Abstract
Prevalence of hepatitis C virus (HCV) infection is high in patients with end-stage renal dysfunction, including patients undergoing hemodialysis (HD). The HCV infection itself can cause glomerulonephritis and puts individuals at increased risk of developing end-stage renal disease; fortunately, successful HCV eradication sometimes restore HCV-related renal dysfunction. Moreover, the prognosis of dialysis patients infected with HCV is significantly worse and the renal allograft survival in HCV-infected patients is also worse than in dialysis patients without HCV infection. If life prognosis is favorable, therefore, anti-HCV therapy is strongly recommended for HCV-infected patients with severe renal dysfunction. The standard therapy for HCV-infected patients with severe renal dysfunction has historically been interferon-based therapy. However, this therapy remains ineffective in achieving high, sustained viral response rates and the rate of adverse events and treatment discontinuation due to treatment-induced adverse events continues to be high in patients with severe renal dysfunction. Safe and effective anti-HCV therapies are urgently needed, and crucial, for patients with severe renal dysfunction. Recently, direct-acting antivirals (DAAs) that specifically target viral proteins have been developed, and these targets include the NS3, NS5A, and NS5B of HCV. Clinical trials have revealed high efficacy and safety of the DAA-based therapies, but patients with severe renal dysfunction were not included in the majority of these trials. However, several recent reports have shown high efficacy and safety for some regimens of DAA combination therapy for HCV-infected patients with severe renal dysfunction. In this review, we discuss novel treatments for HCV-infected patients with severe renal dysfunction and the pharmacokinetics of these drugs.
Keywords: DAAs, HCV, Hemodialysis, CKD
Introduction
Hepatitis C virus (HCV) infection affects approximately 130–150 million people worldwide and is one of the primary factors of liver cirrhosis and hepatocellular carcinoma.1–3 The rate of HCV infection is generally high in patients with end-stage renal dysfunction, including patients on hemodialysis (HD).4,5 However, the reported prevalence of HCV infection among HD patients varies from 5% to approximately 60% in different countries.6–9
HCV infection sometimes causes extrahepatic disorders, including lymphoma, lichen planus, diabetes mellitus and renal dysfunction.10 In addition, HCV infection is the primary cause of mixed cryoglobulinemia, which is known to induce membrane-proliferative glomerulonephritis,11 and causes increased risk of developing end-stage renal disease.12 Successful HCV eradication restores the HCV-related renal dysfunction,13,14 as has been confirmed by a large cohort study. Hsu et al.15 reported that the 8-year cumulative incidence of end-stage renal disease was significantly lower in HCV-infected patients treated with anti-HCV therapy than in the untreated control group (0.15% vs. 1.32%); in addition, the anti-HCV therapy was also found to significantly suppress acute coronary syndrome and ischemic stroke.
The prognosis of HCV-infected patients on HD is also significantly worse than that of non-HCV-infected dialysis patients.16–18 A recent meta-analysis of seven studies involving 11,589 HD patients showed that HCV infection was an independent risk factor of mortality in HD patients.19 In addition, anti-HCV is mandatory in HCV-infected patients who are candidates for kidney transplantation because the chance of renal allograft survival is worse in HCV-infected patients than in non-HCV-infected patients.20 Thus, HCV-infected patients with chronic renal dysfunction have an additional indication for HCV eradication therapy. The Kidney Disease Improvement Global Outcome (KIDIGO) and Japanese Society for Dialysis Therapy (JSDT) highly recommend anti-HCV therapy for dialysis patients with an HCV infection, if life prognosis is favorable.21,22 Moreover, the demographic profile of HCV-infected patients has shown a trend in increasing age, year by year,23,24 and the number of cases with renal dysfunction are expected to increase over time due to age-related decline in the renal function.25
The standard therapy for HCV-infected patients with severe renal dysfunction has historically been interferon (IFN)-based therapy. However, this therapy remains incapable of achieving a high rate of sustained viral response (SVR), even for patients with normal renal function.26–28 Host factors, such as the IL-28B genotype29 or hepatitis viral protein-induced IFN signaling impairment, are considered responsible for this failure.30,31 In addition, IFN-based therapies are associated with adverse events (AEs) and of treatment discontinuation due to treatment-induced AEs, with especially high rates in patients with severe renal dysfunction, as compared to patients with normal renal function. Therefore, safe and effective anti-HCV therapies are urgently needed and critical for patients with severe renal dysfunction, including dialysis patients.
Bartenschlager et al.32 developed an HCV replicon system in 1999, which along with the advancements in the structural analyses of HCV proteins, led to rapid progress in the development of direct-acting antivirals (DAAs). DAAs directly target viral proteins and, as such, mainly consist of three classes for combating HCV infection: those that inhibit the HCV NS3 protein, which has protease activity; those that inhibit the HCV NS5A protein; and those that inhibit the NS5B protein, which has polymerase activity.
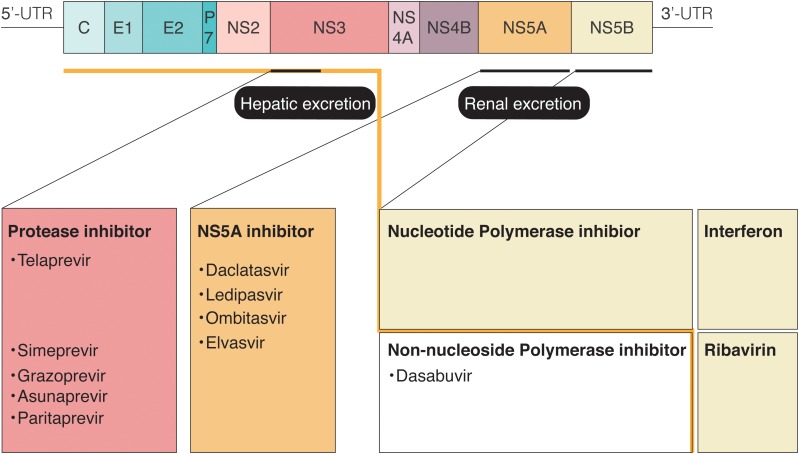
A number of clinical trials have revealed that the IFN-free DAA combination therapies lead to significant improvements in SVR rates and safety;33–37 unfortunately, however, patients with severe renal dysfunction were excluded from the majority of these trials. Additionally, in DAA combination therapy, DAAs-resistant cases,38,39 drug-drug interaction and drug excretion (Fig. 1) should be carefully monitored. Several recent studies have shown efficacy and safety for the IFN-free DAA combination therapy when used in HCV-infected patients with severe renal dysfunction40–42 (Table 1). According to these data, the standard therapy for HCV-infected patients with severe renal dysfunction could change from the traditional IFN-based strategy to the IFN-free DAAs strategy, similar to that used in patients with normal renal function.
Fig. 1. Anti-HCV drugs and excretion.
