Abstract
Background. Neurocognitive disorders remain common among human immunodeficiency virus (HIV)–positive adults, perhaps owing to persistent HIV-1 RNA in cerebrospinal fluid (CSF) during antiretroviral therapy (ART).
Methods. Using a single-copy assay, we measured HIV-1 RNA levels in CSF and plasma specimens from 220 HIV-positive adults who were taking suppressive ART. Fifty-five participants were tested twice.
Results. HIV-1 RNA was detected in 42.3% of CSF and 65.2% of plasma samples. Correlates of higher CSF HIV-1 RNA levels included higher nadir and current CD4+ T-cell counts, a plasma HIV-1 RNA level of ≥ 1 copy/mL, and a lower central nervous system penetration-effectiveness score (model P < .001). Worse neurocognitive performance was associated with discordance in HIV-1 RNA detection between plasma and CSF, lower overall CSF HIV-1 RNA level, and longer ART duration, among others (model P < .001). In the longitudinal subgroup, CSF HIV-1 RNA persisted in most participants (69%) over 7 months.
Conclusions. Low-level HIV-1 RNA in CSF is common during suppressive ART and is associated with low-level HIV-1 RNA in blood, better immune status, and lower ART drug distribution into CSF. The association between HIV-1 RNA discordance and HIV-associated neurocognitive disorder (HAND) may reflect compartmentalization. The relationship between HAND, lower HIV-1 RNA levels in CSF, and lower CD4+ T-cell counts may reflect disturbances in the immune response to HIV-1 in the CNS.
Keywords: HIV, cerebrospinal fluid, cognitive disorders, antiretroviral therapy
Human immunodeficiency virus (HIV)–associated neurocognitive disorder (HAND) is common, with a prevalence ranging from 30% to 70% among HIV-infected adults, including those taking combination antiretroviral therapy (cART) [1–3]. Several explanations may account for this, including advancing age [4, 5], longer duration of exposure to HIV, comorbid conditions [6, 7], and more-advanced immune suppression [8, 9]. Another, nonexclusive explanation for high HAND prevalence among treated individuals is incomplete effectiveness or toxicity of ART in the central nervous system (CNS) [10].
HIV-1 enters the CNS soon after infection and can be protected in this compartment from immune and drug pressure [11, 12]. Autopsy and neuroimaging studies have identified that HIV-1 can localize in the basal ganglia and hippocampus [13, 14], even during the first weeks of infection [15]. Potent ART can reduce the HIV-1 level in blood and cerebrospinal fluid (CSF) below the quantification limit of commercially available assays, but HIV-1 might continue to replicate at low levels, increasing the risk for viral compartmentalization in the CNS [16]. Persistent low-level HIV-1 replication could also lead to glial activation and neuronal injury.
Published reports have identified that low-level HIV-1 is present in CSF in up to 28% of adults taking ART [17, 18] but have not found associations with estimated ART drug distribution into the CNS or neurocognitive outcomes. Limitations of these projects included their small sample size and the assay method. This method required ultracentrifugation of up to 12 mL of CSF and could therefore be prone to inaccuracy. Simpler methods, such as one that uses molecular beacons and does not require ultracentrifugation, might yield different results.
The objectives of this project were to determine how frequently HIV-1 RNA was present at low levels in CSF during suppressive ART and whether low-level HIV-1 RNA in CSF was associated with worse estimated ART drug distribution into the CNS and worse neurocognitive performance.
METHODS
Participants and Procedures
The CNS HIV-1 Antiretroviral Therapy Effects Research (CHARTER) cohort is composed of 1555 HIV-1–infected adults who provided written informed consent for all study procedures. All subjects completed venipuncture, neuromedical assessment, and comprehensive neurocognitive testing. The Human Subjects Protection Committees of each institution approved all procedures. A total of 220 participants were selected for this project on the basis of 4 criteria: use of 3-drug cART, HIV-1 RNA levels of ≤50 copies/mL in plasma and CSF, absence of comorbid conditions of sufficient severity to account for impaired neurocognitive performance [2], and availability of at least 2 mL of plasma and CSF stored at −70°C. Selected participants had been assessed between October 2003 and May 2008. To assess changes in low-level HIV-1 RNA over time, a second CSF sample was assayed in 55 participants whose ART regimen was stable and who had HIV-1 RNA levels of ≤50 copies/mL in plasma and CSF at the second time point.
Laboratory Assessment
HIV-1 infection was diagnosed by an enzyme-linked immunosorbent assay, with confirmation by a Western blot. Routine clinical chemistry panels, complete blood counts, rapid plasma reagin (RPR) tests, hepatitis C virus (HCV) antibody tests, and CD4+ T-cell analysis (by flow cytometry) were performed at each site's Clinical Laboratory Improvement Amendments–certified laboratory. HIV-1 RNA levels were measured centrally in plasma and CSF by reverse-transcription polymerase chain reaction (Roche Amplicor, version 1.5; lower limit of quantification, 50 copies/mL).
To measure HIV-1 RNA levels of <50 copies/mL, a validated single-copy assay procedure that has been used in other studies of HIV-1 RNA detection in CSF was used [19]. The HIV-1 SuperLow Assay (bioMONTR Labs) is a proprietary, modified version of the NucliSENS EasyQ assay (bioMèrieux) and is capable of quantifying HIV-1 levels as low as 1 copy/mL. The standard EasyQ assay was modified by extraction of 2 mL of fluid, using magnetic bead technology (miniMAG system from bioMèrieux). A total of 25 µL of extracted eluate, 20 µL of primer, and 5 µL of 2× enzyme in place of standard kit volumes were used. Molecular beacons targeting the pol/gag region of HIV-1 RNA are used for amplification and detection by isothermal reactions at 41°C. The HIV-1 RNA level was quantified using a proprietary reduction algorithm in conjunction with the NucliSENS EasyQ H HIV-1, version 2.0, Director software [20].
Neuromedical Assessment
This assessment included medical history, structured neurological and medical examination, and body fluid collection. Participants in this project reported use of 57 different ART combinations, the most common of which are summarized in the Supplementary Materials. Four-day ART adherence was estimated by self-report. ART drug distribution into the CNS was estimated by the CNS penetration-effectiveness (CPE) method [21].
