Abstract
Evolving research has provided evidence that noninvasive electrical stimulation (ES) of the eye may be a promising therapy for either preserving or restoring vision in several retinal and optic nerve diseases. In this review, we focus on minimally invasive strategies for the delivery of ES and accordingly summarize the current literature on transcorneal, transorbital, and transpalpebral ES in both animal experiments and clinical studies. Various mechanisms are believed to underlie the effects of ES, including increased production of neurotrophic agents, improved chorioretinal blood circulation, and inhibition of proinflammatory cytokines. Different animal models have demonstrated favorable effects of ES on both the retina and the optic nerve. Promising effects of ES have also been demonstrated in clinical studies; however, all current studies have a lack of randomization and/or a control group (sham). There is thus a pressing need for a deeper understanding of the underlying mechanisms that govern clinical success and optimization of stimulation parameters in animal studies. In addition, such research should be followed by large, prospective, clinical studies to explore the full potential of ES. Through this review, we aim to provide insight to guide future research on ES as a potential therapy for improving vision.
Eye Diseases and Treatment Options
Blindness affects 39 million people worldwide, and its prevalence continuously increases with an aging population (http://www.who.int/mediacentre/factsheets/fs282/en). The leading causes of irreversible blindness in the developed world include glaucoma, age-related macular degeneration (AMD), diabetic retinopathy, and retinitis pigmentosa (RP). Glaucoma refers to a group of ocular diseases that result in damage to the optic nerve, leading to progressive, irreversible loss of retinal ganglion cells,1 and it is the second leading cause of blindness worldwide.2 It is estimated that by 2020, approximately 11 million individuals will be blind from glaucoma.2 Current glaucoma therapies, including medication, laser trabeculoplasty, surgery, or a combination of any of these, are directed solely at lowering intraocular pressure, which slows vision loss for some patients but cannot restore vision loss due to disease. AMD is another progressive disease leading to blindness as a result of photoreceptor cell loss in the macula (ie, the central part of the retina). It primarily affects central vision, which is critical for daily activities, such as reading and driving. AMD is the third leading cause of blindness in the world and the primary cause of visual deficiency in industrialized countries.3 Clinically, AMD is divided into two forms: wet and dry. There are currently several treatment options for wet AMD. However, no established therapy is available for dry AMD, which constitutes 85% to 90% of known cases. Certain treatments, including intravitreal injection of antivascular endothelial growth factor, have shown considerable success in slowing disease progression and reducing the number of blind people worldwide by preventing further neovascularization from wet AMD. However, no well-documented cures exist that can reverse damage that has already been inflicted to the photoreceptors. RP encompasses several genetic defects that result in photoreceptor loss and blindness. The prevalence of RP is reported to be 1:3000 to 1:5000.4 In contrast to AMD, the retina is first affected peripherally, with gradual progression to the central retina. RP targets rod photoreceptors, thus initially causing poor peripheral and night vision.
Although there are no currently available cures for these potentially blinding conditions (eg, glaucoma, RP, and AMD), there are many fields of research that give hope of visual restoration to patients with end-stage eye disease. Visual cycle modulators that mediate the intracellular processing of vitamin A are currently being applied in clinical trials for RP, and initial evidence has shown that they slow progression of disease and may provide better vision in patients with mild-to-moderate disease.5 Gene therapy aims at treating, curing, or preventing disease by delivering genes into the eyes; however, current therapies are primarily experimental, with most human clinical trials still in the research phase.6 For patients only retaining light perception, optogenetic (ie, the use of genetic methods combined with optical technology to achieve retinal neuron function) and stem cell therapies are promising strategies for improving or restoring vision.7
ES in Ophthalmology
The first-reported electrical stimulation (ES) of the eye to generate visual sensations to light was performed in 1755 by Charles LeRoy, who applied an electrical discharge on the eye surface of a patient blind from cataracts. The patient reported that the ES provoked strong flashes of light or phosphene. In 1873, Henri Dor reported the beneficial effects of ES on the eye via treatment of amblyopia, amauroses, glaucoma, retinochoroiditis, and white optic atrophy.8 In 1929, Otfrid Foerster, a German neurosurgeon, reported a neural response to ES in a patient's visual cortex.9 In 1974, the ES of the visual system was shown to elicit visual perceptions using suprathreshold stimulation.10 Thirty years later, the therapeutic potential of subthreshold ES of the retina was identified by Chow et al.11 The authors showed that patients having inactive subretinal chips generating only subthreshold currents exhibited visual improvement in areas far from the site of the electrical device, suggesting a generalized neuroprotective effect of ES. This finding was associated with the expression of neurotrophic factors and sparked a research interest in the effects of ES in animal experiments and thereafter in clinical studies for treating blindness.
ES: Methods of Delivery
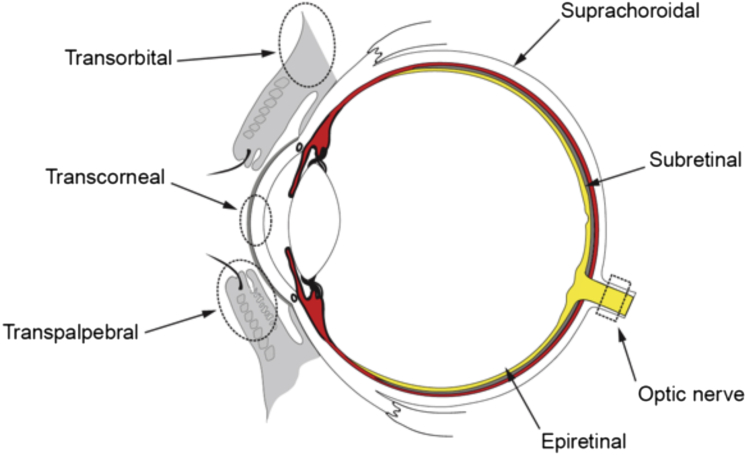
During the past 10 years, there has been a significant increase in research on developing an ES treatment for various eye diseases. The basis of the therapeutic strategy is to stimulate neurons along the visual pathway with electrical current. Presently, there are eight routes by which ES has been delivered to patients with the purpose of improving vision: transpalpebral, transorbital, transcorneal, subretinal, epiretinal, and transchoroidal approaches, as well as the direct stimulation of the optic nerve or brain (Figure 1). Ocular stimulation targets the remaining retinal cells, whereas stimulation of the brain targets the higher visual structures. Although ocular stimulations are minimally invasive and simple to implement, stimulation of the brain is useful when connections between the eye and brain are affected, such as in advanced glaucoma.
Figure 1.
Different routes of electrical stimulation of the eye. The red circle represents choroidea, except for the anterior part of the eye. The gray semicircle represents the retinal pigment epithelial cells, and the yellow semicircle illustrates the retina. Image is courtesy of Håkon Raanes (Norway).
Retinal Prostheses
Retinal prostheses (alias retinal implants or chips) use energy converters to generate electricity to mimic photoreceptor activities. Epiretinal implants electrically target the ganglion cell layer, subretinal implants primarily target the inner nuclear layer of the retina, and transchoroidal implants stimulate the retina from the outer region.12 Patients with RP usually exhibit defective pigment epithelium and photoreceptors, but the inner retina and ganglion cell layer are intact. To restore visual function, retinal prostheses must detect light, converting light energy into electricity and thereby activating downstream retinal neurons. The field of visual prosthetics has progressed significantly since the first implantation in the 1960s13; however, many challenges must still be overcome before it can effectively restore vision in blind patients.12
Minimally Invasive ES
Transpalpebral, transorbital, and transcorneal ES (TES) have the advantage of being minimally invasive, only touching the skin and the cornea, with minor adverse effects reported. The use of these approaches reduces the risk of severe complications compared to retinal prostheses. It is of utmost importance that ES is minimally invasive; therefore, the choice of electrodes must be taken into consideration. In both animal experiments and clinical studies, TES has been widely adapted, and the contact lens electrodes are most commonly used. Until now, three types of electrodes have been used in TES: Dawson-Trick-Litzkow (DTL)-Plus electrodes, electroretinography (ERG)-Jet electrodes, and golden ring electrodes, the last lacking a contact lens. DTL-Plus and ERG-Jet electrodes have been used in clinical studies and only negligibly stimulated different areas of the retina. In animal experiments, the ERG-Jet electrode is mostly used because of the simplicity of the procedure for fastening the electrodes onto animals. Transpalpebral (electrodes placed on the eyelids) and transorbital (electrodes placed at or near the eyeball) ES are other minimally invasive procedures. In the present review, we will systematically discuss the experimental populations and parameters, evaluation methods, major findings, advances, and challenges of the various ES procedures.
