Abstract
Purpose
Human epidermal growth factor receptor 3 (HER3) has been identified as an important component of many receptor tyrosine kinase-driven cancers. LJM716 is a human IgG monoclonal antibody that binds HER3, trapping it in an inactive conformation. In this study, a phase I dose escalation was performed with a primary objective to establish the maximum tolerated dose and/or the recommended dose of LJM716 in Japanese patients with selected advanced solid tumors. Secondary objectives included the evaluation of the safety and tolerability, preliminary antitumor activity, and pharmacokinetics of LJM716 in Japanese patients.
Methods
LJM716 was administered intravenously at doses of 10, 20, or 40 mg/kg once weekly, in 28-day cycles, to 12 patients with HER2-amplified breast cancer or gastric cancer, or with esophageal squamous cell carcinoma or squamous cell carcinoma of the head and neck, regardless of HER2 status.
Results
The maximum tolerated dose was not reached, and the recommended dose was established at 40 mg/kg. No dose-limiting toxicities were observed in the first cycle. The most frequently reported adverse events were diarrhea, fatigue, stomatitis, pyrexia, and paronychia. One unconfirmed partial response was observed in a patient with breast cancer, and 50% of the patients achieved stable disease as the best overall response. Exposure increased with ascending dose, and half-life was estimated to be 11–14 days. No anti-LJM716 antibodies were detected.
Conclusions
LJM716 was well tolerated in Japanese patients, and a degree of tumor shrinkage was observed.
Clinical trial information
ClinicalTrials.gov NCT01911936.
Electronic supplementary material
The online version of this article (doi:10.1007/s00280-016-3214-4) contains supplementary material, which is available to authorized users.
Keywords: HER3, HER2, LJM716, Monoclonal antibody, Phase I
Introduction
Inappropriate activation of the human epidermal growth factor receptor (HER) family of tyrosine kinases has been implicated in a wide variety of different cancers [1]. Activation occurs via homo- or heterodimerization of HER family members, inducing kinase activity and subsequent downstream activation of intracellular signaling pathways. HER3 lacks significant kinase activity [2] but has recently been recognized as an important component of receptor tyrosine kinase-driven tumorigenesis, dimerizing with and activating other HER family members, such as epidermal growth factor receptor (EGFR) and HER2 [3]. Overexpression of HER2 or interaction with the ligand, neuroregulin1 (NRG1), promotes HER2:HER3 heterodimerization and subsequent phosphoinositide 3-kinase signaling, driving tumor cell proliferation and tumor growth [1, 3]. HER3 therefore plays an important role in the development and maintenance of HER2- and NRG1-driven cancers, and represents an attractive target for directed therapy.
The efficacy of HER2-directed therapies has already been demonstrated, and approved drugs include the monoclonal antibodies, trastuzumab and pertuzumab, and the tyrosine kinase inhibitor, lapatinib [4–6]. Although the use of these drugs has vastly improved the treatment options for patients with HER2-driven cancer, problems with resistance have been documented. Resistance can develop through multiple mechanisms, including cross-activation by and compensatory upregulation of other HER family members, including HER3 [7]. As such, targeting HER2 and HER3 simultaneously is hoped to improve response to treatment; indeed, promising outcomes have been reported following combination of HER2-directed therapies with other therapies [8–10]. A number of cancer types are good candidates for HER3-directed therapy. Dysregulated HER2 expression has been documented in esophageal squamous cell carcinoma (ESCC; 31%) [11], metastatic breast cancer (18–20%) [7], and gastric/gastroesophageal junction cancer (15–30%) [12]. In addition to HER2-driven cancers, preclinical data suggest that NRG1-driven cancers, which include a significant proportion of squamous cell carcinomas of the head and neck (SCCHN), are also likely to benefit from HER3-directed therapy [13, 14].
LJM716 is a fully human IgG monoclonal antibody that specifically binds HER3, trapping it in the inactive conformation. LJM716 is uniquely able to block both the ligand-independent and ligand-dependent modes of HER3 activation, and antitumor activity has been demonstrated in both HER2-amplified and NRG1-driven xenograft models [15, 16]. In a recent global phase I clinical trial in predominantly Western Caucasian patients with HER2-overexpressing breast cancer or gastric cancer, and with ESCC or SCCHN, regardless of HER2 status (NCT01598077), single-agent LJM716 was well tolerated up to a recommended dose (RD) of 40 mg/kg [17]. Here, the safety and tolerability of single-agent LJM716 were evaluated in Japanese patients in the same indications.
