Abstract
Background
There is evidence for considerable heterogeneity in the responsiveness to regular physical activity (PA) which might reflect the influence of genetic factors. The aim of this systematic review was to assess whether the response to a PA intervention for measures of body composition and cardiorespiratory fitness is (i) correlated within twin pairs and/or families and (ii) more correlated in monozygotic twins (MZ) compared to dizygotic twins (DZ), which would be consistent with genetic effects.
Methods
We performed electronic database searches, combining key words relating to “physical activity” and “genetics”, in MEDLINE, CINAHL, EMBASE, SPORTS Discuss, AMED, PsycINFO, WEB OF SCIENCE, and SCOPUS from the earliest records to March 2016.
Twin and family studies were included if they assessed body composition and/or cardiorespiratory fitness following a PA intervention, and provided a heritability estimate, maximal heritability estimate, or within MZ twin pair correlation (rMZ).
Data on heritability (twin studies), maximal heritability (family studies), and the rMZ were extracted from included studies, although heritability estimates were not reported as small sample sizes made them uninformative.
Results
After screening 224 full texts, nine twin and five family studies were included in this review. The pooled rMZ in response to PA was significant for body mass index (rMZ = 0.69, n = 58), fat mass (rMZ = 0.58, n = 48), body fat percentage (rMZ = 0.55, n = 72), waist circumference (rMZ = 0.50, n = 27), and VO2max (rMZ = 0.39, n = 48), where “n” represents the total number of twin pairs from all studies. Maximal heritability estimates ranged from 0–21% for measures of body composition, and 22–57% for cardiorespiratory fitness.
Twin studies differed in sample age, baseline values, and PA intervention, although the exclusion of any one study did not affect the results.
Conclusions
Shared familial factors, including genetics, are likely to be a significant contributor to the response of body composition and cardiorespiratory fitness following PA.
Genetic factors may explain individual variation in the response to PA.
Trial Registrations
PROSPERO Registration No CRD42015020056.
Electronic supplementary material
The online version of this article (doi:10.1186/s40798-016-0073-9) contains supplementary material, which is available to authorized users.
Keywords: Genetics, Heritability, Familial aggregation, Physical activity, Body composition, Cardiorespiratory fitness
Key points
Shared familial factors, including genetics, are likely to play a stronger role in the response of body composition when compared to cardiorespiratory fitness.
The response of body mass index, fat mass, and body fat percentage to PA appear to be more dependent on shared familial factors than measures such as waist-to-hip ratio.
These results have implications for the management of conditions which advocate increased levels of PA, since shared familial factors, including genetics, might serve as an explanation for why some people respond more effectively than others in specific measures of PA.
Background
Engagement in regular physical activity (PA) is one of the most important aspects for maintaining optimal health and is recommended for reducing the risk of numerous diseases (including cardiovascular disease) in people of all ages [1–4]. In addition, PA is used as a non-pharmacological treatment option for coronary heart disease [5], osteoporosis [6], rheumatoid arthritis [7], anxiety disorders [8], and a variety of musculoskeletal conditions, including low back pain [9]. Although the benefits of PA are numerous, their positive effects on cardiorespiratory fitness [e.g., maximal oxygen uptake (VO2max)] and measures of body composition [e.g., body mass index (BMI)] [10] deserve special attention, due to their subsequent influence on cardiovascular disease and mortality rates. Cardiorespiratory fitness is a strong and independent risk factor for cardiovascular disease and all-cause mortality [11], with up to 7% of deaths being attributed to low cardiorespiratory fitness [12]. Similarly, high values of body composition measures, such as BMI and waist circumference, are significantly associated with greater all-cause [13] and CVD-related mortality [14].
Although the benefits of PA are clear and substantial, research has demonstrated that genetic factors have a strong influence on PA engagement [15], with the heritability of time-spent in moderate-to-vigorous intensity PA estimated at 47% [16]. In addition, not everyone engaged in PA will benefit to the same extent, with strong evidence for considerable heterogeneity in the responsiveness to regular PA [17–19]. This variation might also reflect the influence of genetic factors.
Twin and family studies are commonly used to investigate the extent to which shared familial factors, including genetics, contribute to the variation of a phenotype. Monozygotic (MZ) twins share 100% of their segregating genes, while dizygotic (DZ) twins share 50% on average. If genes influence a phenotype, we would expect to see a greater correlation for MZ twins than for DZ twins, and if genes are the only influence on a phenotype the ratio should be 2:1, with a heritability estimate of 100%. Smaller differences between the correlations would indicate that shared environmental effects are involved, with the shared environment referring to the exposure to similar environmental (non-genetic) factors within twin pairs (e.g., nutrition, physical activity, childhood experiences, parental beliefs and values, socioeconomic status, etc.). Family studies can estimate maximal heritability using correlations between parent-offspring pairs and siblings (sometimes adjusted for correlation between spouses) [19]. However, unlike heritability estimates from twin studies, these studies are unable to tease apart the contribution from genetic and shared environmental factors. This is because different proportions of genetic sharing are required to separate genetic and shared environmental sources of variation, and in nuclear families parent-offspring pairs and sibling-pairs share equal proportions of their genes (50%). Although we can estimate spouse correlations, we cannot tell whether this correlation is due to shared genes (assortative mating) or shared environmental factors.
The role of both genetic and environmental factors shared within families in the response to a PA intervention has been investigated in a number of studies. MZ twin pairs who completed a standardized PA intervention demonstrated great variation in the amount of weight lost between twin pairs, but only a small amount of variation within twin pairs [20]. In addition, individual differences in the response of VO2max following an exercise program were 2.5 times more variable between families than within families [19]. These results suggest that factors shared within families, including genes, play a role in the response to a PA intervention, although their exact contribution, across measures of body composition and cardiorespiratory fitness, are not well understood. A better understanding of the contribution genetics and shared environmental effects make to people’s response to PA may help health practitioners understand the possible reasons behind individual variation in response to a PA targeted intervention, and why some patients demonstrate a more favorable response.
The aim of this systematic review is to obtain quantitative estimates of twin correlations (both MZ and DZ), heritability (from twin studies), and maximal heritability (from family studies), for measures of body composition and cardiorespiratory fitness in response to a PA intervention.
Methods
Search Strategy
We conducted a systematic review and meta-analysis in accordance with the “Preferred reporting items for systematic reviews and meta-analyses” (PRISMA) statement [21]. The protocol for this systematic review has been registered on PROSPERO (Registration No: CRD42015020056). We performed electronic database searches in MEDLINE, CINAHL, EMBASE, SPORTS Discuss, AMED, PsycINFO, WEB OF SCIENCE, and SCOPUS from the earliest records to May 2015. The search was then updated in March 2016. We used a comprehensive key word search strategy (Additional file 1) combining key words relating to PA (e.g., “physical activi*” OR “exercise” OR “resistance training” etc.) and genetics (e.g., “genetic*” OR “herita*” OR “family resemblance” etc.). The search strategy remained sensitive to capture all outcomes related to body composition and cardiorespiratory fitness. To identify additional studies we performed a hand search of the reference lists from included papers.
Study Selection
Two reviewers (TA and JZ) independently performed the selection of studies and consensus was used to resolve any disagreement. Studies were included if they investigated clinically relevant outcome measures of body composition or cardiorespiratory fitness following a PA or exercise intervention (referred to hereafter as PA interventions) amongst twin pairs and/or family members. Studies investigating a PA intervention in combination with other interventions (e.g., diet) were included. We included randomised controlled trials and case series provided they reported a within MZ twin pair correlation (rMZ), heritability estimate (from a twin study), or maximal heritability estimate (from a family study). Heritability estimates and the rMZ for the response of an intervention (based on change scores) are commonly reported in studies where twin pairs are considered as clusters, with the treatment effect as a fixed variable [22]. To investigate the intra-pair resemblance in the response to PA it is essential that twin pairs participate in an identical intervention. This is similar to the methodology employed in family studies to obtain a maximal heritability estimate (where the variance explained by genetic and shared environmental factors cannot be teased apart). Therefore, we decided not to use methodological quality as part of the inclusion/exclusion criteria as it is not practical to consider items commonly assessed in systematic reviews of randomized controlled trials (such as allocation concealment, blinding, and intention-to-treat) [23] when considering this study design. It is unlikely results from twin and family studies investigating heritability are subject to publication bias, since the contribution of genetics and shared environment is relevant regardless of whether the estimates are small or large. However, we acknowledge the possibility that individual studies may only report results for traits that demonstrate a high heritability. To minimize the risk of reporting bias, we contacted authors when there was data available on body composition and cardiorespiratory fitness but within twin pair correlations were not reported. Observational studies or studies only assessing the heritability of PA engagement, without a PA intervention, were excluded. There was no restriction on the age or gender of participants, nor the type of PA intervention investigated. We included published conference abstracts and dissertations provided they met the inclusion criteria.
Data Extraction
Two reviewers (DS and JZ) independently performed the extraction of data. A standardized data extraction form was used to collect data on participants’ characteristics (age, gender, and zygosity), sample size, prescribed PA intervention (frequency, intensity, duration, and type), co-prescription of other interventions (e.g., diet), outcomes assessed, loss to follow up, and study type.