Animal Experiments
Many research groups have shown favorable effects of ES on the retina and optic nerve using rats, cats, and rabbits as experimental animals. So far, there are no animal studies on transpalpebral ES. However, beneficial effects of ES have been shown in one study using transorbital ES14 and in several animal experiments using TES (Table 1).15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35 These studies can be categorized as follows: i) healthy animals17, 18, 19, 23, 26, 31, 34; ii) transgenic animals22, 28; iii) animals as a disease model for RP21; iv) animals with induced ischemic insult,27, 29, 33 optic nerve crush,15, 16, 20, 32 and transected optic nerve24, 25; and v) animal cells isolated from the retina.35
Table 1.
TES: Animal Experiments
| Reference | Species; disease model | Experimental population; stimulation location | Control group | ES parameters | Evaluation method | Results |
|---|---|---|---|---|---|---|
| Henrich-Noack et al, 201315 | Male hooded rats; induced ONC | 16 rats; cornea; one eye | Other eye | 100 μA, 1 millisecond/phase, directly after ONC and on days 3, 7, 11, 15, 19, and 23 after ONC, 30-second duration; delivered at 10, 12, 9, 11, 8, 10, 9, and 12 Hz with 5-second break between each | ICON; ERG recordings | TES inhibits post-traumatic cell death considerably; TES decreases ONC-associated neuronal shrinkage and swelling, exceptionally in long-term surviving RGCs |
| Henrich-Noack et al, 201316 | Male hooded rats; induced ONC | Eight rats; cornea; both eyes | Eight rats; sham | 100 μA, 20-Hz biphasic square-wave pulses of 1 millisecond/phase, 60-minute duration, stimulated immediately after ONC and on day 11 after induction of ONC | ICON before ONC and on postlesion days 3, 7, and 15; ERG recordings | TES resulted in increased surviving of RGCs; the rate of RGC death was more variable in TES group; changes in morphology of survived neurons were milder in TES group |
| Kanamoto et al, 201517 | Wistar rats | 18 tats; cornea; left eye | Right eye | 50/100/200 μA, 20 Hz, 1-millisecond pulses, 30-minute duration, one session | Enucleated eyes 30 minutes or 24 hours after TES; proteomic analysis of retina | 25 Functional proteins exhibited higher levels of expression in TES group compared with control group |
| Ma et al, 201418 | Healthy adult cats | 13 cats; cornea; right eye | — | In optical imaging experiments: 1.2 mA, 5–100 Hz, 2–40-millisecond pulses/phase, 2-second duration; studying effects of different parameters: 0.24–2.64 mA, 20 Hz, 10-millisecond pulse width per phase, 1.2 mA, 20 Hz, 2–22 millisecond pulse width per phase | Multiwavelength optical imaging of intrinsic signals; electrophysiological recordings; hemoglobin oxygenation; cerebral blood volume | OIS activation regions in the visual cortex were correlative with TES; ascending current intensities and longer pulse width increased the extent of activation; largest response observed at 10–20 Hz; TES led to activation of peripheral retina |
| Mihashi et al, 201119 | Healthy adult cats | Two cats; cornea; both eyes | — | Maximum current up to 1 mA, 20 Hz, 20 paired biphasic pulses of 5 milliseconds | Electrophysiological recordings; fundus images | TES activates the RGCs; TES changes the blood flow |
| Miyake et al, 200720 | Long-Evans rats; induced ONC | Five rats; cornea | Five rats; sham | 500 μA, 20 Hz, pulses, 50-microsecond duration; started immediately after the ONC, applied for 6 hours | VEP; histology | TES instantly increased VEP amplitude, which was weakened by the ONC; increased number of axons in retina observed centrally beyond the crushed region when compared with the control group |
| Morimoto et al, 200721 | RCS rats; model for RP | 18 rats; cornea; left eye | Right eye | 50 or 100 μA, 20 Hz, 1 millisecond, 1-hour duration, one session | ERG recordings; histology | Compared to sham, 100 μA TES increased the thickness of the ONL at each time point; TES resulted in increased number of surviving photoreceptors and inhibited functional reduction of retina |
| Morimoto et al, 201222 | Rhodopsin P347L transgenic rabbits | Six rabbits; cornea; left eye | Right eye | 700 μA, 20 Hz, 10 milliseconds/phase, 1-hour duration, once a week for 6 weeks | ERG recordings; histology; immunohistochemistry | TES led to increased survival of photoreceptors and preserved ERGs |
| Morimoto et al, 201423 | Healthy adult cats | Eight cats; cornea; left eye | — | Biphasic pulses: 0.1, 0.5, 1.0, 2.0 mA, 20 Hz at 20 pulses; pulse durations: 0.5, 1.0, 2.0, 3.0, 5.0, 10.0 milliseconds/phase at 20 Hz at 20 pulses; frequencies of 5, 10, 20, 30, 50 Hz at 5 milliseconds/phase at 20 pulses; duration of 0.5, 1, 4, and 5 milliseconds/phase at 50 Hz | Optical imaging of reflectance changes in retina; electrophysiological recordings from OC | The intensity increased with the frequency, pulse duration, and current intensity; TES resulted in the reflective changes on the optic disk and retinal vessels, and in activation of retinal neurons |
| Morimoto et al, 200524 | Adult Wistar rats; transected ON | 26 rats; cornea; both eyes | 12 Rats; sham | 100 μA, 20 Hz, 3 milliseconds/phase, 1-hour duration, one session | Quantification of RGC density; RT-PCR; Northern blotting; Western blotting; immunohistochemistry | TES increased the number of surviving axotomized RGCs; the electric charge strength had important role for the degree of rescue; IGF-1 was up-regulated in the retina after TES; IGF-1 was first expressed in the Müller cells and then diffused into the inner retinal layer |
| Morimoto et al, 201025 | Wistar rats; transected ON | 36 rats; cornea; left eye | 12 Rats; sham; right eye | 0.5, 1, 2, 3, and 5 milliseconds/phase, with 100 μA, 20 Hz for 60 minutes; 50, 100, 200, 300, and 500 μA with 1 millisecond/phase, 20 Hz, 60 minutes; 0.5, 1, 5, 20, 50, and 100 Hz at 100 μA, 1 millisecond/phase for 60 minutes; 15, 30, and 60 minutes at 100 μA, 1 millisecond/phase, and 20 Hz; repeated stimulations on days 0, 4, 7, and 10 after the transection were compared with a single stimulation 14 days after transection | Quantification of RGC density with fluorescence microscope | The optimal neuroprotective parameters were 1 and 2 milliseconds/phase, 100 and 200 μA, frequency of 1, 5, and 20 Hz; >30 minutes of TES was required to achieve a neuroprotective effect; repeated stimulation was more neuroprotective than a single stimulation 14 days after transection |
| Ni et al, 200926 | SD rats | 332 rats; cornea; both eyes | Light stimulation | 100–500 μA, 20–100 Hz, 3 milliseconds, 1.5 hours, one session; 200 or 300 μA, 20 Hz, 3 milliseconds, one session per 3 days for up to 14 days | ERG recordings; histology; immunohistochemistry; RT-PCR; Western blotting | Post-TES exhibited an enhanced and longer-term protective effect than pre-TES; after TES, expression of Bcl-2 was up-regulated, Bax was down-regulated, and levels of CNTF and BDNF were up-regulated |
| Osako et al, 201327 | SD rats; induced NAION | 19 rats; cornea | 14 Rats; sham | 100 μA, 20 Hz, 1 millisecond/phase, 60-minute duration; TES applied 3 hours after the induction of NAION and was later performed on the 1st, 4th, 7th, 14th, and 28th day | OCT measurements; STR; measurements of the number of surviving RGCs | The use of TES resulted in decreased STR amplitude and the number of RGCs in NAION rats |
| Rahmani et al, 201328 | P23H transgenic rats; mimic autosomal dominant RP | Six rats; cornea, left eye | 10 Rats, sham, 16 weeks of age | 1.5 μA, 5 Hz, 40–50 kΩ; TES applied twice per week, 30-minutes sessions, from 4 to 16 weeks of age | ERG recordings; histology | TES led to preservation of b-wave amplitudes and rod sensitivity; no histological changes were associated with TES treatment |
| Schatz et al, 201229 | SD rats; induced NAION | 15 rats; cornea; right eye | 15 rats; sham; 3 rats for histology | 200 μA, 20 Hz for 1 hour, biphasic stimuli 2 milliseconds/phase (1 millisecond positive, 1 millisecond negative pulse) | ERG recordings; histology; immunohistochemistry; TUNEL assays | TES resulted in lower b-wave; photoreceptor cell death was observed in sham group, primarily in the superior hemiretina; eyes treated with TES exhibited preservation of ONL thickness and resulted in reduced death of photoreceptor cells |
| Sergeeva et al, 201230 | Lister hooded rats; chronically lesioned after ONC | 10 rats; cornea | 10 rats; sham | 100 μA, 1 millisecond/phase, 30-second trains, 10-second breaks between each stimulation; the frequency of 10, 12, 9, 11, 8, 10, 9, and 12 Hz; repeat after 2-minute break | EEG recordings | EEG was not significantly changed in lesioned rats; TES only exhibited the effect by inducing cortical plasticity when the retina can be excited |
| Sergeeva et al, 201531 | Lister hooded rats | 24 rats; cornea; both eyes | — | 300 μA, biphasic 2-millisecond pulses, 1 per second, 20-minute duration | Exposed to flicker light; VEP and EER in visual cortex and superior colliculus | Physiological responses recorded after TES |
