Abstract
Background
Chronic methamphetamine use may lead to changes in reward-related function of the ventral striatum and caudate nucleus. Whether methamphetamine dependent individuals show heightened reactivity to positively valenced stimuli (i.e., positive reinforcement mechanisms), or an exaggerated response to negatively valenced stimuli (i.e., driven by negative reinforcement mechanisms) remains unclear. This study investigated neural functioning of expectancy and receipt for gains and losses in adults with (METH+) and without (METH−) histories of methamphetamine dependence.
Methods
Participants (17 METH+; 23 METH−) performed a probabilistic feedback expectancy task during blood-oxygen level-dependent (BOLD) functional magnetic resonance imaging (fMRI). Participants were given visual cues probabilistically associated with monetary gain, loss, or neutral outcomes. General linear models examined the BOLD response to: (1) anticipation of gains and losses, and (2) gain and loss monetary outcomes.
Results
METH+ had less BOLD response to loss anticipation than METH− in the ventral striatum and posterior caudate. METH+ also showed more BOLD response to loss outcomes than to gain outcomes in the anterior and posterior caudate, whereas METH− did not show differential responses to the valence of outcomes.
Discussion
METH+ individuals showed attenuated neural response to anticipated gains and losses, but their response to loss outcomes was greater than to gain outcomes. A decreased response to loss anticipation, along with a greater response to loss outcomes, suggests an altered ability to evaluate future risks and benefits based upon prior experience, which may underlie suboptimal decision-making in METH+ individuals that increases the likelihood of risky behavior.
Keywords: Methamphetamine, reward, anticipation, receipt, striatum, fMRI
Introduction
Stimulant-using individuals often demonstrate dysfunctional decision-making, which may predate the initiation of use (Leland et al., 2006; Leland and Paulus, 2005; Paulus et al., 2008). Individuals with a history of methamphetamine use (METH+) in particular appear more influenced by the immediately preceding choice (Paulus et al., 2002), show a rigid stimulus-response relationship (Paulus et al., 2003), and are less able to adjust decision-making to short-term versus long-term gains (Gonzalez et al., 2007). METH+ individuals have also shown inefficient cortical processing in delay discounting, as seen by a preference for immediate rewards and no significant changes in cortical activation in association with decision-making difficulty (Monterosso et al., 2007).
One source of this disadvantageous decision-making may be impaired reward processing. Regions engaged during reward processing include the ventral striatum, orbitofrontal cortex, and ventral anterior cingulate (Haber and Knutson, 2010; Wallis and Kennerley, 2011; Volkow et al., 1996). Under normal conditions, neurons within this network respond to various reward-related events (Rolls, 2000; Schoenbaum et al., 1998; Schultz, 1998; Tremblay and Schultz, 1999) and may be modulated by motivation, preference, and expectancy. Dopamine neurons in particular are activated by the rewarding characteristics of a wide range of somatosensory, visual, and auditory stimuli, and have been shown to distinguish between reward and nonreward objects (Romo and Schultz, 1990). Moreover, dopamine innervation may have persistent effects upon corticostriatal synapses; dopamine denervations within the striatum have been shown to reduce the number of dendritic spines (Ingham et al., 1993). In this way, dopaminergic response patterns work in tandem with the striatum to build appropriate behavioral responses based upon contextual information (Nakahara et al., 2004; Reynolds et al., 2001; Satoh et al., 2003; Schultz et al., 1993). Of the brain regions mediating reward, the ventral striatum has been theorized to contribute a central role to reward processing due to massive projections of midbrain dopaminergic neurons into this region (Daniel and Pollmann, 2014).
Functional magnetic resonance imaging (fMRI) studies have separated the component processes involved in reward expectancy (i.e., the incentive salience of a stimulus and motivator to attain a particular goal) and outcome (i.e., the pleasure or disappointment experienced by obtaining or not receiving that target). The ventral striatum is preferentially activated by reward expectation (Knutson et al., 2001; O'Doherty et al., 2002). Its selective activation during reward anticipation has been observed for primary rewards, such as a sweet taste (O'Doherty et al., 2002; Berns et al., 2001; McClure et al., 2003), and secondary rewards, such as money (Knutson et al., 2001; Breiter et al., 2001; Delgado et al., 2000; Elliott et al., 2003; Thut et al., 1997). In contrast, the anterior caudate, which receives projections from the prefrontal cortex and midbrain dopaminergic neurons, may be engaged as part of its role in goal-directed behavior (Balleine and O'Doherty, 2010; Yin and Knowlton, 2006). However, others have reported activation of the caudate to receipt of reward as well (Bischoff-Grethe et al., 2015; Delgado et al., 2000). These activation patterns may be linked to dopamine neurotransmission, as dopamine release in the nucleus accumbens (part of the ventral striatum) increases local blood oxygen level dependent (BOLD) signal through agonism of postsynaptic D1 receptors (Knutson and Gibbs, 2007). Furthermore, this activation may be lateralized. Dopaminergic transmission in response to unpredicted rewards has been shown primarily in the right ventral striatum (Martin-Soelch et al., 2011; Molochnikov and Cohen, 2014). While both the left and right striatum may react similarly to anticipation and outcomes, a right asymmetry has often been reported in humans related to motivational value (Jocham et al., 2009; Spreckelmeyer et al., 2009). Furthermore, this laterality may be related to asymmetries in D2 receptor binding that may be associated with differences in approach and avoidance behavior (Tomer et al., 2014).
While differential reward processing has been reported in abstinent users of nicotine, cocaine, marijuana, and alcohol compared to non-users (Fedota et al., 2015; Goldman, 2002; Galen and Henderson, 1999; Rose et al., 2013; Balodis and Potenza, 2015), few studies have focused on the functional neuroanatomy of reward anticipation and outcome in METH+. In healthy volunteers, acute amphetamine administration decreases ventral striatal response to gain anticipation, but increases response to loss anticipation and gain outcomes during a reward task (Knutson et al., 2004). Prior studies have demonstrated alterations in frontostriatal regions due to long-term methamphetamine use (London et al., 2015). Acute administration of substances of abuse, especially stimulants, produces up to five times greater increase in striatal dopamine than do natural rewards (Chau et al., 2004). Stimulants initially interact with monoamine transporter proteins; cocaine inhibits all three monoamine transporters (dopamine, serotonin, and norepinephrine), while amphetamines additionally increase monoamine release (Koob, 1999; Wise, 1998). Chronic administration of stimulants increases reward thresholds (Koob, 1999; Koob and Le Moal, 2001) due to their effects on dopamine receptors. In particular, the striatum has reduced dopamine receptor binding (Chang et al., 2007; Volkow et al., 2001) and increased gray matter volume (Jernigan et al., 2005). This receptor decrease (believed to reflect hypodopaminergic function) may in part account for subsequent cravings and the progression to dependence (Volkow et al., 2002). Given the role of the striatum in reward anticipation and receipt, one would predict altered striatal function during reward anticipation and receipt in stimulant-dependent individuals, but findings have been mixed. Treatment-seeking cocaine dependent individuals showed less striatal response to reward anticipation (Bustamante et al., 2014), greater response to rewarding outcomes (Jia et al., 2011), or no discernable differences (Patel et al., 2013), relative to healthy subjects. During the Balloon Analog Risk Task, a paradigm known to engage neural substrates for evaluating risk, reward, and decision-making, METH+ individuals showed greater modulation of ventral striatal activity, but less modulation of the dorsolateral prefrontal cortex, compared to controls in relation to risk and reward, suggesting impaired processing of anticipated outcomes (Kohno et al., 2014). Furthermore apathy, which is associated with a loss of motivation,(Levy and Dubois, 2006) and is linked to frontostriatal pathways common to reward processing (Bonelli and Cummings, 2007), is elevated in METH+ (Marquine et al., 2014; Cattie et al., 2012; Gonzalez et al., 2005). Thus, an additional question is whether apathy might play a role in how METH+ individuals respond to reward expectancy and outcomes.
This study examined BOLD response in methamphetamine dependence using a task that separates the expectancy and outcome phases for reward and loss. We hypothesized that recently dependent METH+ individuals would show less ventral striatal activation to reward expectancy and less caudate activation to rewarding outcomes than METH− volunteers. We further hypothesized METH+ would show an attenuated response to punishment expectancy, in keeping with deficits considering negative consequences during risky decision-making. Finally, we hypothesized that greater apathy in METH+ would be associated with reduced response to outcomes for rewards and losses.
Methods
Participants
Seventeen adults with histories of methamphetamine dependence (METH+) and 25 comparison adults (METH−) were recruited through the Translational Methamphetamine AIDS Research Center. METH+ met diagnostic (DSM-IV; American Psychiatric Association, 2000) criteria for lifetime amphetamine dependence including abuse or dependence in the past 18 months as determined by the Composite International Diagnostic Interview (CIDI; Kessler and Ustun, 2004). METH− participants did not meet criteria for lifetime or current methamphetamine abuse or dependence. In terms of non-methamphetamine related substance use, participants were excluded if they met DSM-IV criteria for substance (other than alcohol, marijuana and nicotine) abuse in the prior year or dependence within the preceding five years. Participants who met criteria for lifetime dependence or abuse of marijuana within the last 12 months were enrolled. Those who met lifetime criteria for alcohol abuse within the prior 12 months were enrolled, but were excluded if they met criteria for dependence within the previous 12 months. Nicotine use was not exclusionary, and participants were told not to alter their typical pattern of daily usage. They were asked to refrain from smoking during the break in the scanning session. Breath carbon monoxide levels and the presence of cotinine in urine were assessed on the day of the scan. Participants also were excluded for: positive urine toxicology screen or Breathalyzer for illicit drugs (other than marijuana due to its long-lasting detectability) or recent alcohol use on the day of scan; MRI contraindication; lifetime history of schizophrenia or other psychotic disorder; previous cerebrovascular events as determined by comprehensive neurological exam; head injury with loss of consciousness >30 minutes or neurologic complications; or seizure disorder. Participants were recruited from the San Diego area via flyers and advertisements at community events and drug dependence treatment programs. All participants gave written consent prior to enrollment and again prior to scanning. The University of California, San Diego Human Research Protections Program approved all procedures.