Data Analysis
Data on correlation (r), equality of variances (F), heritability (h 2), and maximal heritability were extracted from included studies. In family studies, “heritability” estimates were derived from the familial correlation model and termed “maximal heritability”, since the model is unable to partition the variance explained by genetic and non-genetic sources shared within families [24]. In twin studies, heritability estimates were calculated from the following formula: h 2 = 2(rMZ–rDZ), where rDZ is the within DZ twin pair correlation. When h 2 was greater than 1 we used rMZ as the heritability estimate, since it is not possible for genetics to contribute more than 100% to the variance of a phenotype. In addition, if there were no data available for DZ twins, we used rMZ as an estimate of the upper bound of heritability (including variance from genetic and shared environmental factors). In cases where the F-ratio was reported but the rMZ was not, we used the following formula to calculate rMZ as described by Haggard: r = (F–1)/(F + 1) [25]. Authors were contacted when required data were not published. When raw data were obtained from twin studies, we attempted to fit variance components models to change scores in order to estimate the rMZ and rDZ simultaneously and formally compare models in which these two parameters were forced to be equal with models in which they were allowed to differ. However, for many phenotypes the models could not be fitted or failed to converge due to small sample sizes (no results shown from these models). Instead, for all phenotypes, and separately for MZ and DZ twin pairs, we performed a one-way (twin pair identifier) analysis of variance with change score as the outcome (calculated from the pre and post-intervention raw data). Specifying change score as a repeated measure within a twin pair in the models enabled calculation of the within twin pair correlation. When possible and applicable, we adjusted the analyses for age, gender, and baseline values [22]. If studies were considered homogenous in terms of outcomes and PA interventions, we performed a meta-analysis using Comprehensive Meta-Analysis Version 3.0. Additionally, if there were enough studies investigating PA interventions of varying durations, the co-prescription of other interventions (e.g., diet), or analyzing data from males and females separately, we sub-grouped our meta-analyses accordingly. If pooling data on heritability/maximal heritability was not possible (from either twin or family studies), we attempted to pool data on the rMZ. Data on correlation and sample size from each study with greater than or equal to four twin pairs (the minimum number of observations allowed to be entered into the software) was used to provide a pooled estimate of the rMZ, 95% confidence interval (CI), and p-value. Heterogeneity between studies was assessed using the I 2 statistic. An I 2 value <25% indicates low heterogeneity between studies. We used fixed-effects where I 2 was <50% and random-effects when I 2 was ≥50% (moderate heterogeneity). We did not display pooled estimates where the I 2 value indicated high heterogeneity (≥75%) [26].
Results
Description of Studies
The comprehensive key word search yielded 27,830 results, with one additional study retrieved from hand searching the reference lists of included studies. After removing duplicates and screening titles and abstracts there were 224 full texts which were screened. A total of 14 studies (nine twin and five family studies) were included in this systematic review, with eight twin studies forming the basis for our meta-analyses (Fig. 1). The nine twin studies included data from a total of 83 complete MZ twin pairs, and 15 complete DZ twin pairs, with no twin pairs used in more than one study (as confirmed by authors named in multiple included studies). The five family studies were based off the same sample of 199 families (which did not include any twin pairs). Although there were numerous twin and family studies similar in design and outcomes, we were unable to pool heritability estimates for any outcomes for two main reasons. First, there were an insufficient number of family studies deriving results from independent samples. Second, although we were able to obtain heritability estimates from three twin studies, these estimates were uninformative since the 95% CI covered the whole range (0,1) (apart from Danis and colleagues who estimated heritability without utilizing DZ twins in its design [27]), and differences between the rMZ and rDZ were not statistically significant (Table 1).Instead, we were able to pool the rMZ for selected outcomes, giving us quantitative estimates of the upper bound of heritability. Included studies that reported more than one outcome measure were used in multiple meta-analyses.
Fig. 1.
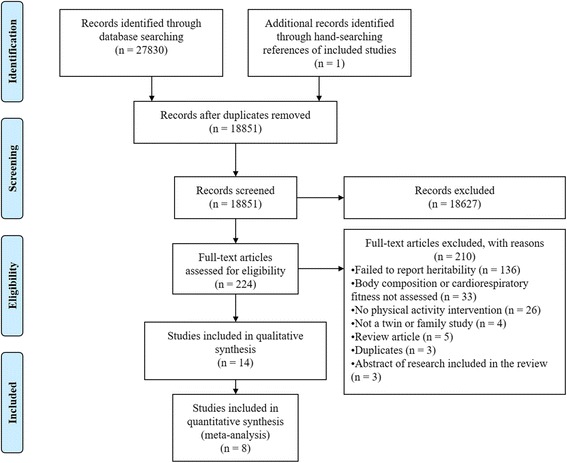
PRISMA flow diagram
Table 1.
Within MZ and DZ twin pair correlations for the response of body composition and cardiorespiratory fitness following a physical activity intervention in twin studies
| Author (year) | Sample | Age [mean (SD)] | Baseline status [mean (SD)] | Within MZ correlation (95% CI) | Within DZ correlation (95% CI) | Between MZ and DZ correlation significanced |
|---|---|---|---|---|---|---|
| Body fat percentage (%) | ||||||
| Hopkins ND (2012)a | 6 MZ (1 male and 5 female) and 6 DZ (2 male and 4 female) twin pairs | MZ: 13.5 (0.8) DZ: 13.4 (0.8) |
MZ: 27.1 (6.9) DZ: 26.0 (11.3) |
0.63 (−0.37 to 0.95) | 0.31 (−0.67 to 0.90) | p = 0.606 |
| Afman G (1988)b | 18 MZ (2 males and 16 females) and 9 DZ (3 males and 6 females) twin pairs | MZ: 19.0 (1.4) DZ: 19.4 (1.8) |
MZ: 21.3 (9.0) DZ: 19.9 (7.2) |
0.61 (0.20 to 0.84) | 0.50 (−0.25 to 0.87) | p = 0.742 |
| Danis A (2003) | 9 MZ male twin pairs | 11–14c | E: 17.8 (4.1) C: 16.8 (2.8) |
* | * | h 2 = 69%** |
| BMI | ||||||
| Hopkins ND (2012)a | 6 MZ (1 male and 5 female) and 6 DZ (2 male and 4 female) twin pairs | MZ: 13.5 (0.8) DZ: 13.4 (0.8) |
MZ: 21.5 (3.5) DZ: 21.9 (3.5) |
0.81 (0.00 to 0.98) | 0.57 (−0.45 to 0.94) | p = 0.557 |
| Afman G (1988)b | 16 MZ (3 males and 13 females) and 6 DZ (2 males and 4 females) twin pairs | MZ:18.6 (1.1) DZ: 19.3 (1.3) |
MZ: 21.9 (1.9) DZ: 22.6 (3.7) |
0.42 (−0.10 to 0.76) | 0.00 (−0.81 to 0.81) | p = 0.485 |
| Weight (kg) | ||||||
| Hopkins ND (2012)a | 6 MZ (1 male and 5 female) and 6 DZ (2 male and 4 female) twin pairs | MZ: 13.5 (0.8) DZ: 13.4 (0.8) |
MZ: 59.0 (11.5) DZ: 58.9 (12.6) |
0.89 (0.28 to 0.99) | 0.00 (−0.81 to 0.81) | p = 0.091 |
| Afman G (1988)b | 19 MZ (3 males and 16 females) and 9 DZ (3 males and 6 females) twin pairs | MZ: 18.7 (1.0) DZ: 19.4 (1.8) |
MZ: 60.4 (10.6) DZ: 67.1 (13.4) |
0.53 (0.10 to 0.79) | 0.13 (−0.58 to 0.73) | p = 0.337 |
| Fat free mass | ||||||
| Hopkins ND (2012)a | 6 MZ (1 male and 5 female) and 6 DZ (2 male and 4 female) twin pairs | MZ: 13.5 (0.8) DZ: 13.4 (0.8) |
MZ: 69.9 (6.8)% DZ: 69.9 (6.8)% |
0.52 (−0.50 to 0.94) | 0.34 (−0.65 to 0.90) | p = 0.785 |
| Afman G (1988)b | 19 MZ (3 males and 16 females) and 9 DZ (3 males and 6 females) twin pairs | MZ: 18.9 (1.4) DZ: 19.4 (1.8) |
MZ: 48.2 (8.1) kg DZ: 53.2 (13.2) kg |
0.40 (−0.07 to 0.72) | 0.18 (−0.55 to 0.75) | p = 1.000 |
| Relative VO2 max (mL.kg−1min−1) | ||||||
| Hopkins ND (2012)a | 6 MZ (1 male and 5 female) and 6 DZ (2 male and 4 female) twin pairs | MZ: 13.5 (0.8) DZ: 13.4 (0.8) |
MZ: 44.4 (8.1) DZ: 45.7 (8.1) |
0.43 (−0.59 to 0.92) | 0.21 (−0.73 to 0.87) | p = 0.763 |
| Afman G (1988)b | 19 MZ (3 males and 16 females) and 9 DZ (3 males and 6 females) twin pairs | MZ: 18.9 (1.4) DZ: 19.4 (1.8) |
MZ: 33.3 (7.3) DZ: 37.1 (8.0) |
0.44 (0.00 to 0.74) | 0.00 (−0.66 to 0.66) | p = 0.324 |
| Danis A (2003) | 9 MZ male twin pairs | 11–14c | E: 52.1 (3.6) C: 54.0 (3.9) |
* | * | h2 = 44%** |
| Absolute VO2 max (L.min−1) | ||||||
| Afman G (1988)b | 20 MZ (3 males and 16 females) and 9 DZ (3 males and 6 females) twin pairs | MZ: 18.9 (1.4) DZ: 19.4 (1.8) |
MZ: 2.0 (0.6) DZ: 2.5 (0.9) |
0.44 (0.00 to 0.74) | 0.00 (−0.66 to 0.66) | p = 0.320 |
| Danis A (2003) | 9 MZ male twin pairs | 11–14c | E: 2.1 (0.4) C: 2.1 (0.4) |
* | * | h 2 = 54%** |
MZ monozygotic, DZ dizygotic, E experimental group, C control group, SD standard deviation, CI confidence interval, h 2 heritability, VO 2 max maximal oxygen uptake, BMI body mass index
*No reported correlation due to a different method used to estimate heritability
**Unable to calculate the standard error and thus present the 95% CI
aWithin twin pair correlations (95% CI) extracted from the publication
bWithin twin pair correlations (95% CI) calculated from raw data
cDid not report a mean age (SD)
dUnable to calculate the within MZ and DZ twin pair correlations for Danis A (2003) due to methodology, so the h 2 is presented instead
The characteristics of the included twin and family studies, including sample size, age, baseline PA status, and PA intervention are described in Tables 2 and 3. The mean age [standard deviation (SD)] of participants ranged from 13 (1) to 39 (2) in twin studies, and 17 to 65 years in family studies. At study entry, participants were mostly sedentary or engaged in light PA but not highly physically trained. Only two twin studies [20, 28] analysed data from twin pairs living apart at the time of enrolment [mean age (SD) 30 (8) and 39 (2), respectively], while another reported that more than 50% of the twin pairs were living together at this time [mean age (SD) 19 (2)] [29]. Every study recruited healthy individuals from the community, except Hainer and colleagues [28] who recruited twin pairs admitted to an obesity unit for a 40-day PA and diet program. The frequency of the PA interventions ranged from three times a week to daily, with the duration ranging from 15 min to 2 h. The exercise intensity ranged from 50 to 97% VO2max, with numerous modes of PA being utilized, including a cycle ergometer, resistance training, walking or running, over a period of 22 days up to 6 months. Two twin studies [29, 30] reported drop outs based on participants failing to complete the training protocol (Table 2), while the included family studies only analyzed data from participants who completed 60 exercise sessions in 21 weeks [31] (Table 3).