Neurobehavioral Assessment
The comprehensive neurocognitive test battery assessed 7 cognitive domains affected by HIV-1 disease [2]. The best available normative standards were used, which adjusted for effects of age, education level, sex, and ethnicity. To classify the presence and severity of neurocognitive impairment, a published objective algorithm was used that requires presence of at least mild impairment in at least 2 cognitive domains, conforms to the Frascati criteria for diagnosing HAND [1], and yielded excellent interrater reliability in prior studies [22]. Neurocognitive performance was then summarized by the global clinical rating, a validated method that integrates relevant information about the 7 neurocognitive domains and yields a value between 1 (for normal performance) and 9 (for severely impaired performance), with a value of 5 indicating definite, mild impairment [23]. Frascati guidelines were also used to classify comorbid neuropsychiatric conditions, the most common of which are summarized in the Supplementary Materials.
Data Analyses
Statistical methods included Pearson correlation, t tests, multivariable regression, and recursive partitioning. Data distributions were inspected, and data were transformed to improve the symmetry of their distributions as needed. When transformation did not adequately improve the distribution of highly skewed data distributions, nonparametric statistical tests were used. When appropriate, the Cohen d value was calculated to estimate effect size [24]. Multivariable regression was performed in 4 stages. First, candidate covariates were screened using a selection α of 0.15. Candidates included age, current and nadir CD4+ T-cell count, estimated duration of HIV-1 disease, duration of the current ART regimen, CPE of the current regimen, total number of previous ART regimens, ART adherence, HCV serostatus, and RPR reactivity status. Second, all candidate covariates that met the selection criterion were included in a full model. Third, the Akaike information criterion (AIC) was used to identify the best model, using stepwise selection. Finally, first-order interactions were evaluated among retained covariates. When statistically significant interactions were found, the nature of the interaction was investigated with recursive partitioning. Analyses were performed in the total sample, as well as in the subgroup that tested negative for HCV and had nonreactive RPR tests (n = 155). Statistical analyses were performed using JMP, version 12 (SAS Institute, Cary, NC).
RESULTS
Demographic and Disease Characteristics
As summarized in Table 1, the median age of participants was 44 years (interquartile range [IQR], 39–50 years) and >75% were men. Subjects were from diverse racial/ethnic backgrounds. Most subjects had been infected for >10 years, had experienced advanced immune suppression in the past, and had an improved immune status during ART. About 25% were taking their initial cART regimen, and >50% had been receiving their current cART regimen for >1 year. Supplementary Materials include a summary of differences between this group and the larger CHARTER cohort.
Table 1.
Demographic and Clinical Characteristics of the Sample, Overall and by Human Immunodeficiency Virus Type 1 (HIV-1) RNA Level in Cerebrospinal Fluid (CSF)
| Characteristic | Overall (n = 220) | By CSF HIV-1 RNA Level, Copies /mL |
P Value | |
|---|---|---|---|---|
| ≥ 1 (n = 93) | <1 (n = 127) | |||
| Demographic | ||||
| Age, y | 44.0 (39–50) | 44.0 (38–51) | 44.0 (40–50) | .31 |
| Female sex | 22.6 | 24.5 | 21.3 | .57 |
| Race/ethnicity | .70 | |||
| Black | 36.2 | 38.3 | 34.6 | |
| Hispanic | 11.3 | 11.7 | 11.2 | |
| White | 50.2 | 48.9 | 51.2 | |
| Other | 2.3 | 1.1 | 3.2 | |
| HIV-1 disease and coinfection | ||||
| Time since HIV-1 diagnosis, mo | 121.5 (69–198) | 111.0 (66–190) | 139.9 (73–202) | .39 |
| CD4+ T-cell count | ||||
| Current, cells/µL | 503.0 (326–728) | 575.5 (348–788) | 451.0 (269–679) | .01a |
| Nadir | ||||
| Overall, cells/µL | 150.0 (36–261) | 176.5 (50–280) | 133.0 (27–225) | .06a |
| <200 cells/µL | 62.4 | 53.2 | 69.3 | .01a |
| Plasma HIV-1 RNA level ≥ 1 copy/mL | 65.2 | 69.2 | 62.2 | .28 |
| AIDS diagnosis | 68.8 | 61.7 | 74.0 | .05a |
| RPR reactive | 7.7 | 7.4 | 7.9 | .90 |
| HCV seropositive | 25.8 | 19.2 | 30.7 | .05a |
| ART | ||||
| Receiving first regimen | 22.2 | 22.3 | 22.0 | .96 |
| Duration of current regimen, mo | 16.1 (7–32) | 19.4 (5–33) | 14.9 (7–31) | .56 |
| Total duration of all regimens, mo | 70.0 (35–106) | 59.6 (32–104) | 73.5 (41–108) | .14a |
| Current regimen | ||||
| NNRTI containing | 44.8 | 46.8 | 43.3 | .60 |
| PI containing | 54.3 | 54.3 | 54.3 | .99 |
| Took ≥ 95% of doses in past 4 d | 91.9 | 92.6 | 91.3 | .74 |
| CPE | ||||
| Overall, mean ± SD | 7.1 ± 1.3 | 6.8 ± 1.2 | 7.2 ± 1.3 | .02a |
| Greater than or equal to median CPE (7) | 72.2 | 59.6 | 71.6 | .06a |
Data are percentage of subjects or median value (interquartile range) unless otherwise specified.
Abbreviations: ART, combination antiretroviral therapy; CPE, central nervous system penetration effectiveness; HCV, hepatitis C virus; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; RPR, rapid plasma reagin.
a Included in multivariable analyses.
