Abstract
Impulsivity is considered a multidimensional construct that encompasses a range of behaviors, including poor impulse control, premature decision‐making, and the inability to delay gratification. In order to determine the extent to which impulsivity and its components share a common network, a voxel‐based lesion‐symptom mapping (VLSM) analysis was performed in a large sample of patients (N = 131) with focal, penetrating traumatic brain injuries (pTBI). Impulsivity was assessed using the Barratt Impulsiveness Scale (BIS‐11), a standard self‐report measure that allows for unique estimates of global impulsivity and its factor analysis‐derived components (e.g., “motor impulsivity”). Heightened global impulsivity was associated with damage to multiple areas in bilateral prefrontal cortex (PFC), left superior, middle and inferior temporal gyrus, and left hippocampus. Moreover, a cluster was identified within the left PFC associated specifically with motor impulsivity (defined as “acting without thinking”). The results were consistent with the existing literature on bilateral prefrontal cortical involvement in behavioral impulsivity, but also provided new evidence for a more complex neuroanatomical representation of this construct, characterized by left‐lateralized temporal and hippocampal involvement, as well as a left‐lateralized prefrontal network specifically associated with motor impulsivity. Hum Brain Mapp 38:656–665, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: impulsivity, motor impulsivity, prefrontal cortex, traumatic brain injury, voxel‐based lesion‐symptom mapping
INTRODUCTION
Impulsivity is a behavior driven by immediate urges and executed without thoughtful deliberation or appropriate regard to consequence [Daruna and Barnes, 1993; Stahl et al., 2014]. Although impulsivity is typically measured as a unitary construct, psychometric evidence has long supported a multidimensional model of this trait [Barratt et al., 1987; Buss and Plomin, 1975; Eysenck and Eysenck, 1977; Gerbing et al., 1987; Revelle, 1997] with distinct subtypes derived from factor analytic methods [Caswell et al., 2015; Dickman, 1990; Eysenck et al., 1985; Lynam et al., 2007; Parker et al., 1993; Patton et al., 1995]. Multidimensional measures of impulsivity, primarily acquired through self‐report methods, have identified a varying number of impulsivity subtypes typically ranging from two to six factors. Identified factors include acting without thinking [i.e., “motor impulsivity”; Patton et al., 1995], also referred to as “urgency” [Lynam et al., 2007], or “spontaneity” [Gerbing et al., 1987; Parker et al., 1993], a failure to plan for future events [i.e., “non‐planning”; Buss and Plomin, 1975; Eysenck and Eysenck, 1977; Patton et al., 1995; Schalling and Åsberg, 1985] or “lack of premeditation” [Lynam et al., 2007] and an inability to focus on the task at hand [i.e., “attentional impulsivity”; Patton et al., 1995] or lack of perseverance/persistence [Buss and Plomin, 1975; Gerbing et al., 1987; Lynam et al., 2007]. While these factors have been psychometrically validated and applied over decades of personality research, remarkably little attention has been given to determining their biological bases. In particular, it is unknown whether unique factors of impulsivity are subserved by distinct, identifiable neural networks, or alternatively, whether these factors map onto networks associated with the general dimension of impulsivity.
Research on the neural networks underlying impulsivity as a general dimension has focused on functional and anatomical imaging approaches. These studies consistently link high self‐reported impulsivity with network‐level changes in the prefrontal cortex (PFC) and temporal lobe. Farr et al. [2012] reported a negative correlation between self‐reported trait impulsivity, as measured by the Barratt Impulsiveness Scale [BIS‐11; Patton et al., 1995] and activation in the right middle frontal cortex and right anterior dorsal insula during the stop‐signal task [Farr et al., 2012], a standard measure of inhibitory control. During this task, regional activation was decreased specifically during the “stop” versus “go” trials among participants with high trait impulsivity, suggesting that these regions play a role in regulating impulsiveness. Studies examining structural differences associated with self‐reported impulsivity have also largely focused on frontal regions of the brain, with recent studies of healthy volunteers reliably reporting links between decreases in regional cortical thickness and increased impulsivity. In a recent study by Schilling et al. [2012], for example, researchers found an inverse correlation between scores on the BIS‐11 [Patton et al., 1995] and cortical thickness in left middle frontal, orbital frontal (OFC) and superior frontal regions. Similarly, Matsuo et al. [2009] reported an inverse correlation between the BIS‐11 and gray matter volumes in bilateral OFC, as well as in left anterior cingulate cortex. Together, these studies suggest that a network of frontotemporal regions, particularly the OFC and middle frontal cortex, play a fundamental role in the expression of reported trait impulsivity.
Although there has been a great deal of neuroimaging research dedicated to outlining the substrates of impulsivity as a general dimension, only a small number of studies have explored the possibility that individual factors of impulsivity may be linked with distinct brain regions. For example, Matsuo et al. [2009] reported that the BIS‐11 subfactor, non‐planning impulsivity (i.e., a lack of thoughtful and careful thinking), was associated with decreased right OFC volume in healthy participants. They also discovered an inverse correlation between self‐reported motor impulsivity and left superior OFC volume in healthy subjects. Similarly, a study by Schilling et al. [2012] found a negative correlation between non‐planning impulsivity and gray matter thickness in both left middle frontal gyrus and right OFC while also discovering a negative correlation between self‐reported motor impulsivity and gray matter thickness in the left middle frontal cortex and superior frontal cortex. Furthermore, attentional impulsivity was associated with decreased gray matter thickness in the left middle frontal gyrus, a region known to mediate sustained attention [Lawrence et al., 2003].
