Abstract
Colorectal (CRCs) and endometrioid (EMCs) cancers in patients with Lynch syndrome exhibit microsatellite instability (MSI) detected by PCR or immunohistochemistry (IHC). While both assays are equally sensitive for CRCs, some suggest that PCR has a higher false-negative rate than IHC in EMCs. We assessed the MSI profiles of 91 EMC and 311 CRC specimens using five mononucleotide repeat markers: BAT25, BAT26, NR21, NR24, and MONO27. EMCs with high MSI (MSI-H) showed a mean left shift of 3 nucleotides (nt), which was significantly different from 6 nt in CRCs. A shift of 1 nt was observed in multiple markers in 76% of MSI-H EMCs, whereas only 12% of MSI-H CRCs displayed a 1-nt shift in one of five markers. IHC against four mismatch repair proteins was performed in 78 EMCs. Loss of staining in one or more proteins was detected in 18 of 19 tumors that were MSI-H by PCR. When EMC tumor cell burden was diluted to <30%, MSI-H was no longer observed in two of three EMCs with a mean nucleotide shift of 1 nt. These results indicate that EMC and CRC MSI profiles are different and that caution should be exercised when interpreting the results, as subtle, 1-nt changes may be missed. These findings provide a potential cause of previously reported discordant MSI and IHC results in EMCs.
CME Accreditation Statement: This activity (“JMD 2017 CME Program in Molecular Diagnostics”) has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of the American Society for Clinical Pathology (ASCP) and the American Society for Investigative Pathology (ASIP). ASCP is accredited by the ACCME to provide continuing medical education for physicians.
The ASCP designates this journal-based CME activity (“JMD 2017 CME Program in Molecular Diagnostics”) for a maximum of 36 AMA PRA Category 1 Credit(s)™. Physicians should claim only credit commensurate with the extent of their participation in the activity.
CME Disclosures: The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
Lynch syndrome (LS) (Online Mendelian Inheritance in Man no. 120435), also referred as hereditary nonpolyposis colorectal cancer, is an autosomal dominant disorder with a relatively common disease prevalence of 1 in 440.1, 2 About 2% to 3% of colorectal cancers (CRCs) and 1% to 2% of endometrioid cancers (EMCs; including endometrial cancer and endometrioid cancer of the ovary) are due to LS.3 LS is a heterogeneous disorder exhibiting reduced penetrance, differences in age of onset, and variability in expression.
In LS patients, the lifetime risks are 50% to 70% for CRC and 40% to 60% for EMC in women, and the overall risk for other associated tumors is increased.4 LS is caused by germline mutations in one of four mismatch repair (MMR) genes or by a deletion in the EPCAM locus affecting the adjacent MMR gene.5 These germline mutations result in defective MMR machinery that leads to microsatellite instability (MSI) throughout the genome and gives rise to tumors. Both Lynch-related and sporadic cancers can manifest MSI. MSI tumors are associated with a better prognosis yet a poor response to adjuvant 5-fluorouracil based chemotherapy.6 Many institutions have adopted an algorithm for universal screening for MSI in all newly diagnosed CRCs and EMCs to identify patients with potential LS.
The two widely used methods of clinical screening for LS are MSI detection by PCR with template DNA extracted from tumor tissue, and immunohistochemistry (IHC) staining using antibodies directed against MMR proteins in tumor tissue sections.7, 8 MSI is characterized by the expansion or contraction of DNA sequences through the insertion or deletion of repeated DNA sequences. If MSI is detected at ≥30% of the loci analyzed, the tumor is considered to have a high frequency of MSI (MSI-H). If MSI is detected at <30% of the loci studied, the tumor has a low frequency of MSI (MSI-L). If MSI is not detected at any locus, the tumor is considered to be microsatellite stable (MSS). MSI-L or MSS status greatly reduces the likelihood of LS in a patient. The absence of nuclear staining of one or more proteins on IHC may detect an abnormal MMR protein and predict the likely mutant gene. In contrast, the MSI PCR assay measures the function of the MMR system, and may identify MSI-H cases caused by missense mutations of MMR genes that may not result in the loss of immunoreactivity and thus could be missed by IHC.9 Both assays have a reported sensitivity of 92% to 93%.3 Initially, MSI testing was performed using two mono- and three dinucleotide polymorphic DNA markers, as presented at a National Cancer Institute 1998 workshop.10 Currently, however, the majority of diagnostic laboratories use a commercial kit containing five mononucleotide markers (see Materials and Methods for details).
While the algorithm for CRC screening may include IHC or MSI or both, it is largely an institutional decision based on cost, expertise, and resources. However, IHC is considered by some to be the preferred method of screening for EMC because of higher false-negative rates reported with MSI testing in EMC tumors. Hampel et al11 reported that 50% of mutS homolog 6 (MSH6) mutant EMCs do not show MSI-H by MSI PCR. Another study documented that 21% of IHC-deficient EMC tumors were MSS by the MSI PCR assay.12 However, a recent study by McConechy et al13 showed 93% concordance between the two methods, with 4% MSI-H EMCs missed by IHC, yet only 1% of IHC-deficient EMCs were missed by the MSI assay. Furthermore, the correlation of MSI status with pathologic features of EMCs is also controversial.14, 15, 16 In the present two-part study, we compared MSI PCR profile patterns between EMCs and CRCs to identify a possible cause of the reported higher false-negative rate with MSI testing in EMCs, and we examined the relationship of pathologic features of EMCs with MSI status.
Materials and Methods
Specimens and Patient Data Collection
Our retrospective study cohort consisted of banked DNA samples from 311 CRCs and 91 EMCs submitted to the Molecular Diagnostics Laboratory, Vanderbilt University Medical Center (Nashville, TN), for clinical MSI testing between June 2012 and January 2015. Hematoxylin and eosin stains from all EMCs were reviewed by the participating pathologist (C.S.) for tumor cellularity by calculating the percentage of tumor nuclei over total nuclei in the tissue section. Pathologic features were collected from pathologic reports during the clinical testing for MSI status. Clinical and follow-up data from the EMC patients were collected from electronic medical records, with Institutional Review Board approval (Vanderbilt University Medical Center Institutional Review Board protocol 160099). All demographic and clinicopathologic data were deidentified. Clinical MSI testing results from EMC and CRC specimens were retrospectively reanalyzed for this study.
