Abstract
Background. Respiratory syncytial virus (RSV) is a leading cause of childhood bronchiolitis and pneumonia, particularly in early infancy. Immunization of pregnant women could boost preexisting immune responses, providing passive protection to newborns through placental transfer of anti-RSV antibody.
Methods. In this first-in-humans clinical trial of a purified recombinant RSV protein F vaccine engineered to preferentially maintain prefusion conformation (RSV-PreF), 128 healthy men 18–44 years old were randomized to one dose of a RSV-PreF vaccine containing 10, 30, or 60 µg of RSV-PreF antigen, with or without alum adjuvant, or control, and followed for one year for safety and immunogenicity outcomes.
Results. Injection site pain was the most common adverse event, reported by up to 81.3% of participants. The highest RSV neutralizing antibody responses were in the 30 µg RSV-PreF/alum, 60 µg RSV-PreF/alum, and 60 µg RSV-PreF/nonadjuvant groups. Responses were evident on day 7, and 30 days after vaccination these participants had RSV-A neutralizing antibody titers of ≥1:512, and >70% had titers of 1:1024, with titers increasing by 3.2–4.9 fold. Responses remained high on day 60 but waned on days 180 and 360.
Conclusions. The RSV-PreF vaccine elicited rapid RSV neutralizing antibody responses in healthy young men, with an acceptable adverse event profile.
Keywords: respiratory syncytial virus, vaccine, maternal immunization, vaccine safety and immunogenicity
(See the editorial commentary by Englund and Chu on pages 4–7.)
Respiratory syncytial virus (RSV) is a leading cause of early childhood lower respiratory tract illness (LRTI), with an estimated 33 million episodes worldwide and 199 000 attributable deaths in low-resource countries each year [1]. In developed countries, RSV bronchiolitis and pneumonia lead to hospital admission in about 2% of infected infants [2, 3]. There are no preventive or therapeutic interventions currently available that adequately address this significant global public health problem.
Maternal immunization is now an accepted method to reduce infant pertussis, tetanus, and influenza [4], although vaccine uptake remains low. The highest burden of childhood RSV-associated illness occurs in the first 6 months of life, and maternal RSV vaccination has thus been identified as a potential strategy to protect the infant [5]. Since most women of child-bearing age would have preexisting antibody from prior infection, a RSV vaccine given during the third trimester of pregnancy would be expected to boost preexisting antibody levels and result in increased passage of anti-RSV antibodies through the placental active-transport mechanism for immunoglobulin G (IgG). Maternal immunization potentially could also protect the infant through a reduced risk of infection transmission from the mother and, possibly, from passive immunity conferred through breast milk. RSV antibodies are known to be transferred efficiently across the placenta [6], and high cord blood RSV antibody levels are associated with a lower incidence of severe RSV-associated LRTI [7, 8].
The RSV F surface glycoprotein, which is highly conserved across A and B subgroup isolates and considered essential in disease pathogenesis [9], is a target for passive immunization with monoclonal antibodies [10], which reduce the risk of RSV-associated hospitalization. There is evidence that the prefusion conformation of the F glycoprotein, rather than the postfusion form, is the main target of naturally induced anti-RSV neutralizing antibody (nAb) in human serum [11] and, thus, would be a preferred vaccine antigen. A specific epitope on the prefusion conformation, site ø (zero), is thought to be one of the major targets of RSV nAb [12] and results in potent neutralizing activity in animal models [13]. In this first-in-humans study, the safety, reactogenicity, and immunogenicity of a RSV vaccine for pregnant women, containing purified recombinant RSV glycoprotein F engineered to preferentially maintain prefusion conformation (RSV-PreF), was evaluated.
METHODS
This was a randomized, controlled, observer-blinded, first-in-humans, phase 1 clinical trial to evaluate the safety and reactogenicity of a single dose of 1 of 6 formulations of an RSV vaccine in 18–44-year-old healthy men at 3 sites in Canada. The study was conducted in 2 sequential steps, with dose escalation in step 2. The study was initiated on 22 July 2013, and day 360 visits were concluded on 16 March 2015. The study (clinical trials registration NCT01905215) was undertaken in compliance with Good Clinical Practice guidelines, the Declaration of Helsinki, and national regulatory requirements and was approved by local or regional institutional review boards at each study site.
Participants
Eligible men were 18–44 years of age at the time of vaccination; healthy, based on medical history and clinical examination; able to comply with the protocol; and gave informed written consent. Women were excluded from participation on the guidance of the regulatory authority, which advised that later testing of this novel product could occur in women of childbearing age. Exclusionary criteria were immunocompromise, a family history of immunodeficiency, autoimmune disease, a malignancy within 5 years, a history of hypersensitivity to latex or any vaccine component, acute illness or fever, participation in another clinical study, receipt or intent to receive another vaccine 30 days previous to or after the study vaccine (with the exception of influenza vaccine, which could be administered ≥15 days before study vaccination), or receipt of either immunoglobulins or blood products within 3 months or previous RSV vaccination or any investigational product within 30 days. Any hematological or biochemical value outside the normal range at the local laboratory that was considered clinically significant by the investigator was also considered exclusionary; participants could be rescreened within 30 days.
