Abstract
Aim: We examined the effects of mild hyperbaric oxygen on the properties of the soleus muscle in rats with metabolic syndrome.
Methods: Five-week-old metabolic syndrome (SHR/NDmcr-cp, cp/cp) rats were divided into normobaric (CP) and mild hyperbaric oxygen (CP-H) groups (n = 5/group). In addition, 5-week-old Wistar rats were assigned as the normobaric control (WR) group (n = 5). The CP-H group was exposed to 1.25 atmospheres absolute with 36% oxygen for 3 h daily for 16 weeks. Succinate dehydrogenase (SDH) activity and mRNA levels of peroxisome proliferator-activated receptor γ coactivator-1α (Pgc-1α) in the soleus muscle were examined. The fiber type composition, cross-sectional areas, and SDH staining intensity in the soleus muscle were also examined.
Results: The CP-H group showed lower fasting and nonfasting blood glucose, glycated hemoglobin, total cholesterol, triglyceride, insulin, and systolic blood pressure levels; higher adiponectin levels; and higher SDH activity and mRNA levels of Pgc-1α in the muscle than the CP group. Compared with the CP group, the CP-H group had a lower percentage of type I fibers and observed type IIA fibers in the muscle. The CP-H group also had higher SDH staining intensity of type I and type IIC fibers in the muscle than the CP group. No differences in these values were observed in the muscles of the WR and CP-H groups.
Conclusion: Mild hyperbaric oxygen inhibited growth-related increase in blood glucose levels and decrease in muscle oxidative capacity of rats with metabolic syndrome because of improved oxidative metabolism.
Keywords: Blood glucose, Muscle fiber, Oxidative metabolism, Pgc-1α mRNA, Soleus muscle
Introduction
Metabolic syndrome is linked to physical inactivity and consumption of a high-fat and high-calorie diet and is characterized by obesity, high blood pressure, increased blood glucose levels, and hyperlipidemia1). Skeletal muscle is the primary site of insulin action and glucose metabolism. Reduced oxidative capacity in skeletal muscle impairs glucose metabolism and increases the risk of development and aggravation of metabolic syndrome2). Metabolic syndrome ultimately develops into lifestyle-related diseases, such as cardiovascular disease, type 2 diabetes, hypertension, and associated complications3–6).
Compared with healthy individuals, obese patients with or without type 2 diabetes have a low percentage of high-oxidative type I fibers and a high percentage of low-oxidative type II fibers, particularly type IIB fibers, in the vastus lateralis and rectus abdominis muscles7–10). Previous studies using animal models11, 12) observed that rats with metabolic syndrome exhibited a low oxidative capacity of the soleus muscle with a decreased percentage of type I fibers and an increased percentage of type IIA fibers compared with normal rats. One of these studies12) showed decreased oxidative enzyme activity in type IIA fibers of rats with metabolic syndrome compared with normal rats. These results indicate a low oxidative capacity of skeletal muscle in humans and animal models with metabolic syndrome.
An elevation in atmospheric pressure accompanied by high oxygen concentration enhances the partial pressure of oxygen and increases blood flow and oxygen, particularly dissolved oxygen, in the plasma13). An increase in both atmospheric pressure and oxygen concentration enhances oxidative enzyme activity in mitochondria and consequently increases oxidative metabolism in cells and tissues. Thus, mild hyperbaric oxygen facilitates oxidative metabolism, particularly the pathways in the mitochondrial TCA cycle, thereby improving the oxidative capacity of skeletal muscles and their fibers. We have demonstrated that mild hyperbaric oxygen at 1.25 atmospheres absolute (ATA) with 36% oxygen enhanced blood flow and increased oxygen levels, thereby improving oxidative metabolism14, 15). We observed that animal models exposed to mild hyperbaric oxygen inhibited and/or improved lifestyle-related diseases, i.e., type 2 diabetes16–19), diabetes-induced cataract20), and hypertension21). In addition, mild hyperbaric oxygen inhibited development and aggravation in arthritis22) and age-related decrease in muscle oxidative capacity23). A clinical study24) showed that mild hyperbaric oxygen reversed the increase in melanin pigmentation induced by ultraviolet B irradiation as well as reduced senile spot size.
Oxidative metabolism is regulated by many factors including peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α)25–27). PGC-1α plays an important role in oxidative metabolism by regulating mitochondrial biogenesis, fiber type composition, and oxidative capacity in skeletal muscle28, 29). Therefore, reduced mRNA levels of Pgc-1α in the skeletal muscle of animal models may induce a low percentage of high-oxidative fibers and a high percentage of low-oxidative fibers, whereas increased mRNA levels of Pgc-1α may induce a shift of fiber types from low oxidative to high oxidative.
We hypothesized that mild hyperbaric oxygen would improve decreased mRNA levels of Pgc-1α and oxidative capacity in the skeletal muscle of animal models with metabolic syndrome. In this study, we focused on fiber characteristics (including type composition, cross-sectional area, and oxidative enzyme activity) and mRNA levels related to oxidative metabolism in the soleus muscle. The soleus muscles have high oxidative capacity and are required to function against gravity, e.g., maintaining posture and walking30), indicating that these muscles function most effectively at relatively low intensity for long durations. We used the SHR/NDmcr-cp [cp/cp] rat as an animal model for metabolic syndrome. Rats with metabolic syndrome have a nonsense mutation in the leptin receptor and develop obesity, high blood pressure and glucose levels, hyperinsulinemia, and hyperlipidemia as adults31, 32
Aim
Humans and animal models with metabolic syndrome have a low oxidative capacity in skeletal muscle. Reduced oxidative capacity in skeletal muscle impairs glucose metabolism and increases the risk of development, or aggravation, of metabolic syndrome. An increase in both atmospheric pressure and oxygen concentration enhances oxidative enzyme activity in mitochondria and consequently increases oxidative metabolism in cells and tissues. Thus, mild hyperbaric oxygen at 1.25 ATA with 36% oxygen would be expected to improve the decreased oxidative capacity of skeletal muscles and their fibers in rats with metabolic syndrome. In this study, we examined the effects of mild hyperbaric oxygen on the properties of the soleus muscle in rats with metabolic syndrome.
Methods
Ethical Statement
All experimental procedures were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals published by the Institutional Animal Use Committee at Kyoto University, Kyoto, Japan.