DNA Preparation
Tumor and paired normal tissue DNA was extracted from three to five 10-μm curls or 5 to 10 unstained slides using the QIAquick PCR Purification Kit (catalog number 28104; Qiagen, Hilden, Germany) and the Qiacube instrument (Qiagen), according to the instructions supplied by the manufacturer.
MSI Testing by PCR and Capillary Electrophoresis
DNA extracted from paraffin-embedded tumor and paired normal tissues was subjected to MSI testing by multiplex PCR using the MSI Analysis System (catalog number MD1641; Promega, Madison, WI). Briefly, this multiplex PCR assay contains fluorescently labeled primers for five mononucleotide repeat markers, BAT25 (SLC7A8), BAT26 (MSH2), NR21 (KIT), NR24 (ZNF2), and MONO27 (MAP4K3), and two pentanucleotide repeat markers, PENTAC and PENTAD. After PCR, amplicons were detected by capillary electrophoresis on the ABI 3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA) and the results were analyzed using GeneMapper software version 4.1 (Applied Biosystems). Allele peaks present in the tumor sample that were not found in the corresponding normal sample (referred to as left or right shift) indicated instability of a marker. MSI status was determined as MSI-H, MSI-L, or MSS, depending on the number of mononucleotide markers demonstrating instability and corresponding to two or more (≥30%), one (<30% but >0%), or zero markers, respectively.
Tumor Cellularity Dilution Study
Representative EMC and CRC tumors with mean absolute nucleotide shifts of 1, 2, 3, 4 and 5 nt(s), sufficient remaining tumor, normal paired DNA, and tumor cellularity ranging from 30% to 80% were used for the tumor cellularity dilution study. Briefly, based on the concentration and the tumor cellularity, banked DNA extracted from paraffin-embedded tumor tissue was diluted using the respective paired normal control DNA from paraffin-embedded tissue to achieve template DNA aliquots with relative tumor cellularity values of 30%, 20% and 10%. MSI profiles of diluted tumor DNA were compared to those of undiluted tumor DNA as well as to MSI profiles of each patient's normal control DNA.
Quantification of MSI Repeat Number Changes
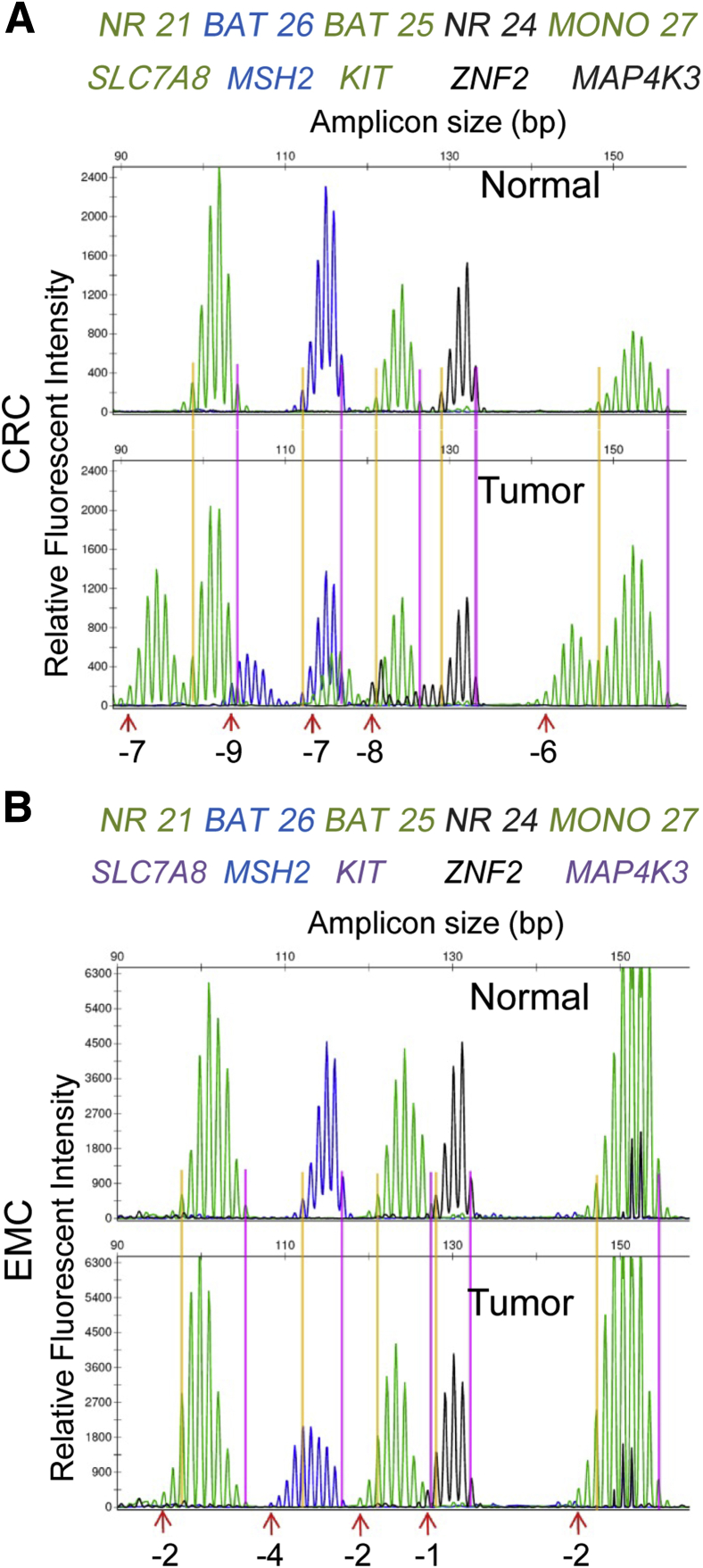
MSI PCR patterns were retrospectively reanalyzed in all tested CRCs and EMCs. Changes in PCR fragment lengths representing changes in mononucleotide repeat numbers were determined in each individual marker by two participating molecular geneticists (Y.W. and C.L.V.-J.) without knowledge of the IHC results. Using the electropherogram from the normal control DNA from each patient, the peak with the highest relative fluorescent intensity value was identified. Subsequently, the leftmost and rightmost peaks with heights of at least 5% that of the highest peak in the normal specimen were identified in each marker and considered as position zero (Figure 1). The difference in peak size (in base pairs) between the leftmost and the rightmost peaks also with a peak height of at least 5% that of the highest peak in tumor DNA specimen and position zero in the normal DNA was defined as the absolute nucleotide shift (Figure 1). The absolute nucleotide shift represents the change in the length of mononucleotide repeats of the tumor DNA compared with that in normal tissue DNA.
