Abstract
Background
Few options beside avoidance of smoking and obesity are available to prevent pancreatic cancer. The association between aspirin use and risk of pancreatic cancer has been inconsistent across studies.
Methods
We performed a population-based study of 761 case and 794 control subjects frequency matched on sex and age during 2006–2011 in Shanghai, China. Participants were asked about episodes of regular use of aspirin, tablets per day or week, and ages that use started and stopped. Data were analyzed by unconditional logistic regression, with adjustments for age, sex, education, body-mass index, years of cigarette smoking, cigarettes smoked per day, Helicobacter pylori CagA seropositivity, ABO blood group, and history of diabetes mellitus. Meta-regression was carried out to summarize the literature.
Results
Ever-regular use of aspirin was associated with lowered risk of pancreatic cancer: odds ratio [OR] = 0.54; 95% CI, 0.40–0.73, P=10−4.2. Risk decreased 8% per each cumulative year of use: ORtrend = 0.92; 95% CI, 0.87–0.97; P=.0034. Across this and 18 published studies of this association, the OR for ever-regular use decreased with increasingly more recent mid-study year, for any aspirin type (Ptrend=10−5.1), and for low-dose aspirin (Ptrend=0.0014).
Conclusion
Regular use of aspirin thus appears to reduce risk of pancreatic cancer by almost half.
Impact
People who take aspirin for prevention of other diseases likely also reduce their risk of pancreatic cancer. Aside from benefits for both cardiovascular disease and certain cancers, long-term aspirin use entails some risks of bleeding complications which necessitates risk-benefit analysis for individual decisions about use.
Keywords: Aspirin, Case-control Studies, Pancreatic Cancer
Introduction
Pancreatic cancer is among the most fatal of all cancer types. By 2022, in the U.S., deaths from pancreatic cancer are expected to exceed all other cancer types except lung (1). While cigarette smoking and long-term obesity/diabetes mellitus are two avoidable causes of pancreatic cancer, most cases of the disease are apparently not preventable. Some conflicting evidence suggests that aspirin use may lower risk and in fact, our study in Connecticut found approximately 50% reduced risk with regular use of either low-dose or regular-dose aspirin (2). During 2006–2011, we carried out a second population-based case-control study of pancreatic cancer, in Shanghai, China, and thus sought to examine whether or not aspirin use was associated with risk in that population.
Materials and Methods
Our Shanghai study has been described in detail elsewhere (3). In brief: from December 2006–January 2011, in 37 Shanghai hospitals, we identified 1,241 newly diagnosed patients with pancreatic cancer and recruited 1,092 (88%), of whom 892 were confirmed to be eligible by review of pathology and clinical records. Patients were identified in each hospital by active, real-time surveillance of admissions for workup of possible pancreatic cancer, and were interviewed in-hospital, generally within 1–2 days of admission. Over the same years, we attempted to contact 1,529 age and sex frequency-matched potentially eligible control subjects randomly selected from the Shanghai Residents Registry, and interviewed 1,067 (70%) at-home. At the in-person questionnaire interviews, after receiving signed informed consent, we obtained venipuncture blood samples from 761 cases and 794 controls. Study subjects were questioned about various standard demographic, lifestyle and medical history factors, as well as specifically asked about all episodes of regular use of aspirin and other non-steroidal anti-inflammation medications (NSAIDs). A show-card listing names of medications of current and past availability was used to promote recall of dates, durations and frequencies of use. Regular use was considered to be use of at least one tablet per week for three months or longer. We did not distinguish regular-dose from low-dose aspirin preparations in recording subject responses. For laboratory analyses, we used commercial enzyme-linked immunosorbent assay kits to determine plasma seropositivity for CagA-positive Helicobacter pylori strain (Ravo Diagnostika p120, Alere GmbH, Köln, Germany) (4). ABO blood group was determined by custom TaqMan genotyping (Applied Biosystems, Inc., Foster City, California) of two functional SNPs, rs8176719 and rs8176746 (3). The study was approved by the human subjects review boards of the Shanghai Cancer Institute and Yale University.
