Abstract
Aim: Atherosclerosis is a chronic inflammatory disease, which leads to thrombosis and acute coronary syndrome. Matrix metalloproteinase-9 (MMP-9) is involved in the stability of the extracellular matrix (ECM) and atherosclerosis plaque. Until now, it is established that lipopolysaccharide (LPS) and norepinephrine (NE) are associated with the pathological process of atherosclerosis. However, the combined effect of LPS and NE on MMP-9 is unclear. We investigated the combined effect of LPS and NE on MMP-9 expression in human monocytes and the mechanism involved in the process.
Methods: THP-1 cells were cultured and treated with LPS and/or NE. MMP-9 and TIMP-1 gene and protein expression were detected by real time PCR and ELISA, respectively. MMP-9 activity was detected by gelatin zymography. Adrenoceptor antagonists and MAPKs inhibitors were used to clarify the mechanism. Pathway-related proteins were detected by Western blot.
Results: We found that NE enhances LPS-induced MMP-9 and TIMP-1 expression as well as MMP-9 activity in THP-1 cells. This effect is reversed by the beta (β)-adrenoceptor antagonist propranolol, extracellular signal-regulated kinases (ERK) inhibitor U0126, and c-Jun N-terminal kinase (JNK) inhibitor SP600125. NE enhances LPS-induced ERK/JNK phosphorylation. NE up-regulates LPS-induced c-Fos expression, which is counteracted by propranolol, U0126, and SP600125. Furthermore, c-Fos silence reverses the effect of NE on MMP-9 activity.
Conclusions: Our results suggest that NE enhances LPS-induced MMP-9 expression through β-adrenergic receptor and downstream ERK/JNK-c-Fos pathway. This study may help us to understand the combined effect and mechanism of NE/LPS on MMP-9 expression.
Keywords: Norepinephrine, Lipopolysaccharide, Matrix metalloproteinase-9, Atherosclerosis
Introduction
Atherosclerosis is recognized as a chronic inflammatory disease. Considerable evidence supports that immune cells, such as monocytes, are involved in this process. Monocytes and macrophages accumulate in the growing lesion, ingest lipids, and contribute to the development of atherosclerosis. Rupture of atherosclerosis plaques, which frequently occurs in regions that contain many monocytes, leads to coronary thrombosis and acute coronary syndrome1).
Rupture of atherosclerosis plaques always occurs when there is severe inflammation response and stress stimulation, which leads to the structure of the thin fibrous cap being destroyed. Matrix metalloproteinase-9 (MMP-9) is involved in this process. MMP-9 and its natural inhibitor, which is called the tissue inhibitor of matrix metalloproteinase-1 (TIMP-1), play an important role in the regulation of extracellular matrix (ECM) metabolism. Studies have shown that MMP-9 is related to plaque instability2). High expression of MMP-9 is observed in atherosclerotic plaques, particularly in the shoulder regions, which are more prone to instability and rupture3, 4).
Lipopolysaccharide (LPS) and norepinephrine (NE) are associated with atherosclerosis. In various tissues, LPS has been shown to accelerate atherosclerosis5). On binding the toll like receptor 4 (TLR4), LPS triggers signaling cascades and upregulates the expression of pro-inflammatory chemokines and cytokines. An activated TLR4-signaling pathway in monocytes plays an important role in the progression of atherosclerotic disease6, 7). In addition, activation of the sympathetic nervous system could contribute to endothelial dysfunction and atherogenesis8). NE, the neurotransmitter released from the sympathetic neurons, contributes to thrombopoiesis and participates in the pathogenesis of atherosclerosis9).
In some clinical scenarios, such as sepsis, plasma levels of both LPS and NE are evaluated. Recent studies have shown that sepsis accelerates atheroma development10). However, the combined effect of LPS and NE on the expression of MMP-9 is unclear. Based on these facts, we intended to clarify whether NE affects LPS-induced MMP-9 expression in human monocytes and the mechanism involved in the process.
Materials and Methods
Reagents and Antibodies
RPMI 1640 medium and Fetal Bovine Serum (FBS) were purchased from Gibco. Penicillin and streptomycin were purchased from HyClone. A lymphocyte separation kit, norepinephrine, propranolol, phentolamine, and lipopolysaccharide (from Escherichia coli, serotype B6:O55) were obtained from Sigma-Aldrich. SB203580 (p38 mitogen-activated protein kinase inhibitor, p38 MAPK inhibitor), U0126 (extracellular signal-regulated kinase inhibitor, ERK1/2 inhibitor), and SP600125 (stress-activated protein kinase/c-Jun N-terminal kinase inhibitor, JNK inhibitor) were obtained from the Beyotime Institute of Biotechnology. All primary and secondary antibodies were purchased from Cell Signaling Technology.
Cell Culture
Primary monocytes from healthy donors were isolated using a lymphocyte separation kit and the density gradient separation method. Informed consent was obtained from all participants. The studies were approved by the Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University. The monocytic cell line THP-1 was obtained from the Cell Bank of Chinese Academy of Science (Shanghai, China). Cells were maintained in RPMI-1640 containing 10% FCS as well as 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2, 95% air. Experiments were performed after a 24 h cell starvation in RPMI-1640 with 1% FBS. These cells were centrifuged and resuspended in fresh RPMI-1640 with 1% FBS and seeded into 24-well plates at a density of 1 × 106 cells/ml for treatment.
ELISA
The levels of MMP-9 and TIMP-1 in the cell supernatants were determined using MMP-9 and TIMP-1 ELISA kits (eBioscience, USA) according to the manufacturer's instructions.