Animals Housing
Five-week-old metabolic syndrome male rats were randomly assigned to either the normobaric (CP) or mild hyperbaric oxygen (CP-H) group (n = 5/group). Wistar male rats were assigned as the normobaric control (WR) group (n = 5). All rats were housed in individual cages and under normobaric conditions (1 ATA with 20.9% oxygen). The room was maintained at 22 ± 2°C with 45% – 55% relative humidity and 12-h light/dark cycle (light from 08:00 to 20:00). All rats were given standard chow (MF, Oriental East Inc., Tokyo, Japan) and water ad libitum. Body weight and food intake were measured biweekly.
Exposure to Mild Hyperbaric Oxygen
The rats in the CP-H group were exposed to 1.25 ATA with 36% oxygen in a mild hyperbaric oxygen chamber (Japan Patent No. 5076067 dated September 7, 2012; Japan Trademark Registration No. 5804129 dated November 6, 2015) for 3 h (11:00 to 14:00) daily from 5 to 21 weeks of age.
Blood Glucose and Glycated Hemoglobin (HbA1c) Analyses
Blood samples were obtained from the tails of conscious rats. Blood glucose levels were measured using a blood glucose meter (GT-1650; Arkray Inc., Kyoto, Japan) after 6 h of fasting biweekly. Nonfasting glucose levels were measured at 21 weeks of age. In addition, HbA1c was measured at 21 weeks of age using a DCA vantage analyzer (Siemens Healthcare Diagnostics Co., Ltd., Germany).
Blood Pressure Analyses
Both diastolic and systolic blood pressure levels were measured at 5, 15, and 21 weeks of age. Blood pressures were determined automatically in conscious rats using the indirect tail-cuff method using a BP-98A sphygmomanometer (Softron Inc., Tokyo, Japan).
Serum Analyses
At 21 weeks of age, rats were anesthetized with sodium pentobarbital (35 mg/kg body weight, i.p.) and blood samples were obtained from the abdominal aorta. Total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride levels were measured as described previously33). Insulin, leptin, and high molecular weight adiponectin levels were measured using a rat enzyme-linked immunosorbent assay kit (Shibayagi Co., Ltd., Gunma, Japan).
Biochemical Analyses
After blood sampling at 21 weeks of age, the soleus muscles were removed bilaterally and wet muscle weights were measured. The soleus muscle of the right leg was rapidly frozen in liquid nitrogen for the measurement of succinate dehydrogenase (SDH) activity11, 12). The muscle was homogenized using a glass tissue homogenizer with 5 volumes of ice-cold 0.3 M phosphate buffer, pH 7.4. Sodium succinate was added to yield a final concentration of 17 mM. The final concentrations of the components of the reaction mixture were as follows: 17 mM sodium succinate, 1 mM sodium cyanide, 0.4 mM aluminum chloride, and 0.4 mM calcium chloride. This reaction mixture was transferred to cuvettes, placed in a spectrophotometer, and reduction in cytochrome c in the mixture was determined by measuring the increase in extinction at 550 nm. SDH activity was calculated from the ferricytochrome c concentration and protein content.
Histochemical Analyses
The soleus muscle of the left leg was divided into distal and proximal portions for histochemical and mRNA analyses, respectively. The distal portion of the muscle was pinned on a corkboard at its approximate in vivo length and rapidly frozen in isopentane that had been cooled with a mixture of dry ice and acetone. The muscle was mounted on a specimen chuck with Tissue-Tek OCT compound (Sakura Finetek Japan Co., Ltd., Tokyo, Japan). Serial transverse sections (16 µm thickness) were cut in a cryostat at −25°C. Some sections were brought to room temperature, air dried, and preincubated in acidic (pH 4.5) or alkaline (pH 10.4) conditions for the assessment of ATPase staining intensity. The fibers in each muscle section were classified as type I (positive response to preincubation at pH 4.5 and negative response to preincubation at pH 10.4), type IIA (negative response to preincubation at pH 4.5 and positive response to preincubation at pH 10.4), and type IIC (positive response to preincubation at pH 4.5 and 10.4)11, 12). The fiber type composition and cross-sectional area (CSA) of approximately 300 fibers in the central region of the muscle were determined.
The sections were stained for 10 min to determine the SDH staining intensity of the fibers. The SDH staining intensity was determined in the 300 aforementioned fibers using a computer-assisted image-processing system (Neuroimaging System, Kyoto, Japan)11, 12). Sectional images were digitized as grayscale images. Each pixel was quantified as 1 of 256 gray levels; a gray level of 0 was equivalent to 100% light transmission, whereas a gray level of 255 was equivalent to 0% light transmission. The mean optical density (OD) of all pixels, which were converted to gray level values, within a fiber was determined using a calibration photographic tablet with 21 steps of gradient-density ranges and the corresponding diffused density values.
mRNA Analyses
Total RNA was extracted from the proximal portion of the muscle using the QuickGene RNA tissue kit SII (Fujifilm, Kanagawa, Japan). Reverse transcription was performed using the High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA). Resultant cDNA samples were stored. Expression levels of peroxisome proliferator-activated receptor α (Pparα) (ppara, Rn00566193_m1), Pparδ/β (ppard, Rn00565707_m1), and Pgc-1α (ppargc1a, Rn00580241_m1) were quantified by TaqMan Gene Expression Assays (Applied Biosystems). Each Taq-Man probe and primer set was validated by performing a quantitative real-time polymerase chain reaction (qPCR) with a series of cDNA template dilutions to obtain standard curves of threshold cycle against relative concentration using the housekeeping gene 18S as an internal standard. All samples and nontemplate control reactions were performed in a 7500 Fast Sequence Detection System (Applied Biosystems). The mRNA levels were normalized to the control (WR) group34, 35).
Statistics
Values were expressed as mean ± SD. One-way ANOVA was used to determine significant differences among the WR, CP, and CP-H groups. When the differences were found to be significant by ANOVA, individual group comparisons were determined using Scheffé's post hoc test. Statistical significance was set at p < 0.05.
Results
Body Weights
The body weights at 19 and 21 weeks of age were greater in the CP and CP-H groups than in the age-matched WR group (Fig. 1).
Fig. 1.
