Abstract
In the current study, we examined the ability of Salmonella enterica serovar Typhimurium to infect the central nervous system and cause meningitis following the natural route of infection in mice. C57BL/6J mice are extremely susceptible to systemic infection by Salmonella Typhimurium because of loss-of-function mutations in Nramp1 (SLC11A1), a phagosomal membrane protein that controls iron export from vacuoles and inhibits Salmonella growth in macrophages. Therefore, we assessed the ability of Salmonella to disseminate to the central nervous system (CNS) after oral infection in C57BL/6J mice expressing either wild-type (resistant) or mutant (susceptible) alleles of Nramp1. In both strains, oral infection resulted in focal meningitis and ventriculitis with recruitment of inflammatory monocytes to the CNS. In susceptible Nramp1−/− mice, there was a direct correlation between bacteremia and the number of bacteria in the brain, which was not observed in resistant Nramp1+/+ mice. A small percentage of Nramp1+/+ mice developed severe ataxia, which was associated with high bacterial loads in the CNS as well as clear histopathology of necrotizing vasculitis and hemorrhage in the brain. Thus, Nramp1 is not essential for Salmonella entry into the CNS or neuroinflammation, but may influence the mechanisms of CNS entry as well as the severity of meningitis.
Meningitis caused by Salmonella enterica serovars is a life-threatening disease with high fatality rates and frequent long-term neuropsychological sequelae.1 Although this is a rare disease in most parts of the world, in sub-Saharan Africa nontyphoidal S. enterica, particularly serovar Typhimurium (Salmonella Typhimurium), is now one of the most common causes of bacterial meningitis.2, 3 In severe cases, Salmonella meningitis can lead to ventriculitis, hydrocephalus, or cerebral abscess.4, 5, 6, 7
Animal models are essential to study of the mechanisms involved in bacterial meningitis, particularly entry into the central nervous system (CNS) and induction of meningeal inflammation. Recently, Salmonella Typhimurium was shown to be able to infect the CNS after oral inoculation in mice.8, 9 In addition, a few animals developed clinical signs of rolling that may be indicative of Salmonella-induced neurological damage.9 These studies indicate that mice may be a useful model to study Salmonella meningitis after the natural oral route of infection. A natural route of infection would allow analysis of the relative rate of Salmonella infection in the CNS, the correlation of brain infection with peripheral infection, and the association of an inflammatory response with infection in the brain. Furthermore, a natural route model would allow for the analysis of how the peripheral host immune response influences the ability of Salmonella to gain access to the brain as well as the development of bacterial meningitis.
In mice, a significant component of innate resistance to Salmonella Typhimurium is because of the natural resistance–associated macrophage protein 1 (Nramp1; alias Slc11a1), a divalent cation transporter expressed in cells of the monocyte/macrophage lineage. NRAMP1 limits the amount of iron within phagosomes and thus restricts replication of vacuolar pathogens, such as Salmonella, within macrophages.10 During the early phase of infection, NRAMP1 restricts the intracellular replication of Salmonella in the reticuloendothelial organs.11 Murine strains with homozygous loss-of-function mutations in Nramp1 (eg, BALB/c and C57BL/6) develop lethal acute infections, whereas mice with functional Nramp1 (eg, Sv129S6) develop a chronic or sustained infection. Resistant mice often show no clinical disease, although Salmonella Typhimurium can persist for long periods within macrophages in the mesenteric lymph nodes.12, 13, 14, 15 Interestingly, Salmonella Typhimurium has been detected in the CNS of both Nramp1+/+ and Nramp1−/− strains of mice.8, 9
To directly examine the influence of Nramp1 on dissemination to the CNS and subsequent meningitis, we infected wild-type C57BL/6J (Nramp1−/−) and Nramp1-reconstituted C57BL/6 (Nramp1+/+) mice with Salmonella Typhimurium by oral gavage. Nramp1+/+ C57BL/6 are more resistant than wild-type C57BL/6 mice13 and provide a model to analyze how a stronger innate immune response may influence Salmonella infection of and damage to the brain. We examined the time course of Salmonella Typhimurium infection in the brain, the localization of bacteria within the CNS, and the neuroinflammatory response to infection. Almost all mice had detectable Salmonella in the CNS and developed mild foci of inflammation in the meninges, although the time course of infection and the histopathology differed between Nramp1−/− and Nramp1+/+ strains. In addition, a few Nramp1+/+ mice developed a more severe CNS infection with clinical signs of neurological disease and histological features consistent with Salmonella-induced meningitis in humans, including ventriculitis and abscesses.
Materials and Methods
Ethics Statement
All animal work was performed in accordance with the Animal Welfare Act and Public Health Service Policy. Animal experiments were performed at the Rocky Mountain Laboratories (Hamilton, MT) or the University of Colorado at Boulder under protocols approved by the Institutional Animal Care and Use Committees (protocol numbers: RML 2014-028, RML 2014-070, UCB 1307.02).
Mice
C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME), 129SvEvTac mice (Taconic Laboratories, Hudson, NY), and C57BL/6 Nramp1G169D mice homozygous for the wild-type Nramp1 (Slc11a1) allele derived from Sv129 mice16 (Dr. Ferric Fang and Dr. Steve Libby, University of Washington, Seattle) were used for these studies. Mice were kept in pathogen-free conditions with free access to food and water, except where noted.
Bacterial Infections
Salmonella enterica serovar Typhimurium wild-type strain SL134417 was grown to stationary phase for 18 hours at 37°C with shaking in Luria-Bertani–Miller broth supplemented with streptomycin (100 μg/mL). Bacteria were pelleted and diluted to the appropriate concentration based on the OD at 600 nm. The actual inoculum size was verified by plating on Luria-Bertani plates for colony-forming units (CFUs). Adult 6- to 8-week-old 129SvEvTac (Taconic Laboratories), C57BL/6 Nramp1G169D (Nramp1−/−), and C57BL/6 Nramp1Gly169 (Nramp1+/+) mice were without food and water for 2 hours before oral inoculation (oral gavage) with Salmonella. The inoculum was administered in 100 μL of sterile buffered pharmaceutical grade saline (Mediatech, Manassas, VA) using a 20-gauge gavage needle. Food and water were restored 1 hour after inoculation. Mice were monitored daily for signs of clinical illness (ie, ruffled fur, hunched posture, or ataxia). Under anesthesia, blood was collected via cardiac puncture before transcardial perfusion with 5 mL of heparin sterile buffered pharmaceutical grade saline (100 U/mL). Tissues (brain, spleen, and liver) were collected in preweighed tubes containing 500 μL sterile buffered pharmaceutical grade saline, homogenized with a Precellys 24 homogenizer, and adjusted for concentration (based on weight) before plating and enumeration of CFUs. For most studies, the brain was divided into two sagittal sections, one half used for CFU counts and the other used for immunohistochemistry or flow cytometry.
Flow Cytometry
Brain tissue was homogenized using a 7-mL dounce homogenizer in 2 mL phosphate-buffered saline (PBS). Homogenate was strained using a 70 μmol/L cell strainer (Bioexpress, Kaysville, UT). The microglia fraction was collected at the 70%/30% interface of a 70%/30%/0% Percoll gradient after centrifugation. Cells were further processed for flow cytometry staining. Fc receptors were blocked using CD16/CD32 Fcγ III/II (clone 2.4G2; BD Biosciences, San Jose, CA). Cells were stained using a combination of the following antibodies: CD11c-PE/Cy7 (clone HL3; BD Biosciences), F4/80-e450 (clone BM8; eBiosciences), CD45- PE (clone 30-F11; BD Biosciences), Ly6C-AF700 (clone AL-21; BD Biosciences), Ly6G-APC/Cy7 (clone 1A8; BD Biosciences,), CD4-APC/Cy7 (clone GK1.5; BD Biosciences), CD8-PE (clone 53-6.7; BD Biosciences), NK1.1-AF700 (clone PK136; BD Biosciences), and CD45R/B220 (clone RA3-6B2; BD Biosciences), for 1 hour on ice, followed by a wash step and fixation in 2% paraformaldehyde for 20 minutes at room temperature. Cells were washed, resuspended in PBS, and processed for flow cytometry analysis. All flow analysis was done on a LSR II cytometer (BD Biosciences) and analyzed using FloJo software version 10.0.7 (FlowJo, Ashland, OR).