Real-time PCR (RT PCR)
Total RNA was isolated from primary monocytes and THP-1 cells using Trizol reagent (Life Technology) and was reverse transcribed using Transcriptor First Strand cDNA Synthesis Kit (Roche, Switzerland). RT PCR was performed with the FastStart Universal SYBR Green Master (Roche), and the reactions were conducted in ABI 7500 FAST RT PCR system. The nucleotide sequences of primers used are shown in Table 1.
Table 1. Primers used for real-time PCR.
| Target | Forward-Primer (5′-3′) | Reverse-Primer (5′-3′) |
|---|---|---|
| MMP-1 | AAAATTACACGCCAGATTTGCC | GGTGTGACATTACTCCAGAGTTG |
| MMP-3 | AGTCTTCCAATCCTACTGTTGCT | TCCCCGTCACCTCCAATCC |
| MMP-9 | TGTACCGCTATGGTTACACTCG | GGCAGGGACAGTTGCTTCT |
| MMP-12 | GGAATCCTAGCCCATGCTTTT | CATTACGGCCTTTGGATCACT |
| TIMP-1 | CTTCTGCAATTCCGACCTCGT | ACGCTGGTATAAGGTGGTCTG |
| β-actin | ATTGGCAATGAGCGGTTC | GGATGCCACAGGACTCCAT |
Western Blot Analysis
THP-1 cells were harvested in RIPA lysis buffer (Bioteke Co, Beijing, China) containing 1 mM phenylmethylsulfonyl fluoride (PMSF) and then centrifuged at 12,000 g for 15 min at 4°C. Whole cell lysates were used for analysis. Equal amounts of protein were separated by SDS-PAGE and transferred onto a PVDF membrane. The membranes were incubated with primary antibodies, followed by incubation with a horseradish peroxidase-conjugated goat anti-rabbit or mouse IgG secondary antibody. Detection was performed with ECL (Thermo). The bands were quantified by optical density ratio using GAPDH as a control.
Assay of MMP-9 by Gelatin Zymography
MMP-9 activity was assessed by gelatin zymography. In brief, we cultured cells (1 × 106 cells/ml) for different treatments, collected culture supernatants, added buffer without reducing the agent to the supernatants, and subjected the supernatants to electrophoresis on 10% SDS-polyacrylamide gels containing 0.1% gelatin. After electrophoresis, we incubated the gels in 1 × Zymogram Renaturing Buffer (LC2670, Life Technology) for 30 min with gentle agitation. To assess gelatinolytic activity, the gels were incubated for 24 h at 37°C in a developing buffer (LC2671, Life Technology) and stained with Coomassie Brilliant Blue solution.
RNA Interference
c-Fos siRNA (catalog number sc-29221) and negative control siRNA (catalog number sc-37007) were purchased from Santa Cruz Biotechnology, Inc. For the transfection procedure, cells were grown to 70%–80% confluence, and c-Fos siRNA and control siRNA were transfected using LipofetamineTM2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. At 24 h post-transfection, the cells received different treatments. Then, cells were harvested for Western blot analyses and gelatin zymography.
Statistical Analysis
Data were expressed as mean ± SD and analyzed using statistical software SPSS 20. The significance of the differences among the two groups was determined by t test. One-way ANOVA followed by Bonferroni's Multiple Comparison was used for analysis of more than two groups. All experiments were repeated at least three times. P values <0.05 were considered statistically significant.
Results
LPS Upregulates MMPs and Tissue Inhibitor of Metalloproteinase-1 (TIMP-1) Expression in Human Monocytes
As shown in Fig. 1A and Fig. 1B, LPS at 1 µg/ml significantly increased the gene expression of several main matrix metalloproteinases (MMP-1, MMP-3, MMP-9, and MMP-12) (P < 0.001) in primary isolated human monocytes and THP-1 cells. LPS could also upregulate TIMP-1 gene and protein expression, which is more obvious at the time point of 12 h and 24 h, respectively (Fig. 1C and Fig. 1D). We also assessed gelatinolytic activity of MMP-9 in the culture supernatants after LPS stimulation. As shown in Fig. 1E, LPS-treated cells had strong gelatinolytic activity 24 h and 48 h post-LPS treatment. In contrast, the activity of MMP-2 was unchanged by LPS treatment.
Fig. 1.
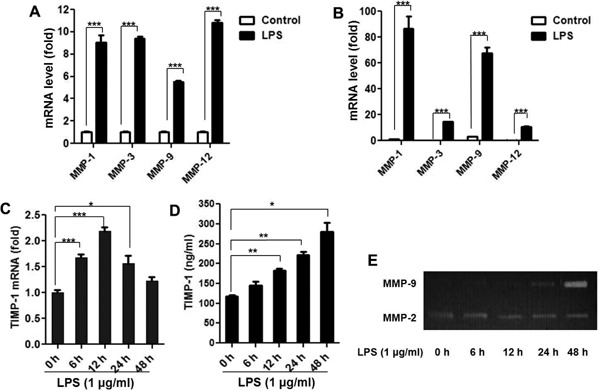
LPS upregulates MMPs and TIMP-1 expression in human monocyte cells
Primary human monocytes and THP-1 cells (1 × 106 cells/ml) were dispensed on 24-well plates until 70%–80% confluent and then treated with LPS (1 µg/ml). The MMPs mRNA level was detected by RT-PCR 3 h after stimulation in (A) primary human monocytes (B) THP-1 cells. TIMP-1 mRNA and protein levels were detected for the indicated time using RT-PCR and ELISA KIT in THP-1 cells (C and D). The cell-free supernatants were assayed for MMP-9 activity by gelatin zymography (E). Data are expressed as mean ± SD from three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
NE Enhances LPS-induced MMP-9 and TIMP-1 Expression
MMP-9 plays an important role in the stability of atherosclerotic plaque. To investigate whether NE could affect LPS-induced TIMP-1 and MMP-9 expression, THP-1 cells were exposed to different concentrations of NE (0.01 µM, 0.1 µM, and 1.0 µM) for 40 min, and then with LPS for another 24 h and 48 h. As shown in Fig. 2B and Fig. 2C, NE enhanced LPS-induced MMP-9 and TIMP-1 secretion at 24 h and 48 h. Furthermore, the effect was more obvious when the concentration of NE was 1.0 µM. NE also enhanced LPS-induced MMP-9 gene expression (Fig. 2A) and gelatinolytic activity (Fig. 2D). However, NE alone could not induce MMP-9 expression. The CCK8 assay showed that neither NE alone (0.01 µM, 0.1 µM, and 1.0 µM) nor NE with LPS affected THP-1 cell viability (Fig. 2E).
