Abstract
Vascular endothelial growth factor (VEGF)-D is capable of inducing angiogenesis and lymphangiogenesis through signaling via VEGF receptor (VEGFR)-2 and VEGFR-3, respectively. Mutations in the FIGF (c-fos–induced growth factor) gene encoding VEGF-D have not been reported previously. We describe a young male with a hemizygous mutation in the X-chromosome gene FIGF (c.352 G>A) associated with early childhood respiratory deficiency. Histologically, lungs showed ectatic pulmonary arteries and pulmonary veins. The mutation resulted in a substitution of valine to methionine at residue 118 of the VEGF-D protein. The resultant mutant protein had increased dimerization, induced elevated VEGFR-2 signaling, and caused aberrant angiogenesis in vivo. Our observations characterize a new subtype of congenital diffuse lung disease, provide a histological correlate, and support a critical role for VEGF-D in lung vascular development and homeostasis.
The most widely used clinicopathologic classification of interstitial and diffuse lung disease prevalent in infancy, the ChILD classification, has provided a framework for naming and understanding pathobiologic processes specifically relevant to pediatric patients.1 Practical limitations in applying the scheme arise when individual cases show disease elements from more than one of the established categories, which are diffuse developmental disorders, lung growth abnormalities, specific conditions of unknown or poorly understood etiology, and surfactant dysfunction disorders.1 Diseases from each of the categories can, in fact, be of unknown etiology, presumably driven by yet undiscovered genetic factors. Genetic discovery can help in characterizing distinct subgroups of disease more precisely and may provide insight into underlying disease biology.
Vascular endothelial growth factors (VEGFs) are signaling proteins that promote angiogenesis and lymphangiogenesis. They play important roles in normal development, carcinogenesis, and nonneoplastic diseases.2, 3, 4 VEGF-D, encoded by the c-fos–induced growth factor (FIGF) gene on chromosome X, is produced as a full-length protein consisting of VEGF homology domain and C- and N- terminal propeptides. C- and N-terminal cleavage generates VEGF homology domain, a mature and highly active form of VEGF-D.5, 6 VEGF-D binds to VEGF receptor (VEGFR)-2 and VEGFR-3 to exert angiogenic and lymphangiogenic activities, respectively. Receptor binding is dependent on the protein isomer and the dimerization level, with increased dimerization leading to augmented VEGFR-2 phosphorylation and activity.7, 8, 9 Furthermore, VEGF-D has been shown to modulate vessel development through sex determining region Y–box (SOX) 18.7 Pathogenic mutations in FIGF have not yet been reported.
Herein, we describe a boy with chronic tachypnea, hypoxemia, intrapulmonary vasculopathy, and a hemizygous FIGF mutation. In vitro and in vivo studies confirmed that the resultant mutant VEGF-D possessed a markedly enhanced ability to activate VEGFR-2 and led to aberrant vessel formation.
Materials and Methods
Oversight
This study was approved by Boston Children's Hospital's (Boston, MA) institutional review board.
Crystal Structure Analysis
Effects of V118M on VEGF-D were analyzed using the crystal structure of VEGF-D (Protein Data Bank ID code 2XV7) as a template.
Histology
Clinical material from biopsy specimens was fixed in formalin, processed routinely, and embedded in paraffin. Immunohistochemical staining was conducted to determine the pattern of CD31 (Ab28364; Abcam, Cambridge, MA), phospho-VEGFR-2 (CC1027; Cell Applications, San Diego, CA), D2-40 (CM266A; BioCare Medical, Concord, CA), and VEGF-D (MAB286; R&D Systems, Minneapolis, MN), as described previously.10
Isolation of Mononuclear Cells from Peripheral Blood
Peripheral blood mononuclear cells were isolated and stained with antibodies against VEGF-D, as described previously.2
Cell Culture
Human umbilical vein endothelial cells, lung lymphatic endothelial cells (Lonza, Walkersville, MD), aortic endothelial cells (Life Technologies, Carlsbad, CA), and embryonic kidney 293 cells (ATCC, Manassas, VA) were cultured, as described previously.11
Stable Transfection
Human embryonic kidney 293 cells were transfected with human nonmutant or mutated VEGF-D plasmid (OriGene Technologies, Rockville, MD) using Lipofectamine 3000 Reagent (Life Technologies), according to the manufacturer's recommendations. Cells were harvested, and culture supernatants were concentrated using Amicon Ultra centrifugal filter units (Millipore, Billerica, MA).