Despite converging evidence linking impulsivity and its factors to frontotemporal brain regions, findings based on structural and functional imaging in healthy participants are limited in their capacity to determine whether a particular brain region is necessary for a specific function [Szczepanski and Knight, 2014]. That is, the methods used to describe the above findings are insufficient to establish causal relationships between structure and function. Thus, it remains unknown whether the frontotemporal regions described above play a causal role in inhibiting impulsive behavior, or whether an increase in impulsive behavior leads to dysregulated prefrontal activity. In order to address these types of questions, research linking impulsive behavior to localized brain damage is crucial. To our knowledge, however, no studies to date have examined the effects of focal brain lesions on impulsivity. Results linking lesion location to subsequent changes in impulsivity would provide valuable information regarding how particular PFC regions are causally involved in this trait.
To address this question, we examined a large group of veterans (N = 131) from the Vietnam Head Injury Study (VHIS) who sustained focal penetrating traumatic brain injuries (pTBI) during combat. We employed a voxel‐based lesion‐symptom mapping (VLSM) analysis to explore the causal role of focal brain lesions on the general dimension of impulsivity (i.e., “global impulsivity”) and three specific factors, namely, motor, non‐planning and attentional impulsivity, as described by the Barratt Impulsiveness Scale [BIS‐11; Patton et al., 1995]. Based on above research linking global impulsivity to decreased function in OFC and middle frontal cortex, we hypothesized that damage to frontal brain regions would be associated with increased scores on this trait. Furthermore, given evidence supporting impulsivity as a multidimensional construct with distinct underlying networks, we expected that impulsivity factors would correspond to regional brain volume loss in areas related to domain‐specific functions such as OFC (non‐planning impulsivity), middle frontal gyrus (attentional impulsivity) and frontotemporal regions, including OFC, middle frontal gyrus, superior frontal gyrus, dorsolateral PFC, and insula [motor impulsivity; Szczepanski and Knight, 2014].
METHODS
Subjects
Participants were 131 brain injured male combat veterans drawn from Phase IV (2009–2012) of the VHIS. The VHIS registry, a multi‐phase longitudinal study, provides a rare opportunity to explore brain‐behavior relationships among a large cohort of combat veterans with focal penetrating brain injuries. Table 1 reports demographic and select neuropsychological results for all subjects included in the present study. The Institutional Review Board at the National Institute of Neurological Disorders and Stroke in Bethesda (MD) approved study procedures, and participants provided written consent for inclusion in the study.
Table 1.
Demographic and neuropsychological data from 131 patients with penetrating TBI
| Measure | M | SD | Normal range |
|---|---|---|---|
| Age (years) | 63.28 | 2.87 | |
| Education (years) | 14.56 | 2.25 | |
| Pre‐injury armed forces qualifications test (%) | 64.35 | 23.14 | |
| Post‐injury armed forces qualifications test (%) | 54.46 | 26.14 | |
| Brain volume loss (%) | 3.05 | 3.78 | |
| Token test | 96.93 | 8.68 | 97–100 |
| Trail making | 8.98 | 4.05 | 9–11 |
| Beck depression inventory (BDI‐II) | 7.88 | 8.15 | 0–19 |
Note. Trail Making refers to the D‐KEFS Number Letter Switching subtest.
Neuropsychological Tests
All participants in Phase IV of the VHIS completed an extensive battery of neuropsychological tests over a 5–7 day period at the National Institute of Neurological Disorders and Stroke (NINDS) in Bethesda, MD. We report a subset of these tests including the following: the Armed Forces Qualification Test [AFQT‐7A, 1960], used to assess pre‐ and post‐injury general intelligence; the Revised Token Test [McNeil and Prescott, 1994] to measure verbal comprehension; the Delis‐Kaplan Executive Function System [D‐KEFS; Delis et al., 2001] Trail Making Test (TMT) to assess set shifting and cognitive flexibility; the Beck Depression Inventory [BDI‐II; Beck et al., 1996] to measure depressive symptoms; and the Frontal Systems Behavioral Scale [FrSBe; Grace and Malloy, 2001] to assess patient awareness.
Participants also completed the Barratt Impulsiveness Scale [BIS‐11; Patton et al., 1995], a 30‐item self‐report measure designed to assess the construct of global impulsivity. The BIS‐11 is extensively used across both research and clinical settings [Stanford et al., 2009]. A previous study using principal component analysis revealed three distinct factors on the BIS‐11, which include motor impulsivity (11 items), non‐planning impulsivity (11 items), and attentional impulsivity [8 items; Patton et al., 1995]. Studies have found these BIS‐11 factors to be sensitive in distinguishing dimensions of impulsivity among various clinical [Dom et al., 2006; Peluso et al., 2007; Swann et al., 2008] and non‐clinical populations [Asahi et al., 2004; Matsuo et al., 2009].