Materials and methods
Study oversight
This was a phase I, open-label, multicenter study (clinicaltrials.gov registry number NCT01911936) [18]. The accrual period, from the first patient visit to the last patient visit, was from September 19, 2013, to March 6, 2015. The study was designed by the sponsor (Novartis Pharmaceuticals Corporation) and performed in accordance with the principles of Good Clinical Practice. The protocol was approved by an Institutional Review Board at each institution, and the study was conducted according to the ethical principles of the Declaration of Helsinki. All patients provided written informed consent before any study procedures.
Patient selection
All patients were aged ≥18 years, had an Eastern Cooperative Oncology Group (ECOG) performance status of ≤2, and had HER2-overexpressing or amplified (HER2+) locally advanced/metastatic breast cancer or gastric cancer, or recurrent or metastatic SCCHN or ESCC, regardless of HER2 status, for which no effective treatment option exists (investigator decision). For breast cancer, patients were required to have tumors with 3+ HER2-overexpression documented by immunohistochemistry or amplification by in situ hybridization; for gastric cancer (including gastroesophageal junction tumors), patients were required to have tumors with immunohistochemistry 2+ or 3+ and amplification by in situ hybridization [19, 20]. Exclusion criteria included patients with untreated or symptomatic central nervous system metastases, other primary malignancies requiring intervention, or prior treatment with an anti-HER3 antibody.
Study objectives
The primary objectives for the study were to determine the maximum tolerated dose (MTD) and/or RD of LJM716 as a single agent when administered intravenously (IV) to Japanese patients with advanced solid tumors. The secondary objectives were to characterize the safety and tolerability, pharmacokinetics (PK), and preliminary antitumor activity of LJM716, and to assess the emergence of antibodies against LJM716.
Study design and treatment plan
During the dose-escalation part, at least 12 patients were planned to be treated in successive cohorts. The starting dose was 10 mg/kg LJM716, followed by 20 and 40 mg/kg, given by IV infusion over 2 h once weekly (QW) in 28-day cycles. An adaptive Bayesian logistic regression model (BLRM) [21] incorporating escalation with overdose control criteria was used to guide dose-escalation decisions [22, 23], and provide support to establish the MTD or RD for LJM716.
Premedication prior to each dose of LJM716 was recommended (650 mg acetaminophen or equivalent and 50 mg IV diphenhydramine or equivalent) to circumvent infusion-related reactions. Dose reductions to ≥10 mg/kg were permitted, as were dose interruptions ≤28 days. LJM716 administration was discontinued in the event of disease progression, unacceptable adverse events (AEs), or as the result of patient or physician decision. It was decided not to open the dose-expansion part of this study.
Toxicity assessments
Safety assessments were carried out based on all AEs and their relationship to the study drug treatment, with regular monitoring of hematology, blood chemistry, and urine analysis, and regular assessment of vital signs, physical condition, body weight, performance status, and cardiac function. AEs were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. Dose-limiting toxicities (DLTs) were defined as AEs or abnormal laboratory values assessed as unrelated to disease progression, intercurrent illness, or concomitant medication, as defined in Supplemental Table 1.
Response assessments
Tumor lesions were assessed by investigators according to Response Evaluation Criteria In Solid Tumors (RECIST) guidelines, version 1.1 [24]. Patients underwent screening computed tomography (CT) scans of the chest, abdomen, and pelvis, and MRI where evaluation by CT was not adequate. Visible skin lesions and easily palpable subcutaneous tumors were measured by physical examination. Screening was performed within 28 days of the first dose. The post-baseline RECIST assessments were performed every two cycles and then at the end of treatment if a scan was not conducted 30 days prior to this.
Pharmacokinetics and immunogenicity
Serum was collected for PK assessments at multiple time points, including serial samplings to calculate PK parameters (Cycle 1 and Cycle 3) and trough samplings (Day 1 of each cycle). The serum concentration of LJM716 was measured using a validated ELISA. PK parameters (maximum observed serum concentration after drug administration [C max], time to C max [T max], area under the curve [AUC], etc.) were determined by a non-compartmental method with a lower limit of quantification of 150 ng/mL. Serum immunogenicity was assessed using an anti-LJM716 antibody test.
