Abstract
Purpose
Cerebral hemispheres represent both structural and functional asymmetry, which differs among right- and left-handers. The left hemisphere is specialized for language and task execution of the right hand in right-handers. We studied the corticospinal tract in right- and left-handers by diffusion tensor imaging and tractography. The present study was aimed at revealing a morphological difference resulting from a region of interest (ROI) obtained by functional MRI (fMRI).
Methods
Twenty-five healthy participants (right-handed: 15, left-handed: 10) were enrolled in our assessment of morphological, functional and diffusion tensor MRI. Assessment of brain fibre reconstruction (tractography) was done using a deterministic algorithm. Fractional anisotropy (FA) and mean diffusivity (MD) were studied on the tractography traces of the reference slices.
Results
We observed a significant difference in number of leftward fibres based on laterality. The significant difference in regard to FA and MD was based on the slices obtained at different levels and the laterality index. We found left hand asymmetry and right hand asymmetry respectively for the MD and FA.
Conclusions
Our study showed the presence of hemispheric asymmetry based on laterality index in right- and left-handers. These results are inconsistent with some studies and consistent with others. The reported difference in hemispheric asymmetry could be related to dexterity (manual skill).
Introduction
Cerebral hemispheric asymmetry was brought up by Hippocrates. He demonstrated how a head wound on one side led to seizures and a hemiplegia of the opposite side of the body (in Adams [1]). Since antiquity, many descriptions of brain hemispheric asymmetry have been given. These regard the gray matter (cerebral cortex [29] and the basal ganglia [35]), as well as the white matter [27, 53].
Handedness is one example of lateralization of cerebral functions [15]. Several studies have shown a difference in hemispheric asymmetry, between right- and left-handers [27, 28], according to the assessed functions. Left hemisphere is attributed to language, gnosia and praxia. Right hemisphere is attributed to visuospatial and visuoconstructive perceptions and attention [20]. Language lateralization related to handedness was described by Broca in 1863 [8], stating that left hemisphere was specialized for language and the dominant hand manual task. Geschwind et al. [20, 21] showed that the left planum temporale was wider than the right one. Thus, these authors put forward an association between brain anatomical asymmetry and handedness.
More recently, in vivo magnetic resonance imaging (MRI) assessment of encephalic volume revealed the gray and white matter asymmetries [22, 39]. These studies were consistent with previous ones revealing a morphological lateralization of different cerebral lobes. Kertesz et al. [28] showed such lateralization by comparing 52 right-handers with 52 left-handers. They put forward an association between left hemispheric dominance and wider right frontal and left occipital lobes. Other studies have found an association between the depth of central sulcus and handedness [3], or a lateralization of lateral sulcus based on planum temporale asymmetry [42]. The encephalon MRI morphometry assessment of central sulcus by Amunts et al. [3] in 31 right-handed and 14 left-handed men, revealed a leftward lateralization of central sulcus in the right-handers. The left-handers showed a less significant lateralization. These authors assumed an association between handedness and a wider cerebral cortex area and likely a more significant cerebral connectivity. In a more recent study [2] by Amunts et al., the leftward lateralization of central sulcus was only observed in men. As a result, a difference in organisation of the cortex related to hand mobility between men and women was reported. Rubens et al. [42] evaluated 36 bodies by cerebral hemisphere photographic methods and assessing the length and shape of the lateral sulcus. The left lateral hemisphere was reported to be longer and more rectilinear that the right one. This could affect the size of neighboring brain structures such as planum temporale. The results of an evaluation of 100 bodies by Geschwind and Levitsky [21], were consistent with those of Rubens et al.: in accordance to the length of lateral sulcus, the left planum temporale was reported to be wider (65% of cases) than the right one. However, Saenger et al. [43] studied brain asymmetries using MRI functional connectivity and gray matter volume in right- and left-handers. They concluded that functional asymmetries are not always concordant with morphological asymmetries.
Some authors also studied the association between handedness, or possible existence of dexterity (manual skill), and lateralization of the white matter [9, 24, 52]. Today, diffusion tensor imaging (DTI) and tractography allow the reconstruction of fibre tracts and even bringing light into the possible association between lateralization of the corticospinal tract (CST) and handedness. Büchel et al. [9] showed an increase in fractional anisotropy (FA) within the precentral gyrus, controlateral to the dominant hand. They revealed the following: an association between cerebral asymmetry and handedness, and the role of DTI in the assessment of the white matter. Westerhausen et al. [52] DTI assessment of the association between cerebral asymmetry and internal capsule asymmetry was conclusive. However, no evident correlation between internal capsule asymmetry and laterality was revealed. In his 2002 literature review, Hammond [24] stated that the dominant hemisphere, controlateral to the dominant hand, represented a better functional organisation via an increase in cortical connectivity of the primary motor area (M1). This could explain the better manual task performance of the dominant hand but does not prove a correlation with the CST volume.
The aim of our study was to evaluate the correlation between handedness and cerebral asymmetry through focusing on hand motor fibres within the CST. Using deterministic tractography, we aimed to highlight a difference between the right- and left- handers. The choice of our method followed the footsteps of a previous study [44] by comparing the following parameters: FA and mean diffusivity (MD).
Material
Subjects
Twenty-five healthy volunteers were enrolled in this study: fifteen right-handers (ten men and five women) and ten left-handers (six men and four women). They had no history of neurological disorders. Age ranged from 22 years to 46 years (mean age 30.8 years) for right-handers and 18 years to 42 years (mean age 29.2) for left-handers. Written informed consent was obtained from each subject and data were handled anonymously. Handedness was determined using a test created by Dellatolas [17] for use on the healthy population in France, adapted from studies by Annett [4] and Oldfield [34]. Index laterality is summarised in table 1.
Table 1.
Score and Laterality of subjects, test from [17], ranged from 0 to 20. R: right-hander (0/20), RA: right-handed ambidextrous (1 to 6/20), L: left-hander (17 to 20/20), LA: left-handed ambidextrous (7 to 16/20).
| Subjects | score | laterality |
|---|---|---|
| 1 | 0 | R |
| 2 | 0 | R |
| 3 | 0 | R |
| 4 | 0 | R |
| 5 | 0 | R |
| 6 | 1 | RA |
| 7 | 0 | R |
| 8 | 0 | R |
| 9 | 0 | R |
| 10 | 0 | R |
| 11 | 0 | R |
| 12 | 0 | R |
| 13 | 0 | R |
| 14 | 0 | R |
| 15 | 0 | R |
| 16 | 20 | L |
| 17 | 11 | LA |
| 18 | 20 | L |
| 19 | 14 | LA |
| 20 | 7 | LA |
| 21 | 13 | LA |
| 22 | 20 | L |
| 23 | 20 | L |
| 24 | 20 | L |
| 25 | 20 | L |
Magnetic Resonance (MR) data acquisition
Brain MR scans were carried out on each volunteer. MRI scans were performed on a Philips Achieva 3T system (Philips Medical Systems, Best, The Netherlands) using an 8-channel head coil. All volunteers were in supine position. To minimise involuntary head motion, bitemporal maintenance points were used.
