Abstract
This perspective paper proposes that endogenous apoplastic fructans in fructan accumulating plants, released after stress-mediated cellular leakage, or increased by exogenous application, can act as damage-associated molecular patterns (DAMPs), priming plant innate immunity through ancient receptors and defense pathways that most probably evolved to react on microbial fructans acting as microbe-associated molecular patterns (MAMPs). The proposed model is placed in an evolutionary perspective. How this type of DAMP signaling may contribute to cross-tolerance and multistress resistance effects in plants is discussed. Besides apoplastic ATP, NAD and fructans, apoplastic polyamines, secondary metabolites, and melatonin may be considered potential players in DAMP-mediated stress signaling. It is proposed that mixtures of DAMP priming formulations hold great promise as natural and sustainable alternatives for toxic agrochemicals.
Keywords: DAMP, fructan, immunity, signaling, stress, tolerance
Introduction: Damp Signaling in Plants and in Animals
Throughout their lifecycle, plants are prone to different sorts of stresses, many of which cause cellular rupture. In case of biotic stress, the recognition of molecular patterns from microbes (microbe-associated molecular patterns, MAMPs) or herbivores (herbivore-associated molecular patterns, HAMPs) is well-known, especially for MAMPs. However, more recently the importance of damaged-self recognition has come to light. The manuscript by Duran-Flores and Heil (2016) highlights the significance of DAMPs (damage-associated molecular patterns) in response to cellular disruption. The role of DAMPs in animals has been proposed as the so-called danger model (Matzinger, 1994). Different molecules were proposed as DAMPs, including extracellular ATP and mitochondrial DNA (Krysko et al., 2011; Crišan et al., 2016). Recently, Martin (2016) proposed members of the IL-1 (interleukin 1) family of cytokines as the canonical DAMPs in animals, indicating how well-studied molecular structures can have a yet unknown function as DAMPs. However, research on DAMP signaling in plants is still in its infancy, although DAMP-mediated signaling was proposed as one of the central players in plant defense priming (Martinez-Medina et al., 2016). As of late, Heil (2012), Heil and Land (2014), Heil et al. (2016) have put the debate on DAMPs into the spotlight by discussing evolutionary benefits as well as features ascribable to DAMPs.
Sugars as Damps: the Case of Plant Fructans
In their latest manuscript, Duran-Flores and Heil (2016) include sucrose (Suc), a central transport and signaling sugar in plants (Smeekens and Hellmann, 2014) for the first time as a DAMP in their scheme, associated with plant defense responses. The recently launched “sweet immunity” concept attempts to explain the role of (sweet) small sugars, and by extension, less sweet carbohydrates with a higher degree of polymerization (DP) in plant innate immunity responses. Considering biotic stress responses, small metabolic sugars are not only a possible food source for the pathogen, but can act as signaling molecules to induce plant defense response (Bolouri Moghaddam and Van den Ende, 2012, 2013), with a central role for the SnRK1 energy sensor (Van den Ende and El-Esawe, 2014; Hulsmans et al., 2016).
Fructans are polysaccharides synthesized in the vacuole of 15% of flowering species (Van den Ende et al., 2004). Fructose moieties are added to Suc by various fructosyltransferases. Different types of fructans are found in plants, depending on the linkage type and branching, including inulins, levans, graminans, and neokestose-type inulins and levans as well as complex, mixed-type fructans from Agave sp., the agavins (Mancilla-Margalli and López, 2006; Valluru and Van den Ende, 2008; Van den Ende, 2013).
Here, we propose a possible role of fructans as DAMPs in fructan accumulating plants. Livingston and Henson (1998) detected an increase in apoplastic fructan content after subzero acclimation in oat (Avena sativa). Their presence in the apoplastic environment after a stress event may suggest a possible role as DAMPs. Recently, it was found that short inulin-type fructans (fructooligosaccharides, FOS) from Arctium lappa or burdock (burdock fructooligosaccharides, BFO) prime plant defenses in different pathosystems. (Wang et al., 2009; Zhang et al., 2009; Sun et al., 2013). Priming, a process believed to occur at the expense of minimal amounts of ATP, brings plants in a “ready-to-go” status, preparing them for a faster and stronger response to future (a)biotic stresses (Conrath, 2015).
Bacterial Fructans Acting as Mamps in Plants?
Although, the above-mentioned plant fructan priming function may involve DAMP signaling in fructan accumulating plants, it is important to realize that fructans are also present in bacteria and fungi. While levan-type fructans are widespread in microorganisms, inulin-type fructans are only found in certain genera of gram-positive bacteria (Toksoy et al., 2016 and references therein). Genera such as Lactobacillus and Streptococcus produce levans extracellularly. In Lactobacillus, production of either levans or inulins has been found in related strains (Ozimek et al., 2006; Anwar et al., 2010). Fructans increase virulence of pathogenic species through mechanisms such as biofilm formation and Ca2+-chelation to suppress host defenses, as reported in Erwinia amylovora (Koczan et al., 2009; Ordax et al., 2010; Ichinose et al., 2013).
Importantly, the DP of these bacterial fructans is much higher than those occurring in plant fructans (Toksoy et al., 2016). Thus, bacterial fructans are likely immobile within the plant cell wall. More likely, FOS derived from their (partial) hydrolysis by plant apoplastic fructan exohydrolases (FEHs) (Van den Ende et al., 2004) may readily diffuse through the plant apoplast to trigger potential defense-related receptors present in the plant plasma membrane (PM). As such, bacterial FOS may be recognized as MAMPs in plants, sensed by so far unidentified receptors.
A Possible Comparison With Fructan-Mediated Immune Signaling in Animals?
