Abstract
Mismatch repair protein deficiency (MMR-D) and high microsatellite instability (MSI-H) are features of Lynch syndrome–associated colorectal carcinomas and have implications in clinical management. We evaluate the ability of a targeted next-generation sequencing panel to detect MMR-D and MSI-H based on mutational phenotype. Using a criterion of >40 total mutations per megabase or >5 single-base insertion or deletion mutations in repeats per megabase, sequencing achieves 92% sensitivity and 100% specificity for MMR-D by immunohistochemistry in a training cohort of 149 colorectal carcinomas and 91% sensitivity and 98% specificity for MMR-D in a validation cohort of 94 additional colorectal carcinomas. False-negative samples are attributable to tumor heterogeneity, and next-generation sequencing results are concordant with analysis of microsatellite loci by PCR. In a subset of 95 carcinomas with microsatellite analysis, sequencing achieves 100% sensitivity and 99% specificity for MSI-H in the combined training and validation set. False-positive results for MMR-D and MSI-H are attributable to ultramutated cancers with POLE mutations, which are confirmed by direct sequencing of the POLE gene and are detected by mutational signature analysis. These findings provide a framework for a targeted tumor sequencing panel to accurately detect MMR-D and MSI-H in colorectal carcinomas.
Mismatch repair protein deficiency (MMR-D) and high microsatellite instability (MSI-H) reflect the mechanism of tumorigenesis in approximately 15% of colorectal carcinomas, which may be sporadic or inherited as part of Lynch syndrome. In MMR-D and MSI-H colorectal carcinomas, defective DNA mismatch repair machinery leads to hypermutation and instability of DNA repeat regions.1
Multiple laboratory tests are available to evaluate the status of the mismatch repair pathway. These include immunohistochemistry (IHC) staining for mismatch repair proteins, PCR to evaluate microsatellite instability, MLH1 promoter methylation analysis, and BRAF sequencing. These tests are performed directly on tumor samples and have prognostic and therapeutic implications for nearly all patients with colorectal carcinoma.2, 3 In addition, the results can be used to guide germline testing if the results indicate an increased risk of Lynch syndrome.4
As next-generation sequencing technology becomes increasingly available for clinical use, we hypothesize that much of the Lynch syndrome–related testing in the anatomical and molecular pathology laboratory may be accomplished by a single assay. Sequencing of tumor specimens can test for mutational patterns characteristic of MMR-D and MSI-H while simultaneously detecting pathogenic alterations and facilitating downstream germline testing.
Herein, we use a cancer gene panel to demonstrate an approach for MMR-D and MSI-H detection by targeted next-generation sequencing, and we correlate the sequencing results with DNA mismatch repair protein IHC and PCR-based microsatellite instability testing in a series of colorectal carcinomas.
Materials and Methods
Patient Selection and Training and Validation Cohorts
Patients were prospectively enrolled via Profile, an institutional cohort study for cancer genotyping.5 All participants provided written informed consent. A total of 243 colorectal adenocarcinomas were tested by targeted next-generation sequencing in the study. Of these, 149 were assigned to the training cohort, and 94 were assigned to the validation cohort based on period of enrollment and hybrid capture method (Library Preparation and Next-Generation Sequencing).
All cases were evaluated for mismatch repair protein expression by IHC. Sixty-two of 149 specimens in the training cohort and 33 of 94 specimens in the validation cohort were also tested by PCR of microsatellite loci. PCR testing was performed clinically as requested by the treating physician. In addition, all cases with abnormal IHC results, except one in the training cohort without available material, were tested by PCR for the purposes of this study. Germline testing results were obtained by review of the medical record. This study was approved by the institutional review board of the Dana Farber Cancer Institute and the Partners Human Research Committee.
Library Preparation and Next-Generation Sequencing
DNA was isolated from formalin-fixed, paraffin-embedded tumor tissue. Paired normal samples were not tested. Corresponding hematoxylin and eosin–stained slides were reviewed and selected for areas with at least 20% tumor nuclei. Tumor-enriched areas were macrodissected from ten 4-μm sections. DNA was isolated using standard extraction methods (Qiagen, Valencia, CA) and quantified using PicoGreen-based dsDNA detection (Life Technologies, Carlsbad, CA). Indexed sequencing libraries were prepared from 50-ng sonically sheared DNA samples using Illumina TruSeq LT reagents (Illumina Inc, San Diego, CA).