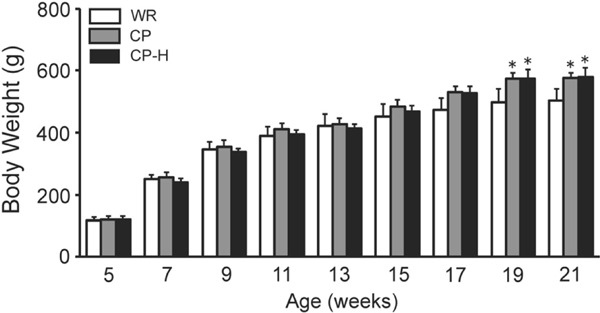
Body weights of the WR, CP, and CP-H groups at each time point studied
WR, normal rats; CP, metabolic syndrome rats; CP-H, metabolic syndrome rats exposed to mild hyperbaric oxygen for 3 h per day. Values are means ± SD for five animals. *p < 0.05 compared with WR.
Food Intake Levels
The food intake levels at 7, 9, and 11 weeks of age were greater in the CP and CP-H groups than in the age-matched WR group (Fig. 2). The food intake levels at 13 and 15 weeks of age were greater in the CP group than in the age-matched WR group.
Fig. 2.
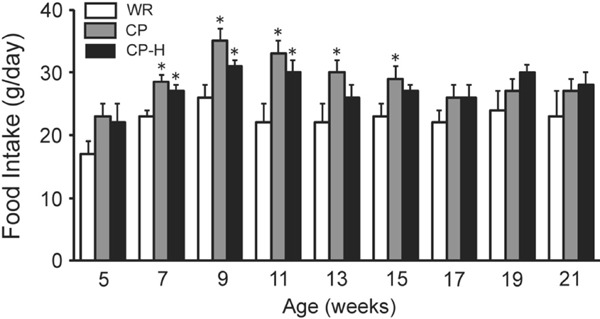
Food intake levels of the WR, CP, and CP-H groups at each time point studied
WR, normal rats; CP, metabolic syndrome rats; CP-H, metabolic syndrome rats exposed to mild hyperbaric oxygen for 3 h per day. Values are means ± SD for five animals. *p < 0.05 compared with WR.
Fasting Blood Glucose Levels
The fasting blood glucose levels from 15 to 21 weeks of age were lower in the CP-H group than in the age-matched WR and CP groups (Fig. 3).
Fig. 3.
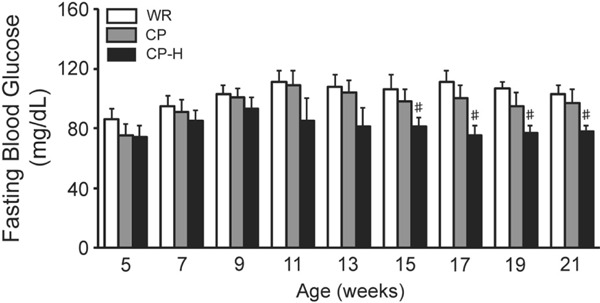
Fasting blood glucose levels of the WR, CP, and CP-H groups at each time point studied
WR, normal rats; CP, metabolic syndrome rats; CP-H, metabolic syndrome rats exposed to mild hyperbaric oxygen for 3 h per day. Values are means ± SD for five animals. #p < 0.05 compared with WR and CP.
Blood Pressure Levels
Both the diastolic and systolic blood pressure levels at 15 and 21 weeks of age were higher in the CP and CP-H groups than in the age-matched WR group (Fig. 4). The systolic blood pressure levels at 21 weeks of age were higher in the CP group than in the age-matched CP-H group.
Fig. 4.
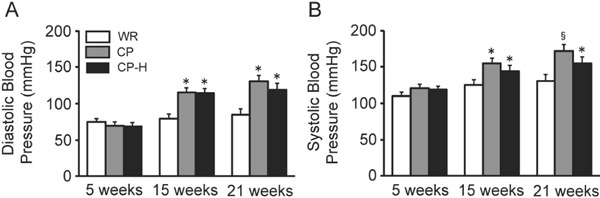
Diastolic (A) and systolic (B) blood pressure levels of the WR, CP, and CP-H groups at 5, 15, and 21 weeks of age
WR, normal rats; CP, metabolic syndrome rats; CP-H, metabolic syndrome rats exposed to mild hyperbaric oxygen for 3 h per day. Values are means ± SD for five animals. *p < 0.05 compared with WR; §p < 0.05 compared with WR and CP-H.
Nonfasting Blood Glucose, HbA1c, Total Cholesterol, HDL Cholesterol, and Triglyceride Levels
The nonfasting blood glucose (Fig. 5A), HbA1c (Fig. 5B), total cholesterol (Fig. 5C), HDL cholesterol (Fig. 5D), and triglyceride (Fig. 5E) levels were the highest in the CP group. All of the above parameters were higher in the CP-H group than in the WR group.
Fig. 5.
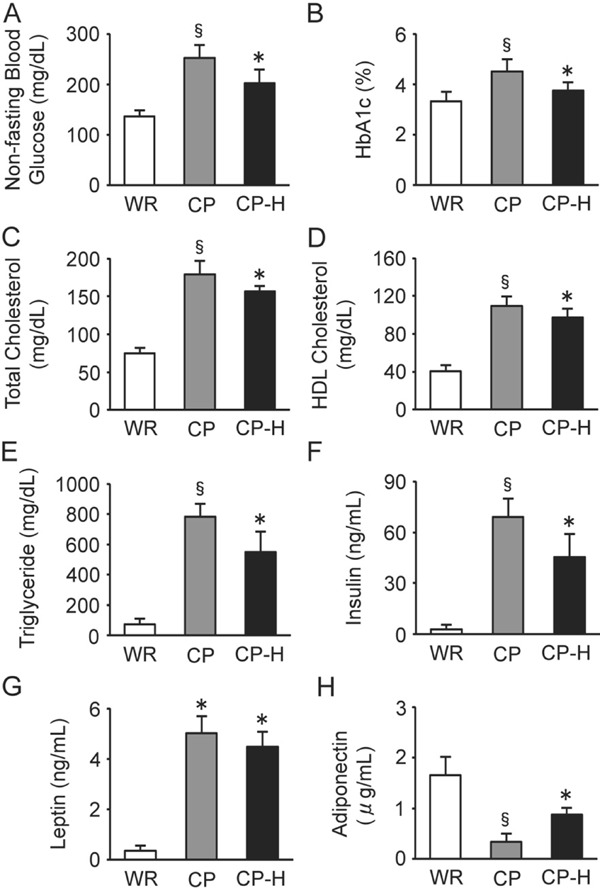
Nonfasting blood glucose (A), HbA1c (B), total cholesterol (C), HDL cholesterol (D), triglyceride (E), insulin (F), leptin (G), and adiponectin (H) levels of the WR, CP, and CP-H groups
WR, normal rats; CP, metabolic syndrome rats; CP-H, metabolic syndrome rats exposed to mild hyperbaric oxygen for 3 h per day; HbA1c, glycated hemoglobin; HDL, highdensity lipoprotein. Values are means ± SD for five animals. *p < 0.05 compared with WR; §p < 0.05 compared with WR and CP-H.
