Abstract
Early pathological descriptions of Crohn disease (CD) argued for a potential defect in lymph transport; however, this concept has not been thoroughly investigated. In mice, poor healing in response to infection-induced tissue damage can cause hyperpermeable lymphatic collecting vessels in mesenteric adipose tissue that impair antigen and immune cell access to mesenteric lymph nodes (LNs), which normally sustain appropriate immunity. To investigate whether analogous changes might occur in human intestinal disease, we established a three-dimensional imaging approach to characterize the lymphatic vasculature in mesenteric tissue from controls or patients with CD. In CD specimens, B-cell–rich aggregates resembling tertiary lymphoid organs (TLOs) impinged on lymphatic collecting vessels that enter and exit LNs. In areas of creeping fat, which characterizes inflammation-affected areas of the bowel in CD, we observed B cells and apparent innate lymphoid cells that had invaded the lymphatic vessel wall, suggesting these cells may be mediators of lymphatic remodeling. Although TLOs have been described in many chronic inflammatory states, their anatomical relationship to preestablished LNs has never been revealed. Our data indicate that, at least in the CD-affected mesentery, TLOs are positioned along collecting lymphatic vessels in a manner expected to affect delivery of lymph to LNs.
In the bowel, the regulation of inflammatory and immune responses must be finely balanced to cope with the large microbial load present in the gut lumen. It is thought that the etiology and pathophysiology of inflammatory bowel disease relate to inflammatory and immune alterations that, in turn, connect with changes in the microbiome or interactions with microbial products.1 A recent study that followed mice for weeks after Yersinia pseudotuberculosis infection revealed that long after the infection was completely cleared, immune dysregulation persisted.2 This persistence was because of tissue insult that lingered and continuously deviated the immune response, normally programmed in draining lymph nodes (LNs), in part by inhibiting dendritic cell trafficking to the LNs. Inhibition of dendritic cell trafficking to LNs has also been found to underlie ileitis in the SAMP1/YitFc mouse model.3 In the case of infection-induced impairment of dendritic cell migration, the inhibition was apparently caused by compromised lymphatic integrity that damaged lymphatic collecting vessels in the mesentery and left them highly hyperpermeable, such that the contents of lymph spilled out into the mesentery, including migratory dendritic cells, rather than progressing to downstream mesenteric LNs.2
An interesting consideration that results from this work is whether related phenomena might contribute to inflammatory bowel disease in humans. Indeed, lymphatic dysfunction has been long discussed, although often overlooked, in Crohn disease (CD).4, 5 In particular, the sites where lymphatic dysfunction may be most relevant are scarcely studies in inflammatory bowel disease (IBD) models. That is, most analysis is performed in the intestinal wall, where lymphatic capillaries take up immune cells and solutes from the interstitium. The study in mice following Yersinia infection implicated the larger lymphatic vessels in the mesentery that interface with LNs. These vessels, called collecting vessels, are not known to take up cells or solutes but instead they function to actively pump lymph, via the action of specialized muscle cells and valves that promote unidirectional flow, along afferent collecting vessels that drain into LNs and then through efferent collecting vessels that emerge from LNs.6 The permeability of the collecting vessels has recently been shown to be regulated by a subset of dendritic cells.7 Furthermore, classic studies in dogs by Adair et al8 revealed another means by which collecting lymphatic vessel hyperpermeability develops. They observed that afferent lymph, with its typical low protein content, is filtered in the LN so that water is absorbed into the venous vasculature and efferent lymph emerges nearly as concentrated as plasma.8 However, increased efferent collecting vessel pressure changed the filtration properties of the LN and ultimately led to markedly leaky afferent lymphatics.9
Lymphatic collecting vessels are surrounded by fat throughout the body. In the mesentery, they run through the copious mesenteric adipose tissue. In CD, this fat expands beyond its usual anatomical restriction to the mesentery, such that during its expansion, it creeps up on to the intestinal wall, giving it the name creeping fat. Indeed, creeping fat is a hallmark of the inflamed CD-affected tissue, but its etiology is unexplained.10 One possibility connected to the discussion of lymphatic vessels is that creeping fat is driven by the spillage into the mesentery of the fatty chylomicrons carried in lymph11 by highly permeable or leaky lymphatic vessels.
Lymphatic vessels in the human mesentery have scarcely been studied, in either normal or diseased states, generating an obstacle to assessing the potential relevance of these studies in mice and dogs to human disease states. Herein, we developed an approach to characterize the human mesenteric lymphatic vasculature in normal and CD-affected mesentery. We identify the existence of tertiary lymphoid organs (TLOs), known to be present in many chronic inflammatory diseases, although not previously described in CD mesentery, as structures that remodel the collecting lymphatic vessel path to and from LNs, supporting the possibility that flow of lymph through the mesentery is altered in human CD.
Materials and Methods
Patient and Specimen Information
We studied tissue specimens from 17 operated on CD patients (11 women and 6 men; mean age = 45 ± 15 years) bearing disease with a diagnosis during adulthood involving stricturing in ileum-involved disease and 6 control tissues. Control tissue was derived from intestinal tissue removed for non-IBD indications, including distal from cancerous lesions or tissue from cadaveric donors. Specimens were provided as paraffin-embedded blocks of the mesentery, or whole fixed tissue was obtained after cases were signed out by pathologists at Washington University School of Medicine (St. Louis, MO). Patient identification was deidentified, and each tissue was assigned a random number by an honest broker (administered through Washington University's Digestive Diseases Research Core Center) who also recorded relevant patient information without identifiers. Use of these tissues and approval for the study was given by the Institutional Review Board of Washington University School of Medicine.