Figure 1.
Differences in microsatellite instability (MSI) profiles between colorectal cancer (CRC) and endometrioid cancer (EMC). A: MSI profile of a representative MSI–high (MSI-H) CRC with its paired normal control. Shifts in microsatellite repeat lengths are labeled at the bottom (eg, gene NR21/SLC7A8, –7 nt). B: MSI profile of a representative MSI-H EMC compared with its paired normal control. Shifts in microsatellite repeat lengths are labeled at the bottom (eg, gene NR21/SLC7A8, –2 nt). Common names and Human Genome Organisation nomenclature of genes containing microsatellite markers are listed.
IHC Analysis
Archived tumor specimens were available for IHC analysis in 78 of 91 EMCs. Five-micron, unstained sections were prepared from formalin-fixed, paraffin-embedded tissue blocks, and immunolabeled for mutL homolog 1 (MLH1), mutS homolog 2 (MSH2), mutS homolog 6 (MSH6), PMS1 homolog 2 (PMS2) after antigen retrieval. Details about antibodies used are listed in Table 1. Stains were independently reviewed by two participating pathologists (C.S. and R.E.) without knowledge of the MSI status from the PCR assay. The absence of nuclear staining in the tumor cells with the presence of a positive internal control in the adjacent morphologically normal cells in one or more of the MMR proteins is consistent with MMR deficiency.
Table 1.
Antibodies Used for IHC Analysis
| Antibody | Clone | Vendor | Working concentration | Antigen retrieval condition | Antibody incubation condition |
|---|---|---|---|---|---|
| MHL1 | M1 | Ventana Medical Systems (Tucson, AZ) | 1.4 μg/mL | 64 Minutes at 95°C; Ultra Cell Conditioning Solution 1 (Ventana Medical Systems) | 36 minutes at 37°C |
| MSH6 | SP93 | Cell Marque (Rocklin, CA) | 1:50 dilution | 52 Minutes at 95°C; Ultra Cell Conditioning Solution 1 (Ventana Medical Systems) | 1 hour at 37°C |
| MSH2 | FE11 | Dako (Carpinteria, CA) | 1:10 dilution | 60 Minutes at 95°C; Retrieval Bond Epitope Retrieval Solution 2 (Leica Microsystems, Buffalo Grove, IL) | 15 minutes at room temperature |
| PMS2 | A16-4 | BD Biosciences (San Jose, CA) | 1:50 dilution | 40 Minutes at 95°C; Retrieval Bond Epitope Retrieval Solution 2 (Leica Microsystems) | 15 minutes at room temperature |
MLH1, mutL homolog 1; MSH2, mutS homolog 2; MSH6, mutS homolog 6; PMS2, PMS1 homolog 2.
Statistical Analysis
The data were analyzed using GraphPad Prism software version 5.01 (GraphPad Software, Inc., La Jolla, CA). The t-test was used for assessing the significance of mean alterations in repeat length (absolute nucleotide shift) in the five mononucleotide markers. The χ2 test was used for assessing the correlation of MSI status with clinicopathologic features. P < 0.05 was considered statistically significant.
Results
Patient Characteristics and MSI Status
Tumor specimens obtained from 311 CRC and 91 EMC patients referred for MSI testing between June 2012 and January 2015 (Table 2) were used for analysis. In the CRC patients, MSI-H, MSI-L, and MSS accounted for 14.8% (46), 0.3% (1), and 84.9% (264) cases, respectively (Table 2). In the EMC patients, MSI-H, MSI-L, and MSS accounted for 22% (20), 1.1% (1), and 76.9% (70) cases, respectively (Table 2). The majority of the MSI-H tumors were unstable at all five mononucleotide loci (Table 3), regardless of tumor type (CRC, 87%; EMC, 85%). Only 11% of CRCs and 10% of EMCs were unstable at four mononucleotide loci. All tumors were unstable at three or more mononucleotide loci. The most frequently unstable markers in CRCs and EMCs were BAT26 and MONO27, respectively (Table 4).
Table 2.
Patient Characteristics
| Characteristic | Cancer type |
|
|---|---|---|
| CRC | EMC | |
| Age, years | ||
| Min | 23 | 30 |
| Max | 91 | 86 |
| Median | 60 | 61 |
| Sex, n (%) | ||
| Female | 135 (43) | 91 |
| Male | 176 (57) | N/A |
| MSI status, % | ||
| MSI-H | 14.8 | 22 |
| MSI-L | 0.3 | 1.1 |
| MSS | 84.9 | 76.9 |
CRC, colorectal cancer; EMC, endometrioid cancer; MSI-H, microsatellite instability–high; MSI-L, microsatellite instability–low; MSS, microsatellite stable.
Table 3.
Prevalence of Unstable Markers in MSI-H CRC and EMC
| Cancer type | # of unstable markers |
|||
|---|---|---|---|---|
| 2 | 3 | 4 | 5 | |
| CRC | 0 | 2 | 11 | 87 |
| EMC | 0 | 5 | 10 | 85 |
Data are expressed as % of patients.
CRC, colorectal cancer; EMC, endometrioid cancer; MSI-H, microsatellite instability–high.
Table 4.