RNA Interference
The sequences for human KDR/VEGFR-2 siRNA (http://ncbi.nlm.nih.gov/nuccore; accession number NM_002253) were designed as previously reported12: sense, 5′-GGAUAUCACUCCGAUGACA[dT][dT]-3′; anti-sense, 5′-UGUCAUCGGAGUGAUAUCC[dT][dT]-3′. Human KDR/VEGFR-2 siRNA and MISSION siRNA Universal Negative Control were synthesized by Sigma-Aldrich (St. Louis, MO). Human aortic endothelial cells were transfected as described previously.11
Western Blotting
Western blotting was conducted as described previously.2 We used antibodies against VEGF-D (MAB2861; R&D Systems), phospho-VEGFR-2 (CC1027; Cell Applications), phospho-VEGFR-3 (CY1115; Cell Applications), VEGFR-2 (9698; Cell Signaling Technology, Danvers, MA), and VEGFR-3 (CB5792; Cell Applications).
Enzyme-Linked Immunosorbent Assay
The levels of VEGF-D in the concentrated culture supernatants were measured as described previously.11
Cell Proliferation Assay
Human aortic endothelial cells and lung lymphatic endothelial cells were treated with concentrated supernatants (final concentration for both nonmutant and VEGF-DV118M, 1 μg/mL). Cell proliferation assay was conducted as described previously.11
Chick Chorioallantoic Membrane Assay
The chick chorioallantoic membrane assay was conducted as previously described.13 Concentrated supernatant containing 2.5 μg of either nonmutant or VEGF-DV118M was loaded onto cortisone acetate-treated filter disks, which were then placed on the avascular regions of the chick chorioallantoic membrane on day 10. Images of the chick chorioallantoic membrane were analyzed by Angiotool software version 0.5 (National Cancer Institute, NIH, Bethesda, MD), as reported.14
Genetic Testing
Whole-exome and mitochondrial genome sequencing was performed on subjects' genomic DNA via next-generation sequencing (XomeDX; GeneDX, Gaithersburg, MD). Mean depth of coverage was 74×. Potentially pathogenic variants were confirmed via deoxy DNA sequencing.
Statistical Analysis
Values and graphs are presented as means ± SEM. A t-test was used to compare two conditions. Multiple comparisons were evaluated by one-way analysis of variance, followed by the Tukey post hoc test. Analyses were performed using GraphPad Prism version 5.0 (GraphPad Software, Inc. La Jolla, CA). P < 0.05 was considered significant.
Results
Clinical Findings
A white male, born at term without complications, had wheezing, respiratory tract infections, otitis media, and intermittent increased work of breathing in his first year. Treatment included albuterol, fluticasone, and antibiotics. By age 20 months, worsened dyspnea prompted referral to our institution, where a walk test showed mild oxygen desaturation, tachypnea, and subcostal retractions. The results of lung auscultation, radiographs, computed tomographic scan, and echocardiogram were normal. Cardiac catheterization demonstrated a mildly elevated cardiac index without pulmonary hypertension or shunt. Symptoms progressed, with worsened oxygen desaturation on walk test and nighttime hypoxia requiring oxygen. Immunological evaluation showed a low Haemophilus influenzae titer, and no other abnormality.
By age 3 years, there was failure to thrive, exertional chest pain, and orthopnea. The results of a chest radiograph demonstrated bilateral hazy lung infiltrates, which improved with diuretics. Anti-angiogenic therapy (thalidomide) yielded no evident improvement. Cardiac catheterization showed normal cardiac index and pulmonary venous oxygen saturation before and during dobutamine administration. By age 4 years, hypersomnia, generalized pallor, periodic perioral cyanosis, tachycardia, and intractable vomiting emerged. Parenteral nutrition was administered for visceral hypersensitivity disorder. The result of a liver ultrasound was normal. Renal ultrasound to rule out potential diuretic-induced nephrocalcinosis showed hilar linear echogenic foci, possibly reflecting abnormal vasculature. Neurological and cognitive function, muscle tone, and strength were normal. The last follow-up, at age 6 years, showed labored breathing and severe hypoxemia with exercise.