| Tagami et al, 200932 | Wistar rats; induced ONC | 36 rats; cornea; both eyes | 12 Rats; sham | 100 μA, 20 Hz, 1 millisecond/phase, 1-hour duration; four protocols: a single application on day 0, two on day 0 and 7; four on day 0, 4, 7, and 10; and daily applications on days 0–12 | Quantification of axonal growth and RGC density; IGF-1 immunostaining of retina | Daily applied TES encourages both survival of RGCs and regeneration of axons after the crush of ON; TES resulted in increased IGF-1 immunoreactivity |
| Wang et al, 201133 | SD rats; ischemic insult | 28 rats; cornea; both eyes | Sham | 300 μA, 20 Hz, 3 milliseconds/phase, 1 hour every 2 days up to 14 days | ERG recordings; histology; confocal laser microscope; Western blotting; immunohistochemistry | Higher density of RGCs in TES treated retinas on day 7 and 14 after ischemia; TES greatly preserved the thickness of separate retinal layers |
| Willmann et al, 201134 | Wild-type brown Norway rats | 17 rats; cornea; right eye | 16 rats; sham | 200 μA, 20 Hz, 1 millisecond/phase, 1-hour duration, one session | Microarray analysis; RT-PCR; histology; morphometric analysis; ERG recordings | 490 Genes exhibited different expression after TES; neuroprotective genes such as Bax or TNF family members were up-regulated; ERG recordings demonstrated physiological retinal function after TES |
| Zhou et al, 201235 | Rat cells | Primary microglia and Müller cells from retinas of SD rat cell line 661W | — | 300, 500, 1000, 1600 μA, 20 Hz, 3 milliseconds, 1 hour, one session | LDH assays; TUNEL assays; immunocytochemistry; RT-PCR; Western blotting | TES led to inhibition of the secretion of IL-1β and TNF-α in microglia, and to increased secretion of BDNF and CNTF in Müller cells; the death rate was reduced by addition of antibodies to IL-1β and TNF-α |
Bcl-2, B-cell lymphoma-2; BDNF, brain-derived neurotrophic factor; CNTF, ciliary neurotrophic factor; EEG, electroencephalogram; EER, electrically evoked response; ERG, electroretinogram; ES, electrical stimulation; ICON, in vivo confocal neuroimaging; IGF-1, insulin growth factor-1; LDH, lactate dehydrogenase; NAION, nonarteritic ischemic optic neuropathy; OC, optic chiasma; OCT, optical coherence tomography; OIS, optical imaging of intrinsic signals; ON, optic nerve; ONC, optic nerve crush; ONL, outer nuclear layer; RCS, Royal College of Surgeons; RGCs, retinal ganglion cells; RP, retinitis pigmentosa; SD, Sprague-Dawley; STR, scotopic threshold response; TES, transcorneal electrical stimulation; VEP, visual evoked potentials; –, not reported.
TES in Animal Models
In 2005, Morimoto et al24 observed that the survival of retinal ganglion cells (RGCs) was preserved from degeneration after transection of the optic nerve in rats, and that the degree of the electric charge was important for the rescue of cells. It was also demonstrated that insulin-like growth factor 1 (IGF-1) in the retina was up-regulated after stimulation. IGF-1 was initially strongly expressed in Müller cells and then also in the inner retina. In another study, the current intensity, frequency, pulse duration, waveform, and number of TES sessions were changed systematically.25 This vigorous approach demonstrated repeated stimulation after optic nerve transection to be the most advantageous. Furthermore, the highest neuroprotective effect on RGCs was achieved using the following stimulation parameters: 1 and 2 milliseconds per phase, 100 and 200 μA, frequency of 1.5 and 20 Hz, and at least a 30-minute duration.
Enhanced survival of RGCs has also been observed after optic nerve crush,15, 16, 20, 32 after light-induced retinal damage,26 and in ischemic rat retinas.33 Tagami et al32 revealed that the increased survival of RGCs was in accordance with the number of TES applications and that the daily application of TES exhibited the most effect. Furthermore, daily TES administration resulted in the up-regulated expression of IGF-1 to a greater extent than a single application of TES. Finally, enhanced regeneration of RGC axons by daily application of TES was completely blocked by a specific antagonist to the IGF-1 receptor, whereas the promotion of surviving RGCs was not.32
In a study involving Royal College of Surgeons rats, a generally used model for inherited retinal degeneration such as RP, the researchers demonstrated that retinal degenerative processes are markedly slowed by TES. TES increased survival of photoreceptors, as suggested by a significantly greater thickness of the outer nuclear layer, preserved ERG b-wave, and impaired retinal function in Royal College of Surgeons rats.21 In a study by Schatz et al,36 TES before mild light exposure temporarily preserved b-wave amplitudes and outer segment length, and reduced photoreceptor cell death after 2 weeks. The effects observed in this study were minor compared to the effects reported by Ni et al,26 where TES sessions were repeated and different electrode configurations were used. In a rabbit model of rhodopsin mutations that mimic RP, TES resulted in both increased photoreceptor survival and improved retinal function.22 In P23H transgenic rats, delivery of TES twice per week for 12 weeks resulted in the significant preservation of photoreceptor sensitivity, whereas no histological changes accompanied the stimulation.28 A possible explanation for the unchanged morphology may be the low electrical intensity applied in this study.
TES and Changes in Brain Activity
The beneficial effects of TES are not limited to rescuing neurons in the retina. Recently, Sergeeva et al31 successfully generated a new method for TES and flicker light stimulation in freely moving rats as well as a setup to record field potentials from the visual cortex and subcortical structures. A considerable advantage of this novel animal model, compared to what was described in previous studies,16, 21, 24, 30 was the possibility to stimulate nonanesthetized rats. Another major advantage of this new animal model was that the rats were freely moving, permitting behavioral testing while simultaneously investigating the electrophysiological effects of stimulation. It was shown that the narcosis strongly influenced brain oscillations and thus may have interfered with TES or its effects on the brain. This was in accordance with a recent study in which TES-induced electroencephalogram after-effects were only achieved while stimulating shallow (late) narcosis but not a deep (early) slow-wave stage.30 The visual responses in the cortex caused by TES were primarily detected in the areas representing the peripheral visual field.18 In this study, the authors showed that the responses were followed by intensive evoked field potentials. When increasing the pulse width or intensity of TES current, the gradual extension of the responsive regions from the cortical areas to areas representing the central visual field was observed.18
Transorbital ES in Animal Experiments
Compared to TES, the therapeutic effects and physiological mechanisms of transorbital alternating current stimulation are less well studied and are poorly understood. To our knowledge, there is only one published animal experiment that studied the effect of transorbital alternating current stimulation and characterized the responses in several compartments of the visual system.14 The findings from this study suggested that early stages of visual processing in generating electrically evoked potentials after transorbital alternating current stimulation were important, further proposing that the coordination of the input from the retina to the tectum and thalamus was an essential, functional mechanism in transorbital alternating current stimulation.14 These suggestions are in accordance with the results presented by Sergeeva et al,30 demonstrating that the structural integrity at early stages of visual processing was essential for the after-effects achieved by TES.
Clinical Studies
The Effects of TES in Clinical Studies
There are 12 published clinical studies on the use of TES (Table 2).36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47 A controlled, randomized, and partially masked short-term study included 24 patients with RP and demonstrated significant improvements in visual field area and b-waves of the scotopic ERG.36 The study divided patients into three groups, depending on the strength of stimulation in relation to the individual phosphene threshold (0%, 66%, or 150%). The results showed that all functional parameters were slightly improved or remained constant in the 150% group, whereas there was no marked tendency in the 66% group. Compared to normal subjects, Morimoto et al41 showed that patients with RP and cone-rod dystrophy demonstrated significantly higher electrical thresholds (ie, T1, threshold current for initial phosphene; T2, threshold for eliciting a phosphene extending into the central field; and P, threshold for a relative pupillary constriction ≥3%). The authors concluded that TES could be used as one of the most essential tests to select candidates for retinal prostheses. In a study by Inomata et al,39 where three patients with long-standing retinal artery occlusion were treated, TES was found to improve both visual acuity and visual field. Moreover, multifocal ERGs demonstrated that the implicit time and amplitudes of all component waves significantly reformed after the TES treatment, suggesting that TES exhibited favorable effects on both the inner and outer retinal neurons in patients with retinal artery occlusion.