Using a custom RNA bait set created by Agilent SureSelect (Agilent Technologies, Santa Clara, CA), libraries were enriched for exons of genes implicated in cancer biology using solution-based hybrid capture. The RNA bait set for the training cohort was from an earlier enrollment period and included a basic panel that covered the coding region of 275 genes, which encompassed 757,787 bp of the genome. The RNA bait set for the validation cohort included an extended panel of 298 genes covering 831,033 bp. Both panels included coverage of DNA mismatch repair genes implicated in Lynch syndrome, including MLH1, PMS2, MSH2, and MSH6, as well as genes frequently mutated in colorectal cancer, including KRAS, BRAF, PIK3CA, APC, and TP53 (complete gene list available in Supplemental Tables S1 and S2). EPCAM was not included in either panel. Massively parallel sequencing was performed using an Illumina HiSeq2500 (Illumina) in fast mode with 100 × 100 paired-end reads.
Pooled sample reads were deconvoluted (demultiplexed) and sorted using Picard version 1.92 and later (Broad Institute, Cambridge, MA). Reads were aligned to the reference sequence b37 edition from the Human Genome Reference Consortium using BWA version 0.5.9 (Broad Institute). Duplicate reads were identified and removed using Picard. The median mean target coverage per sample after removal of duplicate reads was 169×. The alignments were further refined using the Genome Analysis Toolkit version 1.6 and later (Broad Institute) for localized realignment around indel sites. Recalibration of the quality scores was performed using the Genome Analysis Toolkit. Mutation analysis for single-nucleotide variants was performed using MuTect version 1 0.27200 (Broad Institute). Insertions and deletions were called using Indelocator (Broad Institute). Integrative Genomics Viewer version 2.0.16 or later (Broad Institute) was used for visualization and interpretation. Variants were filtered to exclude synonymous variants, known germline variants in the Single Nucleotide Polymorphism database, and variants that occur at a population frequency of >0.1% in the Exome Sequencing Project database. Copy number detection was performed by analysis of fractional coverage of a defined genomic interval compared with pooled normal samples. Structural variant analysis was performed using BreaKmer to detect larger insertions and deletions.6
DNA Mismatch Repair IHC
IHC was conducted after pressure cooker heat–induced epitope retrieval (0.01 mol/L citrate buffer, pH 6.0) on 4-μm thick formalin-fixed, paraffin-embedded tissue sections using mouse anti-MLH1 monoclonal antibody (1:100 dilution; clone ES05; Novocastra, Buffalo Grove, IL), mouse anti-MSH2 monoclonal antibody (1:150 dilution; clone FE11; Calbiochem, San Diego, CA), mouse anti-PMS2 monoclonal antibody (1:50 dilution; clone MRQ-28; Cell Marque, Rocklin, CA), and mouse anti-MSH6 monoclonal antibody (1:50 dilution; clone PU29; Novocastra, Buffalo Grove, IL) using the Envision Plus Detection System (Dako, Carpinteria, CA).
Microsatellite Loci PCR
Microsatellite instability was evaluated by amplification across five different microsatellite loci [four mononucleotide repeats (BAT25, BAT26, BAT40, BAT34c) and one dinucleotide repeat (D18S55)] with fluorescently labeled primers in paired tumor-normal samples. PCR products were analyzed by capillary gel electrophoresis (3130xl Genetic Analyzer, Applied Biosystems, Foster City, CA). Microsatellite instability was defined by alteration in the length of the PCR product in the tumor sample compared with normal. Samples with instability in greater than or equal to two of five loci are classified as MSI-H. Samples with instability in one of five loci are classified as low microsatellite instability. Samples without instability in any of the five loci are classified as microsatellite stable (MSS).
POLE Sanger Sequencing
POLE was not part of the targeted next-generation sequencing panel, and the presence of POLE mutations in exons 9 and 13 was evaluated by Sanger sequencing in select cases. PCR amplification and sequencing primers were designed as follows: POLE exon 9 (forward: 5′-TGCTTATTTTGTCCCCACAG-3′, reverse: 5′-TACTTCCCAGAAGCCACCTG-3′) and POLE exon 13 (forward: 5′-TCTGTTCTCATTCTCCTTCC-3′, reverse: 5′-CGGGATGTGGCTTACGTG-3′). Bidirectional cycle sequencing was performed with the BigDye Terminator version 3.1 Cycle Sequencing Kit (Thermo Fisher, Cambridge, MA). Sequencing products were analyzed by capillary gel electrophoresis (3130xl Genetic Analyzer, Applied Biosystems), and results were visualized with Mutation Surveyor version 3.20 (Softgenetics, State College, PA) and Chromas Lite version 2.1.1 (Technelysium, South Brisbane, Australia).
Statistical Analysis
The Fisher exact test was used to compare differences in categorical variables. The Mann-Whitney test was used to compare distributions of continuous variables. All tests were two-sided with statistical significance set at P < 0.05.