Histological Findings
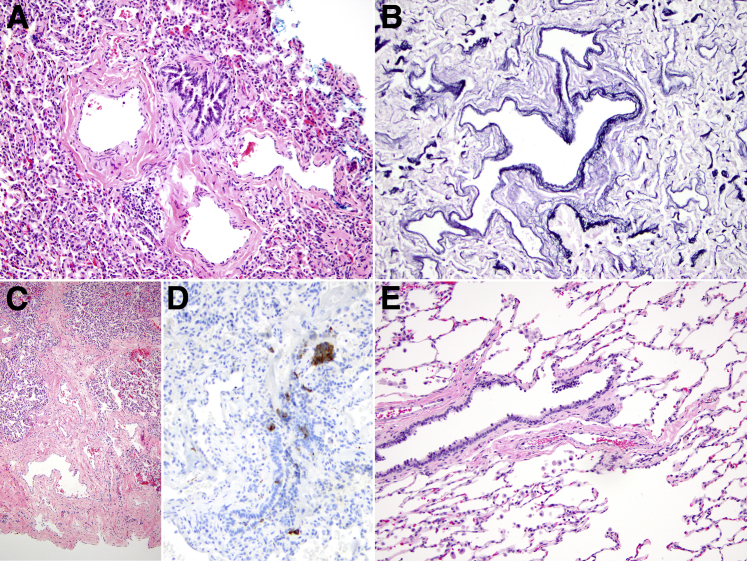
Wedge biopsy samples from both lungs, obtained 9 months apart, showed diffuse dilation and increased tortuosity of pulmonary arteries and veins compared to normal histology (Figure 1, A–C and E). Trichrome and immunostaining with anti-smooth muscle antibodies did not demonstrate muscular hyperplasia or intimal fibrosis. Arteries also showed focal internal elastic lamina fragmentation. Neither capillary dilation nor hemosiderosis was observed. Small airways showed mildly thickened muscular walls. Increased numbers of airway neuroendocrine cells occurred singly and in occasional clusters (Figure 1D). There were mildly increased intra-alveolar macrophages. Pulmonary lymphatic channels showed normal distribution and caliber. In a biopsy of the liver, portal vein radicals and central veins appeared subtly dilated and irregularly contoured, within the limits of evaluation of the single tissue core sampled; there were no appreciable abnormalities of intrahepatic lymphatic channels or sinusoids. Upper gastrointestinal tract mucosa showed no specific pathological features.
Figure 1.
Histological findings in FIGF mutation. A: Pulmonary arteries showed tortuosity and ectasia, imparting a baggy appearance (hematoxylin and eosin stain). B: Their irregular contours were highlighted by elastic tissue staining. C: Low magnification showed ectatic pulmonary veins as well as unremarkable alveolar architecture. D: Frequent airway neuroendocrine cells (highlighted brown by a synaptophysin stain) were present, occurring singly and within clusters. E: In a control patient with normal lung vasculature (pneumonectomy specimen from a 2-year-old boy with an untreated benign lung tumor), the pulmonary arteries are smaller than the corresponding bronchus; they lack tortuosity and do not undulate in and out of the plane of section. Original magnification: ×200 (A, B, and D); ×20 (C); ×100 (E).
Genetic Findings
Whole-exome sequencing identified a hemizygous FIGF variant, c.352 G>A, resulting in a substitution of valine to methionine at residue 118 of the VEGF-D protein (V118M). This variant was not observed in an analysis of approximately 6000 individuals in the National Heart, Lung, and Blood Institute Exome Sequencing Project. In the Exome Aggregation Consortium server and in 86,702 studied alleles, the variant [ExAC Browser (Beta), http://exac.broadinstitute.org/variant/X-15376265-C-T, last accessed September 16, 2016] was found 15 times in heterozygous and 4 times in hemizygote state with a frequency of 173 × 10−6 and 46 × 10−6, respectively15 [ExAC Browser (Beta), http://exac.broadinstitute.org/about, last accessed September 16, 2016]. In silico analysis was performed using SIFT version 1.03 (J. Craig Venter Institute, La Jolla, CA), PANTHER version 11.1 (University of South California, Los Angeles, CA), and SNAP2 (Technische Universität München, Munich, Germany) prediction software. The SIFT score for the substitution was 0 (scores <0.05 suggest functional importance).16 The PANTHER score was 750, indicating that valine at residue 118 has been stable for 750 million years of evolution and that the mutation is probably damaging.17 The SNAP2 prediction analysis indicated a functional mutation as well.18 Secondary findings were either heterozygous changes in autosomal recessive genes (DYSF, CD46, GPR98, RP1, ACADVL, and SLC7A9) or changes in genes that do not fit his phenotype (PMS1). No disease-causing mutations were identified in the mitochondrial genome.
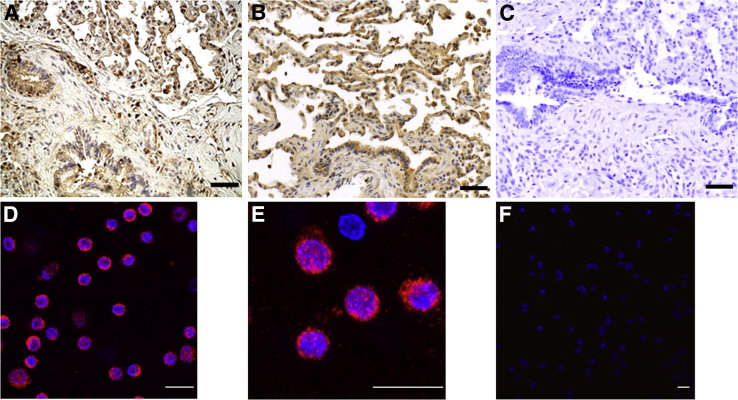
Lung tissue and peripheral blood mononuclear cells showed normal VEGF-D expression (Figure 2). VEGF-D serum level at age 4 years was normal at 162 pg/mL.