The protocol used was the following:
Morphological MRI
T1-weighted sequence in single shot echo-planar. All acquired volumes contained 184 sagittal slices, field of view (FOV): 256x256 mm; acquisition matrix: 256 x 256; voxel size: 1 x 1 x 1 mm3; TE / TR / Flip angle: 4.6 ms / 9.9 ms / 8°; SENSE factor: 2. Total duration of this sequence was 3min53s.
Functional imaging sequence
The fMRI acquisition sequence used gradient echo-planar imaging (EPI) to provide BOLD contrast (blood oxygen-level dependent). Each volume represented comprised 24 contiguous 4 mm axial slices parallel to the AC-PC line, with parameters as follows: FOV: 230 mm; acquisition matrix: 80 x 80; reconstruction matrix after interpolation: 128 x 128; reconstructed voxel size: 1.8 x 1.8 x 4 mm3; TE / TR / Flip angle: 35 ms / 3000 ms / 90°. The acquisition of this 24-slice volume was repeated 62 times. Total duration of each sequence was 3min12s.
The paradigm followed a block design: seven interleaved 30s phases of rest and motor tasks were recorded. The motor tasks consisted of opening and closing the hand. After a period to obtain gradient stabilisation, acquisition of the first two 24-slice volumes was performed (6 s) in three sequences of alternating rest and activation, beginning with rest.
This protocol was performed twice, once for the right hand and once for the left one.
Diffusion weighted imaging (DWI) sequence
The DWI acquisition sequence was an EPI, single shot spin echo Stejskal Tanner sequence, combined with SENSE parallel imaging. Diffusion gradients were applied in 15 noncollinear directions with b= 800 sec/mm2. Parameters were as follows: 60 2-mm slices; FOV: 256 mm; acquisition matrix and reconstructed matrix: 128 x 128; reconstructed voxel size: 2 x 2 x 2 mm3; TE / TR / Flip angle: 64 ms / 10000 ms / 90°; SENSE factor: 3. Total duration of this sequence was 6min.12s.
Method
Data processing and post-processing
Morphological MRI
The MRI T1-weighted sequence was used to obtain a morphological image. This image was used to segment ROIs and to visualize fibre bundles and ROIs on the same image after registration of all sequences.
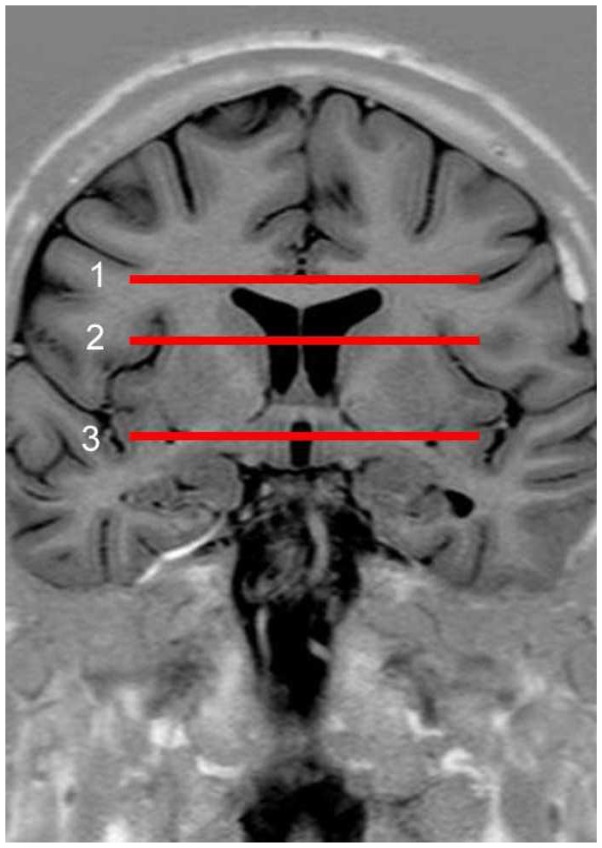
This sequence was used to determinate axial reference slices using fixed landmarks for all subjects. Three reference slices (Figure 1) were localised in corona radiate, internal capsule and diencephalon-mesencephalon junction. Diffusion parameters were recorded on these slices.
Fig 1.
Coronal slice of the brain through the anterior commissure. Reference slices in the brain for the study of traces. Level 1 (corona radiata), dorsal part of corpus callosum. Level 2 (internal capsule), dorsal part of the lenticular nucleus. Level 3 (diencephalon-mesencephalon junction), ventral part of the lenticular nucleus.
Functional MRI
All post-processing calculations were carried out using SPM5 routines (statistical parametrical mapping SPM5 / Wellcome Department of Neurology, Institute of Neurology, London) as well as in-house Matlab scripts (Matlab (2007), The MathWorks Inc). The 24-slice, 62 volumes were automatically realigned with the first to correct head motions. For each volunteer, all volumes were smoothed (smoothness window: 6x6x6). Individual statistical parametric maps were calculated using the general linear model [19] to compare brain activation and stimulation. At the first level, the design matrix included one regressor to model the block paradigm with implicit baseline (“1” for action scans, “0” for rest scans). For each paradigm (left/right), the effect of interest was evaluated with the following contrast: c=1. Anatomical data and fMRI data were normalised and coregistered (nonlinear registration). Coregistered volumes were visually inspected to assess the quality of the registration. The fMRI data were used to segment a part of the superior ROI.
DTI data
Diffusion weighted imaging (DWI) scans were first realigned and corrected for eddy currents distortions. Each DWI image was realigned with the non-weighted image (b=0 s/mm2). For each voxel, a tensor was estimated using a linear regression method [18]. Tensor being a 3x3 symmetric definite positive matrix, it was diagonalised to obtain its eigenvalues (strictly positives) for each voxel, from which fractional anisotropy (FA) and mean diffusivity (MD) maps were derived.
Fibre tracking
DTI fibre tracking was performed using in-house software implementation of conventional fibre tracking algorithms. A deterministic algorithm was used. It was an integration method derived from the fibre assignment by continuous tracking (FACT) method [32], using second-order Runge-Kutta (RK2) [6, 14].
Integration methods use constant integration step size. The FACT method allows the prediction of the position of different points all along the fibre curve. The direction was provided step by step; the step size was 1mm (voxel size). The RK2 method provided an estimation of the trajectory. This trajectory was used to estimate a second, more precise trajectory using one other estimation of this trajectory.
ROI segmentation
We chose to track the CST with a multiple ROI method: the superior ROI (cortical area); the inferior ROI (in the mesencephalon); and an exclusion ROI in the median sagittal plane. The ROIs were segmented by a neuroanatomist.
The superior ROI was segmented using motor activation (fMRI) and the T1-weighted MR images. Since this study focused on the CST, all non-cortical activations (e.g. cerebellar) were discarded. We retain the activation from the fMRI corresponding to the hand motor area using the anatomical landmark of the precentral gyrus described by Yousry et al. [54], and its variations [11]. To focus the superior ROI around the hand motor area, a segmentation algorithm (all non-connected components were discarded) was used to suppress adjacent activations, i.e. contralateral activation, venous artefacts, activations from supplementary motor area. This method allowed the preservation of each subject’s motor activation in the motor area [31] (Figure 2).