Results
Molecular Phenotype of MMR-D in the Training Cohort
Of 149 carcinomas in the training cohort, 13 (8.7%) had loss of nuclear expression of at least one mismatch repair protein and were classified as MMR-D. The remaining 136 carcinomas (91.3%) had intact expression of all four mismatch repair proteins and were classified as mismatch repair protein intact (MMR-I).
Two variables from next-generation sequencing data were selected for analysis: the total mutational burden (substitutions and small insertion and deletion mutations) and the burden of single-nucleotide insertion or deletion mutations (indels) in mononucleotide repeat regions. A repeat region was defined in this study as two or more consecutive nucleotides. These variables were selected to reflect the expected molecular phenotype as a result of DNA repair defects associated with microsatellite instability.
The median total mutational burden in MMR-D carcinomas was 62.0 per megabase (Mb) (range, 13.2 to 277.1 per Mb) compared with 15.8 per Mb (range, 5.3 to 39.6 per Mb) in MMR-I carcinomas (Mann-Whitney P < 0.0001).
The median mutational burden of single-nucleotide indels in repeat regions in MMR-D carcinomas was 11.9 per Mb (range, 0 to 21.1 per Mb) compared with 0 per Mb (mean, 0.5 per Mb; range, 0 to 4.0 per Mb) in MMR-I carcinomas (Mann-Whitney P < 0.0001).
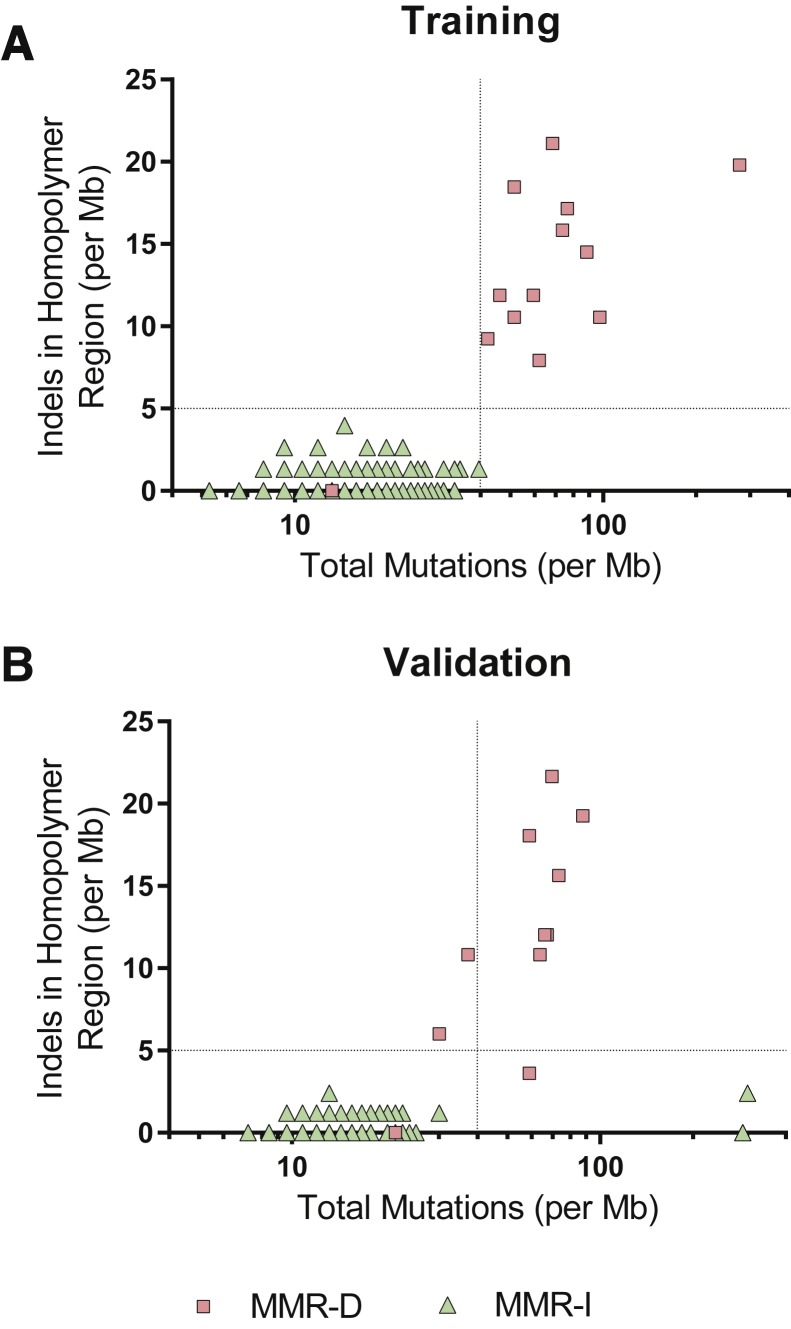
On the basis of these observations, a criterion of total mutations >40 per Mb or indels in repeat regions >5 per Mb was selected for the detection of MMR-D. This criterion achieved 92% sensitivity (n = 12/13) and 100% specificity (n = 136/136) in the training cohort (Figure 1A).