Histopathology
Tissues were fixed in 10% neutral buffered formalin (Leica Biosystems, Buffalo Grove, IL) for a minimum of 7 days before being placed in cassettes and processed with a Leica RM2265 microtome. Embedded tissues are divided into sections (5 μm thick) and dried overnight at 42°C before staining. Samples were deparaffinized and rehydrated using a graded series of xylene, ethanol (100%, 95%, 70%), and PBS. Antigen retrieval was performed in a citrate buffer using a Decloaking Chamber (Biocare Medical, Concord, CA) at 120°C for 20 minutes under high pressure. After PBS wash and blocking (2% donkey serum, 1% bovine serum albumin, 0.1% Triton X-100, 0.05% Tween-20 in PBS), the tissues were stained using rabbit anti-Iba1 (1:200; Wako, Richmond, VA), rabbit anti–glial fibrillary acidic protein (1:500; Agilent, Santa Clara, CA), rabbit anti-CD3 (1:100; Dako), and goat anti-Salmonella common structural antigen (1:500; Kirkegaard & Perry Laboratories, Inc., Gaithersberg, MD) primary antibodies. Secondary antibodies used were Alexa Fluor 488–conjugated chicken anti-rabbit and Alexa Fluor 594–conjugated chicken anti-goat (1:300; Life Technologies, Carlsbad, CA). Samples were mounted with ProLong Gold with DAPI (Life Technologies).
Pathology Scoring
All samples were scored blindly (D.S.) using the following criteria to assess for infiltration of inflammatory cells (ie, neutrophils) and presence of meningitis and/or encephalitis. Score 0, no lesion; 1, focal infiltration of the meninges and/or gray matter by small numbers of lymphocytes, macrophages, and rare neutrophils; 2, focal to multifocal foci in the meninges and gray matter or olfactory bulb that is infiltrated by small numbers of lymphocytes, macrophages, and few neutrophils, may be minimal hemorrhage; 3, multifocal areas of encephalomalacia and meningitis with infiltration by moderate numbers of neutrophils, macrophages, and fewer lymphocytes, multifocal vasculitis with or without fibrin thrombi, multifocal mild hemorrhage, and numerous extracellular bacilli; and 4, coalescing areas of encephalomalacia and meningitis with infiltration by moderate numbers of neutrophils, macrophages, and fewer lymphocytes, multifocal vasculitis with or without fibrin thrombi, multifocal moderate hemorrhage, and numerous extracellular bacilli.
Imaging
Confocal images were captured using ZEN software version 8.1.10 (Carl Zeiss, Thornwood, NY) on a Carl Zeiss LSM710 confocal laser scanning microscope with either a Plan APOCHROMAT 63×/1.4 numerical aperture (NA) or a 20×/0.8 NA objective. Wide field images were acquired using Nikon Elements software ersion 3.2 on a Nikon Eclipse 55i microscope with either a 40×/0.75 NA or a 20×/0.50 NA objective and a Nikon DS-Ri1 digital camera (Nikon, Melville, NY). Slides were also imaged using ScanScope software version 102.0.0.33 (Leica) on either an Aperio ScanScope Fluorescent slide scanner or an Aperio XT Brightfield slide scanner, both equipped with a UPLSAPO 20×/0.75 NA objective (Leica). All image analysis was done with ImageJ (version 2.0.0 W.S. Rasband; NIH, Bethesda, MD) and Adobe Photoshop CS5.1 (Adobe, San Jose, CA).
Statistical Analysis
Graphpad software Statmate version 2.00 (GraphPad, La Jolla, CA) was used to determine group sizes before experimentation, and Graphpad Prism software version 7 was used for all statistical analyses. The tests are indicated in the figure legends.
Results
Detection of Salmonella in the Brain after Oral Infection in Nramp1−/− and Nramp1+/+ Mice
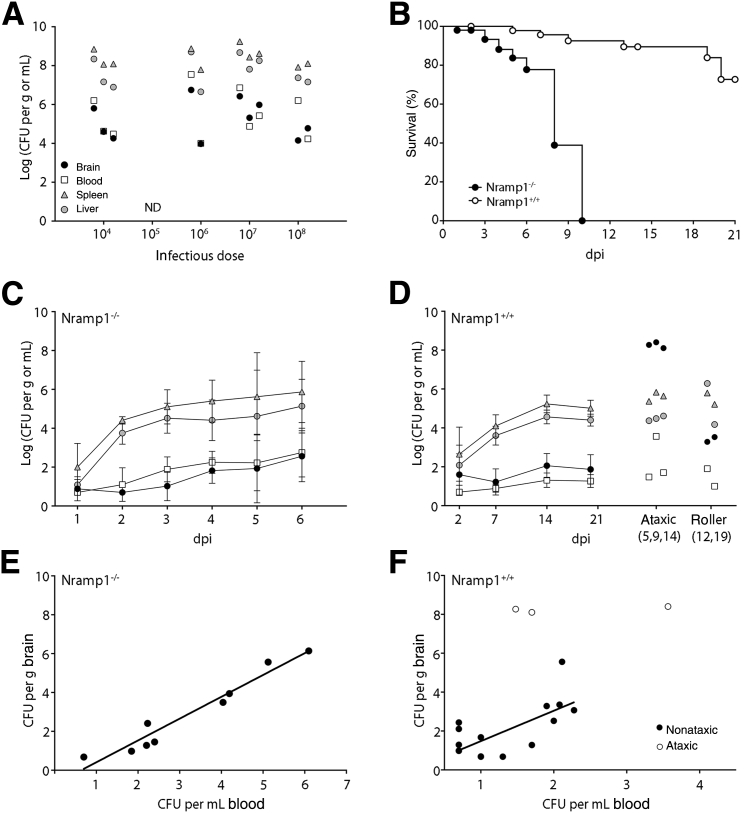
Wild-type strains of Salmonella Typhimurium can disseminate to the brain and cause meningioencephalitis in susceptible mice, but it is not clear how this correlates with systemic infection and whether there is a dose-dependent response.8, 9 To investigate these issues, we infected C57BL/6 (Nramp1−/−) mice by oral gavage with doses ranging from 104 to 109 CFUs. Mice were monitored daily and euthanized when they developed clinical signs of salmonellosis (hunched posture and ruffled fur). No mice showed signs of neurological disease (ataxia, rolling, jumping, twirling, and loss of motor control). To avoid blood contamination of tissues, particularly the brain, transcardial perfusion was performed on all mice immediately before removing tissues. Bacterial loads were examined in the brain, blood, spleen, and liver by plating (Figure 1A). Irrespective of the inoculating dose, all mice (n = 10) with clinical bacteremia had high bacterial loads in the liver (106 to 108 CFUs/g), spleen (107 to 108 CFUs/g), and blood (104 to 107 CFUs/mL). Bacteria (104 to 106 CFUs/g) were also recovered from the brains of all of the symptomatic mice, although at lower levels than those found in the spleen or liver. Thus, bacteremia in C57BL/6 Nramp1−/− mice correlated with infection of the CNS and did not show a dose dependence.
Figure 1.
Salmonella infection and dissemination in Nramp1−/− and Nramp1+/+ mice. A: Organ colonization of Nramp1−/− mice with clinical salmonellosis. Mice were infected orally with 104, 106, 107, or 108Salmonella. Samples were collected on development of clinical disease, up to 10 days postinfection (dpi). B: Percentage survival of Nramp1−/− and Nramp1+/+ mice infected with 108 or 109 colony-forming units (CFUs) Salmonella, respectively. C: Bacterial loads in Nramp1−/− mice infected with 108Salmonella. D: Bacterial loads in Nramp1+/+ mice infected with 109Salmonella. Mice with clinical ataxia or rolling disease are shown separately. E and F: Relationship between bacterial loads in the blood and brain of Nramp1−/− (E) or Nramp1+/+ (F) mice at 6 or 21 days postinfection. Data were fitted using a linear regression model of transformed log-log CFU data. Mice with ataxia are indicated. Data represent means ± SD (C and D). n = 3 (A, 104 and 107Salmonella); n = 2 (A, 106 and 108Salmonella); n = 50 (B, Nramp1−/−); n = 54 (B, Nramp1+/+); n = 3 to 14 mice per time point (C); n = 2 to 20 mice per time point (D).
Although C57BL/6 mice are widely used as a model for systemic salmonellosis, their inability to control Salmonella Typhimurium during the early phase of infection means that they succumb to infection within 2 weeks (Figure 2). In contrast, Nramp1-reconstituted (Nramp1+/+) C57BL/6 mice control early infection, providing a useful model to study how the immune response may influence Salmonella infection of the CNS. To establish a baseline for the meningitis studies, we first compared the course of infection in Nramp1−/− and Nramp1+/+ C57BL/6 mice after oral gavage with 108 or 109 CFUs, respectively. These doses were selected based on initial experiments and on published studies so that 100% of the mice should develop systemic infection.13 Under these conditions, <30% of Nramp1+/+ mice developed clinical signs of disease by day 21, whereas all of the Nramp1−/− mice developed clinical signs of salmonellosis and were euthanized by day 10 (Figure 1B). The difference between these strains is consistent with previous findings13 and provides a model to study the effect of the peripheral immune response on Salmonella infection of the CNS.
