Abstract
Background. Cotrimoxazole (CTX) discontinuation increases malaria incidence in human immunodeficiency virus (HIV)–infected individuals. Rates, quantity, and timing of parasitemia rebound following CTX remain undefined.
Methods. Serial specimens from a trial of HIV-infected individuals receiving antiretroviral treatment (ART) randomized to continue (the CTX arm) or discontinue (the STOP-CTX arm) were examined for malaria parasites by quantitative reverse transcription polymerase chain reaction (PCR). Specimens obtained at enrollment and then quarterly for 12 months and at sick visits were assessed; multiplicity of infection was evaluated by PCR that targeted the polymorphic msp-1/msp-2 alleles.
Results. Among 500 HIV-infected adults receiving ART (median ART duration, 4.5 years), 5% had detectable parasitemia at baseline. After randomization, parasite prevalence increased over time in the STOP-CTX arm, compared with the CTX arm, with values of 4% and <1%, respectively, at month 3, 8% and 2% at month 6, 14% and 2% at month 9, and 22% and 4% at month 12 (P = .0034). The combined mean parasite density at the various time points was higher in the STOP-CTX arm (4.42 vs 3.13 log10 parasites/mL; P < .001). The parasitemia incidence was 42.0 cases per 100 person-years in the STOP-CTX arm and 9.9 cases per 100 person-years in the CTX arm, with an incidence rate ratio of 4.3 (95% confidence interval, 2.7–7.1; P < .001). After enrollment, mixed infections (multiplicity of infection, >1) were only present in the STOP-CTX arm.
Conclusion. Discontinuation of CTX by HIV-infected adults receiving ART resulted in progressive increases in malaria parasitemia prevalence and burden.
Clinical Trials Registration. NCT01425073.
Keywords: HIV, malaria, cotrimoxazole, parasitemia, antiretroviral treatment, adults
In sub-Saharan Africa, human immunodeficiency virus (HIV) continues to be a leading cause of morbidity and mortality. Worldwide, approximately 37 million people are infected with HIV, and 72% of AIDS-related deaths occur in Africa [1]. Strategies to reduce HIV-related morbidity and mortality include the scale-up of antiretroviral therapy (ART), as well as provision of broad-spectrum antibiotics to prevent opportunistic infections.
Cotrimoxazole (CTX), a fixed-dose combination of sulfamethoxazole and trimethoprim, is a widely available, low-cost antibiotic that is used to treat and prevent community-acquired opportunistic infections. The use of CTX is now part of the standard management of people living with HIV. Its value is supported by several studies that have shown its beneficial effects on mortality and morbidity. The drug has a minimal risk of adverse effects among people with early as well as advanced HIV infection [2, 3]. Although CTX is not recommended for malaria prophylaxis, similar to pyrimethamine-sulfadoxine it has antimalarial activity [4, 5]. A study conducted in Uganda among HIV-infected individuals demonstrated that CTX reduced the incidence of malaria and diarrhea by 72% and 35%, respectively [6], while a combination of both ART and CTX prophylaxis resulted in reduction of the malaria incidence rate by 92% [7].
The 2006 World Health Organization (WHO) guidelines recommend CTX for HIV-infected individuals, regardless of clinical stage, with CD4+ T-cell counts of <350 cells/mm3 [8]. For countries with a high prevalence of HIV and limited health infrastructure, the WHO endorses universal CTX for all HIV-infected individuals [8]. These guidelines were created prior to the scale-up of ART. Recent WHO guidelines in 2014 advocate continued CTX therapy, based on data from clinical trials that demonstrated increased malaria incidence following CTX discontinuation despite ART immune reconstitution [9–11]. One of these trials was conducted in Kenya and demonstrated a significantly increased incidence of clinical malaria in individuals randomized to stop CTX therapy (hereafter, the “STOP-CTX arm”), compared with those who continued CTX therapy (hereafter, the “CTX arm”) [10]. Using specimens from this trial, we used molecular methods to detect malaria parasitemia in the 2 cohorts over a 12-month observation period. We characterized the risk associated with stopping CTX therapy by determining parasite density, multiplicity of infecting parasites (MOIs), and rates of new cases of parasitemia.
METHODS
Study, Participants, and Site
Homa Bay, located in the Lake Victoria basin in western Kenya, is holoendemic for malaria. The long rainy season, from late March through May, produces intense malaria transmission from April through August, whereas the short rainy season, from October through December, produces lower transmission intensity from November through January [12]. In 2014, the HIV prevalence in Homa Bay was 26% (higher than the national average of 6%) [13].