Figure 1.
Mismatch repair protein deficiency status compared with mutational burden and single–base pair insertion and deletion mutations in mononucleotide repeat (homopolymer) regions detected by next-generation sequencing in the training (A) and validation (B) data sets. MMR-D, mismatch repair protein deficient; MMR-I, mismatch repair protein intact.
In the single false-negative sample, the patient had two primary colorectal carcinomas and liver metastasis. One of the two primary colorectal carcinomas had loss of MSH2 and MSH6 staining, whereas the other primary carcinoma had intact IHC staining for all four mismatch repair proteins. IHC was not available for the liver metastasis. Sequencing was performed on the metastatic carcinoma in the liver, which revealed a KRAS mutation, a TP53 mutation, two APC frameshift mutations, and copy number changes consistent with carcinogenesis via the chromosomal instability pathway. Microsatellite PCR performed on the liver sample was MSS, consistent with next-generation sequencing results.
Application in the Validation Cohort
Of 94 carcinomas in the validation cohort, 11 (11.7%) were MMR-D, and 83 (88.3%) were MMR-I. Application of the training cohort criterion achieved 91% sensitivity (n = 10/11) and 98% specificity (n = 81/83) in the validation cohort (Figure 1B).
In the single false-negative sample in validation, IHC revealed heterogeneous loss of MSH2 and MSH6 within the carcinoma. Next-generation sequencing results were concordant with MSS status as determined by microsatellite PCR testing. Sequencing identified a TP53 mutation, two APC nonsense mutations, and copy number changes consistent with carcinogenesis via the chromosomal instability pathway. The results were explained by tumor heterogeneity and limited sampling for molecular analysis.
The only two false-positive carcinomas with retained MMR protein expression were determined to be POLE-associated ultramutated colorectal carcinomas (Phenotype of POLE-Associated Ultramutated Colorectal Carcinoma).
We confirmed that using both total mutational rate and the rate of insertion and deletion mutations resulted in better sensitivity than using either variable alone. Using only >40 total mutations per Mb, the assay achieved 73% sensitivity (n = 8/11) and 98% specificity (n = 81/83) in the validation cohort. Using only >5 single-nucleotide indels in repeat regions per Mb, the assay achieved 82% sensitivity (n = 9/11) and 100% specificity (n = 83/83). Other than the carcinoma with heterogenous IHC staining, the only MMR-D carcinoma not detected using single-nucleotide indels in repeat regions had isolated MSH6 deficiency.
Concordance with Microsatellite PCR
Using the criterion established for detecting MMR-D, namely, total mutation burden >40 per Mb or number of indels in repeat regions >5 per Mb, next-generation sequencing achieved 100% sensitivity (n = 11/11) and 100% specificity (n = 51/51) for MSI-H in the training cohort and 100% sensitivity (n = 11/11) and 95% specificity (n = 21/22) for MSI-H in the validation cohort. Sensitivity and specificity for next-generation sequencing–based detection of MSI-H in the combined training and validation cohorts are 100% (n = 22/22) and 99% (n = 72/73), respectively, with the only discordant result attributable to a POLE-mutated colorectal carcinoma.
Phenotype of POLE-Associated Ultramutated Colorectal Carcinoma
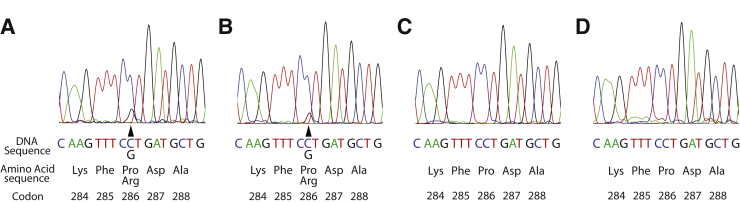
Two carcinomas in the validation cohort were predicted by next-generation sequencing to have MMR-D but had retained mismatch repair protein expression by IHC. One carcinoma was MSS by PCR and the other was MSI-H, with instability observed in two of five microsatellite loci. Both carcinomas had a very high mutation burden (300.8 and 290.0 per Mb) and a low burden of indels in mononucleotide repeat regions (2.4 and 0 per Mb). Sanger sequencing of POLE exons 9 and 13 identified a POLE c.857C>G (p.Pro286Arg) mutation in each sample. As a control, POLE Sanger sequencing was performed for the two MSI-H carcinomas from this study with the highest mutational burden. Both MSI-H carcinomas tested negative for POLE exon 9 or exon 13 mutations (Figure 2).