Patent Blue Tracing
Patent blue dye tracing of lymphatics during surgery was performed with patient and institutional consent from Clinique Universitaire Saint-Luc (Brussels, Belgium) (EudraCT N 2008-001746-12). Following the method as described,12 the surgeon injected a solution of patent blue dye, a dye used for lymphangiography, in the serosa on the anti-mesenteric border of the ileum just before resection.
Whole Mount Imaging
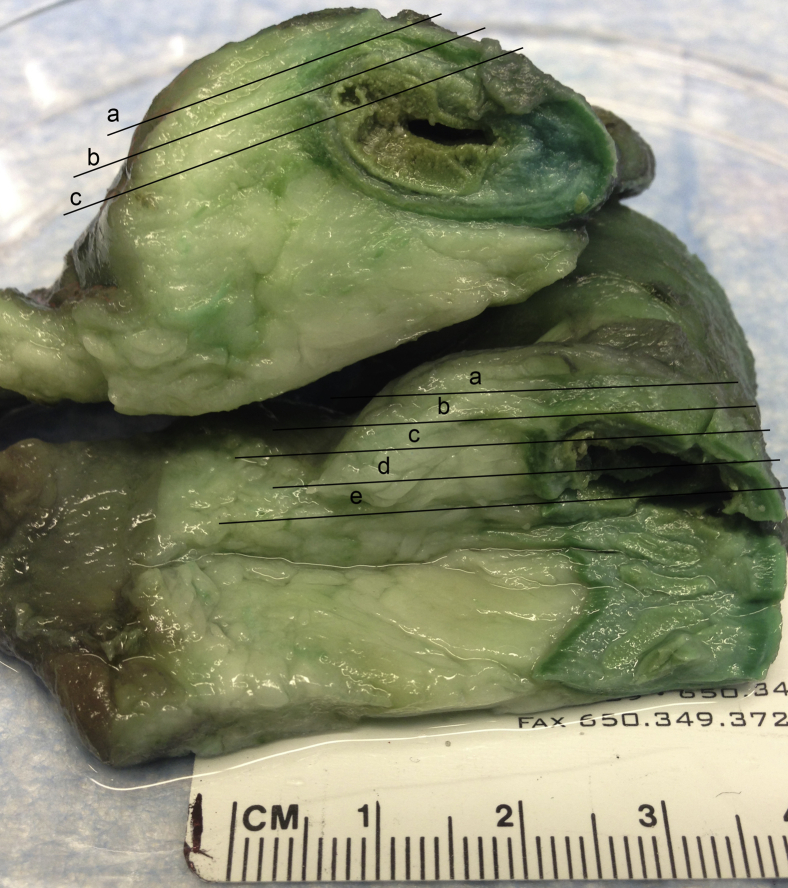
In the course of routine handling by pathologists, tissue derived from surgical resections of CD-affected ileum was fixed in formalin. On transfer to our laboratory, specimens were examined grossly and slices of the mesenteric adipose tissue and intestinal wall were made with the aid of manual tissue holders containing 2-mm cutting grids. Except where noted, slices were made tangential to the serosal surface of mesentery (Figure 1, slices labeled “a” were those containing serosal surface epithelium), and records were kept with respect to location of each slice relative to the terminal ileum and to the serosal surface. Because we observed the highest density of lymphatic capillaries and collecting vessels nearer to the serosal surface, slices along the serosal surface were typically analyzed first, especially those nearer the intestinal border, with some of the border left attached to retain orientation. A typical slice was approximately 2 cm in the x and y directions, respectively, with a z-axis thickness of approximately 2 mm. Up to 60 slices were often made from a single specimen. To select for the tissue sections with lymphatic tissue present, we cleared each slice by dehydration in a graded step of ethanol solutions and transfer to methyl salicylate. This method rendered the fat slices transparent. We then scanned slices using the bright field mode on a Cytation 5 system (Biotek, Winooski, VT) equipped with a light-emitting diode light source. Images from this scan were examined for hints of lymphatic tissue. Selected tissue slices were then rehydrated and immunostained with antibodies diluted in phosphate-buffered saline containing 0.03% to 1% Triton X-100. Slices were dehydrated and cleared in methyl salicylate, then imaged using a SPE confocal system (Leica, Wetzler, Germany). Image processing and analysis used Imaris software version 7.2 (Bitplane, Zurich, Switzerland).
Figure 1.
Depiction of tissue slicing performed to generate material for whole mount imaging. Two segments of ileum are stacked on each other with intestinal lumen on the right and mesenteric fat on the left. The black lines indicate the typical locations at which slices, each labeled a lower case letter a–e, were made for analysis. Slices labeled a were those that contained serosal epithelium and thus contained subserosal lymphatic capillaries. Most collecting vessels were found in slices a or b.