We used unconditional logistic regression methods to estimate odds ratios (ORs) and their 95% confidence intervals (CIs). All analyses were adjusted for the continuous terms age at interview, education category, age-21 body mass index, years of cigarette smoking and number of cigarettes smoked per day, and indicator terms for sex, H. pylori CagA seropositivity, ABO blood group A vs non-A, and history of diabetes mellitus more than 3 years in the past. All P values are two-sided. For the calculation of trends in published odds ratios over calendar time, we used meta-regression of the log odds ratios (5,6). We generally followed the MOOSE guidelines (7) in searching the PubMed, Ovid and EMBASE databases for articles and abstracts on aspirin and cancer in any language published or in-press from the database start through July 25, 2016, as well as in reviewing the reference lists of the obtained articles for additional papers. We did not weight individual studies for quality. We evaluated the adequacy of the time-trend models by calculating, using the method-of-moments variance estimator, the adjusted R2, the proportion of between-study variance explained by the linear covariate (8).
Results
Various demographic and risk-factor characteristics of the cases and controls are presented in Table 1. The subjects were well matched on age at interview and sex. Cases on average had slightly but significantly higher age-21 body mass index than controls, were more likely to carry blood group A and less likely to be H. pylori CagA seropositive, and smoked more cigarettes per day. Cases had a higher frequency of past diagnosis of diabetes mellitus, especially within three years before interview, during which time such diagnoses are considered more likely than not to arise from the developing pancreatic cancers.
Table 1.
Characteristics of pancreatic cancer case patients and population control subjects in urban Shanghai, China, 2006–2011.
| Characteristic | No. of cases (%)a | No. of controls (%)a | Pb |
|---|---|---|---|
| Total number | 761 | 794 | |
| Age at interview, y | |||
| Mean (SD) | 64.9 (9.6) | 64.9 (9.9) | 0.99 |
| 35–49 | 59 (7.8) | 63 (7.9) | |
| 50–59 | 183 (24.0) | 193 (24.3) | |
| 60–69 | 231 (30.4) | 232 (29.2) | |
| 70–79 | 288 (37.8) | 306 (38.5) | |
| Sex | |||
| Male | 435 (57.2) | 460 (57.9) | 0.74 |
| Female | 326 (42.8) | 334 (42.1) | |
| Education | |||
| Primary school or lower | 146 (19.2) | 142 (17.9) | 0.21c |
| Middle or high school | 445 (58.5) | 498 (62.7) | |
| College or higher | 170 (22.3) | 154 (19.4) | |
| BMI at age 21 years, mean (SD) | |||
| Males | 20.6 (2.30) | 20.2 (2.02) | 10−4.4d |
| Females | 20.5 (2.50) | 19.9 (2.34) | |
| History of diabetes mellitus | |||
| Never | 581 (76.3) | 693 (87.3) | |
| Within previous 3 years | 80 (10.5) | 22 (2.8) | 10−10.1c |
| More than 3 years in the past | 100 (13.1) | 79 (9.9) | |
| Helicobacter pylori CagA seropositivity | |||
| No | 319 (41.9) | 257 (32.4) | 10−4.0 |
| Yes | 442 (58.1) | 537 (67.6) | |
| ABO blood groupe | |||
| O | 200 (26.3) | 250 (31.5) | |
| A | 289 (38.0) | 225 (28.3) | 10−3.6 |
| B | 193 (25.4) | 229 (28.8) | 0.70 |
| AB | 79 (10.4) | 90 (11.3) | 0.61 |
| Tobacco use | |||
| Never smoker | 428 (56.3) | 458 (57.7) | 0.55c |
| Former smoker | 97 (12.7) | 109 (13.7) | |
| Current smoker | 236 (31.0) | 227 (28.6) | |
| Cigarettes, years of smoking, mean (SD) | |||
| Among former smokers | 29.2 (14.5) | 30.2 (12.5) | 0.76d |
| Among current smokers | 36.5 (10.5) | 36.0 (10.5) | |
| Cigarettes, frequency per day, mean (SD) | |||
| Among former smokers | 16.7 (10.2) | 14.9 (9.3) | 0.029d |
| Among current smokers | 17.8 (9.2) | 16.1 (9.3) | |
Abbreviations: BMI, body mass index, weight/height2 (kg/m2); SD, standard deviation.