Figure 2.
VEGF-D expression in the lung and peripheral blood of the patient. A: Lung wedge biopsy tissue stained with anti–VEGF-D antibodies showed normal VEGF-D expression in alveolar macrophages and airway epithelial cells. B: Lung tissue from age-matched control showed similar VEGF-D distribution. C: Isotype control. Peripheral blood mononuclear cells were immunoreactive with anti–VEGF-D antibodies (D, low power; E, high power). F: Isotype control. n = 1 (A and C–F); n = 5 (B). Scale bars: 100 μm (A–C); 20 μm (D–F).
VEGF-D V118M Induces Aberrant Angiogenesis in Vitro and in Vivo
Naturally occurring mutations in the human FIGF gene have not been previously identified. However, it has been shown that substitution of cysteine residue at position 117 with alanine increases the biological activity of VEGF-D in vitro. Furthermore, this cysteine residue is considered a critical regulator of VEGF-D dimer stability.9, 19 The valine 118 residue is highly conserved across all members of the VEGF family of proteins, suggesting an essential role in VEGF structure and function as well.19, 20
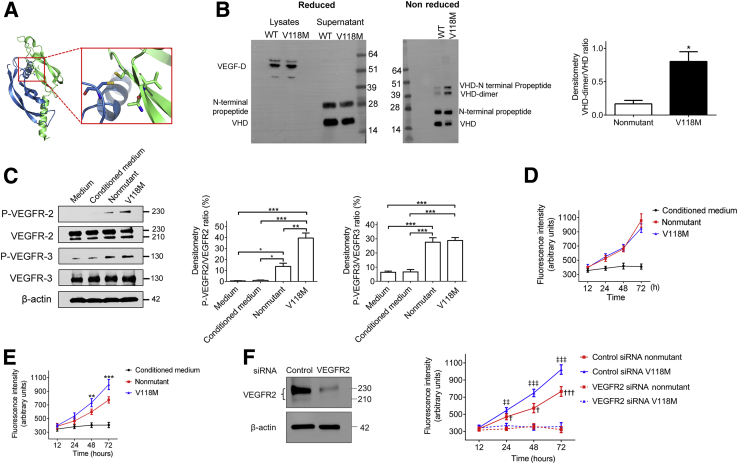
Our modeling indicated that change from a compact and rigid valine 118 to a longer and more flexible methionine would cause clashes within the VEGF-D core, likely leading to rearrangements of residues that participate in dimerization (Figure 3A). Indeed, electrophoresis of cell lysates and cell culture supernatant of stably transfected human embryonic kidney 293 cells under reducing conditions showed that VEGF-DV118M, similar to nonmutant VEGF-D, was present as a full-length form in the lysates and was processed correctly by cleavage of the C- and N-terminal ends in cell culture supernatants6 (Figure 3B). Under nonreducing conditions, however, secreted VEGF-DV118M formed significantly more dimers compared to the nonmutant protein (Figure 3B).
Figure 3.
Effects of nonmutant VEGF-D and VEGF-DV118M. A: Two molecules of the VEGF-D receptor pack in the crystal lattice of 2XV7 with an extensive dimeric interface, which has buried surface area of 2370 Å2. Inset: A close-up of the boxed area, on the dimeric interface with Val 118 in the immediate vicinity of other residues that modulate the dimeric interaction. B: Constructs expressing VEGF-DV118M or nonmutant VEGF-D were transfected into human embryonic kidney 293 (HEK293) cells. VEGF-D protein expressions were validated by Western blot. Left panel: Under reducing conditions, both nonmutant and VEGF-DV118M were present as a full-length form in the lysates of stable transfected cells. In cell culture supernatants from both nonmutant and mutated VEGF-D plasmid transfected HEK293 cells, two forms of VEGF-D were identified: VEGF-D cleaved at the C-terminal regions (upper bands) and VEGF homology domain (VHD; lower bands). Under nonreducing conditions, VEGF-DV118M in cell culture supernatants formed significantly more dimers compared to the nonmutant protein. Right panel: Quantification of the ratio of VHD dimers/VHD monomer. C: Human umbilical vein endothelial cells were incubated with concentrated conditioned medium from stable transfected HEK293 cell lines containing equal amounts of nonmutant and VEGF-DV118M (final VEGF-D concentration, 1 μg/mL). Left panel: Phosphorylation of VEGFR-2 and VEGFR-3 was detected after 30 minutes. Middle and right panels: Quantification of the ratio of p-VEGFR-2/total VEGFR-2 and p-VEGFR-3/total VEGFR-3, respectively. Cell proliferation assay of human lung lymphatic endothelial cells (D) and aortic endothelial cells (HAECs; E) treated with concentrated conditioned medium from stable transfected HEK293 cell lines containing equal amounts of nonmutant and VEGF-DV118M (final VEGF-D concentration, 1 μg/mL). Data represent results of three independent experiments. F: HAECs were transfected with control siRNA or VEGFR-2 siRNA for 48 hours and subsequently incubated with concentrated conditioned medium from stable transfected HEK293 cell lines containing equal amounts of nonmutant and VEGF-DV118M (final VEGF-D concentration, 1 μg/mL). Left panel: Efficiency of siRNA-mediated knockdown of VEGFR-2 was confirmed by Western blotting. Right panel: Cell proliferation was determined at the indicated time points after treatment with nonmutant or VEGF-DV118M. Data represent at least three independent experiments. Data are means ± SEM (D–F). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 (t-test or one-way analysis of variance, as appropriate); †P < 0.05, †††P < 0.001 (control versus VEGFR-2 siRNA transfected cells treated with nonmutant VEGF-D by one-way analysis of variance); ‡‡P < 0.01, ‡‡‡P < 0.001 (control versus VEGFR-2 siRNA transfected cells treated with VEGF-DV118M).