Antibodies and Imaging of Paraffin-Embedded Sections
Antibodies used in this study included goat anti-human podoplanin (R&D Systems, Minneapolis, MN), α-smooth muscle actin (Sigma, St. Louis, MO), CD20 (ab9475; Abcam, Cambridge, UK), CD3 (ab5690; Abcam), IL-33R (B4E6; Mdbioproducts, St. Paul, MN), CD68 (Y1/82A; Biolegend, San Diego, CA), CD138 (af2780; R&D Systems), collagen I (ab34710; Abcam), and peripheral node addressin (MECA-79; BD Pharmingen, San Jose, CA). Nuclei were stained with DAPI. Secondary antibodies used in detection were purchased from Jackson Immunoresearch (West Grove, PA) as conjugates to Cy2, Cy3, Cy5, or horseradish peroxidase. Detection with horseradish peroxidase was used in conjunction with the Opal 7-color Kit from Perkin Elmer (Waltham, MA), following the manufacturer's directions. In addition to use of these reagents in whole mount staining, sections of paraffin-embedded tissues (approximately 5 μm thick) were deparaffinized and then stained using hematoxylin and eosin or were immunostained following a citric acid antigen retrieval (Diva Decloaker; Biocare Medical, Concord, CA) step. Quantification of TLO area, CD20+ area, and tissue area was made using ImageJ2 and Fiji software (NIH, Bethesda, MD; https://imagej.nih.gov/ij/download.html). From either three- or two-dimensional images (paraffin-embedded sections), the measurements were used to estimate the volume of lymphoid tissue, assuming a general spherical shape. The number of cells within the lymphoid tissue was estimated using the assumption that the average cell diameter was 10 μm.
Results
We established a clinical protocol for injection of patent blue dye into the submucosa of bowel loops that were slated for surgical resection, as previously described.12 This dye, like other macromolecules within the interstitium,11 was expected to be cleared from the tissue predominantly through the lymphatic vasculature and thus visibly label major lymphatic drainage from the mucosa. In regions deemed grossly normal, the dye cleared in a manner that would be expected, based on the anatomical location of mesenteric lymphatic collecting vessels that run anti-parallel to the intestinal wall as they drain to LNs13 (Figure 2A). In bowel segments affected by active CD, the blue dye was highly deviated, running along the intestinal wall without entering mesenteric fat or tracking adjacent to the frontier of creeping fat (Figure 2B). These observations were consistent with previous results using protocol12 and suggested altered lymph drainage as a feature of active CD. These observations fueled our curiosity to examine the lymphatic vessels of the mesentery directly.
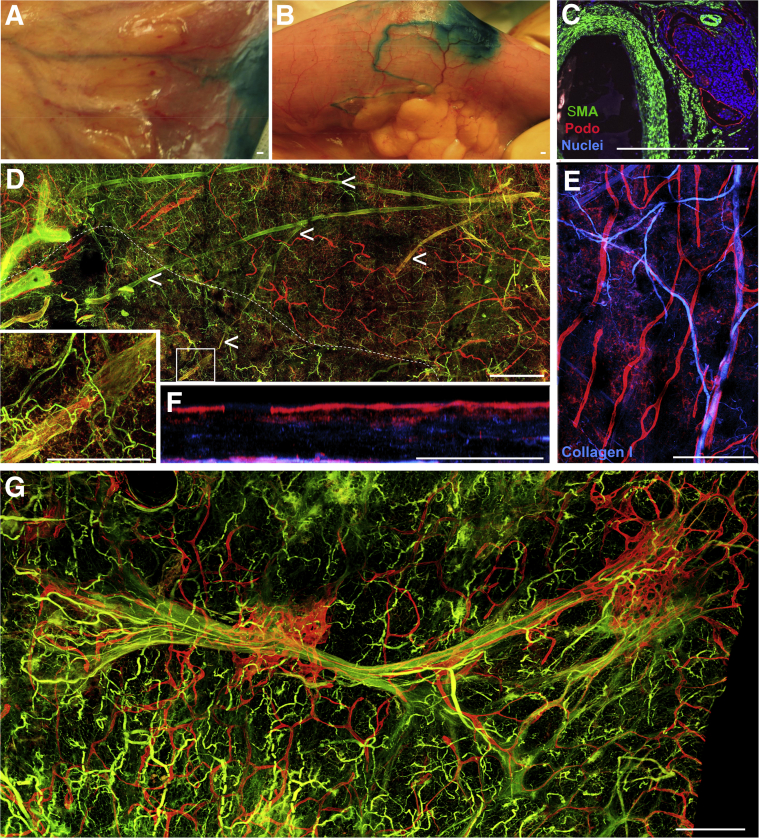
Figure 2.