Values in the table are numbers (percentages) of participants unless indicated otherwise.
P values calculated by chi-square distribution for categorical variables (sex, education, Helicobacter pylori CagA seropositivity, ABO blood group—each group vs group O) and as trends by unconditional logistic regression for continuous variables (age at interview, body mass index at age 21 years, years of smoking, cigarette frequency per day).
P value based on 2 degrees of freedom for homogeneity of risk across three categories.
P value based on 2 degrees of freedom for simultaneous continuous trends in both strata.
For ABO group A vs non-A, the P value is 10–4.2.
Results for aspirin use among study subjects are given in Table 2. All but six of the 230 ever-users used aspirin at least daily. Ever regular use was associated with approximately 50% reduced risk of pancreatic cancer (P=10−4.2). A trend in decreasing risk with duration of use was evident (P=.0034). More than half of all use started within four years of interview. Compared to continuing use, quitting use of aspirin within the recent two years was associated with more than doubled risk of pancreatic cancer, comparable to the risk of never having used it. Magnitudes of associations among case subjects limited to local, regional or distant tumor stages at diagnosis were similar (Table 3). Ever regular use of aspirin was associated with lower risk in women (OR=0.42, 95% CI 0.26 to 0.67) than in men (OR=0.64, 95% CI 0.44 to 0.94); both associations were significant, but not significantly different from each other (P=.16). Analyses of all interviewed subjects, not just those who provided blood samples, yielded similar results (data not shown). Regular non-aspirin NSAID use was reported by only 12 subjects and was not analyzed.
Table 2.
Associations between regular use of aspirin and risk of pancreatic cancera
| Aspirin use | No. of case patientsb n=761 |
No. of control subjectsb n=794 |
OR (95% CI) | P |
|---|---|---|---|---|
| Ever use | ||||
| No | 674 | 651 | Ref. | |
| Yes | 87 | 143 | 0.54 (0.40–0.73) | 10−4.2 |
| Duration of use, y | 3.59 | 4.06 | 0.92 (0.87–0.97) | .0034 |
| Categories of use duration, y | ||||
| Never use | 674 | 651 | Ref. | |
| >0, <2 | 34 | 47 | 0.69 (0.43–1.10) | .12 |
| ≥2, <4 | 23 | 45 | 0.41 (0.24–0.69) | 10−3.0 |
| ≥4 | 30 | 51 | 0.53 (0.33–0.86) | .0099 |
| Time in past since starting use, y | 5.41 | 4.96 | 0.96 (0.92–1.00) | .034 |
| Categories of starting time in past, y | ||||
| Never use | 674 | 651 | Ref. | |
| >0, <2 | 23 | 37 | 0.58 (0.34–1.00) | .051 |
| ≥2, <4 | 22 | 43 | 0.44 (0.26–0.75) | .0027 |
| ≥4 | 42 | 63 | 0.59 (0.39–0.90) | .014 |
| Categories of ending time in past, y | ||||
| Never used | 674 | 651 | 2.30 (1.62–3.26) | 10−5.5 |
| Continuing current use | 57 | 113 | Ref. | |
| >0, <2 | 15 | 15 | 2.36 (1.06–5.25) | .035 |
| ≥2 | 15 | 15 | 2.06 (0.92–4.60) | .077 |
| Age at start of use, y | 62.7 | 64.4 | 0.90 (0.86–0.95)c | 10−4.5 |
| Categories of age at start of use, y | ||||
| Never used | 674 | 651 | Ref. | |
| >0, <60 | 33 | 43 | 0.68 (0.42–1.09) | .11 |
| ≥60, <70 | 32 | 52 | 0.55 (0.34–0.88) | .012 |
| ≥70 | 22 | 48 | 0.39 (0.23–0.68) | 10−3.1 |
| Age at end of use, y | 66.3 | 68.4 | 0.91 (0.87–0.95)c | 10−4.6 |
| Categories of age at end of use, y | ||||
| Never used | 674 | 651 | Ref. | |
| >0, <60 | 19 | 31 | 0.53 (0.29–0.97) | .039 |
| ≥60, <70 | 33 | 42 | 0.69 (0.42–1.11) | .13 |
| ≥70 | 35 | 70 | 0.45 (0.29–0.70) | 10 3.3 |
Unconditional logistic regression models were used to obtain the odds ratios (ORs) and 95% confidence intervals (CIs). All models were adjusted for age at interview (continuous), sex, education category (continuous), body mass index at age 21 (continuous), years of cigarette smoking (continuous), number of cigarettes per day (continuous), H. pylori CagA seropositivity, ABO blood group A vs non-A, and history of diabetes mellitus more than 3 years in the past. Each row in the table is a separate adjusted model.