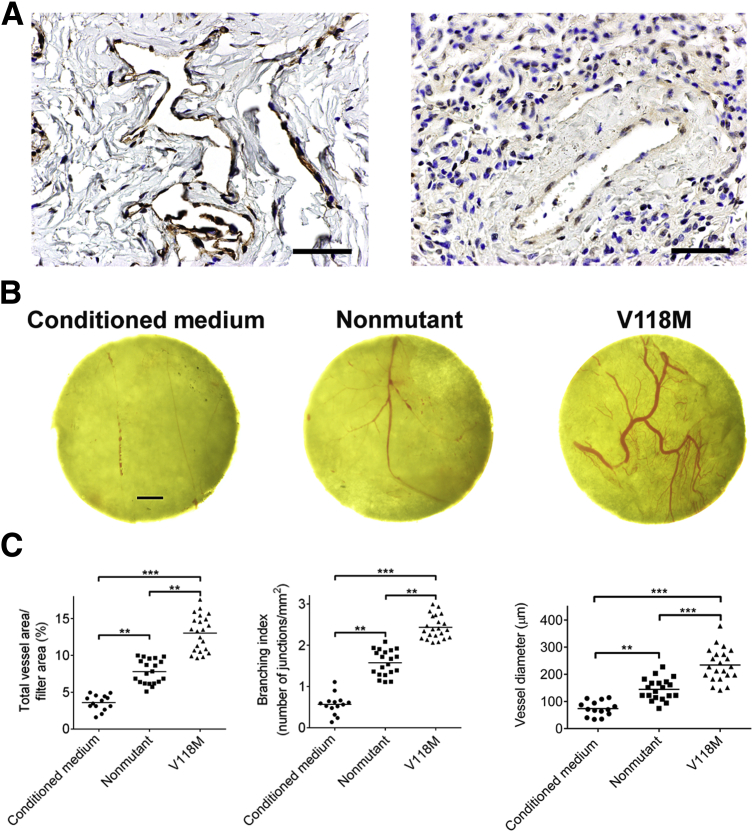
Human umbilical vein endothelial cells were incubated with concentrated conditioned medium containing equal amounts of nonmutant and VEGF-DV118M. VEGF-DV118M elicited much stronger VEGFR-2, but not VEGFR-3, phosphorylation compared to nonmutant (Figure 3C). Furthermore, VEGF-DV118M was more potent than nonmutant in stimulating proliferation of human aortic endothelial cells (expressing VEGFR-2), but not lung lymphatic endothelial cells (expressing VEGFR-3) (Figure 3, D and E). Furthermore, siRNA-mediated knockdown of VEGFR-2 in human aortic endothelial cells rendered these cells unresponsive toward either nonmutant or VEGF-DV118M stimulation (Figure 3F), indicating that both forms of VEGF-D promoted proliferation of human aortic endothelial cells through interaction with VEGFR-2. Furthermore, analysis of lung biopsy of the subject showed increased phospho-VEGFR-2 staining compared to controls (Figure 4A).
Figure 4.
In vivo angiogenic effects of nonmutant VEGF-D and VEGF-DV118M. A: Reactivity with anti–phospho-VEGFR-2 antibodies showing enhanced phospho-VEGFR-2 staining in subject (left panel) compared to control lung (right panel). Nuclei are stained with hematoxylin. B: Filter disks loaded with concentrated conditioned medium from non-transfected human embryonic kidney 293 (HEK293) cells or 2.5 μg of either nonmutant or VEGF-DV118M derived from HEK293 stable cell lines were placed on chick chorioallontoic membranes (CAMs) of 10-day-old chick embryos and collected after 48 hours. Representative photomicrographs of filter disks and the attached CAMs show increased vessel caliber and tortuosity in the presence of VEGF-DV118M. C: Quantification of the area of blood vessels (left panel), branching points (middle panel), and vessel diameter (right panel). ∗∗P < 0.01, ∗∗∗P < 0.001 (by one-way analysis of variance). Scale bars: 100 μm (A); 1 mm (B).
