Abstract
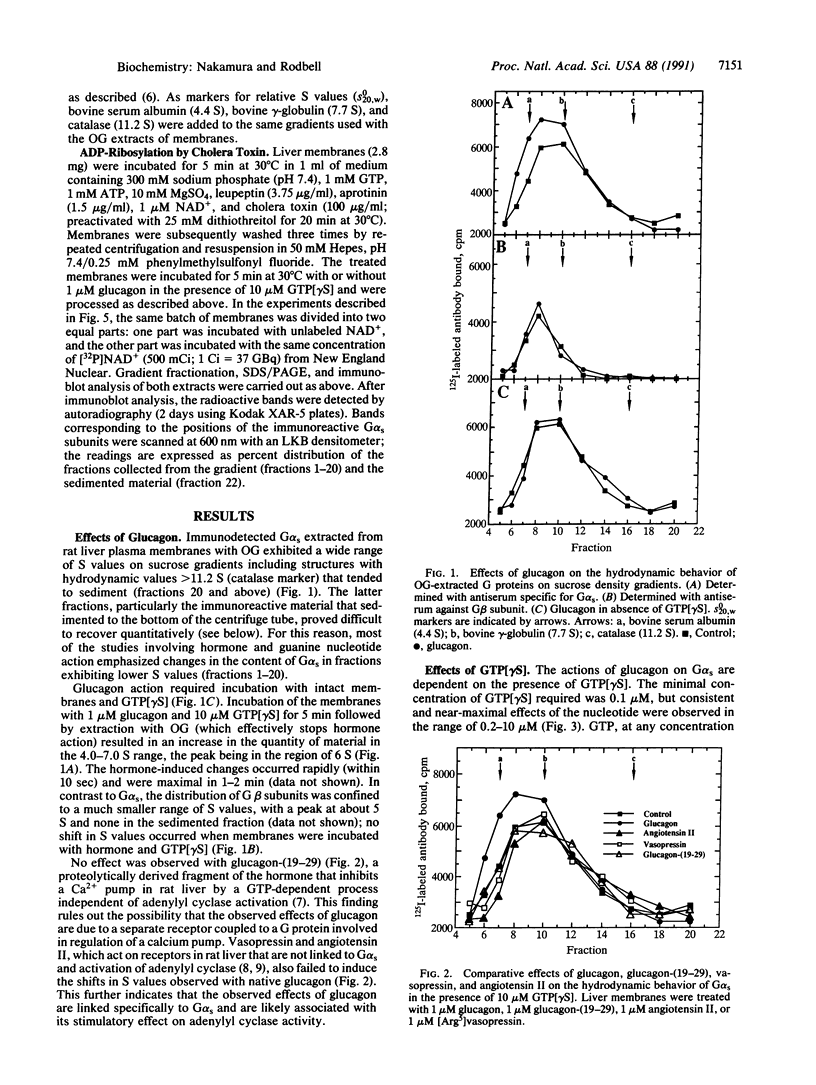
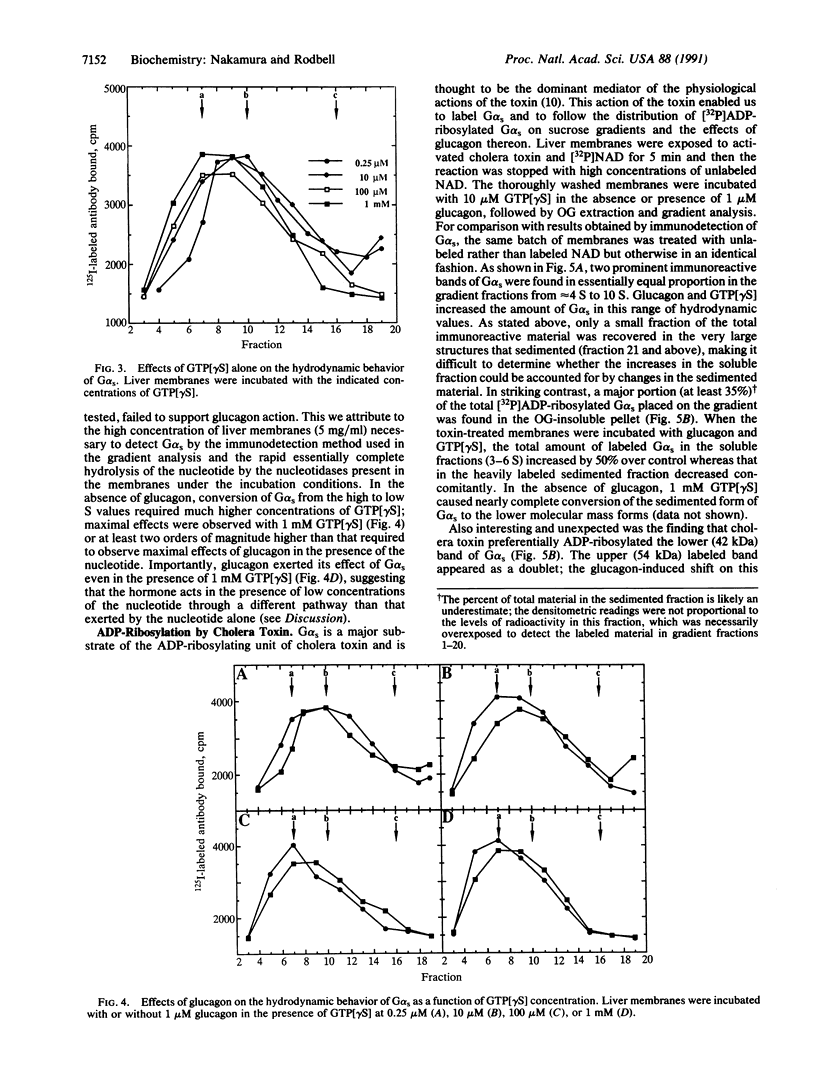
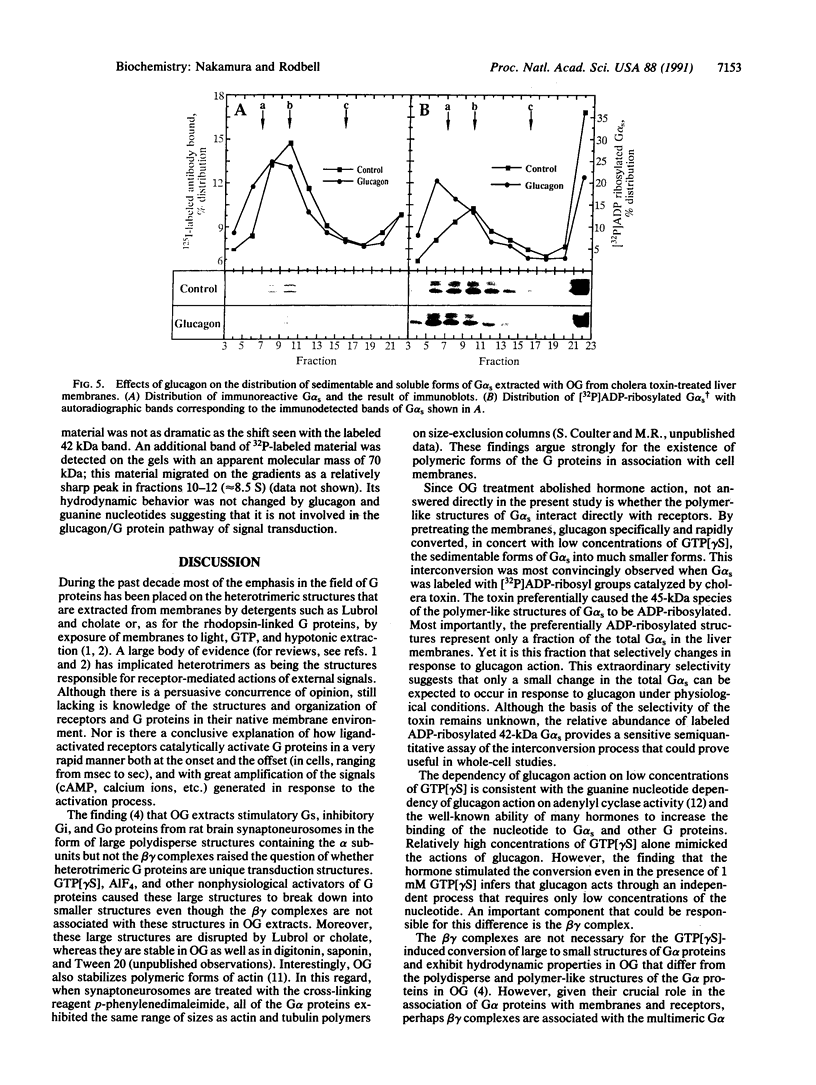
The hydrodynamic behavior of G alpha s, the alpha subunit of the stimulatory guanine nucleotide-binding regulatory protein (G protein), in octyl glucoside extracts of rat liver membranes was investigated. As was previously shown for G proteins similarly extracted from brain synaptoneurosomes, G alpha s behaved as polydisperse structures with S values higher than that of heterotrimeric G proteins. At concentrations of guanosine 5'-[gamma-thio]triphosphate (GTP[gamma S]) greater than 100 microM, incubation with membranes led to smaller structures having S values in the range of 4-5 S. Incubation of liver membranes with glucagon also caused a marked increase in structures having these S values; glucagon action required the presence of low concentrations of GTP[gamma S] (maximal, 10 microM), was rapid (within 10 sec), and was not observed with vasopressin, angiotensin II, or glucagon-(19-29). When G alpha s in its membrane-bound form was [32P]ADP-ribosylated by cholera toxin and the treated membranes were extracted with octyl glucoside, greater than 35% of the labeled G alpha s was found in material that sedimented through sucrose gradients and contained relatively low levels of immunoreactive G alpha s. Glucagon selectively converted the apparently large molecular weight structures to the 4-5 S structures in the presence of GTP[gamma S], even at 1 mM (the maximal effect of the nucleotide alone), when incubated with the toxin-treated membranes. These findings suggest that the glucagon receptor selectively interacts with polymer-like structures of G alpha s and that activation by GTP[gamma S] results in disaggregation. The role of the beta and gamma subunits of G proteins in the hormone-induced process is not clear since the polymer-like structures extracted with octyl glucoside are devoid of beta and gamma subunits.