Figure 2.
Salmonella colonization of the brain of 129S6 (129SvEv) mice. Mice were orally infected with 109Salmonella. Samples were collected 2 weeks after infection. Each symbol represents one mouse. Means and SEM are shown. n = 6. CFU, colony-forming unit.
To examine when Salmonella colonizes the CNS, and how Nramp1 influences this process, we did a time course analysis of infection in Nramp1+/+ and Nramp1−/− mice. Bacterial loads were determined daily, up to day 6, in Nramp1−/− mice and on days 2, 7, 14, and 21 in Nramp1+/+ mice (Figure 1, C and D, and Supplemental Figures S1 and S2). Bacteria were recovered from the liver and spleen of most mice within 1 to 2 days of infection, although, as expected, the levels of bacteria did not peak until after 7 days in the Nramp1+/+ mice. Bacteria were recovered from the blood as early as 2 days postinfection (dpi) in Nramp1−/− mice and 7 dpi in Nramp1+/+ mice. Infection of the brain was slightly delayed compared to blood and peripheral tissues, with >50% of mice having recoverable bacteria in the brain at 4 dpi in Nramp1−/− mice (Supplemental Figure S1) and 14 dpi in Nramp1+/+ mice (Supplemental Figure S2). The bacterial load in the brain, which ranged from undetectable to 106, was consistently lower than that in the spleen or liver for both Nramp1−/− and Nramp1+/+ mice (Figure 1, C and D), with the exception of three Nramp1+/+ mice that had clinical signs of ataxia (Table 1). Thus, bacterial infection of the CNS was observed in most animals for both Nramp1−/− and Nramp1+/+ mice after oral inoculation.
Table 1.
Comparison of Clinical Signs and Histopathology
| Variable | Clinical diagnosis∗ |
Histopathology positive† |
||
|---|---|---|---|---|
| Nramp1−/− | Nramp1+/+ | Nramp1−/− | Nramp1+/+ | |
| Total analyzed | 27 | 20 | 1 | 10 |
| Asymptomatic | 19 | 9 | 1 | 3 |
| Salmonellosis | 8 | 6 | 0 | 3 |
| Roller | 0 | 2 | 0 | 1 |
| Ataxic | 0 | 3 | 0 | 3 |
Mice analyzed include animals at earlier time points (Figure 1C) that had not progressed to clinical disease but were analyzed for histological changes.
Number of animals from Clinical Diagnosis columns that scored positive for clear signs of meningitis or encephalitis by blinded histological examination (D.S.) of hematoxylin and eosin sections.
Salmonella Colonization of the Brain Correlates with Bacteremia in Nramp1−/− but Not Nramp1+/+ Mice
The kinetics of bacterial loads in the brain followed the same trend as bacterial loads in the blood for both Nramp1−/− and Nramp1+/+ mice (Figure 1, C and D). Therefore, to investigate whether there was a direct correlation, we compared bacterial loads in the blood and brain for individual mice. A direct correlation was observed in Nramp1−/− mice (R2 = 0.9450) (Figure 1E); however, no correlation was observed in Nramp1+/+ mice (R2 = 0.4744) (Figure 1F). This suggests that Salmonella levels in the blood may be more important for neuroinvasion in Nramp1−/− mice, compared to Nramp1+/+ mice.
Infection of the CNS in Mice Resistant to Salmonella Bacteremia
Nramp1 is only one of the genes that influences susceptibility to Salmonella infection in mice.18, 13 129S6 mice are more resistant to Salmonella infection than the reconstituted Nramp1+/+ C57BL/6 mice.13 To determine whether other genetic factors may also influence Salmonella infection in the brain, we examined 129S6 mice. However, even in the more resistant 129S6 mice, Salmonella could be readily detected in the brain (Figure 2), suggesting that even restriction factors that prevent bacteremia were not sufficient to prevent Salmonella from entering the CNS.
Salmonella Infection Results in Inflammatory Monocyte Recruitment to the Brain
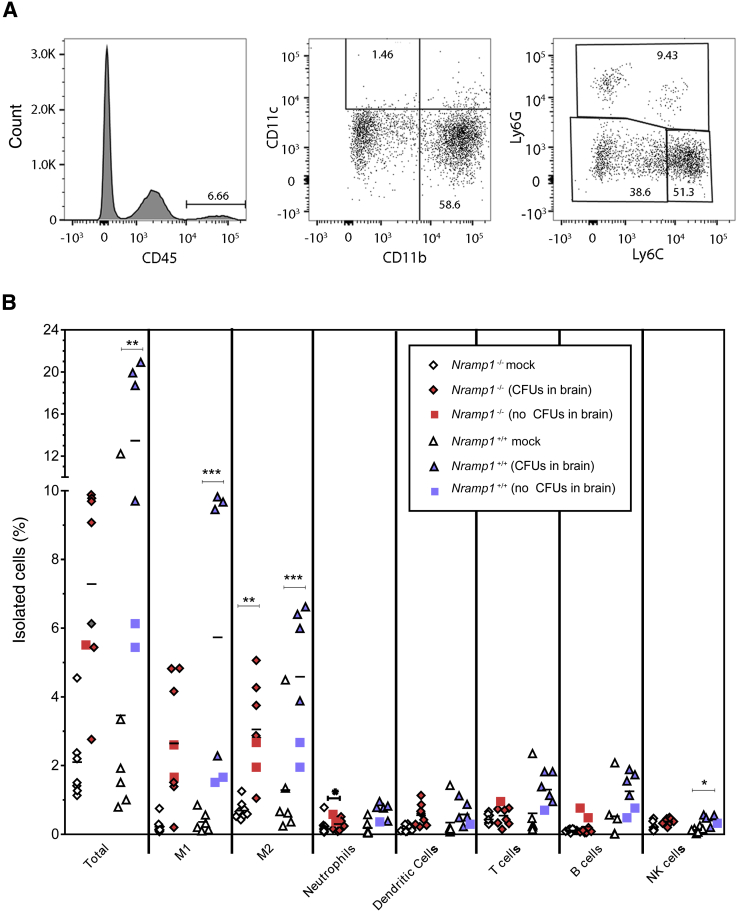
A characteristic feature of bacterial meningitis, in humans and animal models, is the recruitment of inflammatory cells, including monocytes, macrophages, and neutrophils.19, 20 To assess which inflammatory cells are recruited during Salmonella infection, we isolated cells from the brain by Percoll gradient and analyzed them for different cell markers by flow cytometry. We selected 4 and 6 dpi for Nramp1−/− mice and 7 and 14 dpi for Nramp1+/+ mice based on the bacterial loads in the brain at those time points (Figure 1). For each mouse, half of the brain was used for flow cytometry, whereas the other half was used to determine the bacterial load. Infiltrating cells were separated from resident microglia and other brain cells by CD45hi expression (Figure 3A). Cells were then defined as dendritic cells (CD11c+), T cells (CD3+), B cells (CD45R/B220+), natural killer cells (NK1.1+), or myeloid cells (CD11b+). Myeloid cells were further characterized as neutrophils (Ly6G+), inflammatory M1 monocytes (Ly6Chi and Ly6G−), or nonclassic M2 monocytes (Ly6Clo and Ly6G−) (Figure 3A).
Figure 3.
Infiltration of immune cells in the brains of infected Nramp1−/− and Nramp1+/+ mice. Mice were orally infected with Salmonella, and samples were collected on day 6 (Nramp1−/−) and day 14 (Nramp1+/+) after infection. A: Example of gated analysis used for determining M1 and M2 cell phenotypes. B: Percentage of infiltrating immune cells in the brains of infected Nramp1−/− (red symbols) and Nramp1+/+ (blue symbols) mice. Half of the brain was used to estimate the bacterial load [colony-forming units (CFUs)], whereas the other half was processed for flow cytometry. Infected mice, where no CFUs were detected, are indicated for Nramp1−/− (red squares) and Nramp1+/+ (blue squares). Mock-infected Nramp1−/− (white diamonds) and Nramp1+/+ (white triangles) mice were used as controls. Each symbol represents one mouse. n = 8 (B, infected and mock-infected Nramp1−/− mice); n = 6 (B, infected and mock-infected Nramp1+/+ mice). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. NK, natural killer.