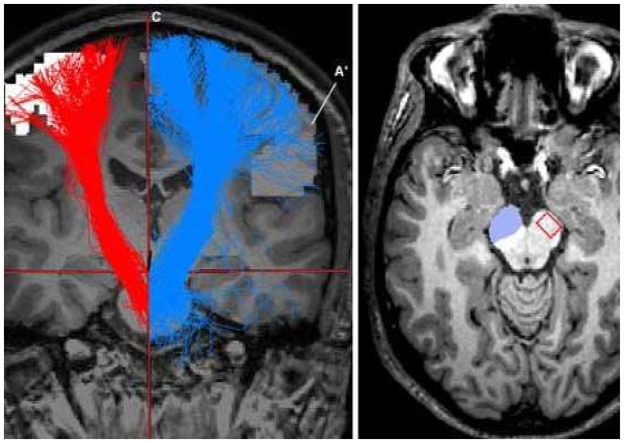
Fig 2.
Coronal slice: tractography visualisation from merged images. Morphological MRI (T1); superior ROI (A and A′); inferior ROI (B); sagittal median plan (C); tractographies (blue and red colours, right- and left- hand fibres respectively). Axial slice: crus cerebri, inferior ROI on left side, location of CST on right side.
The inferior ROI was determined by manual segmentation based on our knowledge of the CST anatomy in the mesencephalon. This ROI was located in the anterior part of the peduncle (crus cerebri) (Figure 2). This segmentation was performed on the T1-weighted MR image with Anatomist software (BrainVISA/Anatomist version 3.0.2).
A third ROI was considered and represented by the median sagittal plane. The fibre reconstruction was done step by step hence this third ROI could not be reached by the fibres.
CST reconstruction by fibre tracking
CST was reconstructed (tractography), using the algorithm based on RK2 previously described, and between ROIs (superior and inferior). The fibres could not go through the sagittal median plane (Figure 2). Two parameters were fixed to stop the tracking: the minimal FA value was set to 0.2 and the maximal angle between two adjacent eigenvectors (deviation angle) was less than 45°.
At each axial, coronal and sagittal slice, we observed and saved the fibre passing points. The latter were called “traces”. Traces were studied on the reference slice, described before, with the point by point mean measurement of FA and MD (Figure 3).
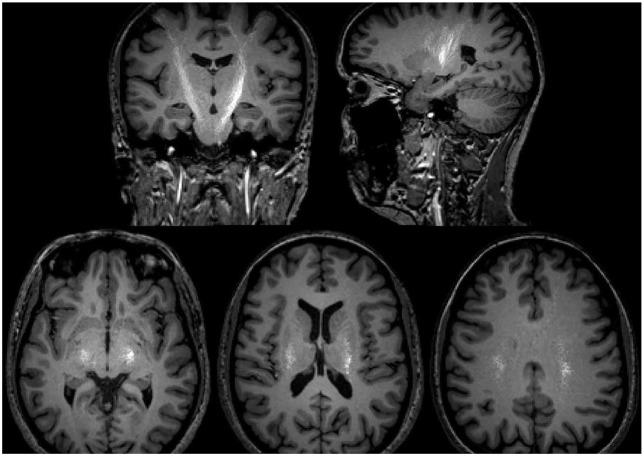
Fig 3.
Tractography traces. Top left: coronal slice, top right: sagittal slice. Bottom: left to right, slice level inferior (1), middle (2), superior (3). In white colour we visualised all points representing the traces with a clear asymmetry in the present case.
Evaluation
Each fibre bundle was visually verified to ensure the quality and the reliability of the images. Asymmetry and laterality were evaluated by several measurements: superior ROI volumes, fibre number for all subjects on both sides, FA and MD on reference slices
Statistical analysis
For statistical analysis, Student t-tests were used for quantitative results (i.e. fibre number and superior ROI volume) and a Spearman correlation test was used to find a correlation between laterality and the measured parameters (FA, MD).
All statistical analyses were conducted using the SAS software (SAS Institute Inc., version 9.2).
Results
ROI volume and laterality
The ROI volumes are displayed in Table 2. No significant difference was observed between left and right regions in right- and left-handers (p=0.83). No significant difference was observed between right (p=0.29), and left (p=0.07) based on laterality.
Table 2.
Quantitative results, ROI volumes (mm3) from both right (r) and left (l) hand fMRI. Right-handers (R) are ranked 1 to 15, left-handers (L) are ranked 16 to 25. SD: Standard Deviation.
| R Subjects | Volume (r) | Volume (l) |
|---|---|---|
| 1 | 11418 | 34339 |
| 2 | 13199 | 17914 |
| 3 | 18094 | 570 |
| 4 | 5450 | 8653 |
| 5 | 64991 | 14706 |
| 6 | 11764 | 23780 |
| 7 | 8042 | 12943 |
| 8 | 37227 | 4661 |
| 9 | 12367 | 11734 |
| 10 | 9284 | 22182 |
| 11 | 25755 | 19876 |
| 12 | 40297 | 48815 |
| 13 | 19418 | 50279 |
| 14 | 10162 | 700 |
| 15 | 19231 | 18376 |
|
| ||
| mean | 20446.6 | 19301.9 |
| median | 13199 | 17914 |
| SD | 15448.7 | 14678.1 |
| L Subjects | Volume (r) | Volume (l) |
|---|---|---|
| 16 | 5087 | 2778 |
| 17 | 14015 | 18080 |
| 18 | 19827 | 18212 |
| 19 | 13119 | 2778 |
| 20 | 18610 | 11077 |
| 21 | 14273 | 7132 |
| 22 | 35858 | 17892 |
| 23 | 8748 | 4189 |
| 24 | 118831 | 16309 |
| 25 | 9497 | 19325 |
|
| ||
| mean | 25786.5 | 11777.2 |
| median | 14144 | 13693 |
| SD | 32033.5 | 6615.5 |
Fibre numbers and laterality
The results of number of fibres are displayed in Table 3. No significant difference was observed for the right hand side (r) or the left hand side (l), in right- (p=0.31) and left-handers (p=0.12). However, a significant difference based on laterality was reported for the left hand side (p=0.03) and none for the right hand side (p=0.18).
Table 3.
