mesocortical dopaminergic systems. Brain Res. 2006;1109:93–107. doi: 10.1016/j.brainres.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 40.Roberts S. Isolation of an internal clock. J Exp Psychol Anim Behav Process. 1981;7:242–268. [PubMed] [Google Scholar]
- 41.Land BB, Narayanan NS, Liu RJ, Gianessi CA, Brayton CE, Grimaldi MD, Sarhan M, Guarnieri DJ, Deisseroth K, Aghajanian GK, et al. Medial prefrontal D1 dopamine neurons control food intake. Nat Neurosci. 2014;17:248–253. doi: 10.1038/nn.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron. 2006;52:921–931. doi: 10.1016/j.neuron.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cools R, D’Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69:e113–125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seong HJ, Carter AG. D1 receptor modulation of action potential firing in a subpopulation of layer 5 pyramidal neurons in the prefrontal cortex. J Neurosci. 2012;32:10516–10521. doi: 10.1523/JNEUROSCI.1367-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends Cogn Sci (Regul Ed) 2014;18:414–421. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujisawa S, Buzsáki G. A 4 Hz oscillation adaptively synchronizes prefrontal, VTA, and hippocampal activities. Neuron. 2011;72:153–165. doi: 10.1016/j.neuron.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen K-H, Okerstrom KL, Kingyon JR, Anderson SW, Cavanagh JF, Narayanan NS. Startle Habituation and Midfrontal Theta Activity in Parkinson’s Disease. J Cogn Neurosci. 2016:1–11. doi: 10.1162/jocn_a_01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Narayanan NS, Rodnitzky RL, Uc EY. Prefrontal dopamine signaling and cognitive symptoms of Parkinson’s disease. Rev Neurosci. 2013;24:267–278. doi: 10.1515/revneuro-2013-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.