Referring to the situation in animals and humans, inulin-type fructans, besides indirectly activating microorganisms in the colon, are believed to be directly recognized by host receptors in the gut system, such as toll-like receptors 2 and 4 (TLR2 and TLR4) (Vogt et al., 2013; Peshev and Van den Ende, 2014; Franco-Robles and López, 2015). This primes innate immunity and contributes to better health. Fructans interact with a lower affinity with TLR2 and TLR4 as compared to bacterial lipo-oligosaccharides (LPS) (Takeuchi et al., 1999).
So far, most research is focused on inulin-type fructans derived from chicory (Cichorium intybus), but other types of plant fructans such as agavins (Agave tequilana, López-Velázquez et al., 2015) and graminans (cereals, Verspreet et al., 2015) are under study. Dietary fructans are degraded by fructan-degrading enzymes from microbes in the colon, since animals lack fructan-degrading enzymes (Capitán-Cañadas et al., 2014; Peshev and Van den Ende, 2014). Dietary supplements of bacterial levans are also known to improve growth and defense responses in different animal species (Li and Kim, 2013; Huang et al., 2015). Anti-tumor and immunomodulatory effects have been ascribed to some bacterial levans (Yoo et al., 2004; Xu et al., 2006). Since animals and humans lack enzymes that can biosynthesize fructans, fructans cannot act as DAMPs. It can be speculated that TLR2 and 4 may both recognize bacterial and plant-derived fructans, although this remains to be proven. Bacterial fructans can be considered as MAMPs in this case. Since TLR2 and TLR4 homologs are absent in plant genomes, it seems that other, so far unidentified fructan receptors were recruited in the evolutionary lineage leading to plants.
Fructan: Mamps, Damps, or Both?
Both MAMPs and DAMPs are currently accepted as immune response inducers (Cook et al., 2015). So are fructans MAMPs, DAMPs, or both? The model that we propose suggests both, with the speculation that an evolutionary event resulted in a switch of fructan perception from MAMP to DAMP in fructan accumulating plants (Figure 1). In animals, the recognition of microbial fructans by PM-localized TLRs has been documented (see above), thus activating innate immunity. The possibility exists that the same holds true in plants, where shorter microbial fructooligosaccharides (mFOS) diffuse through the cell wall acting as MAMPs to activate immune responses. However, a receptor for fructans has not been described so far. Fructans are stored in the vacuole of fructan-accumulating plants. Within the damaged-self context, lysed cells releasing their fructan content may lead to partial fructan degradation in the apoplast. The derived plant fructooligosaccharides (pFOS) are expected to be more mobile, diffusing to neighboring cells where they are possibly sensed by ancient receptors (putatively localized in the PM), that are actually involved in fructan MAMP recognition (Figure 1).
FIGURE 1.
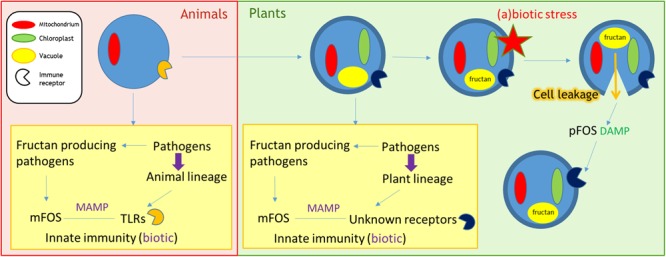
Role of fructans as MAMPs/DAMPs. Pathogens exert evolutionary pressure on host animals/plants. In case of fructan producing pathogens, these mFOS can function as a virulence factor. However, during biotic interactions, they may be recognized as MAMPs by the host. Selection will favor hosts that recognize mFOS through specific receptors that induce immune responses. In animals, microbial fructooligosaccharides (mFOS) from fructan producing pathogens are recognized by TLRs, thereby activating innate immune responses. A similar mechanism may be present in plants, where mFOS from fructan producing phytopathogens bind to currently unknown receptors to induce defense responses. Besides a MAMP recognition mechanism, fructans may also be involved in damaged-self recognition in certain plant species. 15% of flowering species synthesize and store fructans (plant fructooligosaccharides or pFOS) in the vacuole. In these plants, pFOS can be perceived as DAMPs by the unknown fructan receptors involved in MAMP recognition. Cellular rupture after (a)biotic stress will leak the stored pFOS into the apoplastic environment, triggering neighboring cells to upregulate immune responses after pFOS recognition by these receptors. As such, mild abiotic stresses may enhance disease resistance against future pathogen attack. DAMP, damage-associated molecular pattern; MAMP, microbe-associated molecular pattern; mFOS, microbial fructooligosaccharides; pFOS, plant fructooligosaccharides; TLR, toll-like receptor.
Fructans, Damp Signaling, and Cross-Tolerance
Within such framework (Figure 1), mild abiotic stresses may positively influence disease tolerance. If only some cells are damaged, the released mixture of DAMPs (including fructans and other compounds, see below) can prime the surrounding cells, hence priming their native immune system, thus increasing tolerance to a future pathogenic attack. The process in which resistance toward a specific stress is achieved through exposure to another (milder form of) particular stress is known as cross-tolerance. After exposure to a first stress stimulus, the plant is in a primed or hardened state, allowing it to respond to future stresses in a faster and stronger way (Rejeb et al., 2014; Savvides et al., 2016). Some examples can be found in the literature where abiotic stress exposure leads to increased biotic stress resistance. In Arabidopsis thaliana, ozone exposure triggers an induced resistance, associated with the expression of numerous defense-related genes, while drought stress increases resistance to pathogen infection through ROS in Nicotiana benthamiana (Sharma et al., 1996; Ramegowda et al., 2013). Thus, the damaged-self hypothesis and sweet immunity model predict an induction of plant defenses under mild stress conditions. During severe drought, however, Ramegowda and Senthil-Kumar (2015) proposed that massive cellular leakage of nutrients in the apoplast promotes infection. One possible scenario is that promotion of microbial growth by sugars in excess (or any and other nutrients) overrules signaling effects that could lead to increased plant protection.