Quantitative results, number of fibres for RK2 algorithm. Right-handers (R) are ranked 1 to 15, left-handers (L) are ranked 16 to 25. SD: Standard Deviation.
| R Subjects | right hand | left hand |
|---|---|---|
| 1 | 68 | 259 |
| 2 | 162 | 404 |
| 3 | 338 | 73 |
| 4 | 7 | 163 |
| 5 | 203 | 447 |
| 6 | 8 | 234 |
| 7 | 146 | 89 |
| 8 | 395 | 0 |
| 9 | 405 | 42 |
| 10 | 1 | 59 |
| 11 | 446 | 88 |
| 12 | 322 | 475 |
| 13 | 243 | 748 |
| 14 | 67 | 60 |
| 15 | 132 | 86 |
|
| ||
| mean | 196.2 | 215.1 |
| median | 162.0 | 89.0 |
| SD | 149.2 | 206.5 |
| L Subjects | right hand | left hand |
|---|---|---|
| 16 | 1 | 1 |
| 17 | 262 | 215 |
| 18 | 69 | 17 |
| 19 | 31 | 1 |
| 20 | 205 | 194 |
| 21 | 80 | 0 |
| 22 | 899 | 2 |
| 23 | 97 | 92 |
| 24 | 2804 | 156 |
| 25 | 20 | 34 |
|
| ||
| mean | 446.8 | 71.2 |
| median | 97.0 | 34.0 |
| SD | 825.0 | 82.1 |
Fractional anisotropy and laterality
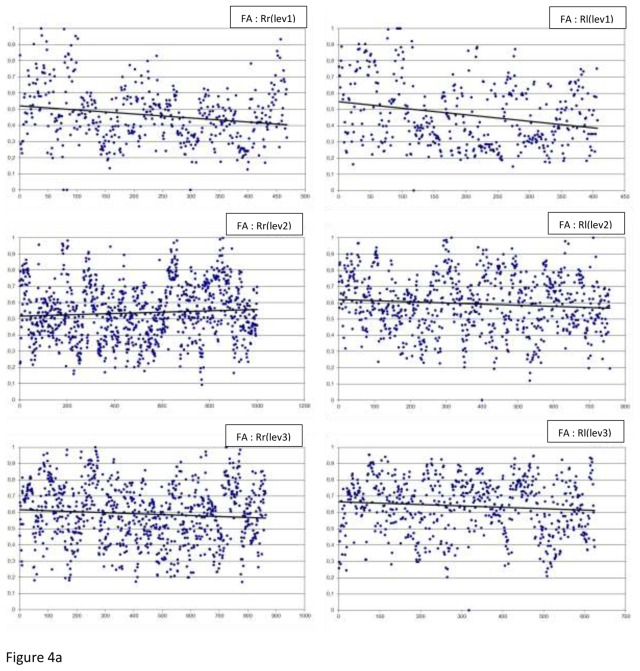
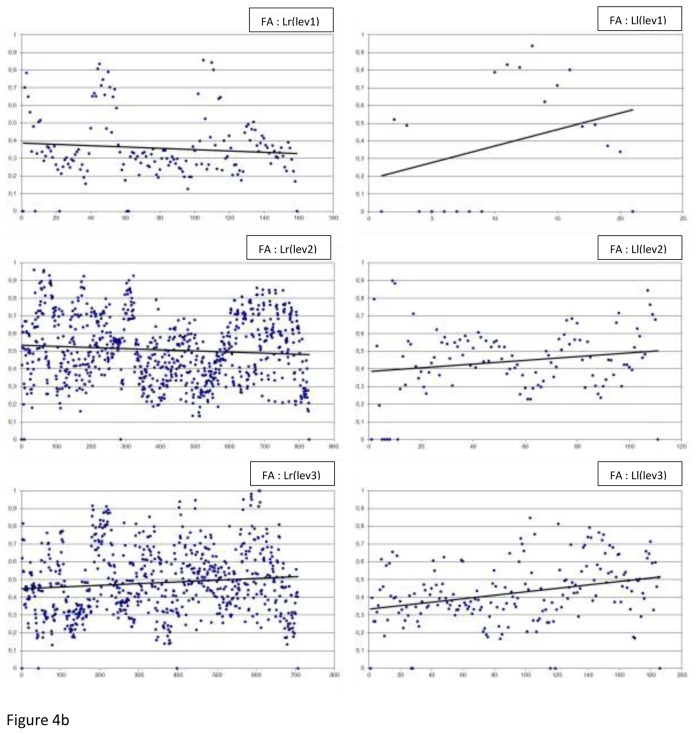
The FA measurements by level, side (r or l) and laterality (R or L) are summarised in Figure 4. Table 4 displays handedness and laterality results, the FA measurements: the minimum, the maximum, the means, the medians and the p values.
Fig 4.
The FA measurements by level (lev), side (r or l) and laterality (R or L), along with the best fitted linear regression plot (a) In Right-handers (R), (b) In Left-handers (L)
Table 4.
Brain asymmetry from laterality, FA results, mean, median, Standard Deviation (SD) and p value (t test). R vs L (right-side): looking for a significant difference between right-handers (R) and left-handers (L) based on laterality.
| RK2 | FA | ||||
|---|---|---|---|---|---|
|
| |||||
| laterality | Right-Handers | Left-Handers | |||
|
| |||||
| hand | right | left | right | left | |
| Level 1 | minimum | 0 | 0 | 0 | 0 |
| maximum | 1 | 1 | 0.85596 | 0.93627 | |
| mean | 0.46223 | 0.46759 | 0.35706 | 0.39016 | |
| median | 0.45054 | 0.42144 | 0.31974 | 0.48064 | |
| p value | 0.671 | 0.6725 | |||
| R vs L (right-side) | <0.0001 | ||||
| R vs L (left-side) | 0.3237 | ||||
|
| |||||
| Level 2 | minimum | 0.09239 | 0 | 0 | 0 |
| maximum | 0.99798 | 1 | 0.95827 | 0.89832 | |
| mean | 0.53480 | 0.59328 | 0.50709 | 0.44397 | |
| median | 0.52552 | 0.59975 | 0.50029 | 0.45063 | |
| p value | <0.0001 | 0.0007 | |||
| R vs L (right-side) | 0.0007 | ||||
| R vs L (left-side) | <0.0001 | ||||
|
| |||||
| Level 3 | minimum | 0.171138 | 0 | 0 | 0 |
| maximum | 1 | 0.95442 | 1 | 0.84667 | |
| mean | 0.58785 | 0.63625 | 0.48280 | 0.42517 | |
| median | 0.59605 | 0.66117 | 0.46439 | 0.40477 | |
| p value | <0.0001 | 0.0001 | |||
| R vs L (right-side) | <0.0001 | ||||
| R vs L (left-side) | <0.0001 | ||||
In regard to asymmetry, we observed a significant difference in the right-handers at 2 of the 3 levels of the slice (level 2: p<0.0001 and level 3: p<0.0001). In fact, the FA mean and median of the above 2 levels were higher for the left hand side. However, in the left-handers, level 2 (p=0.0007) and 3 (p=0.0001) means were higher for the right hand side.
In regard to laterality, very significant differences were observed at the 3 levels of the slice: FA measurement was higher for the right-handers (both sides). The exception to this was reported for the left hand side at level 1 where corona radiata was not significantly different.