Infiltrating (CD45hi) cells were observed in both strains, relative to mock-infected controls (Figure 3B). Mice with detectable CFUs in the CNS generally had a higher percentage of infiltrating cells relative to infected mice without detectable CFUs (Figure 3B). In both strains, the infiltrating cells were composed primarily of M1 and M2 monocytes with lower levels of neutrophils, dendritic cells, T cells, B cells, and natural killer cells (Figure 3B and Supplemental Figure S3). Thus, Salmonella infection resulted in recruitment of inflammatory cells to the brain, primarily M1 and M2 monocytes, in both Nramp1+/+ and Nramp1−/− mice. Interestingly, the presence of NRAMP1, which inhibits the ability of Salmonella to replicate in monocytes, did not appear to substantially affect the cell types in the CNS. Similar to monocyte infiltrate in the brain, we also found increased M1 and M2 monocytes in the blood of both Nramp1+/+ and Nramp1−/− mice (Supplemental Figure S4). Neutrophils were also increased in Nramp1+/+ mice, whereas an increase in natural killer cells was observed in Nramp1−/− mice. The increase in both monocyte populations in the blood correlates with the detection of these cell types in the brain and indicates a proliferation of monocytes from the bone marrow in response to Salmonella infection in both Nramp1+/+ and Nramp1−/− mice.
Histological Analysis Demonstrates More Severe Meningitis and Encephalitis in Nramp1+/+ Mice Compared to Nramp1−/− Mice
In both Nramp1−/− and Nramp1+/+ mice, we observed consistent bacterial infection and inflammatory cell recruitment to the brain after Salmonella infection (Figures 1 and 3), which are indicative of meningitis or encephalitis. Next, hematoxylin and eosin–stained sections of brain tissue were analyzed in a blinded manner (D.S.) by a pathologist using a scoring system of 0 to 4 to rank meningitis and encephalitis (Tables 1 and 2). Only 1 of 27 (approximately 4%) Nramp1−/− mice scored positive for meningitis, whereas 10 of 20 (50%) Nramp1+/+ mice scored positive for meningitis and/or encephalitis. Of the mice that scored positive by histology, four were asymptomatic, three had salmonellosis, one showed signs of rolling, and three had signs of ataxia (Table 2); all had detectable CFUs in the brain. Thus, meningitis and encephalitis were observed in a greater percentage of Nramp1+/+ mice compared to Nramp1−/− mice and were observed in both asymptomatic and symptomatic mice. The three Nramp1+/+ mice with ataxia had the highest bacterial load in their brains (108 CFUs/g) as well as the highest scores for meningitis (4, 2, 4) and encephalitis (4, 4, 4).
Table 2.
Individual Pathology Scores of Mice
| Mouse no. | Nramp1 | Clinical diagnosis | CFU/g | Meningitis∗ | Encephalitis† |
|---|---|---|---|---|---|
| 51-2 | −/− | Asymptomatic | 2.00 × 104 | 1 | 2 |
| 92-4 | +/+ | Asymptomatic | 1.40 × 104 | 1 | 0 |
| 126-2 | +/+ | Asymptomatic | 4.60 × 103 | 2 | 0 |
| 130-1 | +/+ | Asymptomatic | 1.10 × 103 | 0 | 2 |
| 92-3 | +/+ | Salmonellosis | 8.80 × 104 | 1 | 0 |
| 135-2 | +/+ | Salmonellosis | 3.60 × 105 | 0 | 2 |
| 149-1 | +/+ | Salmonellosis | 4.70 × 103 | 0 | 1 |
| 155-1 | +/+ | Roller | 3.40 × 103 | 1 | 0 |
| 93-3 | +/+ | Ataxic | 1.90 × 108 | 4 | 4 |
| 126-1 | +/+ | Ataxic | 2.50 × 108 | 2 | 4 |
| 135-1 | +/+ | Ataxic | 1.30 × 108 | 4 | 4 |
CFU, colony-forming unit; +, positive; −, negative.
Tissue was scored for meningitis based on a ranking of 0 to 4, as described in Materials and Methods.
Tissue was scored for encephalitis based on a ranking of 0 to 4, as described in Materials and Methods.
Immunohistochemistry Analysis Shows Focal Areas of Inflammation in the Meninges and Ventricles of Both Nramp1−/− and Nramp1+/+ Mice
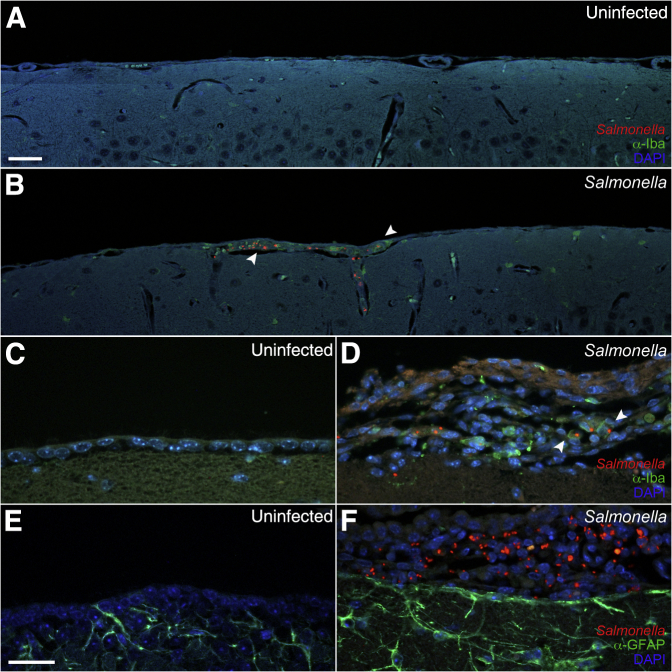
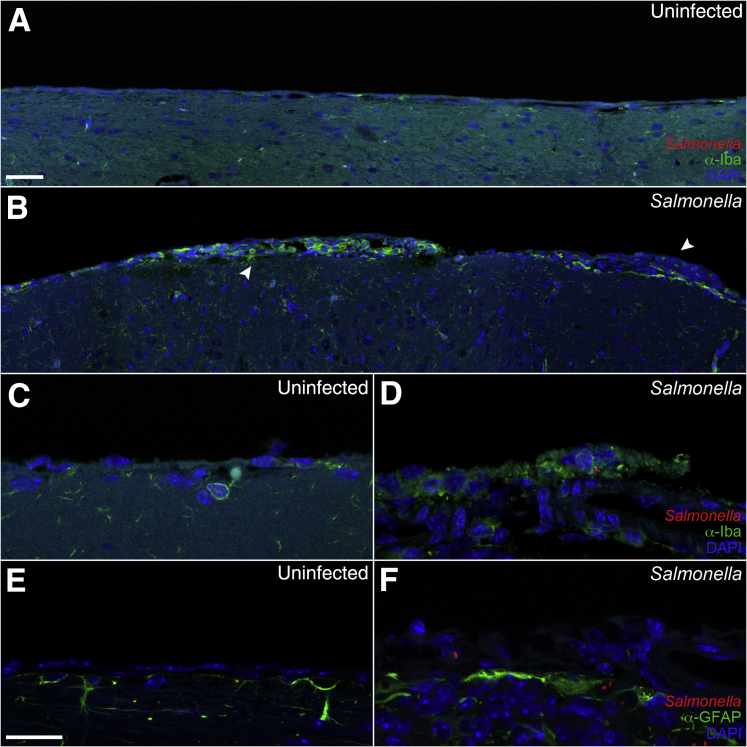
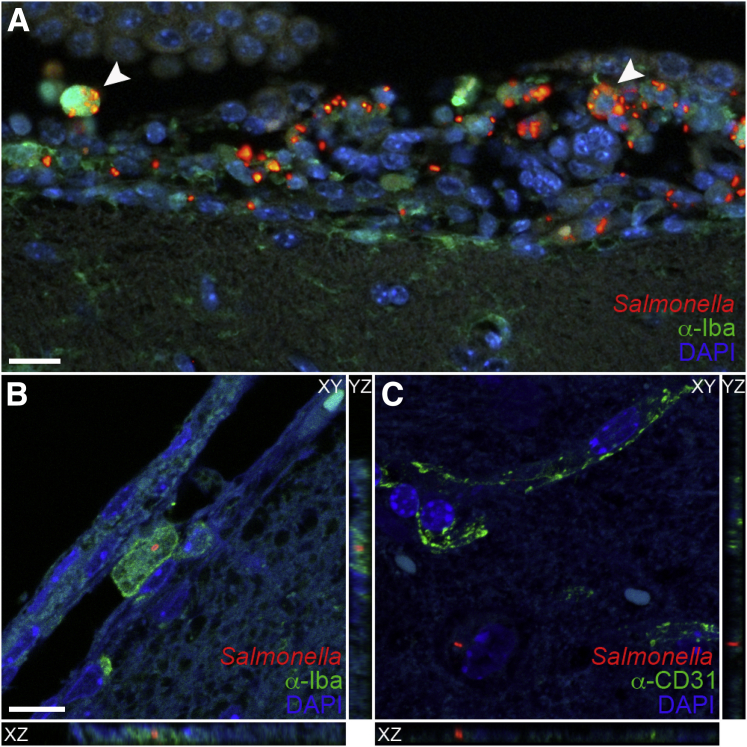
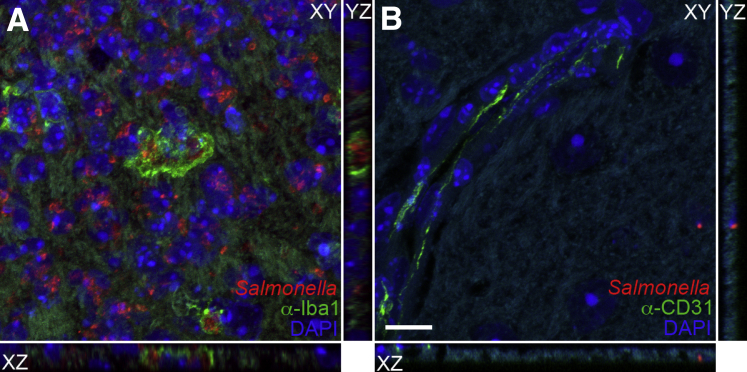
To determine the localization of Salmonella and inflammation in the brain of infected mice, we used immunohistochemistry. Tissue sections were stained with anti-Iba1 to detect microglia and monocytes, anti–glial fibrillary acidic protein to detect astrocytes, anti-CD31 to detect endothelial cells, and anti-lipopolysaccharide to detect Salmonella. Focal areas of inflammation consisting of Iba1+ monocytes/macrophages (green fluorescence) and Salmonella were observed in the meninges and ventricles in most mice with detectable CFUs in the brain (Figures 4 and 5). These mice included animals that were scored negative for meningitis and encephalitis, possibly because of the focal nature of the