In a chick embryo chorioallantoic membrane assay, we observed that conditioned medium containing VEGF-DV118M induced enhanced angiogenesis, with increased sprouting, caliber, and tortuosity compared to its nonmutant counterpart (Figure 4, B and C).
Family Studies
Sequencing of FIGF in the mother and maternal grandfather revealed the same mutation. The grandfather denied childhood respiratory difficulties. His lung function testing at age 60 years revealed a mild mixed restrictive and obstructive ventilatory defect with air trapping. Mild hypoxemia at rest improved to normal range with exercise.
Discussion
To our knowledge, this is the first report of a functional pathological variant in the human FIGF gene. The clinical phenotype, histological abnormalities, and genetic findings in this case provide valuable insights into the biological function of VEGF-D and its role in human health and disease.
Our histological findings fit a novel but predicted phenotype in that the primary manifestation involved the pulmonary vasculature. The histological findings were subtle, and it remains unclear whether the lack of previous descriptions of this disease is because of a rarity of FIGF mutation, the subtlety of histological features, the possibility of less severe phenotypes, or a combination thereof. In fact, the maternal grandfather's mild phenotype could indicate such variable expressivity.
The VEGF family of proteins possesses conserved cysteine residues (137 and 146) that form interchain disulfide bridges. VEGF-D has an additional cysteine 117,19, 21 which interferes with the disulfide bridge, explaining why VEGF-D exists predominantly as a noncovalently linked homodimer.6 Mutations in cysteine 117 result in increased dimer stability and activity,19, 22 without changes in receptor binding affinity.21, 22 In the naturally occurring mutation that we identified (V118M), the change from a more compact and rigid valine to a longer and more flexible methionine might result in rearrangement of the VEGF-D core and generate an increased area of dimer interface. The exact extent of those rearrangements could be further evaluated in structural studies of the system. This explains the increased stability of the protein and its ability to induce increased VEGFR-2 phosphorylation. Not surprisingly, the lack of increase in VEGFR-3 signaling can be explained given the knowledge that the cysteine mutation at residue 117 results in different effects on the receptors, depending on protein concentration.19
Human FIGF is expressed in the lung before birth,23 and in adults in the heart, lung, skeletal muscle, and intestine11, 20; it has recently been shown to play important roles in pulmonary vascular leak in response to hyperoxia.24 The grandfather's respiratory findings may reflect a mild disease phenotype consistent with variable expressivity or incomplete penetrance, and carriers of the gene change may show minimal, if any, phenotype; we cannot rule out as yet unidentified modifying genes or other factors to explain the disparate phenotypic findings between grandfather and grandson. It is possible that low maternal levels of VEGF-DV118M may have contributed to abnormal pulmonary vascular development in utero for the patient. These changes to the pulmonary vessels could be irreversible, explaining the lack of response to thalidomide. Abnormal vasculature in other organs may account for some nonrespiratory clinical findings that were developing as he ages and perhaps because of persistent exposure to mutated VEGF-D produced either locally or by circulating immune cells. Such abnormalities were difficult to confirm in the limited extrapulmonary tissue sampled.
The new disease identified herein is predominantly a pulmonary vascular disease with dominant respiratory symptoms and characteristic genetic findings, and we propose calling it FIGF-associated pulmonary vasculopathy. We believe the intrapulmonary arterial and venous ectasia to be histologically characteristic and likely responsible for the tachypneic, hypoxic, clinical phenotype, perhaps through shunting and aberrant oxygen transfer. FIGF-associated pulmonary vasculopathy appears to be one of many pediatric hypoxic disorders in which airway neuroendocrine cells may be nonspecifically increased,25 and we do not view this as a primary neuroendocrine hyperplasia of infancy. Molecular advances can enhance the understanding of genetic underpinnings, pathogenesis, categorization, and clinicopathologic characteristics of rare pediatric lung diseases. It is possible that mechanisms elucidated in FIGF-associated pulmonary vasculopathy may also shed light on a broader range of diseases involving pulmonary vascular remodeling observed in more common clinical settings.
Acknowledgments
We thank the Exome Aggregation Consortium and the groups that provided exome variant data for comparison; Drs. Bernadette Gochuico and William Gahl for helpful comments and suggestions; and Anthony Lamattina for technical help.