Fig. 2.
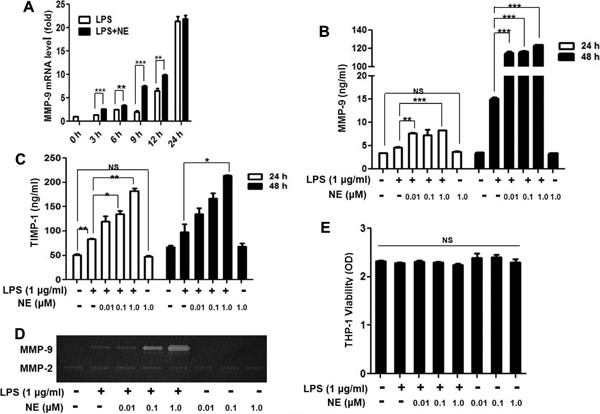
NE enhances LPS-induced MMP-9 and TIMP-1 expression
THP-1 cells were treated with NE (1.0 µM) and LPS (1 µg/ml) for the indicated time, and MMP-9 mRNA level was detected by RT-PCR (A). THP-1 cells were exposed to different concentrations of NE or a vehicle for 40 min, and then with LPS for another 24 h or 48 h. MMP-9 and TIMP-1 expressions were detected by an ELISA kit (B and C). MMP-9 activity was measured by gelatin zymography 48 h after LPS stimulation (D). THP-1 cells viability was detected by CCK8 kit after 48 h stimulation (E). *P < 0.05, **P < 0.01, ***P < 0.001. NS indicates no significant difference.
Contribution of β-adrenergic Receptor Activation to the Effect of MMP-9 Expression by NE in LPSchallenged THP-1 Cells
NE could activate alpha (α)- and beta (β)-adrenergic receptors; hence, we further investigated the role of adrenergic receptors in MMP-9 expression by NE and LPS. THP-1 cells were pre-treated with the β-adrenergic antagonist propranolol (Pro), the α-adrenergic receptor antagonist phentolamine (Pht) for 30 min, and then with NE and LPS. Gene and protein expression of MMP-9 were measured in THP-1 cells and cell supernatants, respectively. As described in Fig. 3A and Fig. 3B, NE significantly increased LPS-induced MMP-9 mRNA (P < 0.001) and protein expression (P < 0.01), which were reversed by pretreatment with propranolol. Furthermore, gelatinolytic activity of MMP-9 enhanced by NE in LPS-challenged THP-1 cells was reversed by propranolol, but not by phentolamine (Fig. 3C).
Fig. 3.
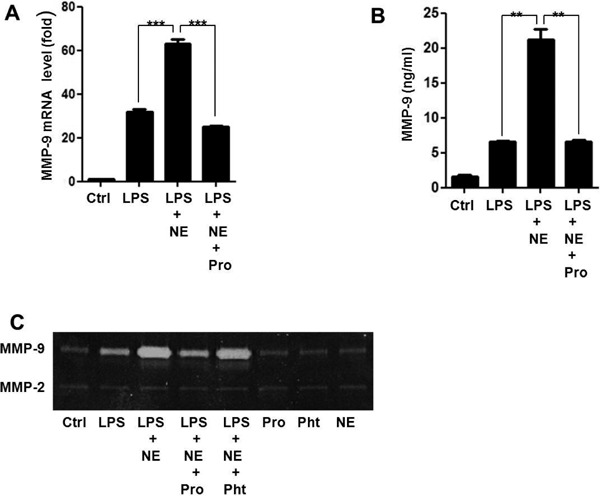
NE enhances LPS-induced MMP-9 expression through β-adrenergic receptor
THP-1 cells were pre-treated with propranolol (Pro), phentolamine (Pht), or a vehicle for 30 min, NE (1.0 µM) for 40 min, and then with LPS for another 3 h for RT-PCR analysis (A) 48 h for ELISA (B) and zymography (C). **P < 0.01, ***P < 0.001.