areas of inflammation. Interestingly, Salmonella in these focal areas was generally more intense in Nramp1−/− mice (Figure 4) than in Nramp1+/+ mice (Figure 5). These focal areas of infection were associated with increased glial fibrillary acidic protein staining at the meningeal layer (Figures 4 and 5), indicating astrocyte activation in response to infection. Salmonella was often found within Iba1+ monocytes in the meningeal layer (Figure 4, Figure 6, A and B, and Figure 7), but extracellular bacteria were occasionally found within the brain parenchyma (Figure 6C and Figure 7) in both Nramp1−/− and Nramp1+/+ mice. Focal areas of meningitis and ventriculitis were observed in both strains of mice, consisting of Salmonella, Salmonella-infected monocytes, and other inflammatory cells. These immunohistochemistry studies correlated with the detection of Salmonella CFUs in the brain of both Nramp1−/− and Nramp1+/+ mice (Figure 1), as well as the increase in monocytes (Figure 3).
Figure 4.
Meningitis in Nramp1−/− mice after Salmonella infection. Brain tissue of Nramp1−/− mice orally infected with Salmonella was fixed and immunostained up to 10 days after infection. A and B: Extended representative images of uninfected (A) and infected (B) brain tissue immunostained for Iba1 (green), Salmonella (red), and DAPI (blue). Arrowheads indicate regions of meningitis. C–F: High-magnification representative images of uninfected (C and E) and infected (D and F) brain tissue immunostained for Salmonella (red), DAPI (blue), and either glial fibrillary acidic protein (GFAP) or Iba1 (green). Scale bars = 40 μm (A–F).
Figure 5.
Meningitis in Nramp1+/+ mice after Salmonella infection. Brain tissue of Nramp1+/+ mice orally infected with Salmonella was fixed and immunostained up to 21 days after infection. A and B: Extended representative images of uninfected (A) and infected (B) brain tissue immunostained for Iba1 (green), Salmonella (red), and DAPI (blue). Arrowheads indicate regions of meningitis. C–F: High-magnification representative images of uninfected (C and E) and infected (D and F) brain tissue immunostained for Salmonella (red), DAPI (blue), and either glial fibrillary acidic protein (GFAP) or Iba1 (green). Scale bars: 40 μm (A and B); 20 μm (C–F).
Figure 6.
Salmonella intracellular infection of the ventricle, meninges, and parenchyma. Brain tissue of Nramp1−/− mice orally infected with Salmonella was fixed and immunostained up to 10 days after infection. A: Representative image of infected ventricular brain tissue immunostained for Iba1 (green), Salmonella (red), and DAPI (blue). Arrowheads indicate examples of intracellular bacteria. B and C: XYZ projections illustrating presence of intracellular and extravascular bacteria in the meninges (B) and parenchyma (C) immunostained for Salmonella (red), DAPI (blue), and either Iba1 (green; B) or CD31 (green; C). XZ or YZ images were acquired in the XY plane with slices taken along the Z-axis. Scale bars: 20 μm (A); 10 μm (B and C).
Figure 7.
Salmonella infection of the brain parenchyma in Nramp1+/+ neurological mice. Brain tissue of Nramp1+/+ mice orally infected with Salmonella was fixed and immunostained at 5, 9, and 14 days after infection. XYZ projections illustrating presence of intracellular and extravascular bacteria in the parenchyma immunostained for Salmonella (red), DAPI (blue), and either Iba1 (green; A) or CD31 (green; B). XZ and YZ images were acquired in the XY plane with slices taken along the Z-axis. Scale bar = 10 μm (A and B).
Pathology Correlates with Neurological Signs of Ataxia in Nramp1+/+ Mice
Of the 53 Nramp1+/+ mice infected with Salmonella, 5 (10%) developed clinical signs of disease clearly distinguishable from systemic salmonellosis (hunched posture and ruffled fur). These fell into two distinct groups. Two mice displayed barrel rolling and spinning behaviors, whereas three displayed classic signs of ataxia, including hind limb tremors, failure to splay legs, grasping of paws, and altered gait. Comparison of the bacterial burdens of these two groups (Figure 1D) revealed that the ataxic mice had much higher bacterial loads in the brain (>108 CFUs/g), whereas the roller mice had loads similar to asymptomatic mice (103 CFUs/g). Remarkably, although the bacterial burden in the brains of ataxic mice was 1000- to 10,000-fold higher than all other mice, the bacterial levels in other organs remained comparable (Figure 1D). Thus, the three Nramp1+/+ animals that developed signs of neurological disease were associated with high bacterial levels in the brain.