Results
Patient characteristics
A total of 12 patients were treated with intravenous LJM716 at doses of 10, 20, and 40 mg/kg QW, between September 19, 2013, and March 6, 2015. The median age of patients was 58 years (range, 33‒75); 8/12 (67%) patients were aged <65 years; 6/12 (50%) were male; 4/12 (33%) had an ECOG performance status of 1; and 1/12 (8%) had an ECOG performance status of 2. Confirmed primary tumor types at enrollment were ESCC [n = 2 (17%)], SCCHN [n = 2 (17%)], HER2-overexpressing breast cancer [n = 6 (50%)], and HER2-overexpressing gastric cancer [n = 2 (17%)]. All patients had stage IV disease at study entry. All patients had received prior antineoplastic therapies (median 6; range, 2–13), including trastuzumab in eight patients. Patient demographics are given in Table 1. The median duration of exposure was 14.0 weeks (range, 4.0–48.1) across all LJM716 doses, and most patients (92%) had an exposure of >4 weeks (Supplemental Table 2). All 12 patients discontinued treatment due to disease progression.
Table 1.
Patient demographics and disease characteristics, by treatment group
| 10 mg/kg QW (n = 3) |
20 mg/kg QW (n = 3) |
40 mg/kg QW (n = 6) |
All patients (N = 12) |
|
|---|---|---|---|---|
| Age, years (median) | 69.0 | 58.0 | 51.5 | 58.0 |
| <65, n (%) | 1 (33) | 2 (67) | 5 (83) | 8 (67) |
| ≥65, n (%) | 2 (67) | 1 (33) | 1 (17) | 4 (33) |
| Sex, n (%) | ||||
| Female | 2 (67) | 1 (33) | 3 (50) | 6 (50) |
| Male | 1 (33) | 2 (67) | 3 (50) | 6 (50) |
| ECOG PS, n (%) | ||||
| 0 | 1 (33) | 1 (33) | 5 (83) | 7 (58) |
| 1 | 2 (67) | 2 (67) | 0 | 4 (33) |
| 2 | 0 | 0 | 1 (17) | 1 (8) |
| Primary site of cancer, n (%) | ||||
| Breast | 2 (67) | 1 (33) | 3 (50) | 6 (50) |
| Esophagus | 0 | 0 | 2 (33) | 2 (17) |
| Head and neck | 0 | 1 (33) | 1 (17) | 2 (17) |
| Gastric | 1 (33) | 1 (33) | 0 | 2 (17) |
| Primary tumor histology, n (%) | ||||
| Squamous cell carcinoma | 0 | 1 (33) | 3 (50) | 4 (33) |
| Adenocarcinoma | 3 (100) | 2 (67) | 3 (50) | 8 (67) |
| Stage at study entry, n (%) | ||||
| IV | 2 (67) | 2 (67) | 5 (83) | 9 (75) |
| IVB | 1 (33) | 1 (33) | 1 (17) | 3 (25) |
ECOG Eastern Cooperative Oncology Group, PS performance status, QW once weekly
Toxicity
No DLTs were reported in the first cycle of the study. Grade 3 pneumonia aspiration observed in one patient (40 mg/kg) during Cycle 2 met the DLT criteria as described in Supplemental Table 1 (other ≥grade 3 non-hematologic toxicity). The MTD was not reached, and the RD was established at 40 mg/kg QW based on the BLRM, safety, tolerability, and PK data. All patients had at least one AE, regardless of study drug relationship. The most frequent AEs in all patients were diarrhea (50%), stomatitis (42%), fatigue, edema peripheral, and pyrexia (33% each). There were no clinically relevant differences in AEs across the study groups. At the RD of 40 mg/kg, the most frequent AEs were diarrhea, pyrexia (50% each), fatigue, nasopharyngitis, anemia, and lymphocyte count decreased (33% each; Table 2). AEs assessed as infusion-related reactions were only observed in the 40 mg/kg dose group (pyrexia in three patients and headache in one patient). Five grade 3/4 AEs were reported: pneumonia aspiration, anemia, neutropenia, hyponatremia, and hypophosphatemia in one patient (8%) each, and decreased lymphocyte count in two patients (17%). Ten patients (83%) had AEs suspected to be study drug-related, and the most common was diarrhea (50%; Supplemental Table 3). Two patients (17%) experienced grade 3/4 AEs suspected to be study drug-related: pneumonia aspiration and neutropenia in one patient (8%) each, and decreased lymphocyte count in two patients (17%). Two patients reported serious AEs: nausea and vomiting, and pneumonia aspiration in one patient (8%) each, of which only pneumonia aspiration was suspected to be study drug-related. No deaths were reported during the study. No AEs that led to study drug discontinuation were reported, and four patients (33%) reported AEs requiring dose interruption: influenza, atrial fibrillation, neutropenia, nasopharyngitis, and pneumonia aspiration in one patient each. No AEs leading to dose reduction were reported.