Table 1. Overview of the efficacy and safety of IFN-free DAAs combination therapies.
| Treatment regimen (treatment duration) | Patients | HCV GT | Number | SVR rate (%) (ITT) | SAE (%) | Treatment discontinued rate (%) | Special notes |
| Grazoprevir/elbasvir (12 weeks) | |||||||
| (Roth et al.)40 | CKD 4/5 including HD | GT1 | 122 | 94.2 (115/122) | 14.4 (16/111) | 0 | Adverse effects were comparable to placebo control group |
| Paritaprevir/ritonavir/ombitasvir, and dasabuvir with or without RBV (12 weeks) | |||||||
| (Pockros et al.)41 | CKD 4/5 including HD | GT1 | 20 | 90 (18/20) | 20 (4/20) | 0 | RBV add-on in patients with GT1a HCV infection |
| Daclatasvir/asunaprevir (24 weeks) | |||||||
| (Suda et al.)42 | HD | GT1 | 21 | 95.5 (20/21) | 5 (1/21) | 5 (1/21) | |
| (Toyoda et al.)72 | HD | GT1b | 28 | 100 (28/28) | 0 | 3.6 (1/28) | |
| (Kawakami et al.)68 | HD | GT1 | 18 | 100 (18/18) | 5.5 (1/18) | 0 | |
| Sofosbuvir-based therapy | |||||||
| SOF/PEG-IFN/RBV, SOF/RBV, SOF/SMV, SOF/SMV/RBV (Saxena et al.)79 |
eGFR < 45 | GT1-6 | 73 | 83 (53/64) | 22 (16/73) | 7 (5/73) | Patients with reduced renal function experienced more frequently, worsening of the renal function and serious adverse effects |
| eGFR < 30 | GT1-3 | 17 | 88 (15/17) | 18 (3/17) | 6 (1/17) | ||
| SOF/SMV (12 weeks) (Nazario et al.)80 | eGFR < 30 including HD | GT1 | 17 | 100 (17/17) | 0 | 0 | |
| SOF/SMV (12 weeks) | |||||||
| SOF/LDV (12 weeks) | |||||||
| (Singh et al.)83 | HD | GT1,3,4 | 8 | 87.5 (7/8) | 0 | 0 | |
| SOF/PEG-IFN/RBV, SOF/RBV, SOF/SMV, SOF/SMV/RBV (Beinhardt et al.)84 |
HD | GT1,3,4 | 10 | 90 (9/10) | 50 (5/10) | 0 | |
| SOF/LDV SOF/SMV SOF/DCV SOF/RBV (12–24 weeks) (Desnoyer et al.)74 |
HD | GT1,2 | 12 | 83% (10/12) | 0 | 0 | |
Abbreviations: DAAs, direct-acting antivirals; HD, hemodialysis; HCV, hepatitis C virus; IFN, interferon; PEG-IFN, pegylated-interferon; SOF, sofosbuvir; RBV, ribavirin; SMV, simeprevir; LDV, ledipasvir; eGFR, estimated glomerular filtration rate; GT, genotype; SVR, sustained viral response; SAE, serious adverse event.
In this review, we discuss the traditional and novel treatments for HCV-infected patients with severe renal dysfunction and the pharmacokinetics of these drugs.
IFN-based therapy
Outline of IFN-based therapy for patients with renal dysfunction
Before the development of DAAs, pegylated (PEG)-IFN monotherapy or in combination with ribavirin (RBV) was the standard therapy for chronic HCV infection. IFN-based therapy was also the standard therapy for patients with renal dysfunction.22 In patients with severe renal dysfunction, the clearance of IFN and RBV is reduced, because these drugs are mainly excreted renally.43 In addition, because of the high molecular weight of INF, HD is unable to remove significant amounts of it. Moreover, patients with renal dysfunction often have anemia or other complications, and INF- or RBV-induced AEs become more problematic.
Efficacy and safety of IFN monotherapy for patients with renal dysfunction
IFN monotherapy
In 2008, Gordon et al.27 reported a meta-analysis of clinical trials using INF monotherapy for hepatitis C treatment in patients on chronic HD. A total of 20 clinical studies between 1966 and 2007 were analyzed. The SVR rates ranged from 19% to 71% and the overall SVR rate was 41% [95% confidence interval (CI): 33–49]. In addition, the overall treatment discontinuation rate was relatively high, at 26% (95% CI: 20–34).
PEG-INF monotherapy
In 2015, Fabrizi et al.44 reported a meta-analysis of clinical trials using PEG-INF monotherapy for hepatitis C treatment in patients on chronic HD. They analyzed 744 patients from 24 clinical studies, including 5 randomized control studies performed between 2006 and 2014. Overall, the estimated SVR was 40% (95% CI: 35–46). The dropout rate was 14% (95% CI: 9–20). The most frequent AEs requiring discontinuation of treatment were hematological and gastrointestinal problems. The authors concluded that the efficacy and safety of PEG-INF monotherapy for dialysis patients with severe renal dysfunction was unsatisfactory.
Efficacy and safety of IFN and RBV for patients with renal dysfunction
In 2014, Fabrizi et al.45 reported a meta-analysis of clinical trials using PEG-INF and RBV for hepatitis C treatment in patients on chronic HD. They analyzed 287 patients from 11 clinical studies, including 2 control studies, performed between 1998 and 2013. Overall, the estimated SVR was 60% (95% CI: 47–71). The dropout rate was 18% (95% CI: 8–35). The most frequent AEs requiring discontinuation of treatment were anemia (11/46, 23%) and infections (6/46, 13%).
However, in some countries, RBV administration is contraindicated in patients with severe renal dysfunction, because RBV is eliminated through the kidney and cannot be eliminated by dialysis.21,43
IFN-based therapy with HCV NS3 protease inhibitors for patients with renal dysfunction
The first-generation protease inhibitors, including telaprevir, boceprevir and the PEG-IFN and RBV combination therapy, could achieve an SVR rate of 75% to 85% in patients with normal renal function.46–48 However, severe AEs, including cutaneous rash,49 anemia and renal impairment,50 were reported. The data regarding the triple antiviral therapy for HCV-infected patients with renal dysfunction are limited,51–55 but the reported SVR rates have varied between 17% and 86% and the dropout rates have varied between 10% and 20%.
IFN-free DAA combination therapy
In the case of DAA administration to patients on HD, special attention should be paid to drug-drug interaction, because these patients usually receive various drugs. Caution is necessary, especially for ritonavir administration, which inhibits CYP3A4.
Grazoprevir and elbasvir combination therapy for HCV-infected patients with renal dysfunction
Grazoprevir is an HCV NS3 protease inhibitor with broad in vitro activity against multiple HCV genotypes and resistance variants.56 Grazoprevir is administered at a dose of 100 mg once a day and is a substrate of CYP3A4, P-gp and OATP. This drug is eliminated mostly through liver, with less than 1% excreted renally.57 Elbasvir is an HCV NS5A inhibitor with potent multiple genotypic antiviral activity in vitro.58 Elbasvir is administered at a dose of 50 mg once a day and it is also a substrate of CYP3A4, P-gp and OATP.57 Similar to grazoprevir, elbasvir is mainly metabolized through the liver, with less than 1% eliminated renally.57
The pharmacokinetics of elbasvir were studied in non-HCV-infected dialysis subjects and subjects with severe renal dysfunction, and then compared with healthy controls.57 The area under the curve (AUC) was 25% higher for the HD subjects and 46% higher for the subjects with severe renal dysfunction, compared with the controls. In the pharmacokinetic analysis of grazoprevir in HCV-infected patients, the AUC was 10% higher for the HD patients and 40% higher for the patients with severe renal impairment, compared to the controls. In addition, elbasvir and grazoprevir are not removed by HD.