Table 2.
Characteristics of twin studies
| Twin studies | |||||
|---|---|---|---|---|---|
| Author (year) | Sample* | Age [mean (SD)] | Baseline physical activity status | Physical activity intervention | Diet intervention |
| Poehlam A (1987) | 6 MZ male twin pairs | 19 (1.3) | Sedentary | F: 22 consecutive days I: 56% VO2 max T: 116 min per day T: Cycle ergometer |
Energy balance deficit of ~4.2 MJ/day |
| Koenigstorfer J (2011) | 6 MZ females twin pairs | 30 (8) | Sedentary | F: 3 times per week (aerobic) and 2 times per week (strength) for 8 weeks I: 68% (±8%) heart rate maximum (aerobic) and 70% of 12 repetition maximum (12RM) T: 45 min each T: Cycle ergometer and strength training (crunches, butterfly crunches, leg press, leg curl, and latissimus pull down) |
Individual counseling for a low fat (25%), hypocaloric diet (5.0–5.8 MJ/day) in accordance with their usual eating patterns and preferences |
| Hopkins ND (2012) | 6 MZ (1 male and 5 female) and 6 DZ (2 male and 4 female) twin pairs | MZ: 13.5 (0.8) DZ: 13.4 (0.8) |
Light and moderate physical activity | F: 3 times per week for 8 weeks I: 65–85% heart rate maximum T: 45 min T: gym-based aerobic exercise |
None |
| Bouchard C (1994) | 7 MZ male twin pairsa | 21.0 (2.7) | Sedentary | F: Twice per day every 9 of 10 days for 93 days I: 50–55%VO2 max T: 60 min T: Cycle ergometer |
Energy balance deficit of ~4.2 MJ/day |
| Hainer V (2000) |
14 MZ female twin pairs | 39 (1.7) | Sedentary | F: Daily for 28 days I: 60%VO2 max T: 20 min T: cycle ergometer aerobic exercises Additional exercise: 4 km walk and 30 min of aerobic exercise |
Hypocaloric diet of 1.6 MJ/day |
| Hamel P (1986) |
6 MZ twin pairs (3 male and 3 female) | 21.2 (3.7) | Not reported | F: 3–5 times per week for 15 weeks I: 60–85% heart rate reserve T: 30–45 min T: Cycle ergometer |
None |
| Prud’Homme D (1984) | 10 MZ twin pairs (4 male and 6 female) | 20.0 (2.9) | None highly trained but some participated in recreational activities | F: 4–5 times per week for 20 weeks I: 60–85% heart rate reserve T: 40–45 min T: Cycle ergometer |
None |
| Afman G (1988) | 19 MZ (3 male and 16 female) and 9 DZ (3 male and 6 female) twin pairsb | MZ: 18.9 (1.4) DZ: 19.4 (1.8) |
Not reported | F: 4 times per week for 11 weeks I: 70–85 heart rate maximum T: 15–45 min T: cycle ergometer and treadmill running |
None |
| Danis A (2003) | 9 MZ male twin pairs | 11–14** | Not participating in sporting activities | F: 3 times per week for 6 months I: 75–97% VO2 max T: 60–90 min T: treadmill running |
None |
MZ monozygotic, DZ dizygotic, MJ mega joules, SD standard deviation, FITT frequency, intensity, time, type
*Twin pairs were generally living together at the time of enrollment, except those in Koenigstorfer J [20] and Hainer V (2000) [28]. Afman G [29] reported that more than 50% of the twin pairs were living together at the time of enrollment
**Did not report a mean age (SD)
a11 MZ twin pairs were initially enrolled but only seven MZ twin pairs completed the exercise protocol (the definition of ‘completing the exercise protocol’ was not outlined)
b34 twin pairs (MZ and DZ) were initially enrolled but only 28 twin pairs (MZ and DZ) completed the protocol (defined as attending 75% or more of the exercise sessions, and having fewer than eight sessions where one twin participated and the co-twin did not)
Table 3.
Characteristics of family studies
| Family Studies (all studies were based on the sample from “The HERITAGE Family Study”) | |||||
|---|---|---|---|---|---|
| Author (year) | Samplea | Age | Baseline physical activity status | Physical activity intervention | Diet intervention |
| Rice T (1999) | 98 Caucasian families (440 individuals) | Parents were less than 65 years old, while offspring ranged from 17–40 years old | Sedentary | F: 3 times per week for 20 weeks I: 55–75% VO2 max T: 30–50 min T: Cycle ergometer |
None. |
| Bouchard C (1999) | 98 Caucasian families (481 individuals) | ||||
| Perusse L (2000) | 99 Caucasian families (483 individuals) | ||||
| Perusse L (2001) | 99 Caucasian families (483 individuals) | ||||
| Gaskill SE (2001) | 100 Caucasian families (339 individuals) and 99 African-American families (172 individuals) | ||||
FITT frequency, intensity, time, type, VO 2 max maximal oxygen uptake
aParticipants needed to complete 60 exercise sessions within 21 weeks to satisfy the protocol and be included in the study
Due to significant between-study variation for the intervention frequency and duration, we were unable to stratify meta-analyses in this way. Instead, we examined the correlations for each outcome to investigate if studies with more frequent bouts of PA, or longer intervention durations reported higher rMZ, but, we were unable to identify any trends. We were able to stratify our meta-analyses by the co-prescription of a diet intervention, and by gender.
Outcomes of Body Composition
There were 11 studies (nine twin studies [20, 27–30, 32–35] and two family studies [24, 36]) which investigated body composition measures and their response following a PA intervention. Pooling of eight twin studies results (excluding Danis and colleagues [27] due to different methodology) suggest there is a significant rMZ across the majority of body composition measures (Table 4). The pooled rMZ was highest for BMI (rMZ = 0.69, 95% CI: 0.49–0.82, n = 58) and the ratio of fat mass to fat free mass (rMZ = 0.69, 95% CI: 0.42–0.85, n = 36) (Fig. 2), where “n” represents the total number of twin pairs from all studies. There were significant pooled rMZ for fat mass (rMZ = 0.58, 95% CI: 0.13–0.83, n = 48), fat free mass (rMZ = 0.57, 95% CI: 0.35–0.73, n = 73) (Fig. 3), body fat percentage (rMZ = 0.55, 95% CI: 0.32–0.72, n = 72), waist circumference (rMZ = 0.50, 95% CI: 0.09–0.77, n = 27) and hip circumference (rMZ = 0.51, 95% CI: 0.11–0.77, n = 27) (Fig. 4). However, the pooled rMZ was lower and not statistically significantly different from 0 for waist-to-hip ratio (rMZ = 0.29, 95% CI: −0.16–0.64, n = 27) (Fig. 5).
Table 4.