Table 2.
Adverse events (≥10%), regardless of study drug relationship, by treatment group
| Preferred term, n (%) | 10 mg/kg QW (n = 3) |
20 mg/kg QW (n = 3) |
40 mg/kg QW (n = 6) |
All patients (N = 12) |
|---|---|---|---|---|
| Diarrhea | 2 (67) | 1 (33) | 3 (50) | 6 (50) |
| Stomatitis | 3 (100) | 1 (33) | 1 (17) | 5 (42) |
| Fatigue | 1 (33) | 1 (33) | 2 (33) | 4 (33) |
| Edema peripheral | 1 (33) | 2 (67) | 1 (17) | 4 (33) |
| Pyrexia | 0 | 1 (33) | 3 (50) | 4 (33) |
| Nausea | 1 (33) | 1 (33) | 1 (17) | 3 (25) |
| Cough | 2 (67) | 0 | 1 (17) | 3 (25) |
| Pruritus | 1 (33) | 1 (33) | 1 (17) | 3 (25) |
| Nasopharyngitis | 0 | 1 (33) | 2 (33) | 3 (25) |
| Paronychia | 2 (67) | 0 | 1 (17) | 3 (25) |
| Anemia | 0 | 1 (33) | 2 (33) | 3 (25) |
| Decreased appetite | 1 (33) | 1 (33) | 1 (17) | 3 (25) |
| Dysphagia | 0 | 1 (33) | 1 (17) | 2 (17) |
| Vomiting | 1 (33) | 1 (33) | 0 | 2 (17) |
| Dyspnea | 0 | 1 (33) | 1 (17) | 2 (17) |
| Rash | 1 (33) | 0 | 1 (17) | 2 (17) |
| Headache | 1 (33) | 0 | 1 (17) | 2 (17) |
| Peripheral sensory neuropathy | 0 | 1 (33) | 1 (17) | 2 (17) |
| Lymphocyte count decreased | 0 | 0 | 2 (33) | 2 (17) |
QW once weekly
Efficacy
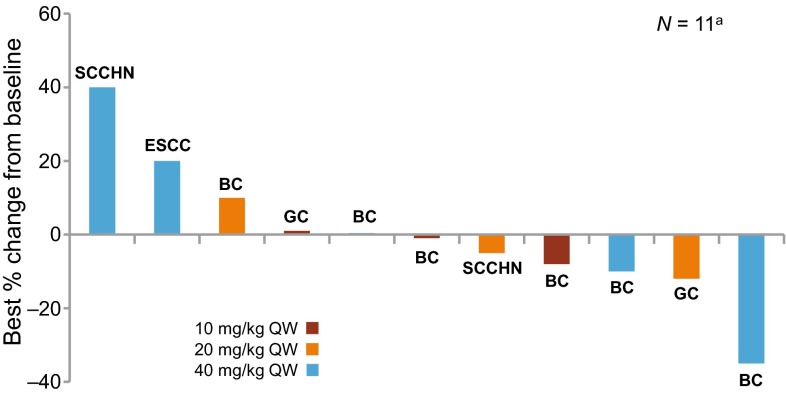
Of all treated patients, six (50%) achieved stable disease and six (50%) had progressive disease (Table 3). None of the patients achieved complete or partial response; however, 4/6 patients with HER2+ breast cancer showed some tumor shrinkage (Fig. 1). One patient with HER2+ breast cancer (40 mg/kg) had an unconfirmed partial response (tumor shrinkage of more than 30% in Cycle 10), followed by subsequent progressive disease.
Table 3.