Vaccines
The RSV-PreF antigen is prepared in Chinese hamster ovary (CHO) cells, in which it is expressed as a soluble, secreted protein. The PreF conformation was characterized by analytical ultracentrifugation, electron microscopy, specific monoclonal antibody (D25) testing, and human sera neutralization inhibition (Blais et al, unpublished data). The study vaccines contained 10, 30, or 60 µg of a recombinant PreF protein antigen, in isotonic saline diluent, with or without alum adjuvant (500 µg of aluminum hydroxide). Vaccines were manufactured by GlaxoSmithKline Vaccines (Rixensart, Belgium). All vaccines were presented as a 0.5-mL single dose. The 3 alum-adjuvanted vaccines were presented as liquid in 0.5-mL monodose vials. These vaccines were turbid in appearance. The 3 PreF plain vaccines were presented in a monodose vial as lyophilized antigen that was reconstituted with diluent and was clear and colorless after reconstitution. The placebo was lyophilized saccharose (20 mg) that was reconstituted in isotonic saline and was clear in appearance.
Study Procedures
At the screening visit, after the consent process, medical history was obtained, physical examination was performed, and blood samples were obtained for safety analysis. Eligible participants were then invited to attend visit 1 within 30 days of the screening visit.
Study group assignment was allocated by an Internet-based central randomization system. The randomization sequence was generated using MATerial Excellence, a software program developed for use in Statistical Analysis System (SAS®) (Cary, North Carolina) by GSK (Rixensart, Belgium). A randomization blocking scheme was used to ensure balance between study groups in each step, and a minimization procedure accounted for age category (18–32 or 33–44 years). Participants and all study personnel, except for an unblinded nurse, whose sole role was to prepare and administer study vaccines, were not aware of treatment assignment.
In step 1, participants were randomly assigned as a ratio of 1:1:1:1:1 to receive a placebo injection or a study vaccine containing either 10 or 30 µg of a recombinant PreF protein antigen, in isotonic saline diluent, with or without alum adjuvant (500 µg of aluminum hydroxide). In step 2, participants were randomly assigned at a ratio of 1:1:1 to receive a study vaccine containing 60 µg of a recombinant PreF protein antigen, in isotonic saline diluent, with or without the alum adjuvant, or placebo (Figure 1).
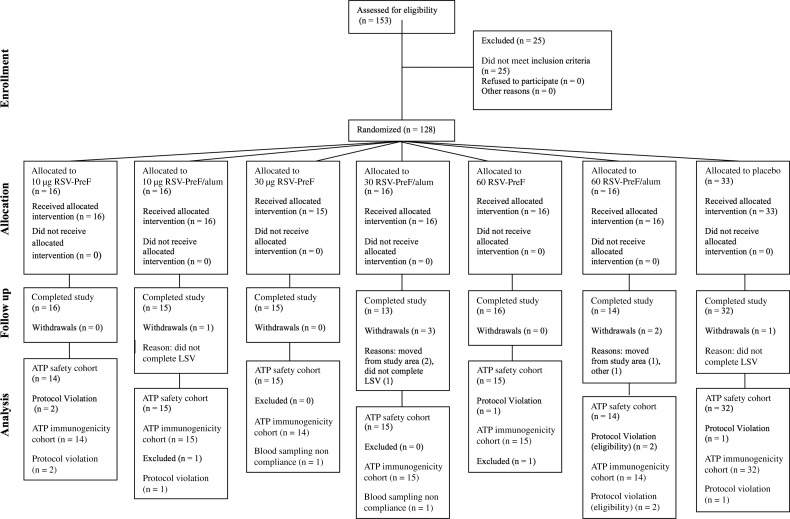
Figure 1.
Flow of participants. Abbreviations: ATP, according to protocol; LSV, last study visit; n, number of participants; RSV-PreF, respiratory syncytial virus engineered to preferentially maintain a prefusion conformation.
One dose of study vaccine was administered intramuscularly in the deltoid region of the nondominant arm. Participants were observed closely for 60 minutes following injection. During the first 3 vaccination days in each step, a maximum of 10 participants were vaccinated each day, at least 60 minutes apart.
At the vaccination visit, participants were instructed to use a diary card for recording any solicited injection site or general adverse events (AEs) and any unsolicited AEs and concomitant medications for 7 days and to bring the diary card to the next visit. A second diary card was provided on day 7 to record, up to day 30, unsolicited AEs and concomitant medications. At each study visit, participants were asked whether there were any changes in their health or AEs since the last visit. Participants attended study sites at the screening visit and on days 0, 7, 30, 60, 180, and 360.
Outcomes
Safety and Reactogenicity
The primary outcome was the assessment of safety and reactogenicity from vaccination up to day 60. A secondary outcome was occurrence of AEs from days 60 to 360. These AEs were solicited local AEs (pain, redness, and swelling) and general AEs (fever, headache, gastrointestinal symptoms, and fatigue) during the day of vaccination and for the following 6 days, occurrence of any abnormal hematological (hemoglobin level, white blood cell, lymphocyte, neutrophil, eosinophil, and platelet count) or biochemical (alanine aminotransferase, aspartate aminotransferase, and creatinine levels) findings at days 0, 7, 30, 60, 180, and 360; any unsolicited AE during the 30 days after vaccination; and the occurrence of any serious adverse events (SAEs), AEs leading to study withdrawal, or investigator-determined clinically significant AEs from days 0 to 360.