CT Acquisition and Lesion Identification
Computer Tomography (CT) scans were acquired for all participants at the Bethesda Naval Hospital, using a General Electric Medical System Light Speed Plus CT scanner in helical mode. All scans were performed during Phase III of the VHIS (2003–2006). Because a large number of these participants had retained intracranial metal shrapnel and surgical clips as a result of their injury, they were unable to undergo magnetic resonance imaging. Structural neuroimaging data were reconstructed with an in‐plane voxel size of 0.4 × 0.4 mm, an overlapping slice thickness of 2.5 mm, and a 1‐mm slice interval. Lesion location and volume loss were obtained using the interactive analysis of brain lesions (ABLe) software implemented in MEDx v3.44 (Medical Numerics) [Makale et al., 2002; Solomon et al., 2007], with enhancements to support the automated anatomical labeling (AAL) atlas [Tzourio‐Mazoyer et al., 2002]. To identify lesion location, each lesion was manually outlined in every slice in native space by a trained neuropsychiatrist. Lesion areas were then reviewed by the primary investigator (JG), who was blind to the results of the neuropsychological evaluations and a consensus was reached on lesion extent. Lesion volume was calculated by summing all traced areas and multiplying by slice thickness. Scans were spatially normalized to a CT template brain image in MNI space [Collins et al., 1994]. Using a 12‐parameter affine fit, spatial normalization was performed using the automated image registration (AIR) algorithm [Woods et al., 1998], which helped to improve registration accuracy.
Statistical Analysis
Behavioral data
All behavioral data were analyzed using IBM SPSS 21 (IBM Corp., Armonk, NY) with the alpha set at 0.05 (two‐tailed). Normality of data was examined using the Kolmogorov‐Smirnov Test, and appropriate parametric (independent samples t‐tests and one‐way analysis of variance [ANOVA]) and non‐parametric (Mann–Whitney U Tests and Kruskal–Wallis tests) statistical tests were employed as needed. To examine patient insight/awareness, we performed a two‐tailed Spearman's correlation between patient and caregiver scores on the FrSBE. Finally, we performed a multiple linear regression analysis with impulsivity scores as the dependent variables and total percent brain volume loss and lesion location as predictor variables.
Voxel‐based lesion‐symptom mapping
Next, a voxel‐based lesion‐symptom mapping (VLSM) analysis was applied, comparing behavioral scores from patients with and without lesions in a single voxel. This generated a t‐statistic representing the probability of the damage in the voxel associated with the observed behavior. In order to have sufficient statistical power and to be able to test regions all over the brain, voxels that did not have at least 4 patients with damage were excluded from the analysis. A lesion density map was created to explore the distribution of lesions by overlapping all spatially normalized lesion images and observing the extent of damage shared at each individual voxel (Fig. 1). Based on this lesion overlay, there was coverage of voxels with at least four lesions in the frontal, temporal and parietal regions. To correct for multiple comparisons, a false discovery rate (FDR) correction of 0.05 was used, and at least 10 adjacent voxels must have been statistically significant for a cluster to be reported. The Automated Anatomical Labeling atlas (AAL) atlas for gray matter [Tzourio‐Mazoyer et al., 2002] and the ICBM DTI‐81 atlas for white matter [Mori et al., 2008] were used to identify significant clusters.
Figure 1.
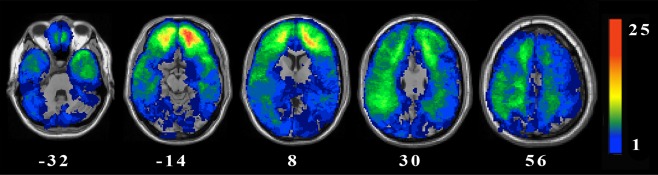
Lesion overlay map of 131 pTBI patients. Values shown in white indicate the z coordinates (MNI) of each axial slice. Maximum lesion overlap occurred primarily in the PFC. Warmer colors indicate greater lesion overlap (units: number of patients with lesion in this region). All images are depicted in radiological convention (the right hemisphere is on the reader's left). [Color figure can be viewed at http://wileyonlinelibrary.com.]
RESULTS
Behavioral Analysis
Several neuropsychological measures were analyzed to ensure that our population scored within normal limits on a set of standard cognitive tasks. These included IQ, language, executive functioning and depression (Table 1). Patients' levels of global impulsivity were assessed via the total score on the BIS‐11 (61.65 ± 10.28, M ± SD), as described in Table 2. Scores on the motor, attentional, and non‐planning factors were 21.58 ± 4.09, 16.15 ± 3.94, and 24.12 ± 4.79, respectively (Table 2). These scores fall within the normal range reported by Patton et al. [1995] and Stanford et al. [2009]. Intercorrelations between the total score and subdomains can be found in Table 3. Across the entire sample, 21 patients (16%) met criteria for “high impulsivity” according to the total score, measured by a score greater than 1 SD above the mean [Patton et al., 1995]. Eighteen patients (14%) met criteria for high motor impulsivity, while fifteen patients (11.5%) met criteria for high attentional impulsivity and twenty‐five patients (19%) met criteria for high non‐planning impulsivity. Heightened impulsivity in more than one subdomain occurred in 15 patients (11.5%). Among them, overlap between motor, attention and non‐planning subdomains occurred equally.
Table 2.
Summary of scores from the Barratt Impulsivity Scale (BIS‐11), N = 131
| Scale | M | SD |
|---|---|---|
| Global impulsivity | 61.65 | 10.28 |
| Motor impulsivity | 21.58 | 4.09 |
| Attentional impulsivity | 16.15 | 3.94 |
| Non‐planning impulsivity | 24.12 | 4.79 |
Table 3.