Several trials showed high efficacy and safety of elbasvir and grazoprevir for HCV-infected patients with various complications.40,59–62 In a phase 3 trial for patients with genotype 1 or 4 HCV and HIV co-infection (C-EDGE CO-INFECTION), when this combination therapy was administered for 12 weeks an SVR rate of 96% was achieved (210/218).63
The C-SURFER is a phase 3 randomized study designed to evaluate the safety and efficacy of grazoprevir and elbasvir combination therapy for genotype 1 HCV-infected patients with severe renal dysfunction (stage 4–5 chronic kidney disease (CKD), including HD patients).40 Two-hundred-and-twenty-four patients with severe renal dysfunction were randomly assigned to receive grazoprevir and elbasvir for 12 weeks (n = 111) or placebo (deferred treatment group, n = 113). In addition, 11 patients were not randomized and received the dual therapy, and they underwent intensive pharmacokinetic evaluation. Of these 235 patients, 179 (76%) needed HD, 122 (52%) had HCV genotype 1a infection, 14 (6%) were cirrhotic, 80 (34%) had diabetes mellitus and 108 (46%) were African American. In patients treated with grazoprevir and elbasvir, the SVR12 rate (ITT analysis) was 94.3% (115/122). And, in the modified full analysis set (excluding patients who failed to receive one or more doses of drug due to issues unrelated to the hepatitis C treatment), the SVR12 rate was 99% (115/116). In the safety analysis, no patients in the grazoprevir and elbasvir therapy group were found to have discontinued because of an AE. On the contrary, in the control group, 5 (4%) patients discontinued the placebo treatment due to AEs. In the grazoprevir and elbasvir therapy group, AEs were reported for 76% of the cases; however, these results were comparable with the placebo group (84%). Thus, the C-SURFER study indicated that grazoprevir and elbasvir for 12 weeks was safe and effective, even in patients infected with HCV genotype 1 and stage 4–5 CKD.
Paritaprevir (PTV)/ritonavir, ombitasvir (OBV), and dasabuvir (DSV) with or without RBV combination therapy for HCV-infected patients with renal dysfunction
OBV is an HCV NS5A inhibitor, PTV is a second-generation NS3 protease inhibitor, ritonavir is the pharmacokinetic enhancer that is a CYP3A inhibitor, and DSV is a non-nucleoside NS5B polymerase inhibitor. PTV, ritonavir and OBV are administered at a dose of 150 mg, 100 mg and 25 mg once a day, respectively. DSV, however, is administered at a dose of 250 mg twice a day. PTV is a substrate of CYP3A4/5, P-gp, OATP1B1 and OATP1B3 and is metabolized mainly through the liver.64 The single-dose pharmacokinetics of PTV were studied in non-HCV-infected subjects with severe renal dysfunction, and compared with subjects with normal renal function. The AUC values increased by 45% in subjects with severe renal dysfunction, compared with the controls.64 Ritonavir is administered to inhibit CYP3A4, resulting in enhancement of the PTV effect. The single-dose pharmacokinetics of ritonavir were studied and the AUC values increased by 114% in subjects with severe renal dysfunction, compared with the control subjects. OBV is metabolized mainly by amide hydrolysis and oxidative metabolism, and biliary excretion is the major elimination route. The result of single-dose pharmacokinetic study of OBV indicated that the AUC values were similar between subjects with severe renal dysfunction and controls. DSV is a substrate of CYP3A4, P-gp, BCRP and organic cation transporter 1, and is mainly metabolized through the liver. The single-dose pharmacokinetics of DSV were studied and the AUC values increased by 50% in subjects with severe renal dysfunction, compared with the control subjects.64
In clinical trials, this regimen showed a high rate of SVR12.36,65,66 However, patients with severe renal dysfunction were not included. Recently, the RUBY-I study investigated the safety and efficacy of these combination therapy in patients with stage 4 or 5 CKD.41 This prospective multicenter study included 20 treatment-naive patients with HCV genotype 1 infection and without cirrhosis. Six patients with CKD stage 4 and 14 patients with CKD stage 5 or who were on HD were included. Fourteen patients (70%) had the IL-28B non-CC genotype and 4 patients (20%) had F3 liver fibrosis. Thirty-three patients with HCV genotype 1a were treated with this combination therapy and RBV and 7 patients with HCV genotype 1b infection were treated with the combination therapy alone. Overall, 90% (18/20) of the patients achieved SVR12 (95% CI: 69.9–97.2). One patient died after finishing the treatment, due to issues unrelated to the treatment, and 1 patient experienced virological relapse. In the safety analysis, all 20 patients were found to have completed the 12 weeks of treatment. Ninety-five percent of the enrolled patients experienced AEs; however, they were mostly mild or moderate and no patient discontinued treatment due to AEs. A common AE was anemia in the RBV add-on group, and RBV therapy was interrupted in 9 patients due to anemia.
Asunaprevir (ASV) and daclatasvir (DCV) combination therapy for HCV infected patients with renal dysfunction
DCV is a first-in-class NS5A inhibitor and has potent pan-genotypic antiviral activity in vitro.67 ASV is a second-generation NS3 protease inhibitor and has antiviral activity against multiple HCV genotypes in vitro. ASV is administered at a dose of 100 mg twice a day, and is metabolized by CYP3A and eliminated mostly in the feces.37 The pharmacokinetics of ASV were studied in non-HCV-infected dialysis subjects and compared with healthy controls.37 The Cmax was 28.6% higher and the AUC was 10.1% lower in dialysis subjects, compared with the controls. Recently, Kawakami et al.68 reported the ASV pharmacokinetics determined in HCV-infected dialysis patients and compared with HCV-infected patients with normal renal function. The AUC from 0 to 6 h (AUC 0–6 h) of ASV was significantly lower in HD patients than in the controls (1345 ± 741 lgh/mL vs. 4769 ± 1964 lgh/mL). DCV is administered at a dose of 60 mg once a day, and is metabolized by CYP3A and eliminated mostly in the feces (88%).69 The pharmacokinetics of DCV were also studied in non-HCV-infected dialysis subjects and compared with healthy controls.69 The AUC was 26.9% higher in dialysis subjects, compared with the controls.