Numbers of subjects for the category variables. For the duration variables, these columns give the mean durations among aspirin ever users; the ORs are per one year of duration, and the P-values represent trend associations.
Odds ratio and confidence limits per a 10-year difference in age among aspirin ever users.
Table 3.
Associations between regular use of aspirin and risk of pancreatic cancer, according to stage of disease and gendera
| Aspirin use | No. of case patientsb n=761 |
No. of control subjectsb n=794 |
OR (95% CI) | P |
|---|---|---|---|---|
| Local stage case patients vs controls: | ||||
| Ever use | ||||
| No | 90 | 651 | Ref. | |
| Yes | 13 | 143 | 0.61 (0.33–1.15) | .13 |
| Duration of use, y | 2.04 | 4.06 | 0.81 (0.67–0.98) | .031 |
| Regional stage case patients vs controls: | ||||
| Ever use | ||||
| No | 386 | 651 | Ref. | |
| Yes | 52 | 143 | 0.56 (0.39–0.80) | .0013 |
| Duration of use, y | 3.65 | 4.06 | 0.92 (0.86–0.99) | .019 |
| Distant stage case patients vs controls: | ||||
| Ever use | ||||
| No | 198 | 651 | Ref. | |
| Yes | 22 | 143 | 0.46 (0.28–0.75) | .0020 |
| Duration of use, y | 4.36 | 4.06 | 0.93 (0.85–1.02) | .11 |
| Male subjects: | ||||
| Ever use | ||||
| No | 378 | 377 | Ref. | |
| Yes | 57 | 83 | 0.64 (0.44–0.94) | .023 |
| Duration of use, y | 3.68 | 4.23 | 0.93 (0.87–1.00) | .045 |
| Female subjects: | ||||
| Ever use | ||||
| No | 296 | 274 | Ref. | |
| Yes | 30 | 60 | 0.42 (0.26–0.67) | 10−3.5 |
| Duration of use, y | 3.41 | 3.82 | 0.89 (0.80–0.99) | .028 |
Unconditional logistic regression models were used to obtain the odds ratios (ORs) and 95% confidence intervals (CIs). All models were adjusted for age at interview (continuous), sex, education category (continuous), body mass index at age 21 (continuous), years of cigarette smoking (continuous), number of cigarettes per day (continuous), H. pylori CagA seropositivity, ABO blood group A vs non-A, and history of diabetes mellitus more than 3 years in the past. Each row in the table is a separate adjusted model.
Numbers of subjects for the category variables. For the duration variables, these columns give the mean durations among aspirin ever users; the ORs are per one year of duration, and the P-values represent trend associations.
Discussion
The pattern of risk associations in Chinese subjects in Shanghai as seen here is remarkably similar to that in our Connecticut study (2). Both studies demonstrated about 50% reduced risk with ever use of aspirin, as well as some evidence of trends of decreasing risk with increasing durations of use. Typical durations of use were shorter in Shanghai than Connecticut; nevertheless, the relative reductions in risk were comparable. Both studies also showed that people who quit using aspirin in the recent two years had about 2–3 fold higher risks compared to individuals continuing on the medication, and that never-users had about double the risk of current users. These observations suggest that aspirin use may be associated with decreased risk of pancreatic cancer, as well as that individuals with developing pancreatic cancer may become increasingly less tolerant of aspirin and thus more likely to terminate use a little before diagnosis. In spite of this possible dual relationship, long-term aspirin use or use 5–10 years or more in the past has been associated with reduced risk (2). Because most observed associations with reduced risk have been for use within a decade of diagnosis, aspirin use may be inferred to slow tumor development rather than prevent initial tumor occurrence (9).
