Abstract
Background. Cognitive impairment persists despite suppression of plasma human immunodeficiency virus (HIV) RNA. Monocyte-related immune activation is a likely mechanism. We examined immune activation and cognition in a cohort of HIV-infected and uninfected women from the Women's Interagency HIV Study (WIHS).
Methods. Blood levels of activation markers, soluble CD163 (sCD163), soluble CD14 (sCD14), CRP, IL-6, and a gut microbial translocation marker (intestinal fatty acid binding protein (I-FABP)) were measured in 253 women (73% HIV-infected). Markers were compared to concurrent (within ± one semiannual visit) neuropsychological testing performance.
Results. Higher sCD163 levels were associated with worse overall performance and worse verbal learning, verbal memory, executive function, psychomotor speed, and fine motor skills (P < .05 for all comparisons). Higher sCD14 levels were associated with worse verbal learning, verbal memory, executive function, and psychomotor speed (P < .05 for all comparisons). Among women with virological suppression, sCD163 remained associated with overall performance, verbal memory, psychomotor speed, and fine motor skills, and sCD164 remained associated with executive function (P < .05 for all comparisons). CRP, IL-6, and I-FABP were not associated with worse cognitive performance.
Conclusions. Monocyte activation was associated with worse cognitive performance, and associations persisted despite viral suppression. Persistent inflammatory mechanisms related to monocytes correlate to clinically pertinent brain outcomes.
Keywords: CD163, CD14, women, HIV infection, cognition disorders, intesticial fatty acid-binding protein (1-19)
Human immunodeficiency virus (HIV)–associated neurocognitive disorder (HAND) is a debilitating condition that affects up to 50% of people living with HIV, despite access to combination antiretroviral therapy (cART) [1]. Understanding the cellular and molecular mechanisms leading to HAND is of clinical importance, particularly among those adherent to treatment, as HAND is associated with loss of employment [2], decreased ability to multitask [3], impaired medication self-management [4], and shorter time to virologic failure. [5] These factors influence health and quality of life [6].
Chronic immune activation is a common theme in describing potential mechanisms underlying persistence of HAND despite suppression of plasma viremia [7]. Two postulated sources of this immune activation are intracellular HIV DNA in tissues and circulating cells (eg, monocytes, lymph nodes, and, potentially, the brain) and gut microbial translocation [8, 9]. However, published work describing HAND is frequently based on the evaluation of study participants who have a mixture of suppressed or unsuppressed plasma HIV RNA levels, a factor that limits our understanding of persistent impairment despite suppressive therapy [10].
Circulation of activated CD14+/CD16+ monocytes containing HIV contribute to chronic immune activation. These monocytes are theorized to carry HIV into the brain [7]. There, HIV proteins and monocyte-derived cytokines and chemokines damage cells and tissue. Regarding gut microbial translocation, this ensues when immune barriers in the gut are markedly depleted within the first month of HIV infection and do not fully recover after initiating cART [11, 12]. This so-called leaky gut allows microbial products to cross into the blood, leading to a low level of chronic immune activation [8].
Chronic immune activation, measured on the basis of plasma and serum biomarkers, is associated with worse cognitive performance, especially when determined using monocyte markers such as soluble CD163 (sCD163) and soluble CD14 (sCD14). sCD163 is a monocyte-associated hemoglobin-haptoglobin complex scavenger receptor cleaved from activated monocytes during inflammation [13]. The plasma sCD163 level is elevated in HIV-infected individuals and is further increased in those with HAND as compared to those without HAND, even in the setting of suppressive cART [14–16]. sCD14 is thought to be an indicator of gut microbial translocation. CD14 binds lipopolysaccharide, part of the cell wall of gram-negative bacteria, which activates the monocyte to cleave and release CD14 as the marker sCD14 [17]. Similar to sCD163, elevated levels of plasma sCD14 are linked to cognitive impairment in HIV-infected individuals [18, 19].
Previous studies linking HAND and monocyte activation have predominantly enrolled men or enrolled disproportionately small numbers of women [16, 20–26]. We recently examined cognition in a large sample of HIV-infected and uninfected women from the Women's Interagency HIV Study (WIHS; n = 1521). HIV-infected women performed worse than HIV-uninfected women in the domains of verbal learning and memory, psychomotor speed, and attention [21]. These findings differ from those described in predominantly male cohorts, in which abnormalities are most frequently described in the domains of learning and executive functioning [1]. Differences in brain outcomes, by sex, have also been suggested in studies identifying increased frequency of ischemic stroke in HIV-infected women versus HIV-infected men [27] and the degree of inflammatory marker resolution following cART initiation, including that for sCD14 [28].