In phase 3 trials for patients with genotype 1b infection, the DCV/ASV combination therapy for 24 weeks achieved a high SVR rate (82–95%).39,70,71 However, data of efficacy and safety for HCV-infected patients with renal dysfunction were not obtained in these clinical trials. This regimen has been approved in several countries and the real-world outcomes have already been reported, including the efficacy and safety of this combination therapy for HD patients.42,68,72 We reported the efficacy and safety of DCV/ASV combination therapy from a study of 21 HCV-infected dialysis patients.42 A total of 95.5% (20/21) of the patients achieved SVR12. Of the 21 patients on dialysis, 3 had NS5A RAVs-Y93H and all of the patients with NS5A RAVs achieved SVR12. On the other hand, 1 patient with NS3 D168E RAVs at baseline experienced relapse at 4 weeks post-treatment. In the safety analysis, 95.5% of the enrolled patients completed the 24 weeks of therapy and no patient had lethal AEs. One patient discontinued treatment due to an elevated alanine aminotransferase (ALT) level. ALT elevations were observed in 14.3% of the patients, and this result is comparable with a phase 3 study conducted in Japan.39
Toyoda et al.72 used propensity score matching to compare the efficacy and safety of the DCV/ASV combination therapy in 28 patients on HD with those of 56 patients without renal dysfunction. They showed that the rate of SVR12 was 100% (28/28) for the dialysis patients (94.6% for the patients with normal renal function) and that serum HCV RNA disappeared significantly earlier in the HD patients. In addition, they showed that treatment-related AEs were comparable between the two groups. Additionally, Kawakami et al.68 analyzed the pharmacokinetics of DCV and ASV in the dialysis setting and showed a high efficacy of this combination therapy for dialysis patients, with an SVR rate of 100% (18/18).
Sofosbuvir-based therapy for HCV-infected patients with renal dysfunction
Sofosbuvir is a potent nucleoside NS5B polymerase inhibitor that has high genetic barrier and high efficacy. Sofosbuvir is usually administered at a dose of 400 mg once a day combined with other DAAs, such as the NS5A inhibitors ledipasvir or DCV, and the NS3 protease inhibitor simeprevir or RBV. Sofosbuvir is firstly metabolized to a pharmacologically active nucleoside analog triphosphate GS-461203.73 Subsequently, GS-461203 is metabolized to the inactive metabolite GS-331007. Importantly, this GS-331007 is mainly excreted through the kidney. The single-dose pharmacokinetics of sofosbuvir were studied in non-HCV subjects with moderate (estimated glomerular filtration rate (eGFR) between 30–50 mL/min/1.73 m2) or severe renal impairment (eGFR less than 30 mL/min/1.73 m2) and in HD subjects, and compared with subjects with normal renal function. The sofosbuvir AUC was 107% and 171% higher in the patients with moderate and severe renal impairment, compared with the control subjects. The GS-331007 AUC was 88% and 451% higher in the patients with moderate and severe renal impairment, respectively. In the dialysis subjects, the sofosbuvir AUC was 28−60% higher, compared to the control subjects, when sofosbuvir was administered before or after HD. The GS-331007 AUC in the dialysis subjects was 1280% or 2070% higher than in the control subjects when sofosbuvir was administered before or after HD.73 Thus, exposure of sofosbuvir and of the metabolite GS-331007 are considered to be quite increased in patients with renal dysfunction. Quite recently, Desnoyer et al.74 reported the pharmacokinetics and safety of a sofosbuvir-containing regime in dialysis patients. They showed that sofosbuvir plasma concentrations were never detectable before and after the HD and, on the contrary to previously reported results in patients with normal renal function, higher GS-331007 plasma concentrations were observed. However, no GS-331007 accumulation was observed, and the regimen was well tolerated generally. Nevertheless, until now sofosbuvir has not been recommended for patients with renal dysfunction, and in some countries it is even contraindicated.75 Further large-number studies are necessary.
Several studies about sofosbuvir-based therapy for patients with renal dysfunction have been recently reported.76–79 Saxena et al.79 reported the outcomes of sofosbuvir-based therapy for patients with renal dysfunction by using the HCV-TARGET database, which is a multicenter, real-world cohort. Of the 1789 enrolled patients, 73 had eGFR of less than 45 mL/min/1.73 m2 (18 patients with eGFR ≤ 30 mL/min/1.73 m2 and 5 patients on dialysis). These patients were compared to 1716 patients with eGFR > 45 mL/min/1.73 m2. The included treatment regimen was sofosbuvir/simeprevir at 40%, sofosbuvir/RBV at 30%, sofosbuvir/PEG-INF/RBV at 18% and sofosbuvir/simeprevir/RBV at 11%; all patients with eGFR ≤ 45 mL/min/1.73 m2 were treated with sofosbuvir 400 mg once a day. Patients with baseline eGFR ≤ 45 mL/min/1.73 m2 had a significantly higher rate of cirrhosis (73%) as compared with the control group (24%). SVR12 was achieved in 53 of the 64 (83%) patients with eGFR < 45 mL/min/1.73 m2; this was comparable to patients with eGFR > 45 mL/min/1.73 m2. In addition, 15 of the 17 (88%) patients with eGFR ≤ 30 mL/min/1.73 m2 and all 5 patients on HD at baseline achieved SVR12. However, in the safety analysis, the patients with eGFR ≤ 45 mL/min/1.73 m2 were found to have experienced significantly higher rates of anemia (31%), worsening of the renal function (10%), and any serious AEs (18%). The authors concluded that patients with renal impairment need close expert monitoring.
On the other hand, Nazario et al.80 reported that 15 dialysis patients and 2 patients with severe renal dysfunction all achieved SVR12, with only mild AEs experienced by patients on the sofosbuvir and simeprevir combination therapy. Singh et al.83 reported that 8 dialysis patients received sofosbuvir-based therapy (4 patients with ledipasvir and 4 patients with simeprevir) and 7 (88%) achieved SVR12. In addition, some studies on sofosbuvir plus RBV or ledipasvir/sofosbuvir in adults with HCV infection and renal insufficiency are still ongoing (NCT01958281). Thus, the results of the clinical trials are expected.
Conclusion
In this review, we described the efficacy, safety and pharmacokinetics of novel anti-HCV drugs in patients with severe renal dysfunction. These data reveal that grazoprevir/elbasvir combination therapy, PTV/ritonavir/OBV with or without DSV therapy, and DCV/ASV combination therapy are highly effective and safe for patients with severe renal dysfunction. Sofosbuvir is highly effective and has high genetic barrier, thus representing one of the key drugs for anti-HCV therapy; however, data on sofosbuvir treatment for patients with severe renal dysfunction are still pending. The results of ongoing clinical trials are expected. Therefore, the use of sofosbuvir to treat HCV-infected patients with eGFR < 30 mL/min/1.73 m2 or on-dialysis remains off-label and should be left to experienced physicians or centers, and administered with full consent of the treated patients.
Timing of HCV treatment in a kidney transplant candidate might depend on the waiting time for the kidney transplant. If the waiting time is lengthy, prompt DAAs therapy is necessary, because of several treatment benefits, including restored liver function.42 On the other hand, if the waiting time is short, because favorable outcomes of either pre- or post-kidney transplant DAAs treatment have been reported,40–42,72,81 both timing could be selected.
According to the emerging data, the standard therapy for HCV-infected patients with severe renal dysfunction will likely change from IFN-based therapy to IFN-free DAAs-based therapy. However, the data to date have been mainly limited to genotype 1-infected patients with severe renal dysfunction. Therefore, if a good prognosis is expected, dialysis patients with genotype 1 HCV infection should be considered for DAAs therapy. In dialysis patients with HCV infection other than genotype 1, except for dialysis patients for whom prompt treatment would be required, waiting for the next-generation DAAs would be the optimal treatment option; this is because clinical trials on the next-generation pan-genotypic DAAs therapy, which are expected to be possible to administer in patients with renal dysfunction, are underway.82 In addition, real world data are still limited. Further investigations are still necessary.