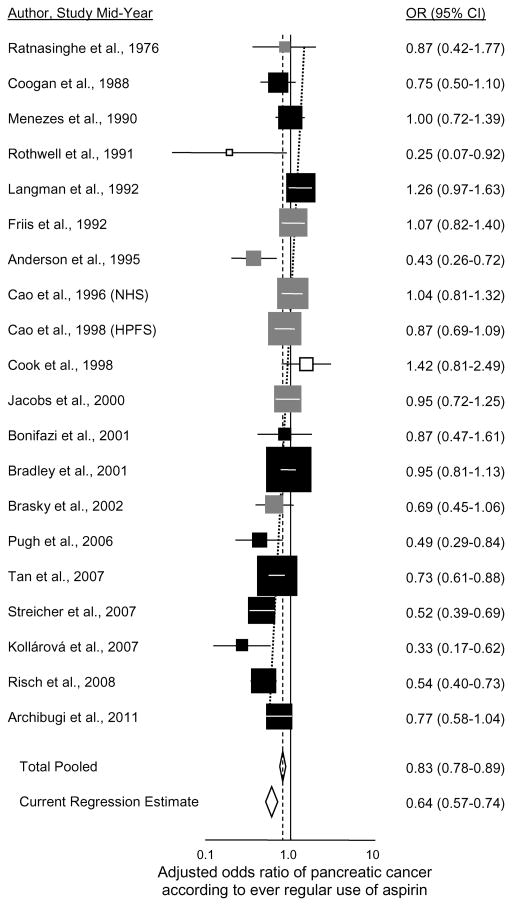
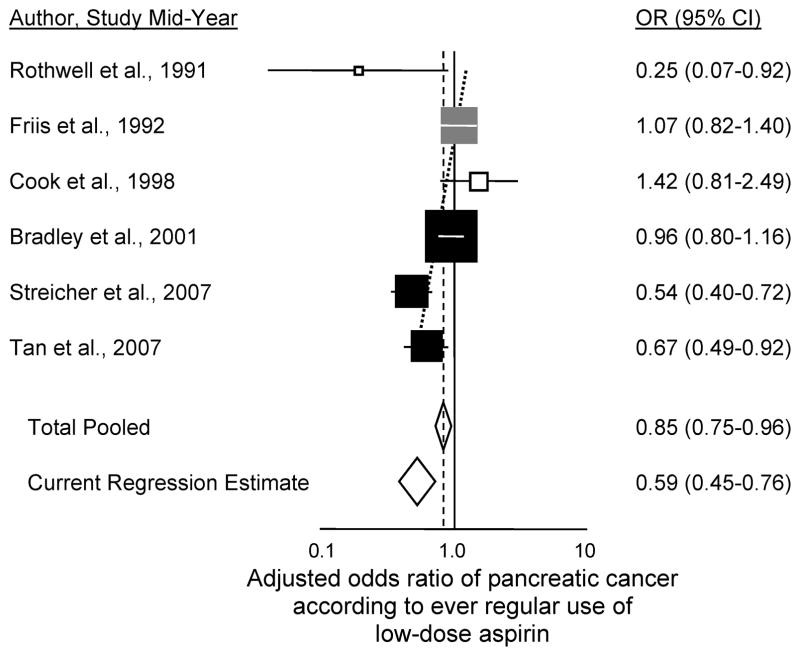
In examining the literature in order to calculate meta-analysis, we found eighteen other studies that have investigated aspirin use and risk of pancreatic cancer (Supplementary Table 1; plotted in Figure 1) (2,10–26). The summary association (OR=0.83; 95% CI 0.78 to 0.89) was not substantially different for the five studies having data for males (OR=0.78; 95% CI 0.67 to 0.90) (2,17,18,23 and the present study) or the eight with data for females (OR=0.84; 95% CI 0.73 to 0.97) (2,10,17,18,20,23,25 and the present study). Among all of the published studies, six have shown significantly reduced risk with use (2,10–14). Of the twelve others (15–26), many of those that began accruing patients in the 1990s or later (and thus that were conducted after the general population introduction of aspirin for cardioprophylaxis) show nonsignificant reduced risk with use. In Figure 1, a trend in the odds ratio for ever regular use of aspirin is evident according to increasingly more recent mid-point of when the aspirin exposures were ascertained in the study, with decline in odds ratio of 2.3% per year (95% CI 1.3% to 3.3%, P=10−5.1; adjusted R2=91%). For a recent study conducted with mid-point in 2011, the predicted odds ratio = 0.64 (95% CI 0.57 to 0.74, P=10−10.2). A similar trend in odds ratio is seen for the six studies specifically examining ever regular use of low-dose aspirin (Figure 2) (2,11,12,18,20,21), with decline in odds ratio of 3.6% per year (95% CI 1.4% to 5.8%, P=0.0014; adjusted R2=84%). The predicted odds ratio for a recent study conducted with exposure mid-point in 2011 would be 0.59 (95% CI 0.45 to 0.76, P=10−4.3). On average, odds ratios for regular use of aspirin and risk of pancreatic cancer have been declining over the past two decades as the general population use of aspirin has increased. We also examined trends in odds ratios according to year of study publication and the results were stronger and more significant, but we felt that mid-year of study accrual better represented the time period of aspirin exposure. Finally, we found similar significant decreasing trends in the odds ratios over time for both ever use of any aspirin and ever use of low-dose aspirin when analyses were limited to case-control studies and to case-control studies involving aspirin information obtained from clinical records rather than subject interviews (data not shown).
Figure 1.