Characterization of the human mesenteric lymphatic vasculature in healthy and Crohn disease (CD)-affected tissue. A and B: Representative images of small bowel loops after injection of patent blue dye in the submucosa during surgical resection. A: An area that showed no evidence of disease. B: An area affected by the development of creeping fat during the course of progressive CD. C: Immunofluorescence analysis that depicts collections of lymphatic vessels in the human CD-affected mesentery nearby blood vessels, with patient referred to surgery because of ileal stenosis from stricturing disease. The lymphatic vessels [podoplanin (Podo), red] encase a collection of nucleated cells stained with DAPI (blue). α-Smooth muscle actin (α-SMA; green) staining failed to identify collecting lymphatic vessels (podoplanin-positive endothelium with α-SMA–positive cells surrounding them) in most paraffin-embedded cross sections. D: Whole mount confocal imaging of large area of mesenteric adipose tissue (ie, cm × cm × mm scale) from a control specimen unaffected by inflammatory bowel disease (IBD) reveals blinded-ended lymphatic capillaries (red) and other lymphatic capillaries near α-SMA–positive blood vessels (left along main image). Tissue depicted below the dotted white line represents an area with mesothelial layer removed. Inset: Region of enlargement, in which the presence of a collecting lymphatic vessel (red + green) is evident. Arrowheads trace this collecting lymphatic vessel branching structure throughout the tissue. E and F: Collagen I (blue) and podoplanin (red) staining depict blood and lymphatic capillaries, respectively, from a control (no IBD) sample distinct from that shown in D. F: A region from E turned to the side to reveal major lymphatic capillary network (red) just below the mesothelial surface and a second lymphatic capillary grouping around the deeper blood vessel. G: Confocal scan of a large area (ie, cm × cm × mm) from a CD-affected mesentery stained for podoplanin (red) and α-SMA (green). Lymphatic capillaries are markedly expanded around the larger blood vessel. The right edge of the image marks the border of the image that was nearest the intestinal wall.
We first turned to whole-mount confocal analysis of fixed ileal tissue, obtained from resection surgical procedures involving the removal of the terminal ileum after case sign-out by pathologists. We were rarely able to identify collecting lymphatic vessels in traditional tissue sections (5 to 10 μm thick) of the mesentery, likely because of the fact that such preparations would include collecting vessels that would be expected to be spaced >1 mm apart. In CD-affected samples, we did, however, observe lymphatic capillary staining in proximity to larger blood vessels and such staining was often associated with a cellular infiltrate (Figure 2C). That is, such inflammatory foci, with associated lymphatics (Figure 2C), were observed in four of nine cases in which only paraffin-embedded sections were accessible in the case. Mesenteric tissue was cut tangentially with respect to the intestinal wall (Figure 1), an orientation that maximized the length of intact blood and lymphatic vessels that are mainly positioned in the mesentery antiparallel to the intestinal wall. The tissue slices that comprised the outermost layer of the mesentery (Figure 1), containing the mesothelium, were the most rich in lymphatic vessel staining. In normal specimens (no IBD diagnosis), blind-ended, podoplanin-positive lymphatic capillaries were abundant just beneath the mesothelium (Figure 2, D–F). Lymphatic capillaries were also enriched in the adventitial walls of blood vessels (Figure 2, D–F). Collecting lymphatic vessels were less prominently stained with markers like podoplanin, but could be seen when the sections were scanned for podoplanin-positive vessels with valves and smooth muscle actin staining (Figure 2D). Working back from the lymphangion highlighted in Figure 2D, a collecting lymphatic vessel branching structure in normal mesentery is marked. In CD specimens prepared using similar approaches, lymphatic capillaries could be seen to have expanded significantly around blood vessels (Figure 2G), fitting with previous literature that reveals lymphatic capillary expansion within the mucosa as well.14 Collecting vessels were routinely difficult to identify in CD specimens, perhaps in part because of their weaker staining intensity in the midst of an expanded lymphatic capillary network.
As our interest was to investigate the lymphatic collecting vessel network and its integrity, we added additional steps in the analysis of tissue that facilitated the identification of collecting lymphatic vessels in the human mesentery, even under disease conditions. These additional steps illuminated a path to investigating pathological changes in the specimens in a more comprehensive manner, as we used a method to examine resected tissue in cm3 scale. In this method, we prepared dozens of tissue slices per tissue specimen with x and y lengths >1 cm each, sliced to 1 to 2 mm thick (z axis). All slices were cleared before immunostaining and subjected to imaging in bright field mode using a strong light-emitting diode light source. Using this approach, lymphoid tissue appeared like dark shadows, including lymph nodes and lymphoid aggregates that were readily visualized along with the vessels that connect them (Figure 3A). Using this approach, all CD resection specimens examined in this manner (n = 8) and no control specimens (n = 3, all no IBD, no cancer) were observed to possess relatively small, dense aggregates (approximately 300 μm without obvious venous feeds) lining along a vessel that led to or from what appeared to be authentic LNs (>1 mm in diameter with clear investiture by venous feeds) (Figure 3A). We became convinced that the vessels connecting these structures were collecting lymphatic vessels, as sectioning through the lymphoid structures would inevitability reveal a few consecutive sections of the vessels that would stain for podoplanin and smooth muscle actin (Figure 3, B and C), reminiscent of the expected staining pattern for collecting lymphatic vessels. In these analyses, collecting vessels were observed to directly feed into ≤300-μm wide spherical collections of cells (Figure 3B) that contained podoplanin-positive cells that appeared morphologically similar to podoplanin-positive stromal cells and lymphatic capillaries, as seen within LNs. Immunostaining for CD3+ T cells and CD20+ B cells revealed that, although authentic LNs contained a T-cell–rich paracortex with multiple B-cell follicles (Figure 3D), these cellular aggregates were typically enriched in B cells, albeit with only a single or only two to three distinct follicles surrounding a central region containing T cells (Figure 3D). The structures we observed were often found with adipocytes from the mesentery serving as their external border (Figure 3E), suggesting that some of the structures lacked a strict capsule. Nonetheless, these collections of lymphocytes contained peripheral node addressin–positive blood vessels (Figure 3E), suggestive of tertiary lymphoid follicles, or TLOs, which are also typically rich in B cells and morphologically arranged similarly as those we have described herein.15 CD68+ cells that would include macrophages were scarce within them (Figure 3F), suggesting that they were not classic granulomas. These observations taken together prompt us to conclude that collecting lymphatic vessels in the mesenteric adipose tissue of CD resection specimens are remodeled in the course of disease to contain TLOs that impinge on their integrity. These TLOs are likely the structures observed in biobank paraffin-embedded and cut sections, as observed in Figure 2. Although we located evidence of TLOs in only four of nine patient specimens where we had access only to paraffin blocks containing mesenteric adipose tissue, the failure to find such structures in all specimens using this traditional approach was likely because of the low probability that a tissue block will be prepared within a collecting lymphatic vessel tract. This outcome is compared with the more comprehensive technique involving the initial light-emitting diode light-based scan, followed by progression to immunofluorescence imaging in 3 dimensions in which TLOs were observed in 5 out of 5 CD cases examined. Thus, we conclude that CD mesentery from patients referred to surgical interventions routinely possess TLO-remodeled collecting lymphatic vessels that affect the tracts that feed to normal LNs.