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaumer L. Transduction of receptor signal into modulation of effector activity by G proteins: the first 20 years or so .... FASEB J. 1990 Nov;4(14):3178–3188. doi: 10.1096/fasebj.4.14.2172060. [DOI] [PubMed] [Google Scholar]
- Bouscarel B., Augert G., Taylor S. J., Exton J. H. Alterations in vasopressin and angiotensin II receptors and responses during culture of rat liver cells. Biochim Biophys Acta. 1990 Dec 10;1055(3):265–272. doi: 10.1016/0167-4889(90)90042-c. [DOI] [PubMed] [Google Scholar]
- Gill D. M. Mechanism of action of cholera toxin. Adv Cyclic Nucleotide Res. 1977;8:85–118. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Heyworth C. M., Whetton A. D., Wong S., Martin B. R., Houslay M. D. Insulin inhibits the cholera-toxin-catalysed ribosylation of a Mr-25000 protein in rat liver plasma membranes. Biochem J. 1985 Jun 15;228(3):593–603. doi: 10.1042/bj2280593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunimoto M., Shibata K., Miura T. Comparison of the cytoskeleton fractions of rat red blood cells prepared with non-ionic detergents. J Biochem. 1989 Feb;105(2):190–195. doi: 10.1093/oxfordjournals.jbchem.a122638. [DOI] [PubMed] [Google Scholar]
- Lotersztajn S., Pavoine C., Brechler V., Roche B., Dufour M., Le-Nguyen D., Bataille D., Pecker F. Glucagon-(19-29) exerts a biphasic action on the liver plasma membrane Ca2+ pump which is mediated by G proteins. J Biol Chem. 1990 Jun 15;265(17):9876–9880. [PubMed] [Google Scholar]