Adjusted odds ratio of pancreatic cancer according to ever regular use of aspirin. Squares are plotted descending in order of increasing mid-years of study subject aspirin-use ascertainment. Black squares denote case-control studies; gray squares cohort studies; white squares randomized controlled trials or their extensions; diamonds denote summary estimates. Risch et al., 2008 refers to the current study. Horizontal lines in each square represent the 95% CI, and the area of each square is proportional to its weight in the analyses. The diagonal dotted line is the regression line of the log odds ratio according to study mid-year, calculated by inverse variance-weighted linear regression of the log odds ratios (8). The Current Regression Estimate is the regression odds ratio predicted at the mid-point of the most recent study (2011).
Figure 2.
Adjusted odds ratio of pancreatic cancer according to ever regular use of low-dose aspirin. Squares are plotted descending in order of increasing mid-years of study subject aspirin-use ascertainment. Black squares denote case-control studies; gray squares cohort studies; white squares randomized controlled trials or their extensions; diamonds denote summary estimates. Horizontal lines in each square represent the 95% CI, and the area of each square is proportional to its weight in the analyses. The diagonal dotted line is the regression line of the log odds ratio according to study mid-year, calculated by inverse variance-weighted linear regression of the log odds ratios (8). The Current Regression Estimate is the regression odds ratio predicted at the mid-point of what would be a recent study as in Figure 1 (2011).
While our study results and the declining odds ratio trends in the literature provide evidence for a beneficial effect of aspirin use on risk of pancreatic cancer, some limitations of this work should be considered. In our Shanghai study, we ascertained aspirin use by self-report, which could have inherent differences between cases and controls, both in our study and in other similar ones. In case-control studies, given that cases generally tend to overreport past exposures relative to controls (27), such differential reporting would not be likely to explain the reduced risks seen here. Evidence of benefit from cohort (10,18,19,22,23,25), randomized trial (12,20) and case-control (13,21,24) studies in which aspirin use information was obtained from clinical databases is weaker but still suggestive, though these studies are mostly older, and the cohort and case-control studies do not reflect more current population frequencies of aspirin use, in which lower odds ratios are seen. Aspirin is an over-the-counter medication for which use may not be well-characterized by standard clinical database information. Analyses of more recent follow-up periods in the cohort studies will be helpful in determining the magnitude of benefit of aspirin use. Finally, various physiological mechanisms for risk reduction with aspirin use have been suggested, and while a number of these mechanisms are plausible, none has yet been convincingly established. We have discussed these mechanisms at length elsewhere (2).