Acknowledgements
This study was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan and the Japan Agency for Medical Research and Development.
Abbreviations
- DAAs
direct-acting antivirals
- HD
hemodialysis
- HCV
hepatitis C virus
- IFN
interferon
- PEG-IFN
pegylated-interferon
- SOF
sofosbuvir
- RBV
ribavirin
- SMV
simeprevir
- LDV
ledipasvir
- eGFR
estimated glomerular filtration rate
- GT
genotype
- SVR
sustained viral response
- SAE
serious adverse event
- PTV
paritaprevir
- OBV
ombitasvir
- DSV
dasabuvir
References
- 1.Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45–S57. doi: 10.1016/j.jhep.2014.07.027. doi: 10.1016/j.jhep.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 2.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 3.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35–S46. doi: 10.1053/jhep.2002.36806. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 4.Iwasa Y, Otsubo S, Sugi O, Sato K, Asamiya Y, Eguchi A, et al. Patterns in the prevalence of hepatitis C virus infection at the start of hemodialysis in Japan. Clin Exp Nephrol. 2008;12:53–57. doi: 10.1007/s10157-007-0005-6. doi: 10.1007/s10157-007-0005-6. [DOI] [PubMed] [Google Scholar]
- 5.Bergman S, Accortt N, Turner A, Glaze J. Hepatitis C infection is acquired pre-ESRD. Am J Kidney Dis. 2005;45:684–689. doi: 10.1053/j.ajkd.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Di Napoli A, Pezzotti P, Di Lallo D, Petrosillo N, Trivelloni C, Di Giulio S, et al. Epidemiology of hepatitis C virus among long-term dialysis patients: a 9-year study in an Italian region. Am J Kidney Dis. 2006;48:629–637. doi: 10.1053/j.ajkd.2006.07.004. doi: 10.1053/j.ajkd.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Fissell RB, Bragg-Gresham JL, Woods JD, Jadoul M, Gillespie B, Hedderwick SA, et al. Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: the DOPPS. Kidney Int. 2004;65:2335–2342. doi: 10.1111/j.1523-1755.2004.00649.x. doi: 10.1111/j.1523-1755.2004.00649.x. [DOI] [PubMed] [Google Scholar]
- 8.Sun J, Yu R, Zhu B, Wu J, Larsen S, Zhao W. Hepatitis C infection and related factors in hemodialysis patients in china: systematic review and meta-analysis. Ren Fail. 2009;31:610–620. doi: 10.1080/08860220903003446. doi: 10.1080/08860220903003446. [DOI] [PubMed] [Google Scholar]
- 9.Alavian SM, Kabir A, Ahmadi AB, Lankarani KB, Shahbabaie MA, Ahmadzad-Asl M. Hepatitis C infection in hemodialysis patients in Iran: a systematic review. Hemodial Int. 2010;14:253–262. doi: 10.1111/j.1542-4758.2010.00437.x. doi: 10.1111/j.1542-4758.2010.00437.x. [DOI] [PubMed] [Google Scholar]
- 10.Gill K, Ghazinian H, Manch R, Gish R. Hepatitis C virus as a systemic disease: reaching beyond the liver. Hepatol Int. 2016;10:415–423. doi: 10.1007/s12072-015-9684-3. doi: 10.1007/s12072-015-9684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabrizi F, Plaisier E, Saadoun D, Martin P, Messa P, Cacoub P. Hepatitis C virus infection, mixed cryoglobulinemia, and kidney disease. Am J Kidney Dis. 2013;61:623–637. doi: 10.1053/j.ajkd.2012.08.040. doi: 10.1053/j.ajkd.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 12.Lee JJ, Lin MY, Chang JS, Hung CC, Chang JM, Chen HC, et al. Hepatitis C virus infection increases risk of developing end-stage renal disease using competing risk analysis. PLoS One. 2014;9:e100790. doi: 10.1371/journal.pone.0100790. doi: 10.1371/journal.pone.0100790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Misiani R, Bellavita P, Fenili D, Vicari O, Marchesi D, Sironi PL, et al. Interferon alfa-2a therapy in cryoglobulinemia associated with hepatitis C virus. N Engl J Med. 1994;330:751–756. doi: 10.1056/NEJM199403173301104. doi: 10.1056/NEJM199403173301104. [DOI] [PubMed] [Google Scholar]
- 14.Tsuge M, Hiramatsu A, Shinohara F, Nakano N, Nakamura Y, Hatooka M, et al. Improvement of renal dysfunction in a patient with hepatitis C virus-related liver cirrhosis by daclatasvir and asunaprevir combination therapy: A case report. Hepatol Res. 2016;46:944–948. doi: 10.1111/hepr.12629. doi: 10.1111/hepr.12629. [DOI] [PubMed] [Google Scholar]
- 15.Hsu YC, Ho HJ, Huang YT, Wang HH, Wu MS, Lin JT, et al. Association between antiviral treatment and extrahepatic outcomes in patients with hepatitis C virus infection. Gut. 2015;64:495–503. doi: 10.1136/gutjnl-2014-308163. doi: 10.1136/gutjnl-2014-308163. [DOI] [PubMed] [Google Scholar]
- 16.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, Miller LG, Daar ES, Gjertson DW, et al. Hepatitis C virus and death risk in hemodialysis patients. J Am Soc Nephrol. 2007;18:1584–1593. doi: 10.1681/ASN.2006070736. doi: 10.1681/ASN.2006070736. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama E, Akiba T, Marumo F, Sato C. Prognosis of anti-hepatitis C virus antibody-positive patients on regular hemodialysis therapy. J Am Soc Nephrol. 2000;11:1896–1902. doi: 10.1681/ASN.V11101896. [DOI] [PubMed] [Google Scholar]
- 18.Goodkin DA, Bragg-Gresham JL, Koenig KG, Wolfe RA, Akiba T, Andreucci VE, et al. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS) J Am Soc Nephrol. 2003;14:3270–3277. doi: 10.1097/01.asn.0000100127.54107.57. [DOI] [PubMed] [Google Scholar]
- 19.Fabrizi F, Takkouche B, Lunghi G, Dixit V, Messa P, Martin P. The impact of hepatitis C virus infection on survival in dialysis patients: meta-analysis of observational studies. J Viral Hepat. 2007;14:697–703. doi: 10.1111/j.1365-2893.2007.00868.x. doi: 10.1111/j.1365-2893.2007.00868.x. [DOI] [PubMed] [Google Scholar]
- 20.Mathurin P, Mouquet C, Poynard T, Sylla C, Benalia H, Fretz C, et al. Impact of hepatitis B and C virus on kidney transplantation outcome. Hepatology. 1999;29:257–263. doi: 10.1002/hep.510290123. doi: 10.1002/hep.510290123. [DOI] [PubMed] [Google Scholar]