In particular, the effects of cold stress on disease resistance have been well-described. Gene expression assays in Vitis amurensis indicate an upregulation of genes involved in innate immune system responses after cold acclimation (Wu et al., 2014; Moyer et al., 2015). Most research has focused on cold hardening and subzero acclimation of fructan accumulating cereals. In wheat (Triticum aestivum), fructan accumulates in response to low temperatures (Meguro-Maoka and Yoshida, 2016) through an increase in enzymatic activity of enzymes involved in fructan biosynthesis (Kawakami and Yoshida, 2002, 2005). Interestingly, the DP of these fructans increases from autumn to winter. Subzero acclimated plants have high contents of graminan-type fructans, characterized by branched structures (Yoshida and Kawakami, 2013). This process is most likely associated with increased apoplastic fructan levels, as observed in subzero acclimated oat (Livingston et al., 1993; Livingston and Henson, 1998), and correlates well with increased tolerance against snow mold infections. Snow molds are fungi with the ability to infect plants under snow at around 0°C (Gaudet and Laroche, 1997). Resistant wheat cultivars display higher fructan levels toward early winter and lower fructan degradation under snow in comparison to susceptible cultivars (Yoshida et al., 1998; Iriki et al., 2005; Nishio et al., 2008; Kawakami and Yoshida, 2012).
Damp Mixtures for Multistress Resistance
Designating fructans as damaged-self signaling molecules in fructan accumulating plants may not be so far-fetched. If the receptors involved are evolutionarily conserved, fructan accumulating plants may sense endogenous fructans as DAMPs, and bacterial fructans as MAMPs. A signaling function for less common sugars, like we propose here for fructans, has been described before. In gentians, gentiobiose appears to be involved in signaling budbreak in overwintering buds (Takahashi et al., 2014). Nevertheless, although we propose fructans as DAMPs in fructan accumulating plants, we must keep in mind that these are only one of many (possible) DAMPs that are released into the apoplast after cellular rupture. Thus, their contribution to priming innate immunity may be limited. Likely, a mixture of DAMPs rather than one released compound will induce an efficient priming.
What are other powerful DAMPs putatively involved in defense priming? Extracellular ATP is a central signaling molecule in plant stress responses, sensed by the PM receptor DORN1 (Cao et al., 2014). Similarly, extracellular NAD was proposed to act as a DAMP in Arabidopsis (Zhang and Mou, 2009; Pétriacq et al., 2016). Polyamines (PAs) such as spermine, spermidine, and putrescine are generally found in plant cells (Hussain et al., 2011; Minocha et al., 2014; Pál et al., 2015) and exogenous application revealed good priming potential (Li et al., 2015; Nahar et al., 2015), suggesting that they may be considered to be candidate DAMPs as well. Accordingly, mild salt stress increases apoplastic PA levels (Moschou et al., 2008). It is well-known that apoplastic PAs play important roles in plant-pathogen interactions, leading to significant changes in host susceptibility to different kinds of pathogens (Marina et al., 2008). Although, these have been explained by hydrogen peroxide-mediated signaling originating from PA oxidation in the apoplast, the view that apoplastic PAs may directly trigger immune receptors in the PM involved in DAMP signaling should be reconsidered.
Similarly, secondary metabolites such as naringenin, quercetin, and rutin may be considered as candidate DAMPs as well. Indeed, exogenous naringenin treatment led to increased drought tolerance (Pourcel et al., 2013), while quercetin and rutin priming led to increased resistance against bacterial pathogens in Arabidopsis (Jia et al., 2010; Yang et al., 2016). A screening of an array of mutants revealed that flavonoids are determinants of freezing tolerance and cold acclimation in Arabidopsis (Schulz et al., 2016). Taken all together, this suggests that some secondary metabolites can be used as signaling compounds to counteract both abiotic and biotic stresses.
Also melatonin, an indoleamine, has a strong priming potential when applied exogenously (Shi et al., 2014). Furthermore, a link between melatonin and sugar metabolism and signaling has been suggested in the context of biotic stress (Zhao et al., 2015). Recently, Jiao et al. (2016) isolated endophytic bacterial strains that live in the plant apoplast and secrete melatonin. Colonization by one such strain protected plants from adverse effects of salt or drought stress through upregulation of intracellular melatonin biosynthesis in the host plant. Thus, apoplastic melatonin levels somehow interact with intracellular melatonin levels, and such mechanisms may be tightly interlinked to damage-self recognition processes originating in the apoplastic continuum under various stresses.
Recently, Bruce et al. (2016) and Savvides et al. (2016) discuss the possibilities of chemical priming on multistress resistance, a popular topic in current research focusing on developing natural and sustainable alternatives for toxic agrochemicals. It is likely, that mixtures of priming agents can lead to synergistic effects and increased multistress tolerance by reflecting to what occurs when a complex mixture of intracellular metabolites is released in the apoplast after cellular rupture. Therefore, future research should focus on the priming efficacy of cocktails of the above-mentioned compounds in combination with different types of fructans from plant and microbial origin.
Fructans and Glucans: a Comparison
The view that fructans act as MAMPs and/or DAMPs may also hold true for other classes of polysaccharides such as β-glucans, containing glucose- instead of fructose moieties. β-1,3- and β-1,6-glucans represent a significant part of fungal cell walls (Dalonso et al., 2015). β-1,3- and β-1,4-glucans are also present in the cell walls of most plants of the Poaceae and in Equisetum, as well as in bryophytes. The highest abundance is found in cereals (Gibeaut et al., 2005; Burton and Fincher, 2009). The recognition of fungal β-glucans by the Dectin-1 receptor in animals was investigated thoroughly. This receptor has been discovered by Brown and Gordon (2001) and downstream responses have been characterized (Brown, 2006; Plato et al., 2015). Recently, Sahasrabudhe et al. (2016a) reported that pre-digestion of oat β-glucan with an endo-glucanase enhances the activation state of the Dectin-1 receptor in human dendritic cells. This observation fits well with the idea that shorter DP glucans, as well as fructans, may be considered as priming agents boosting native immunity both in animals and in plants.