In conclusion, we observed a significant inverse relationship between aspirin use and risk of pancreatic cancer in a large representative sample of Chinese individuals. The pattern of risk reduction was very similar to that seen in other recent studies in the US and elsewhere. While the choice to use aspirin for disease prophylaxis generally depends upon evaluated risks of cardiovascular disease, colorectal cancer, etc., it is likely that such use at least does not increase risk of pancreatic cancer, and very probably appreciably lowers it.
Supplementary Material
Acknowledgments
Financial Support: Supported by grants from the National Cancer Institute (R01 CA114421, to H. A. Risch, H. Yu, and Y.-T. Gao; F31 CA177153, to S. A. Streicher) and by grants from the Science and Technology Commission of Shanghai Municipality (08411954100, to J. Wang, W. Zheng and Y.-T. Gao) and from the Shanghai Cancer Institute (SB10-06, to J. Wang, W. Zheng and Y.-T. Gao).
The authors thank the staff of the 37 Shanghai hospitals for their support in case reporting and recruitment, the review panel clinicians and pathologists for their thorough case evaluations, and Mrs. Lu Sun and the other project staff of the case-control study for their invaluable dedication to the study. The authors assume full responsibility for the analyses and the interpretation of the study data. No funders of the study had any involvement in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Abbreviations
- BMI
body mass index (weight/height2, kg/m2)
- CagA
Helicobacter pylori cytotoxin-associated gene A
- CI
confidence interval
- NSAID
non-steroidal anti-inflammation medication
- OR
odds ratio
Footnotes
Disclosures: There were no apparent or real conflicts of interest for any of the authors.
References
- 1.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 2.Streicher SA, Yu H, Lu L, Kidd MS, Risch HA. Case-control study of aspirin use and risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2014;23:1254–63. doi: 10.1158/1055-9965.EPI-13-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Risch HA, Lu L, Wang J, Zhang W, Ni Q-X, Gao Y-T, et al. ABO blood group and risk of pancreatic cancer: a study in Shanghai and meta-analysis. Am J Epidemiol. 2013;177:1326–37. doi: 10.1093/aje/kws458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Risch HA, Lu L, Kidd MS, Wang J, Zhang W, Ni Q, et al. Helicobacter pylori seropositivities and risk of pancreatic carcinoma. Cancer Epidemiol Biomarkers Prev. 2014;23:172–8. doi: 10.1158/1055-9965.EPI-13-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker WL, White CM, Cappelleri JC, Kluger J, Coleman CI Health Outcomes, Policy, and Economics (HOPE) Collaborative Group. Understanding heterogeneity in meta-analysis: the role of meta-regression. Int J Clin Pract. 2009;63:1426–34. doi: 10.1111/j.1742-1241.2009.02168.x. [DOI] [PubMed] [Google Scholar]
- 6.Higgins JPT, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med. 2004;23:1663–82. doi: 10.1002/sim.1752. [DOI] [PubMed] [Google Scholar]
- 7.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 8.Harbord RM, Higgins JPT. Meta-regression in Stata. Stata J. 2008;8:493–519. [Google Scholar]
- 9.Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–7. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson KE, Johnson TW, Lazovich D, Folsom AR. Association between nonsteroidal anti-inflammatory drug use and the incidence of pancreatic cancer. J Natl Cancer Inst. 2002;94:1168–71. doi: 10.1093/jnci/94.15.1168. [DOI] [PubMed] [Google Scholar]