The Expression of MMP-9 Induced by NE and LPS is Dependent on ERK/JNK
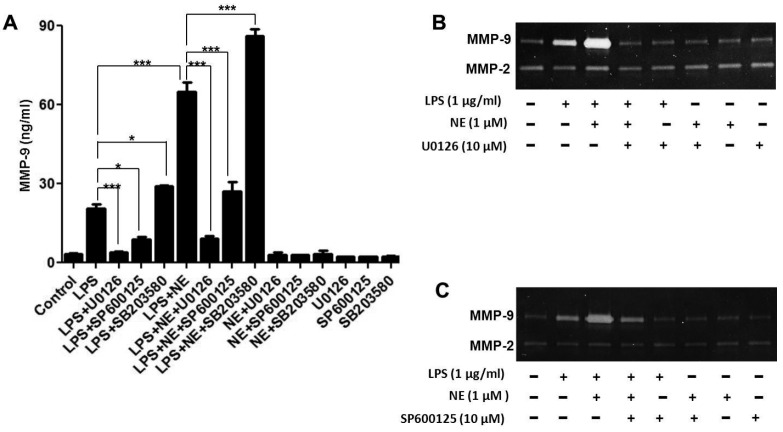
It is well recognized that MAPKs activation is involved in the regulation of LPS-induced MMPs expression. Thus, we investigated the effect of extracellular regulated protein kinases (ERK) inhibitor U0126, c-Jun N-terminal kinase (JNK) inhibitor SP600125, and P38 MAPK inhibitor SB203580 on MMP-9 expression after NE and LPS stimulation. As shown in Fig. 4A, U0126 and SP600125 not only reversed the effect of LPS-induced MMP-9 expression but also counteracted the effect of MMP-9 expression by NE and LPS. In contrast, SB203580 increased MMP-9 expression induced by LPS alone and LPS combined with NE. Furthermore, gelatinolytic activity of MMP-9 enhanced by NE in LPS-challenged THP-1 cells could also be partly reversed by U0126 and SP600125 (Fig. 4B, Fig. 4C). To demonstrate the effect of NE on LPS-induced MAPKs activation, THP-1 cells were exposed to NE (1.0 µmol) for 40 min, and then with LPS for another 30 min. P-ERK, P-JNK, and P-P38 expression were detected by Western blot. As shown in Fig. 5, NE could enhance LPS-induced ERK and JNK phosphorylation as well as inhibit LPS-induced P38 phosphorylation. All the results indicate that JNK/ERK phosphorylation is involved in the expression of MMP-9 induced by NE and LPS.
Fig. 4.
U0126, SP600125 reverse the effect of NE on MMP-9 expression in LPS-Challenged THP-1 cells
After being pre-treated with U0126, SP600125, SB203580, or a vehicle for 30 min, THP-1 cells were stimulated with NE for 40 min, and then with LPS for another 48 h (A) (B) (C). MMP-9 level and enzyme activity were detected by ELISA kit (A) and zymography (B) (C). *P < 0.05, **P < 0.01. ***P < 0.001
Fig. 5.
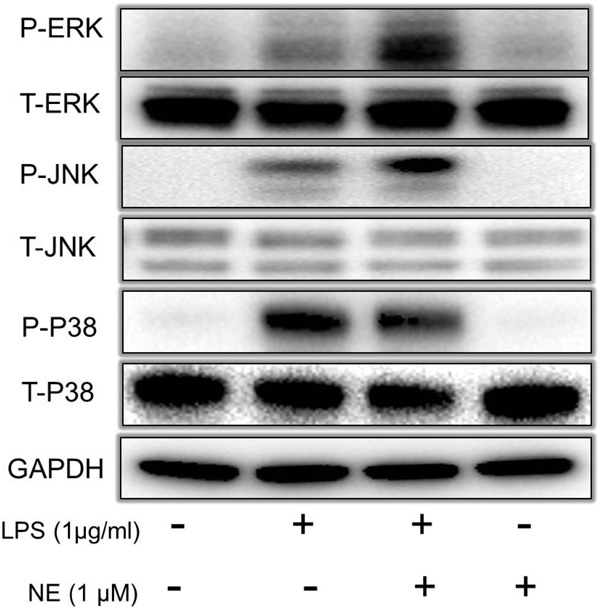
NE enhances LPS-induced ERK/JNK phosphorylation
After being pre-treated with NE or a vehicle for 40 min, THP-1 cells were stimulated with LPS for another 30 min. MAPKs phosphorylation was detected by Western blot. P-ERK, P-JNK, P-P38 indicate phosphorylation of ERK, JNK and P38. T-ERK, T -JNK, T -P38 indicate total of ERK, JNK and P38.
NE Enhances LPS-induced c-Fos Expression, which can be Reversed by Propranolol, U0126, and SP600125
Much evidence indicates that activator protein 1 (AP-1) plays an important role in the expression of MMP-9. We further investigated the effect of LPS and NE on c-Fos expression, which forms the AP-1 transcription factor. c-Fos expression increased immediately after LPS-stimulation (Fig. 6A). NE increased c-Fos expression in LPS-challenged THP-1 cells (Fig. 6B). Then, we investigated the role of propranolol, SP600125, and U0126 on c-Fos expression after NE and LPS stimulation. As shown in Fig. 6C and Fig. 6D, Pro, U0126, and SP600125 pretreatment almost completely inhibited c-Fos expression induced by NE and LPS.
Fig. 6.