Table 2.
TES: Clinical Studies
| Reference | Disease | Experimental population; stimulation location | Control group | ES parameters | Evaluation method | Results |
|---|---|---|---|---|---|---|
| Schatz et al, 201136 | RP | 24 patients; cornea; both eyes | Sham | Intensity 66% and 150% of EPT, 20 Hz, 5-millisecond biphasic pulses, 30 minutes per week, for 6 weeks | VA; VF; ERG recordings; color discrimination; EPTs; blood tests | TES improved VF; all functional parameters were improved or remained constant in 150% group; TES led to decreased desaturated color discrimination and VF mean sensitivity; no marked tendency was observed in 66% group |
| Fujikado et al, 200737 | Normal-sighted | Eight patients | — | 25–250 μA, biphasic pulses at 5–50 Hz, 20 pulses of 10-millisecond duration | Measurement of PR | TES with a frequency of 20 Hz yielded the maximum PR; biphasic pulses with duration of 0.5–1.0 millisecond and a frequency of 20–50 Hz were highly efficient for evoking phosphene |
| Fujikado et al, 200638 | NAION; TON | Eight patients; NAION (n = 3); TON (n = 5); cornea; one eye | Fellow eyes | 300–800 μA, 20 Hz, 10 milliseconds, 30 minutes, one session | EEPR; BCVA; VF | The VA increased in two subjects with NAION and in four subjects with TON |
| Inomata et al, 200739 | RAO | Three patients, two with central and one with branch RAO, cornea; one eye | Fellow eyes | 0–1100 μA until evoking phosphene, 20 Hz, 30-minute duration, once per month for up to 3 months | VA; VF; mfERGs | Improved function of retina in eyes with long-standing RAOs; the VA and mfERGs improved in two cases, and VF was improved in all three cases |
| Kurimoto et al, 201040 | Normal-sighted | 10 patients; cornea; both eyes | Fellow eyes; sham | Up to 150 μA, 20 Hz, 30 minutes, one session | LSFG; SBR; IOP; measurement of blood pressure and pulse rate | Increased the chorioretinal blood flow in normal patients with mild effects on the systemic blood circulation and IOP |
| Morimoto et al, 200641 | RP; CRD | 20 patients; RP (n = 16); CRD (n = 4); cornea | Eight healthy patients | 50 μA–2 mA, biphasic pulses at 20 Hz, 20 pulses of 10-millisecond duration; three electrical current thresholds: T1, T2, and P | EEPR; BCVA; VF; phosphene thresholds | All thresholds significantly higher in patients compared to healthy subjects; T1 and T2 were not correlated with VA, but related to the area and location of the remaining VF; T1 and T2 in RP eyes with EEPR was lower compared to RP eyes without an EEPR |
| Naycheva et al, 201242 | RP; STG; RAO; NAION; POAG | RP (n = 30); STG (n = 14); RAO (n = 20); NAION (n = 16); POAG (n = 17); cornea | 20 Healthy patients | 1 μA–10 mA, 3, 6, 9, 20, 40, and 80 Hz with 10-millisecond biphasic current pulses; 5 millisecond positive, thereafter 5 millisecond negative; periodically switching of the light, every 60–90 seconds | VA; VF; electrophysiology; slit-lamp biomicroscopy; fundus examination; tonometry | EPTs differed between the disease groups; in all groups, EPTs were lowest at 20 Hz; subjects with retinal diseases and across all frequencies exhibited higher EPTs than healthy patients, except in STG at 20 Hz |
| Naycheva et al, 201343 | Central and branch RAO | 13 patients; 12 with central, 1 with branch RAO | Three patients; sham | 5-Milliseconds positive, thereafter 5-milliseconds negative biphasic pulses at 20 Hz, 30 minutes once a week for 6 days, intensity 66% and 150% of EPT | Kinetic and static VFs; VA; full-field and mfERG | The scotopic a-wave slopes increased in 150% treatment group; other parameters in all other groups were unchanged |
| Oono et al, 201144 | Branch RAO | Five patients; two fresh and three long-standing cases; cornea; both eyes | — | 500–900-μA, biphasic pulses at 20 Hz, duration 30 minutes | BCVA; mfERGs; automated static perimetry with HFA | TES reforms the visual function in patients with branch RAO, primarily in long-standing cases |
| Ozeki et al, 201345 | BVMD | One patient; cornea; right eye | — | First treatment: 250 μA/170 μA, 20 Hz, 10 milliseconds/phase, 30-minute duration, two sessions with interval of 1 month; second treatment 2 years later: 160 μA, 20 Hz, 10 milliseconds, 30-minute duration, two sessions with interval of 1 month | BCVA; VF; mfERG | BCVA improved from 20/40–20/30 after 1 month, and from 20/40–20/25 after 6 months; slight improvements for VFs and mfERGs; BCVA decreased to 20/70 after 2 years; second treatment: BCVA improved to 20/30 after 1 month; no improvements in VFs and mfERGs |
| Xie et al, 201146 | Normal-sighted | Six patients; cornea; right eye | — | DTL-Plus corneal electrode and ERG-Jet contact lens electrode; 1.5× EPT current amplitude, 30-minute duration, 2 Hz, 2-millisecond pulse width; after every 5 minutes of continuous TES, stimulation turned off for 30 seconds | PET imaging; FDG activity | The results with both corneal electrodes demonstrated activation of areas in visual cortex that were related to the reported phosphene percept; ERG-Jet was able to generate brighter phosphene percept compared to DTL-Plus; the use of ERG-Jet led to activation of retinotopically mapped primary visual cortex |
| Xie et al, 201247 | RD | Five patients; cornea; right eye | Five healthy patients | First, 30 minutes dark adaptation; 1.5× EPT current amplitude, 2-millisecond pulses, 2 Hz with interpulse of 996 milliseconds, 30-minute duration; after every 5 minutes of continuous TES, stimulation turned off for 30 seconds | PET imaging; FDG activity | EPT current was higher in RD patients compared with control; in both groups, TES and light stimulation resulted in activation of retinotopically mapped primary visual cortex |
BCVA, best-corrected visual acuity; BVMD, best vitelliform macular dystrophy; CRD, cone-rod dystrophy; DTL, Dawson-Trick-Litzkow; EEPR, electrically evoked pupillary response; EPT, electrical phosphene threshold; ERG, electroretinogram; ES, electrical stimulation; FDG, 18F-fluorodeoxyglucose; HFA, Humphrey field analyzer; IOP, intraocular pressure; LSFG, laser speckle flowgraphy; mfERG, multifocal electroretinogram; NAION, nonarteritic ischemic optic neuropathy; PET, positron emission tomography; POAG, primary open-angle glaucoma; PR, pupillary reflex; RAO, retinal artery occlusion, RD, retinal degeneration; RP, retinitis pigmentosa; SBR, square blur rate; STG, Stargardt disease; TES, transcorneal electrical stimulation; TON, traumatic optic neuropathy; VA, visual acuity; VF, visual field; –, not reported.
In another study of eight patients with long-standing or fresh branch retinal artery occlusion, improvements of both multifocal ERG and visual field parameters were reported after TES in long-standing cases, although improvements in the visual field were not observed in patients with fresh occlusion.44 Naycheva et al43 demonstrated that the slopes of the scotopic a-waves significantly increased in patients with central or branch retinal artery occlusion. TES treatment performed >4 months after the onset of nonarteritic ischemic optic neuropathy exhibited improved visual acuity in two of three eyes examined.38 Although visual acuity became impaired some months after the patients stopped receiving ES, after a second TES treatment, the vision improved again. This finding suggests a causal relationship between the treatment and visual improvement. In a recent study using TES, electrical phosphene threshold varied between disease groups.42 Across all tested frequencies (3, 6, 9, 20, 40, and 80 Hz), electrical phosphene thresholds were significantly higher when compared with healthy subjects, except in Stargardt disease patients at 20 Hz. Improvements in visual acuity in a recent case report with best vitelliform macular dystrophy suggest that TES should also be investigated in other cases of retinal dystrophies.45 Furthermore, after TES, the phosphene threshold current was markedly higher in patients with retinal degeneration compared with control subjects.47
It has been shown that TES (up to 150 μA, 20 Hz, 30 minutes, one session) increases choroidal blood flow in normally sighted patients, with minimal effects on the general blood circulation and intraocular pressure.40 The authors suggested that this is probably an important mechanism that underlies the beneficial effects of TES in ischemic retinal diseases. Another study demonstrated that TES at a frequency of 20 Hz evoked pupillary reflexes in subjects with normal vision, whereas biphasic pulse trains at a duration of 0.5 to 1.0 milliseconds and 20- to 50-Hz frequencies evoked phosphene.37 Accordingly, both DTL-Plus and ERG-Jet electrodes showed visual cortex activation in association with the reported phosphene.46
Transorbital and Transpalpebral ES in Clinical Trials
There are six published studies on the use of transorbital ES (Table 3)48, 49, 50, 51, 52, 53 and three on the use of transpalpebral ES (Table 3).54, 55, 56 In seven patients with chronic prechiasmatic visual system damage, it was found that transorbital ES strengthened α band functional connectivity, which was associated with perceptual improvements. Therefore, it is speculated that vision loss in the blind is caused not only by primary tissue damage but also by breakdown of synchronization in brain networks.48 In addition, transorbital ES in patients with optic nerve damage showed significant increases in detection ability, visual field, and visual acuity.49, 50, 51, 52 All three published studies on the use of transpalpebral ES were performed in patients with AMD (Table 3).54, 55, 56 Transpalpebral ES was found to be safe in patients, and the results demonstrated a provisional increase in visual function in some patients54; best-corrected visual acuity was found to be improved in patients with both wet and dry AMD.56
Table 3.