Insulin, Leptin, and Adiponectin Levels
The insulin levels were the highest in the CP group (Fig. 5F). The insulin levels were higher in the CP-H group than in the WR group. The leptin levels were higher in the CP and CP-H groups than in the WR group (Fig. 5G). The adiponectin levels were the lowest in the CP group (Fig. 5H). The adiponectin levels were lower in the CP-H group than in the WR group.
Muscle Weights and SDH Activity
There were no differences in the relative soleus muscle weight among the three groups (Fig. 6A). The SDH activity of the soleus muscle was the lowest in the CP group (Fig. 6B).
Fig. 6.
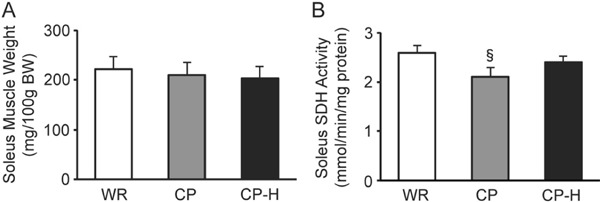
Soleus muscle weights (A) and SDH activity (B) of the WR, CP, and CP-H groups
WR, normal rats; CP, metabolic syndrome rats; CP-H, metabolic syndrome rats exposed to mild hyperbaric oxygen for 3 h per day; SDH, succinate dehydrogenase. Values are means ± SD for five animals. §p < 0.05 compared with WR and CP-H.
Muscle Fiber Properties
The soleus muscles in the WR (Fig. 7A–C) and CP-H (Fig. 7G–I) groups contained three types of fibers: type I, type IIA, and type IIC. In contrast, those in the CP group contained two types of fibers: type I and type IIC (Fig. 7D–F).
Fig. 7.
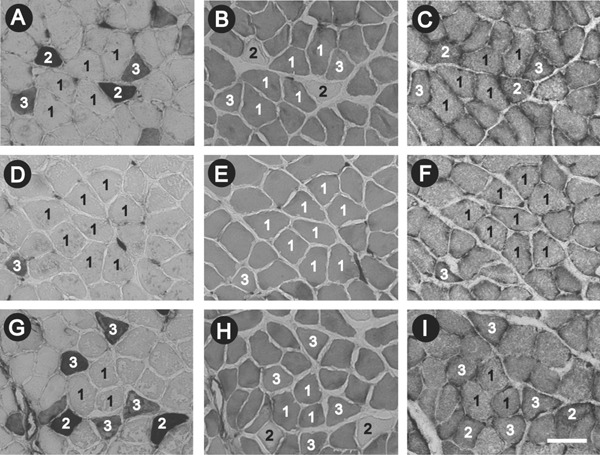
Serial transverse sections of the soleus muscle in the WR (A-C), CP (D-F), and CP-H (G-I) groups stained for ATPase activity following preincubation at pH 10.4 (A, D, G) and pH 4.5 (B, E, H) and for succinate dehydrogenase (SDH) activity (C, F, I)
WR, normal rats; CP, metabolic syndrome rats; CP-H, metabolic syndrome rats exposed to mild hyperbaric oxygen for 3 h per day; 1, type I fiber; 2, type IIA fiber; 3, type IIC fiber. Scale bar on I = 100 µm.
The percentage of type I fibers in the muscle was the highest in the CP group (Fig. 8A). There were no type IIA fibers in the CP group (Fig. 8B). The SDH staining intensity of type I (Fig. 8G) and type IIC (Fig. 8I) fibers was the lowest in the CP group.
Fig. 8.

Percentages of type I (A), type IIA (B), and type IIC (C) fibers, cross-sectional areas of type I (D), type IIA (E), and type IIC (F) fibers, and SDH staining intensity of type I (G), type IIA (H), and type IIC (I) fibers in the soleus muscle of the WR, CP, and CP-H groups
WR, normal rats; CP, metabolic syndrome rats; CP-H, metabolic syndrome rats exposed to mild hyperbaric oxygen for 3 h per day; CSA, cross-sectional area; SDH, succinate dehydrogenase; OD, optical density. Values are means ± SD for five animals. §p < 0.05 compared with WR and CP-H.
Muscle mRNA Levels
The mRNA levels of Pparα were higher in the CP and CP-H groups than in the WR group (Fig. 9A). The mRNA levels of Pparδ/β (Fig. 9B) and Pgc-1α (Fig. 9C) were the lowest in the CP group.
Fig. 9.
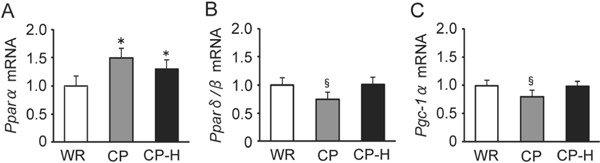
mRNA levels of Pparα (A), Pparδ/β (B), and Pgc-1α (C) in the soleus muscle of the WR, CP, and CP-H groups
WR, normal rats; CP, metabolic syndrome rats; CP-H, metabolic syndrome rats exposed to mild hyperbaric oxygen for 3 h per day; Ppar, peroxisome proliferator-activated receptor; Pgc-1α, peroxisome proliferator-activated receptor γ coactivator-1α. Values are means ± SD for five animals. *p < 0.05 compared with WR; §p < 0.05 compared with WR and CP-H.