In this study, we therefore investigated the relationship between chronic immune activation and cognitive performance in a sample of HIV-infected and uninfected women from the WIHS. Our primary aim was to examine the association between chronic immune activation, using a monocyte activation marker (sCD163) and other markers (C-reactive protein [CRP] and interleukin 6 [IL-6]), and cognitive performance. We hypothesized that higher blood levels of the monocyte activation marker and nonspecific markers were associated with worse cognitive performance. Secondarily, we explored the association between gut microbial translocation and cognitive performance. We examined a monocyte activation marker of gut microbial translocation (sCD14) and a novel marker of gut microbial translocation (intestinal fatty acid binding protein [I-FABP]).
METHODS
Subject Selection
The WIHS is a multicenter prospective observational study of HIV-infected and uninfected women [29]. Women were included if they were able to consent to participate, complete interviews and semiannual visits, and undergo blood sample collection. At the time of enrollment, the women did not have a known diagnosis of dementia. For the current analyses, we evaluated participants enrolled in one of 2 substudies in which pertinent biomarkers were measured: the transient elastography (TE) substudy (n = 337) and the magnetic resonance spectroscopy (MRS)–based steatosis (MRS steatosis) substudy (n = 139). Women could enroll in both substudies. All subjects signed consent forms approved by human subject review boards at their respective sites.
The TE substudy was designed as a nested cohort study to investigate the contributions of HIV monoinfection, hepatitis C virus (HCV) monoinfection, and HIV/HCV coinfection to liver disease, relative to liver disease in women without HIV or HCV infection. Participants were enrolled from October 2010 through December 2012 at the San Francisco, Chicago, and Washington, D. C., sites. All participants were between the ages of 25 and 65 years and negative for hepatitis B virus surface antigen, not pregnant, and not receiving interferon-based therapy. All participants meeting these criteria were approached at their WIHS semiannual visit and asked to participate in the TE substudy. Women who had a body mass index (calculated as the weight in kilograms divided by the height in meters squared) of >35 or evidence of decompensated cirrhosis (eg, ascites, hepatic encephalopathy, or esophageal varices), acute hepatitis, hepatitis flare, or cholestasis were also excluded from the study because of reported interference with the TE measurement. A total of 371 women were approached, of whom 34 did not meet standardized criteria for a valid TE measurement and were excluded.
The MRS steatosis substudy was designed to investigate the contribution of HIV, HCV, and metabolic factors to hepatic steatosis. Participants were enrolled from December 2003 through July 2015 in 2 waves—from 2003 to 2010 (n = 95) and from 2014 to 2015 (n = 44). All women from the San Francisco WIHS site who were capable of undergoing MRS and without hepatitis B virus surface antigenemia, prior HCV treatment, or decompensated cirrhosis were approached. For the current substudy, we selected all participants in either of the 2 substudies (n = 460) described above who completed testing for any of the biomarkers of interest during the semiannual visit before or after the visit when neuropsychological testing was performed (n = 253).
Clinical Characterization
WIHS visits involve comprehensive medical and social evaluations, as well as neuropsychological testing completed in waves such that all women undergo neuropsychological testing over 18 months. Cognitive tests have been described elsewhere [20]. Briefly, they are the Hopkins Verbal Learning Test–Revised, the Stroop Task test, the Trail Making test (parts A and B), the Symbol Digit Modalities Test, the Letter Fluency (F, A, S) test, the Letter Number Sequencing test, the Semantic Fluency (animals) test, and the Grooved Pegboard test. Raw individual test scores were adjusted on the basis of values from a larger sample of HIV-uninfected WIHS women [21, 30–35] and transformed into z scores. All outcome measures for timed tests (the Trail Making, Stroop Task, and Grooved Pegboard tests) were skewed and therefore log transformed before the creation of z scores. z scores were then averaged to create 7 composite indices (verbal learning, verbal memory, attention/concentration, executive functioning, psychomotor speed, verbal fluency, and fine motor skills), as well as an average overall global summary score. Blood samples collected during the semiannual visit before or after the visit when neuropsychological testing was performed were included in the primary analysis. The number of days between biomarker measurement and neuropsychological testing differed slightly by biomarker (eg, a mean [±SD] of −8.85 ± 159 days [range, −329–333 days) for sCD163, compared −6.16 ± 158 days [range, −329–333 days] for sCD14). We then completed sensitivity analyses for those captured on the same day.
Laboratory Measurements
sCD163, sCD14, and I-FABP levels were measured in frozen plasma stored at −70°C, using enzyme-linked immunosorbent assays (ELISAs; the Quantikine ELISA kit [R&D Systems] for sCD163 and sCD14 and the I-FABP ELISA kit [Hycult Biotech] for I-FABP) according to the manufacturer's protocols. CRP and IL-6 levels were measured in frozen sera stored at −70°C using ELISA kits (the BNII nephelometer [Dade Behring] and the Quantikine HS Human IL-6 Immunoassay kit [R&D Systems], respectively) according to the manufacturers’ protocols. HIV RNA levels were measured by the COBAS AmpliPrep/COBAS TaqMan HIV-1 PCR test (Roche Molecular Systems; lower limit of detection, 20 HIV-1 RNA copies/mL) according to the manufacturer's protocols. For sensitivity analyses of women with suppressed HIV RNA levels, we selected 500 copies/mL as the cut point because our goal was to examine correlations in the setting of optimal treatment and because individuals can experience intermittent episodes of detectable viremia (so-called blips) of less clear significance [36].