Figure 2.
A and B:POLE c.857C>G (p.Pro286Arg) mutations were identified in two colorectal carcinomas with high mutational burdens but without increased indels in homopolymer regions (arrowheads indicate affected nucleotide). C and D: The two high microsatellite instability carcinomas with the highest mutational burden were wild type for POLE mutations.
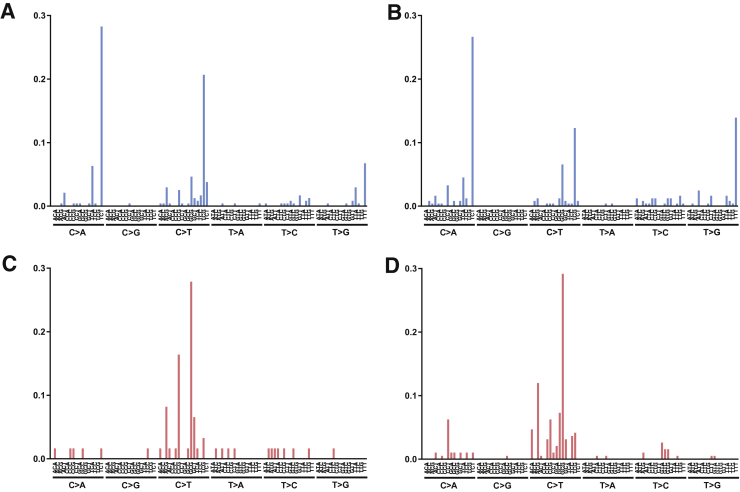
Furthermore, POLE-associated ultramutated colorectal carcinomas had distinctive mutational signatures compared with colorectal carcinomas with MSI-H. Both cases were highly enriched for cytosine to adenine transitions at TpCpT sites. In contrast, carcinomas with MSI-H had a predominance of cytosine to thymine transversions at CpG sites (Figure 3). These findings were consistent with previously described POLE-associated and MSI-H–associated mutational signatures.7
Figure 3.
Mutational signatures of POLE-associated ultramutated colorectal carcinomas (A and B) compared with colorectal carcinomas with high microsatellite instability (C and D).
Gene Mutations in MMR-D Colorectal Carcinomas
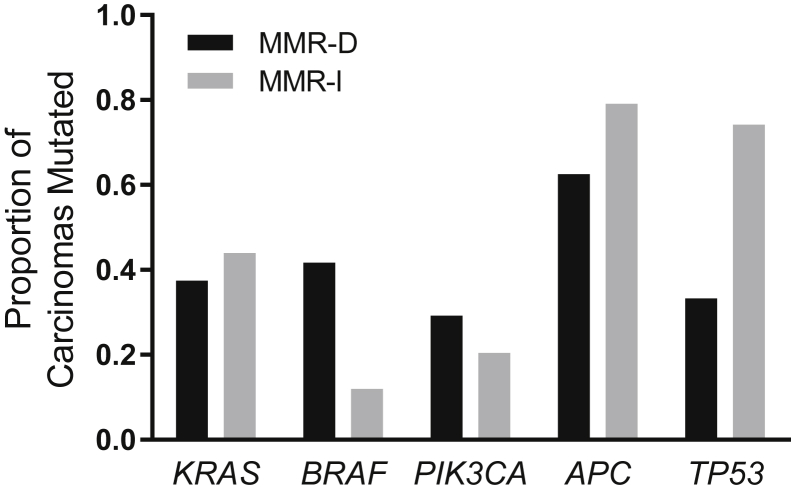
We also evaluated differences in mutational frequencies of specific genes between MMR-D and MMR-I colorectal carcinomas. This analysis was limited to frequently mutated oncogenes and tumor suppressor genes in colorectal cancer: KRAS, BRAF, PIK3CA, APC and TP53 (Figure 4). MMR-D carcinomas were associated with a higher frequency of BRAF mutations (42% versus 12% in MMR-I carcinomas, P = 0.0007) and a lower frequency of TP53 mutations (33% versus 74% in MMR-I tumors, P < 0.0001).
Figure 4.
Commonly mutated colorectal cancer genes in mismatch repair protein deficient (MMR-D) and mismatch repair protein intact (MMR-I) carcinomas. MMR-D carcinomas had a higher frequency of BRAF mutations (P = 0.0007) and lower frequency of TP53 mutations (P < 0.0001).
Detection of Pathogenic Mutations in Patients with Lynch Syndrome
Of the 243 patients in the combined training and validation cohort, 10 had clinical germline testing for Lynch syndrome–associated genes based on abnormal mismatch repair protein IHC or microsatellite PCR results. Seven patients had pathogenic variants in MLH1, PMS2, MSH2 or MSH6, including sequence alterations and copy number changes, identified on germline testing that confirmed a diagnosis of Lynch syndrome. All seven variants were also detected in the next-generation sequencing panel of tumor tissue (Table 1).