Transorbital and Transpalpebral ES: Clinical Studies
| Reference | Disease | Experimental population; stimulation location | Control group | ES parameters | Evaluation method | Results |
|---|---|---|---|---|---|---|
| Bola et al, 201448 | Chronic prechiasmatic visual system damage | Seven patients; skin near the eyeball | Eight patients; sham | Current strength above (125%) phosphene thresholds as reported by the patients; applies for 10 days, 40 minutes each | EEG recording | ES strengthened α band functional connectivity, which was associated with perceptual improvements |
| Fedorov et al, 201149 | OND | 446 patients; upper eye lid | — | Intensity increase stepwise by 10 μA per second, 5 Hz, two to nine pulses; each daily session of 200–250 cycles with five to seven 1-minute breaks, session length 25–40 minutes; 10-day treatment | EEG recording, VF, VA | ES improved VF size in both eyes; VA was significantly increased in both eyes; increased α and θ power in patients who had VF enlargements but no VA change; nonresponders exhibited increased Δ power spectra in occipital and frontal areas |
| Gall et al, 201050 | OND | One patient; skin of the eye lids; both eyes | — | The amplitude was always <600 μA, 10–30 Hz, 10–15 pulses, single duration of 8.7 ± 0.8 milliseconds, 30–40-minute duration, daily for 10 days; intensity increase stepwise by 10 μA per second | VA; VF; static and kinetic perimetry | Detection ability increased; mean perimetric threshold increased |
| Gall et al, 201151 | OND | 24 patients; near the eyeball; both eyes | 18 Patients; sham | Intensity increase stepwise by 10 μA per second, 5 Hz, pulse shape was either square or sinus, two to nine pulses; each session of 200–250 cycles with five to seven 1-minute breaks; 10-day treatment | VF diagnostics with HRP; VA and contrast vision | Detection ability was significantly larger; static and kinetic perimetry provided ES efficacy |
| Sabel et al, 201152 | OND | 12 patients; near the eyeball | 10 Patients; sham | The amplitude of pulses was <1000 μA; current intensity increased stepwise by 10 μA per second; two to nine pulses | HRP; VA; EEG recording | Stimulation resulted in improvement of a VF by 69%; increased temporal procession of visual stimuli |
| Schmidt et al, 201353 | Prechiasmatic partial OND | 18 patients; orbital; both eyes | Six patients; sham | Maximal amplitude <500 μA, 9–37 Hz, two to nine pulses, 10 consecutive days | EEG recording; VF | Enhancement of α activity after ES; the residual VF was significantly improved |
| Anastassiou et al, 201354 | Dry AMD | 12 patients; palpebral; both eyes | 10 Patients; sham | 150–220 μA, 5–80 Hz; 5 consecutive days, two sessions on each day, every session eight spots (40 seconds/spot) | VA testing before treatment, after 5 days, after 4 weeks, after 6 months; macular sensitivity and fixation stability with microperimetry; OCT | Stimulation resulted in improved VA in 7 of 12 patients, with more than five letters; contrast sensitivity showed a similar pattern |
| Chaikin et al, 201555 | Wet and dry AMD | 17 patients; 25 eyes with dry type and 6 eyes with wet type | — | 150 μA; 3–162 Hz; 35-minute session once a week; average number of treatments was 4.8 | IO pressure measurements; OCT; VA | Significant improvements in VA in patients with dry type, but not in wet type; in dry-type subjects, 52% of patients exhibited increased VA, and 26% deterioration |
| Shinoda et al, 200856 | Wet and dry AMD | 21 patients; 16 (27 eyes) with wet type and 5 (7 eyes) with dry type; palpebral; both eyes | — | 800 μA; one 20-minute session was performed four times per day, up to 1 month; 290 Hz for 1 minute, 31 Hz for 2 minutes, 8.9 Hz for 10 minutes, 0.28 Hz for 7 minutes | BCVA before and after 4 weeks; slit-lamp examination; funduscopy; fluorescein angiography; automated static VF testing | All of the patients reported phosphene perception; BCVA improved, but did not reach statistical significance |
AMD, age-related macular degeneration; BCVA, best-corrected visual acuity; EEG, electroencephalogram; ES, electrical stimulation; HRP, high-resolution perimetry; IO, intraocular; OND, optic nerve damage; OCT, optical coherence tomography; VA, visual acuity; VF, visual field; –, not reported.
Possible Mechanisms Underlying the Effects of ES
Despite several studies showing promising results in both animal experiments and clinical studies, there are presently few reports on the mechanisms of action for ES, making this field still poorly understood. Today, we know that after injuries like optic nerve transection, directly injured RGCs are rapidly lost, and so far there are no treatments that can prevent this process. However, the secondary loss of RGCs whose axons were not directly damaged is considerable,57 and the neuroprotective effect of ES is significant in preventing this secondary apoptosis.
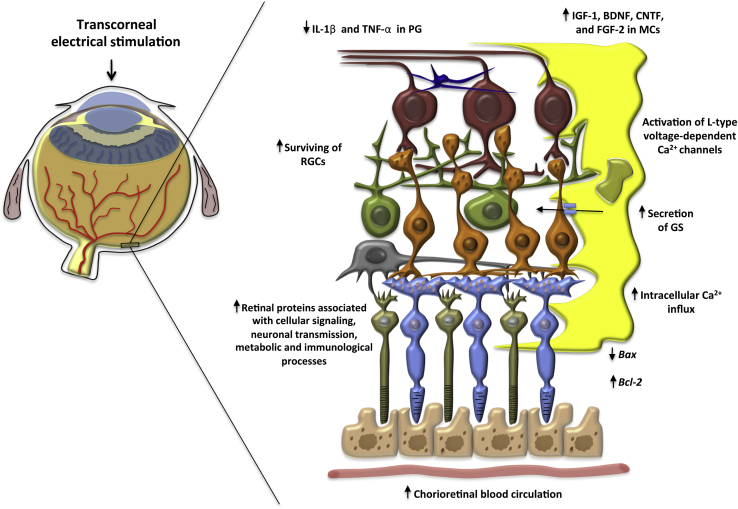
The suggested mechanisms that are believed to underlie the effects of TES are summarized and presented in Figure 2. As in other tissues, it is believed that TES increases the expression of neurotrophic factors. It has been suggested that TES increases the number of surviving RGCs in vivo, probably because of increased levels of IGF-1 produced by Müller cells.24, 58 Another study demonstrated that the expression of mRNA encoding brain-derived neurotrophic factor increased and that intracellular protein levels were found in cultured Müller cells after TES,59 supported by Ni et al.26 The last study also demonstrated that the levels of ciliary nerve trophic factor and expression of Bcl-2 were both increased after TES.26 In cultured retinal Müller cells, Sato et al60 showed that TES also increased the expression of fibroblast growth factor 2, which was also confirmed by Ciavatta et al61 after implantation of microphotodiode arrays in Royal College of Surgeons rats. It has been suggested that L-type calcium channels probably control the release of these growth factors. This was demonstrated when an L-type voltage-dependent calcium channel blocker (nifedipine) was used to inhibit the up-regulation of IGF-1 and brain-derived neurotrophic factor.58, 59
Figure 2.
Possible neuroprotective mechanisms underlying the effects of transcorneal electrical stimulation. The cellular structure in the figure represents an enlarged image of the retina. BDNF, brain-derived neurotrophic factor; CNTF, ciliary nerve trophic factor; FGF, fibroblast growth factor; GS, glutamine synthetase; IGF-1, insulin-like growth factor 1; MCs, Müller cells; PG, primary microglia; RGCs, retinal ganglion cells; TNF-α, tumor necrosis factor-α.