Pooled within monozygotic (MZ) twin pair correlations (95% confidence intervals)
| Outcome | All studies | Studies including a combined physical activity and diet intervention | Studies only including a physical activity intervention |
|---|---|---|---|
| Body fat percentage (%) | 0.55 (0.32–0.72)*** (n = 72) Poehlam A et al. (1987) Koenigstorfer J et al. (2011) Hopkins N et al. (2012) Bouchard C et al. (1994) Hainer V et al. (2000) Hamel P et al. (1986) Prud’Homme D et al. (1984) Afman G et al. (1988) |
0.61 (0.28–0.82)** (n = 33) Poehlam A et al. (1987) Koenigstorfer J et al. (2011) Bouchard C et al. (1994) Hainer V et al. (2000) |
0.49 (0.16–0.73)** (n = 39) Hopkins N et al. (2012) Hamel P et al. (1986) Prud’Homme D et al. (1984) Afman G et al. (1988) |
| BMI | 0.69 (0.49–0.82)*** (n = 58) Koenigstorfer J et al. (2011) Hopkins N et al. (2012) Bouchard C et al. (1994) Hainer V et al. (2000) Prud’Homme D et al. (1984) Afman G et al. (1988) |
0.79 (0.54–0.91)*** (n = 27) Koenigstorfer J et al. (2011) Bouchard C et al. (1994) Hainer V et al. (2000) |
0.58 (0.23–0.79)** (n = 31) Hopkins N et al. (2012) Prud’Homme D et al. (1984) Afman G et al. (1988) |
| Fat free mass (kg) | 0.57 (0.35–0.73)*** (n = 73) Poehlam A et al. (1987) Koenigstorfer J et al. (2011) Hopkins N et al. (2012) Bouchard C et al. (1994) Hainer V et al. (2000) Hamel P et al. (1986) Prud’Homme D et al. (1984) Afman G et al. (1988) |
0.71 (0.43–0.87)*** (n = 33) Poehlam A et al. (1987) Koenigstorfer J et al. (2011) Bouchard C et al. (1994) Hainer V et al. (2000) |
0.43 (0.09–0.68)* (n = 40) Hopkins N et al. (2012) Hamel P et al. (1986) Prud’Homme D et al. (1984) Afman G et al. (1988) |
| Fat mass (kg) | 0.58 (0.13–0.83)* (n = 48) Poehlam A et al. (1987) Koenigstorfer J et al. (2011) Bouchard C et al. (1994) Hainer V et al. (2000) Hamel P et al. (1986) Prud’Homme D et al. (1984) |
0.68 (0.16–0.90)* (n = 33) Poehlam A et al. (1987) Koenigstorfer J et al. (2011) Bouchard C et al. (1994) Hainer V et al. (2000) |
0.27 (−0.36–0.73) (n = 15) Hamel P et al. (1986) Prud’Homme D et al. (1984) |
| Fat mass to fat free mass ratio | 0.69 (0.42–0.85)*** (n = 36) Bouchard C et al. (1994) Hainer V et al. (2000) Hamel P et al. (1986) Prud’Homme D et al. (1984) |
0.82 (0.58–0.93)*** (n = 21) Bouchard C et al. (1994) Hainer V et al. (2000) |
0.30 (−0.33–0.75) (n = 15) Hamel P et al. (1986) Prud’Homme D et al. (1984) |
| Waist circumference (cm) | 0.50 (0.09–0.77)* (n = 27) Koenigstorfer J et al. (2011) Bouchard C et al. (1994) Hainer V et al. (2000) |
– | |
| Hip circumference (cm) | 0.51 (0.11–0.77)* (n = 27) Koenigstorfer J et al. (2011) Bouchard C et al. (1994) Hainer V et al. (2000) |
– | |
| Waist to hip ratio | 0.29 (−0.16–0.64) (n = 27) Koenigstorfer J et al. (2011) Bouchard C et al. (1994) Hainer V et al. (2000) |
– | |
| Sum of skin folds (cm) | 0.67 (0.37–0.85)*** (n = 30) Bouchard C et al. (1994) Hainer V et al. (2000) Prud’Homme D et al. (1984) |
0.73 (0.39–0.89)*** (n = 21) Bouchard C et al. (1994) Hainer V et al. (2000) |
0.49 (−0.26–0.87) (n = 9) Prud’Homme D et al. (1984) |
| Trunk fat | 0.52 (0.12–0.78)* (n = 27) Hopkins N et al. (2012) Bouchard C et al. (1994) Hainer V et al. (2000) |
0.56 (0.13–0.82)* (n = 21) Bouchard C et al. (1994) Hainer V et al. (2000) |
0.30 (−0.68–0.89) (n = 6) Hopkins N et al. (2012) |
| Extremity skin fold (cm) | 0.54 (−0.39–0.92) (n = 21) Bouchard C et al. (1994) Hainer V et al. (2000) |
– | |
| Trunk to extremity ratio | 0.48 (−0.30–0.88) (n = 21) Bouchard C et al. (1994) Hainer V et al. (2000) |
– | |
| Weight (kg) | 0.67 (0.48–0.79)*** (n = 73) Poehlam A et al. (1987) Koenigstorfer J et al. (2011) Hopkins N et al. (2012) Bouchard C et al. (1994) Hainer V et al. (2000) Hamel P et al. (1986) Prud’Homme D et al. (1984) Afman G et al. (1988) |
0.73 (0.47–0.88)*** (n = 33) Poehlam A et al. (1987) Koenigstorfer J et al. (2011) Bouchard C et al. (1994) Hainer V et al. (2000) |
0.61 (0.32–0.79)*** (n = 40) Hopkins N et al. (2012) Hamel P et al. (1986) Prud’Homme D et al. (1984) Afman G et al. (1988) |
| Absolute VO2 max (L.min−1) | 0.38 (0.04–0.64)* (n = 42) Bouchard C et al. (1994) Hamel P et al. (1986) Prud’Homme D et al. (1984) Afman G et al. (1988) |
0.52 (−0.38–0.92) (n = 7) Bouchard C et al. (1994) |
0.36 (−0.01–0.64) (n = 35) Hamel P et al. (1986) Prud’Homme D et al. (1984) Afman G et al. (1988) |
| Relative VO2 max (mL.min−1.kg−1) | 0.39 (0.07–0.64)* (n = 48) Hopkins N et al. (2012) Bouchard C et al. (1994) Hamel P et al. (1986) Prud’Homme D et al. (1984) Afman G et al. (1988) |
0.48 (−0.43–0.91) (n = 7) Bouchard C et al. (1994) |
0.38 (0.04–0.64)* (n = 41) Hopkins N et al. (2012) Hamel P et al. (1986) Prud’Homme D et al. (1984) Afman G et al. (1988) |
n number of twin pairs, VO 2 max maximal oxygen uptake, BMI body mass index
*p < 0.05; **p < 0.01; ***p < 0.001
Fig. 2.
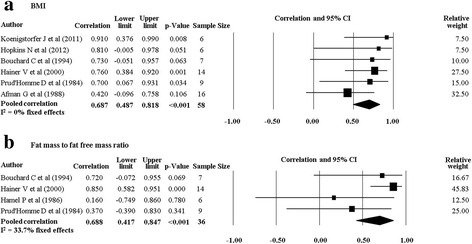
Pooled within monozygotic (MZ) twin pair correlations for BMI and the ratio of fat mass to fat free mass in response to physical activity. CI: confidence interval; sample size; number of twin pairs
Fig. 3.
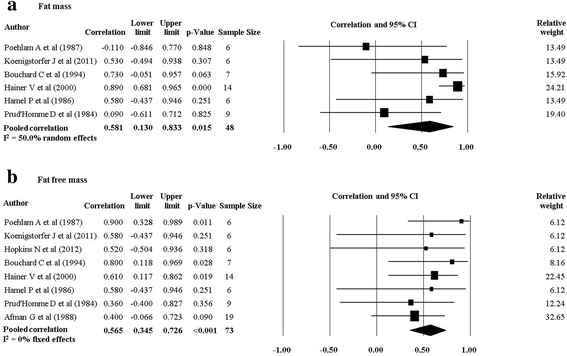
Pooled within monozygotic (MZ) twin pair correlations for fat mass and fat free mass in response to physical activity. CI: confidence interval; sample size; number of twin pairs
Fig. 4.
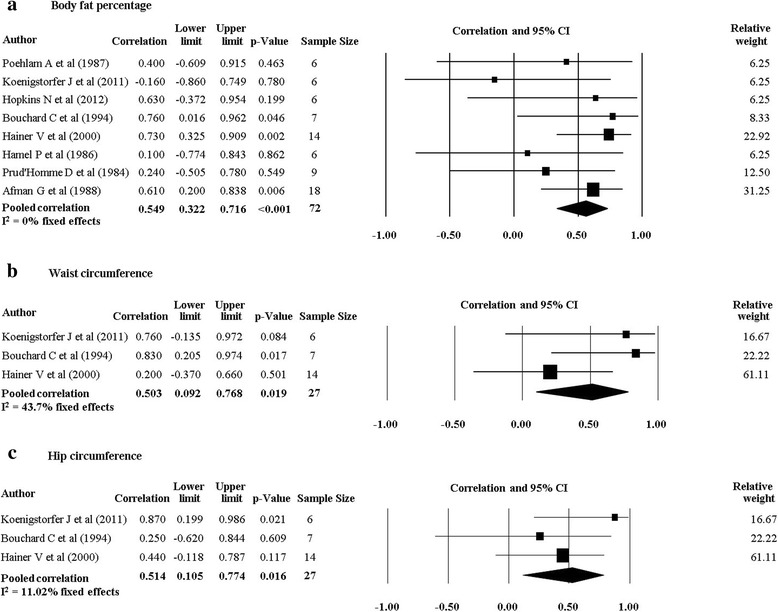
Pooled within monozygotic (MZ) twin pair correlations for body fat percentage, waist circumference and hip circumference in response to physical activity. CI: confidence interval; sample size; number of twin pairs
Fig. 5.

Pooled within monozygotic (MZ) twin pair correlations for waist-to-hip ratio in response to physical activity. CI: confidence interval; sample size; number of twin pairs
When we pooled data from twin studies that included a combined PA and diet intervention (four studies [20, 28, 30, 33], there was a trend for the rMZ to be higher across all measures of body composition compared to twin studies that only involved a PA intervention (four studies [29, 32, 34, 35]) (Table 4). The rMZ for BMI was higher when results were pooled for studies including a combined PA and diet intervention (rMZ = 0.79, 95% CI: 0.54–0.91, n = 27), compared to studies only involving a PA intervention (rMZ = 0.58, 95% CI: 0.23–0.79, n = 31) (Fig. 6), although confidence intervals were wide. Meta-analyses for each outcome were stratified by gender. The rMZ was variable between males and females, depending on the outcome assessed (Table 5), with wide confidence intervals observed for both males and females. The pooled rMZ for the response of fat mass following PA was higher and statistically significant in females (rMZ = 0.85, 95% CI: 0.63–0.94, n = 25) compared to males (rMZ = 0.40, 95% CI: −0.26–0.81, n = 17) (Fig. 7). However, the pooled rMZ for fat free mass was higher in males (rMZ = 0.80, 95% CI: 0.39–0.95, n = 17) compared to females (rMZ = 0.52, 95% CI: 0.19–0.75, n = 38) (Fig. 8), both being statistically significantly different from 0 but not from each other.
Fig. 6.
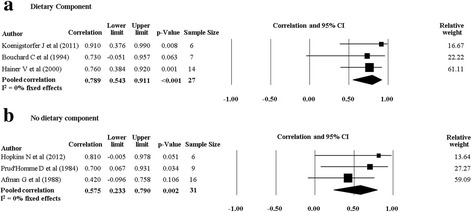
Pooled within monozygotic (MZ) twin pair correlations for BMI in response to physical activity combined with diet, and physical activity without a dietary component. CI: confidence interval; sample size; number of twin pairs
Table 5.