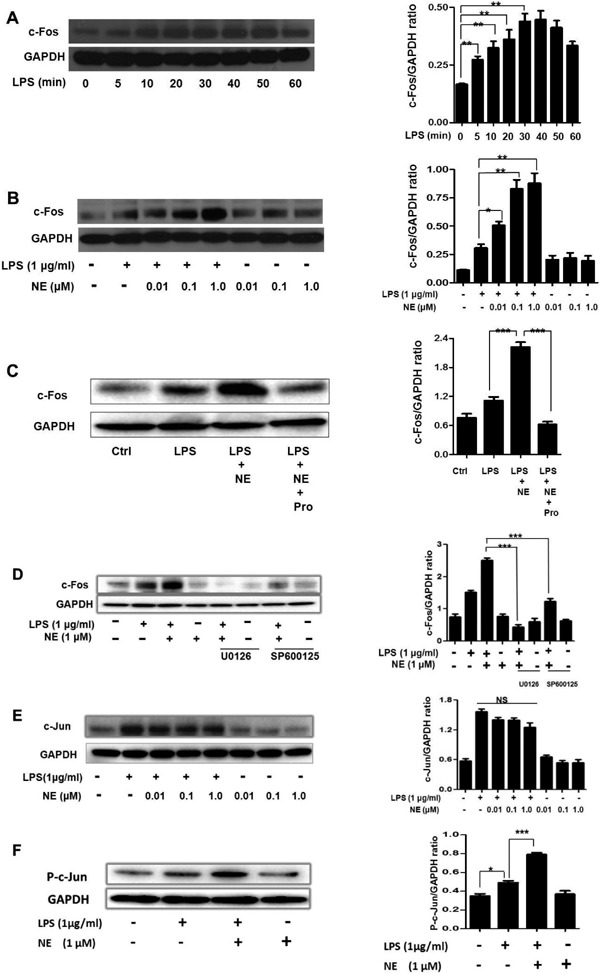
NE enhances LPS-induced c-Fos and P-c-Jun expression. NE/LPS induced c-Fos expression can be reversed by propranolol, U0126, and SP600125
THP-1 cells were incubated with LPS for 0, 5, 10, 20, 30, 40, 50, and 60 min. c-Fos expression was measured by Western blot (A). After being exposed to different concentrations of NE for 40 min, THP-1 cells were stimulated with LPS for another 30 min. c-Fos expression was determined by Western blot (B). Cells were pre-treated with Pro, U0126, SP600125, or a vehicle for 30 min, NE for 40 min, and then with LPS for another 30 min. c-Fos levels were detected by Western blot (C-D). After being exposed to NE for 40 min, THP-1 cells were stimulated with LPS for another 30 min. c-Jun and P-c-Jun were determined by Western blot (E-F). NS indicates no significant difference. *P < 0.05, **P < 0.01, ***P < 0.001.
NE Enhances LPS-induced c-Jun Phosphorylation
c-Jun is another component of AP-1. We investigated the effect of NE/LPS on c-Jun and P-c-Jun expression. LPS increased c-Jun expression. However, different concentrations of NE (0.01 µmol, 0.1 µmol, and 1.0 µmol) failed to increase LPS-induced c-Jun expression (Fig. 6E). Next, phosphorylation of c-Jun was measured by Western blot in NE/LPS treated cells. We found that NE could increase P-c-Jun expression in LPS-challenged THP-1 cells (Fig. 6F).
c-Fos Silencing Reverses the Effect of NE on MMP-9 Activity in LPS-challenged THP-1 Cells
To investigate the role of c-Fos in NE and LPS-induced MMP-9 expression, we used c-Fos siRNA. As shown in Fig. 7A, c-Fos siRNA significantly downregulated c-Fos expression. Moreover, c-Fos silencing partly reversed the increased MMP-9 activity induced by LPS and NE (Fig. 7B).
Fig. 7.
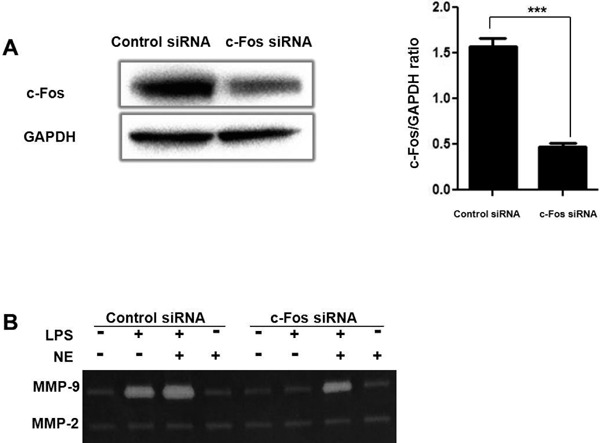
c-Fos silencing reverses the effect of NE on MMP-9 activity in LPS-challenged THP-1 cells
THP-1 cells were transfected with 100 nM control siRNA, or c-Fos siRNA. After 24 h post-transfection, the cells were harvested for Western blot analysis (A). After being treated with NE for 40 min and LPS for another 48 h, MMP-9 activity was measured by gelatin zymography (B). ***P < 0.001.
Discussion
The results of the present study suggest that NE enhances LPS-induced MMP-9 expression through β-adrenergic receptor and downstream ERK/JNK-c-Fos pathway. It is well established that the expression of MMPs plays an important role in the pathology of atherosclerosis diseases11, 12). Recent study has shown that MMP-9, also known as gelatinase, is strongly correlated with plaque instability. MMP-9 is significantly higher in patients with coronary artery disease than in those in the control group13). Over-expressed MMP-9 is accumulated in the shoulder region of the plaque where it is vulnerable to rupture; inhibition of MMP-9 stabilizes the arteries by increasing their collagen content14, 15).
MMP-9 can be secreted by various cells, such as endothelial cells, fibroblasts, and monocytes/macrophages16). Monocytes and macrophages, which are capable of forming foam cells, are protagonists in the process of atherosclerosis. We intended to clarify the effect of NE and LPS on MMP-9 expression in monocyte cells. We found that LPS upregulated MMPs expression in both primary isolated human monocytes and THP-1 cells and also increased MMP-9 activity, which is in accordance with previous reports. We also found that TIMP-1, a natural inhibitor of the MMPs, was upregulated by LPS-stimulation in THP-1 cells, which may be a concomitant response of MMP-9.