Correlates of Low-Level HIV-1 in CSF
Primary Analyses
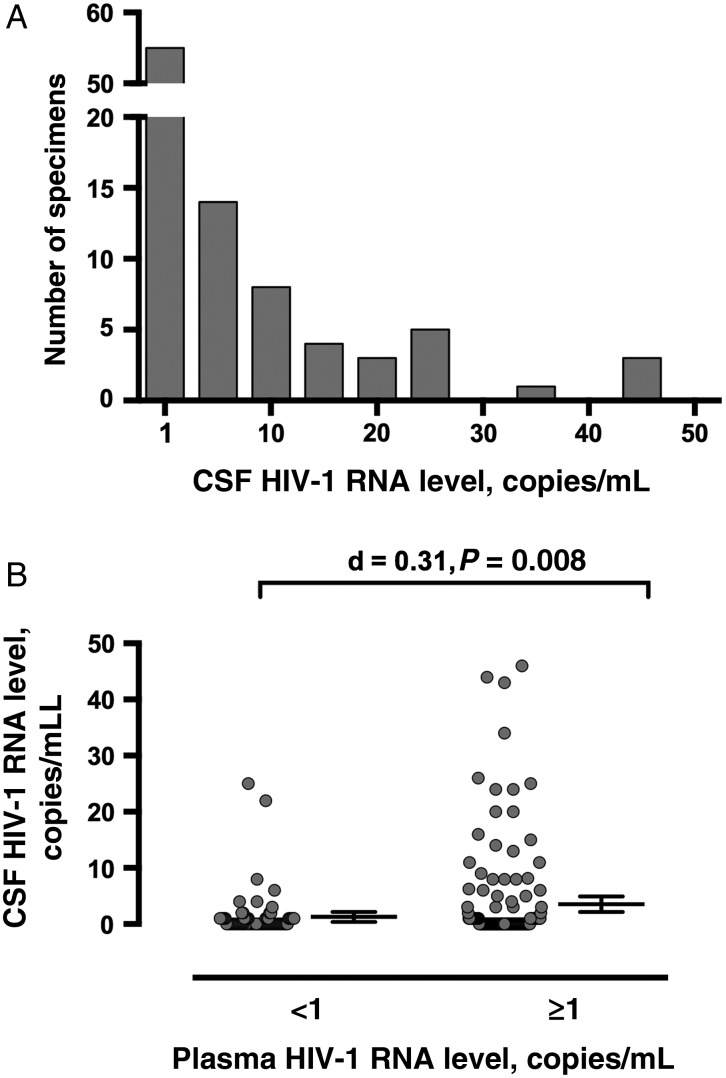
Ninety-three subjects (42.3%) had at least 1 copy/mL of HIV-1 RNA in CSF. Since the distribution of values was highly skewed (median, 0 copies/mL; mean, 2.75 copies/mL; Shapiro-Wilk W = 0.42; Figure 1A), categorical analyses (ie, binary transformation into subjects with <1 copy/mL and those with ≥1 copy/mL) were performed in addition to analyses of continuous HIV-1 RNA values. Table 1 summarizes the differences between participants who had ≥1 copy/mL of HIV-1 in CSF and those who did not.
Figure 1.
A, Distribution of human immunodeficiency virus type 1 (HIV-1) RNA levels among the 93 participants with values of ≥1 copy/mL. B, Although HIV-1 RNA levels in cerebrospinal fluid (CSF) did not correlate with those in plasma (not shown), participants who had HIV-1 RNA levels of ≥1 copy/mL in plasma had higher HIV-1 RNA levels in CSF. For clarity, the bars indicating mean values and 95% confidence intervals are shown next to the data points, rather than superimposed over them.
In the initial stage of multivariable logistic regression modeling of CSF HIV-1 RNA loads of ≥ 1 copy/mL (ie, the binary variable) that included all explanatory variables identified by univariate screening, only CD4+ T-cell count had a parameter estimate P value of < .05 (model R2 = 0.07; P = .01). In the next stage, AIC values selected higher current CD4+ T-cell counts (P = .005), lower CPE values (P = .009), HCV seronegativity (P = .08), and shorter duration of all ART regimens (P = .09) as associated with CSF HIV-1 RNA loads of ≥ 1 copy/mL (model R2 = 0.06; P < .001). Interaction modeling identified that higher CD4+ T-cell counts were only associated with CSF HIV-1 RNA loads of ≥ 1 copy/mL when CPE values were <7 (model R2 = 0.07; P < .001).
In analyses of continuous (instead of binary) CSF HIV-1 RNA levels, higher current CD4+ T-cell counts (P = .05) and lower CPE values (P < .001) were again selected in the best models. The best model also included HIV-1 RNA levels in plasma of ≥1 copy/mL (P = .04), race/ethnicity (higher CSF HIV-1 RNA levels were observed in participants who identified as black or Hispanic; P = .006), and higher nadir CD4+ T-cell counts (P = .08 [model R2 = 0.13; P < .001]). Interaction modeling identified that black or Hispanic participants who took regimens with a lower CPE (ie, those with values of <7) had the highest HIV-1 RNA levels in CSF.
Since HIV-1 RNA levels in CSF were associated with the presence of HIV-1 RNA in plasma (Figure 1B) in the strongest models, we categorized participants into 4 groups on the basis of the presence or absence of HIV-1 RNA in each body fluid. Two categories were concordant (ie, detectable [+; n = 65] or undetectable [−; n = 48] in both fluids) and 2 were discordant (detectable in CSF but undetectable in plasma [CSF+Plasma−; n = 28] or undetectable in CSF and detectable in plasma [CSF−Plasma+; n = 79]).
Secondary Analyses
Supplementary Materials include secondary analyses, including comparisons of CSF HIV-1 RNA with HCV and RPR serostatus, a subgroup analysis in participants who were HCV seronegative and RPR nonreactive, and comparisons with CSF inflammation-associated biomarkers, CSF protein, and CSF leukocytes.
Correlates of Neurocognitive Performance
Primary Analyses
Table 2 summarizes the analysis of global neurocognitive performance. Univariate analyses identified that worse neurocognitive performance was associated with lower CSF HIV-1 RNA levels, contributing neuropsychiatric conditions, HCV seropositivity, AIDS, and longer total duration of ART. Four other variables had P values between .05 and .15 and were included as candidate covariates in multivariable modeling. The initial model identified that the strongest covariates were CSF HIV-1 RNA level (Figure 2A), CSF+Plasma− discordance (Figure 2B), and HCV (model R2 = 0.13; P < .001). The AIC selection model (AIC model 1 in Table 2) identified associations between worse neurocognitive performance and 5 covariates (model R2 = 0.11; P < .001), including both lower CSF HIV-1 RNA level and the presence of CSF+Plasma− discordance. Interaction analysis (AIC model 2 in Table 2; model R2 = 0.18; P < .001) identified that lower HIV-1 RNA levels correlated with worse neurocognitive performance in HCV-seronegative participants (r = −0.25; P = .001) but not in HCV-seropositive participants (r = −0.02; P = .86).