Mean diffusivity and laterality
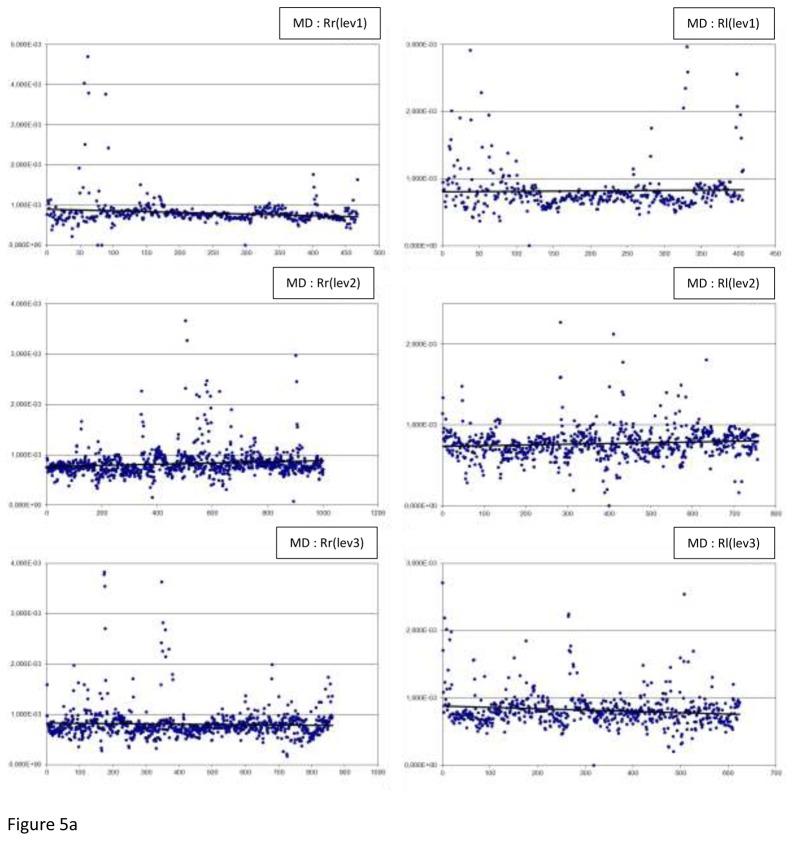
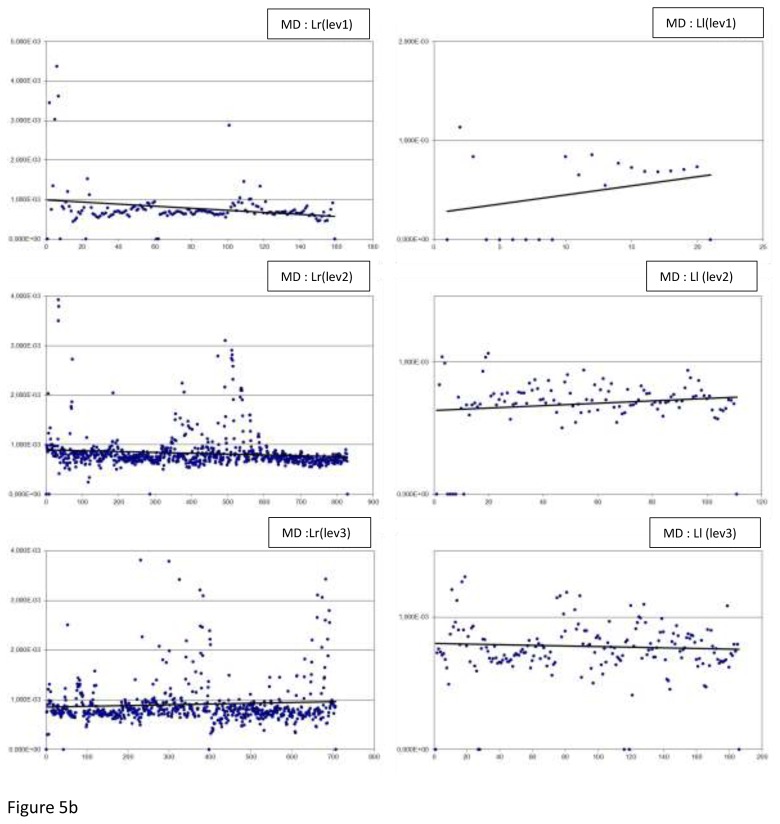
Figure 5 displays all the MD measurements: by level, side (r or l), and laterality (R or L). Table 5 displays the MD results: minimum and maximum, means, medians and p values.
Fig 5.
The MD measurements by level (lev), side (r or l) and laterality (R or L), along with the best fitted linear regression plot (a) In Right-handers (R), (b) In Left-handers (L)
Table 5.
Brain asymmetry from laterality, MD results, mean, median, Standard Deviation (SD) and p value (t test). R vs L (right-side): looking for a significant difference between right-handers (R) and left-handers (L) based on laterality.
| RK2 | MD | ||||
|---|---|---|---|---|---|
|
| |||||
| laterality | Right-Handers | Left-Handers | |||
|
| |||||
| hand | right | left | right | left | |
| Level 1 | minimum | 0 | 0 | 0 | 0 |
| maximum | 0.00469 | 0.00326 | 0.00437 | 0.00114 | |
| mean | 0.00081 | 0.00082 | 0.00078 | 0.00047 | |
| median | 0.00076 | 0.00074 | 0.00068 | 0.00068 | |
| p value | 0.6004 | 0.0111 | |||
| R vs L (right-side) | 0.6162 | ||||
| R vs L (left-side) | <0.0001 | ||||
|
| |||||
| Level 2 | minimum | 0.00008 | 0 | 0 | 0 |
| maximum | 0.00366 | 0.00227 | 0.00392 | 0.00107 | |
| mean | 0.00083 | 0.00077 | 0.00082 | 0.00069 | |
| median | 0.00078 | 0.00076 | 0.00075 | 0.00071 | |
| p value | <0.0001 | <0.0001 | |||
| R vs L (right-side) | 0.8819 | ||||
| R vs L (left-side) | <0.0001 | ||||
|
| |||||
| Level 3 | minimum | 0.00017 | 0 | 0 | 0 |
| maximum | 0.00383 | 0.00271 | 0.00517 | 0.00212 | |
| mean | 0.00082 | 0.00082 | 0.00091 | 0.00078 | |
| median | 0.00076 | 0.00078 | 0.00078 | 0.00075 | |
| p value | 0.9553 | <0.0001 | |||
| R vs L (right-side) | <0.0001 | ||||
| R vs L (left-side) | 0.0868 | ||||
In regard to asymmetry, a significant difference was observed at slice level 2 (p<0.0001) in the right-handers. The mean and median were higher for the right hand side. There was a significant difference at the 3 slice levels in the left-handers: level 1, 2 and 3, p-values were respectively 0.0111, <0.0001, and <0.0001. The means were reported to be higher for the right hand side.
In regard to laterality, significant differences were reported at the 3 slice levels. At the slice level 1 and 2 for the left hand side and level 3 for the right hand side (all 3 levels of slice p<0.0001).
Laterality correlation with FA and MD
A correlation was reported for the FA (r= 0.107; p<0.0001) and MD (r=−0.051; p=0.0199) based on laterality in the left-handers (Table 1).