The randomized trial [10] was conducted between February 2012 and September 2013 at the HIV Treatment and Care Clinic of Homa Bay District Hospital (clinical trials registration NCT01425073), and the details of the study have been described elsewhere [10]. HIV-infected individuals with evidence of immune recovery (ie, an ART duration of ≥18 months and a CD4+ T-cell count of >350 cells/mm3) were randomized to the CTX arm or the STOP-CTX arm. Participants were examined for malaria parasites every 3 months for 12 months and whenever they reported being sick. A total of 50 µL of blood was collected at scheduled and sick visits in tubes containing ethylenediaminetetraacetic acid and blotted on Whatman cellulose filter paper (Sigma-Aldrich, St Louis, Missouri) and transported to the Kenya Medical Research Institute (KEMRI)/Walter Reed Project Basic Science Laboratory in Kisumu, Kenya, where assays were conducted. Each study participant received a unique study identification number that was the only identifier on the samples. All parasitemia assays were conducted while masked to participants’ randomization arm or clinical status. DNA was extracted using QIAamp DNA mini kits (Qiagen, Valencia, California) according to the manufacturer's instructions. The extracted DNA was used to detect malaria parasites by quantitative reverse-transcription polymerase chain reaction (qRT-PCR) analysis and to estimate P. falciparum clonal diversity. The sensitivity of malaria parasite detection by qRT-PCR was <1 parasite/µL [14, 15].
Ethical Considerations
All participants gave informed consent. Consent was written if the participant was literate and denoted by fingerprint if the participant illiterate, with the signature of an independent witness also provided. Scientific and ethical approvals for the study were obtained from the KEMRI Ethical Review Committee (protocol SSC 2077), the Walter Reed Institute of Army Research Human Subject Protection Committee (protocol 1983), and the University of Washington Institutional Review Board.
Detection of Malaria Parasites by qRT-PCR
qRT-PCR for detection of organisms in the genus Plasmodium was performed with forward and reverse primers (PLU3F 5′-GCT-CTT-TCT-TGA-TTT-CTT-GGA-TG-3′ and PLU3R 5′-AGC-AGG-TTA-AGA-TCT-CGT-TCG-3′) and probe (PLU3P 5′-ATGGCCGTTTTTAGTTCGTG-3′) that target the 18S small RNA gene as described previously [14]. To obtain maximum sensitivity, we followed the qRT-PCR approach described by Waitumbi et al, in which both DNA and RNA are used [16]. The RNA was first reverse transcribed, and the resulting complementary DNA (cDNA) and DNA strands are amplified in a subsequent PCR. Briefly, the PCR was performed in a final volume of 10 µL with 2 µL of the template, 5 µL of 2× Qiagen Quatitec probe RT-PCR master mix (Qiagen), 0.4 µL of 0.4 µM probe and primers mix, 0.1 µL of reverse transcriptase enzyme mix, 1.6 µL of 4 µM magnesium chloride, and 1.1 µL of PCR-grade RNAse/DNAse free water. Reactions were performed in a 7500 analytical PCR system thermocycler (Applied Biosystems, CA). The amplification process started with a 30-minute reverse transcription step at 50°C to convert RNA to cDNA. This was followed by heating at 94°C for 10 minutes to inactivate reverse transcriptase and then 45 cycles of 95°C for 15 seconds and 60°C for 1 minute to amplify the target cDNA and genomic DNA. A blood specimen with an estimated parasite count of 469 920 parasites/μL from a donor infected with Plasmodium falciparum was obtained from the National Institute for Biological Standards and Control (Hertfordshire, United Kingdom) and used to generate standards for qRT-PCR analysis. Negative controls included noninfected human DNA and nontemplate control. Subjects were considered free of malaria parasites if the cycle threshold value was >40.
Evaluation of MOI
DNA was amplified in a 2-step nested PCR. In the primary reaction, primers targeting the entire block 2 of msp-1 and block 3 of msp-2 were used as described previously [17]. For this, the PCR was performed in a final volume of 25 µL per reaction. Each reaction tube contained 3 µL of DNA, 15.9 µL of ultra-pure grade RNAse/DNAse-free water (Applied Biosystems), 2.5 µL of 1× MyTaq buffer (Bio-line, United Kingdom), 0.625 µL of 0.25 pm/µL of forward and reverse primers (Applied Biosystems), 2 µL of 2 mM Mgcl2, 0.3 µL of 0.125 mM dNTPs, and 0.1 µL of 0.02 U of MyTaq DNA polymerase (Bio-line). The reactions were performed in the HID Veriti 96-well thermal cycler (Applied Biosystems). The cycle conditions for both msp-1 and msp-2 included initial denaturation at 95°C for 1 minute, followed by 35 cycles of denaturation at 95°C for 15 seconds, annealing at 58°C for 15 seconds, and extension at 72°C for 10 seconds. For positive controls, samples previously confirmed to contain msp-1 (K1, MAD20, and RO33) and msp-2 (FC27 and IC3D7) allelic types were included in all runs. Negative controls were devoid of target DNA.