Statistical Analyses
Before analysis, distributions were examined for all markers and cognitive outcomes. Distributions for sCD14, I-FABP, CRP, and IL-6 levels were skewed and therefore log transformed. For sCD163, 1 sample was >3 SDs above the interquartile range. Because the value was deemed valid, we substituted the outlier value with the next most extreme value in the variable distribution [37].
Multivariable linear regressions in the overall sample were used to examine the relationship between the primary (sCD163 and sCD14) and secondary (CRP, IL-6, and I-FABP) inflammatory markers of interest and cognitive functioning. Adjusted models controlled for the following key covariates: site, HIV and HCV status, antidepressants, depressive symptoms, hypertension, income, and number of previous cognitive test exposures. Follow-up analyses investigated the association between inflammatory markers and cognitive performance among HIV-infected women with viral suppression (HIV RNA level, <500 copies/mL). Subanalyses in the overall sample investigated inflammatory markers and cognitive performance that were measured on the same day. Significance was set at a P value of < .05. All analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC).
RESULTS
Demographic and Clinical Characteristics
Participants averaged 47 years of age and completed an average of 12.9 years of education, and most (67%) were of black, non-Hispanic race/ethnicity (Table 1). A total of 74% were HIV infected (n = 186; 33% were HCV coinfected), and 26% were HIV-uninfected (n = 67; 19% were HCV infected). Among HIV-infected women, 54% had a CD4+ T-lymphocyte count of >500 cells/mm3, and 50% had an undetectable plasma HIV load. The mean nadir CD4+ T-cell count was 261 cells/mm3.
Table 1.
Demographic Characteristics for 253 Participants at Their First Neuropsychological Testing Visit, by Human Immunodeficiency Virus (HIV) Serostatus
| Background Characteristic | HIV Serostatus, No. (%) |
||
|---|---|---|---|
| Uninfected (n = 67) | Infected (n = 186) | P Value | |
| Age, y | 44.15 ± 10.27 | 48.29 ± 8.63 | .002 |
| WRAT-3 reading subtest score | 93.37 ± 17.22 | 91.57 ± 18.11 | .48 |
| Years of education, no. | 12.97 ± 3.38 | 12.94 ± 3.28 | .95 |
| Race/ethnicity | .14 | ||
| African American, non-Hispanic | 44 (66) | 125 (67) | |
| White, non-Hispanic | 10 (15) | 32 (17) | |
| Hispanic | 5 (7) | 21 (11) | |
| Other | 8 (12) | 8 (4) | |
| Annual household incomea | 3.79 ± 2.42 | 3.52 ± 2.41 | .43 |
| Currentb depressive symptoms, CES-D score ≥16 | 18 (27) | 79 (42) | .02 |
| HCV RNA positive | 13 (19) | 61 (32) | .04 |
| Recentc heavyd alcohol use | 16 (24) | 36 (19) | .43 |
| Recentc marijuana/hashish use | 24 (36) | 46 (25) | .08 |
| Smoking status | .53 | ||
| Never | 10 (15) | 39 (21) | |
| Formere | 21 (31) | 58 (31) | |
| Recentc | 36 (54) | 89 (48) | |
| Recentc crack, cocaine, and/or heroin use | 7 (10) | 25 (13) | .53 |
| Diabetesf | 12 (18) | 32 (17) | .90 |
| APRIg | 0.21 (0.13) | 0.32 (0.34) | <.001 |
| Hypertensionh | 17 (25) | 76 (41) | .02 |
| Body mass index | 28.33 ± 5.94 | 26.31 ± 5.64 | .01 |
| Menopausal status | .02 | ||
| Premenopausal | 32 (49) | 54 (31) | |
| Perimenopausal | 6 (9) | 28 (16) | |
| Postmenopausal | 27 (42) | 94 (53) | |
| HIV disease | |||
| CD4+ T-cell count, cells/mm3 | |||
| Nadir | … | 261 (210) | |
| >500 | … | 101 (54) | |
| ≥200 and <500 | … | 63 (34) | |
| <200 | … | 22 (12) | |
| Plasma HIV RNA level, copies/mL | |||
| Undetectablei | … | 94 (50) | |
| <10 000 | … | 68 (37) | |
| ≥10 000 | … | 24 (13) | |
| cART compliance >95% | … | 114 (62) | |
Data are mean value ± SD, no. (%) of women, or median value (interquartile range).