Table 2.
Correlates of Worse Neurocognitive Performance in All 220 Participants
| Correlate | Risk Direction | Univariable Analysis | Full Model | AIC Model 1 | AIC Model 2 |
|---|---|---|---|---|---|
| CSF HIV-1 RNA level | Lower | .002 | .003 | .001 | .06a |
| HCV serostatus | Positive | .01 | .07 | .02 | .02a |
| Neuropsychiatric conditions | Contributing | .02 | .16 | .09 | .05a |
| Duration of all ART regimens | Longer | .03 | .28 | … | … |
| Duration of current ART regimen | Longer | .04 | .14 | .08 | .05a |
| RPR reactivity | Reactive | .07 | .28 | … | … |
| AIDS diagnosis | Present | .11 | .35 | … | … |
| Plasma HIV-1 RNA level | <1 copy/mL | .14 | .72 | … | … |
| CSF-plasma discordance group | CSF+Plasma−b | .14 | .07 | .03 | .04 |
| CPE | … | .18 | … | … | … |
| Nadir CD4+ T-cell count | … | .20 | … | … | … |
| Duration of HIV-1 disease | … | .25 | … | … | … |
| No. of ART regimens | … | .30 | … | … | … |
| PI-containing regimen | … | .90 | … | … | … |
| Current CD4+ T-cell count | … | .91 | … | … | … |
| NNRTI-containing ART regimen | … | .91 | |||
| 4-day ART adherence | … | .99 | … | … | … |
| Multivariable model statistics | … | … | < .001; R2 = 0.13 | < .001; R2 = 0.11 | < .001; R2 = 0.18 |
Data are P values, unless otherwise indicated.
Abbreviations: AIC, Akaike information criterion; ART, antiretroviral therapy; CPE, central nervous system penetration effectiveness; CSF, cerebrospinal fluid; HCV, hepatitis C virus; HIV-1, human immunodeficiency virus type1; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; RPR, rapid plasma reagin.
a Covariate is included in a first-order interaction term. An interaction with HCV was the only one to include CSF HIV RNA level. The others were between HCV and either (1) duration of the current ART regimen or (2) neuropsychiatric conditions.
b HIV-1 RNA level of ≥1 copy/mL in CSF and <1 copy/mL in plasma.
Figure 2.
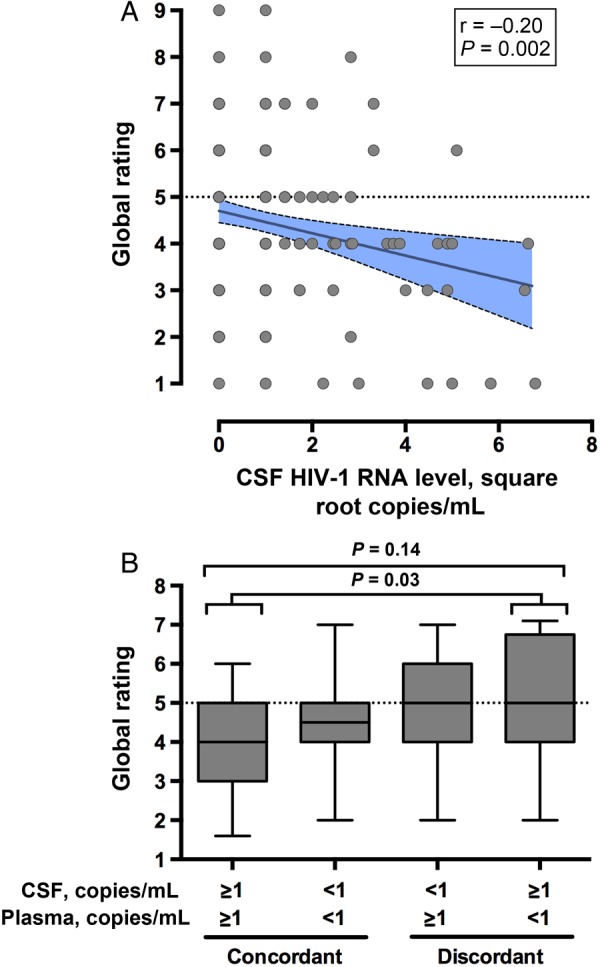
A, Lower human immunodeficiency virus type 1 (HIV-1) RNA levels in cerebrospinal fluid (CSF) correlated with worse global neurocognitive performance. B, The discordant group with an HIV-1 RNA of ≥1 copy/mL in CSF but a level of <1 copy in plasma (the CSF+Plasma− group) had worse neurocognitive performance than the group with an HIV-1 RNA level of ≥ 1 copy/mL in both fluids (the CSF+Plasma+ group). The dashed line indicates the threshold value for impairment (5). Global impairment was present in 60.7% of the CSF+Plasma− group, compared with 41.5% of the CSF+Plasma+ group (P = .09).
Among the 7 cognitive domains assessed, lower HIV-1 RNA levels in CSF were most strongly associated with worse performance in speed of information processing (r = −0.23; P = .004), learning (r = −0.20; P = .02), and working memory (r = −0.20; P = .01). In contrast, CSF + Plasma− discordance was associated with worse performance in 2 other cognitive domains, verbal fluency (P = .03) and possibly executive functioning (P = .09), suggesting that the mechanisms of injury for these 2 conditions are distinct.
Secondary Analyses
Supplementary Materials include comparisons of neurocognitive performance with HCV and RPR status, ART drug classes, and individual ART drugs.