Discussion
Our results highlighted significant differences of FA and MD parameters at three different slice levels. In addition, we revealed a correlation between the laterality index and the FA and MD parameters. We would like to emphasize that in this study, the fMRI images labeled “right” or “left” side refers to the “hand” that was used for motor function assessment, i.e. the right hand side referred to the left hemisphere. Indeed, most of the literature mentioned here reported the cerebral hemisphere on which their study was based.
We selected one of the three ROI that was segmented from functional data taking into account the motor function area of the hand [30]. This study did not show a significant difference either of hemispheric asymmetry or laterality association with the ROI volumes. These results are not consistent with those of the literature reporting asymmetry of the motor functions by fMRI. This could be a bias of our results, given the published evidence that the functional activation is more important at the left motor cortex in right-handers (for the mobility of right and left hand) [23, 29]. This could be explained by the several CST fibres which do not decussate [53]. Other explanation could be the association with the laterality index. Dassonville et al. [16] studied the fMRI motor function of the dominant and non-dominant hand in seven right-handers and six left-handers. They demonstrated that the cortical function is more important for the dominant hand and that it is correlated with the laterality index in both right- and left-handers.
The hemispheric asymmetry revealed in our study was that of the left hand side FA, and that of the right hand side MD (i.e. respectively, the right and left hemisphere). In regard to the FA, we reported a significant difference between the right- and left-handers with a larger FA at right and left in the right-handers. The FA increased based on the laterality index in left-handers (7 to 20/20). There was no evidence of such correlation in the right-handers since our sample consisted of real right-handers except for one slightly ambidextrous (1/20). The FA points out the diffusion process direction [7] and it is influenced by axon myelination, cellular density as well as fibre diameter [46]. Our results are not consistent with those of the reviewed literature and did not conclude a difference in fibre organisation between the right- and left-handers. Sullivan et al. [49], in their study on internal capsule FA in 24 right-handers, found different FA measures in different zones. They stated that this variation of FA was related to the diversity of fibres passing through the internal capsule. Our study took into account an overall FA at each slice level, but by analysing the fibres resulting from RK2 algorithm of CST reconstruction. We would like to emphasize the limitation of our fibres reconstruction method, deterministic tractography, unable to resolve “crossing” and “kissing” fibre bundles within a voxel [12, 40]. Our MD results were similar to the FA ones. However, there was a significant difference at the three slice levels: less homogenous results in regard to only one side at each level. A difference was reported at two slice levels for the left hand side, (i.e. the right hemisphere), being the non-dominant hand in right-handers and less often dominant in left-handers (15% of the cases [38]).
Our DTI and tractography study, at several levels of encephalon, of the white matter tracts run by CST hand motor fibres, did not allow to show a clear significant difference between the right- and left-handers. Several authors [9, 36, 41, 50, 52] have demonstrated an asymmetry between the right and left side of the encephalon. Some authors [41, 50], have reported results consistent with ours in regard to FA. Toosy et al. [50], in their study of 21 cases with amyotrophic lateral sclerosis and 14 controls, showed a gradual decrease of FA on the rostro-caudal axis in the controls. The FA was reported to have more significant difference at the internal capsule of the right hemisphere. The FA decrease was explained by the size of the studied structure within the brainstem and the number of crossing fibres. The above results cannot be compared with ours since we did not study the brainstem. Reich et al. [41], in their 3T scan assessment of CST in 20 volunteers, obtained a correlation between inter-hemispheric number of fibres. This asymmetry varies based on the zone in the nervous system, and an increase in MD asymmetry has been shown in the assessment of hemispheric zone of right CST. This was demonstrated in the study of posterior limb of internal capsule of 60 volunteers (30 right-handers and 30 left-handers) by Westerhausen et al. [52]. These authors showed an increase in left-hand FA and right-hand MD. However, no difference was observed based on laterality. One possible explanation was that the dominant hand muscles to be more innervated than the non-dominant hand muscles. This could explain the dexterity hypothesis [24].
To date, Büchel et al. [9] are the only authors demonstrating an association between handedness and part of the encephalon through an assessment of their second group (28 cases). They found asymmetric FA in the precentral gyrus, controlateral to the dominant hand. In their MRI morphometric evaluation of 56 young right-handed men, Hervé et al. [26] revealed asymmetric gray matter in the precentral and central regions and no neighbouring white matter difference despite the observed left hand side asymmetry. In their fMRI DTI study of arcuate fasciculus in 12 cases (seven right-handers), Vernooij et al. [51] used CST as the reference bundle. They reported leftward asymmetric arcuate fasciculus without any relation with laterality (dominant hand or language), consistent with a previous study on CST (Cicarelli et al. [13]) and contrary to others on motor system [2, 3, 26].
Decussation of CST fibres could be one explanation for not observing an evidence of correlation with laterality. Yakovlev et Rakic [53], in their dissection of fibre bundles in 100 medulla oblonga and 130 spinal cords of foetus and new-borns, demonstrated that in 2/3 of the cases, there were more left hemisphere fibres decussating to the right, and more right hemisphere fibres running to the right of spinal cord. Consequently, the right side of the spinal cord could be considered as the dominant side since it receives more fibres from both sides of the encephalon. Thereafter, Kertesz et Geschwind [27] conducted a similar study by taking into account handedness of the cases, but did not find a correlation with laterality. Consistent results of a more recent study [33] on 70 spinal cord cases revealed right side fibre asymmetry in ¾ of the cases, and more fibres running to the right than left in ¾ of the cases. However, there was no evidence of correlation with handedness, sole a correlation with dexterity was suspected.
Laterality is different between men and women. There are more right-handed women, and thus more (> 25%) non-right-handed men (ambidextrous and left-handers) [47]. Hervé et al. [26] described a less significant planum temporale asymmetry in left-handers men. Shapleske et al. [45] found a significant planum temporale asymmetry for the left hand side. This difference was reported to decrease in left-handers and women. Amunts et al. [2] found that central sulcus was deeper based on laterality only in men (right central sulcus was deeper in 62% of left-handers). Pujol et al. [39] reported hemispheric volume asymmetry on MRI revealing a larger left hemisphere. This hemispheric volume asymmetry was reported to be more significant in men. Powell et al. [37] performed voxel-based statistical analysis on FA maps of 42 right-handers and 40 left handers. Leftward anisotropy was found in arcuate fasciculus regions (greater in right-handers). Studying differences between men and women, they concluded that sex had greater effect than handedness on FA asymmetries. In the previous cited study by Westerhausen et al. [52], a significant difference was reported with an increase in FA in male brains. These authors suggested a difference in CST organisation and structure within internal capsule between men and women. Given the bigger size of men’s brain, the above hypothesis may indicate a variation of CST and thus of parameters such as FA representing its structure. The gender volume asymmetry difference disappeared after matching data for brain size. Catani et al. [10] studied 40 right-handed adults and reported no difference in CST volume and FA parameter between men and women.