Measures
Participants completed the Wide Range Achievement Test-4 as a measure of premorbid intelligence and quality of education (WRAT-4; Wilkinson and Robertson, 2006), and a comprehensive neuropsychological test battery (Heaton et al., 2010) that was summarized as a Global Deficit Score (GDS; Carey et al., 2004; Heaton et al., 1995; Heaton et al., 1994). They also completed the Kalichman Sexual Sensation Seeking Scale (Kalichman et al., 1994); Urgency, Premeditation, Perseverance, and Sensation Seeking Impulsive Behavior Scale (UPPS; Whiteside and Lynam, 2001); Frontal Systems Behavior Scale (Grace and Malloy, 2001); Barratt Impulsivity Scale (BIS; Patton et al., 1995); and Iowa Gambling Task (IGT; Bechara, 2007) during a clinical assessment study visit 68.3 ± 32.7 (mean ± SD) days prior to the MRI visit. On the scan day, participants completed the Beck Depression Inventory (BDI-II; Beck et al., 1996), Profile of Mood State (POMS; Pollock et al., 1979), and, if current tobacco use was endorsed, the Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991). Components of these behavioral scales were used to define frontal systems behavior associated with impulsivity/inhibitory control, sensation seeking, and apathy (Marquine et al., 2014). Two METH− were excluded due to motion artifacts during fMRI that exceeded 3 mm, leaving a final sample of 23 METH− and 17 METH+.
Procedures
Behavioral paradigm
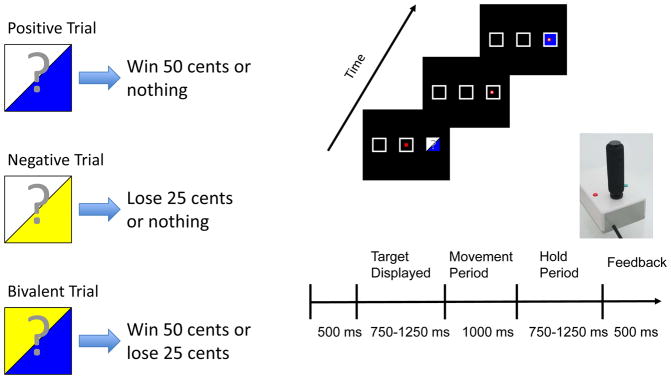
During fMRI, participants performed a probabilistic feedback expectancy task implemented in Presentation software (Neurobehavioral Systems, Albany, CA), where visual cues were paired with monetary outcomes. Cues were projected onto a screen, viewed via an angled mirror mounted on the MRI head coil. Participants used a fiber optic joystick (Current Designs, Philadelphia, PA) to control a gray square cursor. A joystick, rather than a button press, was chosen because it engages greater movement control in terms of musculature, which may contribute to the dorsal striatal response during movements elicited by reward anticipation often reported in animal reward processing studies (Bischoff-Grethe et al., 2015; Hollerman et al., 1998; Kawagoe et al., 1998). Three square targets were presented on a black background. For the first trial of each functional run, participants aligned the cursor to the center target. A target stimulus, represented as one of three visual cues (see Figure 1), was briefly presented for 750–1250 ms in one of three locations. The participants had 1000 ms to align the cursor with the previously illuminated target then hold position for 750–1250 ms, which allowed for better temporal separation of the anticipation and outcome periods. Next, the target was illuminated to indicate financial outcome, and the current location was used as the starting point for the next trial. Four trial types were used: gain trials, in which the stimulus indicated a 50% chance of a gain or neutral monetary outcome; loss trials, indicating a 50% chance of a loss or neutral monetary outcome; bivalent trials, indicating a 50% chance of a gain or loss monetary outcome; and fixation trials, in which no target was illuminated and the participant remained fixated at the current location. Each run contained 63 trials of approximately 4 seconds duration, and each trial type occurred 25% of the time. All participants practiced the task outside the scanner to ensure comprehension of instructions and were informed they would receive additional compensation based on their performance. The monetary amounts (i.e., 50 cents gain, 25 cents loss) were chosen so that participants earned an additional $25 above the standard compensation of $75. When monetary rewards are too small, it can lead to poorer performance than no compensation at all (Gneezy and Rustichini, 2000). In addition, prospect theory suggests that individuals prefer avoiding losses compared to achieving equal gains (Kahneman and Tversky, 1979); although our task involves no decision making, it was important to ensure that monetary gains were salient.
Figure 1.
Examples of the trial types and the timeline for a typical trial. The participant is presented with one of three probabilistic cues; upon extinguishing, the subject uses the fiber optic joystick to move to the target’s location and hold during the delay period. Probabilistic feedback based upon the presented cue is provided for each trial as either a blue filled target indicating a win of 50 cents, a yellow filled target indicating a loss of 25 cents, or a white target indicating neither losses nor gains for that trial.
Imaging
Two functional imaging runs were acquired using T2* weighted echo planar imaging (EPI) on one of two scanning systems: a 3T General Electric Signa HDx (Milwaukee, WI) (252 volumes, TR=2 s, TE=30 ms, flip angle=90°, FOV 24 cm, 64×64 matrix, 3.55×3.55 mm in-plane resolution, 40 3.0 mm [2.6 mm + 0.3 mm gap] ascending interleaved axial slices), or, due to scanner upgrade, a 3T General Electric Discovery MR750 (Milwaukee, WI) (identical parameters as above except 3.75×3.75 mm in-plane resolution, 40 3.0 mm ascending interleaved axial slices). The gradients system and application were not changed during the upgrade, and all post processing and analysis steps were consistent across datasets. High-resolution T1-weighted FSPGR anatomical images (MR750: flip angle=8°, 256×256 matrix, 172 1 mm sagittal slices, TR=8.1 s, TE=3.17 ms, 1×1mm in-plane resolution; Signa HDx: same as above except TR=7.77 s, TE=2.97 ms, and 0.97×0.97 mm in-plane resolution) were acquired to permit activation localization and spatial normalization. EPI-based field maps corrected susceptibility-induced geometric distortions. There were no differences in METH status or other demographic factors based on scanning system employed (see Table 1).
Table 1.
Demographics, drug use, and comorbid psychiatric characteristics by group.
| METH+ (n=17) | METH− (n=23) | ||
|---|---|---|---|
| Characteristic | Mean +/− SD | Mean +/− SD | Statistics a |
| Age at scan (years) | 38.0 ± 8.7 | 38.0 ± 11.9 | t(38)=0.00, p=1.00, g=0.00 |
| Years of education | 12.1 ± 2.0 | 14.4 ± 2.2 | t(36.49)=−3.50, p<0.001, g=−1.1 |
| Wide Range Achievement Test-4 standard score | 100.8 ± 10.5 | 108.1 ± 13.4 | t(37.85)=−1.94, p=0.06, g=−0.6 |
| Beck Depression Inventory (at scan) | 13.9 ± 8.9 | 2.0 ± 3.3 | t(19.25)=5.25, p<0.001, g=1.7 |
| Profile of Mood States (at scan) | 72.6 ± 34.1 | 57.1 ± 15.9 | t(21.15)=1.74, p=0.10, g=0.6 |
| Fagerström Test of Nicotine Dependence Total† | 3.5 ± 3.3 | 1.3 ± 2.3 | t(26.84)=2.30, p=0.03, g=0.7 |
| % Male | 100% | 87% | χ2(n=1)=0.89, p=0.35 |
| % Right-handed | 94% | 96% | χ2(n=1)=0.00, p=1.00 |
| % White | 59% | 70% | χ2(n=2)=3.36, p=0.19 |
| % Latino | 18% | 26% | |
| % African-American | 24% | 4% | |
| % Scanned on 3T General Electric MR750 system | 35% | 57% | χ2(n=1)=1.02, p=0.31 |
| Antisocial personality disorder (lifetime) | 53% | 9% | χ2(n=1)=7.51, p=0.01 |
| Attention-deficit/hyperactivity disorder (lifetime) | 29% | 0% | χ2(n=1)=5.28, p=0.02 |
| Major depressive disorder (lifetime) | 47% | 17% | χ2(n=1)=2.81, p=0.09 |
| Tobacco use (current)‡ | 43% | 14% | χ2(n=1)=6.24, p=0.01 |
| Alcohol dependence (lifetime) | 35% | 13% | χ2(n=1)=1.65, p=0.20 |
| Cannabis dependence (lifetime) | 29% | 0% | χ2(n=1)=3.68, p=0.05 |
| Cocaine dependence (lifetime) | 41% | 0% | χ2(n=1)=8.80, p<0.01 |
| Opiate dependence (lifetime) | 5% | 0% | χ2(n=1)=0.02, p=0.88 |
| Lifetime methamphetamine use in grams | 2461 ± 2954 | N/A | |
| Lifetime methamphetamine use in days | 1913 ± 1551 | N/A | |
| Age of first methamphetamine use (years) | 25.4 ± 8.6 | N/A | |
| Days since last methamphetamine use | 173 ± 160 | N/A | |
| Primary route of methamphetamine use | Smoking | N/A |
Statistical comparisons were by means of Welsh t-tests or χ2 tests for equality of proportions, and Hedge’s g to report effect sizes.