Summary of intercorrelations between subtypes on the Barratt Impulsivity Scale (BIS‐11)
| Measure | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1. Total | – | |||
| 2. Motor | 0.752* | – | ||
| 3. Attention | 0.753* | 0.429* | – | |
| 4. Non‐Planning | 0.810* | 0.445* | 0.443* | – |
*Correlation is significant at the 0.01 level (2‐tailed).
In order to evaluate the validity of using a self‐report measure—rather than one completed by an informant, such as a caregiver—we examined whether patients' reports of their own behavior were correlated with reports provided by a caregiver. Comparisons of self‐report and caregiver report were provided by the Frontal Systems Behavior Scale [FrSBe; Grace and Malloy, 2001], a 46‐item scale that specifically assesses functioning associated with the frontal cortex (i.e., executive functioning, disinhibition, apathy) and includes both a patient and caregiver version. Significant correlations were observed between patient and caregiver reports on both the total FrSBe score (r s = 0.414, P < 0.001) as well as the disinhibition subscale score (r s = 0.484, P < 0.001), thereby supporting the validity of using self‐report measures among this group of patients.
Lesion Analysis
To investigate brain lesions associated with global impulsivity, we applied a whole‐brain VLSM analysis. These results revealed that increased global impulsivity was associated with damage to a broadly distributed network of bilateral prefrontal and left‐lateralized temporal brain regions (see Table 4 and Fig. 2A), including the following structures: left superior frontal gyrus (dorsolateral, orbital, and medial), left middle frontal gyrus (lateral and orbital), left inferior frontal gyrus (triangular and orbital), left anterior cingulate and paracingulate gyri, right middle frontal gyrus (lateral), right superior frontal gyrus (dorsolateral and medial), and right supplementary motor area. Temporal regions associated with global impulsivity included the left temporal gyrus (middle, inferior, and superior) and left temporal pole of the superior and middle temporal gyri. Additionally, lesions in left hippocampus, left parahippocampal gyrus, left fusiform gyrus, and left insula were also associated with increased global impulsivity. The z‐value of these regions ranged from 3.18 to 4.74.
Table 4.
Results from voxel‐based lesion‐symptom analyses showing regions of damage associated with increased global and motor impulsivity
| MNI coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Structure | Hemisphere | Voxels | x | y | z | Z‐value | |
| Global impulsivity | |||||||
| Superior frontal gyrus (dorsolateral, orbital, medial); middle frontal gyrus (lateral, orbital); inferior frontal gyrus (triangular, orbital); insula; anterior cingulate and paracingulate gyri; parahippocampal gyrus; temporal pole: temporal gyrus (superior) | L | 3,736 | −16 | 38 | 24 | 4.74 | |
| Hippocampus; parahippocampal gyrus; fusiform gyrus; temporal gyrus (superior, middle, inferior) | L | 1,760 | −58 | −36 | −20 | 3.86 | |
| Temporal gyrus (superior); temporal pole: temporal gyrus (superior, middle) | L | 12 | −58 | 4 | −14 | 3.44 | |
| Temporal gyrus (superior, middle) | L | 40 | −62 | −2 | −10 | 3.18 | |
| Superior frontal gyrus (dorsolateral) | L | 14 | −32 | 68 | 8 | 3.20 | |
| Superior frontal gyrus (dorsolateral, medial); middle frontal gyrus (lateral); supplementary motor area | R | 513 | 22 | 24 | 36 | 4.04 | |
| Motor impulsivity | |||||||
| Superior frontal gyrus (dorsolateral, orbital, medial); middle frontal gyrus (lateral, orbital), inferior frontal gyrus (triangular, orbital); insula; anterior cingulate and paracingulate gyri | L | 2348 | −20 | 46 | 14 | 4.80 | |
| Superior frontal gyrus (dorsolateral, medial) | L | 16 | −10 | 72 | 20 | 3.64 | |
Note. L, left; R, right; MNI coordinates of peak lesion‐deficit locations. Regions defined using automated anatomical labeling (AAL).
Figure 2.
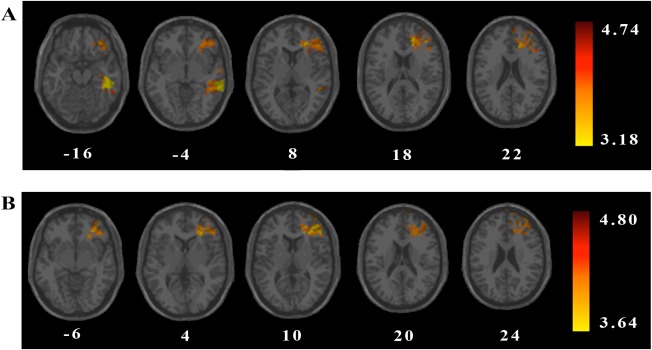
VLSM analysis results depicting areas of damage that were associated with (A) increased global impulsivity (BIS‐11 total impulsivity score) or (B) increased motor impulsivity (BIS‐11 motor impulsivity score). All colored regions showed a significant association between lesion location and BIS‐11 score (1‐tailed, q = 0.025, minimum cluster size = 10 voxels, minimum number of patients = 4), with regions in red corresponding to greater z scores (as shown in the figure legend). Values in white indicate the z coordinates (MNI) of each axial slice. [Color figure can be viewed at http://wileyonlinelibrary.com.]