Pooled within monozygotic (MZ) twin pair correlations (95% confidence intervals)
| Outcome | All studies | Females | Males |
|---|---|---|---|
| Body fat percentage (%) | 0.55 (0.32–0.72)*** (n = 72) Poehlam A et al. (1987) Koenigstorfer J et al. (2011) Hopkins N et al. (2012) Bouchard C et al. (1994) Hainer V et al. (2000) Hamel P et al. (1986) Prud’Homme D et al. (1984) Afman G et al. (1988) |
0.63 (0.36–0.80)*** (n = 41) Koenigstorfer J et al. (2011) Hainer V et al. (2000) Prud’Homme D et al. (1984) Afman G et al. (1988) |
0.58 (−0.04–0.87) (n = 17) Poehlam A et al. (1987) Bouchard C et al. (1994) Prud’Homme D et al. (1984) |
| BMI | 0.69 (0.49–0.82)*** (n = 58) Koenigstorfer J et al. (2011) Hopkins N et al. (2012) Bouchard C et al. (1994) Hainer V et al. (2000) Prud’Homme D et al. (1984) Afman G et al. (1988) |
0.63 (0.36–0.80)*** (n = 41) Koenigstorfer J et al. (2011) Hainer V et al. (2000) Prud’Homme D et al. (1984) Afman G et al. (1988) |
0.63 (−0.13–0.93) (n = 11) Bouchard C et al. (1994) Prud’Homme D et al. (1984) |
| Fat free mass (kg) | 0.57 (0.35–0.73)*** (n = 73) Poehlam A et al. (1987) Koenigstorfer J et al. (2011) Hopkins N et al. (2012) Bouchard C et al. (1994) Hainer V et al. (2000) Hamel P et al. (1986) Prud’Homme D et al. (1984) Afman G et al. (1988) |
0.52 (0.19–0.75)** (n = 38) Koenigstorfer J et al. (2011) Hainer V et al. (2000) Prud’Homme D et al. (1984) Afman G et al. (1988) |
0.80 (0.39–0.95)** (n = 17) Poehlam A et al. (1987) Bouchard C et al. (1994) Prud’Homme D et al. (1984) |
| Fat mass (kg) | 0.58 (0.13–0.83)* (n = 48) Poehlam A et al. (1987) Koenigstorfer J et al. (2011) Bouchard C et al. (1994) Hainer V et al. (2000) Hamel P et al. (1986) Prud’Homme D et al. (1984) |
0.85 (0.63–0.94)*** (n = 25) Koenigstorfer J et al. (2011) Hainer V et al. (2000) Prud’Homme D et al. (1984) |
0.40 (−0.26–0.81) (n = 17) Poehlam A et al. (1987) Bouchard C et al. (1994) Prud’Homme D et al. (1984) |
| Fat mass to fat free mass ratio | 0.69 (0.42–0.85)*** (n = 36) Bouchard C et al. (1994) Hainer V et al. (2000) Hamel P et al. (1986) Prud’Homme D et al. (1984) |
0.85 (0.61–0.95)*** (n = 19) Hainer V et al. (2000) Prud’Homme D et al. (1984) |
0.62 (−0.15–0.92) (n = 11) Bouchard C et al. (1994) Prud’Homme D et al. (1984) |
| Waist circumference (cm) | 0.50 (0.09–0.77)* (n = 27) Koenigstorfer J et al. (2011) Bouchard C et al. (1994) Hainer V et al. (2000) |
0.36 (−0.15–0.72) (n = 20) Koenigstorfer J et al. (2011) Hainer V et al. (2000) |
0.83 (0.21–0.97)* (n = 7) Bouchard C et al. (1994) |
| Hip circumference (cm) | 0.51 (0.11–0.77)* (n = 27) Koenigstorfer J et al. (2011) Bouchard C et al. (1994) Hainer V et al. (2000) |
0.58 (0.13–0.83)* (n = 20) Koenigstorfer J et al. (2011) Hainer V et al. (2000) |
0.25 (−0.62–0.84) (n = 7) Bouchard C et al. (1994) |
| Waist to hip ratio | 0.29 (−0.16–0.64) (n = 27) Koenigstorfer J et al. (2011) Bouchard C et al. (1994) Hainer V et al. (2000) |
0.27 (−0.24–0.66) (n = 20) Koenigstorfer J et al. (2011) Hainer V et al. (2000) |
0.35 (−0.55–0.87) (n = 7) Bouchard C et al. (1994) |
| Sum of skin folds (cm) | 0.67 (0.37–0.85)*** (n = 30) Bouchard C et al. (1994) Hainer V et al. (2000) Prud’Homme D et al. (1984) |
0.78 (0.46–0.92)*** (n = 19) Hainer V et al. (2000) Prud’Homme D et al. (1984) |
0.51 (−0.30–0.89) (n = 11) Bouchard C et al. (1994) Prud’Homme D et al. (1984) |
| Trunk fat | 0.52 (0.12–0.78)* (n = 27) Hopkins N et al. (2012) Bouchard C et al. (1994) Hainer V et al. (2000) |
0.67 (0.22–0.89)** (n = 14) Hainer V et al. (2000) |
0.15 (−0.68–0.81) (n = 7) Bouchard C et al. (1994) |
| Extremity skin fold (cm) | 0.54 (−0.39–0.92) (n = 21) Bouchard C et al. (1994) Hainer V et al. (2000) |
0.78 (0.43–0.93)** (n = 14) Hainer V et al. (2000) |
0.00 (−0.75–0.75) (n = 7) Bouchard C et al. (1994) |
| Trunk to extremity ratio | 0.48 (−0.30–0.88) (n = 21) Bouchard C et al. (1994) Hainer V et al. (2000) |
0.70 (0.27–0.90)** (n = 14) Hainer V et al. (2000) |
0.00 (−0.75–0.75) (n = 7) Bouchard C et al. (1994) |
| Weight (kg) | 0.67 (0.48–0.79)*** (n = 73) Poehlam A et al. (1987) Koenigstorfer J et al. (2011) Hopkins N et al. (2012) Bouchard C et al. (1994) Hainer V et al. (2000) Hamel P et al. (1986) Prud’Homme D et al. (1984) Afman G et al. (1988) |
0.70 (0.46–0.84)*** (n = 41) Koenigstorfer J et al. (2011) Hainer V et al. (2000) Prud’Homme D et al. (1984) Afman G et al. (1988) |
0.45 (−0.20–0.83) (n = 17) Poehlam A et al. (1987) Bouchard C et al. (1994) Prud’Homme D et al. (1984) |
| Absolute VO2 max (L.min−1) | 0.38 (0.04–0.64)* (n = 42) Bouchard C et al. (1994) Hamel P et al. (1986) Prud’Homme D et al. (1984) Afman G et al. (1988) |
0.74 (−0.18–0.97) (n = 21) Prud’Homme D et al. (1984) Afman G et al. (1988) |
0.49 (−0.33–0.89) (n = 11) Bouchard C et al. (1994) Prud’Homme D et al. (1984) |
| Relative VO2 max (mL.min−1.kg−1) | 0.39 (0.07–0.64)* (n = 48) Hopkins N et al. (2012) Bouchard C et al. (1994) Hamel P et al. (1986) Prud’Homme D et al. (1984) Afman G et al. (1988) |
0.51 (0.06–0.79)* (n = 21) Prud’Homme D et al. (1984) Afman G et al. (1988) |
0.40 (−0.43–0.86) (n = 11) Bouchard C et al. (1994) Prud’Homme D et al. (1984) |
n number of twin pairs, VO 2 max maximal oxygen uptake, BMI body mass index
*p < 0.05; **p < 0.01; ***p < 0.001
Fig. 7.
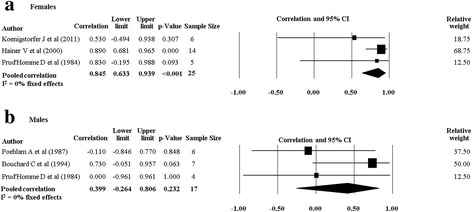
Pooled within monozygotic (MZ) twin pair correlations for fat mass in response to physical activity for females and males. CI: confidence interval; sample size; number of twin pairs
Fig. 8.
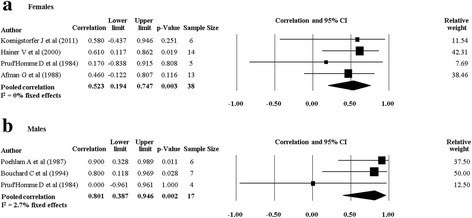
Pooled within monozygotic (MZ) twin pair correlations for fat free mass in response to physical activity for females and males. CI: confidence interval; sample size; number of twin pairs
We were able to extract heritability, and maximal heritability estimates for measures of body composition from three twin studies [27, 29, 32] (with raw data used to generate heritability estimates from one [29]), and two family studies, respectively [24, 36]. However, we did not report the heritability estimates from two twin studies [29, 32], as there were no statistically significant differences between the rMZ and rDZ, making the estimates uninformative (Table 1). Danis and colleagues [27] used different methodology to calculate heritability and we reported the estimates in Table 1. Maximal heritability estimates ranged from 0–21% in family studies, with higher estimates for trunk and extremity skin folds compared to measures of fat mass and waist circumference (Table 6).
Table 6.
Maximal heritability estimates from family studies (includes variance explained by genetic and non-genetic sources shared within families)
| Outcome | Author (year) | Maximal heritability (95% CI) |
|---|---|---|
| Fat mass (kg) | Rice T (1999) | 0%a |
| Trunk skin folds (cm) | Perusse L (2000) | 21% (14 to 28%) |
| Extremity skin folds (cm) | Perusse L (2000) | 15% (5 to 25%) |
| Subcutaneous fat (sum of eight skin folds) (cm) | Perusse L (2000) | 15% (8 to 22%) |
| Trunk to extremity skin fold ratio (adjusted for subcutaneous fat) | Perusse L (2000) | 14% (10 to 18%) |
| Waist circumference (cm) (adjusted for BMI) | Perusse L (2000) | 0%a |
| Absolute VO2 max (L.min−1) | Bouchard C (1999) | 47%a |
| Absolute VO2 max at ventilatory threshold (L.min−1) | Gaskill SE (2001) | Caucasian: 22% (−2 to 46%) African-American: 51 (27% to 75%) |
| Relative VO2 max (mL.min−1.kg−1) | Perusse L (2001) | 50 W: 57%a |
| 60% VO2 max: 23%a | ||
| 80% VO2 max: 44%a |
CI confidence interval, VO 2 max maximal oxygen uptake, W watts, BMI body mass index
aUnable to calculate the standard error and thus present the 95% CI
Outcomes of Cardiorespiratory Fitness
There were nine studies (six twin studies [27, 29, 30, 32, 34, 35] and three family studies [19, 37, 38]) which investigated cardiorespiratory fitness measures and their response following a PA intervention. Pooling of five twin studies results (excluding Danis and colleagues [27] due to different methodology) suggests there are significant pooled rMZ for absolute VO2max (L.min−1) (rMZ = 0.38, 95% CI: 0.04–0.64, n = 42) and relative VO2max (mL.min−1.kg−1) (rMZ = 0.39, 95% CI: 0.07–0.64, n = 48) (Table 4).