Longitudinal Analysis of Low-Level HIV-1 in CSF Over Time
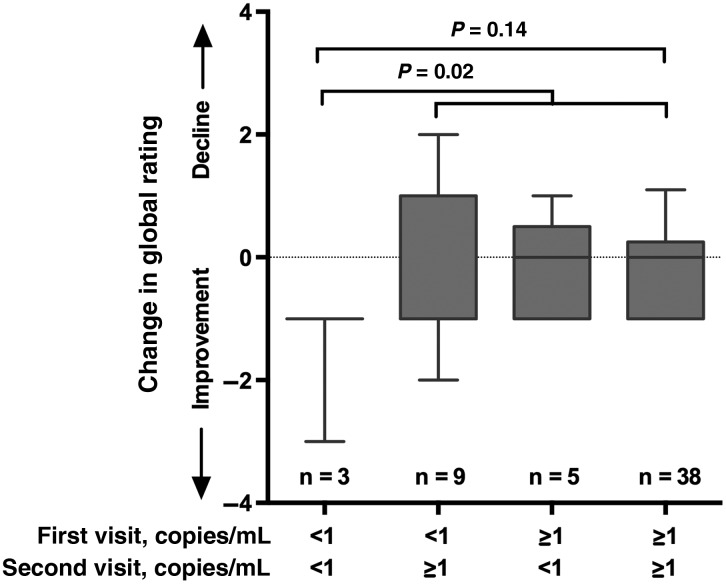
In the 55 subjects who had a second CSF specimen assayed, the median duration between visits was 7 months (IQR, 5.7–8.9 months). Forty-three subjects (78%) had HIV-1 RNA levels in CSF of ≥ 1 copy/mL at their first visit, and, among subjects in this group, HIV-1 RNA levels remained ≥1 copy/mL at the second visit in 38 (88%). Among the 12 subjects who had HIV-1 RNA levels of <1 copy/mL at the first visit, levels in 3 (25%) remained at <1 copy/mL at the second visit. Figure 3 shows the 4 groups defined by the presence or absence of HIV-1 RNA in CSF at the first or second visit against the change in global rating, identifying that the 3 subjects (5%) who maintained HIV-1 RNA levels of <1 copy/mL in CSF had improved neurocognitive performance, compared with those who had HIV-1 RNA levels of ≥1 copy/mL in CSF from at least 1 visit. No secondary analyses were performed in this subgroup because of the small sample size.
Figure 3.
Having a human immunodeficiency virus type 1 (HIV-1) RNA level of < 1 copy/mL in cerebrospinal fluid (CSF) at the first and second visits was associated with improved neurocognitive performance over time.
DISCUSSION
HAND continues to commonly occur, even in adults who are taking suppressive ART [2]. Consistent with this observation, substantial evidence has accumulated that systemic and end-organ inflammation can also persist during virologic suppression, perhaps as a result of production of HIV-1 RNA below the quantification limit of commercial assays [25]. These findings support the need to improve understanding of the mechanisms of CNS injury in the cART era.
In our study of 220 HIV-positive adults with HIV-1 RNA levels of ≤50 copies/mL in both plasma and CSF, we found low-level HIV-1 RNA in the CSF of approximately 4 of 10 participants. This proportion is higher than in prior studies, which have reported proportions up to 28% [17, 18, 26]. Explanations for this apparent disagreement include differences in the assay used, the sample size, and cohort characteristics. Our study is the largest to date, which may make our estimates more representative of clinical populations. While we used a different assay than prior studies, its accuracy has been previously validated in CSF [19]. CHARTER was designed to generalize to US clinical populations, but our study participants were a subgroup of the larger CHARTER cohort. For this reason, selection bias could also explain why our findings differ from those from other reports.
The finding that nearly two thirds of participants had HIV-1 RNA levels in blood of ≥1 copy/mL more closely matches prior reports from HIV-positive adults taking suppressive ART [27]. This low-level circulating HIV-1 is associated with chronic inflammation in both adults who take suppressive ART and in those who spontaneously control HIV [28, 29]. A similar scenario may occur in the CNS, in which low levels of HIV-1 RNA (or proteins) act as immune stimuli, resulting in chronic inflammation and injury of glia and neurons. Lymphocytes and monocytes that migrate into the CNS from the systemic circulation may be the source of low-level HIV-1 RNA in CSF in some cases, but the presence of HIV-1 RNA in CSF in the absence of detectable HIV-1 in blood suggests that viral compartmentalization in the CNS has occurred. Our study showed that approximately 1 in 8 participants (28 of 220 [12.7%]) had discordant HIV-1 RNA findings, with levels ≥1 copy/mL in CSF and <1 copy/mL in blood. Since these groups differ by as little as 1 copy of HIV RNA per milliliter of CSF or blood, the finding could theoretically occur because of differences in specimen processing or assay performance. However, the statistically significant associations between this discordant condition and several other characteristics support that it results from biological processes. Such discordance may be part of a spectrum of conditions recognized in more-severe forms as CSF viral escape, which also occurs in approximately 10% of adults taking suppressive ART [26, 30]. Our study demonstrated that CSF+Plasma− discordance was independently associated with worse global neurocognitive performance, although this association weakened when participants who were either HCV seronegative or RPR nonreactive were excluded. Combined, our findings support that syphilis, HCV infection, and HIV-1 escape in CSF could contribute to HAND in at least a subgroup of adults taking suppressive ART.
Several studies have reported that ART regimens that have greater estimated distribution into the CNS are associated with lower HIV-1 RNA levels in CSF [31, 32]. An important limitation of prior analyses, though, has been inclusion of participants who had plasma HIV-1 RNA levels of >50 copy/mL. Our study directly addresses this limitation by including only participants who were taking ART and had plasma HIV-1 RNA levels of ≤ 50 copy/mL. We again found that higher CPE values were statistically significantly associated with lower HIV-1 RNA levels in CSF, even in multivariable analyses. While reports linking CPE to HIV-1 RNA levels in CSF have had consistent findings, those comparing CPE to neurocognitive performance or neuroimaging findings have not. For instance, some reports have found that regimens yielded higher CPE values were associated with better neurocognitive outcomes [33], while others have found evidence of worse outcomes [34]. While differences in design and power account for at least some of these inconsistencies [35], disagreement between studies could also reflect that HIV-1 RNA levels in CSF relate differently to neurocognitive outcomes during treatment with today's potent regimens than in the past.