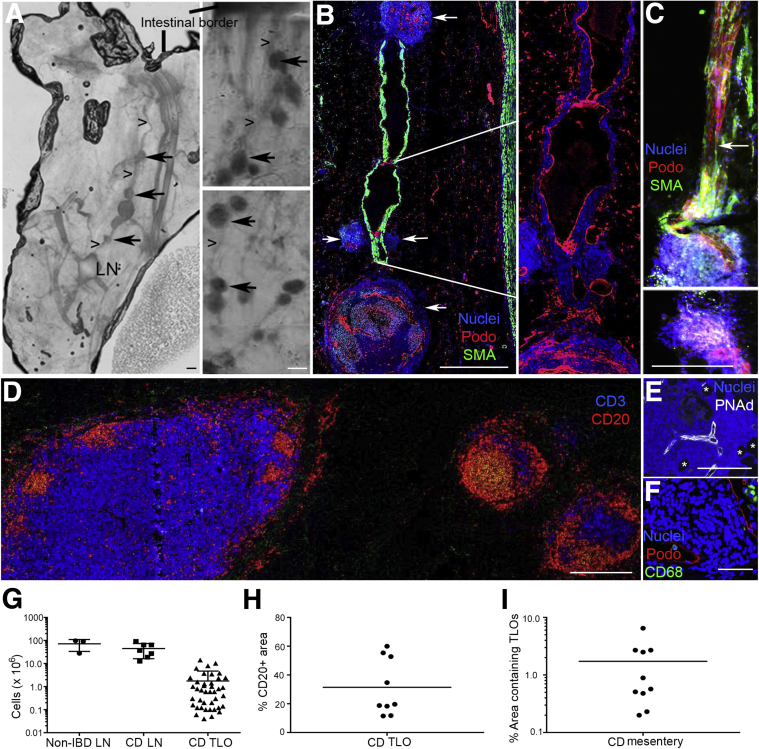
Figure 3.
Improved method to identify tissue specimens containing lymphatic collecting vessels links them to accumulation of tertiary lymphoid organs (TLOs) in Crohn disease (CD) mesentery. In all parts with the exception of D, the images are positioned so that the intestinal border is toward the top of the figure. In D, the intestinal border is on the right of the image. A: Evaluation of methyl salicylate–cleared tissue slices using a light-emitting diode (LED) allows detection of lymph nodes (LNs), lymphoid follicles, and vessels that link them together. Three CD mesenteric specimens shown herein arise from patients with ileal disease that led to surgery because of ileal occlusion in one case, stenosis in another, and fistulizing, perforating disease in the third. Image on the left shows LN with collecting lymphatic vessel (arrowheads) leading back to intestinal wall, with smaller lymphoid collections along the route (arrows). Two images on the right show chains of TLOs that are too small to be visible in the tissue slice when examined by eye, but which are readily identified using LED light scanning. B: Whole mount samples as in A were processed to generate 5-μm paraffin-embedded sections that were immunostained to identify α-smooth muscle actin (α-SMA)–positive vessels and podoplanin (Podo). Data reveal collecting lymphatic vessels interrupted by lymphoid follicle structures (white arrows). Enlargement on the right allows a better view, in the absence of the strong α-SMA staining, of the weaker podoplanin staining that characterizes the lumen of collecting lymphatic vessels. C: Different patient mesenteric fat sample cut in thicker 100-μm sections showing a collecting lymphatic vessel running into a follicle, with some tissue damage generated during delicate preparation. White arrow points to lymphatic collecting vessel valve that defines the direction of flow as being toward the follicle. The lower image, the next 100-μm section in the sequence, shows lymphatic vessels as they wind their way through to the efferent side of the follicle. D: CD20 and CD3 staining to identify follicles as lymphocyte-rich structures. LN on the left, two follicles on the right. Collecting lymphatic vessel that links these structures in a chain is thin enough that it is missing in this 5-μm paraffin section. E and F: Staining of follicle structures for peripheral node addressin (PNAd; E) and CD68 (F). Asterisks in E denote adipocytes. G: Quantification of cell numbers in lymph nodes and TLOs. Each lymph node analysis comes from a different CD specimen. For the TLO analysis, we measured the size of multiple TLOs in the same specimen. The 41 TLO evaluations depicted in the panel arise from TLOs combined from 10 different patients. H: Tissues from nine of the patient samples were stained for CD20, and the fraction of TLO structures positive for CD20 is depicted. I: The TLO area in the mesentery of the same 10 CD patients was quantified relative to the total mesenteric area in cross sections. Each dot in H and I represents data from one patient. Horizontal lines represent the mean value in the respective data column. Scale bars = 300 μm.