- Nakamura S., Rodbell M. Octyl glucoside extracts GTP-binding regulatory proteins from rat brain "synaptoneurosomes" as large, polydisperse structures devoid of beta gamma complexes and sensitive to disaggregation by guanine nucleotides. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6413–6417. doi: 10.1073/pnas.87.16.6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittner R. A., Fain J. N. Vasopressin transiently stimulates phospholipase C activity in cultured rat hepatocytes. Biochim Biophys Acta. 1989 Feb 9;1010(2):227–232. doi: 10.1016/0167-4889(89)90165-1. [DOI] [PubMed] [Google Scholar]
- Rodbell M., Birnbaumer L., Pohl S. L., Krans H. M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. V. An obligatory role of guanylnucleotides in glucagon action. J Biol Chem. 1971 Mar 25;246(6):1877–1882. [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Schlegel W., Kempner E. S., Rodbell M. Activation of adenylate cyclase in hepatic membranes involves interactions of the catalytic unit with multimeric complexes of regulatory proteins. J Biol Chem. 1979 Jun 25;254(12):5168–5176. [PubMed] [Google Scholar]
- Vaillancourt R. R., Dhanasekaran N., Johnson G. L., Ruoho A. E. 2-Azido-[32P]NAD+, a photoactivatable probe for G-protein structure: evidence for holotransducin oligomers in which the ADP-ribosylated carboxyl terminus of alpha interacts with both alpha and gamma subunits. Proc Natl Acad Sci U S A. 1990 May;87(10):3645–3649. doi: 10.1073/pnas.87.10.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Goldstein L. S. One motor, many tails: an expanding repertoire of force-generating enzymes. Cell. 1990 Mar 23;60(6):883–885. doi: 10.1016/0092-8674(90)90334-b. [DOI] [PubMed] [Google Scholar]
- Wessling-Resnick M., Johnson G. L. Allosteric behavior in transducin activation mediated by rhodopsin. Initial rate analysis of guanine nucleotide exchange. J Biol Chem. 1987 Mar 15;262(8):3697–3705. [PubMed] [Google Scholar]