Discussion
Body Weights and Food Intake Levels
In this study, there were differences in the food intake levels between the normal (WR) group and metabolic syndrome (CP and CP-H) groups: the food intake levels from 7 to 11 weeks of age were higher in the CP and CP-H groups than in the age-matched WR group (Fig. 2). In addition, the CP group showed higher food intake levels at 13 and 15 weeks of age than the age-matched WR group. The increased food intake levels by rats with metabolic syndrome until 15 weeks of age correspond with our previous studies11, 12). These studies showed increased body weights during this period. It is therefore plausible that the body weight increases when the quantity of diet increases. In this study, however, there were no differences in the body weight from 7 to 15 weeks of age among the WR, CP, and CP-H groups (Fig. 1). We did not elucidate the reason why increased food intake levels from 7 to 15 weeks of age did not result in the body weight differences between normal and metabolic syndrome rats. Finally, mild hyperbaric oxygen could not inhibit a growth-related excessive increase in the body weight of rats with metabolic syndrome.
Blood Pressure Levels and Plasma Components
Mild hyperbaric oxygen inhibited increased systolic blood pressure levels in rats with metabolic syndrome (Fig. 4B). Mild hyperbaric oxygen also improved increased fasting (Fig. 3) and nonfasting (Fig. 5A) blood glucose, HbA1c (Fig. 5B), total cholesterol (Fig. 5C), triglyceride (Fig. 5E), and insulin (Fig. 5F) levels. These inhibitions and improvements induced by mild hyperbaric oxygen, which increases blood flow and dissolved oxygen, appear to be an adaptive response to counter the detrimental effects of metabolic syndrome-induced hypertension, hyperglycemia, hyperlipidemia, and hyperinsulinemia.
Characteristics of Skeletal Muscles in Humans with Metabolic Syndrome
Obesity, defined as an increase in the mass of adipose tissue, is associated with various metabolic disorders and cardiovascular diseases36). Obese individuals with or without type 2 diabetes contain a low percentage of high-oxidative type I fibers and a high percentage of low-oxidative type II fibers in the vastus lateralis and rectus abdominis muscles7, 8, 10). In particular, obesity is associated with a higher percentage of low-oxidative type IIB fibers in skeletal muscles7, 9). In general, a positive correlation between insulin action and the percentage of type I fibers and a negative correlation with the percentage of type IIB fibers are observed in the vastus lateralis muscle of obese individuals7). Another study8) observed that obesity is inversely related to both in vitro glucose transport and type I fiber population in the vastus lateralis muscle. Furthermore, fiber type composition in the rectus abdominis muscle is related to both in vitro insulin-stimulated glucose transport and body mass index9).
Characteristics of Skeletal Muscles in Rats with Metabolic Syndrome
Previous studies11, 12) observed that rats with metabolic syndrome exhibited low oxidative capacity of the soleus muscle with a decreased percentage of type I fibers and an increased percentage of type IIA and/or type IIC fibers compared with normal rats. One of these studies12) showed a decreased oxidative enzyme activity of type IIA fibers in rats with metabolic syndrome compared with normal rats. In contrast, obese Zucker rats without diabetes exhibited normal fiber type composition in the soleus muscle, whereas obese Zucker rats with type 2 diabetes exhibited a lower percentage of type IIA fibers in the soleus muscle than lean Zucker rats37).
It is suggested that the aggravation of metabolic syndrome exacerbates lifestyle-related diseases, such as type 2 diabetes, and induces changes in fiber types to “no type IIA fibers.” In fact, our previous studies38, 39) showed that adult rats with type 2 diabetes, i.e., nonobese Goto-Kakizaki and obese Otsuka Long-Evans Tokushima fatty rats, exhibit no type IIA fibers in the soleus muscle.
In this study, rats with metabolic syndrome showed decreased SDH activity (Fig. 6B), increased percentage of type I fibers (Fig. 8A), no type IIA fibers (Fig. 8B), and decreased SDH staining intensity of type I (Fig. 8G) and type IIC (Fig. 8I) fibers in the soleus muscle. The decreased oxidative capacity in the skeletal muscle of rats with metabolic syndrome corresponds with our previous findings11, 12, 33, 34).
Skeletal muscle is a major target of insulin-stimulated glucose uptake. Metabolic syndrome is associated with an impaired insulin-stimulated glucose uptake and disposal capacity, which is attributed to insulin resistance in skeletal muscle. Therefore, the altered patterns of fiber types in skeletal muscles of obese individuals and animal models with metabolic syndrome may be linked to impaired glucose tolerance and insulin sensitivity.
Effects of Mild Hyperbaric Oxygen on Muscle Properties
Running restores decreased oxidative capacity in skeletal muscle of rats with metabolic syndrome11). Longer running distances are associated with higher oxidative capacity in skeletal muscle of rats with metabolic syndrome. In contrast, hypertension, hyperglycemia, and hyperlipidemia are aggravated by a high-fat and high-calorie diet. A high-fat and high-calorie diet reduces the muscle oxidative capacity in rats with metabolic syndrome12).
An elevation in atmospheric pressure accompanied by an increase in oxygen concentration enhances the partial pressure of oxygen and increases the levels of dissolved oxygen in the plasma40, 41). These conditions enhance the oxidative capacity of mitochondria, thereby increasing the oxidative metabolism in cells and tissues. Furthermore, an enhancement in atmospheric pressure and oxygen concentration increases carbon dioxide concentration, which in turn facilitates the release of oxygen from hemoglobin and causes vasodilatation. Previous studies14, 15) reported that mild hyperbaric oxygen increases the oxidative capacity in skeletal muscle of rats. In this study, mild hyperbaric oxygen reduced the percentage of type I fibers to normal (Fig. 8A) and improved SDH staining intensity of type I (Fig. 8G) and type IIC (Fig. 8I) fibers in the soleus muscle of rats with metabolic syndrome.
Muscle fiber specification appears to be associated with metabolic syndrome. We conclude that mild hyperbaric oxygen at 1.25 ATA with 36% oxygen improves the muscle oxidative capacity of rats with metabolic syndrome by increasing blood flow and dissolved oxygen.
Effect of Mild Hyperbaric Oxygen on Muscle mRNA Levels
Previous studies26, 42) established that along with the coactivator PGC-1α, PPARs control diverse aspects of aerobic metabolism in skeletal muscle, including fatty acid oxidation, oxidative phosphorylation, and mitochondrial biogenesis. In particular, PPARα and PPARδ/β directly regulate the expression of certain nuclear-encoded mitochondrial genes and are closely related to the regulation of oxidative metabolism via PGC-1α43, 44).