This assessment was completed only in participants who endorsed current use of tobacco.
Two METH+ and 3 METH− participants did not report current levels of tobacco use.
Data Analysis
Behavior analysis
The joystick was sampled at 60 Hz and filtered offline with a 10 Hz Butterworth low pass filter (Butterworth, 1930) to remove jitter. Movement onset and offset were determined with a three-step algorithm (Teasdale et al., 1993). Behavioral responses were analyzed using a linear mixed effects (LME) model from the R (R Development Core Team, 2012) nlme package (Pinheiro et al., 2013). Subject was a random effect, and group (METH−, METH+) and anticipation (gain, loss, and bivalent) were the between subjects factors. Three separate performance measures were examined: response time, defined as the time from when the target was extinguished to onset of joystick movement and reflecting how quickly the participant initiated a response; movement duration, defined as the onset of joystick movement to the time of maximum displacement and reflecting how fast the participant moved the joystick to capture the target; and error commission, defined as trials for which the participant moved before the target was extinguished, moved to the wrong target, or failed to capture the target during the allotted time.
Statistical analyses
Functional images were preprocessed and analyzed using Analysis of Functional NeuroImages (AFNI; Cox, 1996), FSL (Smith et al., 2004), and R statistical packages. EPIs were slice-time and motion-corrected, and aligned to high-resolution anatomical images (Saad et al., 2009). Outlier volumes, identified with AFNI’s 3dToutcount, which were more than 5 times greater than the mean were censored. T1-weighted images were skull-stripped and registered to the MNI-152 atlas using affine transform followed by nonlinear refinement (Andersson et al., 2010; Jenkinson and Smith, 2001). Functional data were aligned to standard space, resampled to 3 mm isotropic voxels, and smoothed with a 4.2 mm FWHM Gaussian kernel.
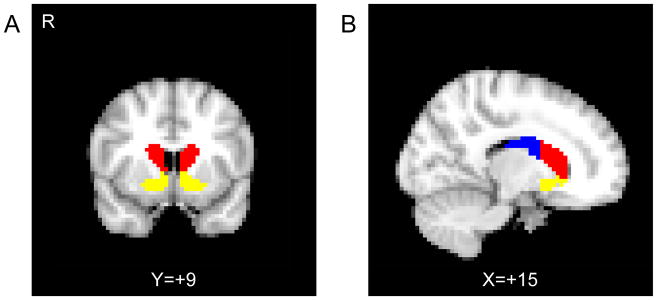
Statistical analyses used two general linear models (GLM) using AFNI’s SPMG basis function within 3dDeconvolve. The first GLM modeled gain, loss, and, bivalent anticipation, and the second modeled gain, neutral, and loss outcomes, with six motion parameters included as covariates of no interest in each model. The fixation trial periods were not explicitly modeled and served as an implicit baseline. Following deconvolution, beta values were converted to percent signal change. The mean percent signal change within each region of interest (ROI) for each event was subjected to LME in R. Striatal ROIs (i.e., the putamen, caudate, and nucleus accumbens in their entirety) were initially extracted from the Harvard-Oxford atlas (Desikan et al., 2006) and were manually edited to define based upon functional distinctions (Martinez et al., 2003). The ventral striatum consisted of the nucleus accumbens, rostroventral caudate, and rostroventral putamen (Fudge et al., 2004; Haber et al., 2006). The caudate nucleus was divided into anterior and posterior regions at the anterior commissure (Figure 2).
Figure 2.
Striatal subregion masks used in the analysis. A) The coronal plane anterior to the plane of the anterior commissure is shown, containing the ventral striatum (yellow) and the anterior caudate (red). B) The sagittal plane showing the division between the anterior caudate (red) and the posterior caudate (blue), as defined by the anterior commissure.
Studies using multisite imaging data have reported that inter-participant variance was 7 to 44 times greater than that generated by site variance (Gountouna et al., 2010; Suckling et al., 2008; Brown et al., 2011; Costafreda et al., 2007), even when group membership was confounded by site (Sutton et al., 2008). This suggests that, despite using two different systems, scanner variance is unlikely to contribute to any task or group-related effects we may observe. As each participant was only scanned once in our study, we cannot separately estimate the effects due to participant or scanner. We therefore included subject, nested within scanner, as a random effect in our linear mixed effect models.
For each linear mixed effects analysis, group (METH−, METH+) was treated as a between-subject factor, and hemisphere (left, right) was treated as a within-subject factor due to potential laterality of the striatal response. The first analysis put anticipation (gain or loss) as the within subjects factor, and a separate analysis used outcome (gain or loss) as the within subject factor. Age may influence response to financial outcomes (Samanez-Larkin et al., 2007) and was therefore included as a covariate. Because there were three ROIs (anterior caudate, posterior caudate, and ventral striatum), Bonferroni correction (alpha=0.05, p=0.0167) was used to mitigate the effects of multiple comparisons. Secondary voxelwise analyses examined group x anticipation and group x outcome. To guard against false positives in voxelwise analyses, Monte-Carlo simulations using 3dClustSim indicated an a priori voxel-wise p < 0.05 in a cluster of 1701 μl (63 connected voxels), resulting in an a posteriori cluster-wise p < 0.05.
The association between apathy and brain activation for gain anticipation, loss anticipation, gain outcomes, and loss outcomes were explored separately within each group and condition. A regression model, with apathy score as the independent variable, bilateral (i.e., signal averaged across hemispheres) and asymmetrical (i.e., left minus right hemisphere average signals) activation as the dependent variables, and age as a covariate, was used to determine if any detected relationships were bilateral or lateralized. Similarly, to assess whether methamphetamine use history influenced findings, we fitted a multiple linear regression model with the log transforms of age (as older age might be associated with reduced reward sensitivity), days since last use, age of first use, and methamphetamine density (i.e., total quantity used /total days used) as predictors of the bilateral and asymmetric activation in each ROI. Finally, we explored the associations of conditions highly comorbid with amphetamine dependence: antisocial personality disorder (ASPD), attention-deficit/hyperactivity disorder (ADHD), nicotine dependence (using FTND), and impulsivity, as these conditions have been known to influence the striatum’s response to rewards (Glenn and Yang, 2012; Plichta and Scheres, 2014; Sweitzer et al., 2016; Perry and Carroll, 2008; Taylor et al., 2016; Verdejo-Garcia et al., 2008). These variables were explored in relation to bilateral and asymmetrical striatal activation to anticipation and outcomes using logistic regression or Pearson’s correlations, respectively. As these were exploratory analyses, no correction for multiple comparisons was performed.
Results
Demographics
Groups were similar in age, gender, handedness, and ethnicity (see Table 1). METH+ participants reported a history of prior treatment. More METH+ participants had a lifetime diagnosis of ASPD or ADHD, and were more likely to use tobacco than METH−. METH+ were less educated and tended to score lower on WRAT-4 Reading (see Table 2), but neurocognitive functions (i.e., GDS or impairment status) were not different between groups. Both groups did not differ on Sensation Seeking, but METH+ had higher Impulsivity/Disinhibition and Apathy scores, and exhibited riskier decision-making on the IGT than METH−. At the scan, METH+ reported more depressed mood on the BDI-II than METH−.
Table 2.
Neurocognitive, frontal systems, and behavioral characteristics by group.
| METH+ | METH− | ||
|---|---|---|---|
| Characteristic | Mean +/− SD | Mean +/− SD | Statistics a |
| Neurocognitive Characteristics | |||
| Global Deficit Score | 0.4 ± 0.3 | 0.3 ± 0.3 | t(35.30)=−0.81, p=0.42 |
| % Impaired on Global Deficit Score | 10% | 10% | χ2(n=1)=0.01, p=0.94 |
| Frontal Systems Behavior | |||
| Sensation Seeking | 55.6 ± 9.0 | 52.3 ± 7.9 | t(31.89)=−1.19, p=0.24, g=−0.4 |
| Impulsivity/Disinhibition | 68.3 ± 11.7 | 49.8 ± 8.6 | t(28.17)=−5.51, p<0.001, g=−1.7 |
| Apathy | 76.9 ± 14.3 | 49.3 ± 7.4 | t(22.42)=−7.26, p<0.001, g=−2.3 |
| Iowa Gambling Task Total (Trials 21-100) | −0.1 ± 25.9 | 20.2 ± 28.0 | t(36.02)=2.37, p=0.02, g=0.7 |
| Urgency, Premeditation, Perseverance, and Sensation Seeking Impulsive Behavior Scale | |||
| Sensation Seeking Scale | 32.8 ± 6.5 | 34.4 ± 7.1 | t(36.26)=0.75, p=−.46, g=0.24 |
| Lack of Premeditation Scale | 25.1 ± 4.8 | 19.2 ± 4.0 | t(30.77)=−4.12, p<0.001, g=−1.3 |
| Urgency Scale | 34.2 ± 6.9 | 21.6 ± 6.4 | t(33.11)=−5.93, p<0.001,g=−1.9 |
| Lack of Perseverance Scale | 23.8 ± 4.7 | 17.9 ± 4.6 | t(34.12)=−3.96, p<0.001, g=−1.3 |
| Kalichman Sexual Sensation Seeking Scale | |||
| Non-Sexual Sensation Seeking Scaled Mean | 2.4 ± 0.7 | 2.3 ± 0.6 | t(30.99)=−0.53, p=0.60, g=−0.2 |
| Sexual Sensation Seeking Scaled Mean | 2.5 ± 0.7 | 1.9 ± 0.6 | t(29.48)=−2.69, p=0.01, g=−0.9 |
| Sexual Compulsivity Scaled Mean | 2.0 ± 0.9 | 1.3 ± 0.5 | t(25.06)=−2.88, p=0.01, g=−0.9 |
| Barratt Impulsivity Scale | |||
| Total | 78.0 ± 13.7 | 57.3 ± 11.7 | t(31.33)=−5.02, p<0.001, g=−1.6 |
| Attentional Subscale | 21.1 ± 4.1 | 14.5 ± 3.5 | t(31.17)=−5.35, p<0.001, g=−1.7 |
| Motor Subscale | 24.6 ± 5.9 | 18.6 ± 4.6 | t(29.41)=−3.54, p<0.001, g=−1.1 |
| Non-Planning Subscale | 32.2 ± 5.3 | 24.2 ± 5.1 | t(33.94)=−4.80, p<0.001, g=−1.5 |
Statistical comparisons were by means of Welsh t-tests or χ2 tests for equality of proportions, and Hedge’s g to report effect sizes.