Most of the literature aiming at determining an association between asymmetry and laterality used a small sample size leading to their results limitation [25]. In addition, it is well known that left-handers are not a homogenous population – their dominant hemisphere is the right hemisphere in 15% of the cases [38] while some could be culturally and environmentally forced into right-handedness. In fact, most of variation in handedness is due to genetic effects [5], the rest being attributable to environmental influences [15]. Assessment of laterality is often done by various methods. It is worth mentioning that some studies have not used any assessment method [25]. For instance, Good et al. [22], conducted their laterality assessment of 67 left- and 398 right-handers based on the dominant hand for writing. Such assessment has its limitation in regard to the impact of laterality [47]. We chose an assessment method derived from studies done on a sample of French population free of neurological pathologies (e.g. our sample) [17].
To conclude, consistent with other studies, we demonstrated a CST hand motor task asymmetry in relation with laterality. In line with the literature [10], our study had its limitations such as our deterministic tractography method and our small size for each group. Our results did not allow us to draw conclusions in terms of laterality index. Thus, we suggest larger scale studies using other assessment methods such as diffusion direction imaging [48], i.e. MRI with a low angle diffusion, to obtain a more precise CST reconstruction in particular in the crossing and kissing fibre zones.
Acknowledgments
The authors would like to warmly thank Zarrin Alavi (MSc), INSERM CIC 0502, for her assistance to realize this manuscript.
Footnotes
Conflict of interest
The authors declare no conflict of interest in the realization of this study.
References
- 1.Adams F. The genuine works of Hippocrates. William Woods; New York: 1849. [Google Scholar]
- 2.Amunts K, Jancke L, Mohlberg H, Steinmetz H, Zilles K. Interhemispheric asymmetry of the human motor cortex related to handedness and gender. Neuropsychologia. 2000;38:304–312. doi: 10.1016/s0028-3932(99)00075-5. [DOI] [PubMed] [Google Scholar]
- 3.Amunts K, Schlaug G, Schleicher A, Steinmetz H, Dabringhaus A, Roland PE, Zilles K. Asymmetry in the human motor cortex and handedness. Neuroimage. 1996;4:216–222. doi: 10.1006/nimg.1996.0073. [DOI] [PubMed] [Google Scholar]
- 4.Annett M. A classification of hand preference by association analysis. Br J Psychol. 1970;61:303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- 5.Annett M. Handedness and cerebral dominance: the right shift theory. J Neuropsychiatry Clin Neurosci. 1998;10:459–469. doi: 10.1176/jnp.10.4.459. [DOI] [PubMed] [Google Scholar]
- 6.Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 7.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 8.Broca P. Localisation des fonctions cérébrales. Siège du langage articulé. Bull Soc Anthropol. 1863;4:200–204. [Google Scholar]
- 9.Büchel C, Raedler T, Sommer M, Sach M, Weiller C, Koch MA. White matter asymmetry in the human brain: a diffusion tensor MRI study. Cereb Cortex. 2004;14:945–951. doi: 10.1093/cercor/bhh055. [DOI] [PubMed] [Google Scholar]
- 10.Catani M, Forkel S, Thiebaut de SM. Asymetry of white matter pathways. 2010. pp. 177–209. [Google Scholar]
- 11.Caulo M, Briganti C, Mattei PA, Perfetti B, Ferretti A, Romani GL, Tartaro A, Colosimo C. New morphologic variants of the hand motor cortex as seen with MR imaging in a large study population. AJNR Am J Neuroradiol. 2007;28:1480–1485. doi: 10.3174/ajnr.A0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciccarelli O, Catani M, Johansen-Berg H, Clark C, Thompson A. Diffusion-based tractography in neurological disorders: concepts, applications, and future developments. Lancet Neurol. 2008;7:715–727. doi: 10.1016/S1474-4422(08)70163-7. [DOI] [PubMed] [Google Scholar]
- 13.Ciccarelli O, Parker GJ, Toosy AT, Wheeler-Kingshott CA, Barker GJ, Boulby PA, Miller DH, Thompson AJ. From diffusion tractography to quantitative white matter tract measures: a reproducibility study. Neuroimage. 2003;18:348–359. doi: 10.1016/s1053-8119(02)00042-3. [DOI] [PubMed] [Google Scholar]
- 14.Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, McKinstry RC, Burton H, Raichle ME. Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci U S A. 1999;96:10422–10427. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corballis MC, Badzakova-Trajkov G, Häberling IS. Right hand, left brain: genetic and evolutionary bases of cerebral asymmetries for language and manual action. WIREs Cogn Sci. 2012;3:1–17. doi: 10.1002/wcs.158. [DOI] [PubMed] [Google Scholar]
- 16.Dassonville P, Zhu XO, Ugurbil K, Kim SG, Ashe J. Functional activation in motor cortex reflects the direction and the degree of handedness. Proc Natl Acad Sci U S A. 1997;94:14015–14018. doi: 10.1073/pnas.94.25.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dellatolas G, De Agostini M, Jallon P, Poncet M, Rey M, Lellouch J. Mesure de la préférence manuelle par autoquestionnaire dans la population française adulte. Revue de Psychologie aplliquée. 1988;38:117–136. [Google Scholar]
- 18.Fillard P, Arsigny V, Pennec X, Hayashi KM, Thompson PM, Ayache N. Measuring brain variability by extrapolating sparse tensor fields measured on sulcal lines. Neuroimage. 2007;34:639–650. doi: 10.1016/j.neuroimage.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 19.Friston KJ, Holmes AP, Worsley KJ, Poline J-P, frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 20.Geschwind N. Cerebral dominance and anatomic asymmetry. N Engl J Med. 1972;287:194–195. doi: 10.1056/NEJM197207272870416. [DOI] [PubMed] [Google Scholar]
- 21.Geschwind N, Levitsky W. Human brain: left-right asymmetries in temporal speech region. Science. 1968;161:186–187. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- 22.Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- 23.Gut M, Urbanik A, Forsberg L, Binder M, Rymarczyk K, Sobiecka B, Kozub J, Grabowska A. Brain correlates of right-handedness. Acta Neurobiol Exp (Wars ) 2007;67:43–51. doi: 10.55782/ane-2007-1631. [DOI] [PubMed] [Google Scholar]
- 24.Hammond G. Correlates of human handedness in primary motor cortex: a review and hypothesis. Neurosci Biobehav Rev. 2002;26:285–292. doi: 10.1016/s0149-7634(02)00003-9. [DOI] [PubMed] [Google Scholar]
- 25.Hatta T. Handedness and the brain: a review of brain-imaging techniques. Magn Reson Med Sci. 2007;6:99–112. doi: 10.2463/mrms.6.99. [DOI] [PubMed] [Google Scholar]