In the secondary reaction, allele-specific primers were used to amplify the K1, MAD-20, and RO33 alleles of msp-1 and the FC27 and IC3D7 alleles of msp-2. For these, the reverse primers were labeled with different fluorescent dyes at the 5′ end, with K1 labeled with NED (yellow), MAD20 labeled with PET (red), RO33 labeled with VIC (green), FC27 labeled with 6-FAM (blue), and ICD37 labeled with VIC (green). All primer sets were obtained from Applied Biosystems (Foster City, CA). The reaction volume for both msp-1 and msp-2 allelic amplification was 25 µL, containing 1 µL of the primary amplicons, 19.5 µL PCR-grade RNAse/DNAse free water, 2.5 µL of 1× MyTaq buffer (Bio-line), 0.3125 µL of 125 nm of each allelic-type primer, 1 µL of 1 mM Mgcl2, 0.315 µL of 125 µM dNTPs, and 0.1 µL of 0.02 U of MyTaq DNA polymerase (Bio-line). The cycle conditions for the msp-1 alleles were as follows: initial denaturation at 95°C for 1 minute, followed by 35 cycles of 95°C for 15 seconds, annealing at 61°C for 15 seconds, and extension at 72°C for 10 seconds. For the msp-2 alleles, the cycle conditions consisted of an initial denaturation step at 95°C for 1 minute, followed by 35 cycles at 95°C for 15 seconds, annealing at 58°C for 15 seconds, and extension at 72°C for 10 seconds. All amplifications were performed in a 96-well thermal cycler (HID Veriti; Applied Biosystems). The PCR products were wrapped in aluminum foil to avoid photobleaching of the fluorescent dyes and stored at −20°C until required. Size fractionation was performed by high-resolution capillary electrophoresis on the 3130 genetic analyzer, with fragment sizing and analysis done using GeneMapper Software v4.0 (Applied Biosystems). Each PCR fragment was considered to represent a P. falciparum population of a single allelic variant. The minimum number of genotypes at each locus was determined for each sample as described previously [18].
Data Analysis
Data were analyzed for the STOP-CTX and CTX arms at enrollment and all scheduled and sick follow-up visits. Scheduled visits where the participants were also sick were included in analyses of data from scheduled and sick visits. Descriptive statistics of malaria parasitemia, parasite burden, and number of genotypes in each allelic family of msp-1 and msp-2 (MOI) were computed. Parasitemia in this study was defined as any parasites detected in both study arms. The number of distinguishable alleles for msp-1 and msp-2 was determined for each isolate, and the largest of these numbers was considered the MOI. The frequency of mixed infections, defined as an MOI of >1, was compared between the 2 study arms. χ2 tests were used to test for differences between study arms in parasitemia prevalence over time. In the subset of visits where parasites were detected, parasite density values were log transformed, and the difference between arms over the follow-up period was tested using a generalized estimating equations model with identity link, independence working correlation structure, and robust standard errors, to handle repeated measures within individuals. Rates of incident parasitemia in the 2 study arms were calculated on the basis of person-time at risk. Time at risk included time from the date of randomization that each participant remained parasite free, completed 1 year of follow-up, or was withdrawn from the study, whichever came first. Participants with parasitemia at enrollment did not contribute person-time to the incident parasitemia analysis. All tests were 2 sided at the 5% significance level.
We used Stata, version 12.1 [19], for all statistical analyses.
RESULTS
Recruitment and Follow-up Visits
As described by Polyak et al [10], participant recruitment commenced in February 2012. Five hundred subjects were randomized into 2 arms of 250 each. Of the 250 enrolled in the CTX arm, 247 had parasite data available at baseline (month 0 [M0]). Upon enrollment, participants were scheduled for follow-up at M3, M6, M9, and M12. Enrollment was complete by August 2012, and follow-up was completed by August 2013. Retention was 98%, with similar follow-up rates in each arm of the randomized controlled trial.