Task Behavior
A group (METH+, METH−) x anticipation (gain, loss, bivalent) LME analysis of selection time revealed no differences between groups (p=0.6), no effect of anticipation (p=0.2), and no interaction (p=0.8). A similar analysis for movement duration found no effect of group (p=0.6) or group x anticipation interaction (p=0.1), but a main effect of anticipation (F(2,73)=4.3, p=0.02). Tukey Honest Significant Difference post hoc comparisons showed that movement duration was slower for bivalent anticipation than gain or loss anticipation (all ps<0.03). Error commission (i.e., initiating a motor response before the target extinguished) did not show an effect of group (p=0.6) or a group x anticipation interaction (p=0.4), but participants were far more likely (F(2,76)=224.2, p<0.001) to commit errors on trials with bivalent anticipation than loss (z=10.2, p<0.001) or gain (z=11.8, p<0.001) anticipation, suggesting that larger differences between potential outcomes was more salient than smaller ones.
Region of interest analyses
Effects of anticipation
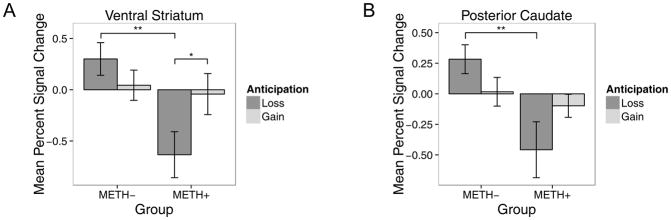
Mean BOLD activation is reported in Table 3. As predicted, a group x anticipation interaction was seen in the ventral striatum (F(1,109)=7.2, p=0.01, g=0.8) and posterior caudate (F(1,114)=7.1, p=0.01, g=0.8). In both regions, METH+ responded less than METH− to loss anticipation (ventral striatum: z=3.4, p=0.004; posterior caudate: z=3.2, p=0.007), and only METH+ tended to have more BOLD response to gain than loss anticipation in the ventral striatum (z=2.5, p=0.05) (see Figure 3). A main effect of group was found for the ventral striatum (F(1,37)=4.7, p=0.04, g=0.7) and the anterior (F(1,38)=9.1, p<0.001, g=0.9) and posterior (F(1,38)=4.2, p=0.05, g=0.6) caudate, but post hoc analyses were not significant (ps > 0.1); therefore this finding will not be interpreted. No main effect of anticipation or hemisphere was detected (all ps > 0.2).
Table 3.
Mean BOLD activation to anticipation trials, by group.
| METH− | METH+ | ||||
|---|---|---|---|---|---|
| ROI | Hemisphere | Gain | Loss | Gain | Loss |
| Ventral Striatum*† | Left | −0.04 ± 0.95 | 0.27 ± 1.18 | −0.23 ± 1.13 | −0.88 ± 1.46 |
| Right | 0.13 ± 1.00 | 0.33 ± 0.98 | 0.14 ± 1.20 | −0.39 ± 1.14 | |
| Anterior Caudate† | Left | 0.09 ± 1.02 | 0.19 ± 0.70 | −0.42 ± 0.72 | −0.89 ± 1.57 |
| Right | 0.21 ± 0.86 | 0.04 ± 1.23 | −0.14 ± 0.58 | −0.58 ± 1.28 | |
| Posterior Caudate*† | Left | 0.02 ± 0.89 | 0.30 ± 0.88 | −0.12 ± 0.55 | −0.38 ± 1.42 |
| Right | 0.01 ± 0.71 | 0.27 ± 0.73 | −0.08 ± 0.57 | −0.53 ± 1.28 | |
Note: BOLD activation reported as mean ± SD. METH−: healthy comparison participants; METH+: participants with a history of methamphetamine dependence. Significant statistical values are reported in main text;
Significant group x anticipation interaction;
Significant main effect of group.
Figure 3.
Bar plots of percent signal change mean ± SEM demonstrating a group x anticipation interaction within the A) ventral striatum and B) posterior caudate. METH+ (n=17) are participants with a history of methamphetamine dependence; METH− (n=23) are healthy comparison participants. *p<0.05; **p<0.01.
Effects of outcome
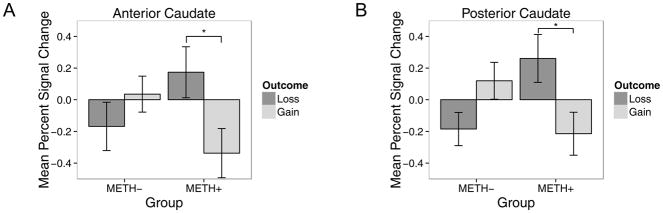
Mean BOLD activation is reported in Table 4. As expected, a group x outcome interaction was seen within the anterior (F(1,114)=8.4, p<0.001, g=0.9) and posterior (F(1,114)=12.7, p<0.001, g=1.1) segments of the caudate (see Figure 4). Post hoc analyses showed that METH+ had more BOLD response to loss than gain outcomes (anterior caudate: z=2.8, p=0.03; posterior caudate: z=2.9, p=0.02). No main effect of group, outcome, or hemisphere were found (all ps > 0.2).
Table 4.
Mean BOLD activation to outcome trials, by group.
| METH− | METH+ | ||||
|---|---|---|---|---|---|
| ROI | Hemisphere | Gain | Loss | Gain | Loss |
| Ventral Striatum | Left | −0.05 ± 0.98 | −0.17 ± 0.74 | 0.26 ± 1.23 | −0.24 ± 0.89 |
| Right | 0.08 ± 1.55 | 0.28 ± 1.05 | 0.25 ± 1.24 | −0.21 ± 0.89 | |
| Anterior Caudate* | Left | −0.05 ± 0.71 | 0.08 ± 0.76 | 0.09 ± 1.05 | −0.46 ± 1.02 |
| Right | −0.29 ± 1.29 | −0.01 ± 0.80 | 0.26 ± 0.84 | −0.22 ± 0.78 | |
| Posterior Caudate* | Left | −0.09 ± 0.73 | 0.15 ± 0.80 | 0.23 ± 0.90 | −0.35 ± 0.95 |
| Right | −0.28 ± 0.70 | 0.09 ± 0.80 | 0.30 ± 0.89 | −0.08 ± 0.59 | |
Note: BOLD activation reported as mean ± SD. METH−: healthy comparison participants; METH+: participants with a history of methamphetamine dependence. Significant statistical values are reported in main text;
Significant group x outcome interaction.
Figure 4.
Bar plots of percent signal change mean ± SEM demonstrating a group x outcome interaction within the A) anterior caudate and B) posterior caudate. METH+ (n=17) are participants with a history of methamphetamine dependence; METH− (n=23) are healthy comparison participants. *p<0.05.
Whole-Brain Voxelwise Analyses
Effects of anticipation
A secondary voxelwise analysis revealed several clusters with a significant group x anticipation interaction, including the left medial frontal gyrus and inferior parietal lobule (see Table 5). Regions exhibiting a main effect of group included the left posterior insula, the right anterior insula, and a cluster that included the right postcentral gyrus and extended into the inferior parietal lobule. Finally, a main effect of anticipation was seen in the left medial frontal and inferior frontal gyri and the right precentral gyrus.
Table 5.