Our analyses also found that lower HIV-1 RNA levels in CSF were associated with worse neurocognitive performance, the direction of which is contrary to our hypothesis. Since higher CPE regimens may sometimes be prescribed for HAND and were associated with lower HIV-1 RNA levels in CSF in this project, one possible explanation for this unexpected, cross-sectional finding is that some participants had preexisting HAND that had not fully responded to ART [36]. Consistent with this, secondary analyses in a subgroup of 109 participants who had been previously assessed supported that neurocognitive impairment was not improving (Supplementary Materials). This could be due to ongoing disturbances in the CNS immune response, which may be particularly prominent in patients whose CD4+ T-cell counts have previously declined to low levels.
The relationship between low CD4+ T-cell counts and low HIV-1 RNA levels provides another clue about how low HIV-1 RNA levels in CSF might predispose to HAND. Multiple studies have found that a low nadir CD4+ T-cell count increases the risk for developing HAND and that this risk appears to persist even after immune recovery [9]. This may reflect persistent disturbances in the CNS immune response, characterized by altered migration and activity of monocytes and lymphocytes that affect HIV clearance and compartmentalization [37–39]. The correlation between lower CSF leukocyte number (Supplementary Materials) and lower HIV-1 RNA level may indicate that fewer activated, replication-competent cells are being pushed into the CNS from the periphery and/or that fewer are being pulled into the CNS by HIV replication in CNS-resident cells [40]. While an overly robust immune response could injure the brain [41], the absence of an adequate response could favor development of compartmentalization [39], could deprive the brain of neurotrophic factors that would support recovery from HAND [42], and could worsen control in the CNS of other pathogens, such as Treponema, HCV, cytomegalovirus, and Toxoplasma.
Another possible and nonmutually exclusive explanation is ART neurotoxicity, either directly, via neuronal or glial injury [32, 43], or indirectly, via metabolic or vascular disease [44, 45]. The observed association between worse neurocognitive performance and the combination of lower HIV-1 RNA levels in CSF (as an indicator of more-potent ART) and longer durations of ART support this conclusion. Weighing against the explanation of ART neurotoxicity is the absence of an association between worse neurocognitive performance and either higher CPE values or use of individual ART drugs with known neurotoxicity, such as efavirenz [46, 47]. Subgroup analyses, while modest in scope, also supported that lower HIV-1 RNA levels in CSF may be beneficial, such as the longitudinal analysis or the correlation with lower levels of some inflammation biomarkers in CSF (data not shown: interleukin 6, ρ = 0.49 [P = .01]; TNF-α, ρ = 0.36 [P = .07]). Any of these effects (ART-unresponsive HAND, disturbed CNS immune response, and ART toxicity) could affect patients with contributing neuropsychiatric conditions to a greater extent than those with minimal neuropsychiatric comorbidities, consistent with our findings and the concept of cognitive reserve or vulnerability [48].
The discussion thus far has focused on the detrimental effects of undetectable HIV-1 RNA levels in CSF, but the converse might also be true. Could the presence of low-level HIV-1 RNA in CSF protect from HAND? If low-level HIV-1 RNA reflects persistent HIV-1 replication, then it might be due to the presence of drug resistance mutations. If these drug resistance mutations include those that reduce viral fitness, such as M184V, then this could be associated with better neurocognitive outcomes, as we have previously found [49]. If persistent HIV-1 replication also stimulates a more effective immune response that reduces the size of the CNS reservoir, then the combination of reduced viral fitness and viral clearance from the CNS should be beneficial. The current analysis does not include data to directly test this hypothesis.
The strengths of our study include its large sample size, the comprehensiveness of the assessments, and the sensitivity of the single-copy assay. Its weaknesses include its cross-sectional design of the primary study, the post hoc nature of some of the analyses, the inconsistent direction of the cross-sectional and longitudinal analyses, the small longitudinal sample size, and the heterogeneity of the cohort, including nearly 30% having either a positive result of HCV antibody testing or a reactive RPR test. Our selected study population may also be less generalizable to clinical populations than the larger CHARTER cohort. In addition, our best multivariable models had relatively modest coefficients of determination, indicating that they explained <20% of variation in viral or neurocognitive outcomes. Validation of our findings in an independent cohort is essential.
If correct, our findings support a complex approach for HAND management in which clinicians should consider factors such as ART drug characteristics, CD4+ T-cell counts, neuropsychiatric conditions, and coinfections. The best approach may be the use of ART regimens that balance sufficient potency in the CNS with the absence of neurotoxicity, similar to those currently recommended by the US Department of Health and Human Services [50]. Ultimately, randomized controlled trials of ART regimens will be needed to inform the clinical management of HAND.
STUDY GROUP MEMBERS
CHARTER is affiliated with the Johns Hopkins University, the Icahn School of Medicine at Mount Sinai, the University of California–San Diego, the University of Texas–Galveston, the University of Washington–Seattle, Washington University–St. Louis, and the University of California–San Diego (headquarters) and includes the following: Robert K. Heaton, PhD, and Scott Letendre MD, directors; I. Grant, MD, J. Allen McCutchan, MD, Ronald J. Ellis, MD, PhD, and Thomas D. Marcotte, PhD, codirectors; Donald Franklin Jr, center manager; Ronald J. Ellis, MD, PhD (Neuromedical Component); David M. Smith, MD, Scott Letendre, MD, and Brookie Best, PharmD (Laboratory and Virology Component); Robert K. Heaton, PhD, J. Hampton Atkinson, MD, and Matthew Dawson (Neurobehavioral Component); Christine Fennema-Notestine, PhD, and Sarah Archibald (Imaging Component); Clint Cushman (Data Management Unit); Ian Abramson, PhD, and Florin Vaida, PhD (Statistics Unit); and Thomas D. Marcotte, PhD (Protocol Coordinating Component). The Johns Hopkins University site includes Ned Sacktor (principal investigator [PI]) and Justin McArthur; the Icahn School of Medicine at Mount Sinai site includes Susan Morgello, MD (co-PI), David Simpson, MD (co-PI), Letty Mintz, NP, Cheuk Tang, PhD, and Thomas Naidich, MD; the University of California–San Diego site includes J. Allen McCutchan, MD (PI); the University of Washington–Seattle site includes Ann Collier, MD (co-PI), Christina Marra, MD (co-PI), Kenneth Maravilla, MD, K. C. Stegbauer, PhD, and Trudy Jones, MN, ARNP; the University of Texas–Galveston site includes Benjamin Gelman, MD, PhD (PI), Eleanor Head, RN, BSN, and Gregory Chaljub, MD; and the Washington University–St. Louis site includes David Clifford, MD (PI), Muhammad Al-Lozi, MD, and Mengesha Teshome, MD.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Financial support. This work was supported by the National Institutes of Health (grants N01 MH22005 [principal investigator {PI}, I. G.], R01 MH107345 [PIs, R. K. H. and S. L. L.], K24 MH097673 [PI, S. L. L.], and K23 MH095679 [PI, A. M. A.]).