A secondary set of VLSM analyses was conducted to examine whether lesion location was associated with each of the three impulsivity factors: motor impulsivity, non‐planning impulsivity, and attentional impulsivity. Results revealed a significant relationship between increased motor impulsivity (i.e., acting quickly without thinking) and damage to select prefrontal brain structures (see Table 4 and Fig. 2B), including the following structures: left middle frontal gyrus (lateral and orbital), left inferior frontal gyrus (orbital and triangular), left superior frontal gyrus (dorsolateral, medial, and orbital), left anterior cingulate and paracingulate gyri, and left insula. No significant lesion effects for the non‐planning or attentional impulsivity factors were observed. The z‐value of these regions ranged from 3.64 to 4.80.
In addition to gray matter damage, both global and motor impulsivity were also associated with damage to surrounding white matter tracts (Table 5). Self‐reported global impulsivity was associated with lesions to the left corona radiata (anterior), left genu of the corpus callosum, left posterior thalamic radiation, left sagittal striatum, left superior longitudinal fasciculus, and right corona radiata (anterior and superior). White matter fibers in these areas project extensively throughout frontotemporal regions, aiding communication between structures associated with impulse control. Self‐reported motor impulsivity was associated with damage primarily in the left anterior corona radiata. White matter damage was not associated with non‐planning or attentional impulsivity factors.
Table 5.
Results from voxel‐based lesion‐symptom analyses showing regions of white‐matter damage associated with increased global and motor impulsivity
| Structure | Hemisphere | Voxels | MNI coordinates | Z‐value | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Global impulsivity | |||||||
| Corona radiata (anterior); genu of the corpus callosum | L | 3,736 | −16 | 38 | 24 | 4.74 | |
| Posterior thalamic radiation; saggital striatum; superior longitudinal fasciculus | L | 1,760 | −58 | −36 | −20 | 3.86 | |
| Corona radiata (anterior and superior) | R | 513 | 22 | 24 | 36 | 4.04 | |
| Motor impulsivity | |||||||
| Corona radiata (anterior) | L | 2,348 | −20 | 46 | 14 | 4.8 | |
Note. L, left; R, right; MNI coordinates of peak lesion‐deficit locations. Regions defined using automated anatomical labeling (AAL).
In order to determine whether the above results might be accounted for by differences in total percent brain volume loss, we performed additional post hoc analyses to examine the role of this potential confound (Lê et al., 2014]. First, Mann–Whitney U tests were conducted to detect potential differences in total percent brain volume loss between patients with lesions in locations associated with heightened global or motor impulsivity (as previously identified in the VLSM analyses) and patients without lesions in these locations. Results from these analyses revealed significant differences between the two groups, both when lesions associated with global impulsivity were examined (U = 1059, P = 0.000) as well as lesions associated with motor impulsivity (U = 998, P = 0.000). Given these findings, two multiple linear regression analyses were subsequently performed, in order to assess the extent to which total percent brain volume loss accounted for the relationship between lesion location and global or motor impulsivity. Both models provided significant estimates of global and motor impulsivity (global: [F 2,128 = 9.55, P < 0.001, R2 = 0.13, R2 adjusted = 0.116]; motor: [F 2,128 = 6.46, P < 0.01, R2 = 0.09, R2 adjusted = 0.078]). Most importantly, lesion location itself significantly predicted both heightened global impulsivity (β = 6.46, P < 0.000,) as well as heightened motor impulsivity (β = 0.23, P < 0.01), while total percent brain volume loss did not (global impulsivity: β = 0.25, P > 0.05; motor impulsivity: β = 0.14, P > 0.05). These analyses thus demonstrate that total percent brain volume loss did not largely account for the relationship between lesion location and global or motor impulsivity.
DISCUSSION
Our results revealed that global impulsivity was defined by a complex network largely concentrated within bilateral prefrontal and left temporal regions. In addition, we observed increased motor impulsivity (i.e., acting without thinking) following damage to a subset of left‐lateralized prefrontal regions. Attentional impulsivity and non‐planning impulsivity were not significantly associated with any specific brain regions across subjects. Critically, the use of a VLSM approach allowed us to examine the causal nature of these relationships, with results indicating that bilateral frontotemporal and left prefrontal regions played a causal role in inhibiting impulsive behavior. To our knowledge, this is the first study linking lesion location to impulsivity, and thus may provide valuable insight into the direction of the associative relationships previously described by imaging studies examining this trait.
As hypothesized, damage to the OFC and middle frontal cortex led to increased global impulsivity, replicating results from structural and functional imaging research that have linked these regions to self‐reported impulsivity in healthy participants [Farr et al., 2012; Matsuo et al., 2009; Schilling et al., 2012]. Moreover, our results are consistent with previous studies that have shown increased disinhibition among patients with damage to frontotemporal regions [Ghashghaei et al., 2007; Goswami et al., 2015; Snowden et al., 2001]. Given that connections between the temporal lobe and frontal cortex make up a network involved in behavior regulation, damage to these areas is likely to result in impaired behavioral control [Brothers, 2002]. For example, patients with frontotemporal lobar degeneration are often severely disinhibited and engage in behaviors that grossly violate social norms [Piguet et al., 2011; Williams et al., 2005]. Induced by atrophy of the mesial‐frontal cortex and temporal lobe, these patients fail to successfully inhibit thoughts, urges and actions. Thus, the present study extends previous findings that demonstrate how damage to analogous frontotemporal regions disrupts a large cortical network that contributes to global impulsivity.