Voxelwise analysis of variance showing a significant main effect of group, anticipation (gain, loss), and the group x anticipation interaction.
| Center mass MNI Coordinates | ||||||||
|---|---|---|---|---|---|---|---|---|
| Structure | Hemisphere | BA | Volume (μL) | X | Y | Z | mean F | mean Hedge's g |
| Group | ||||||||
| Caudate/Medial Frontal Gyrus/Posterior Cingulate | L | 24/23 | 52,947 | −8 | 10 | 21 | 6.4 | 0.8 |
| Thalamus/Posterior Insula | L | 13 | 8,937 | −27 | −32 | 6 | 6.3 | 0.8 |
| Inferior Parietal Lobule | L | 40 | 8,532 | −33 | −42 | 53 | 6.2 | 0.8 |
| Postcentral Gyrus/Inferior Parietal Lobule | R | 2/40 | 6,750 | 47 | −28 | 40 | 6.3 | 0.8 |
| Superior Temporal Gyrus | L | 37 | 3,672 | −45 | −49 | 11 | 6.7 | 0.8 |
| Anterior Insula | R | 13 | 3,537 | 35 | 3 | 1 | 5.5 | 0.7 |
| Lingual Gyrus | R | 18 | 3,348 | 2 | −77 | 1 | 6.0 | 0.8 |
| Superior Frontal Gyrus | R | 9 | 3,024 | 34 | 34 | 35 | 6.9 | 0.8 |
| Precentral Gyrus | R | 6 | 2,187 | 29 | −15 | 61 | 5.9 | 0.8 |
| Anticipation | ||||||||
| Medial Frontal Gyrus | L | 9 | 2,187 | −7 | 43 | 17 | 5.7 | 0.7 |
| Precentral Gyrus | R | 6 | 2,025 | 41 | 11 | 40 | 6.9 | 0.8 |
| Inferior Frontal Gyrus | L | 45 | 1,755 | −40 | 30 | 0 | 5.4 | 0.7 |
| Group x Anticipation | ||||||||
| Middle Frontal Gyrus | L | 9 | 4,968 | −35 | 28 | 11 | 5.7 | 0.7 |
| Medial Frontal Gyrus | L | 24 | 4,887 | −6 | 42 | 18 | 5.9 | 0.8 |
| Inferior Frontal Gyrus | L | 44 | 3,483 | −43 | 7 | 22 | 6.0 | 0.8 |
| Inferior Parietal Lobule | L | 40 | 2,214 | −59 | −32 | 33 | 6.1 | 0.8 |
| Supramarginal Gyrus | R | 40 | 1,890 | 34 | −38 | 37 | 5.7 | 0.7 |
| Superior Parietal Lobule | L | 7 | 1,728 | −26 | −58 | 48 | 5.1 | 0.7 |
Note: Coordinates are in RAI format. BA: Brodmann's area; METH−: healthy comparison participants; METH+: participants with a history of methamphetamine dependence.
Effects of outcome
Only the left precentral and lingual gyri demonstrated a group x outcome interaction (see Table 6). A main effect of group was found within the bilateral lentiform nucleus extending into the thalamus, as well as the left middle and medial frontal gyri. Only one cluster within the left precentral gyrus demonstrated a main effect of outcome.
Table 6.
Voxelwise analysis of variance showing a significant main effect of group, outcome (gain, loss), and the group x outcome interaction.
| Center mass MNI Coordinates | ||||||||
|---|---|---|---|---|---|---|---|---|
| Structure | Hemisphere | BA | Volume (μL) | X | Y | Z | mean F | mean Hedge's g |
| Group | ||||||||
| Lentiform Nucleus/Thalamus | R | 10,989 | −24 | −2 | 2 | 5.9 | 0.8 | |
| Lentiform Nucleus/Thalamus | L | 10,584 | 20 | 5 | 5 | 6.5 | 0.8 | |
| Middle Frontal Gyrus | L | 9 | 6,804 | 38 | −11 | 30 | 5.6 | 0.7 |
| Medial Frontal Gyrus | L | 6/32 | 5,481 | 11 | −28 | 40 | 6.2 | 0.8 |
| Cuneus | R | 18 | 2,133 | −7 | 90 | 8 | 5.6 | 0.7 |
| Middle Frontal Gyrus | L | 10 | 2,052 | 35 | −39 | 16 | 5.9 | 0.8 |
| Uncus | L | 28 | 1,701 | 27 | −4 | −30 | 6.2 | 0.8 |
| Outcome | ||||||||
| Precentral Gyrus | L | 6 | 3,051 | 16 | 23 | 63 | 6.2 | 0.8 |
| Group x Outcome | ||||||||
| Lingual Gyrus | L | 18 | 3,726 | 26 | 89 | −12 | 5.5 | 0.7 |
| Precentral Gyrus | L | 4 | 2,052 | 22 | 27 | 61 | 5.6 | 0.7 |
Note: Coordinates are in RAI format. BA: Brodmann's area; METH−: healthy comparison participants; METH+: participants with a history of methamphetamine dependence.
Relationships to Other Measures
Effects of apathy
In METH+, lower responses to gain anticipation in the bilateral ventral striatum (β=−13.1, t(13)=−3.1, p=0.008) and anterior caudate (β=−13.7, t(13)=−2.4, p=0.01) predicted higher apathy scores. In METH−, lower BOLD responses in the ventral striatum and anterior caudate to loss anticipation (ventral striatum: β=−4.1, t(17)=−2.2, p=0.04; anterior caudate: β=−5.0, t(19)=−2.3, p=0.03) and to gain outcomes (ventral striatum: β=−5.0, t(18)=−3.9, p=0.001; anterior caudate: β=−6.4, t(19)=−3.3, p=0.004) predicted higher apathy scores, suggesting a relationship between even mild levels of apathy and reduced motivation.
Effects of impulsivity
In METH−, higher scores on the IGT, which reflect “safer” decision-making, predicted a lower response to gain anticipation in the anterior caudate, with the right responding less than the left (β=−39.2, t(19)=−2.4, p=0.03). METH− with higher BIS total scores had higher BOLD responses to gain outcomes in the ventral striatum, with the left responding more than the right (β=6.24, t(18)=3.4, p=0.003), and higher BOLD responses to loss outcomes in the anterior (β=7.6, t(19)=2.4, p=0.03) and posterior caudate (β=7.22, t(19)=2.1, p=0.05). In METH+, lower IGT scores, reflecting riskier decision-making, predicted a higher response to loss outcomes in the ventral striatum, with the left responding more than the right (β=−19.8, t(12)=−2.4, p=0.02). METH+ with higher total BIS scores had lower responses to loss outcomes in the ventral striatum (β=−11.56, t(12)=−2.7, p=0.02). Together, these findings suggest that METH+ may ignore negative experiences when making decisions.
Effects of methamphetamine use history
Longer abstinence (range 9 to 539 days) was associated with less response to gain outcomes in the bilateral posterior (β= −0.33, p=0.04) and anterior caudate (β= −0.37, p=0.05), and later age of first methamphetamine use was linked to more response to loss anticipation (β= 1.21, p=0.01). Methamphetamine use density was not related to either anticipation or outcome related responses, suggesting that findings may be related to trait effects of meth use history rather than neurotoxic effect of methamphetamine exposure.
Effects of age
Older METH+ individuals responded less to loss anticipation in the anterior caudate, with the left hemisphere responding less than the right (β= −0.05, p=0.03), and more bilateral posterior caudate response to gain outcomes (β= 0.07, p=0.02). Age was not linked to duration of abstinence in METH+, suggesting age-related changes in reward processing are independent of use intensity.
Effects of tobacco use and mental health
Current and lifetime ASPD and ADHD diagnoses were not linked to bilateral or asymmetric striatal brain response to anticipation or outcomes for METH+. Within METH+, higher Fagerström total scores tended to be associated with a greater left-right difference in the posterior caudate for gain anticipation (r(17)=0.49, p=0.05). Within METH−, higher breath carbon monoxide levels at the time of scan was associated with a greater left-right difference in the anterior caudate for gain anticipation (r(23)=0.44, p=0.04). There was no association between breath carbon monoxide levels and BOLD response in METH+, suggesting findings were not associated with either acute or chronic tobacco use.
Discussion
This study yielded three main results. First, the METH+ group exhibited less response to loss anticipation in the ventral striatum than controls. Second, METH+ exhibited more response to loss outcomes than to gain outcomes in the caudate. Finally, greater apathy in METH+ was associated with less anterior caudate and ventral striatal response to gain anticipation. These findings are consistent with our hypotheses and suggest differences in the neural substrates involved in the processing of anticipation and receipt of potential rewards and losses in methamphetamine dependent individuals. An altered ability to evaluate future risks and benefits may underlie the altered decision-making seen in these individuals, and increase the likelihood of future risky behavior.
METH+ had an attenuated response in the ventral striatum to loss anticipation. The ventral striatum has long been noted for its role in the expectancy of potential rewards, as demonstrated in both animals (Schultz, 2006) and humans (Knutson et al., 2001; Breiter et al., 2001). However, this region has also been shown to be responsive to potential loss (Cooper et al., 2009; Salamone, 1994; Jensen et al., 2003), suggesting a broader role in motivation and decision-making by way of its connections with the ventromedial prefrontal cortex, anterior cingulate, and other areas associated with decision-making (Haber et al., 2006). Importantly, studies of decision-making have shown that METH+ individuals tend to make poor choices despite negative consequences (Gowin et al., 2013; Gowin et al., 2014; Hoffman et al., 2006). Our findings suggest a reduced sensitivity to loss anticipation that may contribute to this behavior. Although we did not detect group differences for outcomes in the ventral striatum, we detected differences for each group’s relationships for risky decision-making and impulsivity with the BOLD response to loss outcomes in the ventral striatum. While METH− with higher impulsivity showed greater BOLD response to loss outcomes, METH+ with higher impulsivity showed reduced BOLD response, suggesting an impaired ability to interpret negative experiences.
In addition to reduced striatal response to the potential for future loss, the caudate showed strong response to loss outcomes in METH+. Greater sensitivity to loss outcomes, coupled with less sensitivity to anticipation, suggests an impaired ability to form action-outcome associations, particularly when the action may lead to a negative consequence. That is, although METH+ may experience negative outcomes intensely, they may be less able to apply that experience to future behavior. Future studies could test this interpretation, using probabilistic association learning tasks that include both positive and negative reinforcement (Mattfeld et al., 2011) and examine the learning curves.