CTX Discontinuation Was Associated With a High Burden of Malaria Parasites
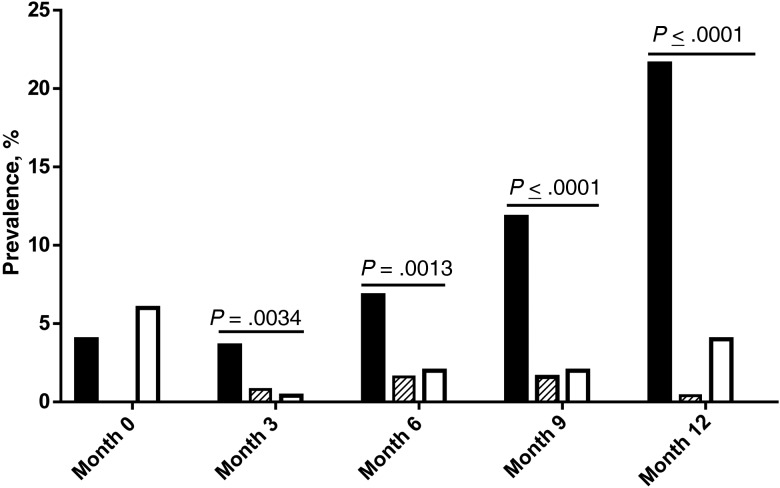
Of 500 enrolled participants who were receiving CTX at baseline, 498 had parasite data available, of whom 25 (5%) had detectable malaria parasites. The 5% parasitemia prevalence did not differ significantly between those randomized to the STOP-CTX arm (11 of 250 [4%]) and those randomized to the CTX arm (14 of 247 [6%]; P = .53). In participants with parasitemia at baseline, the mean log10 parasite density did not differ between the STOP-CTX arm (2.42 log10 parasites/mL) and the CTX arm (2.37 log10 parasites/mL; P = .90). As shown in Figure 1, the mean parasitemia prevalence increased steadily in the STOP-CTX arm (4% [11/248] at M3, 8% [21/249] at M6, and 14% [33/244] at M9), with a >5-fold increase by M12 (22% [54/245]). In comparison, the CTX arm had a parasitemia prevalence of <1% (1/248) at M3, 2% (5/247) at M6, 2% (5/245) at M9, and 4% (10/245) at M12 (P ≤ .0034 for each of the 4 comparisons). Of 119 parasitemia events at scheduled visits in the STOP-CTX arm, only 11 (9.2%) were associated with clinical symptoms. In addition, 16 other malaria events were detected at sick visits. In comparison, none of the 24 parasitemia events after enrollment in the CTX arm was associated with clinical malaria. Despite the marked increase in parasitemia prevalence in the STOP-CTX arm, 80% (108/135) of the parasitemia events were asymptomatic.
Figure 1.
Malaria parasite point prevalence among patients continuing cotrimoxazole (CTX) therapy (the CTX arm) and those ceasing CTX therapy (the STOP-CTX arm). Black filled bars represent asymptomatic cases in the STOP-CTX arm, and striped bars represent clinical cases in the STOP-CTX arm. Open bars represents asymptomatic cases in the CTX arm. Significant difference in the overall (clinical and asymptomatic) parasitemia prevalence between the study arms were detected after enrollment. No clinical cases were detected in the CTX arm. χ2 tests were used to test for differences between study arms in parasitemia prevalence over time.
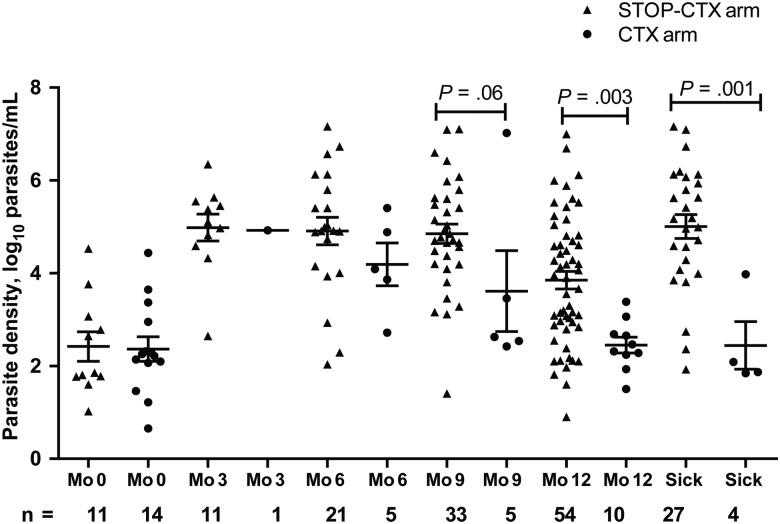
Figure 2 shows the parasite density for all visits where parasites were detected. The STOP-CTX arm had higher mean log10 parasite densities than the CTX arm, with values of 4.98 and 4.92 log10 parasites/mL, respectively, at M3, 4.91 and 4.19 log10 parasites/mL at M6, 4.85 and 3.61 log10 parasites/mL at M9, 3.85 and 2.45 log10 parasites/mL at M12, and 5.01 and 2.44 log10 parasites/mL at sick visits due to malaria. When all postenrollment visits were combined, the mean log10 parasite density was 4.42 log10 parasites/mL in the STOP-CTX arm and 3.13 log10 parasites/mL in the CTX arm, and the mean difference (1.29 log10 parasites/mL; 95% confidence interval [CI], .74–1.85 log10 parasites/mL) was statistically significantly higher in the STOP CTX arm (P < .001).
Figure 2.
Scatterplot showing malaria parasite density at enrollment, quarterly visits, and sick visits among patients continuing cotrimoxazole (CTX) therapy (the CTX arm) and those ceasing CTX therapy (the STOP-CTX arm). Data are limited to patients with parasites detected. Triangles represent the STOP-CTX arm, whereas circles represent the CTX arm. At all visits after enrollment, the STOP-CTX arm had more malaria parasite–infected subjects and a higher mean parasite density than those in the CTX arm. Differences in parasite density between arms over the follow-up period was tested using a generalized estimating equations model with identity link, independence working correlation structure, and robust standard errors to handle repeated measures within individuals. Error bars represent means with standard errors of the mean.