In addition to identifying brain regions that subserve global impulsivity, the present study reveals a neural network that may serve a critical role in inhibiting behaviors specific to motor impulsivity. In line with our predictions, lesions in frontotemporal regions, including the OFC, middle frontal gyrus, superior frontal gyrus, dorsolateral PFC, and insula were associated with increased motor impulsivity. These results are similar to functional neuroimaging studies that have associated self‐reported motor impulsivity in healthy participants with increased activity in these regions during standard inhibitory response tasks [Asahi et al., 2004; Farr et al., 2012]. Our results also replicate the only two structural imaging studies specifically examining motor impulsivity [Matsuo et al., 2009; Schilling et al., 2012], both of which report a left‐lateralized prefrontal network (left OFC, left middle frontal gyrus, left superior frontal gyrus) associated with this factor.
Damage to white matter tracts was also found to contribute to heightened self‐reported global and motor impulsivity. Lesions extending into bilateral anterior corona radiata, genu of the corpus callosum, posterior thalamic radiation, sagittal striatum, and superior longitudinal fasciculus were associated with increased global impulsivity, while damage to projection fibers primarily in the left anterior corona radiata led to heightened reports of motor impulsivity. These results are supported by evidence from a recent study [Depue et al., 2015] confirming that white matter integrity within the PFC is critical for successfully inhibiting irrelevant stimuli and stopping programmed motor responses.
The present study did not find evidence that non‐planning impulsivity or attentional impulsivity were significantly associated with a specific neural signature. While two previous studies did observe distinct structural differences associated with all three impulsivity factors in a sample of healthy participants [Matsuo et al., 2009; Schilling et al., 2012], other studies have failed to identify brain regions specifically associated with non‐planning and attentional impulsivity [e.g., Asahi et al., 2004]. Given that all three factors have comparable reliability [e.g., Stahl et al., 2014], and that variability between the three factors did not significantly differ in our sample, the explanation for our lack of findings is not likely to involve potential psychometric issues associated with the non‐planning and attentional factors. Instead, it is possible that the motor impulsivity factor is subserved by a more reliable cognitive process than the non‐planning and attentional factors. This interpretation may be supported by a recent study [Caswell et al., 2015] indicating that motor impulsivity is the only factor on the BIS‐11 scale to correlate significantly with the stop‐signal task, a standard measure of inhibitory control that is considered to capture the most basic and fundamental components of inhibitory processing [Logan, 1994]. Like the stop‐signal task, which also reliably recruits inferior frontal gyrus, it may be that the motor impulsivity factor is mediated by a basic inhibitory process that is reliably associated with this well‐defined neural network.
Despite the unique opportunity to sample a large, relatively homogenous set of patients with focal brain lesions, we acknowledge specific limitations to the present study. First, our sample was composed entirely of older adult male combat veterans. Therefore, it may be difficult to interpret how our results might generalize to a more diverse population, particularly given that impulsivity has been associated with distinct brain structures in men versus women [Diekhof et al., 2012]. Furthermore, our use of a self‐report measure in this patient population could be concerning, as there is evidence to suggest that some TBI patients may lack insight or awareness regarding their conditions, potentially weakening the validity of their responses [Kelley et al., 2014; Robertson and Schmitter‐Edgecombe, 2015]. As explained above, this concern was addressed by an additional analysis, which made use of available data from a measure that incorporates both patients' and caretakers' reports of the patients' behavioral changes following frontal lobe injury (FrSBe). Within our sample, patient and caregiver scores on both the disinhibition subscale and the total score of this measure were significantly correlated, suggesting that our patients had insight regarding their reported behaviors.
Furthermore, there is evidence to suggest self‐report methods may be more reliable than laboratory tasks in assessing trait impulsivity [Stahl et al., 2014]. This may be because self‐report measures have the ability to assess stable personality traits as opposed to state‐dependent behaviors. In fact, while positive correlations have been found between both self‐report and traditional laboratory measures, they are often weak and inconsistent [Caswell et al., 2015; Reynolds et al., 2006] suggesting that they may be measuring subtly different aspects of impulsivity [Moeller et al., 2001; Snowden and Gray, 2011; Stahl et al., 2014]. For example, self‐report questionnaires allow researchers to measure an individual's behavior within various social contexts [Aichert et al., 2012], while traditional laboratory‐based measures of impulsivity explore specific inhibitory functions in response to stimulus‐driven cues. Furthermore, correlations are weak even among multiple laboratory‐based measures, such as the stop‐signal task and delay‐discounting tasks, indicating that while these processes may share overlapping features, the brain regions supporting these functions (i.e., from valuation of response options to initiation of purposeful “stopping”) may be distinct [Dalley et al., 2011]. As a result, self‐report data may afford researchers an opportunity to measure impulsivity as a behavioral consequence of underlying inhibitory failures.
In summary, our findings add to existing literature supporting prefrontal cortical involvement in behavioral impulsivity, but more importantly, also provide new evidence for a predominantly left‐lateralized network specific to motor impulsivity. Future studies could directly examine the possible role of semantic processing in impulsivity, for example, by evaluating this trait specifically among patients with semantic processing deficits such as expressive aphasia. In addition, future studies might also aim to determine whether methods of targeting and enhancing regional activity—for example, using non‐invasive brain stimulation—might be effective in improving performance on tasks related to global and motor impulse control [Hogeveen et al., 2016]. With a better understanding of the neural systems responsible for both producing and inhibiting impulsive behaviors, we will not only develop a more complete picture of the inhibitory control system, but also aid in the development of new, targeted therapeutic strategies for strengthening this system and improving behavioral impulse control.