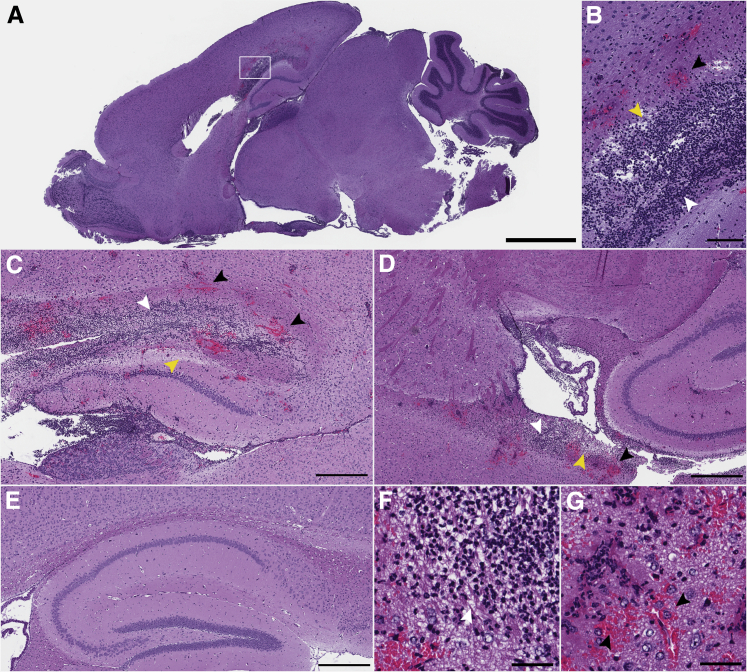
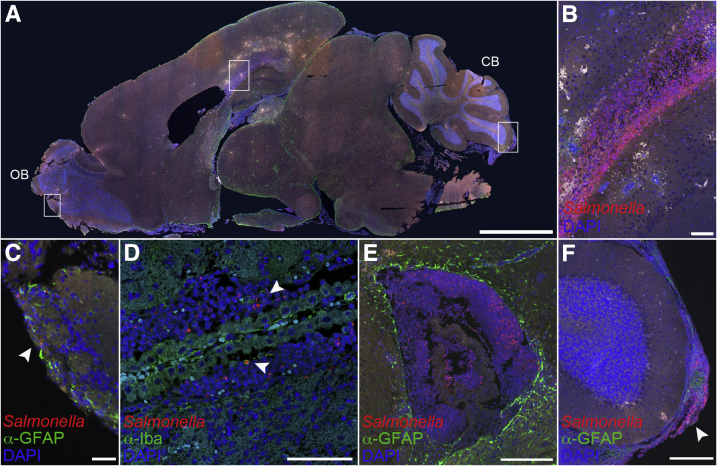
Imaging of brain tissue from the three ataxic animals revealed multiple areas of necrotizing vasculitis often occluded by fibrin thrombi (Figure 8). Brains from these three mice contained periventricular malacia associated with infiltration of large numbers of intact and degenerate neutrophils, which were not observed in uninfected controls (Figure 8). Similar signs of inflammation were observed in the lateral and third ventricles (Figure 8). Thus, ataxic mice had histological changes characteristic of Gram-negative bacillary meningoencephalitis.21 Fluorescence immunohistochemistry and confocal microscopy revealed that the severe histological changes in the ataxic Nramp1+/+ mice were associated with areas of bacterial infection. Salmonella was observed in the olfactory bulb (Figure 9A), periventricular areas (Figure 9B), ventricles (Figure 9, C and D), and meninges (Figure 9, E and F). Most Salmonella was found in the areas of periventricular malacia and neutrophil infiltration (Figure 9, B–D), but was not directly observed around blood vessels associated with necrotizing vasculitis (areas of red blood cell infiltration as shown by white cells) (Figure 9B). Thus, Salmonella infection was widespread in ataxic mice, although primarily localized to the ventricles, periventricular areas, and meninges.
Figure 8.
Neutrophil infiltration, focal hemorrhaging, and ventriculitis in the brains of ataxic Nramp1+/+ mice. Brains from mice with clinical signs of ataxia after infection with Salmonella were stained with hematoxylin and eosin. A: Low-magnification image showing neutrophil infiltration and hemorrhaging (boxed area), magnified in B. Malacia with neutrophil infiltrate and focal hemorrhaging in the corpus callosum (B and C) and lateral ventricle (D). E: Image of hippocampus and corpus callosum from an uninfected mouse for comparison. F and G: Infiltration and vascular thrombosis. Black arrowheads indicate regions of hemorrhage; yellow arrowheads, regions of tissue clearing; and white arrowheads, regions of cellular infiltrate. Scale bars: 2 mm (A); 200 μm (B); 500 μm (C–E); 50 μm (F and G).
Figure 9.
Bacterial accumulation and macrophage infiltration in the brains of ataxic Nramp1+/+ mice. Brains from mice with clinical signs of ataxia after infection with Salmonella were immunostained. A: Low-magnification image of infected brain tissue immunostained for glial fibrillary acidic protein (GFAP; green), Salmonella (red), and DAPI (blue). Boxed regions illustrate bacteria in the corpus collosum, olfactory bulb, and meningies and are magnified in B, C, and F, respectively. For orientation purposes, the olfactory bulb (OB) and cerebellum (CB) are labeled. Representative image of Salmonella in the corpus callosum (B), olfactory bulb (C), ventricle (D and E), and cerebellar meninges (F) of a neurological mouse immunostained for either Iba1 or GFAP (green), Salmonella (red), and DAPI (blue). Arrowheads represent intracellular bacteria present in a monocyte (D) and presence of bacterial meningitis (F). Scale bars: 2 mm (A); 100 μm (B–D); 200 μm (E and F).
Immunohistochemistry of brain tissue sections from the two roller mice did not show significant bacteria (Supplemental Figure S5A), consistent with the lack of severe neurological pathology (Table 1) and the low CFU counts (Figure 1D). Because signs of spinning and barrel rolling can be indicative of otitis media (ear infection), we analyzed one of the roller mice for Salmonella infection in the inner ear. Bacterial infection was readily detected in the ear, as determined by fluorescent labeling for Salmonella (Supplemental Figure S5, B, C, and E). Histological examination revealed monocytes in the ear canal (Supplemental Figure S5, D and F), as confirmed by immunohistochemistry staining for Iba1 (Supplemental Figure S5E). Thus, the clinical sign of barrel rolling was associated with an acute inner ear infection, rather than CNS infection.
Discussion
Gram-negative meningitis is a serious health concern, with high mortality and long-term sequelae. However, there are only a few animal models that reconstitute the natural route of infection of the CNS, making it difficult to fully understand the progression of the disease. In the current study, we compared the development of meningitis in highly susceptible Nramp1−/− and more resistant Nramp1+/+ mice following a natural route of Salmonella infection. In both strains, meningitis was observed with detectable levels of Salmonella in the CNS and an influx of monocytes and other immune cells in the brain. In addition, a small number of Nramp1+/+ animals developed clinical neurological disease characterized by ataxia. The pathology observed in these mice was similar to that reported in human neurological disease cases of Salmonella meningitis, including ventriculitis and hemorrhage.6, 22 Thus, oral infection of Salmonella results in meningitis in both susceptible and resistant strains of mice, with a more severe form of meningitis occurring infrequently in resistant mice. The development of the more severe form of meningitis could be linked to the persistent infection in Nramp1+/+ mice, which may allow more opportunity for Salmonella to cross the blood-brain or blood–cerebrospinal fluid (CSF) barriers and gain better access to the CSF. Once Salmonella infects the CSF, it may be able to evade the immune system and replicate to high enough levels to then induce the severe damage observed in the brains in the ataxic Nramp1+/+ mice. Future studies on how Salmonella gains access to the CSF and whether the CSF environment allows for uncontrolled replication of Salmonella remain important to answer questions that need to be addressed.
Both Salmonella and Escherichia coli K1, the most common cause of neonatal Gram-negative bacillary meningitis in the United States, are enteropathogens that enter the CNS through hematogenous spread. The mechanism of bacterial entry into the CNS is not well understood. Experimental models for Gram-negative meningitis are limited. In most cases, adult animals do not reliably develop meningitis unless the pathogen is inoculated directly into the CSF or by intracranial installation. Although these models have yielded important information about the factors leading to, and the effects of, meningeal inflammation, they bypass the natural dissemination of the bacteria from the vascular system to the central nervous system.
An important aspect of the current study was the ability to follow bacterial dissemination from the gastrointestinal tract via the blood into the CNS. Our studies suggest that macrophages may play an important role in this process for Salmonella. One of the primary host factors that restrict intracellular survival of Salmonella in macrophages is Nramp1. We found that bacteremia levels correlated strongly with brain CFUs in Nramp1−/− mice, but not in Nramp1+/+ mice. Salmonella-infected monocytes were also readily detected in the brains of Nramp1−/− mice, consistent with the inability of Nramp1−/− monocytes to effectively suppress Salmonella growth.16, 23 These Salmonella-infected monocytes were not as readily detected in the brains of Nramp1+/+ mice, despite higher numbers of cells in the CNS. Thus, Nramp1−/− and Nramp1+/+ mice provide two distinct models of Salmonella meningitis, with strong differences in the ability of monocytes to control the Salmonella infection. Comparison of these two models will provide a better understanding of how monocytes mediate meningitis in immunocompetent and immunocompromised hosts.
Studies in humans and animal models for E. coli K1 have indicated that a high degree of bacteremia is a prerequisite for invasion of the CNS,24, 25 with at least 105 CFUs/mL required in adult animals.26 However, herein, brain neuroinvasion of Salmonella was observed when the blood levels were at least 2 logs lower, approximately 103 CFUs/mL in Nramp1−/− mice and <102 CFUs/mL in Nramp1+/+ mice. Thus, in this model, Salmonella may have a lower bacteremia threshold for entry into the CNS. Whether or not this lower requirement is because of a role for monocyte/macrophage trafficking of bacteria into the CNS remains to be determined. In our model, Nramp1+/+ mice, which have lower bacteremia levels, had greater infiltration of monocytes in the brain than Nramp1−/− mice. Thus, there was no direct correlation between monocytes in the CNS and bacteremia with Salmonella infection. However, it is unclear if there is a percentage of monocytes infected with Salmonella that differs between Nramp1+/+ and Nramp1−/− mice, which could influence the contribution of infected monoctyes to Salmonella infection in the brain.