Potential conflicts of interest. S. L. L. reports that research funding has been provided to the University of California–San Diego on his behalf by Gilead Sciences, ViiV Healthcare, and Merck and that he has also been paid for providing educational lectures or participating on advisory boards by Cipla, Gilead Sciences, Janssen, Merck, and ViiV Healthcare. A. C. C. reports that research funding or supplies have been provided to the University of Washington–Seattle on her behalf by Schering Plough, Merck, and Roche Molecular Systems; that she has received compensation for educational lectures by the International Antiviral Society–USA and by Merck for participation in a data safety and monitoring committee; and that she or her immediate family formerly owned stock in Abbott Laboratories, Bristol-Myers-Squibb, Johnson & Johnson, and Pfizer. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: for the CHARTER Group, Robert K. Heaton, Scott Letendre, I. Grant, J. Allen McCutchan, Ronald J. Ellis, Thomas D. Marcotte, Donald Franklin, Ronald J. Ellis, David M. Smith, Scott Letendre, Brookie Best, Robert K. Heaton, J. Hampton Atkinson, Matthew Dawson, Christine Fennema-Notestine, Sarah Archibald, Clint Cushman, Ian Abramson, Florin Vaida, Thomas D. Marcotte, Ned Sacktor, Justin McArthur, Susan Morgello, David Simpson, Letty Mintz, Cheuk Tang, Thomas Naidich, J. Allen McCutchan, Ann Collier, Christina Marra, Kenneth Maravilla, K. C. Stegbauer, Trudy Jones, Benjamin Gelman, Eleanor Head, Gregory Chaljub, David Clifford, Muhammad Al-Lozi, and Mengesha Teshome
References
- 1.Antinori A, Arendt G, Becker JT et al. . Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007; 69:1789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heaton RK, Clifford DB, Franklin DR Jr et al. . HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010; 75:2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simioni S, Cavassini M, Annoni JM et al. . Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 2010; 24:1243–50. [DOI] [PubMed] [Google Scholar]
- 4.Becker JT, Lopez OL, Dew MA, Aizenstein HJ. Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS 2004; 18(suppl 1):S11–8. [PubMed] [Google Scholar]
- 5.Valcour V, Shikuma C, Shiramizu B et al. . Age, apolipoprotein E4, and the risk of HIV dementia: the Hawaii Aging with HIV Cohort. J Neuroimmunol 2004; 157:197–202. [DOI] [PubMed] [Google Scholar]
- 6.Letendre SL, Cherner M, Ellis RJ et al. . The effects of hepatitis C, HIV, and methamphetamine dependence on neuropsychological performance: biological correlates of disease. AIDS 2005; 19(suppl 3):S72–8. [DOI] [PubMed] [Google Scholar]
- 7.Taylor MJ, Letendre SL, Schweinsburg BC et al. . Hepatitis C virus infection is associated with reduced white matter N-acetylaspartate in abstinent methamphetamine users. J Int Neuropsychol Soc 2004; 10:110–3. [DOI] [PubMed] [Google Scholar]
- 8.Ellis RJ, Badiee J, Vaida F et al. . CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS 2011; 25:1747–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munoz-Moreno JA, Fumaz CR, Ferrer MJ et al. . Nadir CD4 cell count predicts neurocognitive impairment in HIV-infected patients. AIDS Res Hum Retroviruses 2008; 24:1301–7. [DOI] [PubMed] [Google Scholar]
- 10.Letendre SL, Ellis RJ, Everall I, Ances B, Bharti A, McCutchan JA. Neurologic complications of HIV disease and their treatment. Top HIV Med 2009; 17:46–56. [PMC free article] [PubMed] [Google Scholar]
- 11.Ellis RJ, Gamst AC, Capparelli E et al. . Cerebrospinal fluid HIV RNA originates from both local CNS and systemic sources. Neurology 2000; 54:927–36. [DOI] [PubMed] [Google Scholar]
- 12.Garcia F, Niebla G, Romeu J et al. . Cerebrospinal fluid HIV-1 RNA levels in asymptomatic patients with early stage chronic HIV-1 infection: support for the hypothesis of local virus replication. AIDS 1999; 13:1491–6. [DOI] [PubMed] [Google Scholar]
- 13.Shapshak P, Segal DM, Crandall KA et al. . Independent evolution of HIV type 1 in different brain regions. AIDS Res Hum Retroviruses 1999; 15:811–20. [DOI] [PubMed] [Google Scholar]
- 14.Wiley CA, Soontornniyomkij V, Radhakrishnan L et al. . Distribution of brain HIV load in AIDS. Brain Pathol 1998; 8:277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paul R, Cohen R, Navia B, Tashima K. Relationships between cognition and structural neuroimaging findings in adults with human immunodeficiency virus type-1. Neurosci Biobehav Rev 2002; 26:353–9. [DOI] [PubMed] [Google Scholar]
- 16.Gisslen M, Hagberg L, Rosengren L et al. . Defining and evaluating HIV-related neurodegenerative disease and its treatment targets: a combinatorial approach to use of cerebrospinal fluid molecular biomarkers. J Neuroimmune Pharmacol 2007; 2:112–9. [DOI] [PubMed] [Google Scholar]
- 17.Spudich S, Lollo N, Liegler T, Deeks SG, Price RW. Treatment benefit on cerebrospinal fluid HIV-1 levels in the setting of systemic virological suppression and failure. J Infect Dis 2006; 194:1686–96. [DOI] [PubMed] [Google Scholar]
- 18.Yilmaz A, Svennerholm B, Hagberg L, Gisslen M. Cerebrospinal fluid viral loads reach less than 2 copies/ml in HIV-1-infected patients with effective antiretroviral therapy. Antivir Ther 2006; 11:833–7. [PubMed] [Google Scholar]
- 19.Santos JR, Munoz-Moreno JA, Molto J et al. . Virological efficacy in cerebrospinal fluid and neurocognitive status in patients with long-term monotherapy based on lopinavir/ritonavir: an exploratory study. PLoS One 2013; 8:e70201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McClernon DR, Ramsey E, Clair MS. Magnetic silica extraction for low-viremia human immunodeficiency virus type 1 genotyping. J Clin Microbiol 2007; 45:572–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Letendre S, FitzSimons C, Ellis RJ et al. . Correlates of CSF viral loads in 1,221 volunteers of the CHARTER cohort [abstract 172]. Presented at: 17th Conference on Retroviruses and Opportunistic Infections, San Francisco, CA, 2010. [Google Scholar]