Frequency of Instability of Five Mononucleotide Markers in MSI-H CRC and EMC
| Cancer type | Mononucleotide marker |
||||
|---|---|---|---|---|---|
| NR21 | BAT26 | BAT25 | NR24 | MONO27 | |
| CRC | 93 | 98 | 96 | 89 | 96 |
| EMC | 95 | 90 | 85 | 86 | 100 |
Data are expressed as % of patients.
CRC, colorectal cancer; EMC, endometrioid cancer; MSI-H, microsatellite instability–high.
EMCs Exhibited Smaller Repeat Number Changes than CRCs
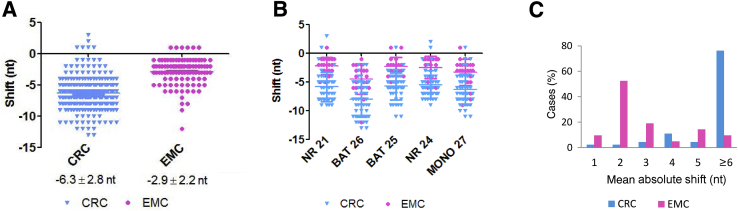
A visible and quantifiable difference in the MSI profiles between CRC and EMC was observed. In CRC tumors, mononucleotide markers exhibited a significant left shift compared with the subtle left shift often observed in MSI-H EMCs (Figure 1). In both CRCs and EMCs, the majority of mononucleotide markers displayed a left shift, indicating a deletion of microsatellite repeats. For example, –7 in the CRC from Figure 1 refers to a left shift of 7 nt, which represents deletion of seven mononucleotide repeats. Only rare tumors have markers with a right shift of 1 or 2 nt, indicated by a positive value (Figure 2A). Absolute nucleotide shifts in all five markers in all MSI-H cases were quantified and are shown in Figure 2A. CRCs demonstrated more left shift (means ± SD, −6.3 ± 2.8 nt) than did EMCs (means ± SD, −2.9 ± 2.2 nt). All five makers exhibited similar changes in repeat numbers within the same tumor type (Figure 2B).
Figure 2.
Differences in colorectal cancer (CRC) and endometrioid cancer (EMC) nucleotide shifts observed in microsatellite instability (MSI) profiles. A: Alterations in repeat length for all loci in all MSI-high CRC (cyan; n = 46) and EMC (magenta; n = 20) are plotted in the graph. B: Alterations in repeat length in five individual mononucleotide markers are plotted in the graph. C: The distribution of mean absolute shifts in repeat lengths of five markers per tumor differs significantly between CRC (cyan) and EMC (magenta). The mean nucleotide shifts in EMC (magenta) are significantly smaller than those of CRC (cyan) in all markers. P < 0.0001.
The mean changes in repeat number in all five markers in each tumor were calculated and plotted as the mean absolute shift (Figure 2C). There was a significant difference in the distributions of mean absolute shift between CRCs and EMCs (P < 0.0001). Approximately 43% of EMCs had a mean absolute shift of 2 nt. Collectively, there were 53% of EMC tumors with a mean absolute shift of 1 or 2 nt. In contrast, almost 80% of CRC tumors had a mean absolute shift of 6 nt or more. These results suggest that the change in repeat number in MSI-H EMCs is significantly smaller and may be missed if not examined carefully.
MSI PCR and IHC Correlation
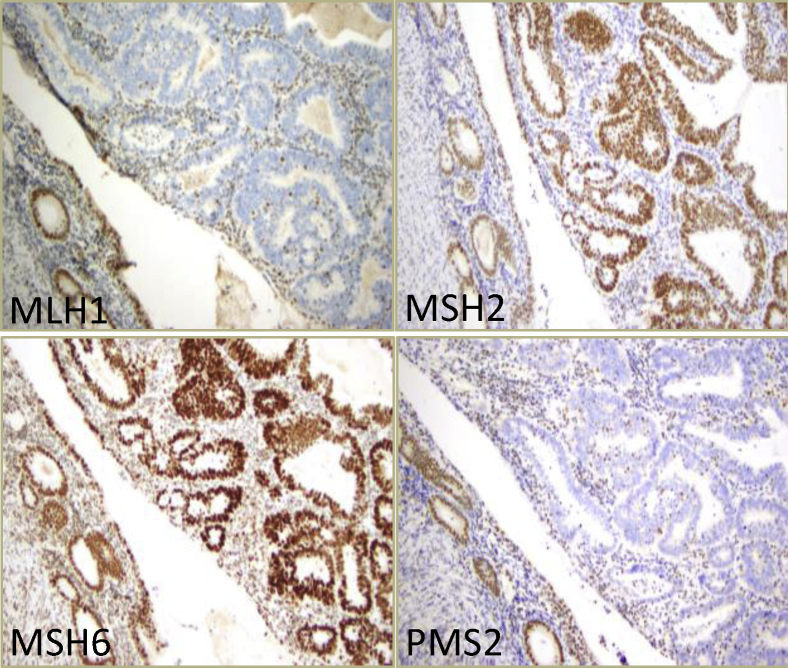
IHC against the four MMR proteins MLH1, MSH2, MSH6, and PMS2 was performed on 78 available endometrial tumors (Table 5). Sixty tumors (77%) exhibited a normal staining pattern, indicating MSS. Eighteen tumors (23%) showed loss of immunoreactivity in one (Supplemental Figure S1) or two MMR proteins, with the majority displaying dual loss of MLH1 and PMS2 (Figure 3). All 18 tumors with loss of MMR protein(s) exhibited MSI-H by PCR, whereas 1 MSI-H tumor by PCR retained immunoreactivity of all four MMR proteins, but with some tumor cells showing weaker expressions of MLH1 and PMS2 compared with those of MSH2 and MSH6 (Supplemental Figure S2). Overall, the sensitivity values were 95% (18/19) with IHC and 100% (19/19) with the PCR assay (Table 6).
Table 5.