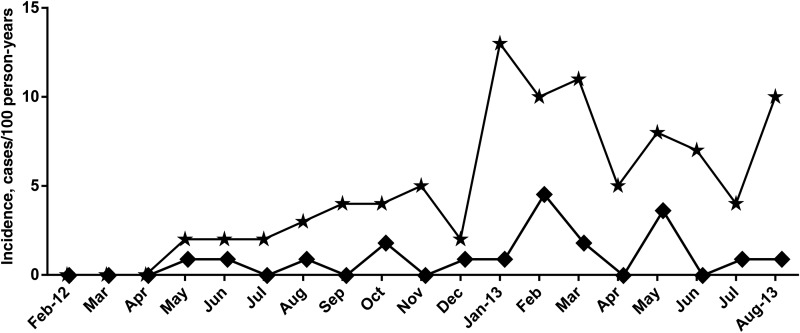
Figure 3 shows parasitemia incidents over the study period that started in February 2012. Over the 12-month follow-up period, 474 study subjects had 232 person-years of follow-up in the CTX arm and 215 person-years of follow-up in the STOP-CTX arm, during which 24 new parasitemia events developed in the CTX arm, compared with 90 in the STOP-CTX arm. The rate of parasitemia in the CTX arm was 9.9 cases per 100 person-years. compared with 42.0 cases per 100 person-years in the STOP-CTX arm, yielding an incidence rate ratio of 4.3 (95% CI, 2.7–7.1; P < .0001). As shown in Figure 3, in the STOP-CTX arm, new parasitemia events were highest 1 year after the start of the study (January–March 2013) and August 2013.
Figure 3.
Malaria parasitemia incidence rates by calendar month among patients continuing cotrimoxazole (CTX) therapy (the CTX arm) and those ceasing CTX therapy (the STOP-CTX arm). Stars represent new malaria parasitemia events in the STOP-CTX arm, and diamonds represent the CTX arm. The rate of new infections was higher in the STOP-CTX arm and peaked one year after the start of the study (January to March 2013) and then in August 2013 that coincided with increasing time after cessation of CTX therapy.
MOIs
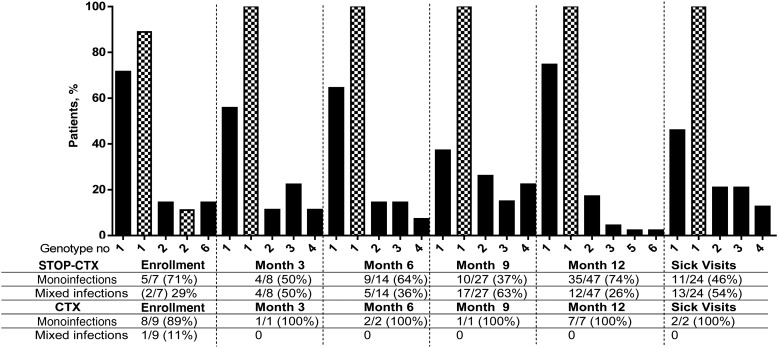
Of the Plasmodium-positive blood samples, genotyping was successful in 64% (7 of 11) at M0, 89% (8 of 9) at M3, 82% (14 of 17) at M6, 93% (27 of 29) at M9, 89% (47 of 53) at M12, and 89% (24 of 27) for sick visits in the STOP-CTX arm. For the CTX arm, genotyping was less successful, with values of 64% (9 of 14) at M0, 100% (1 of 1) at M3, 40% (2 of 5) at M6, 25% (1 of 4) at M9, 70% (7 of 10) at M12, and 50% (2 of 4) for sick visits. The lower success in genotyping specimens from the CTX arm is likely due to low parasite density, compared with the STOP-CTX arm (Figure 2).
The number of distinguishable alleles for msp-1 and msp-2 was determined, and the largest of these numbers was considered the MOI of that sample. The distribution of MOIs at enrollment, at quarterly visits, and during malaria sick visits is shown in Figure 4. The frequency of mixed infections, defined as an MOI of >1, increased steadily throughout the year in the STOP-CTX arm, with values of 28% (2 of 7) at enrollment, 44% (4 of 9) at M3, 36% (5 of 14) at M6, 52% (15 of 29) at M9, and 26% (12 of 47) at M12, and was highest (54% [13 of 24] at sick visits. In comparison, mixed infections in the CTX arm were only detected at enrollment (11% [1 of 9]), and all subsequent visits, including the sick visit, had single infections (Figure 4).
Figure 4.
Multiplicity of infections at enrollment, quarterly visits, and malaria sick visits among patients continuing cotrimoxazole (CTX) therapy (the CTX arm) and those ceasing CTX therapy (the STOP-CTX arm). Black bars represent the STOP-CTX arm, whereas hatched bars represent the CTX arm. After enrollment, all infections in the CTX arm were monoinfections. More mixed infections were observed in individuals who stopped CTX therapy.