In plants, only a few examples of β-glucan recognition are present. In soybean (Glycine max) it has been shown that a β-glucan binding protein can recognize β-glucans of the oomycete Phytophthora megasperma (Fliegmann et al., 2004). In a recent manuscript, analysis of key enzymes in β-1,6-glucan biosynthesis in Colletotrichum graminicola revealed a downregulation of this biosynthesis pathway in biotrophic hyphae in order to attenuate immune responses of the host (Oliveira-Garcia and Deising, 2016). Besides their possible function as MAMPs, these β-glucans could also function as DAMPs in cereals.
Other examples include arabinoxylans, which increase phagocytosis in macrophages and induce anti-inflammatory effects (Ghoneum and Matsuura, 2004; Kang et al., 2016). Accordingly, arabinoxylan activates Dectin-1 and modulates particulate β-glucan-induced Dectin-1 activation (Sahasrabudhe et al., 2016b).
Conclusion
While research on DAMP signaling in plants is still in an early phase, this perspective paper proposes possibilities for new and inventive experiments. The potential role of microbial fructans as MAMP in plants and plant fructans as DAMP in fructan accumulating plants is explained and compared to the case of glucans. While microbial fructan perception in animals has been characterized, the situation in plants is still unclear and identification of a fructan receptor requires further investigation. We propose that through such evolutionary ancient mechanism, plant-derived fructans, as potential DAMPs, may prime the immune system of fructan accumulating plants. Within this framework, the role of DAMP signaling in multistress resistance is discussed and other potential DAMPs, such as PAs and secondary metabolites, may be important players in (a)biotic stress tolerance as well. The potential use of mixtures of DAMPs for priming requires further investigation and may provide promising alternatives for toxic agrochemicals.
Author Contributions
MV and WVdE defined the perspective. MV wrote the first draft, input was provided by ŁPT and WVdE.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
WVdE and ŁPT are supported by funds of FWO Vlaanderen.
Abbreviations
- ATP
adenosine triphosphate
- BFO
burdock fructooligosaccharides
- DAMP
damage-associated molecular pattern
- DORN1
does not respond to nucleotides 1
- DP
degree of polymerization
- FEH
fructan exohydrolase
- FOS
fructooligosaccharides
- HAMP
herbivore-associated molecular pattern
- IL-1
interleukin 1
- LPS
lipo-oligosaccharides
- MAMP
microbe-associated molecular pattern
- mFOS
microbial fructooligosaccharides
- NAD
nicotinamide adenine dinucleotide
- PA
polyamine
- pFOS
plant fructooligosaccharides
- PM
plasma membrane
- ROS
reactive oxygen species
- SNF1
sucrose non-fermenting 1
- SnRK1
SNF1-related kinase 1
- Suc
sucrose
- TLR
toll-like receptor
References
- Anwar M. A., Kralj S., Piqué A. V., Leemhuis H., van der Maarel M. J. E. C., Dijkhuizen L. (2010). Inulin and levan biosynthesis by probiotic Lactobacillus gasseri strains: characterization of three novel fructansucrase enzymes and their fructan products. Microbiol 156 1264–1274. 10.1099/mic.0.036616-0 [DOI] [PubMed] [Google Scholar]
- Bolouri Moghaddam M. R., Van den Ende W. (2012). Sugars and plant innate immunity. J. Exp. Bot. 63 3989–3998. 10.1093/jxb/ers129 [DOI] [PubMed] [Google Scholar]
- Bolouri Moghaddam M. R., Van den Ende W. (2013). Sweet immunity in the plant circadian regulatory network. J. Exp. Bot. 64 1439–1449. 10.1093/jxb/ert046 [DOI] [PubMed] [Google Scholar]
- Brown G. D. (2006). Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat. Rev. Immun. 6 33–43. 10.1038/nri1745 [DOI] [PubMed] [Google Scholar]
- Brown G. D., Gordon S. (2001). A new receptor for β-glucans. Nature 413 36–37. 10.1038/35092620 [DOI] [PubMed] [Google Scholar]
- Bruce T. J. A., Smart L. E., Birch A. N. E., Blok V. C., MacKenzie K., Guerrieri E., et al. (2016). Prospects for plant defence activators and biocontrol in IPM – Concepts and lessons learnt so far. Crop Prot. 10.1016/j.cropro.2016.10.003 [DOI] [Google Scholar]
- Burton R. A., Fincher G. B. (2009). (1,3;1,4)-β-D-glycans in cell walls of the Poaceae, lower plants, and fungi: a tale of two linkages. Mol. Plant 2 873–882. 10.1093/mp/ssp063 [DOI] [PubMed] [Google Scholar]
- Cao Y., Tanaka K., Nguyen C. T., Stacey G. (2014). Extracellular ATP is a central signaling molecule in plant stress responses. Curr. Opin. Plant Biol. 20 82–87. 10.1016/j.pbi.2014.04.009 [DOI] [PubMed] [Google Scholar]
- Capitán-Cañadas F., Ortega-González M., Guadix E., Zarzuelo A., Suárez M. D., de Medina F. S., et al. (2014). Prebiotic oligosaccharides directly modulate proinflammatory cytokine production in monocytes via activation of TLR4. Mol. Nutr. Food Res. 58 1098–1110. 10.1002/mnfr.201300497 [DOI] [PubMed] [Google Scholar]
- Conrath U. (2015). Priming for enhanced defense. Annu. Rev. Plant Pathol. 53 97–119. 10.1146/annurev-phyto-080614-120132 [DOI] [PubMed] [Google Scholar]