PGC-1α, which was originally identified as a nuclear receptor coactivator, is a member of a family of transcription coactivators that play a central role in the regulation of glucose/fatty acid metabolism, mitochondrial synthesis, vascularization, proteolysis, and apoptosis25, 27). PGC-1α is expressed at high levels in cells and tissues where mitochondria are abundant and oxidative metabolism is at a high level, such as in the brain, brown adipose tissue, the heart, kidney, liver, and skeletal muscle29, 45). PGC-1α is also believed to be essential for fatty acid oxidation as it interacts with PPARα to promote the transcription of nuclear genes that encode mitochondrial fatty acid oxidation enzymes46).
Skeletal muscle fiber properties, including type composition and oxidative enzyme activity, are regulated by PGC-1α28, 45, 47). An upregulation of PGC-1α in transgenic mice under the influence of a muscle creatine kinase promoter results in a shift of fiber types from low oxidative to high oxidative45). We suggest that an increase in mRNA levels of Pgc-1α induced by mild hyperbaric oxygen restores decreased muscle oxidative capacity and can result in improved glucose tolerance and insulin resistance.
The soleus muscle of rats with metabolic syndrome exhibited lower mRNA levels of Pparδ/β than that of normal rats33). Another study11) showed that the soleus muscle of rats with metabolic syndrome exhibits low mRNA levels of Pparδ/β and Pgc-1α and high mRNA levels of Pparα. In this study, the soleus muscle of rats with metabolic syndrome exhibited higher mRNA levels of Pparα (Fig. 9A) and lower mRNA levels of Pparδ/β (Fig. 9B) and Pgc-1α (Fig. 9C) than that of normal rats; thus, the soleus muscle of rats with metabolic syndrome have a low capacity for fatty acid oxidation.
In general, type I and type IIA fibers in the hind limb muscles of normal rats have a relatively high oxidative enzyme activity, whereas type IIB fibers have a relatively low oxidative enzyme activity48–50). The rat soleus muscle contains type I, type IIA, and type IIC fibers; type IIA and type IIC fibers have a higher oxidative enzyme activity than type I fibers49, 50). In this study, the soleus muscle of rats with metabolic syndrome was comprised of type I and type IIC fibers, whereas those of the other two groups had high-oxidative type IIA and IIC fibers as well as low-oxidative type I fibers (Figs. 7, 8A–C). The mRNA levels of Pgc-1α in the soleus muscle were lower in rats with metabolic syndrome than in normal rats (Fig. 9C). In contrast, mild hyperbaric oxygen improved muscle SDH activity (Fig. 6B) and fiber SDH staining intensity of type I (Fig. 8G) and type IIC (Fig. 8I) fibers and induced type shifts of fibers from low-oxidative type I to high-oxidative type IIA (Fig. 8A, B). The reduced mRNA levels of Pgc-1α in the soleus muscle of rats with metabolic syndrome may be related to the low percentage of high oxidative fibers and high percentage of low oxidative fibers. Thus, it appears that one mechanism for increased muscle oxidative capacity induced by mild hyperbaric oxygen is due to an enhancement in the mRNA levels of Pgc-1α.
Our previous study11) observed a high proportion of type I fibers in the soleus muscle of exercised rats with metabolic syndrome and a concurrent increase in the SDH staining intensity of type I fibers. The study11) was unique in that the running distance of exercised rats with metabolic syndrome positively correlated with muscle SDH activity and mRNA levels of Pgc-1α. Conversely, in this study, mild hyperbaric oxygen reduced a percentage of type I fibers to normal (Fig. 8A). It is widely known that endurance exercise causes a steady increase in blood flow. However, the increase in blood flow following an endurance exercise is induced mostly in active skeletal muscles but not in internal organs. In addition, because of the increased atmospheric pressure and oxygen concentration, mild hyperbaric oxygen has an advantage in that it can increase the amount of dissolved oxygen in the plasma, which does not occur with endurance exercise. It is suggested that there are some differences in mechanism(s) for changes in fiber properties induced by endurance exercise versus mild hyperbaric oxygen. However, we did not elucidate the reason for these changes in skeletal muscle induced by endurance exercise and mild hyperbaric oxygen.
Conclusion
Mild hyperbaric oxygen at 1.25 ATA with 36% oxygen inhibited a growth-related increase in blood glucose levels and decrease in muscle oxidative capacity of rats with metabolic syndrome via improved oxidative metabolism induced by increased blood flow and dissolved oxygen.
Sources of Funding
This study was supported by the Uehara Memorial Foundation, Japan.
Conflicts of Interest
None.