DISCUSSION
Many studies have established the clinical relevance of continuing the use of CTX to reduce the risk of malaria in HIV-infected adults [9–11]. In this study, the use of molecular methods allowed evaluation and characterization of the asymptomatic parasitemia that would not be evaluable by other malaria diagnostic methods or in subjects without clinical symptoms. We demonstrate progressively increasing parasitemia prevalence, incidence rate ratio, and MOI among ART-treated HIV-infected adults who discontinued CTX, compared with those who continued CTX. In addition, at each scheduled visit, parasite density was higher in the STOP-CTX arm as compared to the CTX arm. Despite provision and use of insecticide-treated nets and immune recovery following a median of 4.5 years of ART use, >20% of individuals had malaria parasitemia 1 year after CTX discontinuation. Parasitemia prevalence increased steadily from quarter to quarter, with marked increases noted by 9 months after cessation. At enrollment, 95% had no malaria parasitemia, illustrating the ability of CTX prophylaxis to control parasitemia. This is consistent with earlier studies that demonstrated a protective efficacy rate of up to 92% for CTX prophylaxis [6, 7]. The 5% of individuals with parasitemia at baseline may not have been adherent to CTX, as suggested by a much lower parasitemia prevalence later in follow-up among individuals in the CTX arm. Alternatively, some may have had CTX-resistant parasites. Resistance to CTX by Streptococcus pneumoniae attributable to the same genes that code for antifolate resistance in malaria parasites (dihydropteroate synthase and dihydrofolate reductase) has been reported in Europe [20] and Uganda [21]. A previous study in Kisumu by Hamel et al [22], showed that CTX retains antimalarial efficacy even in regions where antifolate resistance to malaria is high [23, 24], such as western Kenya [25]. We attribute the 5% parasitemia at enrollment to nonadherence. Participants received biweekly telephone calls to reinforce adherence, and this may explain the drop in parasitemia prevalence to <1% by M3 in the CTX arm.
We found that parasitemia prevalence in the STOP-CTX arm increased steadily every quarter to 22% (a >5-fold increase) by M12 after enrollment. Given that CTX has a short half-life (10–18 hours), within 2 weeks of stopping the drug, there would be no expected residual CTX prophylactic effect [26]. Our data demonstrate the period required for reconstitution of a malaria parasite reservoir in a previously aparasitemic population. Eighty percent of parasitemias were asymptomatic, suggesting insufficient parasitemia to cause disease. Thus, our cohort of immune-reconstituted adults receiving ART (for >4 years and with a CD4+ T-cell count >350 cells/mm3) appears similar to their HIV-uninfected semi-immune counterparts [27].
As previously reported for this randomized controlled trial, individuals who developed clinical malaria did not have severe infections [10]. CTX may provide a community benefit in decreasing the parasitemic reservoir and subsequent infections in children or in HIV-infected individuals not receiving ART. However, given a comparable malaria risk in ART-recipient HIV-infected adults and HIV-uninfected adults and the potential, despite the availability of ART, for antibiotic resistance with widespread CTX use when CTX therapy is continued indefinitely in settings where the HIV prevalence is high, it will be important to model the long-term community and individual risk/benefit of CTX to inform policy.
Parasite density in the CTX arm decreased throughout follow-up, demonstrating the efficacy of CTX in controlling parasitemia. In the STOP-CTX arm, parasite density, although higher at each scheduled visit as compared to that in CTX group, did not increase over time (Figure 2).
The rate of acquisition of new malaria parasitemias was evaluated in the STOP-CTX and CTX arms (Figure 3). Over the observation period, the STOP-CTX arm had a significantly higher incidence of parasitemia (42.0 cases per 100 person-years in the STOP-CTX vs 9.9 cases per 100 person-years in the CTX arm). In the STOP-CTX arm, new parasitemia events were highest 1 year after the start of the study (January–March 2013) and August 2013. We think that the highest parasitemia incidences, seen in February 2013 and August 2013, are a consequence of the cumulative increases in parasitemia prevalence with increasing time after CTX cessation. As also in Figure 1, this process is gradual, increases steadily, and took 1 year to yield a PCR-detectable parasitemia prevalence of 22% in the STOP-CTX population.
We were unable to compare the incidence of parasitemia in the study population to that in HIV-uninfected adults in the same region because we did not enroll a concurrent control group of HIV-uninfected individuals. However, the 22% asymptomatic parasitemia prevalence at M12 in the STOP-CTX arm is comparable to the 20.9% cross-sectional prevalence of asymptomatic malaria in adults living in western Kenya in 2015 [28].
We noted more mixed infections among individuals who stopped CTX therapy than among those who continued CTX therapy (Figure 4). The highest frequency (63%) was detected at M9. In comparison, after enrollment, all infections in the CTX arm were monoinfections. After enrollment, 40% of the malaria parasite infections detected in the STOP-CTX arm had a cumulative mean MOI of 1.8, similar to the mean MOI of 2 observed over a 1-year period in the study by Baliraine et al in western Kenya [29]. The reported MOI by Baliraine et al involved younger individuals (15–17 years old), among whom only a subset (0.9%) would be expected to have HIV infection, based on prevalence data for western Kenya [30]. This is consistent with the hypothesis that HIV-infected adults restore their malaria semi-immune status during ART.