Table 1.
Mismatch Repair Gene Mutations Detected by Tumor and Germline Sequencing in Lynch Syndrome–Associated Colorectal Carcinomas
| Mismatch repair IHC | MSI PCR | Total mutations per Mb | Single–base pair indels in repeat regions, per Mb | Tumor sequencing | Allele fraction, % | Germline sequencing |
|---|---|---|---|---|---|---|
| MSH2 and MSH6 loss | MSI-H | 97.7 | 10.6 | MSH2 c.1861C>T (p.R621*), MSH2 c.1165C>T (p.R389*), MSH6 c.845dupT (p.V282fs), MSH6 c.3103C>T (p.R1035*) | 16 26 39 24 |
MSH6 c.845dupT (p.V282fs) |
| PMS2 loss | MSI-H | 88.4 | 14.5 | PMS2 c.137G>T (p.S46I) | 60 | PMS2 c.137G>T (p.S46I) |
| MSH2 and MSH6 loss | MSI-H | 73.4 | 15.4 | MSH2 c.1684G>T (p.E562*) | 71 | MSH2 c.1684G>T (p.E562*) |
| MSH2 and MSH6 loss | MSI-H | 66.2 | 12.0 | MSH2 c.2131C>T (p.R711*), MSH2 c.1251dupT (p.I418fs) | 45 20 |
MSH2 c.2131C>T (p.R711*) |
| PMS2 loss | MSI-H | 59.4 | 11.8 | PMS2 exon 1–5 deletion | NA | PMS2 exon 1–5 deletion |
| MSH6 loss | MSI-H | 59.0 | 3.6 | MSH6 c.3939_3957dup19 (p.Arg1318fs), PMS2 c.2347G>A (p.V783I) | NA | MSH6 c.3939_3957dup19 (p.Arg1318fs) |
| MLH1 and PMS2 loss | MSI-H | 37.3 | 10.8 | MLH1 exon 13 deletion | NA | MLH1 exon 13 deletion |
IHC, immunohistochemistry; MSI, microsatellite instability; MSI-H, high microsatellite instability; NA, not applicable.
Discussion
Lynch syndrome confers an increased lifetime risk of cancer development in affected individuals and is implicated in the pathogenesis of approximately 3% of colorectal cancers. A diagnosis of Lynch syndrome has clinical implications for the patient and family members, and many laboratories have instituted universal screening for Lynch syndrome in newly diagnosed colorectal carcinomas using immunohistochemical staining of DNA mismatch repair genes or microsatellite instability PCR or both.8 Abnormal results may be evaluated by additional testing with MLH1 promoter methylation analysis or BRAF gene sequencing.9 This algorithmic approach requires a complex clinical decision tree and may require a single sample to be tested by multiple assays. We hypothesize that much of the testing performed for Lynch syndrome screening may be streamlined with a single next-generation sequencing assay.
Prior work using next-generation sequencing to predict microsatellite status has focused on targeted sequencing of known microsatellite loci10 or analysis of microsatellite regions using new informatics tools.11, 12, 13 We find that the mutational phenotype from a standard informatics pipeline restricted to coding regions of genes is also able to detect microsatellite instability with high sensitivity and specificity.
In the validation cohort, the only discordant results compared with microsatellite PCR testing are found to be due to carcinomas harboring POLE p.Pro286Arg mutations. POLE p.Pro286Arg is an exon 9 hotspot mutation in the exonuclease domain of POLE and has been associated with an elevated rate of mutation in vitro14 and an ultramutated phenotype in human cancer.15 In our analysis, the POLE-associated ultramutated phenotype can be resolved by POLE gene sequencing and mutational signature analysis.
Our results are comparable to an analysis by Stadler et al,16 who observed that mutational burden alone is highly sensitive and specific for MMR-D in a similar targeted next-generation sequencing panel. Compared with their assay, we perform sequencing on tumor-only samples without paired normal tissue and rely on the informatics pipeline to filter common germline events. As a result, the assay by Stadler et al16 is expected to be more specific for the detection of true somatic events.
We find that tumor-only sequencing is equally effective in detecting MMR-D with the additional benefits of decreasing sequencing cost, simplifying the laboratory workflow and making testing available to patients in situations in which paired normal DNA may be difficult to obtain. Furthermore, we identify recurrent insertion and deletion mutations in repeat regions as a key phenotypic feature of MMR-D, which may be more specific for MSI-H compared with other mechanisms of hypermutation, such as POLE mutation. We also observe a low rate of discrepancy between MMR-D and MSI-H attributable to tumor heterogeneity, and we validate next-generation sequencing against microsatellite PCR, the current molecular gold standard. We describe the resolution of MSI-H from POLE-associated ultramutated carcinomas through analysis of mutational signatures. Finally, we find that our sequencing panel detects pathogenic mutations in DNA mismatch repair genes, which can guide downstream germline testing.