Abbreviations: cART, combination antiretroviral therapy; CES-D, Center for Epidemiological Studies Depression; HCV, hepatitis C virus; WRAT-3, Wide Range Achievement Test.
a Categorized using an ordinal scale, as follows: 1, ≤$6000; 2, $6001–$12 000; 3, $12 001–$18 000; 4, $18 001–$24 000; 5, $24 001–30 000; 6, $30 001–$36 000; 7, $36 001–$75 000; and 8, >$75 000.
b Defined as within the past week.
c Defined as within 6 months of the most recent WIHS visit.
d Defined as >7 drinks/week.
e Defined as any previous use, but not in the past 6 months.
f Defined as having a self-reported diagnosis, having a fasting glucose of over 125 mg/dL, or being on diabetic medications.
g The aspartate aminotransferase to platelet ratio index (APRI) was calculated as [(actual AST level)/(upper limit of normal AST level)] × [100/platelet count].
h Defined as any indication of hypertension (ie, systolic blood pressure of ≥140 mm Hg, diastolic blood pressure of ≥90 mm Hg, self-report, or use of antihypertensive medication).
i Defined as ≤48 copies/mL.
Higher Plasma sCD163 and sCD14 Levels Were Associated With Worse Cognitive Performance, Even Among Virally Suppressed Women
Higher levels of sCD163 and sCD14 were associated with worse cognitive performance in both unadjusted and adjusted models of the overall sample (Table 2). Specifically, higher sCD163 levels were associated with worse overall cognitive performance (β = −0.31, P < .001) and worse performance in the domains of verbal learning, verbal memory, executive function, and psychomotor speed (P < .001 for all comparisons), plus fine motor skills (P = .01). Although there was no association between sCD14 levels and overall cognitive performance (P = .07), higher sCD14 levels were associated with worse performance in verbal learning, executive function, and psychomotor speed (P < .01 for all comparisons), plus verbal memory (P = .04). No significant interactions were found between HIV status and sCD163 or sCD14 (data not shown). Adding in diabetes or liver fibrosis (aspartate transaminase to platelet ratio index) as a covariate in the models did not change the pattern of effects.
Table 2.
Associations Between Cognitive Performance and Plasma Levels of Soluble CD163 (sCD163) and Soluble CD14 (sCD14) in the Overall Sample
| Cognitive Domain | sCD163 Levela |
sCD14 Levela |
||||
|---|---|---|---|---|---|---|
| No. | Unadjusted β | Adjusted β | No. | Unadjusted β | Adjusted β | |
| Global summary score | 186 | −0.24b | −0.30b | 188 | −0.15c | −0.13d |
| Verbal learning | 214 | −0.27b | −0.28b | 216 | −0.25b | −0.20e |
| Verbal memory | 213 | −0.25b | −0.33b | 215 | −0.15c | −0.15c |
| Attention/concentration | 202 | −0.15c | −0.11 | 204 | −0.06 | −0.02 |
| Executive function | 194 | −0.24b | −0.31b | 196 | −0.20e | −0.20e |
| Psychomotor speed | 213 | −0.29b | −0.31b | 215 | −0.23b | −0.20e |
| Verbal fluency | 214 | −0.12 | −0.12 | 216 | −0.11 | −0.08 |
| Fine motor skills | 210 | −0.21e | −0.21c | 212 | −0.14c | −0.10 |
Two fewer subjects had sCD163 levels measured than subjects who had sCD14 levels measured.
a β values denote standardized parameter estimates from regression models. Adjusted β values are from multivariable regression models with the following key covariates: site, human immunodeficiency virus (HIV) and hepatitis C virus (HCV) status, antidepressants, depressive symptoms, hypertension, income, and number of cognitive tests previously exposed to.
b P < .001.
c P < .05.
d P > .05 and P < .10.
e P < .01.