In conclusion, the data presented here demonstrate that discontinuation of CTX prophylaxis by individuals with HIV during ART results in a progressive increase in the prevalence of asymptomatic malaria parasitemia, an increase in rates of multiple strains, and a higher parasite burden. This is in addition to increased episodes of clinical malaria noted in our previous publication [10]. Taken together, our data suggest that most HIV-infected patients who are receiving ART and have immune reconstitution are semi-immune to malaria and that continued CTX use may provide a community benefit in decreasing the parasitemic reservoir and subsequent infections in susceptible populations.
Notes
Acknowledgments. We thank the participants in the Homa Bay community; the Homa Bay District Hospital and clinic staff; the PSC and the local Médecins Sans Frontières staff, for their collaboration; officials in the KEMRI and Kenyan Ministry of Health; our community advisory board; the KEMRI–University of Washington study team; the University of Washington Department of Global Health; the US Army Medical Research Unit–Kenya; the US Military HIV Research Program, for its support; and the members of the independent data monitoring safety board, Drs Bryan Shepherd (chair), John T. Brooks, James Campbell, Moses Kamya, Bernhards Ogutu, and Sten Vermund, for their expertise and guidance.
Disclaimer. The US government has the right to retain a nonexclusive, royalty-free license, and any copyright covering this article. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting true views of the Department of the Army, Department of the Navy, or Department of Defense.
Financial support. This work was supported by the Armed Forces Health Surveillance Branch and its Global Emerging Infections Surveillance and Response section (ProMIS identifiers CO519_11_KY and CO608_12_KY), the Merle A. Sande Award in International Infectious Diseases, Infectious Disease Society of America; and the Eunice Kenndy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH; grant K24-HD054314 to G. J. S.); and the National Institute of Allergy and Infectious Diseases, the National Cancer Institute, the National Institute of Mental Health, the National Institute on Drug Abuse, the NICHD, the National Heart, Lung, and Blood Institute, the National Institute on Aging, the National Institute of General Medical Sciences, and the National Institute of Diabetes and Digestive Kidney Diseases, NIH (award P30AI027757).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.UNAIDS. HIV/AIDS Fact sheet 2016. http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf. Accessed 25 October 2016.
- 2.Anglaret X, Chêne G, Attia A et al. . Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Côte d'Ivoire: a randomised trial. Lancet 1999; 353:1463–8. [DOI] [PubMed] [Google Scholar]
- 3.Wiktor SZ, Sassan-Morokro M, Grant AD et al. . Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Cote d'Ivoire: A randomised controlled trial. Lancet 1999; 353:1469–75. [DOI] [PubMed] [Google Scholar]
- 4.Walker A, Ford D, Gilks C et al. . Daily co-trimoxazole prophylaxis in severely immunosuppressed HIV-infected adults in Africa started on combination antiretroviral therapy: an observational analysis of the DART cohort. Lancet ; 2010; 375:1278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manyando C, Njunju EM, D'Alessandro U, Van Geertruyden J-P. Safety and efficacy of co-trimoxazole for treatment and prevention of Plasmodium falciparum malaria: a systematic review. PLoS One 2013; 8:e56916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watera C, Todd J, Muwonge R et al. . Feasibility and effectiveness of cotrimoxazole prophylaxis for HIV-1-infected adults attending an HIV/AIDS clinic in Uganda. JAIDS J Acquir Immune Defic Syndr 2006; 42:373–8. [DOI] [PubMed] [Google Scholar]
- 7.Mermin J, Lule J, Ekwaru JP et al. . Effect of co-trimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda. Lancet 2004; 364:1428–34. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization (WHO). Guidelines on co-trimoxazole prophylaxis for HIV-related infections among children, adolescents and adults. Geneva, Switzerland: WHO, 2006. http://www.who.int/hiv/pub/guidelines/ctxguidelines.pdf. Accessed 27 August 2015.
- 9.World Health Organization (WHO). Guidelines on post-exposure prophylaxis for HIV and the use of co-trimoxazole prophylaxis for HIV-related infections among adults, adolescents and children: recommendations for a public health approach. Geneva, Switzerland: WHO, 2014. [PubMed]
- 10.Polyak CS, Yuhas K, Singa B et al. . Cotrimoxazole prophylaxis discontinuation among antiretroviral-treated HIV-1-infected adults in Kenya: a randomized non- inferiority trial. PLoS Med 2016; 13:361:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell JD, Moore D, Degerman R et al. . HIV-infected Ugandan adults taking antiretroviral therapy with CD4 counts >200 cells/ L who discontinue cotrimoxazole prophylaxis have increased risk of malaria and diarrhea. Clin Infect Dis 2012; 54:1204–11. [DOI] [PubMed] [Google Scholar]
- 12.Division of Malaria Control, Ministry of Public Health and Sanitation. 2010 Kenya malaria indicator survey, 2010. http://dhsprogram.com/pubs/pdf/MIS7/MIS7.pdf. Accessed 21 November 2015.