In primary microglia isolated from retinas of Sprague-Dawley rats, the inhibitory effects of ES on the secretion of IL-1β and tumor necrosis factor-α were confirmed, as well as favorable effects on the production of brain-derived neurotrophic factor and ciliary nerve trophic factor in Müller cells35 and the down-regulation of the proapoptopic gene Bax.26 Willmann et al34 also showed that TES led to clear changes in the expression of neuroprotective genes and that different genes may be expressed, depending on whether TES was applied to a healthy or diseased retina. Other effects of ES, investigated using a rat model of retinal ischemia, included increased secretion of glutamine synthetase from Müller cells, suggested to improve glutamate-mediated neuroexcitotoxicity.33 Furthermore, ES may also promote neuronal survival by increasing intracellular Ca2+ influx, which can cause neuronal cell depolarization and increase intracellular cyclic adenosine monophosphate levels.62 TES also was found to improve choroidal blood flow in normal-sighted patients, with mild effects on the systemic blood circulation and intraocular pressure.40
The expression of retinal genes and the levels of retinal proteins associated with TES have recently been investigated. Willmann et al34 investigated the effect of TES on mRNA expression, retinal structure, and function. The authors demonstrated that TES induced differential the expression of 490 genes in the retina, including Bax and members of the tumor necrosis factor family. Physiological retinal function and intact structural properties were maintained for up to 35 hours after stimulation.34 A proteomic study by Kanamoto et al17 demonstrated that 25 proteins were up-regulated after TES, including cellular signaling proteins, proteins associated with neuronal transmission, metabolic proteins, immunological factors, and structural proteins. The pattern of differentially expressed proteins was altered at 30 minutes and 24 hours after TES, and the authors suggested that TES induced acute and chronic changes in protein expression. It was further proposed that the acute changes were direct and transient effects induced by electric shock to neural cells, whereas the chronic changes were indirect and secondary effects in mRNA expression or protein signaling pathways.17
Complications and Safety Profile
There are >120 articles on the use of invasive retinal prostheses for ES, making these approaches substantially more studied than other recently described, less invasive techniques. Both transpalpebral and transorbital approaches have the advantage of being minimally invasive (only touching the skin), with minor adverse effects reported. In a clinical study, it was reported that 1 of 21 patients developed contact dermatitis after transpalpebral ES.56 One investigation reported light frontal headache after transorbital ES in 3 of 18 patients.53 Furthermore, among 12 patients studied, 5 had some sensations while under the stimulation electrodes and 1 had spontaneous phosphene.52 TES touches only the cornea, which substantially reduces the risk of severe complications compared to retinal implants. The adverse effects were infrequent and mild for both DTL-Plus and ERG-Jet electrodes, with few patients reporting either foreign-body sensations with the DTL-Plus electrode36 or mild transient corneal punctate keratopathy on slit-lamp examination after the use of the ERG-Jet electrode.38 Schatz et al36 also reported minor conjunctiva irritation after intraocular pressure measurements. Other clinical TES studies reported slight corneal superficial punctuate keratopathy,41 transient superficial keratitis,39 and foreign-body sensations in three patients.43 Adverse effects after TES in animal studies have not been reported; however, one study addressed this concern and reported no negative effects up to 35 hours after application.34 All other methods will, by nature of their invasiveness, be prone to various complications, depending on the location. The excellent safety profile is likely to be substantially lower in future clinical settings.
Challenges and Future Perspectives
A high number of recent reports demonstrate improvements in visual function by ES, which are expected to translate into large multicenter trials. The results from studies using different models of experimental animals have demonstrated favorable effects of ES on both the retina and optic nerve. However, the considerable problem with clinical studies so far has been the absence of randomization, a sham control, and inclusion of few patients. It is known that intraindividual changes in vision parameters vary significantly, common in neurorehabilitation; this makes it even more challenging in clinical trials. Another problem is that researchers all over the world experience the enormous advertising of stimulation prostheses and electrodes from many manufacturers because of considerable engineering and technical advances, making selection more difficult. As transpalpebral ES treatment is the only current treatment to show improved visual function in patients with AMD, more research to unravel the mechanisms behind its effects in an animal model is warranted. Such data are likely to yield clues on potential mechanisms of action using a transpalpebral approach as well as on how the advantageous effects on visual function in general can be further improved.
Stimulation parameters, such as current intensity, frequency, duration, and number of sessions, have been shown to vary in accordance with type of pathology and species.33, 38 For example, in rats, the proposed current intensity of TES for protection of photoreceptors (300 μA, 3 milliseconds per phase) is higher than that for survival of RGCs (100 μA, 1 millisecond per phase).26, 30 However, in humans, the threshold intensity required to elicit phosphene in both the central and peripheral visual fields is generally between 300 and 900 μA.44 Moreover, a study using positron emission tomography demonstrated that TES resulted in the activation of both retinotopographically matched primary visual cortex and visual perception in both normal-sighted persons and subjects with retinal degenerative disease.44 However, the threshold current required to evoke phosphene is significantly lower in normal-sighted persons compared to patients with retinal degenerative disease.47 Optimizing the ES parameters needed to achieve the ideal balance between the beneficial effects and adverse effects is required.
Conclusion
ES with the purpose of restoring limited vision and/or treating blindness has undergone enormous scientific advances during the past 10 years. The amount of evidence for restoring visual function in various diseases using ES is rapidly increasing. We have currently reached a stage where ES has moved into clinical practice. Its favorable safety profile is likely to boost progress in ES for treating eye diseases. Still, there is a pressing need for a deeper understanding of the underlying mechanisms that govern clinical success. The optimization of stimulation parameters in animal studies, followed by large, prospective, clinical studies to explore the full potential of ES, is still required.
Acknowledgment
We thank Håkon Raanes for making Figure 1.
Footnotes
Supported by the Department of Oral Biology (University of Oslo), the Department of Medical Biochemistry (Oslo University Hospital), and NIH/National Eye Institute grants R41 EY025913, R01 EY025259, and P30 EY03790-33.
D.F.C. and T.P.U. contributed equally as senior authors.
Disclosures: None declared.
References
- 1.Leske M.C., Heijl A., Hussein M., Bengtsson B., Hyman L., Komaroff E., Early Manifest Glaucoma Trial Group Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 2.Quigley H.A., Broman A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman H.R., Chan C.C., Ferris F.L., 3rd, Chew E.Y. Age-related macular degeneration. Lancet. 2008;372:1835–1845. doi: 10.1016/S0140-6736(08)61759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guadagni V., Novelli E., Piano I., Gargini C., Strettoi E. Pharmacological approaches to retinitis pigmentosa: a laboratory perspective. Prog Retin Eye Res. 2015;48:62–81. doi: 10.1016/j.preteyeres.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Maeda T., Dong Z., Jin H., Sawada O., Gao S., Utkhede D., Monk W., Palczewska G., Palczewski K. QLT091001, a 9-cis-retinal analog, is well-tolerated by retinas of mice with impaired visual cycles. Invest Ophthalmol Vis Sci. 2013;54:455–466. doi: 10.1167/iovs.12-11152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solinis M.A., Del Pozo-Rodriguez A., Apaolaza P.S., Rodriguez-Gascon A. Treatment of ocular disorders by gene therapy. Eur J Pharm Biopharm. 2015;95:331–342. doi: 10.1016/j.ejpb.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Garg S.J., Federman J. Optogenetics, visual prosthesis and electrostimulation for retinal dystrophies. Curr Opin Ophthalmol. 2013;24:407–414. doi: 10.1097/ICU.0b013e328363829b. [DOI] [PubMed] [Google Scholar]