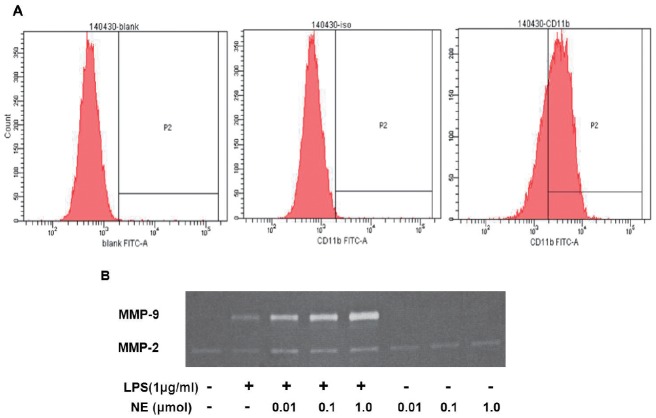
Although many previous reports have investigated the interaction between NE and LPS, they mainly focused on the effect of the cytokines release by NE and LPS17, 18). Speidl et al. have demonstrated that catecholamines could potentiate LPS-induced expression of MMP-9 expression in U937 cells19); however, little is known about the mechanism and pathway involved in this process. In the present study, we found that NE increased LPS-induced MMP-9 gene expression as well as the protein level and gelatinolytic activity in another monocytic cell line, THP-1 cells. However, NE alone could not induce MMP-9 and TIMP-1 expression in our experiment, which is inconsistent with Speidl's reports. This could be explained by the fact that various cells have different responses to NE; furthermore, the concentration of NE used in these two studies also had minor differences (1 µM and 1 µg/ml). Furthermore, we used macrophages derived from THP-1 cells for zymography detection. We found that NE could also enhance LPS-induced MMP-9 activity in macrophages (Supplemental Fig. 1). In addition, we found that NE increased LPS-induced TIMP-1 expression. Mittal et al. have proposed that an extracellular stimulus may cause a concordant increase in MMPs and TIMP20). We speculate that NE/LPS-induced AP-1 activation also upregulates TIMP-1 expression. We believe this effect is very important. Meanwhile, extracellular stimulus increases MMP-9 and TIMP-1 expression to maintain ECM balance.
Supplemental Fig. 1.
NE enhances LPS-induced MMP-9 activity in the THP-1 derived macrophage
THP-1 cells were incubated with PMA (100 ng/ml) for 72 h. A CD11b-FITC antibody was used to detect macrophages (A). After being exposed to different concentrations of NE or a vehicle for 40 min, and then to LPS for another 48 h, MMP-9 activity was measured by gelatin zymography (B)
NE could activate α- and β-adrenergic receptors. Previous studies have shown that different receptor stimulations exert different effects21). Thus, we intended to clarify which type of receptor was involved in the MMP-9 production process. We found that the β-adrenergic receptor inhibitor propranolol nearly completely inhibited NE-induced MMP-9 (1) gene expression, (2) the protein level, and (3) gelatinolytic activity, whereas phentolamine, an α-adrenergic receptor inhibitor, had no effect on MMP-9 activity. Hori et al. proved that administration of isoprenaline (β-adrenoceptor agonist) could increase MMP-9 expression22). Another study showed that atenolol (β-1 adrenoceptor antagonist) attenuates the MMP-9 increment by Angiotensin II23). All these studies indicate that β-adrenergic receptors play an important role in MMP-9 expression.
Accumulating evidence indicates that LPS increases MMP-9 production by activating MAPKs in various cells24, 25). We employed MAPKs inhibitors to clarify the pathway involved in NE- and LPS-challenged THP-1 cells. We found that ERK inhibitor U0126, JNK inhibitor SB600125, reduced MMP-9 expression, and gelatinolytic activity not only induced by LPS but also by LPS combined with NE. We also found that NE could enhance LPS-induced ERK and JNK phosphorylation. This suggests that ERK and JNK phosphorylation is essential in NE/LPS-induced MMP-9 expression. On the contrary, P38 inhibitor SB203580 increased MMP-9 expression by LPS and NE/LPS. Furthermore, NE inhibits P38 phosphorylation evoked by LPS. It seems that inhibition of P38 activity may contribute to MMP-9 expression in our study. However, other studies have shown P38 kinase phosphorylation is essential in the process of LPS-induced MMP-9 expression26). The inconsistent results may be due to the different cell types used. Previous studies also showed that there is a crosstalk between P38 and the ERK pathway27, 28); inhibition of P38 phosphorylation enhances ERK phosphorylation29). It is reasonable to speculate that inhibition of P38 in LPS and NE challenged THP-1 cells enhanced the ERK signaling pathway, which resulted in the overexpression of MMP-9.
The most prominent transcription factors implicated in MMP-9 gene activation are AP-1 and NF-κB30). We detected NE/LPS induced NF-κB P-P65 and P65 expression (Supplemental Fig. 2). However, we found that NE could not enhance LPS-induced P65 activation. It seems that the NF-κB pathway is not involved in the mechanism. c-Fos and c-Jun are two important components of the AP-1 transcription factor. In our study, we found that LPS increased c-Fos expression, and NE enhanced this effect. We also employed c-Fos siRNA to clarify its role in MMP-9 expression. As is shown, c-Fos knockdown partly abolished MMP-9 gelatinolytic activity which is evoked by NE and LPS. Interestingly, when we pre-treated THP-1 cells with ERK and JNK inhibitors, the augmentation of c-Fos expression is alleviated. When pre-treated with propranolol, as we expected, the NE-induced augmentation of c-Fos expression in LPS-challenged cells is also abolished. However, NE could not affect LPS-induced c-Jun expression at all. But we found that NE could also increase P-c-Jun expression in LPS-challenged THP-1 cells. It seems that c-Jun activation is also involved in NE/LPS induced MMP-9 expression. This result could also explain why c-Fos knowdown did not completely abolish MMP-9 gelatinolytic activity in Fig. 7B. However, more studies are needed to clarify the role and mechanism of P-c-Jun in NE/LPS induced MMP-9 expression.