- Cook D. E., Mesarich C. H., Thomma B. P. (2015). Understanding plant immunity as a surveillance system to detect invasion. Annu. Rev. Phytopathol. 53 541–563. 10.1146/annurev-phyto-080614-120114 [DOI] [PubMed] [Google Scholar]
- Crisan T. O., Netea M. G., Joosten L. A. B. (2016). Innate immune memory: implications for host responses to damage-associated molecular patterns. Eur. J. Immunol. 46 817–828. 10.1002/eji.201545497 [DOI] [PubMed] [Google Scholar]
- Dalonso N., Goldman G. H., Gern R. M. M. (2015). β-(1(3),(1(6)-glucans: medicinal activities, characterization, biosynthesis and new horizons. Appl. Microbiol. Biotechnol. 99 7893–7906. 10.1007/s00253-015-6849-x [DOI] [PubMed] [Google Scholar]
- Duran-Flores D., Heil M. (2016). Sources of specificity in plant damaged-self recognition. Curr. Opin. Plant Biol. 32 77–87. 10.1016/j.pbi.2016.06.019 [DOI] [PubMed] [Google Scholar]
- Fliegmann J., Mithöfer A., Wanner G., Ebel J. (2004). An ancient enzyme domain hidden in the putative β-glucan elicitor receptor of soybean may play an active part in the perception of pathogen-associated molecular patterns during broad host resistance. J. Biol. Chem. 279 1132–1140. 10.1074/jbc.M308552200 [DOI] [PubMed] [Google Scholar]
- Franco-Robles E., López M. G. (2015). Implication of fructans in health: immunomodulatory and antioxidant mechanisms. Sci. World J. 2015:289267 10.1155/2015/289367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet D. A., Laroche A. (1997). “Winter survival of cereals parasitized by snow mold,” in Plant Cold Hardiness eds Li H., Chen T. H. H. (Berlin: Springer; ) 331–342. 10.1007/978-1-4899-0277-1_31 [DOI] [Google Scholar]
- Ghoneum M., Matsuura M. (2004). Augmentation of macrophage phagocytosis by modified arabinoxylan rice bran (MGN-3/biobran). Int. J. Immunopathol. Pharmacol. 17 283–292. [DOI] [PubMed] [Google Scholar]
- Gibeaut D. M., Pauly M., Bacic A., Fincher G. B. (2005). Changes in cell wall polysaccharides in developing barley (Hordeum vulgare) coleoptiles. Planta 221 729–738. 10.1007/s00425-005-1481-0 [DOI] [PubMed] [Google Scholar]
- Heil M. (2012). Damaged-self recognition as a general strategy for injury detection. Plant Sign. Behav. 7 576–580. 10.4161/psb.19921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M., Land W. G. (2014). Danger signals–damaged-self recognition across the tree of life. Front. Plant Sci. 5:578 10.3389/fpls.2014.00578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M., Land W. G., Tör M. (2016). Editorial: wound recognition across the tree of life. Front. Plant Sci. 7:319 10.3389/fpls.2016.01319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M. Y., Chang C. I., Chang C. C., Tseng L. W., Pan C. L. (2015). Effects of dietary levan on growth performance, nonspecific immunity, pathogen resistance and body composition of orange-spotted grouper (Epinephelus coioides H.). Aquacult. Res. 46 2752–2767. 10.1111/are.12430 [DOI] [Google Scholar]
- Hulsmans S., Rodriguez M., De Coninck B., Rolland F. (2016). The SnRK1 energy sensor in plant biotic interactions. Trends Plant Sci. 21 648–661. 10.1016/j.tplants.2016.04.008 [DOI] [PubMed] [Google Scholar]
- Hussain S. S., Ali M., Ahmad M., Siddique K. H. M. (2011). Polyamines: natural and engineered abiotic and biotic stress in plants. Biotechnol. Adv. 29 300–311. 10.1016/j.biotechadv.2011.01.003 [DOI] [PubMed] [Google Scholar]
- Ichinose Y., Taguchi F., Mukaihara T. (2013). Pathogenicity and virulence factors of Pseudomonas syringae. J. Gen. Plant Pathol. 79 285–296. 10.1007/s10327-013-0452-8 [DOI] [Google Scholar]
- Iriki N., Nishio Z., Kawakami A., Yoshida M., Kuroki M., Funtov K., et al. (2005). Fructan content in Aegilops cylindrica and its relationship to snow mold resistance and freezing tolerance. Plant Prod. Sci. 8 563–566. 10.1626/pps.8.563 [DOI] [Google Scholar]
- Jia Z., Zou B., Wang X., Qui J., Ma H., Gou Z., et al. (2010). Quercetin-induced H2O2 mediates the pathogen resistance against Pseudomonas syringae pv. tomato DC3000 in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 396 522–527. 10.1016/j.bbrc.2010.04.131 [DOI] [PubMed] [Google Scholar]
- Jiao J., Ma Y., Chen S., Liu C., Song Y., Qin Y., et al. (2016). Melatonin-producing endophytic bacteria from grapevine roots promote the abiotic stress-induced production of endogenous melatonin in their hosts. Front. Plant Sci. 7:1387 10.1189/fpls.2016.01387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H., Lee M. G., Lee J. K., Choi Y., Choi Y. S. (2016). Enzymatically-processed wheat bran enhances macrophage activity and has in vivo anti-inflammatory effects in mice. Nutrients 8:188 10.3390/nu8040188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami A., Yoshida M. (2002). Molecular characterization of sucrose:sucrose 1-fructosyltransferase and sucrose:fructan 6-fructosyltransferase associated with fructan accumulation in winter wheat during cold hardening. Biosci. Biotech. Biochem. 66 2297–2305. 10.1271/bbb.66.2297 [DOI] [PubMed] [Google Scholar]