IHC Staining Pattern Results
| Staining pattern | Cases, n | Cases, % |
|---|---|---|
| MLH1−/PMS2− | 15 | 19.2 |
| MSH2−/MSH6− | 1 | 1.3 |
| MSH6− | 1 | 1.3 |
| PMS2− | 1 | 1.3 |
| All retained | 60 | 76.9 |
| Total | 78 | 100 |
IHC, immunohistochemistry analysis; MLH1, mutL homolog 1; MSH2, mutS homolog 2; MSH6 mutS homolog 6; PMS2, PMS1 homolog 2.
Figure 3.
Representative immunohistochemistry analysis images of microsatellite instability–high in endometrioid cancer tumor tissue with loss of mutL homolog 1 (MLH1) and PMS1 homolog 2 (PMS2), but with retained immunoreactivity for mutS homolog 2 (MSH2) and mutS homolog 6 (MSH6).
Table 6.
Sensitivity of IHC and MSI PCR
| Screening method | True MSI-H | True MSS |
|---|---|---|
| IHC | ||
| IHC-deficient | 18 | 0 |
| IHC-intact | 1 | 59 |
| IHC sensitivity | 18/(18 + 1) = 95% | |
| MSI PCR | ||
| MSI PCR positive | 19 | 0 |
| MSI PCR negative | 0 | 59 |
| MSI PCR sensitivity | 19/19 (100%) | |
IHC, immunohistochemistry analysis; MSI-H, microsatellite instability–high; MSS, microsatellite stable.
Efficiency of MSI Detection Based on Tumor Cellularity
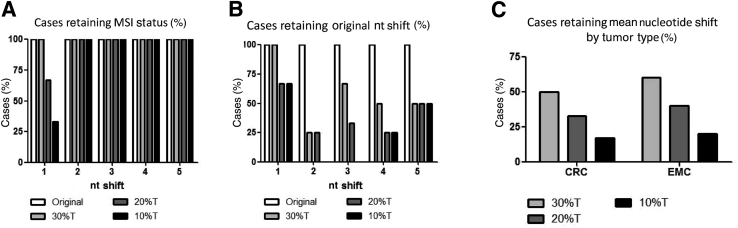
MSI testing on DNA of tumor tissue requires comparison of DNA extracted from adjacent normal tissue or peripheral blood. The proportion of tumor nuclei to total nuclei (percent tumor cell burden) in the DNA extracted from tumor tissue can affect the ability to detect a small nucleotide shift and thereby compromise the sensitivity of the MSI PCR assay. We therefore selected representative tumors from CRC and EMC groups with a mean absolute shift of 1, 2, 3, 4 or 5 nt. Tumor DNA was diluted with paired normal DNA to reach relative tumor cellularity values of 30%, 20%, and 10%. Only the tumors with a mean absolute shift of 1 nt showed a change in MSI status when tumor cellularity was 20% or less (Figure 4A).
Figure 4.
Nucleotide shift in repeat lengths and microsatellite instability (MSI) status are sensitive to tumor cellularity. A: Only tumors with an original mean absolute nucleotide shift of 1 nt lose their MSI-high status when the tumor cellularity is ≤20%. B: Percentages of cases retaining original alterations in repeat length are plotted based on original mean absolute nucleotide shifts. C: Both colorectal cancers (CRCs) and endometrioid cancers (EMCs) show a reduction in mean nucleotide shift when tumor cellularity is ≤20%. T, tumor.
In our study, 10% of MSI-H EMCs had a mean absolute shift of 1 nt, which could potentially be missed if the tumor cellularity is <30% (Figure 2C). In contrast, there were only 2% CRCs with a mean absolute shift of 1 nt (Figure 2C). Thus, the detection of MSI by PCR in EMCs is dependent on accurate assessment of tumor cellularity to prevent false-negative results. When the tumor cellularity was <30% in both tumor types, although up to 75% cases exhibited smaller shifts of 2 to 5 nt (Figure 4, B and C), most of these changes did not affect their reported MSI status. Taken together, these data suggest that the cutoff for tumor cellularity should be ≥30% of tumor nuclei in both EMCs and CRCs.
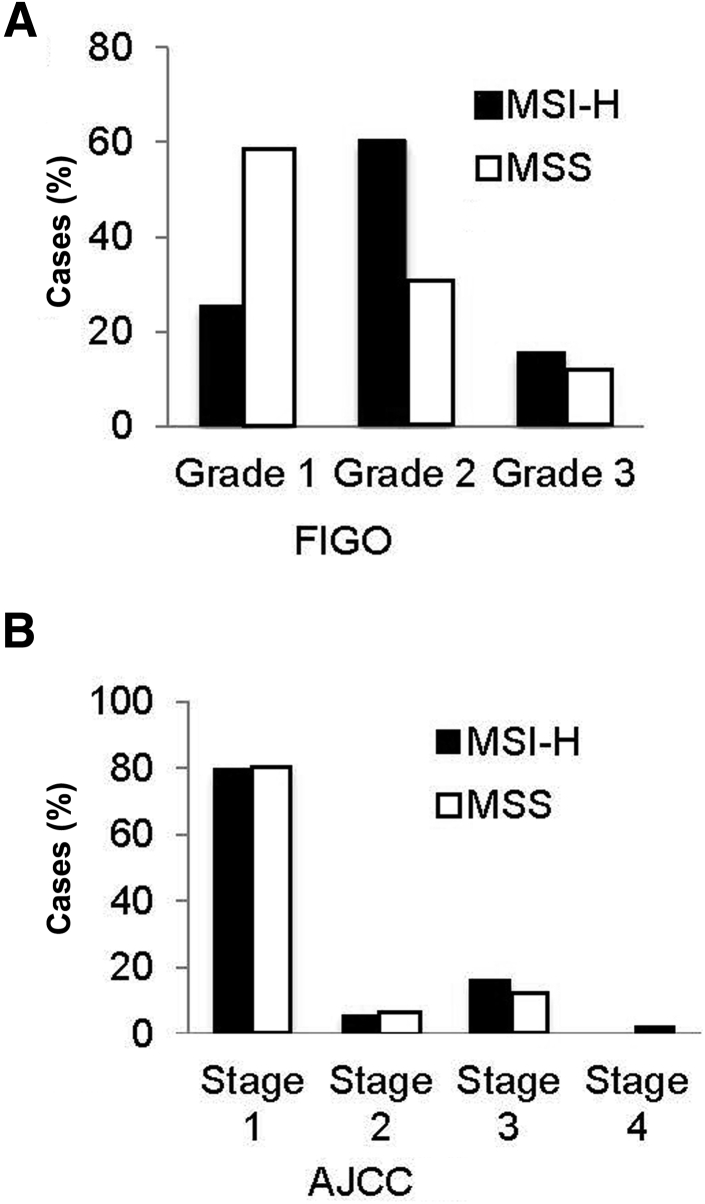
MSI Status Associates with International Federation of Gynecology and Obstetrics Grade in EMCs
MSI status was significantly associated with International Federation of Gynecology and Obstetrics grade (Figure 5A). MSS EMCs were most often grade 1, whereas MSI-H EMCs were typically grade 2. However, MSI status did not correlate with age at diagnosis or other pathologic characteristics, such as American Joint Committee on Cancer stage (Figure 5B), tumor site, histologic type, cervix involvement, lymph-vascular invasion, tumor margin, or myometrial invasion.