Supplemental Fig. 2.
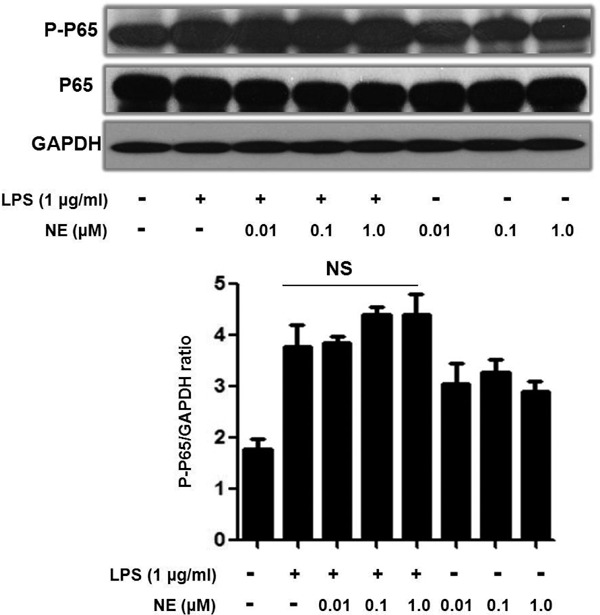
NE did not enhance LPS-induced NF-κB P65 phosphorylation
After being exposed to NE for 40 min, THP-1 cells were stimulated with LPS for another 30 min. P-P65 and P-65 expression were determined by Western blot
Conclusions
In conclusion, we demonstrated that LPS and NE, two important factors in the pathologic process of atherosclerosis, have a synergistic effect on the expression of MMP-9. This effect is associated with the β-adrenergic receptor, and the ERK/JNK-c-Fos pathway.
Acknowledgements
This work was supported by grants from the National Nature Science Foundation of China (No. 81170167, 81200191 and 81400277)
Conflict of Interest (COI)
All authors disclose no conflict of interest including any financial, personal or other relationships with other people or organizations that could inappropriately influence, or be perceived to influence, their work.
References
- 1). Shah PK, Falk E, Badimon JJ, Fernandez-Ortiz A, Mailhac A, Villareal-Levy G, Fallon JT, Regnstrom J, Fuster V. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation 1995; 92: 1565-1569 [PubMed] [Google Scholar]
- 2). Heo SH, Cho CH, Kim HO, Jo YH, Yoon KS, Lee JH, Park JC, Park KC, Ahn TB, Chung KC, others Plaque rupture is a determinant of vascular events in carotid artery atherosclerotic disease: involvement of matrix metalloproteinases 2 and 9. J Clin Neurol 2011; 7: 69-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). De Paoli F, Eeckhoute J, Copin C, Vanhoutte J, Duhem C, Derudas B, Dubois-Chevalier J, Colin S, Zawadzki C, Jude B, others The neuron-derived orphan receptor 1 (NOR1) is induced upon human alternative macrophage polarization and stimulates the expression of markers of the M2 phenotype. Atherosclerosis 2015; 241: 18-26 [DOI] [PubMed] [Google Scholar]
- 4). Stintzing S, Heuschmann P, Barbera L, Ocker M, Jung A, Kirchner T, Neureiter D. Overexpression of MMP9 and tissue factor in unstable carotid plaques associated with Chlamydia pneumoniae, inflammation, and apoptosis. Ann Vasc Surg 2005; 19: 310-319 [DOI] [PubMed] [Google Scholar]
- 5). Andoh Y, Ogura H, Satoh M, Shimano K, Okuno H, Fujii S, Ishimori N, Eshima K, Tamauchi H, Otani T, others Natural killer T cells are required for lipopolysaccharide-mediated enhancement of atherosclerosis in apolipoprotein E-deficient mice. Immunobiology 2013; 218: 561-569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). den Dekker WK, Cheng C, Pasterkamp G, Duckers HJ. Toll like receptor 4 in atherosclerosis and plaque destabilization. Atherosclerosis 2010; 209: 314-320 [DOI] [PubMed] [Google Scholar]
- 7). Schoneveld AH, Hoefer I, Sluijter JP, Laman JD, de Kleijn DP, Pasterkamp G. Atherosclerotic lesion development and Toll like receptor 2 and 4 responsiveness. Atherosclerosis 2008; 197: 95-104 [DOI] [PubMed] [Google Scholar]
- 8). Chistiakov DA, Ashwell KW, Orekhov AN, Bobryshev YV. Innervation of the arterial wall and its modification in atherosclerosis. Auton Neurosci 2015 [DOI] [PubMed] [Google Scholar]
- 9). Chen S, Du C, Shen M, Zhao G, Xu Y, Yang K, Wang X, Li F, Zeng D, Chen F, others Sympathetic stimulation facilitates thrombopoiesis by promoting megakaryocyte adhesion, migration, and proplatelet formation. Blood 2016; 127: 1024-1035 [DOI] [PubMed] [Google Scholar]
- 10). Kaynar AM, Yende S, Zhu L, Frederick DR, Chambers R, Burton CL, Carter M, Stolz DB, Agostini B, Gregory AD, others Effects of intra-abdominal sepsis on atherosclerosis in mice. Crit Care 2014; 18: 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Vacek TP, Rehman S, Neamtu D, Yu S, Givimani S, Tyagi SC. Matrix metalloproteinases in atherosclerosis: role of nitric oxide, hydrogen sulfide, homocysteine, and polymorphisms. Vasc Health Risk Manag 2015; 11: 173-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Hopps E, Caimi G. Matrix metalloproteases as a pharmacological target in cardiovascular diseases. Eur Rev Med Pharmacol Sci 2015; 19: 2583-2589 [PubMed] [Google Scholar]
- 13). Noji Y, Kajinami K, Kawashiri MA, Todo Y, Horita T, Nohara A, Higashikata T, Inazu A, Koizumi J, Takegoshi T, others Circulating matrix metalloproteinases and their inhibitors in premature coronary atherosclerosis. Clin Chem Lab Med 2001; 39: 380-384 [DOI] [PubMed] [Google Scholar]