ACKNOWLEDGMENTS
The authors would like to thank the National Institute of Neurological Disorders and Stroke and the National Naval Medical Center for providing their facilities and supporting this research along with the Vietnam veterans who donated their time and energy to participating in this study. We would also like to thank G. J. Solomon, V. Raymont, S. Bonifant, B. Cheon, C. Ngo, A. Greathouse, K. Reding, and G. Tasick for the testing and evaluation of participants.
REFERENCES
- AFQT‐7A (1960): Department of Defense Form 1293, March 1.
- Aichert DS, Wöstmann NM, Costa A, Macare C, Wenig JR, Möller HJ, Rubia K, Ettinger U (2012): Associations between trait impulsivity and prepotent response inhibition. J Clin Exp Neuropsychol 34:1016–1032. [DOI] [PubMed] [Google Scholar]
- Asahi S, Okamoto Y, Okada G, Yamawaki S, Yokota N (2004): Negative correlation between right prefrontal activity during response inhibition and impulsiveness: A fMRI study. Eur Arch Psychiatry Clin Neurosci 254:245–251. [DOI] [PubMed] [Google Scholar]
- Barratt ES, Pritchard WS, Faulk DM, Brandt ME (1987): The relationship between impulsiveness subtraits, trait anxiety, and visual N100 augmenting/reducing: A topographic analysis. Pers Individ Dif 8:43–51. [Google Scholar]
- Beck AT, Steer RA, Brown GK (1996): Beck Depression Inventory‐II. San Antonio: Psychological Corporation. [Google Scholar]
- Brothers L (2002): The social brain: A project for integrating primate behavior and neurophysiology in a new domain. Found Social Neurosci 367–384. [Google Scholar]
- Buss AH, Plomin R (1975): A Temperament Theory of Personality Development. New York: Wiley‐Interscience. [Google Scholar]
- Caswell AJ, Bond R, Duka T, Morgan MJ (2015): Further evidence of the heterogeneous nature of impulsivity. Pers Individ Dif 76:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC (1994): Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18:192–205. [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW (2011): Impulsivity, compulsivity, and top‐down cognitive control. Neuron 69:680–694. [DOI] [PubMed] [Google Scholar]
- Daruna JH, Barnes PA (1993): A neurodevelopmental view of impulsivity In: McCown WG, Johnson JL, Shure MB, editors. The Impulsive Client: Theory, Research, and Treatment. Washington, DC: American Psychological Association; pp 23–37. [Google Scholar]
- Delis DC, Kaplan E, Kramer JH (2001): Delis‐Kaplan Executive Function System. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Depue B, Orr J, Smolker H, Naaz F, Banich M (2015): The organization of right prefrontal networks reveals common mechanisms of inhibitory regulation across cognitive, emotional, and motor processes. Cereb Cortex 26:1634–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman SJ (1990): Functional and dysfunctional impulsivity: Personality and cognitive correlates. J Pers Soc Psychol 58:95. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Keil M, Obst KU, Henseler I, Dechent P, Falkai P, Gruber O (2012): A functional neuroimaging study assessing gender differences in the neural mechanisms underlying the ability to resist impulsive desires. Brain Res 1473:63–77. [DOI] [PubMed] [Google Scholar]
- Dom G, Hulstijn W, Sabbe B (2006): Differences in impulsivity and sensation seeking between early‐ and late‐onset alcoholics. Addict Behav 31:298–308. [DOI] [PubMed] [Google Scholar]
- Eysenck SB, Eysenck HJ (1977): The place of impulsiveness in a dimensional system of personality description. Br J Soc Clin Psychol 16:57–68. [DOI] [PubMed] [Google Scholar]
- Eysenck SB, Pearson PR, Easting G, Allsopp JF (1985): Age norms for impulsiveness, venturesomeness and empathy in adults. Pers Individ Dif 6:613–619. [Google Scholar]
- Farr OM, Hu S, Zhang S, Chiang‐shan RL (2012): Decreased saliency processing as a neural measure of Barratt impulsivity in healthy adults. NeuroImage 63:1070–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbing DW, Ahadi SA, Patton JH (1987): Toward a conceptualization of impulsivity: Components across the behavioral and self‐report domains. Multivar Behav Res 22:357–379. [DOI] [PubMed] [Google Scholar]
- Ghashghaei H, Hilgetag C, Barbas H (2007): Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. NeuroImage 34:905–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami R, Dufort P, Tartaglia M, Green R, Crawley A, Tator CH, Wennberg R, Mikulis DJ, Keightley M, Davis KD (2015): Frontotemporal correlates of impulsivity and machine learning in retired professional athletes with a history of multiple concussions. Brain Struct Funct 221:1911–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace J, Malloy PF (2001): Frontal Systems Behavior Scale: Professional Manual. Lutz, FL: Psychological Assessment Resources Inc. [Google Scholar]
- Hogeveen J, Grafman J, Aboseria M, David A, Bikson M, Hauner K (2016): Effects of high‐definition and conventional tDCS on response inhibition. Brain Stimul. [DOI] [PubMed] [Google Scholar]
- Kelley E, Sullivan C, Loughlin JK, Hutson L, Dahdah MN, Long MK, Schwab KA, Poole JH (2014): Self‐awareness and neurobehavioral outcomes, 5 years or more after moderate to severe brain injury. J Head Trauma Rehabil 29:147–152. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein E (2003): Multiple neuronal networks mediate sustained attention. J Cogn Neurosci 15:1028–1038. [DOI] [PubMed] [Google Scholar]
- Lê K, Coelho C, Mozeiko J, Krueger F, Grafman J (2014): Does brain volume loss predict cognitive and narrative discourse performance following traumatic brain injury?. Am J Speech‐Lang Pathol 23:S271–S284. [DOI] [PubMed] [Google Scholar]
- Logan GD (1994): On the ability to inhibit thought and action: A users' guide to the stop signal paradigm In: Dagenbach D, Carr T, editors. Inhibitory Processes in Attention, Memory and Language. San Diego: Academic Press; pp 189–239. [Google Scholar]
- Lynam D, Smith G, Cyders M, Fischer S, Whiteside S (2007): The UPPS‐P: A multidimensional measure of risk for impulsive behavior. Unpublished technical report.