- 21.Akiba T, Hora K, Imawari M, Sato C, Tanaka E, Izumi N, et al. 2011 Japanese Society for Dialysis Therapy guidelines for the treatment of hepatitis C virus infection in dialysis patients. Ther Apher Dial. 2012;16:289–310. doi: 10.1111/j.1744-9987.2012.01078.x. doi: 10.1111/j.1744-9987.2012.01078.x. [DOI] [PubMed] [Google Scholar]
- 22.Kidney Disease: Improving Global Outcomes (KDIGO) KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl. 2008:S1–S99. doi: 10.1038/ki.2008.81. doi: 10.1038/ki.2008.81. [DOI] [PubMed] [Google Scholar]
- 23.Davis GL, Albright JE, Cook SF, Rosenberg DM. Projecting future complications of chronic hepatitis C in the United States. Liver Transpl. 2003;9:331–338. doi: 10.1053/jlts.2003.50073. doi: 10.1053/jlts.2003.50073. [DOI] [PubMed] [Google Scholar]
- 24.Taura N, Hamasaki K, Nakao K, Ichikawa T, Nishimura D, Goto T, et al. Aging of patients with hepatitis C virus-associated hepatocellular carcinoma: long-term trends in Japan. Oncol Rep. 2006;16:837–843. [PubMed] [Google Scholar]
- 25.Walker WG, Neaton JD, Cutler JA, Neuwirth R, Cohen JD. Renal function change in hypertensive members of the Multiple Risk Factor Intervention Trial. Racial and treatment effects. The MRFIT Research Group. JAMA. 1992;268:3085–3091. [PubMed] [Google Scholar]
- 26.Fabrizi F, Dixit V, Messa P, Martin P. Interferon monotherapy of chronic hepatitis C in dialysis patients: meta-analysis of clinical trials. J Viral Hepat. 2008;15:79–88. doi: 10.1111/j.1365-2893.2007.00907.x. doi: 10.1111/j.1365-2893.2007.00907.x. [DOI] [PubMed] [Google Scholar]
- 27.Gordon CE, Uhlig K, Lau J, Schmid CH, Levey AS, Wong JB. Interferon treatment in hemodialysis patients with chronic hepatitis C virus infection: a systematic review of the literature and meta-analysis of treatment efficacy and harms. Am J Kidney Dis. 2008;51:263–277. doi: 10.1053/j.ajkd.2007.11.003. doi: 10.1053/j.ajkd.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Ayaz C, Celen MK, Yuce UN, Geyik MF. Efficacy and safety of pegylated-interferon alpha-2a in hemodialysis patients with chronic hepatitis C. World J Gastroenterol. 2008;14:255–259. doi: 10.3748/wjg.14.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105–1109. doi: 10.1038/ng.449. doi: 10.1038/ng.449. [DOI] [PubMed] [Google Scholar]
- 30.Tsunematsu S, Suda G, Yamasaki K, Kimura M, Izumi T, Umemura M, et al. Hepatitis B virus X protein impairs α-interferon signaling via up-regulation of suppressor of cytokine signaling 3 and protein phosphatase 2A. J Med Virol. 2017;89:267–275. doi: 10.1002/jmv.24643. doi: 10.1002/jmv.24643. [DOI] [PubMed] [Google Scholar]
- 31.Suda G, Sakamoto N, Itsui Y, Nakagawa M, Tasaka-Fujita M, Funaoka Y, et al. IL-6-mediated intersubgenotypic variation of interferon sensitivity in hepatitis C virus genotype 2a/2b chimeric clones. Virology. 2010;407:80–90. doi: 10.1016/j.virol.2010.07.041. doi: 10.1016/j.virol.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 32.Lohmann V, Körner F, Koch J, Herian U, Theilmann L, Bartenschlager R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 33.Mizokami M, Yokosuka O, Takehara T, Sakamoto N, Korenaga M, Mochizuki H, et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. Lancet Infect Dis. 2015;15:645–653. doi: 10.1016/S1473-3099(15)70099-X. doi: 10.1016/S1473-3099(15)70099-X. [DOI] [PubMed] [Google Scholar]
- 34.Omata M, Nishiguchi S, Ueno Y, Mochizuki H, Izumi N, Ikeda F, et al. Sofosbuvir plus ribavirin in Japanese patients with chronic genotype 2 HCV infection: an open-label, phase 3 trial. J Viral Hepat. 2014;21:762–768. doi: 10.1111/jvh.12312. doi: 10.1111/jvh.12312. [DOI] [PubMed] [Google Scholar]
- 35.Kumada H, Hayashi N, Izumi N, Okanoue T, Tsubouchi H, Yatsuhashi H, et al. Simeprevir (TMC435) once daily with peginterferon-α-2b and ribavirin in patients with genotype 1 hepatitis C virus infection: The CONCERTO-4 study. Hepatol Res. 2015;45:501–513. doi: 10.1111/hepr.12375. doi: 10.1111/hepr.12375. [DOI] [PubMed] [Google Scholar]
- 36.Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973–1982. doi: 10.1056/NEJMoa1402869. doi: 10.1056/NEJMoa1402869. [DOI] [PubMed] [Google Scholar]
- 37.Bristol-Myers Squibb Company. Sunvepra capsules (asunaprevir) Japanese Prescribing Information. 2014.
- 38.Ito J, Suda G, Yamamoto Y, Nagasaka A, Furuya K, Kumagai K, et al. Prevalence and characteristics of naturally occurring sofosbuvir resistance-associated variants in patients with hepatitis C virus genotype 1b infection. Hepatol Res. 2016;46:1294–1303. doi: 10.1111/hepr.12685. doi: 10.1111/hepr.12685. [DOI] [PubMed] [Google Scholar]
- 39.Kumada H, Suzuki Y, Ikeda K, Toyota J, Karino Y, Chayama K, et al. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology. 2014;59:2083–2091. doi: 10.1002/hep.27113. doi: 10.1002/hep.27113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roth D, Nelson DR, Bruchfeld A, Liapakis A, Silva M, Monsour H, Jr, et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015;386:1537–1545. doi: 10.1016/S0140-6736(15)00349-9. doi: 10.1016/S0140-6736(15)00349-9. [DOI] [PubMed] [Google Scholar]
- 41.Pockros PJ, Reddy KR, Mantry PS, Cohen E, Bennett M, Sulkowski MS, et al. Efficacy of direct-acting antiviral combination for patients with hepatitis C virus genotype 1 infection and severe renal impairment or end-stage renal disease. Gastroenterology. 2016;150:1590–1598. doi: 10.1053/j.gastro.2016.02.078. doi: 10.1053/j.gastro.2016.02.078. [DOI] [PubMed] [Google Scholar]
- 42.Suda G, Kudo M, Nagasaka A, Furuya K, Yamamoto Y, Kobayashi T, et al. Efficacy and safety of daclatasvir and asunaprevir combination therapy in chronic hemodialysis patients with chronic hepatitis C. J Gastroenterol. 2016;51:733–740. doi: 10.1007/s00535-016-1162-8. doi: 10.1007/s00535-016-1162-8. [DOI] [PubMed] [Google Scholar]
- 43.REBETOL® (ribavirin USP) capsules, for oral use. 2016. Merck & Co., Inc. Available from: https://www.merck.com/product/usa/pi_circulars/r/rebetol/rebetol_pi.pdf .