There was one twin study which investigated the response of cardiorespiratory fitness following a combined PA and diet intervention [30] and four twin studies which investigated the response of cardiorespiratory fitness following an isolated PA intervention [29, 32, 34, 35]. The rMZ for absolute and relative VO2max in the study (n = 7) which combined PA with diet (rMZ = 0.52, 95% CI: −0.38–0.92, and rMZ = 0.48, 95% CI: −0.43–0.91, respectively) was higher than the pooled rMZ from the studies which only investigated a PA intervention (rMZ = 0.36, 95% CI: −0.01–0.64, n = 35, and rMZ = 0.38, 95% CI: 0.04–0.64, n = 41, respectively) (Fig. 9) although the confidence intervals overlapped, and the rMZ from the individual study was not statistically significantly different from 0 (with 95% CIs generated from the meta-analysis software). Meta-analyses for absolute and relative VO2max were stratified by gender, with the pooled rMZ being higher in females (Table 5). The pooled rMZ for the response of absolute VO2max following PA was 0.74 in females (n = 21) and 0.49 in males (n = 11), although neither were statistically significantly different from 0 (Fig. 10).
Fig. 9.
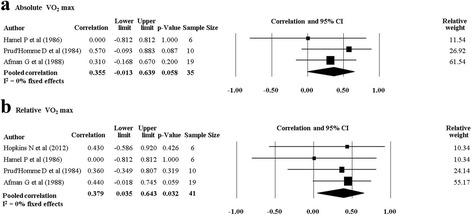
Pooled within monozygotic (MZ) twin pair correlations for absolute and relative maximal oxygen uptake (VO2 max) in response to physical activity without a dietary component. CI: confidence interval; sample size; number of twin pairs
Fig. 10.
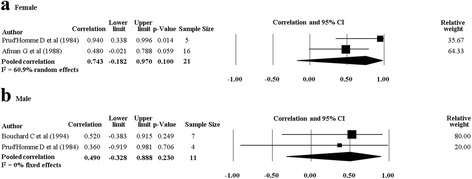
Pooled within monozygotic (MZ) twin pair correlations for absolute maximal oxygen uptake (VO2 max) in response to physical activity for females and males. CI: confidence interval; sample size; number of twin pairs
Heritability estimates for the response of VO2max from two twin studies [29, 32] were not reported, as there were no statistically significant differences between the rMZ and rDZ (Table 1). Danis and colleagues [27] used different methodology to calculate heritability and we reported the estimates in Table 1 .Maximal heritability estimates from the three included family studies [19, 37, 38] were variable, ranging from 22–57% depending on race and when VO2max was measured (e.g., ventilatory threshold, pre-determined power levels, etc.) (Table 6).
Discussion
Our results demonstrate consistent evidence that shared familial factors (whether genetic or environmental) play a role in the response of body composition and cardiorespiratory fitness following PA, despite varying on the outcome being assessed, particularly when results were stratified by gender. The pooled rMZ were generally >0.5, and the bulk of most CIs also exceeded 0.5. Shared familial factors appear to play a larger role in the response of body composition when compared to cardiorespiratory fitness, and may have more influence on the response for most outcomes when considering a combined PA and diet intervention.
Heritability Estimates and the Within MZ Twin Pair Correlation
Only a few studies included DZ twins (n = 2) [29, 32], so we pooled the rMZ to provide an estimate of the upper bound of heritability. Traditionally, twin and family studies investigating the heritability of a phenotype (e.g., PA engagement [15, 16], BMI [39], and chronic pain [40]) have done so using a cross-sectional design, with twin studies dividing the variance of a phenotype into components or proportions due to additive genetic factors (heritability), shared environmental factors, and unique environmental factors. Our pooled estimates represent the upper bound of heritability, including variance from additive genetic and shared environmental factors. However, our study investigated how shared familial factors influence the response to PA, with the rMZ derived from the change in outcome status following an intervention. Since interventions were implemented over a specified timeframe, with training parameters controlled, it has been suggested that unique and shared environmental factors would make minor contributions to the variance of the response to PA [22], resulting in a rMZ that would give a close estimate of heritability. However, family studies included in this review found significant correlations between spouses for the response of body composition [38] and cardiorespiratory fitness [19, 24] following a PA intervention. Although some suggest this indicates a greater influence of shared environmental factors [19], this correlation may equally be due to shared genes (assortative mating), so without making strong assumptions as to which is occurring in spouses, this is unlikely to indicate a greater influence of shared environmental factors.
Shared Familial Influence on Changes of Body Composition
Factors shared within MZ twin pairs appear to play a strong role in the response of BMI (pooled rMZ = 0.69) following a PA intervention (Fig. 2), although they appear to be less influential in the response of other outcomes (e.g., waist-to-hip ratio) (Fig. 5). Although we were unable to pool heritability estimates, our pooled rMZ for the response of BMI following PA appears to be within the range of previous studies reporting the cross-sectional heritability of BMI (ranging from 47–90% in twins studies [39]). However, other cross-sectional studies have reported heritability estimates for waist circumference (66%) and body fat percentage (68%) [41] that appear to be slightly higher than our rMZ in response to exercise (pooled rMZ = 0.50 and 0.55, respectively), especially considering our results represent the upper bound of heritability. Therefore, by comparing our results to those of previous investigations, it appears the genetic influences on an individual’s body composition (cross-sectional association) might be different, and perhaps higher, than the way their body composition responds to PA.
Previous cross-sectional twin studies have reported gender differences for the heritability of body composition, although they appear to vary depending on the outcome of interest. A twin study by Schousboe and colleagues [42] reported that males have higher heritability estimates compared to females for body fat percentage (63 and 59%, respectively), sum of skin folds (65 and 61%, respectively), waist circumference (61 and 48%, respectively) and waist-to-hip ratio (22 and 10%, respectively). However, other studies have reported higher heritability estimates in females across a variety of body composition measures [43, 44]. The variability between genders for the heritability of body composition has been supported in various twin studies, regardless of the sample size, methods of analyses or ethnicity [43, 45–47]. Our results extend the understanding that gender influences the role shared familial factors, including genes, play in the variation of body composition (cross-sectional association), and suggests gender influences how shared familial factors influence the response of body composition measures following PA. In particular, shared familial factors appear to have a greater influence on changes in fat mass for females engaged in PA (Fig. 7) and fat free mass for males engaged in PA (Fig. 8). Therefore, to better understand how both genetics and shared environmental factors impact an individual’s response to PA, it may be important to take into consideration the gender of the individual, and the outcome of interest.
Shared Familial Influence on Changes in Cardiorespiratory Fitness
The heritability of VO2max assessed in cross-sectional studies ranges from 40–71% in twin studies [41, 48] and has been reported at 50% (maximal heritability) in the HERITAGE Family Study [49]. Our pooled rMZ were 0.38 and 0.39 for absolute and relative VO2max, respectively, and appear to be smaller than heritability estimates for an individual’s pre-training VO2max, although the CIs for our results include the cross-sectional estimates. This suggests genetics may be more influential in determining an individual’s cardiorespiratory fitness, compared to their fitness response following PA, although the biological explanation for this is unclear.
The point estimates of the rMZ for the response of cardiorespiratory fitness following PA appear slightly greater in females (Fig. 10), although the CIs for both the male and female correlations cover almost all the possible range of values due to small sample sizes in the original studies. Similarly, existing studies investigating the heritability of cardiorespiratory fitness have been limited in their ability to analyze the effect of gender due to small sample sizes [41], and single gender cohorts [48]. Therefore, our results should be viewed as preliminary with this area deserving attention in future studies.
Strengths and Limitations
Our study demonstrated considerable strengths in its design. First, previous studies have predominantly focussed on investigating the heritability of PA engagement (cross-sectional association) [15], without considering how genetics and shared environmental factors impact an individual’s response to PA. From a health-care perspective, it may be more important to investigate how genetics and environmental factors influence the response to PA. It is likely the response to PA would be more dependent on unique environmental factors, such as training parameters (frequency, intensity, duration, type), adherence, therapeutic alliance, and many more. However, neither training frequency nor duration appeared to influence the rMZ for either body composition or cardiorespiratory fitness, which may suggest the role genetics plays in response to PA is independent of these parameters. Quantifying the influence of genetics and environmental factors on the response to PA may serve to explain why certain individuals do not respond as well to a structured PA program across a variety of outcomes, with implications for how we can modify the training environment to achieve a positive response. Second, twin studies which have investigated how genetics influence the response to PA have been limited in their ability to draw firm conclusions due to small sample sizes. Small sample sizes of the included studies explain cases where our pooled CIs were wide, even though we were able to pool results for up to 83 MZ twin pairs, improving the precision around these estimates. To obtain 95% CIs of sufficiently small width to be informative (e.g., a total width of 0.1), in studies that include only MZ twins, approximately 400 twin pairs are required if the correlation is moderately high (0.7), and greater than 1000 twin pairs if the correlation is 0.4. For studies including both MZ and DZ twins, 150 twin pairs of each zygosity would be required to detect a significant difference (p = 0.05) between rMZ = 0.7 and rDZ = 0.5, with 80% power. If both correlations are lower (e.g., rMZ = 0.5 and rDZ = 0.3), 275 twins pairs of each zygosity would be required. Many of the studies which reported heritability or maximal heritability also failed to report confidence intervals for their estimates, or provide sufficient information to enable these to be estimated accurately (Tables 1 and 6). Although point estimates are available, there is clearly a substantial information difference between a heritability of 47% with a 95% CI of 44–50% and the same heritability with a 95% CI of 10–85%, and we expect that studies included in this review are more like to the second situation, limiting the utility of the reported estimates. Third, raw data were used to re-analyse previously reported correlations in four twin studies [29, 30, 34, 35] and adjust for age, gender (if applicable), and baseline values. This provided a more precise estimate for quantifying the role genetics plays in the response to PA.