- 11.Tan XL, Reid Lombardo KM, Bamlet WR, Oberg AL, Robinson DP, Anderson KE, et al. Aspirin, nonsteroidal anti-inflammatory drugs, acetaminophen, and pancreatic cancer risk: a clinic-based case-control study. Cancer Prev Res. 2011;4:1835–41. doi: 10.1158/1940-6207.CAPR-11-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 13.Pugh TFG, Little M, Carey F, Metcalfe M, Robinson R, Clark A, et al. Aspirin, NSAIDS, calcium-channel blockers and statins in the aetiology of pancreatic cancer: preliminary results from a case-control study in two centres in the UK. Gut. 2011;60(Suppl 1):A81. [Google Scholar]
- 14.Kollárová H, Azeem K, Tomášková H, Procházka V, Martínek A, Shonová O, et al. Zdravotní stav a karcinom pankreatu [Health status and pancreatic cancer] Gastroenterol Hepatol (Prague) 2013;67:154–61. [Google Scholar]
- 15.Bonifazi M, Gallus S, Bosetti C, Polesel J, Serraino D, Talamini R, et al. Aspirin use and pancreatic cancer risk. Eur J Cancer Prev. 2010;19:352–4. doi: 10.1097/CEJ.0b013e32833b48a4. [DOI] [PubMed] [Google Scholar]
- 16.Coogan PF, Rosenberg L, Palmer JR, Strom BL, Zauber AG, Stolley PD, et al. Nonsteroidal anti-inflammatory drugs and risk of digestive cancers at sites other than the large bowel. Cancer Epidemiol Biomarkers Prev. 2000;9:119–23. [PubMed] [Google Scholar]
- 17.Menezes RJ, Huber KR, Mahoney MC, Moysich KB. Regular use of aspirin and pancreatic cancer risk. BMC Public Health. 2002;2:18. doi: 10.1186/1471-2458-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friis S, Sorensen HT, McLaughlin JK, Johnsen SP, Blot WJ, Olsen JH. A population-based cohort study of the risk of colorectal and other cancers among users of low-dose aspirin. Br J Cancer. 2003;88:684–8. doi: 10.1038/sj.bjc.6600760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ratnasinghe LD, Graubard BI, Kahle L, Tangrea JA, Taylor PR, Hawk E. Aspirin use and mortality from cancer in a prospective cohort study. Anticancer Res. 2004;24:3177–84. [PubMed] [Google Scholar]
- 20.Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Low-dose aspirin in the primary prevention of cancer. The women’s health study: A randomized controlled trial. JAMA. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 21.Bradley MC, Hughes CM, Cantwell MM, Napolitano G, Murray LJ. Non-steroidal anti-inflammatory drugs and pancreatic cancer risk: a nested case-control study. British J Cancer. 2010;102:1415–21. doi: 10.1038/sj.bjc.6605636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobs EJ, Newton CC, Gapstur SM, Thun MJ. Daily aspirin use and cancer mortality in a large US cohort. J Natl Cancer Inst. 2012;104:1208–17. doi: 10.1093/jnci/djs318. [DOI] [PubMed] [Google Scholar]
- 23.Cao Y, Nishihara R, Wu K, Wang M, Ogino S, Willett WC, et al. Population-wide impact of long-term use of aspirin and the risk for cancer. JAMA Oncol. 2016;2:762–9. doi: 10.1001/jamaoncol.2015.6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langman MJ, Cheng KK, Gilman EA, Lancashire RJ. Effect of anti-inflammatory drugs on overall risk of common cancer: case-control study in general practice research database. BMJ. 2000;320:1642–6. doi: 10.1136/bmj.320.7250.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brasky TM, Liu J, White E, Peters U, Potter JD, Walter RB, et al. Non-steroidal anti-inflammatory drugs and cancer risk in women: results from the Women’s Health Initiative. Int J Cancer. 2014;135:1869–83. doi: 10.1002/ijc.28823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Archibugi L, Maisonneuve P, Piciucchi M, Valente R, Delle Fave G, Capurso G. Statins but not aspirin nor their combination have a chemopreventive effect on pancreatic cancer occurrence. Pancreas. 2015;44:1359. [Google Scholar]
- 27.Weinstock MA, Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE. Recall (report) bias and reliability in the retrospective assessment of melanoma risk. Am J Epidemiol. 1991;133:240–5. doi: 10.1093/oxfordjournals.aje.a115868. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.