Using this approach, we quantified the number of cells within TLOs compared with authentic lymph nodes from non-IBD specimens or CD-affected specimens. The means ± SD cellularity of individual lymph nodes was 72 ± 38 million in non-IBD specimens and 45 ± 29 million in CD lymph nodes, whereas the TLOs individually contained 1.8 ± 2.9 million cells (Figure 3G). Some patients' TLOs averaged up to 60% CD20+ B cells, but the means ± SD was lower, at 29% ± 18% B cells (Figure 3H), highlighting the relatively high diversity in composition of these structures. The fractional area that the TLO-like structures occupied in the CD specimens was 1.7% ± 1.9% of the mesenteric fat volume within 3 cm of the ileal wall (Figure 3I).
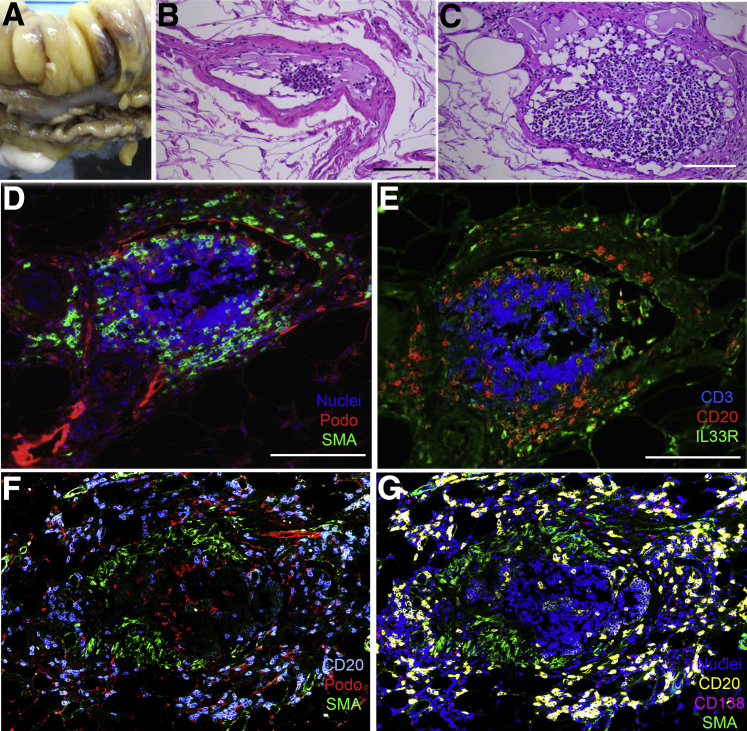
From some specimens, we prepared tissue from the adipose compartment that expands during disease, the so-called creeping fat (Figure 4A). We identified collecting lymphatic vessels in the mesenteric fat associated with creeping fat, as we observed vessels having a mural wall and the presence of lymphocytes but no red blood cells in the lumen (Figure 4, B–C). These vessels furthermore had a podoplanin-positive endothelial lining (Figure 4D). CD20+ B cells and IL-33R+ CD3− cells, possibly innate lymphoid cells,16 had localized within the mural wall, whereas CD3+ T cells were confined to the lumen (Figure 4E). These observations caused us to wonder whether B cells and perhaps the IL-33R+ cells might be active participants in remodeling the collecting lymphatic vessels. It was not uncommon to find TLOs that had a central ring of α-smooth muscle actin–positive cells just beneath a ring of podoplanin-positive cells (Figure 4F), possibly indicative of the anatomical arrangement of an earlier collecting lymphatic vessel embedded within the TLO before remodeling. Again, CD20+ cells were organized on the outside of the α-smooth muscle actin–positive structure, whereas the more compact collection of T lymphocytes was inside. We identified a few scattered CD138+ B cells, likely plasma cells, on the margins of the structures (Figure 4G), consistent with the possibility of antibody production locally in the structures.
Figure 4.
B cells and IL-33R+ CD3− cells localize to the collecting lymphatic vessel walls associated with creeping fat. A: Portion of the small bowel bearing relocalized, or creeping, fat from a patient receiving ileal resection because of Crohn disease complications. Adipose tissue was cut so as to bisect lobes of the fat, in the opposite orientation to the usual direction we cut the tissue (Figure 1). B and C: Hematoxylin and eosin sections from these lobes showed the presence of collecting lymphatic vessels. The two parts show the same vessel at different points. D and E: Adjacent sections from the block used in C were stained to identify nucleated cells, podoplanin (Podo), and α-smooth muscle actin (α-SMA; D) or CD3, CD20, and IL-33R (E). F and G: Analysis of a tertiary lymphoid organ from different patient as above. Seven-color confocal scanning was performed with the display of data in two parts. Scale bars = 50 μm.