- 26.Hervé PY, Crivello F, Perchey G, Mazoyer B, Tzourio-Mazoyer N. Handedness and cerebral anatomical asymmetries in young adult males. Neuroimage. 2006;29:1066–1079. doi: 10.1016/j.neuroimage.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 27.Kertesz A, Geschwind N. Patterns of pyramidal decussation and their relationship to handedness. Arch Neurol. 1971;24:326–332. doi: 10.1001/archneur.1971.00480340058006. [DOI] [PubMed] [Google Scholar]
- 28.Kertesz A, Polk M, Black SE, Howell J. Anatomical asymmetries and functional laterality. Brain. 1992;115(Pt 2):589–605. doi: 10.1093/brain/115.2.589. [DOI] [PubMed] [Google Scholar]
- 29.Kim SG, Ashe J, Hendrich K, Ellermann JM, Merkle H, Ugurbil K, Georgopoulos AP. Functional magnetic resonance imaging of motor cortex: hemispheric asymmetry and handedness. Science. 1993;261:615–617. doi: 10.1126/science.8342027. [DOI] [PubMed] [Google Scholar]
- 30.Kim YH, Kim DS, Hong JH, Park CH, Hua N, Bickart KC, Byun WM, Jang SH. Corticospinal tract location in internal capsule of human brain: diffusion tensor tractography and functional MRI study. Neuroreport. 2008;19:817–820. doi: 10.1097/WNR.0b013e328300a086. [DOI] [PubMed] [Google Scholar]
- 31.Lotze M, Erb M, Flor H, Huelsmann E, Godde B, Grodd W. fMRI evaluation of somatotopic representation in human primary motor cortex. Neuroimage. 2000;11:473–481. doi: 10.1006/nimg.2000.0556. [DOI] [PubMed] [Google Scholar]
- 32.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 33.Nathan PW, Smith MC, Deacon P. The corticospinal tracts in man. Course and location of fibres at different segmental levels. Brain. 1990;113(Pt 2):303–324. doi: 10.1093/brain/113.2.303. [DOI] [PubMed] [Google Scholar]
- 34.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 35.Peterson BS, Riddle MA, Cohen DJ, Katz LD, Smith JC, Leckman JF. Human basal ganglia volume asymmetries on magnetic resonance images. Magn Reson Imaging. 1993;11:493–498. doi: 10.1016/0730-725x(93)90468-s. [DOI] [PubMed] [Google Scholar]
- 36.Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CA, Barker GJ, Noppeney U, Koepp MJ, Duncan JS. Hemispheric asymmetries in language-related pathways: a combined functional MRI and tractography study. Neuroimage. 2006;32:388–399. doi: 10.1016/j.neuroimage.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 37.Powell JL, Parkes L, Kemp GJ, Sluming V, Barrick TR, Garcia-Finana M. The effect of sex and handedness on white matter anisotropy: a diffusion tensor magnetic resonance imaging study. Neuroscience. 2012;207:227–242. doi: 10.1016/j.neuroscience.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Pujol J, Deus J, Losilla JM, Capdevila A. Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology. 1999;52:1038–1043. doi: 10.1212/wnl.52.5.1038. [DOI] [PubMed] [Google Scholar]
- 39.Pujol J, Lopez-Sala A, Deus J, Cardoner N, Sebastian-Galles N, Conesa G, Capdevila A. The lateral asymmetry of the human brain studied by volumetric magnetic resonance imaging. Neuroimage. 2002;17:670–679. [PubMed] [Google Scholar]
- 40.Qazi AA, Radmanesh A, O’Donnell L, Kindlmann G, Peled S, Whalen S, Westin CF, Golby AJ. Resolving crossings in the corticospinal tract by two-tensor streamline tractography: Method and clinical assessment using fMRI. Neuroimage. 2009;47(Suppl 2):T98–106. doi: 10.1016/j.neuroimage.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reich DS, Smith SA, Jones CK, Zackowski KM, van Zijl PC, Calabresi PA, Mori S. Quantitative characterization of the corticospinal tract at 3T. AJNR Am J Neuroradiol. 2006;27:2168–2178. [PMC free article] [PubMed] [Google Scholar]
- 42.Rubens AB, Mahowald MW, Hutton JT. Asymmetry of the lateral (sylvian) fissures in man. Neurology. 1976;26:620–624. doi: 10.1212/wnl.26.7.620. [DOI] [PubMed] [Google Scholar]
- 43.Saenger VM, Fernando FA, Martinez-Gudino ML, Alcauter S. Hemispheric asymmetries of functional connectivity and grey matter volume in the default mode network. Neuropsychologia. 2012;50:1308–1315. doi: 10.1016/j.neuropsychologia.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 44.Seizeur R, Wiest-Daessle N, Prima S, Maumet C, Ferre JC, Morandi X. Corticospinal tractography with morphological, functional and diffusion tensor MRI: a comparative study of four deterministic algorithms used in clinical routine. Surg Radiol Anat. 2012;34:709–719. doi: 10.1007/s00276-012-0951-x. [DOI] [PubMed] [Google Scholar]
- 45.Shapleske J, Rossell SL, Woodruff PW, David AS. The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance. Brain Res Brain Res Rev. 1999;29:26–49. doi: 10.1016/s0165-0173(98)00047-2. [DOI] [PubMed] [Google Scholar]
- 46.Shimony JS, McKinstry RC, Akbudak E, Aronovitz JA, Snyder AZ, Lori NF, Cull TS, Conturo TE. Quantitative diffusion-tensor anisotropy brain MR imaging: normative human data and anatomic analysis. Radiology. 1999;212:770–784. doi: 10.1148/radiology.212.3.r99au51770. [DOI] [PubMed] [Google Scholar]
- 47.Sommer IEC. Sex differences in handedness, brain asymmetry, and language lateralization. Information Processing in the Cerebral Hemispheres. 2010:277–312. [Google Scholar]
- 48.Stamm A, Perez P, Barillot C. Technical report. INRIA; 2011. Diffusion Directions Imaging (DDI) [Google Scholar]
- 49.Sullivan EV, Zahr NM, Rohlfing T, Pfefferbaum A. Fiber tracking functionally distinct components of the internal capsule. Neuropsychologia. 2010;48:4155–4163. doi: 10.1016/j.neuropsychologia.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toosy AT, Werring DJ, Orrell RW, Howard RS, King MD, Barker GJ, Miller DH, Thompson AJ. Diffusion tensor imaging detects corticospinal tract involvement at multiple levels in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2003;74:1250–1257. doi: 10.1136/jnnp.74.9.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vernooij MW, Smits M, Wielopolski PA, Houston GC, Krestin GP, van der LA. Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right- and left-handed healthy subjects: a combined fMRI and DTI study. Neuroimage. 2007;35:1064–1076. doi: 10.1016/j.neuroimage.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 52.Westerhausen R, Huster RJ, Kreuder F, Wittling W, Schweiger E. Corticospinal tract asymmetries at the level of the internal capsule: is there an association with handedness? Neuroimage. 2007;37:379–386. doi: 10.1016/j.neuroimage.2007.05.047. [DOI] [PubMed] [Google Scholar]
- 53.Yakovlev P, Rakic P. Patterns of decussation of bulbar pyramids and distribution of pyramidal tracts on two sides of spinal cord. Trans Am Neurol Assoc. 1966;81:366–367. [Google Scholar]
- 54.Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120(Pt 1):141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]