Figure 5.
Microsatellite instability (MSI) status in endometrioid cancer (EMC) correlates to some clinicopathologic features. A: MSI status correlates with the International Federation of Gynecology and Obstetrics (FIGO) grade of EMC with microsatellite-stable (MSS) tumors more often grade 1 and MSI-high (MSI-H) tumors more often grade 2 (χ2P = 0.0394). B: MSI status does not correlate with the American Joint Committee on Cancer (AJCC) stage of EMC. P = 0.9297 (χ2).
Discussion
It is important to identify EMC patients with LS because they have significantly elevated lifetime risks for additional malignancies, including 40% to 60% for CRC and 9% to 12% for ovarian cancers.14 With a diagnosis of LS confirmed by MMR germline mutation analysis, an appropriate surveillance plan for the patient can be developed. In addition, the establishment of LS in the proband coupled with germline MMR mutation identification enables mutation testing for at-risk family members and appropriate clinical management in mutation-positive carriers. The National Comprehensive Cancer Network guideline Uterine Neoplasm recommends that physicians “consider screening with IHC and MSI for inherited MMR gene mutations in patients <50 years and those with a significant family history of endometrial and/or colorectal cancers.”15,pp.UN-1 Thus, screening for LS in EMC patients has become the standard of practice, and accurate identification of these patients is important for their clinical management and that of other members in the family. Regarding initial testing for MSI in EMCs, the Society for Gynecologic Oncology clinical practice statement on EMC LS screening states, “IHC is the most cost-effective of the tumor studies and is widely available in most pathology laboratories.” [Society of Gynecologic Oncology. SGO Clinical Practice Statement: Screening for Lynch syndrome in endometrial cancer 2014. Available at https://www.sgo.org/clinical-practice/guidelines/screening-for-lynchsyndrome-in-endometrial-cancer (accessed July 2016).] Whether the PCR or the IHC assay or both are used for identifying LS in patients is dependent on costs, sensitivity of the assay, and the collaboration between the clinical and laboratory teams at the treating institution.
In 2002, it was reported that EMCs in LS patients showed false-negative results on radioactive PCR amplification of microsatellite repeats followed by gel electrophoresis.16 The same phenomenon was observed on fluorescent PCR amplification of two mono- and three dinucleotide markers and capillary electrophoresis.17, 18 With MSI testing available at our institution and IHC testing recently implemented, we sought to optimize our screening algorithm, which relied on determining whether these previously reported findings were applicable to the five mononucleotide marker–based MSI commercial kit in use by our laboratory and in most clinical laboratories for CRC and EMC testing.16, 17, 18 In this process, we identified and quantified distinct differences in MSI profiles between cohorts of CRC and EMC patients referred for clinical MSI testing (Table 2). Interestingly, unstable microsatellite markers in CRCs showed a statistically larger shift and thus a larger number of deleted mononucleotides compared with the nucleotide shift changes and deleted mononucleotide numbers in EMCs (Figures 1 and 2). Furthermore, these subtle 1-nt differences can be missed if the tumor cellularity within the specimen is <30%, resulting in false-negative results in the subset of EMCs exhibiting small nucleotide shifts (Figure 4). The findings from our study emphasize the critical need for accurate tumor cellularity assessment before the initiation of MSI testing, and demonstrate the potential of inaccurate assessment of this task. Furthermore, while most clinical laboratories are accustomed to looking at clearly obvious, large nucleotide shifts in CRCs, small and subtle changes observed in some EMCs may be overlooked. Similarly, if robust PCR amplification with high relative fluorescence intensity is not observed, small, subtle, 1-nt shifts may also not be observed. With knowledge of these findings and the confirmation that MSI detection by PCR is as sensitive as that by IHC, laboratories should consider the use of this technology as an alternative to IHC when developing their testing algorithms and be assured that the sensitivity of this assay is not any less than that of IHC.
While MSI testing is at least as sensitive to IHC testing, there are limitations to this assay. If paired normal tissue is not available for comparison or the tumor cellularity is insufficient (<30%), IHC testing should be the preferred method. In addition, IHC allows for the specific identification of the defective MMR protein(s). However, IHC can be intact in MSI-H tumors with germline mutations when the specific mutation(s) does not disrupt antibody-reactive epitope,19 and IHC might not be the ideal method for detecting EMC in treated patients, as chemoradiation can reduce MMR protein expression in otherwise MSS tumors.20
There are several possible mechanisms underlying the difference in MSI profiles seen in MSI-H EMCs and CRCs that, in part, may be attributable to differences in the biology of the two different types of epithelium.21, 22 First, like normal colonic and endometrial epithelium, MSI-H CRCs may have more rapid turnover and more rounds of DNA replication compared with those of EMCs, consequently leading to a larger nucleotide shift compared with that in MSI-H EMCs. Second, almost 80% of EMCs in this cohort were American Joint Committee on Cancer stage 1 and early-stage tumors; as such, they may have undergone fewer rounds of DNA replication and therefore may have exhibited a smaller nucleotide shift. However, we did not see a significant correlation between tumor stage and the number of nucleotide shifts in MSI-H EMCs, but that finding could have been due to our small sample size. Finally, complete loss of two functional MMR alleles may occur in a later stage of cancer development in EMCs than in CRCs. Alternatively, differences in MMR machinery during DNA replication in EMCs and CRCs may exist. Further studies using MMR-deficient cell lines and a larger cohort of cases representing different stages of disease are needed to clarify the mechanisms of these differences.