Multiple myeloid cell types have been shown to contribute to inflammation and pathogenesis in the brain, including resident microglia, M1 inflammatory monocytes, M2 nonclassic monocytes, and resident brain macrophages. In this model, both M1 and M2 infiltrating monocytes were increased in both strains of mice, even in animals with almost undetectable bacteria in the brain. M1 and M2 monocyte populations may have distinct functions in meningitis, as M1 monocytes are considered to be more proinflammatory, whereas M2 monocytes are considered to be anti-inflammatory, as evidenced by their production of anti-inflammatory cytokines, such as IL-10.27 In E. coli K1 studies, depletion of circulating M1 monocytes resulted in more severe meningitis, but this was because of peripheral control of bacteremia rather than a direct effect within the CNS.28 As Nramp1−/− monocytes have limited ability to clear bacterial infections, the role of these cells in Salmonella meningitis may be mostly damaging. In contrast, Nramp1+/+ monocytes may have both protective and pathogenic roles in the disease process.
In conclusion, we found that oral inoculation of Salmonella, the natural route of infection, results in meningitis in both resistant and susceptible strains of mice. This meningitis includes the presence of Salmonella in the CNS as well as the recruitment of inflammatory cells to the brain. This provides a unique opportunity to dissect out these processes and gain a better understanding of the underlying mechanisms of disease progression that leads to Gram-negative bacterial meningitis.
Acknowledgments
We thank Suzette A. Priola, Byron Caughey, and Brent Race for critical reading of the manuscript; and Dan Long, Nancy Kurtz, and Aaron B. Carmody for technical assistance with experiments.
Footnotes
Supported in part by the National Institute of Allergy and Infectious Diseases Intramural Program and in part by NIH grant R01AI095395.
T.J.B., T.S., and T.A.N. contributed equally to this work.
Disclosures: None declared.
Current address of T.J.B., Department of Biomedical Sciences, Western Michigan University Homer Stryker M.D. School of Medicine, Kalamazoo, MI.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2016.09.002.
Supplemental Data
Salmonella infection and dissemination in Nramp1−/− mice. Nramp1−/− mice were orally infected with Salmonella and observed daily for signs of clinical illness. Brain, blood, liver, and spleen samples were collected up to 10 days after infection and processed for colony-forming units (CFUs). Mice with no detectable CFUs are shown with open symbols, with the limit of detection shown as a dotted line. Data represent individual mice. n = 3 to 14 mice per time point.
Salmonella infection and dissemination in Nramp1+/+ mice. Nramp1+/+ mice were orally infected with Salmonella and observed daily for signs of clinical and neurological illness. Brain, blood, liver, and spleen samples were collected up to 21 days after infection and processed for colony-forming units (CFUs). Mice showing rolling disease phenotype are highlighted in green. Mice collected with neurological disease are shown separately. Mice with no detectable CFUs are shown with open symbols, with the limit of detection shown as a dotted line. Data represent individual mice. n = 3 to 20 mice per time point.
Percentage of infiltrating immune cells in the brain over time in Nramp1−/− and Nramp1+/+ mice orally infected with Salmonella. Samples were collected on days 4 and 6 or days 7 and 14 postinfection (dpi), respectively. Nramp1−/− (red/orange) and Nramp1+/+ (blue/light blue) mice or mock infected (pink/white). Significance was determined by a one-way analysis of variance. Data represent means ± SD. n = 4 to 8 mice per time point. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 (one-way analysis of variance). DC, dendritic cell; NK, natural killer.
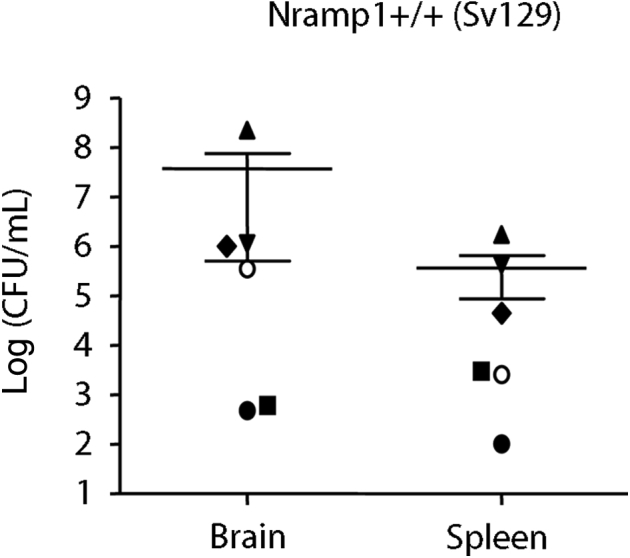
Percentage of different immune cells in the spleen in Nramp1−/− and Nramp1+/+ mice orally infected with Salmonella at 6 or 14 days after infection, respectively. Brain tissue from the mouse was used to estimate the bacterial load [colony-forming units (CFUs)] in the brain. Infected mice, where no CFUs were detected, are indicated as squares, whereas mice with CFUs detected are shown as triangles. Mock-infected Nramp1−/− and Nramp1+/+ mice were used as controls. Each symbol represents one mouse. NK, natural killer.
Immunofluorescence and hematoxylin and eosin (H&E) staining in Nramp1+/+ mice exhibiting rolling symptoms after Salmonella infection. Brain and skull tissue of Nramp1+/+ mice orally infected with Salmonella was fixed and either H&E or immunostained at 12 or 19 days after infection. A: Representative image of Salmonella-infected tissue from a mouse exhibiting rolling symptoms immunostained for glial fibrillary acidic protein (GFAP; green), Salmonella (red), and DAPI (blue). For orientation purposes, the olfactory bulb (OB) and cerebellum (CB) are labeled. B: Low-magnification image of skull tissue from the same mouse immunostained for Iba1 (green), Salmonella (red), and DAPI (blue). Arrowheads indicate location of eye (upper) and ear (lower). C–E: Representative images of Salmonella (C and E) and cellular infiltrate in the inner ear canal illustrating substantial otitis media. Arrowheads indicate location of bacterial staining. F: Low-magnification image of H&E-stained skull tissue. Arrowheads indicate location of eye (upper) and ear (lower). Scale bars: 3 mm (A); 5 mm (B); 400 μm (C); 100 μm (D); 50 μm (E); 5 mm (F).