Best overall response in all disease types (investigator assessed)
| Best overall response, n (%) | 10 mg/kg QW (n = 3) |
20 mg/kg QW (n = 3) |
40 mg/kg QW (n = 6) |
All patients (N = 12) |
|---|---|---|---|---|
| Complete response | 0 | 0 | 0 | 0 |
| Partial response | 0 | 0 | 0 | 0 |
| Stable disease | 3 (100) | 1 (33) | 2 (33) | 6 (50) |
| Unconfirmed CR/PR | 0 | 0 | 1 (17) | 1 (8) |
| Progressive disease | 0 | 2 (67) | 4 (67) | 6 (50) |
| Unknown | 0 | 0 | 0 | 0 |
Data cut off: March 6, 2015
CR complete response, PR partial response, QW once weekly
Fig. 1.
Best percentage change from baseline in target lesions by treatment group. BC breast cancer, ESCC esophageal squamous cell carcinoma, GC gastric cancer, QW once weekly, SCCHN squamous cell carcinoma of the head and neck. aThe number of patients was 11 because one patient with ESCC did not have target lesions
Pharmacokinetic studies and immunogenicity
The PK parameters for Cycle 1 and Cycle 3 are given in Table 4, and these were comparable to those seen previously in a global study in predominantly Western Caucasian patients [17]. The exposure of LJM716 increased as the dose increased. Upon a power model analysis of the dose–exposure relationship for AUC from time zero to the time of the last quantifiable concentration (AUC0–last) and C max in Cycle 1, dose-proportionality in the dose range 10–40 mg/kg was suggested with slope estimates of 0.82 (90% confidence interval [CI] 0.53–1.12) and 0.85 (90% CI 0.65–1.04), respectively, near to unity. There was 2.8–3.4-fold accumulation at steady state in Cycle 3 after repeated weekly doses, based on AUC after the first doses of Cycles 1 and 3. The effective half-life of LJM716 was estimated to be 11–14 days, based on the accumulation. All patients were tested for antidrug antibodies against LJM716, but antibodies were not detected in any of the samples.
Table 4.
Pharmacokinetic parameters for LJM716 (Cycle 1; Cycle 3)
| AUC0–last (h·μg/mL) | C min (μg/mL) | C max (μg/mL) | T max (h) | |
|---|---|---|---|---|
| Cycle 1 | Mean (SD) | Median (range) | ||
| 10 mg/kg (n = 3) |
18,700 (7100) |
65.8 (27.3) |
195 (53.9) |
4.38 (2.83‒9.57) |
| 20 mg/kg (n = 3) |
33,700 (5120) |
137 (27.8) |
362 (53.4) |
4.63 (2.07‒9.65) |
| 40 mg/kg (n = 6) |
59,000 (21,500) |
243 (100) |
628 (136) |
3.75 (2.02‒9.65) |
| Cycle 3 a | Individual data | |||
| 10 mg/kg (n = 2) |
35,300; 62,400 | 185; 280 | 268; 615 | 9.50; 9.53 |
| 40 mg/kg (n = 1) |
243,000 | 1210 | 2130 | 4.73 |
AUC 0–last area under the curve from time zero to the time of the last quantifiable concentration, C max maximum observed serum concentration after drug administration, C min minimum drug serum concentration, SD standard deviation, T max time to reach C max
aThe number of available patients was limited in Cycle 3
Discussion
This study evaluated the safety and tolerability of IV-administered LJM716 in Japanese patients with solid tumors. The RD of LJM716 was established at 40 mg/kg QW; the same as that determined in a global clinical trial in Western Caucasian patients [17]. The MTD was not reached during the course of this study. LJM716 was well tolerated with a manageable safety profile, with observed toxicities largely grade 1 or 2. The most frequently observed AE was diarrhea, which is seen with most other HER family inhibitors [25–27]. Diarrhea in this study was mild. Similarly, skin toxicities were generally less frequent and milder than seen with other HER family inhibitors [6, 28]. The fact that these toxicities were mild (grade 1 or 2) might be considered to be due to the specificity of LJM716 for HER3; more severe AEs could be expected to result from off-target effects. Similarly, more severe AEs are seen in combination studies, with grade 3 gastrointestinal problems and skin toxicities being reported in trials combining HER2 or HER3-targeted mAbs with EGFR inhibitors [29–34]. Of 12 patients treated with LJM716, only one serious AE suspected to be drug induced was reported. This was aspiration-induced pneumonia in a patient with SCCHN. While the cause for this is unknown, serious bacterial pneumonia has been reported in two other studies with HER3 mAbs [27, 33]. Overall, LJM716 was found to have a favorable safety profile.