- 8.Dor H. Beiträge zur Electrotherapie der Augenkrankheiten. Albrecht von Graefes Arch Klin Exp Ophthalmol. 1873;19:532. [Google Scholar]
- 9.Foerster O. Beitrage zur pathophysiologie der sehbahn und der spehsphare. J Psychol Neurol. 1929;39:435–463. [Google Scholar]
- 10.Dobelle W.H., Mladejovsky M.G. Phosphenes produced by electrical stimulation of human occipital cortex, and their application to the development of a prosthesis for the blind. J Physiol. 1974;243:553–576. doi: 10.1113/jphysiol.1974.sp010766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow A.Y., Chow V.Y., Packo K.H., Pollack J.S., Peyman G.A., Schuchard R. The artificial silicon retina microchip for the treatment of vision loss from retinitis pigmentosa. Arch Ophthalmol. 2004;122:460–469. doi: 10.1001/archopht.122.4.460. [DOI] [PubMed] [Google Scholar]
- 12.Lorach H., Marre O., Sahel J.A., Benosman R., Picaud S. Neural stimulation for visual rehabilitation: advances and challenges. J Physiol Paris. 2013;107:421–431. doi: 10.1016/j.jphysparis.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Brindley G.S., Lewin W.S. The sensations produced by electrical stimulation of the visual cortex. J Physiol. 1968;196:479–493. doi: 10.1113/jphysiol.1968.sp008519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foik A.T., Kublik E., Sergeeva E.G., Tatlisumak T., Rossini P.M., Sabel B.A., Waleszczyk W.J. Retinal origin of electrically evoked potentials in response to transcorneal alternating current stimulation in the rat. Invest Ophthalmol Vis Sci. 2015;56:1711–1718. doi: 10.1167/iovs.14-15617. [DOI] [PubMed] [Google Scholar]
- 15.Henrich-Noack P., Lazik S., Sergeeva E., Wagner S., Voigt N., Prilloff S., Fedorov A., Sabel B.A. Transcorneal alternating current stimulation after severe axon damage in rats results in “long-term silent survivor” neurons. Brain Res Bull. 2013;95:7–14. doi: 10.1016/j.brainresbull.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Henrich-Noack P., Voigt N., Prilloff S., Fedorov A., Sabel B.A. Transcorneal electrical stimulation alters morphology and survival of retinal ganglion cells after optic nerve damage. Neurosci Lett. 2013;543:1–6. doi: 10.1016/j.neulet.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Kanamoto T., Souchelnytskyi N., Kurimoto T., Ikeda Y., Sakaue H., Munemasa Y., Kiuchi Y. Proteomic study of retinal proteins associated with transcorneal electric stimulation in rats. J Ophthalmol. 2015;2015:492050. doi: 10.1155/2015/492050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Z., Cao P., Sun P., Li L., Lu Y., Yan Y., Chen Y., Chai X. Optical imaging of visual cortical responses evoked by transcorneal electrical stimulation with different parameters. Invest Ophthalmol Vis Sci. 2014;55:5320–5331. doi: 10.1167/iovs.14-14600. [DOI] [PubMed] [Google Scholar]
- 19.Mihashi T., Okawa Y., Miyoshi T., Kitaguchi Y., Hirohara Y., Fujikado T. Comparing retinal reflectance changes elicited by transcorneal electrical retinal stimulation with those of optic chiasma stimulation in cats. Jpn J Ophthalmol. 2011;55:49–56. doi: 10.1007/s10384-010-0906-x. [DOI] [PubMed] [Google Scholar]
- 20.Miyake K., Yoshida M., Inoue Y., Hata Y. Neuroprotective effect of transcorneal electrical stimulation on the acute phase of optic nerve injury. Invest Ophthalmol Vis Sci. 2007;48:2356–2361. doi: 10.1167/iovs.06-1329. [DOI] [PubMed] [Google Scholar]
- 21.Morimoto T., Fujikado T., Choi J.S., Kanda H., Miyoshi T., Fukuda Y., Tano Y. Transcorneal electrical stimulation promotes the survival of photoreceptors and preserves retinal function in Royal College of Surgeons rats. Invest Ophthalmol Vis Sci. 2007;48:4725–4732. doi: 10.1167/iovs.06-1404. [DOI] [PubMed] [Google Scholar]
- 22.Morimoto T., Kanda H., Kondo M., Terasaki H., Nishida K., Fujikado T. Transcorneal electrical stimulation promotes survival of photoreceptors and improves retinal function in rhodopsin P347L transgenic rabbits. Invest Ophthalmol Vis Sci. 2012;53:4254–4261. doi: 10.1167/iovs.11-9067. [DOI] [PubMed] [Google Scholar]
- 23.Morimoto T., Kanda H., Miyoshi T., Hirohara Y., Mihashi T., Kitaguchi Y., Nishida K., Fujikado T. Characteristics of retinal reflectance changes induced by transcorneal electrical stimulation in cat eyes. PLoS One. 2014;9:e92186. doi: 10.1371/journal.pone.0092186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morimoto T., Miyoshi T., Matsuda S., Tano Y., Fujikado T., Fukuda Y. Transcorneal electrical stimulation rescues axotomized retinal ganglion cells by activating endogenous retinal IGF-1 system. Invest Ophthalmol Vis Sci. 2005;46:2147–2155. doi: 10.1167/iovs.04-1339. [DOI] [PubMed] [Google Scholar]
- 25.Morimoto T., Miyoshi T., Sawai H., Fujikado T. Optimal parameters of transcorneal electrical stimulation (TES) to be neuroprotective of axotomized RGCs in adult rats. Exp Eye Res. 2010;90:285–291. doi: 10.1016/j.exer.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Ni Y.Q., Gan D.K., Xu H.D., Xu G.Z., Da C.D. Neuroprotective effect of transcorneal electrical stimulation on light-induced photoreceptor degeneration. Exp Neurol. 2009;219:439–452. doi: 10.1016/j.expneurol.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Osako T., Chuman H., Maekubo T., Ishiai M., Kawano N., Nao I.N. Effects of steroid administration and transcorneal electrical stimulation on the anatomic and electrophysiologic deterioration of nonarteritic ischemic optic neuropathy in a rodent model. Jpn J Ophthalmol. 2013;57:410–415. doi: 10.1007/s10384-012-0203-y. [DOI] [PubMed] [Google Scholar]
- 28.Rahmani S., Bogdanowicz L., Thomas J., Hetling J.R. Chronic delivery of low-level exogenous current preserves retinal function in pigmented P23H rat. Vision Res. 2013;76:105–113. doi: 10.1016/j.visres.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 29.Schatz A., Arango-Gonzalez B., Fischer D., Enderle H., Bolz S., Rock T., Naycheva L., Grimm C., Messias A., Zrenner E., Bartz-Schmidt K.U., Willmann G., Gekeler F. Transcorneal electrical stimulation shows neuroprotective effects in retinas of light-exposed rats. Invest Ophthalmol Vis Sci. 2012;53:5552–5561. doi: 10.1167/iovs.12-10037. [DOI] [PubMed] [Google Scholar]
- 30.Sergeeva E.G., Fedorov A.B., Henrich-Noack P., Sabel B.A. Transcorneal alternating current stimulation induces EEG “aftereffects” only in rats with an intact visual system but not after severe optic nerve damage. J Neurophysiol. 2012;108:2494–2500. doi: 10.1152/jn.00341.2012. [DOI] [PubMed] [Google Scholar]
- 31.Sergeeva E.G., Henrich-Noack P., Gorkin A.G., Sabel B.A. Preclinical model of transcorneal alternating current stimulation in freely moving rats. Restor Neurol Neurosci. 2015;33:761–769. doi: 10.3233/RNN-150513. [DOI] [PubMed] [Google Scholar]
- 32.Tagami Y., Kurimoto T., Miyoshi T., Morimoto T., Sawai H., Mimura O. Axonal regeneration induced by repetitive electrical stimulation of crushed optic nerve in adult rats. Jpn J Ophthalmol. 2009;53:257–266. doi: 10.1007/s10384-009-0657-8. [DOI] [PubMed] [Google Scholar]
- 33.Wang X., Mo X., Li D., Wang Y., Fang Y., Rong X., Miao H., Shou T. Neuroprotective effect of transcorneal electrical stimulation on ischemic damage in the rat retina. Exp Eye Res. 2011;93:753–760. doi: 10.1016/j.exer.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 34.Willmann G., Schaferhoff K., Fischer M.D., Arango-Gonzalez B., Bolz S., Naycheva L., Rock T., Bonin M., Bartz-Schmidt K.U., Zrenner E., Schatz A., Gekeler F. Gene expression profiling of the retina after transcorneal electrical stimulation in wild-type Brown Norway rats. Invest Ophthalmol Vis Sci. 2011;52:7529–7537. doi: 10.1167/iovs.11-7838. [DOI] [PubMed] [Google Scholar]