- Kawakami A., Yoshida M. (2005). Fructan:fructan 1-fructosyltransferase, a key enzyme for biosynthesis of graminan oligomers in hardened wheat. Planta 223 90–104. 10.1007/s00425-005-0054-6 [DOI] [PubMed] [Google Scholar]
- Kawakami A., Yoshida M. (2012). Graminan breakdown by fructan exohydrolase induced in winter wheat inoculated with snow mold. J. Plant Physiol. 169 294–302. 10.1016/j.jplph.2011.09.003 [DOI] [PubMed] [Google Scholar]
- Koczan J. M., McGrath M. J., Zhao Y., Sundin G. W. (2009). Contribution of Erwinia amylovora exopolysaccharides amylovoran and levan to biofilm formation: implications in pathogenicity. Phytopathol 99 1237–1244. 10.1094/PHYTO-99-11-1237 [DOI] [PubMed] [Google Scholar]
- Krysko D. V., Agostinis P., Krysko O., Garg A. D., Bachert C., Lambrecht B. N., et al. (2011). Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 32 157–164. 10.1016/j.it.2011.01.005 [DOI] [PubMed] [Google Scholar]
- Li J., Hu L., Zhang L., Pan X., Hu X. (2015). Exogenous spermidine is enhancing tomato tolerance to salinity-alkalinity stress by regulating chloroplast antioxidant system and chlorohyll metabolism. BMC Plant Biol. 15:303 10.1186/s12870-015-0699-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Kim I. H. (2013). Effects of levan-type fructan supplementation on growth performance, digestibility, blood profile, fecal microbiota, and immune responses after lipopolysaccharide challenge in growing pigs. J. Anim. Sci. 91 5336–5343. 10.2527/jas2013-6665 [DOI] [PubMed] [Google Scholar]
- Livingston D. P., Henson C. A. (1998). Apoplastic sugars, fructans, fructan exohydrolase, and invertase in winter oat: responses to second-phase cold hardening. Plant Physiol. 116 403–408. 10.1104/pp.116.1.403 [DOI] [Google Scholar]
- Livingston D. P., Knievel D. P., Gildow F. E. (1993). Oligomer accumulation in stems during cold hardening and their in vitro synthesis in a crude enzyme extract. New Phytol. 127 27–36. 10.1111/j.1469-8137.1994.tb04256.x [DOI] [PubMed] [Google Scholar]
- López-Velázquez G., Parra-Ortiz M., Mora I. D. D., García-Torres I., Enríquez-Flores S., Alcántara-Ortigoza M. A., et al. (2015). Effects of fructans from mexican agave in newborns fed with infant formula: a randomized controlled trial. Nutrients 7 8939–8951. 10.3390/nu7115442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancilla-Margalli N. A., López M. G. (2006). Water-soluble carbohydrates and fructan structure patterns from Agave and Dasylirion species. J. Agric. Food Chem. 54 7832–7839. 10.1021/jf060354v [DOI] [PubMed] [Google Scholar]
- Marina M., Maiale S. J., Rossi F. R., Romero M. F., Rivas E. I., Garriz A., et al. (2008). Apoplastic polyamine oxidation plays different roles in local responses of tobacco to infection by the necrotrophic fungus Sclerotinia sclerotiorum and the biotrophic bacterium Pseudomonas viridiflava. Plant Physiol. 147 2164–2178. 10.1104/pp.108.122614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. J. (2016). Cell death and inflammation: the case for IL-1 family cytokines as the canonical DAMPS of the immune system. FEBS J. 283 2599–2615. 10.1111/febs.13775 [DOI] [PubMed] [Google Scholar]
- Martinez-Medina A., Flors V., Heil M., Mauch-Mani B., Pieterse C. M. J., Pozo M. J., et al. (2016). Recognizing plant defense priming. Trends Plant Sci. 21 818–822. 10.1016/j.tplants.2016.07.009 [DOI] [PubMed] [Google Scholar]
- Matzinger P. (1994). Tolerance, danger, and the extended family. Annu. Rev. Immunol. 12 991–1045. 10.1146/annurev.iy.12.040194.005015 [DOI] [PubMed] [Google Scholar]
- Meguro-Maoka A., Yoshida M. (2016). Analysis of seasonal expression levels of wheat fructan exohydrolase (FEH) genes regulating fructan metabolism involved in wintering ability. J. Plant Physiol. 191 54–62. 10.1016/j.jplph.2015.12.001 [DOI] [PubMed] [Google Scholar]
- Minocha R., Majumdar R., Minocha S. C. (2014). Polyamines and abiotic stress in plants: a complex relationship. Front. Plant Sci. 5:175 10.3389/fpls.2014.00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschou P. N., Paschalidis K. A., Delis I. D., Andriopoulou A. H., Lagiotis G. D., Yakoumakis D. I., et al. (2008). Spermidine exodus and oxidation in the apoplast induced by abiotic stress is responsible for H2O2 signatures that direct tolerance responses in tobacco. Plant Cell 20 1708–1724. 10.1105/tpc.108.059733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer M. M., Londo J., Gadoury D. M., Cadle-Davidson L. (2015). Cold stress-induced disease resistance (SIDR): indirect effects of low temperatures on host-pathogen interactions and disease progress in the grapevine powdery mildew pathosystem. Eur. J. Plant Pathol. 144 695–705. 10.1007/s10658-015-0745-1 [DOI] [Google Scholar]