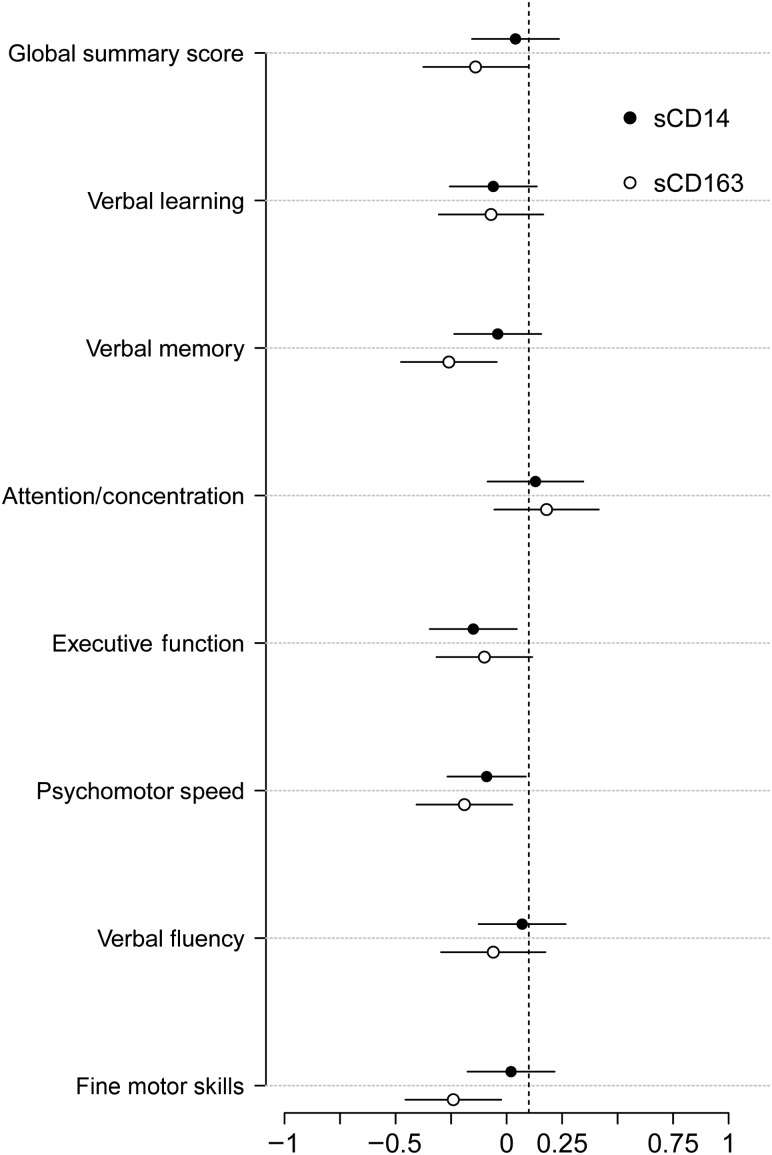
Associations in women with viral suppression were consistent with the findings in the overall sample. Higher levels of sCD163 were associated with worse overall cognitive performance (β = −0.24, P = .04) and worse performance in the domains of verbal memory (P = .003), psychomotor speed (P = .01), and fine motor skills (P = .004; Figure 1). Higher levels of sCD14 were only associated with worse performance of executive function (P = .02) and marginally worse performance of psychomotor speed (P = .05; Figure 1). Adding the nadir CD4+ T-cell count into the model did not change the pattern of results.
Figure 1.
Associations between plasma soluble CD163 (sCD163) and sCD14 and cognitive performance in virally suppressed HIV-infected women. Circles denote the standardized β weight from the regression model, and the line denotes the 95% confidence interval around the β weight. Lines not crossing 0 are significant. For sCD163, significance was noted for the global summary score (β = −0.24, P = .04), verbal memory (β = −0.36, P = .003), psychomotor speed (β = −0.28, P = .01), and fine motor skills (β = −0.34, P = .004). For sCD14, significance was noted for executive function (β = −0.25, P = .02), and a trend for significance was noted for psychomotor speed (β = −0.19, P = .05). The sample size range was 100–115.
We conducted sensitivity analyses to determine whether our patterns of results were confounded by the duration between the completion of assays and neuropsychological tests. Specifically, we examined associations in a subset of women (HIV infected and uninfected) from whom a blood specimen was collected on the same day as neuropsychological testing (the number evaluated ranged between 43 and 53 per analysis; Supplemental Table 1). Consistent with analyses in the overall sample, there was an inverse pattern of associations between biomarker levels and cognitive performance. However, significance varied across associations. There were significant associations between sCD163 and verbal learning (P = .007) and verbal memory (P = .03). With respect to sCD14, there were significant associations with verbal learning (P = .007), verbal memory (P < .001), and fine motor skills (P = .03) and marginal associations with overall performance (P = .06) and executive function (P = .06).
Serum CRP, Serum IL-6, and Plasma I-FABP Levels and Cognitive Performance
CRP was not associated with cognitive performance in the overall sample; however, higher serum CRP levels were associated with better verbal memory in the subset of women with viral suppression (β = 0.22, P = .04; Supplemental Table 2). Serum IL-6 and plasma I-FABP levels were not associated with overall cognitive performance or with any specific cognitive domain (Supplemental Tables 3 and 4).