Discussion
In this study, we describe lymphatic vessel expansion in the mesentery associated with CD. When traditional histological approaches were taken, only a minority of specimens were observed to bear lymphoid collections in the mesentery. However, when we developed a more comprehensive technique to identify lymphatic vessel tracts that directly feed to lymph nodes in CD samples, we identified the inflammatory lymphocyte-rich structures resembling TLOs in every patient sample.
An interesting concept has been put forward that TLOs form to mediate local immunity when lymph drainage to regional lymph nodes is impaired.17 This fits well with evidence and discussion in CD that lymph drainage is impaired.4, 14, 18 TLO formation as a feature of CD has been discussed previously.19 However, because of the techniques applied herein and the fact that we have extended our analysis into the tissue—the mesenteric adipose tissue—that houses collecting lymphatic vessels, we have, for the first time, been able to associate TLOs with collecting lymphatic vessels, which are the major lymphatic vessels that normally supply LNs. Thus, our findings suggest that TLOs, at least in CD but perhaps much more widely, are anatomically located so as not only to take over immunological roles when lymph flow is poor,17 but to physically participate in altering the course of immunological communication with the draining LN. In contrast to lymphatic capillaries, which readily expand during lymphangiogenesis associated with inflammation, collecting vessels are not known to expand. Thus, processes that remodel these vessels may greatly affect communication between LNs and the tissue, such as the intestinal lamina propria, that they normally drain. Even if TLOs perform some of the immunological functions performed by LNs,17 the types of responses that are coordinated in the two structures may not be fully overlapping. It is furthermore possible that the structural alterations we describe herein to human collecting lymphatic vessels render them obstructed or permeable in a manner similar to the outcomes described in mice infected by Y. pseudotuberculosis2 or dogs that have been treated to have elevated output pressures in LNs.9 We speculate that this, in turn, may promote leakage of chylomicrons11 that could drive the genesis of creeping fat in CD. Given that two different mouse models have linked chronic inflammatory changes in the intestine to failed dendritic cell migration to lymph nodes,2, 3 it is especially compelling to consider that the TLOs may alter dendritic cell access to draining lymph nodes.
However, a limitation of this study is its observational nature. The concepts raised by this study will require that techniques to track lymph transport in the intestine of humans be undertaken and that suitable mouse models be identified. Although one can draw potential parallels between our study and the Y. pseudotuberculosis model, the latter was not reported to be associated with ectopic TLOs per se. Identification of a mouse model that does develop such structures in association with chronic ileitis would be especially useful to test the functional relationship between the presence of such structures, lymph transport, immunity, and disease progression. Spahn et al20 earlier reported that TLO-like structures formed along the colon in mice treated with dextran sodium sulfate and lacking lymph nodes and Peyer's patches. Interestingly, a follow-up study revealed that while the standard protocol using dextran sodium sulfate was not effective at producing TLOs in the colon of mice, a protocol in which dextran sodium sulfate was given 7 days and then the mice returned to drinking water over subsequent days did produce numerous TLO-like structures, even in the absence of retinoic acid receptor–related orphan receptor γτ–dependent lymphoid tissue inducer cells.21 Indeed, the structures were rich in B cells, and the B cells appeared to perform the function of lymphoid inducer cells in a lymphotoxin β receptor–dependent manner.21 B cells aggravated disease pathology but facilitated the containment of intestinal microbes.21 As we herein reveal the prominent presence of TLO-like structures in the CD-affected mesentery, these mouse studies—which did not yet examine relationship of TLOs to mesenteric lymphatics—take on greater relevance and potentially provide a framework for future translational studies intent on better understanding the pathological basis of CD.
Acknowledgment
We thank Darren Nix (Digestive Diseases Research Core Center, Washington University School of Medicine, St. Louis, MO) for assistance in tissue acquisition.
Footnotes
Supported by Rainin Foundation Breakthrough and Innovator Awards, NIH Pioneer Award DP1DK109668 (G.J.R.), and NIH P30 DK52574 (Digestive Diseases Research Core Center of Washington University School of Medicine).
Disclosures: None declared.