Abbreviations
ATA, atmosphere absolute; CSA, cross-sectional area; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; OD, optical density; PGC-1α, peroxisome proliferator-activated receptor γ coactivator-1α; PPAR, peroxisome proliferator-activated receptor; SDH, succinate dehydrogenase
References
- 1). Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, Egusa G, Hiro T, Hirobe K, Iida M, Kihara S, Kinoshita M, Maruyama C, Ohta T, Okamura T, Yamashita S, Yokode M, Yokote K: Metabolic syndrome. J Atheroscler Thromb, 2014; 21: 1-5 [DOI] [PubMed] [Google Scholar]
- 2). Kelley DE, Goodpaster BH, Wing RR, Simoneau JA: Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol Endocrinol Metab, 1999; 277: E1130-E1141 [DOI] [PubMed] [Google Scholar]
- 3). Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS: Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA, 2003; 289: 76-99 [DOI] [PubMed] [Google Scholar]
- 4). Ballantyne CM, Hoogeveen RC, McNeill AM, Heiss G, Schmidt MI, Duncan BB, Pankow JS: Metabolic syndrome risk for cardiovascular disease and diabetes in the ARIC study. Int J Obes, 2008; 32: S21-S24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Abdul-Ghani MA, DeFronzo RA: Pathogenesis of insulin resistance in skeletal muscle. J Biomed Biotechnol, 2010; 10.1155/2010/476279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Reaven GM: The metabolic syndrome: time to get off the merry-go-round? J Intern Med, 2011; 269: 127-136 [DOI] [PubMed] [Google Scholar]
- 7). Lillioja S, Young AA, Culter CL, Ivy JL, Abbott WG, Zawadzki JK, Yki-Järvinen H, Christin L, Secomb TW, Bogardus C: Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest, 1987; 80: 415-424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Wade AJ, Marbut MM, Round JM: Muscle fibre type and aetiology of obesity. Lancet, 1990; 335: 805-808 [DOI] [PubMed] [Google Scholar]
- 9). Hickey MS, Carey JO, Azevedo JL, Houmard JA, Pories WJ, Israel RG, Dohm GL: Skeletal muscle fiber composition is related to adiposity and in vitro glucose transport rate in humans. Am J Physiol Endocrinol Metab, 1995; 268: 453-457 [DOI] [PubMed] [Google Scholar]
- 10). Gaster M, Staehr P, Beck-Nielsen H, Schrøder HD, Handberg A: GLUT4 is reduced in slow muscle fibers of type 2 diabetic patients: is insulin resistance in type 2 diabetes a slow, type 1 fiber disease? Diabetes, 2001; 50: 1324-1329 [DOI] [PubMed] [Google Scholar]
- 11). Nagatomo F, Fujino H, Kondo H, Kouzaki M, Gu N, Takeda I, Tsuda K, Ishihara A: The effects of running exercise on oxidative capacity and PGC-1α mRNA levels in the soleus muscle of rats with metabolic syndrome. J Physiol Sci, 2012; 62: 105-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Nagatomo F, Fujino H, Kondo H, Takeda I, Tsuda K, Ishihara A: High-fat diet-induced reduction of peroxisome proliferator-activated receptor γ coactivator-1α messenger RNA levels and oxidative capacity in the soleus muscle of rats with metabolic syndrome. Nutr Res, 2012; 32: 144-151 [DOI] [PubMed] [Google Scholar]
- 13). Ishihara A, Nagatomo F, Fujino H, Kondo H: Exposure to mild hyperbaric oxygen increases blood flow and resting energy expenditure but not oxidative stress. J Sci Res Reports, 2014; 3: 1886-1896 [Google Scholar]
- 14). Ishihara A, Kawano F, Okiura T, Morimatsu F, Ohira Y: Hyperbaric exposure with high oxygen concentration enhances oxidative capacity of neuromuscular units. Neurosci Res, 2005; 52: 146-152 [DOI] [PubMed] [Google Scholar]
- 15). Matsumoto A, Okiura T, Morimatsu F, Ohira Y, Ishihara A: Effects of hyperbaric exposure with high oxygen concentration on the physical activity of developing rats. Dev Neurosci, 2007; 29: 452-459 [DOI] [PubMed] [Google Scholar]
- 16). Yasuda K, Aoki N, Adachi T, Tsujimoto G, Gu N, Matsunaga T, Kikuchi N, Tsuda K, Ishihara A: Hyperbaric exposure with high oxygen concentration inhibits growthassociated increase in the glucose level of diabetic Goto-Kakizaki rats. Diabetes Obes Metab, 2006; 8: 714-715 [DOI] [PubMed] [Google Scholar]
- 17). Matsumoto A, Nagatomo F, Yasuda K, Tsuda K, Ishihara A: Hyperbaric exposure with high oxygen concentration improves altered fiber types in the plantaris muscle of diabetic Goto-Kakizaki rats. J Physiol Sci, 2007; 57: 133-136 [DOI] [PubMed] [Google Scholar]
- 18). Yasuda K, Adachi T, Gu N, Matsumoto A, Matsunaga T, Tsujimoto G, Tsuda K, Ishihara A: Effects of hyperbaric exposure with high oxygen concentration on glucose and insulin levels and skeletal muscle-fiber properties in diabetic rats. Muscle Nerve, 2007; 35: 337-343 [DOI] [PubMed] [Google Scholar]
- 19). Gu N, Nagatomo F, Fujino F, Takeda I, Tsuda K, Ishihara A: Hyperbaric oxygen exposure improves blood glucose level and muscle oxidative capacity in rats with type 2 diabetes. Diabetes Technol Ther, 2010; 12: 125-133 [DOI] [PubMed] [Google Scholar]
- 20). Nagatomo F, Roy RR, Takahashi H, Edgerton VR, Ishihara A: Effect of exposure to hyperbaric oxygen on diabetes-induced cataracts in mice. J Diabetes, 2011; 3: 301-308 [DOI] [PubMed] [Google Scholar]
- 21). Nagatomo F, Fujino H, Takeda I, Ishihara A: Effects of hyperbaric oxygenation on blood pressure levels of spontaneously hypertensive rats. Clin Exp Hypertens, 2010; 32: 193-197 [DOI] [PubMed] [Google Scholar]
- 22). Nagatomo F, Gu N, Fujino H, Okiura T, Morimatsu F, Takeda I, Ishihara A: Effects of exposure to hyperbaric oxygen on oxidative stress in rats with type II collagen-induced arthritis, Clin Exp Med, 2010; 10: 7-13 [DOI] [PubMed] [Google Scholar]
- 23). Nishizaka T, Nagatomo F, Fujino H, Nomura T, Sano T, Higuchi K, Takeda I, Ishihara A: Hyperbaric oxygen exposure reduces age-related decrease in oxidative capacity of the tibialis anterior muscle in mice. Enzyme Res, 2010; 10.4061/2010/824763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Nishizaka T, Nomura T, Sano T, Higuchi K, Nagatomo F, Ishihara A: Hyperbaric oxygen improves UVB irradiation-induced melanin pigmentation and diminishes senile spot size. Skin Res Tech, 2011; 17: 332-338 [DOI] [PubMed] [Google Scholar]