- 44.Fabrizi F, Dixit V, Messa P, Martin P. Pegylated interferon mono-therapy of chronic hepatitis C in the dialysis population: systematic review and meta-analysis. Ther Apher Dial. 2015;19:611–621. doi: 10.1111/1744-9987.12318. doi: 10.1111/1744-9987.12318. [DOI] [PubMed] [Google Scholar]
- 45.Fabrizi F, Dixit V, Messa P, Martin P. Antiviral therapy (pegylated interferon and ribavirin) of hepatitis C in dialysis patients: meta-analysis of clinical studies. J Viral Hepat. 2014;21:681–689. doi: 10.1111/jvh.12276. doi: 10.1111/jvh.12276. [DOI] [PubMed] [Google Scholar]
- 46.Kumada H, Toyota J, Okanoue T, Chayama K, Tsubouchi H, Hayashi N. Telaprevir with peginterferon and ribavirin for treatment-naive patients chronically infected with HCV of genotype 1 in Japan. J Hepatol. 2012;56:78–84. doi: 10.1016/j.jhep.2011.07.016. doi: 10.1016/j.jhep.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 47.Poordad F, McCone J, Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207–1217. doi: 10.1056/NEJMoa1009482. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suda G, Yamamoto Y, Nagasaka A, Furuya K, Kudo M, Chuganji Y, et al. Serum granulysin levels as a predictor of serious telaprevir-induced dermatological reactions. Hepatol Res. 2015;45:837–845. doi: 10.1111/hepr.12421. doi: 10.1111/hepr.12421. [DOI] [PubMed] [Google Scholar]
- 50.Karino T, Ozeki I, Hige S, Kimura M, Arakawa T, Nakajima T, et al. Telaprevir impairs renal function and increases blood ribavirin concentration during telaprevir/pegylated interferon/ribavirin therapy for chronic hepatitis C. J Viral Hepat. 2014;21:341–347. doi: 10.1111/jvh.12162. doi: 10.1111/jvh.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dumortier J, Guillaud O, Gagnieu MC, Janbon B, Juillard L, Morelon E, et al. Anti-viral triple therapy with telaprevir in haemodialysed HCV patients: is it feasible? J Clin Virol. 2013;56:146–149. doi: 10.1016/j.jcv.2012.10.009. doi: 10.1016/j.jcv.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Wiegand J, Maasoumy B, Buggisch P, Buslau A, Schiefke I, Berg T, et al. Letter: Telaprevir triple therapy in chronic hepatitis C genotype 1 patients receiving haemodialysis. Aliment Pharmacol Ther. 2014;39:1342–1344. doi: 10.1111/apt.12748. doi: 10.1111/apt.12748. [DOI] [PubMed] [Google Scholar]
- 53.Knapstein J, Galle PR, Zimmermann T. Antiviral triple therapy with boceprevir in a chronic hepatitis C haemodialysis patient awaiting kidney re-transplantation. Dig Liver Dis. 2014;46:88–89. doi: 10.1016/j.dld.2013.08.133. doi: 10.1016/j.dld.2013.08.133. [DOI] [PubMed] [Google Scholar]
- 54.de Kanter CT, den Hollander JG, Verweij-van Wissen CP, Burger DM. Telaprevir pharmacokinetics in a hepatitis C virus infected patient on haemodialysis. J Clin Virol. 2014;60:431–432. doi: 10.1016/j.jcv.2014.05.008. doi: 10.1016/j.jcv.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 55.Mehawej M, Rostaing L, Alric L, Del Bello A, Izopet J, Kamar N. Boceprevir-based triple antiviral therapy for chronic hepatitis C virus infection in kidney-transplant candidates. J Transplant. 2015;2015:159795. doi: 10.1155/2015/159795. doi: 10.1155/2015/159795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Summa V, Ludmerer SW, McCauley JA, Fandozzi C, Burlein C, Claudio G, et al. MK-5172, a selective inhibitor of hepatitis C virus NS3/4a protease with broad activity across genotypes and resistant variants. Antimicrob Agents Chemother. 2012;56:4161–4167. doi: 10.1128/AAC.00324-12. doi: 10.1128/AAC.00324-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.ZEPATIER™ (elbasvir and grazoprevir) tablets, for oral use. 2016. Merck & Co., Inc. Available from: https://www.merck.com/product/usa/pi_circulars/z/zepatier/zepatier_pi.pdf .
- 58.Liu R, Curry S, McMonagle P, Yeh WW, Ludmerer SW, Jumes PA, et al. Susceptibilities of genotype 1a, 1b, and 3 hepatitis C virus variants to the NS5A inhibitor elbasvir. Antimicrob Agents Chemother. 2015;59:6922–6929. doi: 10.1128/AAC.01390-15. doi: 10.1128/AAC.01390-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Forns X, Gordon SC, Zuckerman E, Lawitz E, Calleja JL, Hofer H, et al. Grazoprevir and elbasvir plus ribavirin for chronic HCV genotype-1 infection after failure of combination therapy containing a direct-acting antiviral agent. J Hepatol. 2015;63:564–572. doi: 10.1016/j.jhep.2015.04.009. doi: 10.1016/j.jhep.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 60.Lawitz E, Gane E, Pearlman B, Tam E, Ghesquiere W, Guyader D, et al. Efficacy and safety of 12 weeks versus 18 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin for hepatitis C virus genotype 1 infection in previously untreated patients with cirrhosis and patients with previous null response with or without cirrhosis (C-WORTHY): a randomised, open-label phase 2 trial. Lancet. 2015;385:1075–1086. doi: 10.1016/S0140-6736(14)61795-5. doi: 10.1016/S0140-6736(14)61795-5. [DOI] [PubMed] [Google Scholar]
- 61.Sulkowski M, Hezode C, Gerstoft J, Vierling JM, Mallolas J, Pol S, et al. Efficacy and safety of 8 weeks versus 12 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin in patients with hepatitis C virus genotype 1 mono-infection and HIV/hepatitis C virus co-infection (C-WORTHY): a randomised, open-label phase 2 trial. Lancet. 2015;385:1087–1097. doi: 10.1016/S0140-6736(14)61793-1. doi: 10.1016/S0140-6736(14)61793-1. [DOI] [PubMed] [Google Scholar]
- 62.Buti M, Gordon SC, Zuckerman E, Lawitz E, Calleja JL, Hofer H, et al. Grazoprevir, elbasvir, and ribavirin for chronic hepatitis C virus genotype 1 infection after failure of pegylated interferon and ribavirin with an earlier-generation protease inhibitor: final 24-week results from C-SALVAGE. Clin Infect Dis. 2016;62:32–36. doi: 10.1093/cid/civ722. doi: 10.1093/cid/civ722. [DOI] [PubMed] [Google Scholar]
- 63.Rockstroh JK, Nelson M, Katlama C, Lalezari J, Mallolas J, Bloch M, et al. Efficacy and safety of grazoprevir (MK-5172) and elbasvir (MK-8742) in patients with hepatitis C virus and HIV co-infection (C-EDGE CO-INFECTION): a non-randomised, open-label trial. Lancet HIV. 2015;2:e319–e327. doi: 10.1016/S2352-3018(15)00114-9. doi: 10.1016/S2352-3018(15)00114-9. [DOI] [PubMed] [Google Scholar]
- 64.VIEKIRA PAK (ombitasvir, paritaprevir, and ritonavir tablets;dasabuvir tablets), co-packaged for oral use. 2016. AbbVie Inc., available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206619lbl.pdf .