E.B., Y.C., A.C., P.S., S.O.V., and S.Y.E.-C. contributed to study design, data collection, data analysis, data interpretation, generation of figures, and drafting of the manuscript; J.M.S., X.A., D.M., and R.H. contributed to study design, data collection, data analysis, and data interpretation; all authors have revised the article for important intellectual content and approved the final version.
Footnotes
Supported by NIH grant R21 HL119902 (S.Y.E.-C.), the Department of Defense grant TS130031 (S.Y.E.-C.) and the Anne Levine LAM Research Fund (S.Y.E.-C.).
E.B. and Y.C. contributed equally.
S.O.V. and S.Y.E.-C. contributed equally as senior authors.
Disclosures: None declared.
Current address of E.B., Division of Pulmonary and Allergy, UMass Memorial Medical Center, Worcester, MA.
References
- 1.Deutsch G.H., Young L.R., Deterding R.R., Fan L.L., Dell S.D., Bean J.A., Brody A.S., Nogee L.M., Trapnell B.C., Langston C., Albright E.A., Askin F.B., Baker P., Chou P.M., Cool C.M., Coventry S.C., Cutz E., Davis M.M., Dishop M.K., Galambos C., Patterson K., Travis W.D., Wert S.E., White F.V. Diffuse lung disease in young children: application of a novel classification scheme. Am J Respir Crit Care Med. 2007;176:1120–1128. doi: 10.1164/rccm.200703-393OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Chemaly S., Pacheco-Rodriguez G., Malide D., Meza-Carmen V., Kato J., Cui Y., Padilla P.I., Samidurai A., Gochuico B.R., Moss J. Nuclear localization of vascular endothelial growth factor-D and regulation of c-Myc-dependent transcripts in human lung fibroblasts. Am J Respir Cell Mol Biol. 2014;51:34–42. doi: 10.1165/rcmb.2013-0417OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janer J., Lassus P., Haglund C., Paavonen K., Alitalo K., Andersson S. Pulmonary vascular endothelial growth factor-C in development and lung injury in preterm infants. Am J Respir Crit Care Med. 2006;174:326–330. doi: 10.1164/rccm.200508-1291OC. [DOI] [PubMed] [Google Scholar]
- 4.Okizaki S., Ito Y., Hosono K., Oba K., Ohkubo H., Kojo K., Nishizawa N., Shibuya M., Shichiri M., Majima M. Vascular endothelial growth factor receptor type 1 signaling prevents delayed wound healing in diabetes by attenuating the production of IL-1beta by recruited macrophages. Am J Pathol. 2016;186:1481–1498. doi: 10.1016/j.ajpath.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 5.McColl B.K., Paavonen K., Karnezis T., Harris N.C., Davydova N., Rothacker J., Nice E.C., Harder K.W., Roufail S., Hibbs M.L., Rogers P.A., Alitalo K., Stacker S.A., Achen M.G. Proprotein convertases promote processing of VEGF-D, a critical step for binding the angiogenic receptor VEGFR-2. FASEB J. 2007;21:1088–1098. doi: 10.1096/fj.06-7060com. [DOI] [PubMed] [Google Scholar]
- 6.Stacker S.A., Stenvers K., Caesar C., Vitali A., Domagala T., Nice E., Roufail S., Simpson R.J., Moritz R., Karpanen T., Alitalo K., Achen M.G. Biosynthesis of vascular endothelial growth factor-D involves proteolytic processing which generates non-covalent homodimers. J Biol Chem. 1999;274:32127–32136. doi: 10.1074/jbc.274.45.32127. [DOI] [PubMed] [Google Scholar]
- 7.Duong T., Koltowska K., Pichol-Thievend C., Le Guen L., Fontaine F., Smith K.A., Truong V., Skoczylas R., Stacker S.A., Achen M.G., Koopman P., Hogan B.M., Francois M. VEGFD regulates blood vascular development by modulating SOX18 activity. Blood. 2014;123:1102–1112. doi: 10.1182/blood-2013-04-495432. [DOI] [PubMed] [Google Scholar]
- 8.Harris N.C., Davydova N., Roufail S., Paquet-Fifield S., Paavonen K., Karnezis T., Zhang Y.F., Sato T., Rothacker J., Nice E.C., Stacker S.A., Achen M.G. The propeptides of VEGF-D determine heparin binding, receptor heterodimerization, and effects on tumor biology. J Biol Chem. 2013;288:8176–8186. doi: 10.1074/jbc.M112.439299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leppanen V.M., Jeltsch M., Anisimov A., Tvorogov D., Aho K., Kalkkinen N., Toivanen P., Yla-Herttuala S., Ballmer-Hofer K., Alitalo K. Structural determinants of vascular endothelial growth factor-D receptor binding and specificity. Blood. 2011;117:1507–1515. doi: 10.1182/blood-2010-08-301549. [DOI] [PubMed] [Google Scholar]