The intensity grading scheme for solicited AEs is seen in Supplementary Table 1. Unsolicited AEs were assigned to the categories of mild (easily tolerated by the participant, causing minimal discomfort, and not interfering with everyday activities; grade 1), moderate (sufficiently discomforting to interfere with normal everyday activities; grade 2), or severe (preventing normal, everyday activities; grade 3) by the investigator. Grading of intensity of laboratory parameters was based on Food and Drug Administration guidance [14].
Study holding rules were in place. All SAEs were reported to an internal Safety Review Committee in addition to local, institutional, and national authorities, as required.
Immunogenicity
Secondary objectives of the study were to evaluate humoral immune responses to a single dose of vaccine 7, 30, and up to 60 days after vaccination and the persistence of responses from days 60 to 360, using neutralizing titers against RSV serotypes A and B and enzyme linked immunosorbent assay (ELISA) antibody titers against RSV F at days 0, 7, 30, 60, 180, and 360. The anti-F protein IgG ELISA is an indirect ELISA allowing the detection and quantitation of specific IgG antibodies directed against the RSV F protein in human serum samples; the antigen used for the ELISA is the same PreF protein used for immunization. Antigens are purified from a CHO cell expression system and are coated by passive adsorption onto 96-well microplates. After a washing and a blocking step, serial 2-fold dilutions of test sera, controls, and a reference standard are incubated to allow specific binding of antibodies directed against the F protein antigens. Bound IgGs are detected by addition of a goat anti-human IgG antibody conjugated to horseradish peroxidase. After a washing step, the horseradish peroxidase substrate solution (TMB/H2O2) is added, and a colored product develops proportionally to the amount of anti-F protein IgG antibody present in the test serum. The color is quantified by reading the optical densities at 450–620 nm, using a spectrophotometer. Antibody concentrations of individual serum and control samples are determined after interpolation from the reference standard curve, using a 4-parameter equation, and are expressed in arbitrary ELISA units (ELU) per milliliter. Palivizumab-competing antibodies (PCAs) were also evaluated at these time points, based on the method of Glenn et al.
As a tertiary objective, the study sought to evaluate whether the vaccine induced immune responses to host cell proteins. These results are published separately.
Hematological and biochemical tests were performed at local laboratories. Serologic assays were performed at GSK Laboratories, Laval, Canada (Blais et al, unpublished data).
Sample Size and Statistical Analysis
This was a first-in-humans study, and therefore there were no previous estimates of the frequency of AEs or of immune responses. As the primary outcome was safety, the sample size was based on the likely precision around estimates of the percentage of participants in each vaccine group with symptoms following vaccination. With 16 participants per group, the lower limit (LL) of the 95% confidence interval (CI) on an AE incidence of 25.0% would be 7.3%, and the upper limit (UL) would be 52.4%. The corresponding UL and LL on the 95% CI for symptoms frequencies of 50.0% and 75.0% in a group of 16 participants would be 24.7% and 75.3%, and 47.6% and 92.7%, respectively.
Safety analysis was conducted on the total vaccinated cohort, defined as all participants who received study vaccine, and their demographic characteristics were described. The percentage of participants with at least 1 AE, with a severe AE or an SAE following vaccine, were tabulated, with an exact 95% CI. The number and percentage of participants with a hematologic or biochemistry result below or above the local laboratory range were tabulated. Unsolicited AEs were classified according to the Medical Dictionary for Regulatory Activities (MeDRA®, International Conference on Harmonization).
The immunogenicity analysis was performed on the according-to-protocol cohorts for immunogenicity (on days 0, 7, 30, and 60) and persistence (on days 180 and 360), which included participants who did not develop an exclusionary medical condition or receive an exclusionary concomitant medication during the respective follow-up periods and for whom at least 1 appropriately timed anti-RSV serologic response was available. For each anti-RSV assay, the percentage of participants, and associated exact 95% CIs, with values above the cutoff were determined, as were geometric mean concentrations (GMCs), geometric mean titers (GMTs), and their respective 95% CIs. Reverse cumulative distribution curves were created using data from before vaccination and 7, 30, 60, 180, and 360 days after vaccination. The geometric mean of the fold increase from baseline to days 7, 30, 60, 180, and 360 was calculated, with respective 95% CIs, and the percentage of participants in each study group with various fold increases was tabulated. For the sake of this analysis, Vaccine Response (VR) was defined as at least a 4-fold increase from pre-vaccination if pre-vaccination NA titre <7 log2 (128), at least a 3-fold increase from pre-vaccination if pre-vaccination NA titre in [7–8] log2, at least a 2.5-fold increase from pre-vaccination if pre-vaccination NA titre in [10] log2, and at least 1-fold increase from pre-vaccination if pre-vaccination NA titre >10 log2 (1024), and was reported at Day 30, 60, 180 and 360.
Between-group GMT ratios at each time point after vaccination were calculated using an analysis of covariance model on the log10 transformation of the titers, including the vaccine group as a fixed effect and the prevaccination titer as the regressor. Seropositivity for antibody was determined as follows: anti–RSV-PreF antibody concentration, ≥10 ELU/mL; anti–RSV-A nAb titer, ≥8 ED60; anti–RSV-B nAb titer, ≥6 ED60; and PCA, ≥3.34 µg/mL.