Although we hypothesized that METH+ would show an attenuated response to gain anticipation compared to METH− in the ventral striatum, we did not detect significant group differences. Rather, we found that METH+ showed attenuated loss anticipation compared to METH−, and that the METH+, but not METH− showed differences in their response to gain anticipation vs. loss anticipation. There are ongoing questions in the field as to whether the ventral striatum codes for valence (i.e., gains vs. losses) or salience (i.e., regardless of valence). An increasing number of neuroimaging studies suggest that the ventral striatum may respond to the hedonic salience or arousal, rather than valence alone (Field et al., 2015; Jensen et al., 2007; Zink et al., 2006). This is also supported in the animal literature, where striatal neurons that code positive value were found near neurons coding negative value (Reynolds and Berridge, 2002; Faure et al., 2010). Thus, it is possible that loss anticipation was as arousing as gain anticipation. This is reflected by the fact that the METH+ differentiated gain and loss anticipation, whereas the METH− group did not. This is also supported by prospect theory, which suggests that avoiding a loss may be more relevant than obtaining a gain (Kahneman and Tversky, 1979). Alternatively, a lack of robust differences could potentially be due to variability across individuals in their neural response to gain anticipation.
Consistent with other studies (Cattie et al., 2012; Marquine et al., 2014), METH+ reported overall higher apathy levels than METH−. Elevated apathy in METH+ has been associated with declines in everyday functioning (Cattie et al., 2012), supporting the possibility of impaired striatal function. While mild apathy can be present in otherwise healthy individuals, severe apathy has been linked to striatal (Mendez et al., 1989; Bhatia and Marsden, 1994) and prefrontal cortex (Levy and Dubois, 2006) dysfunction. In our study, METH+ with lower BOLD responses to gain anticipation had higher levels of apathy, suggesting apathy may have had an effect on reward motivation. Given the role of dopamine in mediating motivation and reward sensitivity (Schultz, 2006) and the effects of methamphetamine use on dopamine binding (Chang et al., 2007; Volkow et al., 2001), elevated apathy may in part reflect poor dopaminergic signaling. This has implications for goal-directed behavior, as impairments in associating context with the value of a given behavior might not only impair one’s desire to complete the action, but also the ability to evaluate the consequences of the action.
Other than length of abstinence and onset age, substance use patterns and comorbid diagnoses were not associated with anticipation or outcome. Abstinence duration has been associated with increased availability of dopaminergic transporters in the striatum (Volkow et al., 2001), suggesting at least partial recovery of function and also greater ability to remain abstinent over more days and presumably more relapse opportunities. It’s possible that this improvement may lead to more responsiveness to positive outcomes within the caudate. Although the research on age and methamphetamine use in humans is sparse, animal studies suggest it may cause greater neuronal damage in older animals than younger ones (Bowyer et al., 1993). Our data suggest that older METH+ had lower BOLD responses to loss anticipation in the anterior caudate and higher BOLD responses to gain outcomes in the posterior caudate that was independent of use duration. Future studies should examine whether METH use in older individuals might impair reward processing and decision making than in younger individuals.
Our findings differed from prior studies of reward in those with histories of cocaine use, which reported altered striatal response to gain but not loss, anticipation (Bustamante et al., 2014), or outcome (Jia et al., 2011). Although methamphetamine is similar to cocaine in inhibiting the reuptake of dopamine by dopaminergic transporters (Koob et al., 1998), it is unique in that it promotes the release of dopamine via reverse transport (Sulzer et al., 2005), is more widely distributed in both cortical and subcortical areas, and takes longer to clear from gray matter (Fowler et al., 2008). Repeated exposure to methamphetamine has been shown to cause neurodegeneration of striatal axon terminals (Ares-Santos et al., 2013); elevated levels of dopamine and other neurotransmitters due to methamphetamine use may ultimately lead to oxidative stress and long lasting damage in brain tissue (Volz et al., 2007). These differences in the mechanisms of action and recovery may partly explain the effects seen with methamphetamine relative to cocaine use.
This study has several limitations. First, the cognitive assessment visit occurred on average 2 months prior to the scan visit, which may affect interpretation based on temporal stability of the measures. While the GDS and some of the frontal systems behavior measures have good temporal stability for time periods less than 3 months (Cysique et al., 2011; Woods et al., 2006; Beck et al., 1995), the POMS vigor subscale and the motivation-related questions from the BDI-II used to determine apathy have less stability over time. Overall, the METH+ participants met criteria for depression at both visits, and the Frontal Systems Behavior Scale has been reported to be stable (Velligan et al., 2002), supporting chronically elevated apathy in METH+. Therefore, the apathy composite measure combined stable measures from the cognitive assessment visit with the more state-based measures (i.e., POMS, BDI-II) from the imaging visit. Future studies should conduct the cognitive assessment closer to the imaging visit. Second, most METH+ reported current tobacco use, whereas few METH− did. It is possible that effects may be at least partly attributed to tobacco, although post hoc analyses found little evidence of its influence on our findings. Third, in several ROIs, the METH+ percent signal change was below zero, which can be difficult to interpret. Negative BOLD signal could be related to decreased cerebral blood flow, in turn caused by suppression of neural activity (Shmuel et al., 2006; Devor et al., 2007). As separate GLMS for anticipation and outcome were conducted, it’s possible the “negative” activation was due to other non-modeled events contained within the implicit baseline. Alternatively, other studies have reported negative BOLD signal in the striatum in response to aversive stimuli, and which may be due to endogenous dopamine transmission (Shih et al., 2009). An open question is whether D2 receptor availability in the METH+ group played a role in the observed negative BOLD signal. Fourth, nearly all participants were men, so results may not generalize to women. Finally, a potential limitation was the use of separate scanners during data collection. In running our ROI analyses, we failed to find statistical differences whether we nested subject within scanner and used this term as a random effect in the model, used scanner as a covariate, or did not include scanner at all. As noted in the methods, a number of publications in the literature (Gountouna et al., 2010; Suckling et al., 2008; Brown et al., 2011; Costafreda et al., 2007) suggest that multisubject variance far outweighs multisite variance, although multisite variance is certainly a concern (Friedman et al., 2008). However, the study by Friedman et al. (2008) was focused on intersite variance across 10 magnets that differed by field strength, manufacturer, head coil and/or acquisition sequence. Our study used the same field strength, manufacturer, head coils, and sequences for data collection, save for a need to make a modest change to slice thickness on the MR750 due to its incompatibility with thinner EPI slices at the time of data collection. Friedman et al. (2008) also studied the same participants at each site, thereby vastly reducing intersubject variability. Importantly, our methods followed the guidelines described by Glover et al. (2012), which suggest that scanner effects be included in the group level model, and that a mixed effects approach should be used.
This study demonstrated that METH+ have attenuated neural response to potential gains and losses in areas associated with reward processing. However, these individuals also showed greater brain response to loss outcomes than gain outcomes within the caudate. Thus, METH individuals do not process the expectation of negative events as much but – instead – process the occurrence of these events more intensely. These results are consistent with an impaired ability to evaluate future risks and benefits that may increase the likelihood of future risky behavior.
Acknowledgments
This research was supported by NIH grant P50-DA026306 (IG). The Authors declare that there is no conflict of interest.