- 25). Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM: Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell, 1999; 98: 115-124 [DOI] [PubMed] [Google Scholar]
- 26). Wende AR, Huss JM, Schaeffer PJ, Giguere V, Kelly DP: PGC-1α coactivates PDK4 gene expression via the orphan nuclear receptor ERRα: a mechanism for transcriptional control of muscle glucose metabolism. Mol Cell Biol, 2005; 25: 10684-10694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Liang H, Ward WF: PGC-1α: a key regulator of energy metabolism. Adv Physiol Educ, 2006; 30: 145-151 [DOI] [PubMed] [Google Scholar]
- 28). Schuler M, Ali F, Chambon C, Duteli D, Borner JM, Tardivel A: PGC-1α expression is controlled in skeletal muscles by PPARβ, whose ablation results in fiber-type switching, obesity, and type 2 diabetes. Cell Metab, 2006; 4: 407-414 [DOI] [PubMed] [Google Scholar]
- 29). Miura S, Kai Y, Kamei Y, Ezaki O: Isoform-specific increases in murine skeletal muscle peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) mRNA in response to β2-adrenergic receptor activation and exercise. Endocrinology, 2008; 149: 4527-4533 [DOI] [PubMed] [Google Scholar]
- 30). Hennig R, Lømo T: Firing patterns of motor units in normal rats. Nature, 1985; 314: 164-166 [DOI] [PubMed] [Google Scholar]
- 31). Takaya K, Ogawa Y, Hiraoka J, Hosoda J, Yamori Y, Nakao K: Nonsense mutation of leptin receptor in the obese spontaneously hypertensive Koletsky rat. Nat Genet, 1996; 14: 130-131 [DOI] [PubMed] [Google Scholar]
- 32). Friedman JE, Ishizuka T, Liu S, Farrell CJ, Bedol D, Koletsky RJ, Kaung HL, Ernsberger P: Reduced insulin receptor signaling in the obese spontaneously hypertensive Koletsky rat. Am J Physiol Endocrinol Metab, 1997; 273: E1014-E1023 [DOI] [PubMed] [Google Scholar]
- 33). Nagatomo F, Gu N, Fujino H, Takeda I, Tsuda K, Ishihara A: Skeletal muscle characteristics of rats with obesity, diabetes, hypertension, and hyperlipidemia. J Atheroscler Thromb, 2009; 16: 576-585 [DOI] [PubMed] [Google Scholar]
- 34). Nagatomo F, Fujino H, Kondo H, Gu N, Takeda I, Ishioka N, Tsuda K, Ishihara A: PGC-1α mRNA level and oxidative capacity of the plantaris muscle in rats with metabolic syndrome, hypertension, and type 2 diabetes. Acta Histochem Cytochem, 2011; 44: 73-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Nagatomo F, Fujino H, Kondo H, Suzuki H, Kouzaki M, Takeda I, Ishihara A: PGC-1α and FOXO1 mRNA levels and fiber characteristics of the soleus and plantaris muscles in rats after hindlimb unloading. Histol Histopathol, 2011; 26: 1545-1553 [DOI] [PubMed] [Google Scholar]
- 36). Novo S, Balbarini A, Belch JJ, Bonura F, Clement DL, Diamantopoulos E, Fareed J, Norgren L, Poredos P, Rotzocil K: The metabolic syndrome: definition, diagnosis and management. Int Angiol, 2008; 27: 220-231 [PubMed] [Google Scholar]
- 37). Adachi T, Kikuchi N, Yasuda K, Anahara R, Gu N, Matsunaga T, Yamamura T, Mori C, Tsujimoto G, Tsuda K, Ishihara A: Fibre type distribution and gene expression levels of both succinate dehydrogenase and peroxisome proliferator-activated receptor-γ coactivator-1α of fibres in the soleus muscle of Zucker diabetic fatty rats. Exp Physiol, 2006; 92: 449-455 [DOI] [PubMed] [Google Scholar]
- 38). Yasuda K, Ishihara A, Adachi T, Shihara N, Seino Y, Tsuda K: Growth-related changes in skeletal muscle fiber type and insulin resistance in diabetic Otsuka Long-Evans Tokushima fatty rats. Acta Histochem Cytochem, 2001; 34: 371-382 [Google Scholar]
- 39). Yasuda K, Nishikawa W, Iwanaka N, Nakamura E, Seino Y, Tsuda K, Ishihara A: Abnormality in fibre type distribution of soleus and plantaris muscles in non-obese diabetic Goto-Kakizaki rats. Clin Exp Pharmacol Physiol, 2002; 29: 1001-1008 [DOI] [PubMed] [Google Scholar]
- 40). Tibbles PM, Edelsberg JS: Hyperbaric-oxygen therapy. New Engl J Med, 1996; 334: 1642-1648 [DOI] [PubMed] [Google Scholar]
- 41). Leach RM, Rees PJ, Wilmshurst P: ABC of oxygen: hyperbaric oxygen therapy. BMJ, 1998; 317: 1140-1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, Evans RM: Peroxisome-proliferator-activated receptor δ activates fat metabolism to prevent obesity. Cell, 2003; 113: 159-170 [DOI] [PubMed] [Google Scholar]
- 43). Puigserver P, Spiegelman BM: Peroxisome proliferator-activated 1α (PGC-1α): transcriptional coactivator and metabolic regulator. Endocr Rev, 2003; 24: 78-90 [DOI] [PubMed] [Google Scholar]
- 44). Kelly DP, Scarpulla RC: Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev, 2004; 18: 357-368 [DOI] [PubMed] [Google Scholar]
- 45). Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM: Transcriptioal co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature, 2002; 418: 797-801 [DOI] [PubMed] [Google Scholar]
- 46). Vega RB, Huss JM, Kelly DP: The coactivator PGC-1α cooperates with peroxisome proliferator-activated receptor α in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol, 2000; 20: 1868-1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47). Puigserver P: Tissue-specific regulation of metabolic pathways through the transcriptional coactivator PGC-1α. Int J Obes, 2005; 29: S5-S9 [DOI] [PubMed] [Google Scholar]
- 48). Smith D, Green H, Thomson J, Sharratt M: Oxidative potential in developing rat diaphragm, EDL, and soleus muscle fibers. Am J Physiol, 1988; 254 (Cell Physiol 23): 661-668 [DOI] [PubMed] [Google Scholar]
- 49). Nakatani T, Nakashima T, Kita T, Hirofuji C, Itoh K, Itoh M, Ishihara A: Succinate dehydrogenase activities of fibers in the rat extensor digitorum longus, soleus, and cardiac muscles. Arch Histol Cytol, 1999; 62: 393-399 [DOI] [PubMed] [Google Scholar]
- 50). Nakatani T, Nakashima T, Kita T, Ishihara A: Cell size and oxidative enzyme activity of type-identified fibers in rat hindlimb muscles: a review. Acta Histochem Cytochem, 2003; 36: 105-114 [Google Scholar]