- Nahar M., Hasanuzzaman M., Alam M. M., Fujita M. (2015). Exogenous spermidine alleviates low temperature injury in mung bean (Vigna radiata L.) seedlings by modulating ascorbate-glutathione and glyoxalase pathway. Int. J. Mol. Sci. 16 30117–30132. 10.3390/ijms161226220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio Z., Iriki N., Takata K., Ito M., Tabiki T., Murray T. D. (2008). Influence of cold-hardening and soil matric potential on resistance to speckled snow mold in wheat. Plant Dis. 92 1021–1025. 10.1094/PDIS-92-7-1021 [DOI] [PubMed] [Google Scholar]
- Oliveira-Garcia E., Deising H. B. (2016). Attenuation of PAMP-triggered immunity in maize requires down-regulation of the key β-1,6-glucan synthesis genes KRE5 and KRE6 in biotrophic hyphae of Colletotrichum graminicola. Plant J. 87 355–375. 10.1111/tpj.13205 [DOI] [PubMed] [Google Scholar]
- Ordax M., Marco-Noales E., López M. M., Biosca E. G. (2010). Exopolysaccharides favor the survival of Erwinia amylovora under copper stress through different strategies. Res. Microbiol. 161 549–555. 10.1016/j.resmic.2010.05.003 [DOI] [PubMed] [Google Scholar]
- Ozimek L. K., Kralj S., van der Maarel M. J. E. C., Dijkhuizen L. (2006). The levansucrase and inulosucrase enzymes of Lactobacillus reuteri 121 catalyse processive and non-processive transglycosylation reactions. Microbiol 152 1187–1196. 10.1099/mic.0.28484-0 [DOI] [PubMed] [Google Scholar]
- Pál M., Szalai G., Janda T. (2015). Speculation: polyamines are important in abiotic stress signaling. Plant Sci. 237 16–23. 10.1016/j.plantsci.2015.05.003 [DOI] [PubMed] [Google Scholar]
- Peshev D., Van den Ende W. (2014). Fructans: prebiotics and immunomodulators. J. Funct. Foods 8 348–357. 10.1016/j.jff.2014.04.005 [DOI] [Google Scholar]
- Pétriacq P., Ton J., Patrit O., Tcherkez G., Gakière B. (2016). NAD acts as an integral regulator of multiple defense layer. Plant Physiol. 172 1465–1479. 10.1104/pp.16.00780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plato A., Hardison S. E., Brown G. D. (2015). Pattern recognition receptors in antifungal immunity. Sem. Immunopath. 37 97–106. 10.1007/s00281-014-0462-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourcel L., Irani N. G., Koo A. J., Bohorquez-Restrepo A., Howe G. A., Grotewold E. (2013). A chemical complementation approach reveals genes and interactions of flavonoids with other pathways. Plant J. 1 383–397. 10.1111/tpj.12129 [DOI] [PubMed] [Google Scholar]
- Ramegowda V., Senthil-Kumar M. (2015). The interactive effects of simultaneous biotic and abiotic stresses on plants: mechanistic understanding from drought and pathogen combination. J. Plant Physiol. 176 47–54. 10.1016/j.jplph.2014.11.008 [DOI] [PubMed] [Google Scholar]
- Ramegowda V., Senthil-Kumar M., Ishiga Y., Kaundal A., Udayakumar M., Mysore K. S. (2013). Drought stress acclimation imparts tolerance to Sclerotinia sclerotiorum and Pseudomonas syringae in Nicotiana benthamiana. Int. J. Mol. Sci. 14 9497–9513. 10.3390/ijms14059497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejeb I. B., Pastor V., Mauch-Mani B. (2014). Plant responses in simultaneous biotic and abiotic stress: molecular mechanisms. Plants 3 458–475. 10.3390/plants3040458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahasrabudhe N. M., Schols H. A., Faas M. M., de Vos P. (2016a). Arabinoxylan activates Dectin-1 and modulates particulate β-glucan-induced Dectin-1 activation. Mol. Nutr. Food Res. 60 458–467. 10.1002/mnfr.201500582 [DOI] [PubMed] [Google Scholar]
- Sahasrabudhe N. M., Tian L., van den Berg M., Bruggeman G., Bruininx E., Schols H. A., et al. (2016b). Endo-glucanase digestion of oat β-glucan enhances Dectin-1 activation in human dendritic cells. J. Funct. Foods 21 104–112. 10.1016/j.jff.2015.11.037 [DOI] [Google Scholar]
- Savvides A., Ali S., Tester M., Fotopoulos V. (2016). Chemical priming of plants against multiple abiotic stresses: mission possible? Trends Plant Sci. 21 329–340. 10.1016/j.tplants.2015.11.003 [DOI] [PubMed] [Google Scholar]
- Schulz E., Tohge T., Zuther E., Fernie A. R., Hincha D. K. (2016). Flavonoids are determinants of freezing tolerance and cold acclimation in Arabidopsis thaliana. Sci. Rep. 6:34027 10.1038/srep34027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma Y. K., León J., Raskin I., Davis K. R. (1996). Ozone-induced responses in Arabidopsis thaliana: the role of salicylic acid in the accumulation of defense-related transcripts and induced resistance. Proc. Natl. Acad.Sci. U.S.A. 93 5099–5104. 10.1073/pnas.93.10.5099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Jiang C., Ye T., Tan D., Reiter R. J., Zhang H., et al. (2014). Comparative physiological, metabolomics, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L.) Pers.] by exogenous melatonin. J. Exp. Bot. 66 681–694. 10.1093/jxb/eru373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S., Hellmann H. A. (2014). Sugar sensing and signaling in plants. Front. Plant Sci. 5:113 10.3389/fpls.2014.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F., Zhang P., Guo M., Yu W., Chen K. (2013). Burdock fructooligosaccharide induces fungal resistance in postharvest Kyoho grapes by activating the salicylic acid-dependent pathway and inhibiting browning. Food Chem. 138 539–546. 10.1016/j.foodchem.2012.10.058 [DOI] [PubMed] [Google Scholar]