LJM716 demonstrated dose-dependent exposure, with an estimated half-life of 11–14 days, and PK parameters similar to those observed in the global study and in line with other therapeutic antibodies. The AUC/C max observed at the RD was sufficient to achieve effective systemic drug levels, based on preclinical studies. In the preclinical study, 1–10 nM LJM716 suppressed growth of HER2-overexpressing or NRG-stimulated cell lines [15]. In this study, the mean C max was 243 μg/mL (equivalent to ~1–2 μM based on a typical molecular weight of ~150 kDa for a human IgG mAb) for the 40 mg/kg dose group at Cycle 1, and significantly higher at Cycle 3.
Although no confirmed complete or partial responses were observed during the course of the study, tumor shrinkage of more than 30% (unconfirmed partial response) was observed in one patient with HER2+ breast cancer (40 mg/kg group), and tumor shrinkage was also observed in three of the other five patients with HER2+ breast cancer across all doses. A total of 50% of the patients achieved stable disease. Five of the six patients with HER2+ breast cancer showing stable disease or an unconfirmed partial response had documented progression with the most recent prior regimen, which contained trastuzumab, and the sixth patient received trastuzumab as part of the second most recent treatment regimen. Further investigation is needed to confirm whether LJM716 will be an efficacious treatment option for patients with breast cancer who are trastuzumab resistant.
Future development of HER3-directed therapies may benefit from the consideration of appropriate biomarkers. NRG1 has been validated as a predictive biomarker for response to the HER3-directed mAbs AV-203 and patritumab in preclinical and in clinical studies, respectively [35, 36]. In contrast, no correlation was seen between tumor inhibition and HER3 levels [35].
Although the single-agent LJM716 antitumor activity observed here was less extensive than that observed in preclinical xenograft studies, these same studies established that efficacy was significantly improved by combination of LJM716 with other HER2-directed therapies [15, 16]. Consistent with this, there is an increasing body of clinical evidence demonstrating that the efficacy of HER2/HER3-directed therapies is improved by combination with other anti-HER antibodies [9, 37], tyrosine kinase inhibitors [33, 34], and/or chemotherapy [8, 10]. For example, in a phase II trial in which 100% of the patients progressed on pertuzumab single-agent therapy, a clinical benefit rate (stable disease + partial response + complete response ≥6 months) of 41% was achieved following subsequent combination therapy with trastuzumab [37]. It is noteworthy that the addition of LJM716 to trastuzumab resulted in increased inhibition of pAKT and improved in vitro efficacy exceeding that achieved by the combination of trastuzumab with pertuzumab [15]. Based on these observations, the future development of LJM716 is likely to focus on combination with other therapies, and a phase I study of LJM716 in combination with trastuzumab in patients with HER2-overexpressing breast or gastric cancer is currently ongoing (NCT01602406).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the participating patients, their families, all study co-investigators, and research coordinators. Medical editorial assistance was provided by Laura Hilditch, Ph.D., and was funded by Novartis Pharmaceuticals Corporation.
Funding
This study was funded by Novartis Pharmaceuticals Corporation.