References
- 1.de Souza H.S., Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13–27. doi: 10.1038/nrgastro.2015.186. [DOI] [PubMed] [Google Scholar]
- 2.Fonseca D.M., Hand T.W., Han S.J., Gerner M.Y., Glatman Zaretsky A., Byrd A.L., Harrison O.J., Ortiz A.M., Quinones M., Trinchieri G., Brenchley J.M., Brodsky I.E., Germain R.N., Randolph G.J., Belkaid Y. Microbiota-dependent sequelae of acute infection compromise tissue-specific immunity. Cell. 2015;163:354–366. doi: 10.1016/j.cell.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mikulski Z., Johnson R., Shaked I., Kim G., Nowyhed H., Goodman W., Chodaczek G., Pizarro T.T., Cominelli F., Ley K. SAMP1/YitFc mice develop ileitis via loss of CCL21 and defects in dendritic cell migration. Gastroenterology. 2015;148:783–793e5. doi: 10.1053/j.gastro.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Kruiningen H.J., Colombel J.F. The forgotten role of lymphangitis in Crohn's disease. Gut. 2008;57:1–4. doi: 10.1136/gut.2007.123166. [DOI] [PubMed] [Google Scholar]
- 5.von der Weid P.Y., Rehal S., Ferraz J.G. Role of the lymphatic system in the pathogenesis of Crohn's disease. Curr Opin Gastroenterol. 2011;27:335–341. doi: 10.1097/MOG.0b013e3283476e8f. [DOI] [PubMed] [Google Scholar]
- 6.Randolph G.J., Angeli V., Swartz M.A. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 7.Ivanov S., Scallan J.P., Kim K.W., Werth K., Johnson M.W., Saunders B.T., Wang P.L., Kuan E.L., Straub A.C., Ouhachi M., Weinstein E.G., Williams J.W., Briseno C., Colonna M., Isakson B.E., Gautier E.L., Forster R., Davis M.J., Zinselmeyer B.H., Randolph G.J. CCR7 and IRF4-dependent dendritic cells regulate lymphatic collecting vessel permeability. J Clin Invest. 2016;126:1581–1591. doi: 10.1172/JCI84518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adair T.H., Moffatt D.S., Paulsen A.W., Guyton A.C. Quantitation of changes in lymph protein concentration during lymph node transit. Am J Physiol. 1982;243:H351–H359. doi: 10.1152/ajpheart.1982.243.3.H351. [DOI] [PubMed] [Google Scholar]
- 9.Adair T.H., Guyton A.C. Modification of lymph by lymph nodes, III: effect of increased lymph hydrostatic pressure. Am J Physiol. 1985;249:H777–H782. doi: 10.1152/ajpheart.1985.249.4.H777. [DOI] [PubMed] [Google Scholar]
- 10.Colombel J.F., Watson A.J., Neurath M.F. The 10 remaining mysteries of inflammatory bowel disease. Gut. 2008;57:429–433. doi: 10.1136/gut.2007.122192. [DOI] [PubMed] [Google Scholar]
- 11.Randolph G.J., Miller N.E. Lymphatic transport of high-density lipoproteins and chylomicrons. J Clin Invest. 2014;124:929–935. doi: 10.1172/JCI71610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tonelli F., Giudici F., Liscia G. Is lymphatic status related to regression of inflammation in Crohn's disease? World J Gastrointest Surg. 2012;4:228–233. doi: 10.4240/wjgs.v4.i10.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmid-Schonbein G.W. Microlymphatics and lymph flow. Physiol Rev. 1990;70:987–1028. doi: 10.1152/physrev.1990.70.4.987. [DOI] [PubMed] [Google Scholar]
- 14.Rahier J.F., De Beauce S., Dubuquoy L., Erdual E., Colombel J.F., Jouret-Mourin A., Geboes K., Desreumaux P. Increased lymphatic vessel density and lymphangiogenesis in inflammatory bowel disease. Aliment Pharmacol Ther. 2011;34:533–543. doi: 10.1111/j.1365-2036.2011.04759.x. [DOI] [PubMed] [Google Scholar]
- 15.Ruddle N.H. Lymphatic vessels and tertiary lymphoid organs. J Clin Invest. 2014;124:953–959. doi: 10.1172/JCI71611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim B.S., Wang K., Siracusa M.C., Saenz S.A., Brestoff J.R., Monticelli L.A., Noti M., Tait Wojno E.D., Fung T.C., Kubo M., Artis D. Basophils promote innate lymphoid cell responses in inflamed skin. J Immunol. 2014;193:3717–3725. doi: 10.4049/jimmunol.1401307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thaunat O., Kerjaschki D., Nicoletti A. Is defective lymphatic drainage a trigger for lymphoid neogenesis? Trends Immunol. 2006;27:441–445. doi: 10.1016/j.it.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Rahier J.F., Dubuquoy L., Colombel J.F., Jouret-Mourin A., Delos M., Ferrante M., Sokol H., Hertogh G.D., Salleron J., Geboes K., Desreumaux P. Decreased lymphatic vessel density is associated with postoperative endoscopic recurrence in Crohn's disease. Inflamm Bowel Dis. 2013;19:2084–2090. doi: 10.1097/MIB.0b013e3182971cec. [DOI] [PubMed] [Google Scholar]
- 19.Sura R., Colombel J.F., Van Kruiningen H.J. Lymphatics, tertiary lymphoid organs and the granulomas of Crohn's disease: an immunohistochemical study. Aliment Pharmacol Ther. 2011;33:930–939. doi: 10.1111/j.1365-2036.2011.04605.x. [DOI] [PubMed] [Google Scholar]
- 20.Spahn T.W., Herbst H., Rennert P.D., Lugering N., Maaser C., Kraft M., Fontana A., Weiner H.L., Domschke W., Kucharzik T. Induction of colitis in mice deficient of Peyer's patches and mesenteric lymph nodes is associated with increased disease severity and formation of colonic lymphoid patches. Am J Pathol. 2002;161:2273–2282. doi: 10.1016/S0002-9440(10)64503-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lochner M., Ohnmacht C., Presley L., Bruhns P., Si-Tahar M., Sawa S., Eberl G. Microbiota-induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORgamma t and LTi cells. J Exp Med. 2011;208:125–134. doi: 10.1084/jem.20100052. [DOI] [PMC free article] [PubMed] [Google Scholar]