MSI-H CRCs have a well-documented association with a number of pathologic features, such as mucinous differentiation, signet ring cell morphology, medullary features, Crohn-like lymphoid reaction, and abundant tumor-infiltrating lymphocytes.23 In contrast, there have been limited and controversial studies regarding the correlation of MSI status with clinicopathologic features in EMC. Two studies from 2006 in a relatively large-scale cohort of EMC patients showed significant associations between tumor MSI-H and advanced tumor stage, myometrial invasion, and improved survival.24, 25 However, a later study, in 2014, showed no associations of MSI status with survival, tumor grade, tumor stage, or histologic examination findings in 109 patients.26 In our study, a correlation between International Federation of Gynecology and Obstetrics grade and MSI status was observed. However, MSI status did not correlate with age at diagnosis or other pathologic characteristics, such as American Joint Committee on Cancer stage, tumor site, histologic type, cervix involvement, lymph-vascular invasion, tumor margin, or myometrial invasion, potentially due to our limited study cohort. Therefore, additional, larger-scale studies are needed to support these associations.
Footnotes
Supported by grants NIH/NIDDK5P30 DK058404-13 and NIH/NCI5P50 CA095103-13 (C.S.).
Disclosures: None declared.
Current address of Y.W., Department of Human Genetics, EGL Genetics Laboratory, Emory University, Atlanta, Georgia.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.jmoldx.2016.07.008.
Supplemental Data
A and B: Representative immunohistochemistry analysis images of a microsatellite instability–high endometrioid cancer with loss of PMS2 only (A) and loss of mutS homolog 6 (MSH6) loss (B). MLH, human mutL homolog; PMS, postmeiotic segregation increased S. cerevisiae 2.
Immunohistochemistry (IHC) analysis and microsatellite instability (MSI) PCR profile of the one endometrioid cancer case with discrepant results. A: IHC shows expression of all four mismatch repair proteins; however, some tumor cells appear to have rather weak expressions of human mutL homolog 1 (MLH1) and postmeiotic segregation increased S. cerevisiae 2 (PMS2). B: MSI profile shows instability in three of the five mononucleotide repeat markers. MSH, mutS homolog.
References
- 1.Kohlmann W, Gruber SB: Lynch syndrome. In GeneReviews [Internet]. Copyright University of Washington, Seattle. 2014. Available at https://www.ncbi.nlm.nih.gov/books/NBK1211. (last revised May 22, 2014)
- 2.Lynch H.T., Shaw M.W., Magnuson C.W., Larsen A.L., Krush A.J. Hereditary factors in cancer. Study of two large Midwestern kindreds. Arch Intern Med. 1966;117:206–212. [PubMed] [Google Scholar]
- 3.Tafe L.J., Riggs E.R., Tsongalis G.J. Lynch syndrome presenting as endometrial cancer. Clin Chem. 2014;60:111–121. doi: 10.1373/clinchem.2013.206888. [DOI] [PubMed] [Google Scholar]
- 4.Cragun D., DeBate R.D., Vadaparampil S.T., Baldwin J., Hampel H., Pal T. Comparing universal Lynch syndrome tumor-screening programs to evaluate associations between implementation strategies and patient follow-through. Genet Med. 2014;16:773–782. doi: 10.1038/gim.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sehgal R., Sheahan K., O'Connell P.R., Hanly A.M., Martin S.T., Winter D.C. Lynch syndrome: an updated review. Genes (Basel) 2014;5:497–507. doi: 10.3390/genes5030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guillotin D., Martin S.A. Exploiting DNA mismatch repair deficiency as a therapeutic strategy. Exp Cell Res. 2014;329:110–115. doi: 10.1016/j.yexcr.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Samowitz W.S. Evaluation of colorectal cancers for Lynch syndrome: practical molecular diagnostics for surgical pathologists. Mod Pathol. 2015;28 Suppl 1:S109–S113. doi: 10.1038/modpathol.2014.127. [DOI] [PubMed] [Google Scholar]
- 8.Beamer L.C., Grant M.L., Espenschied C.R., Blazer K.R., Hampel H.L., Weitzel J.N., MacDonald D.J. Reflex IHC analysis and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J Clin Oncol. 2012;30:1058–1063. doi: 10.1200/JCO.2011.38.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Technology Assessment Committee, College of American Pathologists. Prognostic uses of MSI testing. Perspectives on emerging technology report 2011. Available at http://www.cap.org/apps/docs/committees/technology/microsatellite_testing.pdf (accessed July 2016)
- 10.Boland C.R., Thibodeau S.N., Hamilton S.R., Sidransky D., Eshleman J.R., Burt R.W., Meltzer S.J., Rodriguez-Bigas M.A., Fodde R., Ranzani G.N., Srivastava S. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 11.Hampel H., Panescu J., Lockman J., Sotamaa K., Fix D., Comeras I., LaJeunesse J., Nakagawa H., Westman J.A., Prior T.W., Clendenning M., de la Chapelle A., Frankel W., Penzone P., Cohn D.E., Copeland L., Eaton L., Fowler J., Lombardi J., Dunn P., Bell J., Reid G., Lewandowski G., Vaccarello L. Comment on: screening for Lynch Syndrome (Hereditary Nonpolyposis Colorectal Cancer) among Endometrial Cancer Patients. Cancer Res. 2007;67:9603. doi: 10.1158/0008-5472.CAN-07-2308. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson S.E., Aronson M., Pollett A., Eiriksson L.R., Oza A.M., Gallinger S., Lerner-Ellis J., Alvandi Z., Bernardini M.Q., MacKay H.J., Mojtahedi G., Tone A.A., Massey C., Clarke B.A. Performance characteristics of screening strategies for Lynch syndrome in unselected women with newly diagnosed endometrial cancer who have undergone universal germline mutation testing. Cancer. 2014;120:3932–3939. doi: 10.1002/cncr.28933. [DOI] [PubMed] [Google Scholar]