Compliance with ethical standards
Conflict of interest
Shunji Takahashi received research funding from Novartis; Taito Esaki received research funding from Eli Lilly, Taiho, Novartis, Daiichi Sankyo, AstraZeneca, Bayer, Merck Serono, Ono, Boehringer, MSD, and Astellas; Yoshinori Ito received research funding from Chugai, Novartis, Parexel, and EPS; Shunji Takahashi received honoraria from Novartis; Taito Esaki received honoraria from Yakult, Chugai, Eli Lilly, Taiho, and Merck Serono; Yoshinori Ito received honoraria from Chugai, Novartis, Esai, and Taiho; Tomoyuki Kakizume, Takeshi Tajima, Hiromi Takeuchi, and Heiko Maacke are employees of Novartis; Takayuki Kobayashi, Junichi Tomomatsu, Hisanobu Oda, and Tatsuhiro Kajitani have no conflict of interest. The study was designed under the responsibility of and funded by Novartis; Novartis collected and analyzed the data and contributed to the interpretation of the study. All authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
References
- 1.Amin DN, Campbell MR, Moasser MM. The role of HER3, the unpretentious member of the HER family, in cancer biology and cancer therapeutics. Semin Cell Dev Biol. 2010;21:944–950. doi: 10.1016/j.semcdb.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jura N, Shan Y, Cao X, Shaw DE, Kuriyan J. Structural analysis of the catalytically inactive kinase domain of the human EGF receptor 3. Proc Natl Acad Sci USA. 2009;106:21608–21613. doi: 10.1073/pnas.0912101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci USA. 2003;100:8933–8938. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 5.Agus DB, Akita RW, Fox WD, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–137. doi: 10.1016/S1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 6.Gomez HL, Doval DC, Chavez MA, et al. Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J Clin Oncol. 2008;26:2999–3005. doi: 10.1200/JCO.2007.14.0590. [DOI] [PubMed] [Google Scholar]
- 7.Gagliato DM, Jardim DL, Marchesi MS, Hortobagyi GN. Mechanisms of resistance and sensitivity to anti-HER2 therapies in HER2+ breast cancer. Oncotarget. 2016 doi: 10.18632/oncotarget.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baselga J, Cortes J, Kim S-B, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baselga J, Gelmon KA, Verma S, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol. 2010;28:1138–1144. doi: 10.1200/JCO.2009.24.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 11.Kono K, Mimura K, Fujii H, Shabbir A, Yong WP, Jimmy So A. Potential therapeutic significance of HER-family in esophageal squamous cell carcinoma. Ann Thorac Cardiovasc Surg. 2012;18:506–513. doi: 10.5761/atcs.ra.12.01995. [DOI] [PubMed] [Google Scholar]
- 12.Ang YL, Yong WP, Tan P. Translating gastric cancer genomics into targeted therapies. Crit Rev Oncol Hematol. 2016;100:141–146. doi: 10.1016/j.critrevonc.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Wilson TR, Lee DY, Berry L, Shames DS, Settleman J. Neuregulin-1-mediated autocrine signaling underlies sensitivity to HER2 kinase inhibitors in a subset of human cancers. Cancer Cell. 2011;20:158–172. doi: 10.1016/j.ccr.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Zhang K, Jones L, Lim S, et al. Loss of trop2 causes ErbB3 activation through a neuregulin-1-dependent mechanism in the mesenchymal subtype of HNSCC. Oncotarget. 2014;5:9281–9294. doi: 10.18632/oncotarget.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garner AP, Bialucha CU, Sprague ER, et al. An antibody that locks HER3 in the inactive conformation inhibits tumor growth driven by HER2 or neuregulin. Cancer Res. 2013;73:6024–6035. doi: 10.1158/0008-5472.CAN-13-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garrett JT, Sutton CR, Kurupi R, et al. Combination of antibody that inhibits ligand-independent HER3 dimerization and a p110alpha inhibitor potently blocks PI3K signaling and growth of HER2+ breast cancers. Cancer Res. 2013;73:6013–6023. doi: 10.1158/0008-5472.CAN-13-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynolds KL, Juric D, Baselga J, et al. A phase I study of LJM716 in patients with esophageal squamous cell carcinoma, head and neck cancer, or HER2-overexpressing metastatic breast or gastric/gastroesophageal junction cancer (abstract) J Clin Oncol. 2014;32(suppl 15):2517. [Google Scholar]
- 18.Esaki T, Oda H, Kajitani T, et al. Phase I study of the safety and tolerability of LJM716 in Japanese patients with advanced solid tumors (abstract) Mol Cancer Ther. 2015;14(suppl 2):C120. doi: 10.1158/1535-7163.TARG-15-C120. [DOI] [Google Scholar]
- 19.Wolff AC, Hammond MEH, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 20.Wolff AC, Hammond MEH, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18–43. doi: 10.5858/2007-131-18-ASOCCO. [DOI] [PubMed] [Google Scholar]