References
- American Psychiatric Association. Diagnostic & Statistical Manual of Mental Disorders: DSM:VI-TR, 4th edition. Washington, DC: 2000. [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. Non-linear registration, aka spatial normalisation 2010 [Google Scholar]
- Ares-Santos S, Granado N, Moratalla R. The role of dopamine receptors in the neurotoxicity of methamphetamine. J Intern Med. 2013;273:437–453. doi: 10.1111/joim.12049. [DOI] [PubMed] [Google Scholar]
- Balleine BW, O'Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis IM, Potenza MN. Anticipatory reward processing in addicted populations: a focus on the monetary incentive delay task. Biological Psychiatry. 2015;77:434–444. doi: 10.1016/j.biopsych.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Iowa Gambling Task Professional Manual. Boca Raton, FL: Psychological Assessment Resources; 2007. [Google Scholar]
- Beck A, Steer R, Brown G. Manual for the Beck Depression Inventory – II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Beck KH, Thombs DL, Mahoney CA, et al. Social context and sensation seeking: gender differences in college student drinking motivations. Int J Addict. 1995;30:1101–1115. doi: 10.3109/10826089509055830. [DOI] [PubMed] [Google Scholar]
- Berns GS, McClure SM, Pagnoni G, et al. Predictability modulates human brain response to reward. Journal of Neuroscience. 2001;21:2793–2798. doi: 10.1523/JNEUROSCI.21-08-02793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia KP, Marsden CD. The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain. 1994;117:859–876. doi: 10.1093/brain/117.4.859. [DOI] [PubMed] [Google Scholar]
- Bischoff-Grethe A, Buxton RB, Paulus MP, et al. Striatal and Pallidal Activation during Reward Modulated Movement Using a Translational Paradigm. Journal of the International Neuropsychological Society. 2015;21:399–411. doi: 10.1017/S1355617715000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli RM, Cummings JL. Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci. 2007;9:141–151. doi: 10.31887/DCNS.2007.9.2/rbonelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer JF, Gough B, Slikker W, Jr, et al. Effects of a cold environment or age on methamphetamine-induced dopamine release in the caudate putamen of female rats. Pharmacol Biochem Behav. 1993;44:87–98. doi: 10.1016/0091-3057(93)90284-z. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, et al. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Brown GG, Mathalon DH, Stern H, et al. Multisite reliability of cognitive BOLD data. Neuroimage. 2011;54:2163–2175. doi: 10.1016/j.neuroimage.2010.09.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante JC, Barros-Loscertales A, Costumero V, et al. Abstinence duration modulates striatal functioning during monetary reward processing in cocaine patients. Addiction biology. 2014;19:885–894. doi: 10.1111/adb.12041. [DOI] [PubMed] [Google Scholar]
- Butterworth S. On the Theory of Filter Amplifiers. Experimental Wireless & the Wireless Engineer. 1930;7:536–541. [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. Journal of Clinical and Experimental Neuropsychology. 2004;26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Cattie JE, Woods SP, Iudicello JE, et al. Elevated neurobehavioral symptoms are associated with everyday functioning problems in chronic methamphetamine users. J Neuropsychiatry Clin Neurosci. 2012;24:331–339. doi: 10.1176/appi.neuropsych.11080192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, et al. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102(Suppl 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Chau DT, Roth RM, Green AI. The neural circuitry of reward and its relevance to psychiatric disorders. Current Psychiatry Reports. 2004;6:391–399. doi: 10.1007/s11920-004-0026-8. [DOI] [PubMed] [Google Scholar]
- Cooper JC, Hollon NG, Wimmer GE, et al. Available alternative incentives modulate anticipatory nucleus accumbens activation. Social Cognitive and Affective Neuroscience. 2009;4:409–416. doi: 10.1093/scan/nsp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, Vencio RZ, et al. Multisite fMRI reproducibility of a motor task using identical MR systems. J Magn Reson Imaging. 2007;26:1122–1126. doi: 10.1002/jmri.21118. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Franklin D, Jr, Abramson I, et al. Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change. J Clin Exp Neuropsychol. 2011;33:505–522. doi: 10.1080/13803395.2010.535504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel R, Pollmann S. A universal role of the ventral striatum in reward-based learning: evidence from human studies. Neurobiol Learn Mem. 2014;114:90–100. doi: 10.1016/j.nlm.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, et al. Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Devor A, Tian P, Nishimura N, et al. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. Journal of Neuroscience. 2007;27:4452–4459. doi: 10.1523/JNEUROSCI.0134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Newman JL, Longe OA, et al. Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: a parametric functional magnetic resonance imaging study. Journal of Neuroscience. 2003;23:303–307. doi: 10.1523/JNEUROSCI.23-01-00303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure A, Richard JM, Berridge KC. Desire and dread from the nucleus accumbens: cortical glutamate and subcortical GABA differentially generate motivation and hedonic impact in the rat. PLoS One. 2010;5:e11223. doi: 10.1371/journal.pone.0011223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedota JR, Sutherland MT, Salmeron BJ, et al. Reward Anticipation Is Differentially Modulated by Varenicline and Nicotine in Smokers. Neuropsychopharmacology. 2015;40:2038–2046. doi: 10.1038/npp.2015.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field BA, Buck CL, McClure SM, et al. Attentional Modulation of Brain Responses to Primary Appetitive and Aversive Stimuli. PLoS One. 2015;10:e0130880. doi: 10.1371/journal.pone.0130880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Logan J, et al. Fast uptake and long-lasting binding of methamphetamine in the human brain: comparison with cocaine. Neuroimage. 2008;43:756–763. doi: 10.1016/j.neuroimage.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman L, Stern H, Brown GG, et al. Test-retest and between-site reliability in a multicenter fMRI study. Human Brain Mapping. 2008;29:958–972. doi: 10.1002/hbm.20440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge J, Breitbart M, McClain C. Amygdaloid inputs define a caudal component of the ventral striatum in primates. Journal of Comparative Neurology. 2004;476:330–347. doi: 10.1002/cne.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galen LW, Henderson MJ. Validation of cocaine and marijuana effect expectancies in a treatment setting. Addict Behav. 1999;24:719–724. doi: 10.1016/s0306-4603(98)00110-5. [DOI] [PubMed] [Google Scholar]
- Glenn AL, Yang Y. The potential role of the striatum in antisocial behavior and psychopathy. Biological Psychiatry. 2012;72:817–822. doi: 10.1016/j.biopsych.2012.04.027. [DOI] [PubMed] [Google Scholar]
- Glover GH, Mueller BA, Turner JA, et al. Function biomedical informatics research network recommendations for prospective multicenter functional MRI studies. J Magn Reson Imaging. 2012;36:39–54. doi: 10.1002/jmri.23572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gneezy U, Rustichini A. Pay enough or don't pay at all. Quarterly Journal of Economics. 2000;115:791–810. [Google Scholar]
- Goldman MS. Expectancy and risk for alcoholism: the unfortunate exploitation of a fundamental characteristic of neurobehavioral adaptation. Alcohol Clin Exp Res. 2002;26:737–746. [PubMed] [Google Scholar]
- Gonzalez R, Bechara A, Martin EM. Executive functions among individuals with methamphetamine or alcohol as drugs of choice: preliminary observations. J Clin Exp Neuropsychol. 2007;29:155–159. doi: 10.1080/13803390600582446. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Vassileva J, Bechara A, et al. The influence of executive functions, sensation seeking, and HIV serostatus on the risky sexual practices of substance-dependent individuals. Journal of the International Neuropsychological Society : JINS. 2005;11:121–131. doi: 10.1017/s1355617705050186. [DOI] [PubMed] [Google Scholar]
- Gountouna VE, Job DE, McIntosh AM, et al. Functional Magnetic Resonance Imaging (fMRI) reproducibility and variance components across visits and scanning sites with a finger tapping task. Neuroimage. 2010;49:552–560. doi: 10.1016/j.neuroimage.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Gowin JL, Mackey S, Paulus MP. Altered risk-related processing in substance users: imbalance of pain and gain. Drug Alcohol Depend. 2013;132:13–21. doi: 10.1016/j.drugalcdep.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowin JL, Stewart JL, May AC, et al. Altered cingulate and insular cortex activation during risk-taking in methamphetamine dependence: losses lose impact. Addiction. 2014;109:237–247. doi: 10.1111/add.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace J, Malloy P. Frontal Systems Behavior Scale. Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc; 2001. [Google Scholar]
- Haber SN, Kim KS, Mailly P, et al. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. Journal of Neuroscience. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, et al. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Butters N, et al. The HNRC 500--neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. Journal of the International Neuropsychological Society. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Kirson D, Velin RA, et al. The utility of clinical ratings for detecting cognitive change in HIV infection. In: Grant I, Martin A, editors. Neuropsychology of HIV Infection. New York: Oxford University Press; 1994. pp. 188–206. [Google Scholar]
- Hoffman WF, Moore M, Templin R, et al. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology (Berl) 2006;188:162–170. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Tremblay L, Schultz W. Influence of reward expectation on behavior-related neuronal activity in primate striatum. Journal of Neurophysiology. 1998;80:947–963. doi: 10.1152/jn.1998.80.2.947. [DOI] [PubMed] [Google Scholar]
- Ingham CA, Hood SH, van Maldegem B, et al. Morphological changes in the rat neostriatum after unilateral 6- hydroxydopamine injections into the nigrostriatal pathway. Experimental Brain Research. 1993;93:17–27. doi: 10.1007/BF00227776. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jensen J, McIntosh AR, Crawley AP, et al. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40:1251–1257. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Jensen J, Smith AJ, Willeit M, et al. Separate brain regions code for salience vs. valence during reward prediction in humans. Human Brain Mapping. 2007;28:294–302. doi: 10.1002/hbm.20274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Archibald SL, et al. Effects of methamphetamine dependence and HIV infection on cerebral morphology. American Journal of Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Jia Z, Worhunsky PD, Carroll KM, et al. An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biological Psychiatry. 2011;70:553–560. doi: 10.1016/j.biopsych.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jocham G, Klein TA, Neumann J, et al. Dopamine DRD2 polymorphism alters reversal learning and associated neural activity. Journal of Neuroscience. 2009;29:3695–3704. doi: 10.1523/JNEUROSCI.5195-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D, Tversky A. Prospect theory - Analysis of decision under risk. Econometrica. 1979;47:263–291. [Google Scholar]
- Kalichman SC, Johnson JR, Adair V, et al. Sexual sensation seeking: scale development and predicting AIDS-risk behavior among homosexually active men. Journal of personality assessment. 1994;62:385–397. doi: 10.1207/s15327752jpa6203_1. [DOI] [PubMed] [Google Scholar]