- 14). Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res 2002; 90: 251-262 [PubMed] [Google Scholar]
- 15). Jones CB, Sane DC, Herrington DM. Matrix metalloproteinases: a review of their structure and role in acute coronary syndrome. Cardiovasc Res 2003; 59: 812-823 [DOI] [PubMed] [Google Scholar]
- 16). Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin Cancer Biol 2010; 20: 161-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Yu X, Jia B, Wang F, Lv X, Peng X, Wang Y, Li H, Wang Y, Lu D, Wang H. alpha(1) adrenoceptor activation by norepinephrine inhibits LPS-induced cardiomyocyte TNF-alpha production via modulating ERK1/2 and NF-kappaB pathway. J Cell Mol Med 2014; 18: 263-273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Huang J, Zhang Y, Wang C, Zhou J, Ma Q, Wang X, Shen X, Jiang C. Enhanced Phosphorylation of MAPKs by NE Promotes TNF-?? Production by Macrophage Through ?? Adrenergic Receptor. Inflammation 2012; 35: 527-534 [DOI] [PubMed] [Google Scholar]
- 19). Speidl WS, Toller WG, Kaun C, Weiss TW, Pfaffenberger S, Kastl SP, Furnkranz A, Maurer G, Huber K, Metzler H, others Catecholamines potentiate LPS-induced expression of MMP-1 and MMP-9 in human monocytes and in the human monocytic cell line U937: possible implications for peri-operative plaque instability. Faseb J 2004; 18: 603-605 [DOI] [PubMed] [Google Scholar]
- 20). Mittal B, Mishra A, Srivastava A, Kumar S, Garg N. Matrix metalloproteinases in coronary artery disease. Adv Clin Chem 2014; 64: 1-72 [DOI] [PubMed] [Google Scholar]
- 21). Rietz A, Spiers JP. The relationship between the MMP system, adrenoceptors and phosphoprotein phosphatases. Brit J Pharmacol 2012; 166: 1225-1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Hori Y, Kunihiro S, Sato S, Yoshioka K, Hara Y, Kanai K, Hoshi F, Itoh N, Higuchi S. Doxycycline attenuates isoproterenol-induced myocardial fibrosis and matrix metalloproteinase activity in rats. Biol Pharm Bull 2009; 32: 1678-1682 [DOI] [PubMed] [Google Scholar]
- 23). Senzaki H, Paolocci N, Gluzband YA, Lindsey ML, Janicki JS, Crow MT, Kass DA. beta-blockade prevents sustained metalloproteinase activation and diastolic stiffening induced by angiotensin II combined with evolving cardiac dysfunction. Circ Res 2000; 86: 807-815 [DOI] [PubMed] [Google Scholar]
- 24). Arai K, Lee SR, Lo EH. Essential role for ERK mitogen-activated protein kinase in matrix metalloproteinase-9 regulation in rat cortical astrocytes. Glia 2003; 43: 254-264 [DOI] [PubMed] [Google Scholar]
- 25). Underwood DC, Osborn RR, Bochnowicz S, Webb EF, Rieman DJ, Lee JC, Romanic AM, Adams JL, Hay DW, Griswold DE. SB 239063, a p38 MAPK inhibitor, reduces neutrophilia, inflammatory cytokines, MMP-9, and fibrosis in lung. Am J Physiol Lung Cell Mol Physiol 2000; 279: L895-L902 [DOI] [PubMed] [Google Scholar]
- 26). Woo CH, Lim JH, Kim JH. Lipopolysaccharide induces matrix metalloproteinase-9 expression via a mitochondrial reactive oxygen species-p38 kinase-activator protein-1 pathway in Raw 264.7 cells. J Immunol 2004; 173: 6973-6980 [DOI] [PubMed] [Google Scholar]
- 27). Xiao YQ, Malcolm K, Worthen GS, Gardai S, Schiemann WP, Fadok VA, Bratton DL, Henson PM. Cross-talk between ERK and p38 MAPK mediates selective suppression of pro-inflammatory cytokines by transforming growth factor-beta. J Biol Chem 2002; 277: 14884-14893 [DOI] [PubMed] [Google Scholar]
- 28). Liu W, Chang L. Caffeine induces matrix metalloproteinase-2 (MMP-2) and MMP-9 down-regulation in human leukemia U937 cells via Ca2+/ROS-mediated suppression of ERK/c-fos pathway and activation of p38 MAPK/c-jun pathway. J Cell Physiol 2010; 224: 775-785 [DOI] [PubMed] [Google Scholar]
- 29). Lai WC, Zhou M, Shankavaram U, Peng G, Wahl LM. Differential regulation of lipopolysaccharide-induced monocyte matrix metalloproteinase (MMP)-1 and MMP-9 by p38 and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinases. J Immunol 2003; 170: 6244-6249 [DOI] [PubMed] [Google Scholar]
- 30). Dziembowska M, Wlodarczyk J. MMP9: a novel function in synaptic plasticity. Int J Biochem Cell Biol 2012; 44: 709-713 [DOI] [PubMed] [Google Scholar]