- 21.Rogatko A, Schoeneck D, Jonas W, Tighiouart M, Khuri FR, Porter A. Translation of innovative designs into phase I trials. J Clin Oncol. 2007;25:4982–4986. doi: 10.1200/JCO.2007.12.1012. [DOI] [PubMed] [Google Scholar]
- 22.Babb J, Rogatko A, Zacks S. Cancer phase I clinical trials: efficient dose escalation with overdose control. Stat Med. 1998;17:1103–1120. doi: 10.1002/(SICI)1097-0258(19980530)17:10<1103::AID-SIM793>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 23.Neuenschwander B, Branson M, Gsponer T. Critical aspects of the Bayesian approach to phase I cancer trials. Stat Med. 2008;27:2420–2439. doi: 10.1002/sim.3230. [DOI] [PubMed] [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 25.Al-Dasooqi N, Gibson R, Bowen J, et al. HER2 targeted therapies for cancer and the gastrointestinal tract. Curr Drug Targets. 2009;10:537–542. doi: 10.2174/138945009788488440. [DOI] [PubMed] [Google Scholar]
- 26.LoRusso P, Janne PA, Oliveira M, et al. Phase I study of U3-1287, a fully human anti-HER3 monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res. 2013;19:3078–3087. doi: 10.1158/1078-0432.CCR-12-3051. [DOI] [PubMed] [Google Scholar]
- 27.Wakui H, Yamamoto N, Nakamichi S, et al. Phase 1 and dose-finding study of patritumab (U3-1287), a human monoclonal antibody targeting HER3, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2014;73:511–516. doi: 10.1007/s00280-014-2375-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piccart-Gebhart M, Holmes E, Balsega J, et al. Adjuvant lapatinib and trastuzumab for early human epidermal growth factor receptor 2–positive breast cancer: results from the randomized phase III adjuvant lapatinib and/or trastuzumab treatment optimization trial. J Clin Oncol. 2016;34:1034–1042. doi: 10.1200/JCO.2015.62.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Assenat E, Azria D, Mollevi C, et al. Dual targeting of HER1/EGFR and HER2 with cetuximab and trastuzumab in patients with metastatic pancreatic cancer after gemcitabine failure: results of the “THERAPY” phase 1–2 trial. Oncotarget. 2015;6:12796–12808. doi: 10.18632/oncotarget.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arteaga CL, O’Neill A, Moulder SL, et al. A Phase I-II study of combined blockade of the ErbB receptor network with trastuzumab and gefitinib in patients with HER2 (ErbB2) overexpressing metastatic breast cancer. Clin Cancer Res. 2008;14:6277–6283. doi: 10.1158/1078-0432.CCR-08-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubinson DA, Hochster HS, Ryan DP, et al. Multi-drug inhibition of the HER pathway in metastatic colorectal cancer: results of a phase I study of pertuzumab plus cetuximab in cetuximab-refractory patients. Investig New Drugs. 2014;32:113–122. doi: 10.1007/s10637-013-9956-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ring A, Wheatley D, Hatcher H, et al. Phase I study to assess the combination of afatinib with trastuzumab in patients with advanced or metastatic HER2-positive breast cancer. Clin Cancer Res. 2015;21:2737–2744. doi: 10.1158/1078-0432.CCR-14-1812. [DOI] [PubMed] [Google Scholar]
- 33.Nishio M, Horiike A, Murakami H, et al. Phase I study of the HER3-targeted antibody patritumab (U3-1287) combined with erlotinib in Japanese patients with non-small cell lung cancer. Lung Cancer. 2015;88:275–281. doi: 10.1016/j.lungcan.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Lassen UN, Cervantes Ruiperez A, Fleitas T, et al. Phase IB trial of RG7116, a glycoengineered monoclonal antibody targeting HER3, in combination with cetuximab or erlotinib in patients with advanced/metastatic tumors of epithelial cell origin expressing HER3 protein (abstract) Ann Oncol. 2014;25(suppl 4):iv147. [Google Scholar]
- 35.Meetze K, Vincent S, Tyler S, et al. Neuregulin 1 expression is a predictive biomarker for response to AV-203, an ERBB3 inhibitory antibody, in human tumor models. Clin Cancer Res. 2015;21:1106–1114. doi: 10.1158/1078-0432.CCR-14-2407. [DOI] [PubMed] [Google Scholar]
- 36.Mendell J, Freeman DJ, Feng W, et al. Clinical translation and validation of a predictive biomarker for patritumab, an anti-human epidermal growth factor receptor 3 (HER3) monoclonal antibody, in patients with advanced non-small cell lung cancer. EBioMedicine. 2015;2:264–271. doi: 10.1016/j.ebiom.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cortés J, Fumoleau P, Bianchi GV, et al. Pertuzumab monotherapy after trastuzumab-based treatment and subsequent reintroduction of trastuzumab: activity and tolerability in patients with advanced human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30:1594–1600. doi: 10.1200/JCO.2011.37.4207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.