DISCUSSION
In this study, our primary aim was to examine the association between chronic immune activation, using a monocyte activation marker (sCD163) and other markers (CRP and IL-6), and cognitive performance in a sample of HIV-infected and uninfected women from the WIHS. Secondarily, we explored the association between gut microbial translocation, using a monocyte activation marker (sCD14) and a novel marker of gut microbial translocation (I-FABP), and cognitive performance. We found that elevated plasma sCD163 and sCD14 levels but not CRP, IL-6, or I-FABP levels were associated with worse cognitive performance in multiple cognitive domains. Notably, these associations remained present among women with suppressed plasma HIV RNA levels. The selective associations of sCD163 and sCD14 suggest that the association between inflammation and cognitive performance in the context of HIV infection are driven by monocyte lineage activation.
These findings support the current theory that chronic immune activation contributes to the development of HAND and confirms the association among HIV-infected women, as well as those with suppressed plasma HIV RNA levels [10]. Other studies, largely comprising men, have reported similar associations of plasma sCD163 and sCD14 [14, 15, 18, 19]. Our study extends this line of work by demonstrating a lack of association with other markers of inflammation that are less tightly linked to monocyte activation. Furthermore, these findings are in a cohort of women whose race and ethnicity distribution reflects that of women living with HIV in the United States [38]. This is pertinent given that there are sex-associated alterations in immune activation in the setting of HIV infection [16, 28, 39]. Our data are less representative of HIV-infected men in the United States.
Inflammation was associated with multiple rather than select cognitive domains in the present study. One possibility is that inflammation may reduce the efficiency of cognitive processing. Consequently, multiple cognitive tests may be impacted. The more robust findings for sCD163 as compared to sCD14 suggests that the impact is more likely due to specific monocyte infection than gut microbial translocation. This is consistent with prior studies linking HIV DNA in CD14+ reservoirs to cognition, brain atrophy, and brain inflammation through cytokine analyses and magnetic resonance spectroscopy [40, 41].
This study also informs clinical practice in cases where successfully treated patients achieve viral suppression yet have cognitive symptoms. Given that there were no significant interactions between the monocyte activation markers and HIV status, one possibility is that similar pathways exist in HIV-infected and uninfected women. However, the comorbidity of HCV infection and the smaller sample of HIV-uninfected women caution against such conclusions. The repeated analyses of only women with HIV infection and viral suppression, however, strengthen our work. Our work is particularly strong in that women in this cohort have substantial comorbidities, yet we continue to identify an association between HIV-related inflammation and cognitive performance. This finding questions premature conclusions that the persistence of HAND is due to past brain injury (before viral suppression) or simply to the presence of comorbidity. The findings support the conclusion that cART insufficiently arrests the chronic inflammatory mechanisms underlying cognitive changes in the current treatment era.
The known fluctuating presentation of HAND led us to limit the time between neuropsychological testing and plasma biomarker measurement to within 1 semiannual visit of each other. To be certain that the time window did not affect the findings, we separately analyzed associations between neuropsychological testing and biomarker measurement completed on the same day. Fewer associations were statistically significant, likely because of small sample sizes; however, the direction and magnitude of effects remained consistent with the larger model, adding confidence that our main findings are sound.
The finding that higher levels of plasma I-FABP are not associated with worse cognitive performance in this sample is counter to the theory that gut injury underlying microbial translocation contributes to worse cognitive performance. I-FABP is a marker of enterocyte damage and therefore of gut microbial translocation [42]. As such, we anticipated that associations would reflect those found for sCD14, a touted marker of gut integrity [43]. Prior studies have reported higher levels of plasma I-FABP in HIV-infected individuals [28, 44, 45], but no studies have investigated the relationship of I-FABP and cognitive performance in the context of HIV infection. Our inconsistent finding may be attributed to the specificity of sCD14 to gut damage rather than general monocyte activation or the degree to which I-FABP represents a propensity for inflammation related to enterocyte damage. With these thoughts, gut microbial translocation, a source of chronic immune activation, may be better marked by sCD14 than I-FABP. Further characterization of I-FABP in the context of HIV infection is necessary.
This study has a few limitations. First, we cannot determine causality, because this study was cross-sectional. However, prior studies have provided evidence that inflammation has a role in the progression of cognitive impairment [46, 47]. These findings give us confidence that inflammation influences cognition during HAND, rather than the reverse. Second, we assessed cognitive performance but did not have the capacity to diagnosis HAND, owing to current WIHS methods. Thus, it is unclear whether the degree of neuropsychological changes noted in association with biomarkers would be sufficient to influence the presence or absence of HAND. A third limitation, also influenced by WIHS methods, is the limitations of power for the sensitivity analyses of same-day neuropsychological testing and biomarker measurement. Few participants complete all testing on the same day, owing to time constraints.
In conclusion, higher levels of plasma sCD163 and sCD14 but not serum CRP, serum IL-6, or plasma I-FABP were associated with worse cognitive performance in a cohort of women from the WIHS. Furthermore, we demonstrated that these associations persist among women with suppressed plasma HIV RNA levels. Our findings extend conclusions of prior studies by suggesting that monocyte activation contributes to cognitive impairment in HIV-infected women in the era of cART.