- 13.McConechy M.K., Talhouk A., Li-Chang H.H., Leung S., Huntsman D.G., Gilks C.B., McAlpine J.N. Detection of DNA mismatch repair (MMR) deficiencies by IHC analysis can effectively diagnose the microsatellite instability (MSI) phenotype in endometrial carcinomas. Gynecol Oncol. 2014;137:306–310. doi: 10.1016/j.ygyno.2015.01.541. [DOI] [PubMed] [Google Scholar]
- 14.Lancaster J.M., Powell C.B., Chen L.M., Richardson D.L. Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2015;136:3–7. doi: 10.1016/j.ygyno.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Koh W.-J., Greer B.E., Abu-Rustum N.R., Apte S.M., Campos S.M., Chan J., Cho K.R., Cohn D., Crispens M.A., DuPont N., Eifel P.J., Nickles Fader A., Fisher C.M., Gaffney D.K., George S., Han E., Huh W.K., Lurain J.R., III, Martin L., Mutch D., Remmenga S.W., Reynolds R.K., Small W., Jr., Teng N., Tillmanns T., Valea F.A., McMillian N., Miranda Hughes M., National Comprehensive Cancer Network Uterine neoplasms, version 1.2014. J Natl Compr Canc Netw. 2014;12:248–280. doi: 10.6004/jnccn.2014.0025. [DOI] [PubMed] [Google Scholar]
- 16.Kuismanen S.A., Moisio A.L., Schweizer P., Truninger K., Salovaara R., Arola J., Butzow R., Jiricny J., Nystrom-Lahti M., Peltomaki P. Endometrial and colorectal tumors from patients with hereditary nonpolyposis colon cancer display different patterns of microsatellite instability. Am J Pathol. 2002;160:1953–1958. doi: 10.1016/S0002-9440(10)61144-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim T.M., Laird P.W., Park P.J. The landscape of microsatellite instability in colorectal and endometrial cancer genomes. Cell. 2013;155:858–868. doi: 10.1016/j.cell.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreira A.M., Westers H., Wu Y., Niessen R.C., Olderode-Berends M., van der Sluis T., van der Zee A.G., Hollema H., Kleibeuker J.H., Sijmons R.H., Hofstra R.M. Do microsatellite instability profiles really differ between colorectal and endometrial tumors? Genes Chromosomes Cancer. 2009;48:552–557. doi: 10.1002/gcc.20664. [DOI] [PubMed] [Google Scholar]
- 19.Muller A., Giuffre G., Edmonston T.B., Mathiak M., Roggendorf B., Heinmoller E., Brodegger T., Tuccari G., Mangold E., Buettner R., Ruschoff J. Challenges and pitfalls in HNPCC screening by microsatellite analysis and IHC analysis. J Mol Diagn. 2004;6:308–315. doi: 10.1016/S1525-1578(10)60526-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shia J., Zhang L., Shike M., Guo M., Stadler Z., Xiong X., Tang L.H., Vakiani E., Katabi N., Wang H., Bacares R., Ruggeri J., Boland C.R., Ladanyi M., Klimstra D.S. Secondary mutation in a coding mononucleotide tract in MSH6 causes loss of immunoexpression of MSH6 in colorectal carcinomas with MLH1/PMS2 deficiency. Mod Pathol. 2013;26:131–138. doi: 10.1038/modpathol.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshman E., Booth C., Potten C.S. The intestinal epithelial stem cell. Bioessays. 2002;24:91–98. doi: 10.1002/bies.10028. [DOI] [PubMed] [Google Scholar]
- 22.Gargett C.E., Nguyen H.P., Ye L. Endometrial regeneration and endometrial stem/progenitor cells. Rev Endocr Metab Disord. 2012;13:235–251. doi: 10.1007/s11154-012-9221-9. [DOI] [PubMed] [Google Scholar]
- 23.Setaffy L., Langner C. Microsatellite instability in colorectal cancer: clinicopathological significance. Pol J Pathol. 2015;66:203–218. doi: 10.5114/pjp.2015.54953. [DOI] [PubMed] [Google Scholar]
- 24.Black D., Soslow R.A., Levine D.A., Tornos C., Chen S.C., Hummer A.J., Bogomolniy F., Olvera N., Barakat R.R., Boyd J. Clinicopathologic significance of defective DNA mismatch repair in endometrial carcinoma. J Clin Oncol. 2006;24:1745–1753. doi: 10.1200/JCO.2005.04.1574. [DOI] [PubMed] [Google Scholar]
- 25.Honore L.H., Hanson J., Andrew S.E. Microsatellite instability in endometrioid endometrial carcinoma: correlation with clinically relevant pathologic variables. Int J Gynecol Cancer. 2006;16:1386–1392. doi: 10.1111/j.1525-1438.2006.00535.x. [DOI] [PubMed] [Google Scholar]
- 26.Kanopiene D., Smailyte G., Vidugiriene J., Bacher J. Impact of microsatellite instability on survival of endometrial cancer patients. Medicina (Kaunas) 2014;50:216–221. doi: 10.1016/j.medici.2014.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A and B: Representative immunohistochemistry analysis images of a microsatellite instability–high endometrioid cancer with loss of PMS2 only (A) and loss of mutS homolog 6 (MSH6) loss (B). MLH, human mutL homolog; PMS, postmeiotic segregation increased S. cerevisiae 2.
Immunohistochemistry (IHC) analysis and microsatellite instability (MSI) PCR profile of the one endometrioid cancer case with discrepant results. A: IHC shows expression of all four mismatch repair proteins; however, some tumor cells appear to have rather weak expressions of human mutL homolog 1 (MLH1) and postmeiotic segregation increased S. cerevisiae 2 (PMS2). B: MSI profile shows instability in three of the five mononucleotide repeat markers. MSH, mutS homolog.