- Kawagoe R, Takikawa Y, Hikosaka O. Expectation of reward modulates cognitive signals in the basal ganglia. Nature Neuroscience. 1998;1:411–416. doi: 10.1038/1625. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Ustun TB. The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) International journal of methods in psychiatric research. 2004;13:93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Bjork JM, Fong GW, et al. Amphetamine modulates human incentive processing. Neuron. 2004;43:261–269. doi: 10.1016/j.neuron.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, et al. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Gibbs SE. Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology (Berl) 2007;191:813–822. doi: 10.1007/s00213-006-0686-7. [DOI] [PubMed] [Google Scholar]
- Kohno M, Morales AM, Ghahremani DG, et al. Risky decision making, prefrontal cortex, and mesocorticolimbic functional connectivity in methamphetamine dependence. JAMA Psychiatry. 2014;71:812–820. doi: 10.1001/jamapsychiatry.2014.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. The role of the striatopallidal and extended amygdala systems in drug addiction. Ann N Y Acad Sci. 1999;877:445–460. doi: 10.1111/j.1749-6632.1999.tb09282.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Leland DS, Arce E, Feinstein JS, et al. Young adult stimulant users' increased striatal activation during uncertainty is related to impulsivity. Neuroimage. 2006;33:725–731. doi: 10.1016/j.neuroimage.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leland DS, Paulus MP. Increased risk-taking decision-making but not altered response to punishment in stimulant-using young adults. Drug Alcohol Depend. 2005;78:83–90. doi: 10.1016/j.drugalcdep.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cerebral Cortex. 2006;16:916–928. doi: 10.1093/cercor/bhj043. [DOI] [PubMed] [Google Scholar]
- London ED, Kohno M, Morales AM, et al. Chronic methamphetamine abuse and corticostriatal deficits revealed by neuroimaging. Brain Research. 2015;1628:174–185. doi: 10.1016/j.brainres.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquine MJ, Iudicello JE, Morgan EE, et al. "Frontal systems" behaviors in comorbid human immunodeficiency virus infection and methamphetamine dependency. Psychiatry Res. 2014;215:208–216. doi: 10.1016/j.psychres.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Soelch C, Szczepanik J, Nugent A, et al. Lateralization and gender differences in the dopaminergic response to unpredictable reward in the human ventral striatum. Eur J Neurosci. 2011;33:1706–1715. doi: 10.1111/j.1460-9568.2011.07642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- Mattfeld AT, Gluck MA, Stark CE. Functional specialization within the striatum along both the dorsal/ventral and anterior/posterior axes during associative learning via reward and punishment. Learning & Memory. 2011;18:703–711. doi: 10.1101/lm.022889.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Berns GS, Montague PR. Temporal prediction errors in a passive learning task activate human striatum. Neuron. 2003;38:339–346. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Adams NL, Lewandowski KS. Neurobehavioral changes associated with caudate lesions. Neurology. 1989;39:349–354. doi: 10.1212/wnl.39.3.349. [DOI] [PubMed] [Google Scholar]
- Molochnikov I, Cohen D. Hemispheric differences in the mesostriatal dopaminergic system. Front Syst Neurosci. 2014;8:110. doi: 10.3389/fnsys.2014.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso JR, Ainslie G, Xu J, et al. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Human Brain Mapping. 2007;28:383–393. doi: 10.1002/hbm.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara H, Itoh H, Kawagoe R, et al. Dopamine neurons can represent context-dependent prediction error. Neuron. 2004;41:269–280. doi: 10.1016/s0896-6273(03)00869-9. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, Deichmann R, Critchley HD, et al. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Patel KT, Stevens MC, Meda SA, et al. Robust changes in reward circuitry during reward loss in current and former cocaine users during performance of a monetary incentive delay task. Biological Psychiatry. 2013;74:529–537. doi: 10.1016/j.biopsych.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Frank L, et al. Decision making by methamphetamine-dependent subjects is associated with error-rate-independent decrease in prefrontal and parietal activation. Biological Psychiatry. 2003;53:65–74. doi: 10.1016/s0006-3223(02)01442-7. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack NE, Zauscher BE, et al. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26:53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Lovero KL, Wittmann M, et al. Reduced behavioral and neural activation in stimulant users to different error rates during decision making. Biological Psychiatry. 2008;63:1054–1060. doi: 10.1016/j.biopsych.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM, DebRoy S, et al. R package version 3.1–109 ed. 2013. nlme: Linear and Nonlinear Mixed Effects Models. [Google Scholar]
- Plichta MM, Scheres A. Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neuroscience and Biobehavioral Reviews. 2014;38:125–134. doi: 10.1016/j.neubiorev.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock V, Cho DW, Reker D, et al. Profile of Mood States: the factors and their physiological correlates. The Journal of nervous and mental disease. 1979;167:612–614. doi: 10.1097/00005053-197910000-00004. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing [Internet] Vienna, Austria: R Fondation for Statistical Computing; 2012. [Google Scholar]
- Reynolds JN, Hyland BI, Wickens JR. A cellular mechanism of reward-related learning. Nature. 2001;413:67–70. doi: 10.1038/35092560. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste "liking"/"disliking" reactions, place preference/avoidance, and fear. Journal of Neuroscience. 2002;22:7308–7320. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cerebral Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Romo R, Schultz W. Dopamine neurons of the monkey midbrain: Contingencies of responses to active touch during self-initiated arm movements. Journal of Neurophysiology. 1990;63:592–606. doi: 10.1152/jn.1990.63.3.592. [DOI] [PubMed] [Google Scholar]
- Rose EJ, Ross TJ, Salmeron BJ, et al. Acute nicotine differentially impacts anticipatory valence- and magnitude-related striatal activity. Biological Psychiatry. 2013;73:280–288. doi: 10.1016/j.biopsych.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Glen DR, Chen G, et al. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. Neuroimage. 2009;44:839–848. doi: 10.1016/j.neuroimage.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD. The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behav Brain Res. 1994;61:117–133. doi: 10.1016/0166-4328(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Gibbs SE, Khanna K, et al. Anticipation of monetary gain but not loss in healthy older adults. Nature Neuroscience. 2007;10:787–791. doi: 10.1038/nn1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T, Nakai S, Sato T, et al. Correlated coding of motivation and outcome of decision by dopamine neurons. Journal of Neuroscience. 2003;23:9913–9923. doi: 10.1523/JNEUROSCI.23-30-09913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nature Neuroscience. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annual Review of Psychology. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Schultz W, Ljungberg T, Apicella P, et al. Primate dopamine neurons: From movement to motivation and back. In: Mano N, Hamada I, DeLong MR, editors. Role of the Cerebellum and Basal Ganglia in Voluntary Movement. Amsterdam: Elsevier Science Publishers; 1993. pp. 89–97. [Google Scholar]
- Shih YY, Chen CC, Shyu BC, et al. A new scenario for negative functional magnetic resonance imaging signals: endogenous neurotransmission. The Journal of Neuroscience. 2009;29:3036–3044. doi: 10.1523/JNEUROSCI.3447-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, et al. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nature Neuroscience. 2006;9:569–577. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Spreckelmeyer KN, Krach S, Kohls G, et al. Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Social Cognitive and Affective Neuroscience. 2009;4:158–165. doi: 10.1093/scan/nsn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suckling J, Ohlssen D, Andrew C, et al. Components of variance in a multicentre functional MRI study and implications for calculation of statistical power. Human Brain Mapping. 2008;29:1111–1122. doi: 10.1002/hbm.20451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, et al. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Sutton BP, Goh J, Hebrank A, et al. Investigation and validation of intersite fMRI studies using the same imaging hardware. J Magn Reson Imaging. 2008;28:21–28. doi: 10.1002/jmri.21419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer MM, Geier CF, Addicott MA, et al. Smoking Abstinence-Induced Changes in Resting State Functional Connectivity with Ventral Striatum Predict Lapse During a Quit Attempt. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor EM, Murphy A, Boyapati V, et al. Impulsivity in abstinent alcohol and polydrug dependence: a multidimensional approach. Psychopharmacology (Berl) 2016;233:1487–1499. doi: 10.1007/s00213-016-4245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale N, Bard C, Fleury M, et al. Determining movement onsets from temporal series. Journal of Motor Behavior. 1993;25:97–106. doi: 10.1080/00222895.1993.9941644. [DOI] [PubMed] [Google Scholar]
- Thut G, Schultz W, Roelcke U, et al. Activation of the human brain by monetary reward. Neuroreport. 1997;8:1225–1228. doi: 10.1097/00001756-199703240-00033. [DOI] [PubMed] [Google Scholar]
- Tomer R, Slagter HA, Christian BT, et al. Love to win or hate to Lose? Asymmetry of dopamine D2 receptor binding predicts sensitivity to reward versus punishment. Journal of Cognitive Neuroscience. 2014;26:1039–1048. doi: 10.1162/jocn_a_00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Velligan DI, Ritch JL, Sui D, et al. Frontal Systems Behavior Scale in schizophrenia: relationships with psychiatric symptomatology, cognition and adaptive function. Psychiatry Res. 2002;113:227–236. doi: 10.1016/s0165-1781(02)00264-0. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neuroscience and Biobehavioral Reviews. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. Journal of Neuroscience. 2001;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Gatley SJ, et al. PET evaluation of the dopamine system of the human brain. Journal of Nuclear Medicine. 1996;37:1242–1256. [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. Role of dopamine in drug reinforcement and addiction in humans: results from imaging studies. Behav Pharmacol. 2002;13:355–366. doi: 10.1097/00008877-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Volz TJ, Fleckenstein AE, Hanson GR. Methamphetamine-induced alterations in monoamine transport: implications for neurotoxicity, neuroprotection and treatment. Addiction. 2007; 102(Suppl 1):44–48. doi: 10.1111/j.1360-0443.2007.01771.x. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Kennerley SW. Contrasting reward signals in the orbitofrontal cortex and anterior cingulate cortex. Annals of the New York Academy of Sciences. 2011;1239:33–42. doi: 10.1111/j.1749-6632.2011.06277.x. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30:669–689. [Google Scholar]
- Wilkinson GS, Robertson GJ. Wide Range Achievement Test 4 Professional Manual. Lutz, FL: Psychological Assessment Resources; 2006. [Google Scholar]
- Wise RA. Drug-activation of brain reward pathways. Drug Alcohol Depend. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]
- Woods SP, Childers M, Ellis RJ, et al. A battery approach for measuring neuropsychological change. Arch Clin Neuropsychol. 2006;21:83–89. doi: 10.1016/j.acn.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nature Reviews Neuroscience. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Chappelow J, et al. Human striatal activation reflects degree of stimulus saliency. Neuroimage. 2006;29:977–983. doi: 10.1016/j.neuroimage.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]