- 65.Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983–1992. doi: 10.1056/NEJMoa1402338. doi: 10.1056/NEJMoa1402338. [DOI] [PubMed] [Google Scholar]
- 66.Zeuzem S, Jacobson IM, Baykal T, Marinho RT, Poordad F, Bourlière M, et al. Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1604–1614. doi: 10.1056/NEJMoa1401561. doi: 10.1056/NEJMoa1401561. [DOI] [PubMed] [Google Scholar]
- 67.Gao M. Antiviral activity and resistance of HCV NS5A replication complex inhibitors. Curr Opin Virol. 2013;3:514–520. doi: 10.1016/j.coviro.2013.06.014. doi: 10.1016/j.coviro.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 68.Kawakami Y, Imamura M, Ikeda H, Suzuki M, Arataki K, Moriishi M, et al. Pharmacokinetics, efficacy and safety of daclatasvir plus asunaprevir in dialysis patients with chronic hepatitis C: pilot study. J Viral Hepat. 2016;23:850–856. doi: 10.1111/jvh.12553. doi: 10.1111/jvh.12553. [DOI] [PubMed] [Google Scholar]
- 69.Daklinza (daclatasvir) tablets prescibing information. Tokyo, Japan: Bristol-Myers KK; 2014. [Google Scholar]
- 70.Kumada H, Suzuki F, Suzuki Y, Toyota J, Karino Y, Chayama K, et al. Randomized comparison of daclatasvir + asunaprevir versus telaprevir + peginterferon/ribavirin in Japanese hepatitis C virus patients. J Gastroenterol Hepatol. 2016;31:14–22. doi: 10.1111/jgh.13073. doi: 10.1111/jgh.13073. [DOI] [PubMed] [Google Scholar]
- 71.Manns M, Pol S, Jacobson IM, Marcellin P, Gordon SC, Peng CY, et al. All-oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: a multinational, phase 3, multicohort study. Lancet. 2014;384:1597–1605. doi: 10.1016/S0140-6736(14)61059-X. doi: 10.1016/S0140-6736(14)61059-X. [DOI] [PubMed] [Google Scholar]
- 72.Toyoda H, Kumada T, Tada T, Takaguchi K, Ishikawa T, Tsuji K, et al. Safety and efficacy of dual direct-acting antiviral therapy (daclatasvir and asunaprevir) for chronic hepatitis C virus genotype 1 infection in patients on hemodialysis. J Gastroenterol. 2016;51:741–747. doi: 10.1007/s00535-016-1174-4. doi: 10.1007/s00535-016-1174-4. [DOI] [PubMed] [Google Scholar]
- 73.SOVALDI® (sofosbuvir) tablets, for oral use. 2015. Gilead Sciences, Inc., available from: http://www.gilead.com/∼/media/Files/pdfs/medicines/liver-disease/sovaldi/sovaldi_pi.pdf .
- 74.Desnoyer A, Pospai D, Lê MP, Gervais A, Heurgué-Berlot A, Laradi A, et al. Pharmacokinetics, safety and efficacy of a full dose sofosbuvir-based regimen given daily in hemodialysis patients with chronic hepatitis C. J Hepatol. 2016;65:40–47. doi: 10.1016/j.jhep.2016.02.044. doi: 10.1016/j.jhep.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 75.Asahina Y, Izumi N, Hiromitsu K, Kurosaki M, Koike K, Suzuki F, et al. JSH Guidelines for the Management of Hepatitis C Virus Infection: A 2016 update for genotype 1 and 2. Hepatol Res. 2016;46:129–165. doi: 10.1111/hepr.12645. doi: 10.1111/hepr.12645. [DOI] [PubMed] [Google Scholar]
- 76.Hundemer GL, Sise ME, Wisocky J, Ufere N, Friedman LS, Corey KE, et al. Use of sofosbuvir-based direct-acting antiviral therapy for hepatitis C viral infection in patients with severe renal insufficiency. Infect Dis (Lond) 2015;47:924–929. doi: 10.3109/23744235.2015.1078908. doi: 10.3109/23744235.2015.1078908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perumpail RB, Wong RJ, Ha LD, Pham EA, Wang U, Luong H, et al. Sofosbuvir and simeprevir combination therapy in the setting of liver transplantation and hemodialysis. Transpl Infect Dis. 2015;17:275–278. doi: 10.1111/tid.12348. doi: 10.1111/tid.12348. [DOI] [PubMed] [Google Scholar]
- 78.Perumpail RB, Wong RJ, Pham EA, Higgins JP, Daugherty TJ, Ahmed A. A new standard of care? standard dose sofosbuvir in an HCV-infected liver transplant recipient undergoing hemodialysis. Dig Dis Sci. 2016;61:39–41. doi: 10.1007/s10620-015-3756-z. doi: 10.1007/s10620-015-3756-z. [DOI] [PubMed] [Google Scholar]
- 79.Saxena V, Koraishy FM, Sise ME, Lim JK, Schmidt M, Chung RT, et al. Safety and efficacy of sofosbuvir-containing regimens in hepatitis C-infected patients with impaired renal function. Liver Int. 2016;36:807–816. doi: 10.1111/liv.13102. doi: 10.1111/liv.13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nazario HE, Ndungu M, Modi AA. Sofosbuvir and simeprevir in hepatitis C genotype 1-patients with end-stage renal disease on haemodialysis or GFR <30 ml/min. Liver Int. 2016;36:798–801. doi: 10.1111/liv.13025. doi: 10.1111/liv.13025. [DOI] [PubMed] [Google Scholar]
- 81.Scalea JR, Barth RN, Munivenkatappa R, Philosophe B, Cooper M, Whitlow V, et al. Shorter waitlist times and improved graft survivals are observed in patients who accept hepatitis C virus+ renal allografts. Transplantation. 2015;99:1192–1196. doi: 10.1097/TP.0000000000000479. doi: 10.1097/TP.0000000000000479. [DOI] [PubMed] [Google Scholar]
- 82.Asselah T, Boyer N, Saadoun D, Martinot-Peignoux M, Marcellin P. Direct-acting antivirals for the treatment of hepatitis C virus infection: optimizing current IFN-free treatment and future perspectives. Liver Int. 2016;36(Suppl 1):47–57. doi: 10.1111/liv.13027. doi: 10.1111/liv.13027. [DOI] [PubMed] [Google Scholar]
- 83.Singh T, Guirguis J, Anthony S, Rivas J, Hanouneh IA, Alkhouri N. Sofosbuvir-based treatment is safe and effective in patients with chronic hepatitis C infection and end stage renal disease: a case series. Liver Int. 2016;36:802–806. doi: 10.1111/liv.13078. doi: 10.1111/liv.13078. [DOI] [PubMed] [Google Scholar]
- 84.Beinhardt S, Al Zoairy R, Ferenci P, Kozbial K, Freissmuth C, Stern R, et al. DAA-based antiviral treatment of patients with chronic hepatitis C in the pre-and postkidney transplantation setting. Transpl Int. 2016;29:999–1007. doi: 10.1111/tri.12799. doi: 10.1111/tri.12799. [DOI] [PubMed] [Google Scholar]