RESULTS
The total vaccinated cohort comprised 128 participants, and 121 completed the study. Participant flow is seen in Figure 1. The according-to-protocol cohort was 119 for analysis of immunogenicity and 114 and 110 for analysis of the persistence of immune responses at days 180 and 360, respectively.
The median age of participants in the total vaccinated cohort was 32 years, and the geographic heritage and/or ancestry of 60.9% was white–Caucasian/European. Age, heritage, and ethnographic characteristics did not differ across study groups (Table 1).
Table 1.
Demographic Characteristics at Enrollment in the Total Vaccinated Cohort, Overall and by Vaccine Formulation Received or Control Group
| Characteristic | 10 µg RSV-PreF |
30 µg RSV-PreF |
60 µg RSV-PreF |
Controla 1 (n = 17) |
Controla 2 (n = 16) |
Overall (n = 128) |
|||
|---|---|---|---|---|---|---|---|---|---|
| Without Alum (n = 16) | With Alum (n = 16) | Without Alum (n = 15) | With Alum (n = 16) | Without Alum (n = 16) | With Alum (n = 16) | ||||
| Age at vaccination, y | |||||||||
| Mean ± SD | 32.1 ± 7.8 | 32.1 ± 7.4 | 33.7 ± 6.6 | 32.3 ± 7.2 | 31.1 ± 7.9 | 32.8 ± 6.2 | 30.2 ± 8.7 | 30.8 ± 7.5 | 31.8 ± 7.3 |
| Median (range) | 32.0 (20.0– 43.0) | 32.5 (20.0– 41.0) | 32.0 (21.0– 43.0) | 31.5 (22.0– 44.0) | 30.5 (20.0– 43.0) | 31.0 (21.0– 42.0) | 29.0 (18.0– 45.0) | 32.5 (20.0– 44.0) | 32.0 (18.0– 45.0) |
| Geographic ancestry, no. (%) | |||||||||
| African Heritage/African American | 1 (6.3) | 2 (12.5) | 0 (0.0) | 2 (12.5) | 4 (25.0) | 1 (6.3) | 0 (0.0) | 1 (6.3) | 11 (8.6) |
| American Indian or Alaskan Native | 0 (0.0) | 1 (6.3) | 0 (0.0) | 1 (6.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.6) |
| Asian | |||||||||
| Central/South Asian heritage | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| East Asian heritage | 0 (0.0) | 1 (6.3) | 0 (0.0) | 1 (6.3) | 0 (0.0) | 0 (0.0) | 2 (11.8) | 1 (6.3) | 5 (3.9) |
| Japanese heritage | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Southeast Asian heritage | 3 (18.8) | 1 (6.3) | 2 (13.3) | 2 (12.5) | 5 (31.3) | 8 (50.0) | 2 (11.8) | 6 (37.5) | 29 (22.7) |
| Native Hawaiian or Other Pacific Islander | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| White | |||||||||
| Arabic/North African heritage | 1 (6.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.9) | 0 (0.0) | 2 (1.6) |
| Caucasian/European heritage | 11 (68.8) | 11 (68.8) | 13 (86.7) | 10 (62.5) | 6 (37.5) | 7 (43.8) | 12 (70.6) | 8 (50.0) | 78 (60.9) |
| Other | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (6.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.8) |
Abbreviations: n, number of participants; RSV-PreF, respiratory syncytial virus engineered to preferentially maintain a prefusion conformation; SD, standard deviation; y, years.
a Saccharose NaCl.
Safety and Reactogenicity
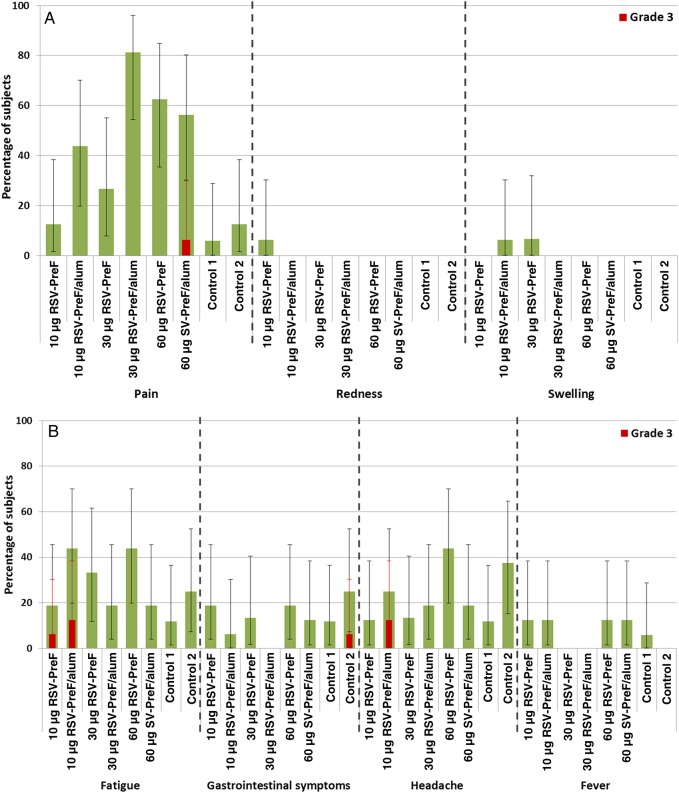
Pain was reported by up to 81.3% of RSV-F vaccine recipients (Figure 2), with a single participant in the group that received 60 µg of RSV-PreF plus alum (ie, the 60-alum group) reporting severe (grade 3) pain. One participant reported redness, and 1 participant each who received 10-alum or 30 µg of antigen without alum (ie, the 30-plain group) reported swelling. The most common systemic AE was fatigue and was reported in up to 43.8% of RSV F vaccine recipients, with 3 reporting severe fatigue, compared with 11.8%–25.0% of controls (33). Fever (body temperature, ≥37.5°C) occurred in 8 of 112 vaccine participants (7.0%) and 1 of 16 controls (6.0%). One participant in the 10-alum group had a temperature of >38.5°C; no participant had a temperature of >39.5°C.