- 10.Cui Y., Wilder J., Rietz C., Gigliotti A., Tang X., Shi Y., Guilmette R., Wang H., George G., Nilo de Magaldi E., Chu S.G., Doyle-Eisele M., McDonald J.D., Rosas I.O., El-Chemaly S. Radiation-induced impairment in lung lymphatic vasculature. Lymphat Res Biol. 2014;12:238–250. doi: 10.1089/lrb.2014.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui Y., Osorio J.C., Risquez C., Wang H., Shi Y., Gochuico B.R., Morse D., Rosas I.O., El-Chemaly S. Transforming growth factor-beta1 downregulates vascular endothelial growth factor-D expression in human lung fibroblasts via the Jun NH2-terminal kinase signaling pathway. Mol Med. 2014;20:120–134. doi: 10.2119/molmed.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan B., Latek R., Hossbach M., Tuschl T., Lewitter F. siRNA Selection Server: an automated siRNA oligonucleotide prediction server. Nucleic Acids Res. 2004;32:W130–W134. doi: 10.1093/nar/gkh366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks P.C., Montgomery A.M., Cheresh D.A. Use of the 10-day-old chick embryo model for studying angiogenesis. Methods Mol Biol. 1999;129:257–269. doi: 10.1385/1-59259-249-X:257. [DOI] [PubMed] [Google Scholar]
- 14.Zudaire E., Gambardella L., Kurcz C., Vermeren S. A computational tool for quantitative analysis of vascular networks. PLoS One. 2011;6:e27385. doi: 10.1371/journal.pone.0027385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lek M., Karczewski K.J., Minikel E.V., Samocha K.E., Banks E., Fennell T., Exome Aggregation Consortium Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar P., Henikoff S., Ng P.C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073–1081. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 17.Tang H., Thomas P.D. PANTHER-PSEP: predicting disease-causing genetic variants using position-specific evolutionary preservation. Bioinformatics. 2016;32:2230–2232. doi: 10.1093/bioinformatics/btw222. [DOI] [PubMed] [Google Scholar]
- 18.Hecht M., Bromberg Y., Rost B. Better prediction of functional effects for sequence variants. BMC Genomics. 2015;16(Suppl 8):S1. doi: 10.1186/1471-2164-16-S8-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toivanen P.I., Nieminen T., Viitanen L., Alitalo A., Roschier M., Jauhiainen S., Markkanen J.E., Laitinen O.H., Airenne T.T., Salminen T.A., Johnson M.S., Airenne K.J., Yla-Herttuala S. Novel vascular endothelial growth factor D variants with increased biological activity. J Biol Chem. 2009;284:16037–16048. doi: 10.1074/jbc.M109.001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Achen M.G., Jeltsch M., Kukk E., Makinen T., Vitali A., Wilks A.F., Alitalo K., Stacker S.A. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4) Proc Natl Acad Sci U S A. 1998;95:548–553. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leppanen V.M., Prota A.E., Jeltsch M., Anisimov A., Kalkkinen N., Strandin T., Lankinen H., Goldman A., Ballmer-Hofer K., Alitalo K. Structural determinants of growth factor binding and specificity by VEGF receptor 2. Proc Natl Acad Sci U S A. 2010;107:2425–2430. doi: 10.1073/pnas.0914318107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anisimov A., Alitalo A., Korpisalo P., Soronen J., Kaijalainen S., Leppanen V.M., Jeltsch M., Yla-Herttuala S., Alitalo K. Activated forms of VEGF-C and VEGF-D provide improved vascular function in skeletal muscle. Circ Res. 2009;104:1302–1312. doi: 10.1161/CIRCRESAHA.109.197830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada Y., Nezu J., Shimane M., Hirata Y. Molecular cloning of a novel vascular endothelial growth factor, VEGF-D. Genomics. 1997;42:483–488. doi: 10.1006/geno.1997.4774. [DOI] [PubMed] [Google Scholar]
- 24.Sato T., Paquet-Fifield S., Harris N.C., Roufail S., Turner D.J., Yuan Y., Zhang Y.F., Fox S.B., Hibbs M.L., Wilkinson-Berka J.L., Williams R.A., Stacker S.A., Sly P.D., Achen M.G. Vegf-d promotes pulmonary oedema in hyperoxic acute lung injury. J Pathol. 2016;239:152–161. doi: 10.1002/path.4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurland G., Deterding R.R., Hagood J.S., Young L.R., Brody A.S., Castile R.G., Dell S., Fan L.L., Hamvas A., Hilman B.C., Langston C., Nogee L.M., Redding G.J. An official American Thoracic Society clinical practice guideline: classification, evaluation, and management of childhood interstitial lung disease in infancy. Am J Respir Crit Care Med. 2013;188:376–394. doi: 10.1164/rccm.201305-0923ST. [DOI] [PMC free article] [PubMed] [Google Scholar]