- Takahashi H., Imamura T., Konno N., Takeda T., Fujita K., Konishi T., et al. (2014). The gentio-oligosaccharide gentiobiose functions in the modulation of bud dormancy in the herbaceous perennial Gentiana. Plant Cell 26 3949–3963. 10.1105/tpc.114.131631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O., Hoshino K., Kawai T., Sanjo H., Takada H., Ogawa T., et al. (1999). Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11 443–451. 10.1016/S1074-7613(00)80119-3 [DOI] [PubMed] [Google Scholar]
- Toksoy E., Hernández L., Combie J. (2016). Review of levan polysaccharide: from a century of past experiences to future prospects. Biotech. Adv. 34 827–844. 10.1016/j.biotechadv.2016.05.002 [DOI] [PubMed] [Google Scholar]
- Valluru R., Van den Ende W. (2008). Plant fructans in stress environments: emerging concepts and future prospects. J. Exp. Bot. 59 2905–2916. 10.1093/jxb/ern164 [DOI] [PubMed] [Google Scholar]
- Van den Ende W. (2013). Multifunctional fructans and raffinose family oligosaccharides. Front. Plant Sci. 4:247 10.3389/fpls.2013.00247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Ende W., De Coninck B., Van Laere A. (2004). Plant fructan exohydrolases: a role in signaling and defense? Trends Plant Sci. 9 523–528. 10.1016/j.tplants.2004.09.008 [DOI] [PubMed] [Google Scholar]
- Van den Ende W., El-Esawe S. (2014). Sucrose signaling pathways leading to fructan and anthocyanin accumulation: a dual function in abiotic and biotic stress responses? Env. Exp. Bot. 108 4–13. 10.1016/j.envexbot.2013.09.017 [DOI] [Google Scholar]
- Verspreet J., Dornez E., Van den Ende W., Delcour J. A., Courtin C. M. (2015). Cereal grain fructans: structure, variability and potential health effects. Trends Food Sci. Technol 43 32–42. 10.1016/j.tifs.2015.01.006 [DOI] [Google Scholar]
- Vogt L., Ramasamy U., Meyer D., Pullens G., Venema K., Faas M. M., et al. (2013). Immune modulation by different types of β2(1 fructans is Toll-like receptor dependent. PLoS ONE 8:e68367 10.1371/journal.pone.0068367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Feng G., Chen K. (2009). Defense responses of harvested tomato fruit to burdock fructooligosaccharide, a novel potential elicitor. Postharv. Biol. Technol. 52 110–116. 10.1016/j.postharvbio.2008.09.002 [DOI] [Google Scholar]
- Wu J., Zhang Y., Yin L., Qu J., Lu J. (2014). Linkage of cold acclimation and disease resistance through plant-pathogen interaction pathway in Vitis amurensis grapevine. Funct. Integr. Gen. 14 741–755. 10.1007/s10142-014-0392-1 [DOI] [PubMed] [Google Scholar]
- Xu Q., Yajima T., Saito K., Ohshima Y., Yoshikai Y. (2006). Levan (β-2,6-fructan), a major fraction of fermented soybean mucilage displays immunostimulating properties via Toll-like receptor 4 signalling: induction of interleukin-12 production and suppression of T-helper type 2 response and immunoglobulin E production. Clin. Exp. All. 36 94–101. 10.1111/j.1365-2222.2006.02401.x [DOI] [PubMed] [Google Scholar]
- Yang W., Xu X., Li Y., Wang Y., Li M., Wang Y., et al. (2016). Rutin-mediated priming of plant resistance to three bacterial pathogens initiating early SA signal pathway. PLoS ONE 11:e0146910 10.1371/journal.pone.0146910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S. H., Yoon E. J., Cha J., Lee H. G. (2004). Antitumor activity of levan polysaccharides from selected microorganisms. Int. J. Biol. Macromol. 34 37–41. 10.1016/j.ijbiomac.2004.01.002 [DOI] [PubMed] [Google Scholar]
- Yoshida M., Abe J., Moriyama M., Kuwabara T. (1998). Carbohydrate levels among winter wheat cultivars varying in freezing tolerance and snow mold resistance during autumn and winter. Physiol. Plant. 103 8–16. 10.1034/j.1399-3054.1998.1030102.x [DOI] [Google Scholar]
- Yoshida M., Kawakami A. (2013). “Molecular analysis of fructan metabolism associated with freezing tolerance and snow mold resistance of winter wheat,” in Plant and Microbe Adaptations to Cold in a Changing World eds Imai R., Yoshida M., Matsumoto N. (Berlin: Springer; ) 231–244. 10.1007/978-1-4614-8253-6_20 [DOI] [Google Scholar]
- Zhang P. Y., Wang J. C., Liu S. H., Chen K. S. (2009). A novel burdock fructooligosaccharide induces changes in the production of salicylates, activates defence enzymes and induces systemic acquired resistance to Colletotrichum orbiculare in cucumber seedlings. J. Phytopathol. 157 201–207. 10.1111/j.1439-0434.2008.01465.x [DOI] [Google Scholar]
- Zhang X., Mou Z. (2009). Extracellular pyridine nucleotides induce PR gene expression and disease resistance in Arabidopsis. Plant J. 57 302–312. 10.1111/j.1365-313X.2008.03687.x [DOI] [PubMed] [Google Scholar]
- Zhao H. B., Xu L. F., Su T., Jiang Y., Hu L. Y., Ma F. W. (2015). Melatonin regulates carbohydrate metabolism and defenses against Pseudomonas syringae pv. tomato DC3000 infection in Arabidopsis thaliana. J. Pin. Res. 59 109–119. 10.1111/jpi.12245 [DOI] [PubMed] [Google Scholar]