Figure 2.
Solicited injection site adverse events (A) and general adverse events (B) during the 7 days after vaccination—total vaccinated cohort. Grade 3 (severe) pain was defined as pain that is significant at rest and prevents normal everyday activities. Redness and/or swelling were considered present if the greatest surface diameter of each was >100 mm. A grade 3 (severe) systemic adverse event was defined as an event that prevents normal everyday activities. Saccharose NaCl served as the control. Error bars depict 95% confidence intervals. Abbreviation: RSV-PreF, respiratory syncytial virus engineered to preferentially maintain a prefusion conformation.
At least 1 unsolicited symptom was reported by 18.8%–37.5% of participants, depending on study group. One non–vaccine-related SAE (traumatic left knee dislocation) was reported in the 60-plain group. One medically attended visit, deemed by the local investigator as unrelated to vaccination, was observed in the 10-alum group. Use of antipyretic therapy in the 7 days after vaccination ranged from 6.3% (in the 60-alum group) to 31.3% (in the 60-plain group) in vaccinated participants. No placebo recipient took antipyretic medication.
Immunogenicity
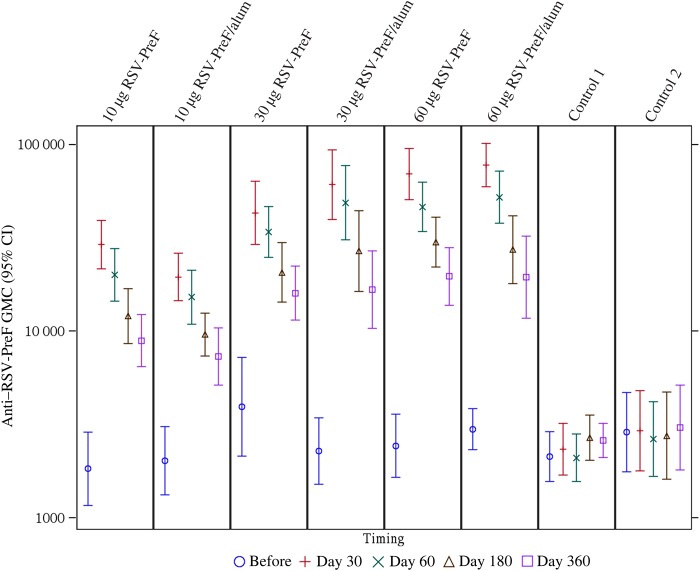
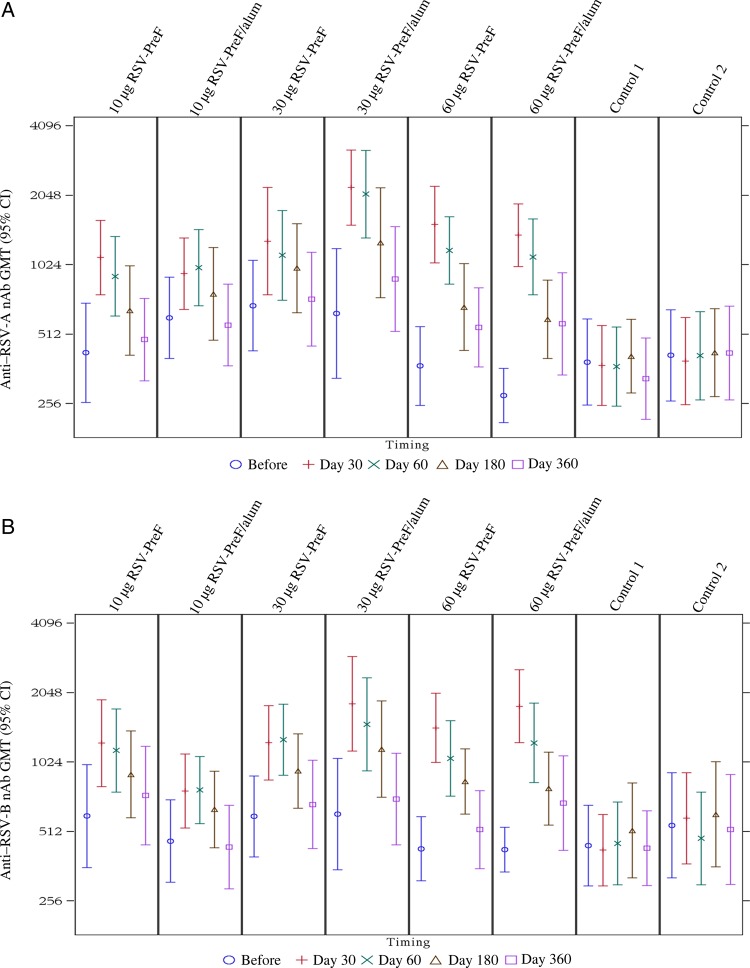
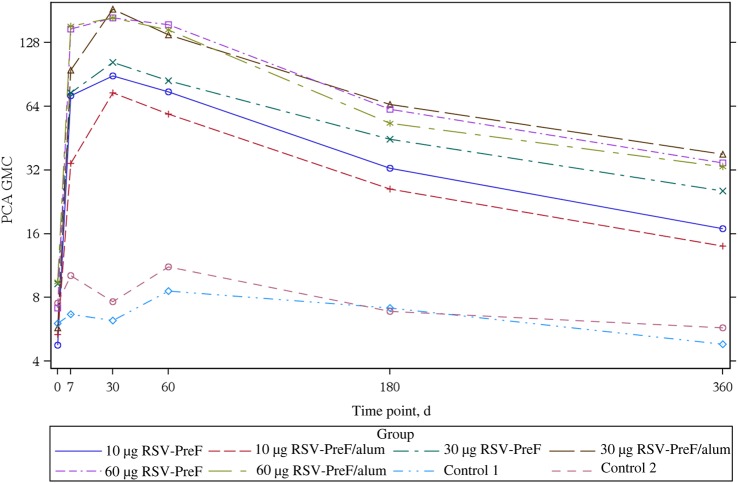
All study participants were RSV seropositive at baseline. The GMCs, by study group, for anti–RSV-PreF antibody and GMTs for anti–RSV-A and anti–RSV-B nAb up to day 360 are seen in Figures 3 and 4. In all vaccine groups, titers were increased on days 7 and 30, but titers remained unchanged in controls. The highest boost responses were seen in the 30-alum, 60-alum, or 60-plain groups, in which, 30 days after vaccination, all participants had anti–RSV nAb titers of ≥1:512, >70.0% had titers of ≥1024, and titers increased 3.2–4.9 fold. Anti–RSV nAb titers for RSV-A and RSV-B strains were similar. The highest Vaccine Response rates were observed on day 30 in the 30-alum and 60-alum groups (85.7% and 84.6%, respectively; Table 2).
Figure 3.