STUDY GROUP MEMBERS
The WIHS sites and principal investigators are as follows: Chicago WIHS, Mardge Cohen and Audrey French; Metropolitan Washington WIHS, Mary Young and Seble Kassaye; Connie Wofsy Women's HIV Study, Northern California, Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien; WIHS Data Management and Analysis Center, Stephen Gange and Elizabeth Golub; and Southern California WIHS, Alexandra Levine and Marek Nowicki (WIHS I–IV).
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Disclaimer. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Institutes of Health (grant K24-MH-098759 to V. G. V.), the National Institute of Allergy and Infectious Diseases (grants UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590 to the WIHS), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant UO1-HD-32632 to the WIHS), the National Cancer Institute (to the WIHS), the National Institute on Drug Abuse (to the WIHS), the National Institute on Deafness and Other Communication Disorders (to the WIHS), the National Center for Research Resources (UCSF-CTSI grant UL1 RR024131 to the WIHS), the National Institute of Mental Health (grant K01-MH-098798 to L. H. R.), and the National Institute of Allergy and Infectious Diseases (grant K24 AI 108516 to P. C. T.).
Potential conflicts of interest. V. G. V. served as a consultant for ViiV Healthcare and Merck on topics related to aging and HIV. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Heaton RK, Franklin DR, Ellis RJ et al. . HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011; 17:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rueda S, Raboud J, Mustard C, Bayoumi A, Lavis JN, Rourke SB. Employment status is associated with both physical and mental health quality of life in people living with HIV. AIDS Care 2011; 23:435–43. [DOI] [PubMed] [Google Scholar]
- 3.Scott JC, Woods SP, Vigil O et al. . A neuropsychological investigation of multitasking in HIV infection: implications for everyday functioning. Neuropsychology 2011; 25:511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waldrop-Valverde D, Jones DL, Gould F, Kumar M, Ownby RL. Neurocognition, health-related reading literacy, and numeracy in medication management for HIV infection. AIDS Patient Care STDS 2010; 24:477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Letendre S, Ellis RJ, Deutsch R et al. . Correlates of time-to-loss-of-viral-response in CSF and plasma in the CHARTER cohort: CPE Score predicts CSF suppression. Presented at: Conference on Retroviruses and Opportunistic Infections, San Francisco, CA, 2010. [Google Scholar]
- 6.Tozzi V, Balestra P, Murri R et al. . Neurocognitive impairment influences quality of life in HIV-infected patients receiving HAART. Int J STD AIDS 2004; 15:254–9. [DOI] [PubMed] [Google Scholar]
- 7.Rao VR, Ruiz AP, Prasad VR. Viral and cellular factors underlying neuropathogenesis in HIV associated neurocognitive disorders (HAND). AIDS Res Ther 2014; 11:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenchley JM, Price DA, Schacker TW et al. . Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12:1365–71. [DOI] [PubMed] [Google Scholar]
- 9.Chomont N, El-Far M, Ancuta P et al. . HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 2009; 15:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou L, Saksena NK. HIV associated neurocognitive disorders. Infect Dis Rep 2013; 5(suppl 1):e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guadalupe M, Reay E, Sankaran S et al. . Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol 2003; 77:11708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ananworanich J, Schuetz A, Vandergeeten C et al. . Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One 2012; 7:e33948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moller HJ. Soluble CD163. Scand J Clin Lab Invest 2012; 72:1–13. [DOI] [PubMed] [Google Scholar]
- 14.Burdo TH, Lentz MR, Autissier P et al. . Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis 2011; 204:154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS 2013; 27:1387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alcaide ML, Parmigiani A, Pallikkuth S et al. . Immune activation in HIV-infected aging women on antiretrovirals--implications for age-associated comorbidities: a cross-sectional pilot study. PLoS One 2013; 8:e63804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Triantafilou M, Triantafilou K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol 2002; 23:301–4. [DOI] [PubMed] [Google Scholar]
- 18.Lyons JL, Uno H, Ancuta P et al. . Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J Acquir Immune Defic Syndr 2011; 57:371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ancuta P, Kamat A, Kunstman KJ et al. . Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One 2008; 3:e2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maki PM, Cohen MH, Weber K et al. . Impairments in memory and hippocampal function in HIV-positive vs HIV-negative women: a preliminary study. Neurology 2009; 72:1661–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maki PM, Rubin LH, Valcour V et al. . Cognitive function in women with HIV: findings from the Women's Interagency HIV Study. Neurology 2015; 84:231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wojna V, Skolasky RL, Hechavarria R et al. . Prevalence of human immunodeficiency virus-associated cognitive impairment in a group of Hispanic women at risk for neurological impairment. J Neurovirol 2006; 12:356–64. [DOI] [PubMed] [Google Scholar]