Notably, our approach for MMR-D and MSI-H screening of using mutational patterns rather than individual gene mutations may be applied to sequencing panels with different gene combinations as long as the panels are of sufficient scale to estimate mutational burden with statistical power. In many cases, laboratories already using next-generation sequencing for cancer may be able to evaluate mismatch repair pathway status using existing data at minimal additional cost.
In addition to providing an accurate measure of mismatch repair pathway status, next-generation sequencing allows for quantification of the degree of mismatch repair deficiency or the overall mutational burden. Increased neoantigen load has been strongly linked to clinical response to immune checkpoint inhibitors in multiple cancer types, including MSI-H colorectal carcinomas,17 and MSI-H colorectal carcinomas harbor immune microenvironments favorable to checkpoint blockade.18 Beyond evaluation of the mismatch repair pathway, sequencing identifies clinically actionable gene mutations. Pathway-activating mutations in KRAS and BRAF predict lack of response to anti-EGFR antibody therapy in patients with advanced colorectal cancer.19, 20 Given the increasing availability of pathway-specific targeted therapies, next-generation sequencing may guide enrollment into clinical trials. As these advances broaden the indication for the sequencing of cancer genomes, it is rational to use data derived from a single test to fulfill multiple clinical roles, including the evaluation of hereditary cancer risk.
In summary, the mutational phenotype of colorectal carcinomas by targeted next-generation sequencing, as defined by the burden of total mutations and single-nucleotide indels in repeat regions, can accurately detect MMR-D and MSI-H compared with current methods. Next-generation sequencing may be a useful tool for colorectal carcinomas by providing a means of generating molecular results to guide therapeutic decisions while simultaneously enabling risk assessment for Lynch syndrome.
Footnotes
Supported by the Department of Pathology, Brigham and Women's Hospital, Dana Farber Cancer Institute, and NIH grant R35 CA197735 (S.O.).
Disclosures: None declared.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.jmoldx.2016.07.010.
Supplemental Data
References
- 1.Lynch H.T., Snyder C.L., Shaw T.G., Heinen C.D., Hitchins M.P. Milestones of Lynch syndrome: 1895–2015. Nat Rev Cancer. 2015;15:181–194. doi: 10.1038/nrc3878. [DOI] [PubMed] [Google Scholar]
- 2.Ribic C.M., Sargent D.J., Moore M.J., Thibodeau S.N., French A.J., Goldberg R.M., Hamilton S.R., Laurent-Puig P., Gryfe R., Shepherd L.E., Tu D., Redston M., Gallinger S. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lochhead P., Kuchiba A., Imamura Y., Liao X., Yamauchi M., Nishihara R., Qian Z.R., Morikawa T., Shen J., Meyerhardt J.A., Fuchs C.S., Ogino S. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J Natl Cancer Inst. 2013;105:1151–1156. doi: 10.1093/jnci/djt173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giardiello F.M., Allen J.I., Axilbund J.E., Boland C.R., Burke C.A., Burt R.W., Church J.M., Dominitz J.A., Johnson D.A., Kaltenbach T., Levin T.R., Lieberman D.A., Robertson D.J., Syngal S., Rex D.K. Guidelines on genetic evaluation and management of lynch syndrome: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2014;147:502–526. doi: 10.1053/j.gastro.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 5.MacConaill L.E., Garcia E., Shivdasani P., Ducar M., Adusumilli R., Breneiser M., Byrne M., Chung L., Conneely J., Crosby L., Garraway L., Gong X., Hahn W., Hatton C., Kantoff P., Kluk M., Kuo F., Jia Y., Joshi R., Longtine J., Manning A., Palescandolo E., Sharaf N., Sholl L., van Hummelen P., Wade J., Wollinson B.M., Zepf D., Rollins B.J., Lindeman N.I. Prospective enterprise-level molecular genotyping of a cohort of cancer patients. J Mol Diagn. 2014;16:660–672. doi: 10.1016/j.jmoldx.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abo R.P., Ducar M., Garcia E.P., Thorner A.R., Rojas-Rudilla V., Lin L., Sholl L.M., Hahn W.C., Meyerson M., Lindeman N.I., Van Hummelen P., MacConnaill L.E. BreaKmer: detection of structural variation in targeted massively parallel sequencing data using Kmers. Nucleic Acids Res. 2014;43:e19. doi: 10.1093/nar/gku1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexandrov L.B., Nik-Zainal S., Wedge D.C., Aparicio S.A., Behjati S., Biankin A.V. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hampel H., Frankel W.L., Martin E., Arnold M., Khanduja K., Kuebler P., Nakagawa H., Sotamaa K., Prior T.W., Westman J., Panescu J., Fix D., Lockman J., Comeras I., de la Chapelle A. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352:1851–1860. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 9.Weisenberger D.J., Siegmund K.D., Campan M., Young J., Long T.I., Faasse M.A., Kang G.H., Widschwendter M., Weener D., Buchanan D., Koh H., Simms L., Barker M., Leggett B., Levine J., Kim M., French A.J., Thibodeau S.N., Jass J., Haile R., Laird P.W. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787–793. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 10.Gan C., Love C., Beshay V., Macrae F., Fox S., Waring P., Taylor G. Applicability of next generation sequencing technology in microsatellite instability testing. Genes (Basel) 2015;6:46–59. doi: 10.3390/genes6010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niu B., Ye K., Zhang Q., Lu C., Xie M., McLellan M.D., Wendl M.C., Ding L. MSIsensor: microsatellite instability detection using paired tumor-normal sequence data. Bioinformatics. 2014;30:1015–1016. doi: 10.1093/bioinformatics/btt755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salipante S.J., Scroggins S.M., Hampel H.L., Turner E.H., Pritchard C.C. Microsatellite instability detection by next generation sequencing. Clin Chem. 2014;60:1192–1199. doi: 10.1373/clinchem.2014.223677. [DOI] [PubMed] [Google Scholar]
- 13.Huang M.N., McPherson J.R., Cutcutache I., Teh B.T., Tan P., Rozen S.G. MSIseq: software for assessing microsatellite instability from catalogs of somatic mutations. Sci Rep. 2015;5:13321. doi: 10.1038/srep13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kane D.P., Shcherbakova P.V. A common cancer-associated DNA polymerase E mutation causes an exceptionally strong mutator phenotype, indicating fidelity defects distinct from loss of proofreading. Cancer Res. 2014;74:1895–1901. doi: 10.1158/0008-5472.CAN-13-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Church D.N., Briggs S.E.W., Palles C., Domingo E., Kearsey S.J., Grimes J.M., Gorman M., Martin L., Howarth K.M., Hodgson S.V., NSECG Collaborators. Kaur K., Taylor J., Tomlinson I.P. DNA polymerase E and Δ exonuclease domain mutations in endometrial cancer. Hum Mol Genet. 2013;22:2820–2828. doi: 10.1093/hmg/ddt131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stadler Z.K., Battaglin F., Middha S., Hechtman J.F., Tran C., Cercek A., Yaeger R., Segal N.H., Varghese A.M., Reidy-Lagunes D.L., Kemeny N.E., Salo-Mullen E.E., Ashraf A., Weiser M.R., Garcia-Aguilar J., Robson M.E., Offit K., Arcila M.E., Berger M.F., Shia J., Solit D.B., Saltz L.B. Reliable detection of mismatch repair deficiency in colorectal cancers using mutational load in next-generation sequencing panels. J Clin Oncol. 2016;34:2141–2147. doi: 10.1200/JCO.2015.65.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le D.T., Uram J.N., Wang H., Bartlett B.R., Kemberling H., Eyring A.D., Skora A.D., Luber B.S., Azad N.S., Laheru D., Biedrzycki B., Donehower R.C., Zaheer A., Fisher G.A., Crocenzi T.S., Lee J.J., Duffy S.M., Goldberg R.M., de la Chapelle A., Koshiji M., Bhaijee F., Huebner T., Hruban R.H., Wood L.D., Cuka N., Pardoll D.M., Papadopoulos N., Kinzler K.W., Zhou S., Cornish T.C., Taube J.M., Anders R.A., Eshleman J.R., Vogelstein B., Diaz L.A. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Llosa N.J., Cruise M., Tam A., Wicks E.C., Hechenbleikner E.M., Taube J.M., Blosser R.L., Fan H., Wang H., Luber B.S., Zhang M., Papadopoulos N., Kinzler K.W., Vogelstein B., Sears C.L., Anders R.A., Pardoll D.M., Housseau F. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43–51. doi: 10.1158/2159-8290.CD-14-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lièvre A., Bachet J.B., Le Corre D., Boige V., Landi B., Emile J.F., Côté J.F., Tomasic G., Penna C., Ducreux M., Rougier P., Penault-Llorca F., Laurent-Puig P. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 20.Di Nicolantonio F., Martini M., Molinari F., Sartore-Bianchi A., Arena S., Saletti P., De Dosso S., Mazzucchelli L., Frattini M., Siena S., Bardelli A. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705–5712. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.