Our study has a few limitations which need to be considered when interpreting the results. First, samples from included twin studies differed in their age, baseline values, PA interventions, and diet interventions. Furthermore, one study recruited twin pairs admitted to an obesity unit for a 40-day physical activity and diet program [28], a sample not representative of the general population. However, we conducted a number of sensitivity analyses and the exclusion of any single study did not significantly affect the results for any of the outcomes (Additional file 2). In addition, we performed separate meta-analyses for studies which included a diet intervention, to better understand how the difference between interventions impacted our results. Second, two twin studies [29, 30] reported drop outs on the basis of twin pairs failing to complete the training protocol (Table 2), while the family studies only analyzed data from participants who completed the training protocol (Table 3). We acknowledge that this may limit the generalizability of the results, as participants who completed the training protocol are likely to be more motivated to engage in PA than the general population. Third, although using a classical twin design to estimate heritability is a widely reported method to investigate how genetics contributes to the variation of a phenotype, it does have some limitations, and together with the fact that individual twin studies had small sample sizes, is the reason we did not focus our results on these estimates. The use of self-reported zygosity measures, based on the difficulty of being told apart by parents, is often criticized. MZ twins who differ in their height and weight can be mistakenly classified as DZ twins when using self-reported measures, resulting in an underestimation of heritability [50]. However, only one study included in this review assessed zygosity using only a self-reported questionnaire [32], with another failing to describe how zygosity was assessed [20]. The remaining twin studies (n = 7) verified questionnaire-based zygosity through DNA mapping. In addition, not considering the genotype-environment interaction is a limitation of the classical twin design, since genetic factors can influence an individual’s choice/exposure to the environment. However, studies included in this review utilized a controlled training environment, reducing the likelihood that an individual’s genetics would impact their environment for the experimental period. Furthermore, the use of heritability as a measure, although widely reported, has some limitations; it is dependent on the modeling of the mean, on the amount of variance and measurement error (which may be larger in studies of changes in outcomes compared with cross-sectional studies of outcomes [51]) and on the total variation within a population, which may differ between populations and between the same population measured at different times [52]. Finally, when estimated from classic twin studies, this estimate depends on the assumption that environments are shared to the same extent by MZ and DZ pairs—an assumption that is rarely considered or tested in practice [53].
Clinical Implications
The results of this current investigation are consistent with a substantial influence of genes on the response of body composition and cardiorespiratory fitness following PA. These results have implications for conditions which utilize PA as a management strategy, for example, diabetes, and low back pain. If an individual’s response to a PA intervention is partially dictated by genetic factors this could potentially explain why some individuals fail to respond to increased PA. This has implications for changing the modifiable training environment to achieve a desired effect (e.g., increased intensity, frequency, or duration), or excluding people who demonstrate a poor response to reduce treatment costs and consumer disappointment. Furthermore, if genetic factors are involved in the poor response to PA as an intervention, this has implications for the selection of alternative management strategies, or a modification to the outcome investigated, since individuals who show a low training response to one parameter (e.g., VO2max) might in fact respond positively to another (e.g., BMI).
Research linking genetic markers to a specific phenotype (quantitative trait locus analysis) have aided the mechanistic understanding of how genetics influence the response of body composition and cardiorespiratory fitness following PA, although more genetic research needs to be done. A family study investigated over 300,000 single-nucleotide polymorphisms (SNPs) and identified 21 SNPs which accounted for 49% of the variance in the response of VO2max following a PA intervention, with one SNP (rs6552828) accounting for ~6% of the variance [54]. The variance explained by these 21 SNPs is similar to the maximal heritability of VO2max response from the family study included in this review (47%), although this study observed significant spouse correlations which some consider consistent with shared environmental effects, thereby reducing the variance explained by genetics [19]. Similarly, nine SNPs were found to explain 20% of the variance of submaximal heart rate in response to PA, with one SNP (rs2253206) accounting for ~5% of the variance [55]. Earlier studies have identified candidate genes that are strongly linked to or associated with the response of BMI, fat mass, fat-free mass, and body fat percentage following a PA intervention [56]. For example, the insulin-like growth factor-1 (IGF-1) gene marker was strongly linked to response of fat-free mass following PA [57], with linkage also present for a polymorphism in the S100A gene [56] (predominantly found in slow-twitch skeletal and cardiac muscle fibers [58]). Research identifying genetic markers is promising and may aid the prediction of how an individual’s body composition and cardiorespiratory fitness will respond following PA, although it is essential these results are replicated in larger samples, and through a variety of genetic analyses before definite conclusions are reached [59, 60]. Furthermore, research investigating practical and cost-effective methods to identify those who will respond positively to a PA intervention would be of significant interest from a public health and clinical perspective. For example, information regarding how family members have previously responded to PA may help to predict how an individual will respond to a similar intervention, potentially reducing the need for costly genetic testing.
Conclusions
Shared familial factors, including genetics, are likely to be significant contributors to the response of several markers of body composition and cardiorespiratory fitness following PA. Shared familial factors may play a stronger role in the response of body composition when compared to cardiorespiratory fitness, and may be more influential in dictating the response for measures of BMI, fat mass, and body fat percentage, compared to waist-to-hip ratio. The influence shared familial factors have on the response to PA may be different in males and females, with such factors having a greater influence on changes in fat mass for females, and fat-free mass for males. In addition, shared familial factors appear to be more influential in dictating the response of body composition and cardiorespiratory fitness when PA is combined with diet.
These results have implications for the management of conditions which advocate increased levels of PA, since genetic factors might serve as an explanation for why some people respond more effectively than others in specific measures of PA. To further quantify the role genetics and environmental factors play in the response to PA future research should focus on adequately powered studies including both MZ and DZ twins, and the replication of existing genome-wide association studies to identify important genetic markers for the response to PA.
Acknowledgments
Funding
There was no funding for this study. KJS is funded in part by a Centre of Research Excellence Grant from the National Health and Medical Research Council of Australia.
Authors’ Contributions
All authors critically revised the manuscript for important intellectual content and approved the final manuscript. Please find below a detailed description of the role of each author. JRZ contributed to the conception and design, acquisition, and assembly of data, analysis and interpretation of data, drafting and revision of the manuscript and final approval of the version to be published. DHS contributed to the conception and design, interpretation of data and results, drafting and revision of the manuscript, and final approval of the version to be published. TBA contributed to the conception and design, acquisition and assembly of data, drafting and revision of the manuscript, and final approval of the version to be published. KS contributed to the conception and design, acquisition and assembly of data, revision of the manuscript, and final approval of the version to be published. AB contributed to the conception and design, interpretation of data, drafting and revision of the manuscript, and final approval of the version to be published. PHF contributed to the conception and design, analysis and interpretation of data, drafting and revision of the manuscript, and final approval of the version to be published. All authors read and approved the final manuscript.
Competing Interests
Zadro JR, Shirley D, Andrade TB, Scurrah KJ, Bauman A, and Ferreira PH declare that they have no competing interests.
Additional files
Search strategy. (DOCX 18 kb)
Sensitivity analysis excluding one study at a time. (JPG 363 kb)
References
- 1.Vogel T, Brechat PH, Leprêtre PM, et al. Health benefits of physical activity in older patients: a review. Int J Clin Pract. 2009;63:303–20. doi: 10.1111/j.1742-1241.2008.01957.x. [DOI] [PubMed] [Google Scholar]
- 2.Sherrington C, Whitney JC, Lord SR, et al. Effective exercise for the prevention of falls: a systematic review and meta-analysis. J Am Geriatr Soc. 2008;56:2234–43. doi: 10.1111/j.1532-5415.2008.02014.x. [DOI] [PubMed] [Google Scholar]
- 3.Janssen I, Leblanc AG. Systematic review of the health benefits of physical activity and fitness in school-aged children and youth. Int J Behav Nutr Phys Act. 2010;7:40. doi: 10.1186/1479-5868-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1435–45. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- 5.Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116:682–92. doi: 10.1016/j.amjmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Nikander R, Sievanen H, Heinonen A, et al. Targeted exercise against osteoporosis: a systematic review and meta-analysis for optimising bone strength throughout life. BMC Med. 2010;8:47. doi: 10.1186/1741-7015-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metsios GS, Stavropoulos-Kalinoglou A, Veldhuijzen van Zanten JJCS, et al. Rheumatoid arthritis, cardiovascular disease and physical exercise: a systematic review. Rheumatol. 2008;47:239–48. doi: 10.1093/rheumatology/kem260. [DOI] [PubMed] [Google Scholar]
- 8.Herring MP, O’Connor PJ, Dishman RK. The effect of exercise training on anxiety symptoms among patients: a systematic review. Arch Intern Med. 2010;170:321–31. doi: 10.1001/archinternmed.2009.530. [DOI] [PubMed] [Google Scholar]