Geometric mean concentrations (GMCs) of anti–respiratory syncytial virus prefusion (RSV-PreF) antibodies before vaccination and on days 30, 60, 180, and 360 after vaccination, by study vaccine group—adapted according-to-protocol (ATP) cohort for immunogenicity. The adapted ATP cohort is as follows: the analysis on the time points up to day 60 comprised the ATP cohort for immunogenicity (n = 119), and the analysis on day 180 and day 360 comprised the ATP cohort for persistence (n = 114). Saccharose NaCl served as the control. Error bars depict 95% confidence intervals (CIs).
Figure 4.
Anti–respiratory syncytial virus A (RSV-A) and anti–RSV-B neutralizing antibody (nAb) geometric mean titers (GMTs), by study group. Abbreviation: CI, confidence interval.
Table 2.
Vaccine Response Rates at Days 30 to 360
| Vaccine Formulation, Day | Subjects With Response, Proportion (%) | Subjects With Titer >1:1024, No. (%) |
|---|---|---|
| 10 µg RSV-PreF | ||
| 30 | 9/14 (64.3) | 6 (42.9) |
| 60 | 5/14 (35.7) | 6 (42.9) |
| 180 | 3/14 (21.4) | 2 (21.4) |
| 360 | 1/14 (7.1) | 2 (14.3) |
| 10 µg RSV-PreF/alum | ||
| 30 | 3/15 (20.0) | 8 (53.3) |
| 60 | 5/15 (33.3) | 8 (53.3) |
| 180 | 3/15 (20.0) | 6 (40.0) |
| 360 | 0/14 (0.0) | 2 (14.3) |
| 30 µg RSV-PreF | ||
| 30 | 7/14 (50.0) | 9 (64.3) |
| 60 | 6/14 (42.9) | 8 (57.1) |
| 180 | 5/15 (33.3) | 7 (46.7) |
| 360 | 4/15 (26.7) | 6 (40.0) |
| 30 µg RSV-PreF/alum | ||
| 30 | 12/14 (85.7) | 12 (85.7) |
| 60 | 10/14 (71.4) | 13 (92.9) |
| 180 | 6/13 (46.2) | 7 (53.8) |
| 360 | 2/12 (16.7) | 4 (33.3) |
| 60 µg RSV-PreF | ||
| 30 | 10/15 (66.7) | 11 (73.3) |
| 60 | 7/15 (46.7) | 9 (60.0) |
| 180 | 3/14 (21.4) | 3 (21.4) |
| 360 | 2/14 (14.3) | 2 (14.3) |
| 60 µg RSV-PreF/alum | ||
| 30 | 11/13 (84.6) | 10 (76.9) |
| 60 | 10/14 (71.4) | 7 (50.0) |
| 180 | 4/12 (33.3) | 3 (25.0) |
| 360 | 3/11 (27.3) | 2 (18.2) |
Vaccine Response were defined as having increased by at least 4-fold from the prevaccination neutralizing antibody (nAb) titer if the prevaccination titer was <7 log2 (ie, <128), by at least 3-fold if the titer was 7–8 log2 (ie, 128–256), by at least 2.5-fold if the titer was 10 log2 (ie, 1024), and by at least 1-fold if the titer was >10 log2 (ie, >1024).