References
- 1.Ramakrishnan M., Ulland A.J., Steinhardt L.C., Moïsi J.C., Were F., Levine O.S. Sequelae due to bacterial meningitis among African children: a systematic literature review. BMC Med. 2009;7:47. doi: 10.1186/1741-7015-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feasey N.A., Dougan G., Kingsley R.A., Heyderman R.S., Gordon M.A. Invasive non-typhoidal salmonella disease: an emerging and neglected tropical disease in Africa. Lancet. 2012;379:2489–2499. doi: 10.1016/S0140-6736(11)61752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swann O., Everett D.B., Furyk J.S., Harrison E.M., Msukwa M.T., Heyderman R.S., Molyneux E.M. Bacterial meningitis in Malawian infants less than 2 months of age: etiology and susceptibility to World Health Organization first-line antibiotics. Pediatr Infect Dis J. 2014;33:560–565. doi: 10.1097/INF.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu H.M., Huang W.Y., Lee M.L., Yang A.D., Chaou K.P., Hsieh L.Y. Clinical features, acute complications, and outcome of Salmonella meningitis in children under one year of age in Taiwan. BMC Infect Dis. 2011;11:30. doi: 10.1186/1471-2334-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryan J.P., Scheld W.M. Therapy of experimental meningitis due to Salmonella-enteritidis. Antimicrob Agents Chemother. 1992;36:949–954. doi: 10.1128/aac.36.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee W.S., Puthucheary S.D., Omar A. Salmonella meningitis and its complications in infants. J Paediatr Child Health. 1999;35:379–382. doi: 10.1046/j.1440-1754.1999.00387.x. [DOI] [PubMed] [Google Scholar]
- 7.Oliveira C.R., Morriss M.C., Mistrot J.G., Cantey J.B., Doern C.D., Sánchez P.J. Brain magnetic resonance imaging of infants with bacterial meningitis. J Pediatr. 2014;165:134–139. doi: 10.1016/j.jpeds.2014.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bollen W.S., Gunn B.M., Mo H., Lay M.K., Curtiss R., III Presence of wild-type and attenuated Salmonella enterica strains in brain tissues following inoculation of mice by different routes. Infect Immun. 2008;76:3268–3272. doi: 10.1128/IAI.00244-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wickham M.E., Brown N.F., Provias J., Finlay B.B., Coombes B.K. Oral infection of mice with Salmonella enterica serovar Typhimurium causes meningitis and infection of the brain. BMC Infect Dis. 2007;7:65–71. doi: 10.1186/1471-2334-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wessling-Resnick M. Nramp1 and other transporters involved in metal withholding during infection. J Biol Chem. 2015;290:18984–18990. doi: 10.1074/jbc.R115.643973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vidal S., Tremblay M.L., Govoni G., Gauthier S., Sebastiani G., Malo D., Skamene E., Olivier M., Jothy S., Gros P. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J Exp Med. 1995;182:655–666. doi: 10.1084/jem.182.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monack D.M., Mueller A., Falkow S. Persistent bacterial infections: the interface of the pathogen and the host immune system. Nat Rev Microbiol. 2004;2:747–765. doi: 10.1038/nrmicro955. [DOI] [PubMed] [Google Scholar]
- 13.Brown D.E., Libby S.J., Moreland S.M., McCoy M.W., Brabb T., Stepanek A., Fang F.C., Detweiler C.S. Salmonella enterica causes more severe inflammatory disease in C57/BL6 Nramp1G169 mice than Sv129S6 mice. Vet Pathol. 2013;50:867–876. doi: 10.1177/0300985813478213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown D.E., McCoy M.W., Pilonieta M.C., Nix R.N., Detweiler C.S. Chronic murine typhoid fever is a natural model of secondary hemophagocytic lymphohistiocytosis. PLoS One. 2010;5:e9441. doi: 10.1371/journal.pone.0009441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsolis R.M., Xavier M.N., Santos R.L., Bäumler A.J. How to become a top model: impact of animal experimentation on human Salmonella disease research. Infect Immun. 2011;79:1806–1814. doi: 10.1128/IAI.01369-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Govoni G., Vidal S., Gauthier S., Skamene E., Malo D., Gros P. The Bcg/Ity/Lsh locus: genetic transfer of resistance to infections in C57BL/6J mice transgenic for the Nramp1 Gly169 allele. Infect Immun. 1996;64:2923–2929. doi: 10.1128/iai.64.8.2923-2929.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoiseth S.K., Stocker B.A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 18.Loomis W.P., Johnson M.L., Brasfield A., Blanc M.P., Yi J., Miller S.I., Cookson B.T., Hajjar A.M. Temporal and anatomical host resistance to chronic Salmonella infection is quantitatively dictated by Nramp1 and influenced by host genetic background. PLoS One. 2014;9:e111763. doi: 10.1371/journal.pone.0111763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diab A., Abdalla H., Li H.L., Shi F.D., Zhu J., Höjberg B., Lindquist L., Wretlind B., Bakhiet M., Link H. Neutralization of macrophage inflammatory protein 2 (MIP-2) and MIP-1α attenuates neutrophil recruitment in the central nervous system during experimental bacterial meningitis. Infect Immun. 1999;67:2590–2601. doi: 10.1128/iai.67.5.2590-2601.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mildner A., Djukic M., Garbe D., Wellmer A., Kuziel W.A., Mack M., Nau R., Prinz M. Ly-6G+CCR2- myeloid cells rather than Ly-6ChighCCR2+ monocytes are required for the control of bacterial infection in the central nervous system. J Immunol. 2008;181:2713–2722. doi: 10.4049/jimmunol.181.4.2713. [DOI] [PubMed] [Google Scholar]
- 21.Bentlin M.R., Ferreira G.L., Rugolo L. Neonatal meningitis according to the microbiological diagnosis: a decade of experience in a tertiary center. Arq Neuropsiquiatr. 2010;68:882–887. doi: 10.1590/s0004-282x2010000600010. [DOI] [PubMed] [Google Scholar]
- 22.Johan A.J., Hung L.C., Norlijah O. Salmonella enteritidis ventriculitis. Southeast Asian J Trop Med Public Health. 2013;44:456–459. [PubMed] [Google Scholar]
- 23.Fritsche G., Nairz M., Libby S.J., Fang F.C., Weiss G. Slc11a1 (Nramp1) impairs growth of Salmonella enterica serovar typhimurium in macrophages via stimulation of lipocalin-2 expression. J Leukoc Biol. 2012;92:353–359. doi: 10.1189/jlb.1111554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dietzman D.E., Fischer G.W., Schoenknecht F.D. Neonatal Escherichia coli septicemia–bacterial counts in blood. J Pediatr. 1974;85:128–130. doi: 10.1016/s0022-3476(74)80308-2. [DOI] [PubMed] [Google Scholar]
- 25.Kim K.S. Escherichia coli translocation at the blood-brain barrier. Infect Immun. 2001;69:5217–5222. doi: 10.1128/IAI.69.9.5217-5222.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie Y., Kim K.J., Kim K.S. Current concepts on Escherichia coli K1 translocation of the blood-brain barrier. FEMS Immunol Med Microbiol. 2004;42:271–279. doi: 10.1016/j.femsim.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Italiani P., Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ribes S., Regen T., Meister T., Tauber S.C., Schütze S., Mildner A., Mack M., Hanisch U.K., Nau R. Resistance of the brain to Escherichia coli K1 infection depends on MyD88 signaling and the contribution of neutrophils and monocytes. Infect Immun. 2013;81:1810–1819. doi: 10.1128/IAI.01349-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Salmonella infection and dissemination in Nramp1−/− mice. Nramp1−/− mice were orally infected with Salmonella and observed daily for signs of clinical illness. Brain, blood, liver, and spleen samples were collected up to 10 days after infection and processed for colony-forming units (CFUs). Mice with no detectable CFUs are shown with open symbols, with the limit of detection shown as a dotted line. Data represent individual mice. n = 3 to 14 mice per time point.
Salmonella infection and dissemination in Nramp1+/+ mice. Nramp1+/+ mice were orally infected with Salmonella and observed daily for signs of clinical and neurological illness. Brain, blood, liver, and spleen samples were collected up to 21 days after infection and processed for colony-forming units (CFUs). Mice showing rolling disease phenotype are highlighted in green. Mice collected with neurological disease are shown separately. Mice with no detectable CFUs are shown with open symbols, with the limit of detection shown as a dotted line. Data represent individual mice. n = 3 to 20 mice per time point.
Percentage of infiltrating immune cells in the brain over time in Nramp1−/− and Nramp1+/+ mice orally infected with Salmonella. Samples were collected on days 4 and 6 or days 7 and 14 postinfection (dpi), respectively. Nramp1−/− (red/orange) and Nramp1+/+ (blue/light blue) mice or mock infected (pink/white). Significance was determined by a one-way analysis of variance. Data represent means ± SD. n = 4 to 8 mice per time point. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 (one-way analysis of variance). DC, dendritic cell; NK, natural killer.
Percentage of different immune cells in the spleen in Nramp1−/− and Nramp1+/+ mice orally infected with Salmonella at 6 or 14 days after infection, respectively. Brain tissue from the mouse was used to estimate the bacterial load [colony-forming units (CFUs)] in the brain. Infected mice, where no CFUs were detected, are indicated as squares, whereas mice with CFUs detected are shown as triangles. Mock-infected Nramp1−/− and Nramp1+/+ mice were used as controls. Each symbol represents one mouse. NK, natural killer.
Immunofluorescence and hematoxylin and eosin (H&E) staining in Nramp1+/+ mice exhibiting rolling symptoms after Salmonella infection. Brain and skull tissue of Nramp1+/+ mice orally infected with Salmonella was fixed and either H&E or immunostained at 12 or 19 days after infection. A: Representative image of Salmonella-infected tissue from a mouse exhibiting rolling symptoms immunostained for glial fibrillary acidic protein (GFAP; green), Salmonella (red), and DAPI (blue). For orientation purposes, the olfactory bulb (OB) and cerebellum (CB) are labeled. B: Low-magnification image of skull tissue from the same mouse immunostained for Iba1 (green), Salmonella (red), and DAPI (blue). Arrowheads indicate location of eye (upper) and ear (lower). C–E: Representative images of Salmonella (C and E) and cellular infiltrate in the inner ear canal illustrating substantial otitis media. Arrowheads indicate location of bacterial staining. F: Low-magnification image of H&E-stained skull tissue. Arrowheads indicate location of eye (upper) and ear (lower). Scale bars: 3 mm (A); 5 mm (B); 400 μm (C); 100 μm (D); 50 μm (E); 5 mm (F).