- 9.Heneweer H, Staes F, Aufdemkampe G, et al. Physical activity and low back pain: a systematic review of recent literature. Eur Spine J. 2011;20:826–45. doi: 10.1007/s00586-010-1680-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodpaster BH, Delany JP, Otto AD, et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. JAMA. 2010;304:1795–802. doi: 10.1001/jama.2010.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei M, Kampert JB, Barlow CE, et al. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA. 1999;282:1547–53. doi: 10.1001/jama.282.16.1547. [DOI] [PubMed] [Google Scholar]
- 12.Sui X, Li H, Zhang J, et al. Percentage of deaths attributable to poor cardiovascular health lifestyle factors: findings from the Aerobics Center Longitudinal Study. Epidemiol Res Int. 2013;2013:9. [DOI] [PMC free article] [PubMed]
- 13.Flegal KM, Kit BK, Orpana H, et al. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flegal KM, Graubard BI, Williamson DF, et al. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–37. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 15.De Vilhena e Santos DM, Katzmarzyk PT, Seabra AF, et al. Genetics of physical activity and physical inactivity in humans. Behav Genet. 2012;42:559–78. doi: 10.1007/s10519-012-9534-1. [DOI] [PubMed] [Google Scholar]
- 16.den Hoed M, Brage S, Zhao JH, et al. Heritability of objectively assessed daily physical activity and sedentary behavior. Am J Clin Nutr. 2013;98:1317–25. doi: 10.3945/ajcn.113.069849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouchard C, Rankinen T. Individual differences in response to regular physical activity. Med Sci Sports Exerc. 2001;33:S446-51. doi: 10.1097/00005768-200106001-00013. [DOI] [PubMed] [Google Scholar]
- 18.Lortie G, Simoneau JA, Hamel P, et al. Responses of maximal aerobic power and capacity to aerobic training. Int J Sports Med. 1984;5:232–6. doi: 10.1055/s-2008-1025911. [DOI] [PubMed] [Google Scholar]
- 19.Bouchard C, An P, Rice T, et al. Familial aggregation of VO(2max) response to exercise training: results from the HERITAGE family study. J Appl Physiol. 1999;87:1003–8. doi: 10.1152/jappl.1999.87.3.1003. [DOI] [PubMed] [Google Scholar]
- 20.Koenigstorfer J, Schmidt WFJ. Effects of exercise training and a hypocaloric diet on female monozygotic twins in free-living conditions. Physiol Behav. 2011;104:838–44. doi: 10.1016/j.physbeh.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9, w64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 22.Bouchard C, Perusse L, Leblanc C. Using MZ twins in experimental research to test for the presence of a genotype-environment interaction effect. Acta Genet Med Gemellol. 1990;39:85–9. doi: 10.1017/S0001566000005596. [DOI] [PubMed] [Google Scholar]
- 23.Maher CG, Sherrington C, Herbert RD, et al. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys Ther. 2003;83:713–21. [PubMed] [Google Scholar]
- 24.Perusse L, Rice T, Province MA, et al. Familial aggregation of amount and distribution of subcutaneous fat and their responses to exercise training in the HERITAGE family study. Obes Res. 2000;8:140–50. doi: 10.1038/oby.2000.15. [DOI] [PubMed] [Google Scholar]
- 25.Haggard E. Intraclass correlation and the analysis of variance. New York: Dryden Press; 1958. p. 171. [Google Scholar]
- 26.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danis A, Kyriazis Y, Klissouras V. The effect of training in male prepubertal and pubertal monozygotic twins. Eur J Appl Physiol. 2003;89:309–18. doi: 10.1007/s00421-002-0785-z. [DOI] [PubMed] [Google Scholar]
- 28.Hainer V, Stunkard AJ, Kunesova M, et al. Intrapair resemblance in very low calorie diet-induced weight loss in female obese identical twins. Int J Obes Relat Metab Disord. 2000;24:1051–7. doi: 10.1038/sj.ijo.0801358. [DOI] [PubMed] [Google Scholar]
- 29.Afman G, Adams T, Fisher G, et al. Influence of genetics and exercise training on the heart: a 22-week study of monozygous and dizygous twins [Manuscript of a Journal Article]: Brigham Young University. 1988. [Google Scholar]
- 30.Bouchard C, Tremblay A, Despres JP, et al. The response to exercise with constant energy intake in identical twins. Obes Res. 1994;2:400–10. doi: 10.1002/j.1550-8528.1994.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 31.Skinner JS, Wilmore KM, Krasnoff JB, et al. Adaptation to a standardized training program and changes in fitness in a large, heterogeneous population: the HERITAGE Family Study. Med Sci Sports Exerc. 2000;32:157–61. doi: 10.1097/00005768-200001000-00023. [DOI] [PubMed] [Google Scholar]
- 32.Hopkins ND, Stratton G, Cable NT, et al. Impact of exercise training on endothelial function and body composition in young people: a study of mono- and di-zygotic twins. Eur J Appl Physiol. 2012;112:421–7. doi: 10.1007/s00421-011-1993-1. [DOI] [PubMed] [Google Scholar]
- 33.Poehlman ET, Tremblay A, Marcotte M, et al. Heredity and changes in body composition and adipose tissue metabolism after short-term exercise-training. Eur J Appl Physiol Occup Physiol. 1987;56:398–402. doi: 10.1007/BF00417766. [DOI] [PubMed] [Google Scholar]
- 34.Hamel P, Simoneau J, Lortie G, et al. Heredity and muscle adaption to endurance training. Med Sci Sports Exerc. 1986;18:690–6. doi: 10.1249/00005768-198612000-00015. [DOI] [PubMed] [Google Scholar]
- 35.Prud’Homme D, Bouchard C, Leblanc C. Sensitivity of maximal aerobic power to training is genotype-dependent. Med Sci Sports Exerc. 1984;16:489–93. doi: 10.1249/00005768-198410000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Rice T, Hong Y, Perusse L, et al. Total body fat and abdominal visceral fat response to exercise training in the HERITAGE Family Study: Evidence for major locus but no multifactorial effects. Metab Clin Exp. 1999;48:1278–86. doi: 10.1016/S0026-0495(99)90268-8. [DOI] [PubMed] [Google Scholar]
- 37.Gaskill SE, Rice T, Bouchard C, et al. Familial resemblance in ventilatory threshold: The HERITAGE Family Study. Med Sci Sports Exerc. 2001;33:1832–40. doi: 10.1097/00005768-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Perusse L, Gagnon J, Province MA, et al. Familial aggregation of submaximal aerobic performance in the heritage family study. Med Sci Sports Exerc. 2001;33:597–604. doi: 10.1097/00005768-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 39.Elks CE, den Hoed M, Zhao JH, et al. Variability in the heritability of body mass index: a systematic review and meta-regression. Front Endocrinol. 2012;3:29. doi: 10.3389/fendo.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hocking LJ, Morris AD, Dominiczak AF, et al. Heritability of chronic pain in 2195 extended families. Eur J Pain. 2012;16:1053–63. doi: 10.1002/j.1532-2149.2011.00095.x. [DOI] [PubMed] [Google Scholar]
- 41.Mustelin L, Latvala A, Pietilainen KH, et al. Associations between sports participation, cardiorespiratory fitness, and adiposity in young adult twins. J App Physiol (Bethesda, Md : 1985) 2011;110:681–6. doi: 10.1152/japplphysiol.00753.2010. [DOI] [PubMed] [Google Scholar]
- 42.Schousboe K, Visscher PM, Erbas B, et al. Twin study of genetic and environmental influences on adult body size, shape, and composition. Int J Obes Relat Metab Disord. 2004;28:39–48. doi: 10.1038/sj.ijo.0802524. [DOI] [PubMed] [Google Scholar]
- 43.Poulsen P, Vaag A, Kyvik K, et al. Genetic versus environmental aetiology of the metabolic syndrome among male and female twins. Diabetologia. 2001;44:537–43. doi: 10.1007/s001250051659. [DOI] [PubMed] [Google Scholar]
- 44.Zillikens MC, Yazdanpanah M, Pardo LM, et al. Sex-specific genetic effects influence variation in body composition. Diabetologia. 2008;51:2233–41. doi: 10.1007/s00125-008-1163-0. [DOI] [PubMed] [Google Scholar]
- 45.Korkeila M, Kaprio J, Rissanen A, et al. Effects of gender and age on the heritability of body mass index. Int J Obes. 1991;15:647–54. [PubMed] [Google Scholar]
- 46.Schousboe K, Willemsen G, Kyvik KO, et al. Sex differences in heritability of BMI: a comparative study of results from twin studies in eight countries. Twin Res. 2003;6:409–21. doi: 10.1375/136905203770326411. [DOI] [PubMed] [Google Scholar]
- 47.Korkeila M, Kaprio J, Rissanen A, et al. Consistency and change of body mass index and weight. A study on 5967 adult Finnish twin pairs. Int J Obes Relat Metab Disord. 1995;19:310–7. [PubMed] [Google Scholar]
- 48.Bouchard C, Lesage R, Lortie G. Aerobic performance in brothers, dizygotic and monozygotic twins. Med Sci Sports Exerc. 1986;18:639–46. doi: 10.1249/00005768-198612000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Bouchard C, Daw EW, Rice T, et al. Familial resemblance for VO2max in the sedentary state: the HERITAGE family study. Med Sci Sports Exerc. 1998;30:252–8. doi: 10.1097/00005768-199802000-00013. [DOI] [PubMed] [Google Scholar]
- 50.Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet. 1997;27:325–51. doi: 10.1023/A:1025635913927. [DOI] [PubMed] [Google Scholar]
- 51.Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
- 52.Hopper JL. Variance components for statistical genetics: applications in medical research to characteristics related to human diseases and health. Stat Methods Med Res. 1993;2:199–223. doi: 10.1177/096228029300200302. [DOI] [PubMed] [Google Scholar]
- 53.Hopper JL. Why ‘common environmental effects’ are so uncommon in the literature In: Spector T, Snieder H, MacGregor AJ, editors. Advances in Twin and Sib-pair Analysis 137 Euston Rd, London, NW1, 2AA: Greewich Medical Media Ltd.; 2000. p. 151–66.
- 54.Bouchard C, Sarzynski MA, Rice TK, et al. Genomic predictors of the maximal O(2) uptake response to standardized exercise training programs. J Appl Physiol (1985) 2011;110:1160–70. doi: 10.1152/japplphysiol.00973.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rankinen T, Argyropoulos G, Rice T, et al. CREB1 is a strong genetic predictor of the variation in exercise heart rate response to regular exercise: the HERITAGE Family Study. Circ Cardiovasc Genet. 2010;3:294–9. doi: 10.1161/CIRCGENETICS.109.925644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chagnon YC, Rice T, Perusse L, et al. Genomic scan for genes affecting body composition before and after training in Caucasians from HERITAGE. J Appl Physiol (1985) 2001;90:1777–87. doi: 10.1152/jappl.2001.90.5.1777. [DOI] [PubMed] [Google Scholar]
- 57.Sun G, Gagnon J, Chagnon YC, et al. Association and linkage between an insulin-like growth factor-1 gene polymorphism and fat free mass in the HERITAGE Family Study. Int J Obes Relat Metab Disord. 1999;23:929–35. doi: 10.1038/sj.ijo.0801021. [DOI] [PubMed] [Google Scholar]
- 58.Haimoto H, Hosoda S, Kato K. Differential distribution of immunoreactive S100-alpha and S100-beta proteins in normal nonnervous human tissues. Lab Invest. 1987;57:489–98. [PubMed] [Google Scholar]
- 59.Bouchard C, Antunes-Correa LM, Ashley EA, et al. Personalized preventive medicine: genetics and the response to regular exercise in preventive interventions. Prog Cardiovasc Dis. 2015;57:337–46. doi: 10.1016/j.pcad.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kitsios G, Zintzaras E. Genomic convergence of genome-wide investigations for complex traits. Ann Hum Genet. 2009;73:514–9. doi: 10.1111/j.1469-1809.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