Abbreviation: RSV-PreF, respiratory syncytial virus engineered to preferentially maintain a prefusion conformation.
Responses by all immunogenicity measures decreased at day 60 and waned further at day 180. At day 360, anti–RSV-PreF antibody concentrations (based on ELISA) and PCA concentrations remained higher than at baseline, as did nAb responses, in the 30-alum, 60-alum, and 60-plain groups. GMT kinetics are seen in Figures 4 and 5.
Figure 5.
Palivizumab competing antibody (PCA) geometric mean concentration (GMC) days 0–360, by study group. Abbreviation: RSV-PreF, respiratory syncytial virus engineered to preferentially maintain a prefusion conformation.
DISCUSSION
In this first-in-humans study of a candidate subunit RSV vaccine engineered to maintain the prefusion conformation of glycoprotein F, which is thought to present the most potent epitope, acceptable reactogenicity and rapid and robust immune responses were observed. Temporary injection-site pain and fatigue, mostly mild in intensity, were the most common AEs in all study groups. No clear differences were observed in terms of safety and reactogenicity between the vaccine formulations, and no vaccine-related SAEs or withdrawals occurred. It is reassuring that postimmunization fever was uncommon and of low grade with this vaccine intended for use in pregnant women, as fever in pregnancy has been associated with adverse outcomes [15].
Immune responses to the RSV PreF vaccine, demonstrated by detection of nAb to RSV-A and RSV-B subtypes, RSV F ELISA antibody, and PCA to the F glycoprotein, were highest in the 2 alum-adjuvanted formulations (30-alum and 60-alum) and the 60-plain group. Notably, all vaccine preparations were associated with robust immune responses. The nAb responses were consistent across A and B subtypes. The robust PCA responses confirm that the RSV F ELISA antibodies generated by the vaccine are binding to epitope in the antigenic site of the F protein of RSV-A that is associated with palivizumab efficacy. Further, all measures of immunogenicity demonstrated responses to a single dose of vaccine as early as 7 days after vaccination. This early immune response suggests that this response was amnestic in nature. Given the small sample size of this study, it is difficult to determine which vaccine candidate is preferred for further clinical development, although a trend for slightly higher immune responses was observed in the higher-dose vaccine groups. Furthermore, the added value of the alum in the vaccine could not be determined through this study. Therefore, further development of the RSV-PreF vaccine is underway in women of childbearing age, using the higher doses of the RSV-PreF vaccine with and without alum.
Although immune responses persisted, as expected, a decline in immune responses was seen in all study groups between days 60 and 180 and, further, by day 360. The data also seem to suggest that the magnitude of the decline in immune responses did not vary according to antigen dose or the presence or absence of alum adjuvant. One year after a single dose of vaccine, nAb titers for the 60-alum, 30-alum, and 60-plain groups were higher than at baseline, as were PreF ELISA-determined and PCA responses.
It is not known whether these serologic measures correlate with clinical protection. An immunologic correlate of clinical protection would ideally be determined during efficacy trials. While a trough level of 40 µg/mL of PCA was correlated with a high level of protection against severe disease in infants who received passive immunoprophylaxis [16], it is not yet known what specific level of anti–RSV PreF nAb transplacentally transferred to infants might correlate with protection from significant RSV disease in early infancy. Every 2-fold increase in anti-RSV nAb is associated with a significantly reduced risk of RSV-associated hospitalization [17]. Assessment of RSV vaccine immunogenicity in adults must take into account prior immune response, since adults are expected to have been previously exposed and have baseline titers. We noted variation in baseline titers, which makes assessment of vaccine immunogenicity more complicated.
Given waning of antibody over the year after immunization, timing of vaccination will need to be carefully considered. Administration of a maternal RSV vaccine would need to be timed to take advantage of the placental active-transport mechanism that begins at gestation weeks 28 to 32, to occur late enough in pregnancy to ensure that titers had not waned prior to delivery, and to align with time points for other aspects of obstetric care. Potentially, a vaccine delivered during the third trimester could accomplish this goal.
A few RSV F vaccine candidates intended for maternal immunization to protect infants are in preclinical or early phase clinical development [18]. In a phase 2 trial of a postfusion RSV F vaccine in adults and women of childbearing age, mean neutralizing antibody titers 28 days after a single immunization increased across different formulations by a factor 2.0 to 3.9 (RSV-A) and 1.2 to 1.8 (RSV-B) [19]. In that same study, an acceptable safety profile was observed after a single and two consecutive vaccine administrations. A phase 3 study of this vaccine in pregnant women is underway (clinical trials registration NCT02247726). The study reported here was not powered to detect differences between groups in reactogenicity or immunogenicity or to select the final formulation or schedule. Larger studies in diverse populations of women of childbearing age, before and during the RSV season, are ongoing to evaluate these outcomes.
In summary, a phase 1 study of a prefusion RSV F vaccine intended for maternal vaccination to protect young infants demonstrated acceptable reactogenicity. One dose of all formulations produced a brisk immune response.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Dr Benjamin Lasko, Martine Douha, Catena Lauria, Anne-Sophie Perraux, Marie-Pierre David, Marta Picciolato, Nathalie Michelet, Laura Maria Scurtu, Julie Todoroff, and study participants, for their contributions.