- 23.Richardson JL, Martin EM, Jimenez N et al. . Neuropsychological functioning in a cohort of HIV infected women: importance of antiretroviral therapy. J Int Neuropsychol Soc 2002; 8:781–93. [DOI] [PubMed] [Google Scholar]
- 24.Richardson JL, Nowicki M, Danley K et al. . Neuropsychological functioning in a cohort of HIV- and hepatitis C virus-infected women. AIDS 2005; 19:1659–67. [DOI] [PubMed] [Google Scholar]
- 25.Durvasula RS, Miller EN, Myers HF, Wyatt GE. Predictors of neuropsychological performance in HIV positive women. J Clin Exp Neuropsychol 2001; 23:149–63. [DOI] [PubMed] [Google Scholar]
- 26.Cohen RA, Boland R, Paul R et al. . Neurocognitive performance enhanced by highly active antiretroviral therapy in HIV-infected women. AIDS 2001; 15:341–5. [DOI] [PubMed] [Google Scholar]
- 27.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr 2012; 60:351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krebs SJ, Slike BM, Sithinamsuwan P et al. . Sex differences in soluble markers vary before and after the initiation of antiretroviral therapy in chronically HIV infected individuals. AIDS 2016; 30:1533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bacon MC, von Wyl V, Alden C et al. . The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 2005; 12:1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manly JJ, Smith C, Crystal HA et al. . Relationship of ethnicity, age, education, and reading level to speed and executive function among HIV+ and HIV− women: the Women's Interagency HIV Study (WIHS) Neurocognitive Substudy. J Clin Exp Neuropsychol 2011; 33:853–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubin LH, Sundermann EE, Cook JA et al. . Investigation of menopausal stage and symptoms on cognition in human immunodeficiency virus-infected women. Menopause 2014; 21:997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubin LH, Pyra M, Cook JA et al. . Post-traumatic stress is associated with verbal learning, memory, and psychomotor speed in HIV-infected and HIV-uninfected women. J Neurovirol 2016; 22:159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubin LH, Cook JA, Weber KM et al. . The association of perceived stress and verbal memory is greater in HIV-infected versus HIV-uninfected women. J Neurovirol 2015; 21:422–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valcour V, Rubin LH, Tien P et al. . Human immunodeficiency virus (HIV) modulates the associations between insulin resistance and cognition in the current combination antiretroviral therapy (cART) era: a study of the Women's Interagency HIV Study (WIHS). J Neurovirol 2015; 21:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valcour VG, Rubin LH, Obasi MU et al. . Liver fibrosis linked to cognitive performance in HIV and hepatitis C. J Acquir Immune Defic Syndr 2016; 72:266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee PK, Kieffer TL, Siliciano RF, Nettles RE. HIV-1 viral load blips are of limited clinical significance. J Antimicrob Chemother 2006; 57:803–5. [DOI] [PubMed] [Google Scholar]
- 37.Tabachnick BG, Fidell LS. Using multivariate statistics. 4th ed Boston: Allyn & Bacon, 2000. [Google Scholar]
- 38.CDC. HIV Surveillance Report. 2014:123.
- 39.Meier A, Chang JJ, Chan ES et al. . Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med 2009; 15:955–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kallianpur KJ, Valcour VG, Lerdlum S et al. . HIV DNA in CD14+ reservoirs is associated with regional brain atrophy in patients naive to combination antiretroviral therapy. AIDS 2014; 28:1619–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valcour VG, Ananworanich J, Agsalda M et al. . HIV DNA reservoir increases risk for cognitive disorders in cART-naive patients. PLoS One 2013; 8:e70164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pelsers MMAL, Namiot Z, Kisielewski W et al. . Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin Biochem 2003; 36:529–35. [DOI] [PubMed] [Google Scholar]
- 43.Lien E, Aukrust P, Sundan A, Muller F, Froland SS, Espevik T. Elevated levels of serum-soluble CD14 in human immunodeficiency virus type 1 (HIV-1) infection: correlation to disease progression and clinical events. Blood 1998; 92:2084–92. [PubMed] [Google Scholar]
- 44.Steele AK, Lee EJ, Vestal B et al. . Contribution of intestinal barrier damage, microbial translocation and HIV-1 infection status to an inflammaging signature. PLoS One 2014; 9:e97171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hunt PW, Sinclair E, Rodriguez B et al. . Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 2014; 210:1228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gorelick PB. Role of inflammation in cognitive impairment: results of observational epidemiological studies and clinical trials. Ann N Y Acad Sci 2010; 1207:155–62. [DOI] [PubMed] [Google Scholar]
- 47.Simen AA, Bordner KA, Martin MP, Moy LA, Barry LC. Cognitive dysfunction with aging and the role of inflammation. Ther Adv Chronic Dis 2011; 2:175–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.