- 35.Zhou W.T., Ni Y.Q., Jin Z.B., Zhang M., Wu J.H., Zhu Y., Xu G.Z., Gan D.K. Electrical stimulation ameliorates light-induced photoreceptor degeneration in vitro via suppressing the proinflammatory effect of microglia and enhancing the neurotrophic potential of Muller cells. Exp Neurol. 2012;238:192–208. doi: 10.1016/j.expneurol.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 36.Schatz A., Rock T., Naycheva L., Willmann G., Wilhelm B., Peters T., Bartz-Schmidt K.U., Zrenner E., Messias A., Gekeler F. Transcorneal electrical stimulation for patients with retinitis pigmentosa: a prospective, randomized, sham-controlled exploratory study. Invest Ophthalmol Vis Sci. 2011;52:4485–4496. doi: 10.1167/iovs.10-6932. [DOI] [PubMed] [Google Scholar]
- 37.Fujikado T., Morimoto T., Kanda H., Kusaka S., Nakauchi K., Ozawa M., Matsushita K., Sakaguchi H., Ikuno Y., Kamei M., Tano Y. Evaluation of phosphenes elicited by extraocular stimulation in normals and by suprachoroidal-transretinal stimulation in patients with retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol. 2007;245:1411–1419. doi: 10.1007/s00417-007-0563-z. [DOI] [PubMed] [Google Scholar]
- 38.Fujikado T., Morimoto T., Matsushita K., Shimojo H., Okawa Y., Tano Y. Effect of transcorneal electrical stimulation in patients with nonarteritic ischemic optic neuropathy or traumatic optic neuropathy. Jpn J Ophthalmol. 2006;50:266–273. doi: 10.1007/s10384-005-0304-y. [DOI] [PubMed] [Google Scholar]
- 39.Inomata K., Shinoda K., Ohde H., Tsunoda K., Hanazono G., Kimura I., Yuzawa M., Tsubota K., Miyake Y. Transcorneal electrical stimulation of retina to treat longstanding retinal artery occlusion. Graefes Arch Clin Exp Ophthalmol. 2007;245:1773–1780. doi: 10.1007/s00417-007-0610-9. [DOI] [PubMed] [Google Scholar]
- 40.Kurimoto T., Oono S., Oku H., Tagami Y., Kashimoto R., Takata M., Okamoto N., Ikeda T., Mimura O. Transcorneal electrical stimulation increases chorioretinal blood flow in normal human subjects. Clin Ophthalmol (Auckland, NZ) 2010;4:1441–1446. doi: 10.2147/OPTH.S14573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morimoto T., Fukui T., Matsushita K., Okawa Y., Shimojyo H., Kusaka S., Tano Y., Fujikado T. Evaluation of residual retinal function by pupillary constrictions and phosphenes using transcorneal electrical stimulation in patients with retinal degeneration. Graefes Arch Clin Exp Ophthalmol. 2006;244:1283–1292. doi: 10.1007/s00417-006-0260-3. [DOI] [PubMed] [Google Scholar]
- 42.Naycheva L., Schatz A., Rock T., Willmann G., Messias A., Bartz-Schmidt K.U., Zrenner E., Gekeler F. Phosphene thresholds elicited by transcorneal electrical stimulation in healthy subjects and patients with retinal diseases. Invest Ophthalmol Vis Sci. 2012;53:7440–7448. doi: 10.1167/iovs.12-9612. [DOI] [PubMed] [Google Scholar]
- 43.Naycheva L., Schatz A., Willmann G., Bartz-Schmidt K.U., Zrenner E., Rock T., Gekeler F. Transcorneal electrical stimulation in patients with retinal artery occlusion: a prospective, randomized, sham-controlled pilot study. Ophthalmol Ther. 2013;2:25–39. doi: 10.1007/s40123-013-0012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oono S., Kurimoto T., Kashimoto R., Tagami Y., Okamoto N., Mimura O. Transcorneal electrical stimulation improves visual function in eyes with branch retinal artery occlusion. Clin Ophthalmol (Auckland, NZ) 2011;5:397–402. doi: 10.2147/OPTH.S17751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ozeki N., Shinoda K., Ohde H., Ishida S., Tsubota K. Improvement of visual acuity after transcorneal electrical stimulation in case of Best vitelliform macular dystrophy. Graefes Arch Clin Exp Ophthalmol. 2013;251:1867–1870. doi: 10.1007/s00417-013-2341-4. [DOI] [PubMed] [Google Scholar]
- 46.Xie J., Wang G.J., Yow L., Cela C.J., Humayun M.S., Weiland J.D., Lazzi G., Jadvar H. Modeling and percept of transcorneal electrical stimulation in humans. IEEE Trans Biomed Eng. 2011;58:1932–1939. doi: 10.1109/TBME.2010.2087378. [DOI] [PubMed] [Google Scholar]
- 47.Xie J., Wang G.J., Yow L., Humayun M.S., Weiland J.D., Cela C.J., Jadvar H., Lazzi G., Dhrami-Gavazi E., Tsang S.H. Preservation of retinotopic map in retinal degeneration. Exp Eye Res. 2012;98:88–96. doi: 10.1016/j.exer.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bola M., Gall C., Moewes C., Fedorov A., Hinrichs H., Sabel B.A. Brain functional connectivity network breakdown and restoration in blindness. Neurology. 2014;83:542–551. doi: 10.1212/WNL.0000000000000672. [DOI] [PubMed] [Google Scholar]
- 49.Fedorov A., Jobke S., Bersnev V., Chibisova A., Chibisova Y., Gall C., Sabel B.A. Restoration of vision after optic nerve lesions with noninvasive transorbital alternating current stimulation: a clinical observational study. Brain Stimul. 2011;4:189–201. doi: 10.1016/j.brs.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 50.Gall C., Fedorov A.B., Ernst L., Borrmann A., Sabel B.A. Repetitive transorbital alternating current stimulation in optic neuropathy. NeuroRehabilitation. 2010;27:335–341. doi: 10.3233/NRE-2010-0617. [DOI] [PubMed] [Google Scholar]
- 51.Gall C., Sgorzaly S., Schmidt S., Brandt S., Fedorov A., Sabel B.A. Noninvasive transorbital alternating current stimulation improves subjective visual functioning and vision-related quality of life in optic neuropathy. Brain Stimul. 2011;4:175–188. doi: 10.1016/j.brs.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 52.Sabel B.A., Fedorov A.B., Naue N., Borrmann A., Herrmann C., Gall C. Non-invasive alternating current stimulation improves vision in optic neuropathy. Restor Neurol Neurosci. 2011;29:493–505. doi: 10.3233/RNN-2011-0624. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt S., Mante A., Ronnefarth M., Fleischmann R., Gall C., Brandt S.A. Progressive enhancement of alpha activity and visual function in patients with optic neuropathy: a two-week repeated session alternating current stimulation study. Brain Stimul. 2013;6:87–93. doi: 10.1016/j.brs.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 54.Anastassiou G., Schneegans A.L., Selbach M., Kremmer S. Transpalpebral electrotherapy for dry age-related macular degeneration (AMD): an exploratory trial. Restor Neurol Neurosci. 2013;31:571–578. doi: 10.3233/RNN-130322. [DOI] [PubMed] [Google Scholar]
- 55.Chaikin L., Kashiwa K., Bennet M., Papastergiou G., Gregory W. Microcurrent stimulation in the treatment of dry and wet macular degeneration. Clin Ophthalmol (Auckland, NZ) 2015;9:2345–2353. doi: 10.2147/OPTH.S92296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shinoda K., Imamura Y., Matsuda S., Seki M., Uchida A., Grossman T., Tsubota K. Transcutaneous electrical retinal stimulation therapy for age-related macular degeneration. Open Ophthalmol J. 2008;2:132–136. doi: 10.2174/1874364100802010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levkovitch-Verbin H., Quigley H.A., Martin K.R., Zack D.J., Pease M.E., Valenta D.F. A model to study differences between primary and secondary degeneration of retinal ganglion cells in rats by partial optic nerve transection. Invest Ophthalmol Vis Sci. 2003;44:3388–3393. doi: 10.1167/iovs.02-0646. [DOI] [PubMed] [Google Scholar]
- 58.Sato T., Fujikado T., Morimoto T., Matsushita K., Harada T., Tano Y. Effect of electrical stimulation on IGF-1 transcription by L-type calcium channels in cultured retinal Muller cells. Jpn J Ophthalmol. 2008;52:217–223. doi: 10.1007/s10384-008-0533-y. [DOI] [PubMed] [Google Scholar]
- 59.Sato T., Fujikado T., Lee T.S., Tano Y. Direct effect of electrical stimulation on induction of brain-derived neurotrophic factor from cultured retinal Muller cells. Invest Ophthalmol Vis Sci. 2008;49:4641–4646. doi: 10.1167/iovs.08-2049. [DOI] [PubMed] [Google Scholar]
- 60.Sato T., Lee T.S., Takamatsu F., Fujikado T. Induction of fibroblast growth factor-2 by electrical stimulation in cultured retinal Mueller cells. Neuroreport. 2008;19:1617–1621. doi: 10.1097/WNR.0b013e3283140f25. [DOI] [PubMed] [Google Scholar]
- 61.Ciavatta V.T., Chrenek M., Wong P., Nickerson J.M., Pardue M.T. Growth factor expression following implantation of microphotodiode arrays in RCS rats. Invest Ophthalmol Vis Sci. 2006;47:3177. [Google Scholar]
- 62.Morimoto T., Miyoshi T., Fujikado T., Tano Y., Fukuda Y. Electrical stimulation enhances the survival of axotomized retinal ganglion cells in vivo. Neuroreport. 2002;13:227–230. doi: 10.1097/00001756-200202110-00011. [DOI] [PubMed] [Google Scholar]