All authors participated in the design, implementation or analysis, or interpretation of the study and in the development of this manuscript. All authors had full access to the data and gave final approval before submission. J. M. L. drafted the manuscript and was responsible for submission of the publication.
J. M. L. holds the Canadian Institutes of Health Research–GlaxoSmithKline Chair in Pediatric Vaccinology at Dalhousie University (Halifax, Canada).
Financial support. This trial was supported by GlaxoSmithKline Biologicals SA. GlaxoSmithKline Biologicals SA was involved in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals SA paid for open access of the article. The authors received no remuneration for the development of the present manuscript.
Potential conflicts of interest. GlaxoSmithKline Biologicals was involved in all stages of the study conduct and analysis and paid for open access of the article, and the authors received no remuneration from GlaxoSmithKline Biologicals for the development of the present manuscript. J. M. L., S. A. H., and S. A. M. have received research funding from GSK, Immunovaccine, Sanofi Pasteur, Pfizer, Pan Provincial Vaccine Enterprise (Prevent), Novavax, the Public Health Agency of Canada, and the Canadian Institutes of Health Research. M. L., L. F., W. D., I. D., and J.-F. T. are employees of the GSK group of companies. W. D. and I. D. own stock/share options or restricted shares in the GSK group of companies. J.-F. T. has a patent application supporting the use of the GSK PreF antigen. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Nair H, Nokes DJ, Gessner BD et al. . Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010; 375:1545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou H, Thompson WW, Viboud CG et al. . Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993-2008. Clin Infect Dis 2012; 54:1427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langley JM, LeBlanc JC, Smith B, Wang EE. Increasing incidence of hospitalization for bronchiolitis among Canadian children, 1980-2000. J Infect Dis 2003; 188:1764–7. [DOI] [PubMed] [Google Scholar]
- 4.Englund JA. Maternal immunization—promises and concerns. Vaccine 2015; 33:6372–3. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO). The Global Vaccine and Immunization Research Forum (GVIRF). 2015. http://www.who.int/immunization/research/forums_and_initiatives/gvirf/en/ Accessed 12 October 2015.
- 6.Munoz FM, Piedra PA, Glezen WP. Safety and immunogenicity of respiratory syncytial virus purified fusion protein-2 vaccine in pregnant women. Vaccine 2003; 21:3465–7. [DOI] [PubMed] [Google Scholar]
- 7.Eick A, Karron R, Shaw J et al. . The role of neutralizing antibodies in protection of American Indian infants against respiratory syncytial virus disease. Pediatr Infect Dis J 2008; 27:207–12. [DOI] [PubMed] [Google Scholar]
- 8.Stensballe LG, Ravn H, Kristensen K et al. . Respiratory syncytial virus neutralizing antibodies in cord blood, respiratory syncytial virus hospitalization, and recurrent wheeze. J Allergy Clin Immunol 2009; 123:398–403. [DOI] [PubMed] [Google Scholar]
- 9.McLellan JS, Ray WC, Peeples ME. Structure and function of respiratory syncytial virus surface glycoproteins. Curr Top Microbiol Immunol 2013; 372:83–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998; 102:531–7. [PubMed] [Google Scholar]
- 11.Magro M, Mas V, Chappell K et al. . Neutralizing antibodies against the preactive form of respiratory syncytial virus fusion protein offer unique possibilities for clinical intervention. Proc Natl Acad Sci U S A 2012; 109:3089–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLellan JS, Chen M, Joyce MG et al. . Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 2013; 342:592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham BS, Modjarrad K, McLellan JS. Novel antigens for RSV vaccines. Curr Opin Immunol 2015; 35:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. Department of Health and Human Services FaDA. Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. Washington DC: Department of Health and Human Services, 2007. [Google Scholar]
- 15.Dreier JW, Andersen AM, Berg-Beckhoff G. Systematic review and meta-analyses: fever in pregnancy and health impacts in the offspring. Pediatrics 2014; 133:e674–88. [DOI] [PubMed] [Google Scholar]
- 16.Subramanian KN, Weisman LE, Rhodes T et al. . Safety, tolerance and pharmacokinetics of a humanized monoclonal antibody to respiratory syncytial virus in premature infants and infants with bronchopulmonary dysplasia. MEDI-493 Study Group. Pediatr Infect Dis J 1998; 17:110–5. [DOI] [PubMed] [Google Scholar]
- 17.Piedra PA, Jewell AM, Cron SG, Atmar RL, Glezen WP. Correlates of immunity to respiratory syncytial virus (RSV) associated-hospitalization: establishment of minimum protective threshold levels of serum neutralizing antibodies. Vaccine 2003; 21:3479–82. [DOI] [PubMed] [Google Scholar]
- 18.PATH. RSV vaccine snapshot. 2015. http://sites.path.org/vaccinedevelopment/files/2015/07/RSV-snapshot-July2015.pdf Accessed 12 October 2015.
- 19.Glenn GM, Fries LF, Thomas DN et al. . A randomized, blinded, controlled, dose-ranging study of a respiratory syncytial virus recombinant fusion (F) nanoparticle vaccine in healthy women of childbearing age. J Infect Dis 2016; 213:411–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.