- 13.National AIDS Control Council, National AIDS and STI Control Programme. Kenya HIV County Profiles. 2014; 150 http://www.nacc.or.ke/attachments/article/464/KenyaCountyProfilesBook_Nov_print.pdf. Accessed 10 February 2015.
- 14.Kamau E, Tolbert LS, Kortepeter L et al. . Development of a highly sensitive genus-specific quantitative reverse transcriptase real-time PCR assay for detection and quantitation of plasmodium by amplifying RNA and DNA of the 18S rRNA genes. J Clin Microbiol 2011; 49:2946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waitumbi JN, Anyona SB, Hunja CW et al. . Impact of RTS,S/AS02A and RTS,S/AS01B on genotypes of P. falciparum in adults participating in a malaria vaccine clinical trial. PLoS One 2009; 4:e7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waitumbi JN, Gerlach J, Afonina I et al. . Malaria prevalence defined by microscopy, antigen detection, DNA amplification and total nucleic acid amplification in a malaria-endemic region during the peak malaria transmission season. Trop Med Int Health 2011; 16:786–93. [DOI] [PubMed] [Google Scholar]
- 17.Liljander A, Wiklund L, Falk N et al. . Optimization and validation of multi-coloured capillary electrophoresis for genotyping of Plasmodium falciparum merozoite surface proteins (msp1 and 2). Malar J 2009; 8:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waitumbi JN, Anyona SB, Hunja CW et al. . Impact of RTS, S / AS02 A and RTS, S / AS01 B on genotypes of P. falciparum in adults participating in a Malaria vaccine clinical trial. PLoS One 2009; 4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stata 12. College Station, TX: StataCorp, 2011.
- 20.Schmitz FJ, Perdikouli M, Beeck A, Verhoef J, Fluit AC. Resistance to trimethoprim-sulfamethoxazole and modifications in genes coding for dihydrofolate reductase and dihydropteroate synthase in European Streptococcus pneumoniae isolates. J Antimicrob Chemother 2001; 48:935–6. [DOI] [PubMed] [Google Scholar]
- 21.Wilén M, Buwembo W, Sendagire H, Kironde F, Swedberg G. Cotrimoxazole resistance of Streptococcus pneumoniae and commensal streptococci from Kampala, Uganda. Scand J Infect Dis 2009; 41:113–21. [DOI] [PubMed] [Google Scholar]
- 22.Hamel MJ, Greene C, Chiller T et al. . Does cotrimoxazole prophylaxis for the prevention of HIV-associated opportunistic infections select for resistant pathogens in Kenyan adults? Am J Trop Med Hyg 2008; 79:320–30. [PubMed] [Google Scholar]
- 23.Kamya MR, Gasasira AF, Achan J et al. . Effects of trimethoprim-sulfamethoxazole and insecticide-treated bednets on malaria among HIV-infected Ugandan children. AIDS 2007; 21:2059–66. [DOI] [PubMed] [Google Scholar]
- 24.Laufer MK, Plowe CV. Cotrimoxazole prophylaxis and malaria in Africa: Have the important questions been answered? Am J Trop Med Hyg 2006; 75:373–4. [PubMed] [Google Scholar]
- 25.Juma DW, Omondi AA, Ingasia L et al. . Trends in drug resistance codons in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase genes in Kenyan parasites from 2008 to 2012. Malar J 2014; 13:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masters PA, O'Bryan TA, Zurlo J. Trimethoprim-sulfamethoxazole revisited. Arch Intern Med 2003; 163:402–10. [DOI] [PubMed] [Google Scholar]
- 27.Pinkevych M, Petravic J, Chelimo K, Kazura JW, Moormann AM, Davenport MP. The dynamics of naturally acquired immunity to Plasmodium falciparum infection. PLoS Comput Biol 2012; 8:e1002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lo E, Zhou G, Oo W, Afrane Y, Githeko A, Yan G. Low Parasitemia in submicroscopic infections significantly impacts malaria diagnostic sensitivity in the highlands of Western Kenya. PLoS One 2015; 10:e0121763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baliraine FN, Afrane YA, Amenya DA et al. . A cohort study of Plasmodium falciparum infection dynamics in Western Kenya Highlands. BMC Infect Dis 2010; 10:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng'eno B, Mwangi A, Ng'ang'a L et al. . Burden of HIV infection among children aged 18 months to 14 years in Kenya: results from a nationally representative population-based cross-sectional survey. JAIDS J Acquir Immune Defic Syndr 2014; 66(suppl 1):S82–8. [DOI] [PMC free article] [PubMed] [Google Scholar]