- Makale M, Solomon J, Patronas NJ, Danek A, Butman JA, Grafman J (2002): Quantification of brain lesions using interactive automated software. Behav Res Methods Instrum Comput 34:6–18. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Nicoletti M, Nemoto K, Hatch JP, Peluso MA, Nery FG, Soares JC (2009): A voxel‐based morphometry study of frontal gray matter correlates of impulsivity. Hum Brain Mapp 30:1188–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil MM, Prescott TE (1994): Revised Token Test. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J (2001): The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat 21:193–198. [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga A, Pike B, Neto PR, Evans A, Zhang J, Huang H, Miller MI, Zijl P, Mazziotta J (2008): Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage 40:570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JD, Bagby RM, Webster CD (1993): Domains of the impulsivity construct: A factor analytic investigation. Pers Individ Dif 15:267–274. [Google Scholar]
- Patton JH, Stanford MS, Barratt ES (1995): Factor structure of the Barratt impulsiveness scale. J Clin Psychol 51:768–774. [DOI] [PubMed] [Google Scholar]
- Peluso M, Hatch J, Glahn D, Monkul E, Sanches M, Najt P, Bowden CL, Barratt ES, Soares JC (2007): Trait impulsivity in patients with mood disorders. J Affect Disord 100:227–231. [DOI] [PubMed] [Google Scholar]
- Piguet O, Hornberger M, Mioshi E, Hodges JR (2011): Behavioural‐variant frontotemporal dementia: Diagnosis, clinical staging, and management. Lancet Neurol 10:162–172. [DOI] [PubMed] [Google Scholar]
- Revelle W (1997): Extraversion and Impulsivity: The Lost Dimension, Vol. 189 Amsterdam: Pergamon/Elsevier Science. [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, & de Wit H (2006): Dimensions of impulsive behavior: Personality and behavioral measures. Pers Individ Dif 40:305–315. [Google Scholar]
- Robertson K, Schmitter‐Edgecombe M (2015): Self‐awareness and traumatic brain injury outcome. Brain Injury 29:848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalling D, Åsberg M (1985): Biological and psychological correlates of impulsiveness and monotony avoidance In: Strelau J, Farley FH, Gale A, editors. The Biological Bases of Personality and Behaviour. Washington, DC: Hemisphere Publishing Corporation. [Google Scholar]
- Schilling C, Kühn S, Romanowski A, Schubert F, Kathmann N, Gallinat J (2012): Cortical thickness correlates with impulsiveness in healthy adults. NeuroImage 59:824–830. [DOI] [PubMed] [Google Scholar]
- Snowden J, Bathgate D, Varma A, Blackshaw A, Gibbons Z, Neary D (2011): Impulsivity and psychopathy: Associations between the Barrett Impulsivity Scale and the Psychopathy Checklist revised. Psychiatry Res 187:414–417. [DOI] [PubMed] [Google Scholar]
- Snowden RJ, Gray NS (2001): Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry 70:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon J, Raymont V, Braun A, Butman JA, Grafman J (2007): User‐friendly software for the analysis of brain lesions (ABLe). Comput Methods Programs Biomed 86:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl C, Voss A, Schmitz F, Nuszbaum M, Tüscher O, Lieb K, Klauer KC (2014): Behavioral components of impulsivity. J Exp Psychol: Gen 143:850. [DOI] [PubMed] [Google Scholar]
- Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH (2009): Fifty years of the Barratt Impulsiveness Scale: An update and review. Pers Individ Dif 47:385–395. [Google Scholar]
- Swann AC, Steinberg JL, Lijffijt M, Moeller FG (2008): Impulsivity: Differential relationship to depression and mania in bipolar disorder. J Affect Disord 106:241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanski SM, Knight RT (2014): Insights into human behavior from lesions to the prefrontal cortex. Neuron 83:1002–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, . . . Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Williams GB, Nestor PJ, Hodges JR (2005): Neural correlates of semantic and behavioural deficits in frontotemporal dementia. NeuroImage 24:1042–1051. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC (1998): Automated image registration: I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr 22:139–152. [DOI] [PubMed] [Google Scholar]