- 22.Carey CL, Woods SP, Gonzalez R et al. . Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol 2004; 26:307–19. [DOI] [PubMed] [Google Scholar]
- 23.Blackstone K, Moore DJ, Franklin DR et al. . Defining neurocognitive impairment in HIV: deficit scores versus clinical ratings. Clin Neuropsychol 2012; 26:894–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed Hillsdale, N.J: The Erlbaum Group, 1988. [Google Scholar]
- 25.Marcotte TD, Deutsch R, Michael BD et al. . A Concise Panel of Biomarkers Identifies Neurocognitive Functioning Changes in HIV-Infected Individuals. J Neuroimmune Pharmacol 2013; 8:1123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eden A, Fuchs D, Hagberg L et al. . HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis 2010; 202:1819–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng L, Bosch RJ, Chan ES et al. . Predictors of residual viraemia in patients on long-term suppressive antiretroviral therapy. Antivir Ther 2013; 18:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013; 39:633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pereyra F, Lo J, Triant VA et al. . Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS 2012; 26:2409–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Canestri A, Lescure FX, Jaureguiberry S et al. . Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis 2010; 50:773–8. [DOI] [PubMed] [Google Scholar]
- 31.Letendre S, Marquie-Beck J, Capparelli E et al. . Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol 2008; 65:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marra CM, Zhao Y, Clifford DB et al. . Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS 2009; 23:1359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carvalhal A, Gill MJ, Letendre SL et al. . Central nervous system penetration effectiveness of antiretroviral drugs and neuropsychological impairment in the Ontario HIV Treatment Network Cohort Study. J Neurovirol 2016; 22:349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caniglia EC, Cain LE, Justice A et al. . Antiretroviral penetration into the CNS and incidence of AIDS-defining neurologic conditions. Neurology 2014; 83:134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cysique LA, Waters EK, Brew BJ. Central nervous system antiretroviral efficacy in HIV infection: a qualitative and quantitative review and implications for future research. BMC Neurol 2011; 11:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellis RJ, Letendre S, Vaida F et al. . Randomized trial of central nervous system-targeted antiretrovirals for HIV-associated neurocognitive disorder. Clin Infect Dis 2014; 58:1015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schrier RD, Hong S, Crescini M et al. . Cerebrospinal fluid (CSF) CD8+ T-cells that express interferon-gamma contribute to HIV associated neurocognitive disorders (HAND). PLoS One 2015; 10:e0116526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shiramizu B, Ananworanich J, Chalermchai T et al. . Failure to clear intra-monocyte HIV infection linked to persistent neuropsychological testing impairment after first-line combined antiretroviral therapy. J Neurovirol 2012; 18:69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith DM, Zarate S, Shao H et al. . Pleocytosis is associated with disruption of HIV compartmentalization between blood and cerebral spinal fluid viral populations. Virology 2009; 385:204–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shacklett BL, Cox CA, Wilkens DT et al. . Increased adhesion molecule and chemokine receptor expression on CD8+ T cells trafficking to cerebrospinal fluid in HIV-1 infection. J Infect Dis 2004; 189:2202–12. [DOI] [PubMed] [Google Scholar]
- 41.Lescure FX, Moulignier A, Savatovsky J et al. . CD8 encephalitis in HIV-infected patients receiving cART: a treatable entity. Clin Infect Dis 2013; 57:101–8. [DOI] [PubMed] [Google Scholar]
- 42.Otten U, Gadient RA. Neurotrophins and cytokines--intermediaries between the immune and nervous systems. Int J Dev Neurosci 1995; 13:147–51. [DOI] [PubMed] [Google Scholar]
- 43.Robertson K, Liner J, Meeker RB. Antiretroviral neurotoxicity. J Neurovirol 2012; 18:388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Becker JT, Kingsley L, Mullen J et al. . Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology 2009; 73:1292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCutchan JA, Marquie-Beck JA, Fitzsimons CA et al. . Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology 2012; 78:485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ciccarelli N, Fabbiani M, Di Giambenedetto S et al. . Efavirenz associated with cognitive disorders in otherwise asymptomatic HIV-infected patients. Neurology 2011; 76:1403–9. [DOI] [PubMed] [Google Scholar]
- 47.Decloedt EH, Maartens G. Neuronal toxicity of efavirenz: a systematic review. Expert Opin Drug Saf 2013; 12:841–6. [DOI] [PubMed] [Google Scholar]
- 48.Morgan EE, Woods SP, Smith C, Weber E, Scott JC, Grant I. Lower cognitive reserve among individuals with syndromic HIV-associated neurocognitive disorders (HAND). AIDS Behav 2012; 16:2279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hightower GK, Letendre SL, Cherner M et al. . Select resistance-associated mutations in blood are associated with lower CSF viral loads and better neuropsychological performance. Virology 2009; 394:243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf Accessed 13 July 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.