Abstract
Increasing evidence points to inflammation as one of the key players in diabetes-mediating adverse effects to the neuronal and vascular components of the retina. Sustained inflammation induces biochemical and molecular changes, ultimately contributing to retinal complications and vision loss in diabetic retinopathy. In this review, we describe changes involving metabolic abnormalities secondary to hyperglycemia, oxidative stress, and activation of transcription factors, together with neuroglial alterations in the diabetic retina. Changes in biochemical pathways and how they promote pathophysiologic developments involving proinflammatory cytokines, chemokines, and adhesion molecules are discussed. Inflammation-mediated leukostasis, retinal ischemia, and neovascularization and their contribution to pathological and clinical stages leading to vision loss in diabetic retinopathy (DR) are highlighted. In addition, potential treatment strategies involving fibrates, connexins, neuroprotectants, photobiomodulation, and anti-inflammatory agents against the development and progression of DR lesions are reviewed. The importance of appropriate animal models for testing novel strategies against DR lesions is discussed; in particular, a novel nonhuman primate model of DR and the suitability of rodent models are weighed. The purpose of this review is to highlight our current understanding of the pathogenesis of DR and to summarize recent advances using novel approaches or targets to investigate and inhibit the retinopathy.
Diabetic retinopathy (DR) is a major complication of diabetes and is the leading cause of visual impairment and blindness among working-age adults.1 Patients with DR may lose sight as a result of the development of diabetic macular edema (DME) and/or proliferative diabetic retinopathy.
The progression of advanced DR can be inhibited by laser-induced photocoagulation,2 but this procedure may destroy parts of the retina. Intravitreal injections of anti–vascular endothelial growth factor therapies3, 4, 5 or corticosteroids6, 7 can also appreciably diminish retinal neovascularization and retinal edema, but these injections require frequent visits to a physician and have only transitory beneficial effects in approximately half of treated patients.3 Use of corticosteroids may have adverse effects, leading to cataract formation and increased intraocular pressure in a significant number of patients8; therefore, its clinical use is limited.
The early stages of DR can be inhibited by improvement of glycemic control using either insulin or oral agents,9, 10 but this control remains difficult or impossible for many diabetics to achieve and maintain. Thus, there has been considerable effort to identify specific pharmacological targets to inhibit the development of retinopathy. Inhibitors of protein kinase C, aldose reductase (AR), nonenzymatic glycation, and vascular endothelial growth factor comprise just a few of the candidates that have been investigated as therapeutic targets against DR, but anti–vascular endothelial growth factor therapy has been unique among pharmacological approaches in showing efficacy in diabetic patients. Therapies developed for conditions other than diabetes or retinopathy, such as blood pressure medications11 and fibrates,12 also are reported to have beneficial effects on DR, although again only in a subset of diabetic patients. Thus, available therapies for DR are not equally effective in all patients.
Targets of DR Lesions for Which Treatment Is Needed
Major causes of clinically significant vision loss due to diabetes are generally accepted to be vascular in origin and include retinal edema and preretinal lesions, such as neovascularization, fibrovascular membranes, and hemorrhages. Vascular permeability, local ischemia, and preretinal neovascularization thus have a clear relationship to visual impairment in DR, and are appropriate targets for DR treatment.
Retinal neurons also are adversely affected in diabetes. They become dysfunctional, as evidenced by diabetes-induced changes in electroretinogram, contrast sensitivity, visual acuity, and color sensitivity, and these defects can impair the quality of vision. However, whether the functional defects or the death of retinal neurons contributes to clinically meaningful loss of vision in diabetes is not yet clear. Although many publications attribute the adverse effects of diabetes to neurodegeneration, it is not clear that cell death is the culprit, as opposed to less obvious metabolic or functional defects within remaining neurons. Thus, retinal neurons also might be a therapeutic target to inhibit the retinopathy and accompanying visual impairment or loss. More important, evidence is accumulating that a specialized kind of retinal neuron (photoreceptors) plays an important role in the diabetes-induced degeneration of retinal capillaries, which can subsequently lead to retinal neovascularization. These topics are discussed below.
Mechanisms Implicated in the Pathogenesis of DR
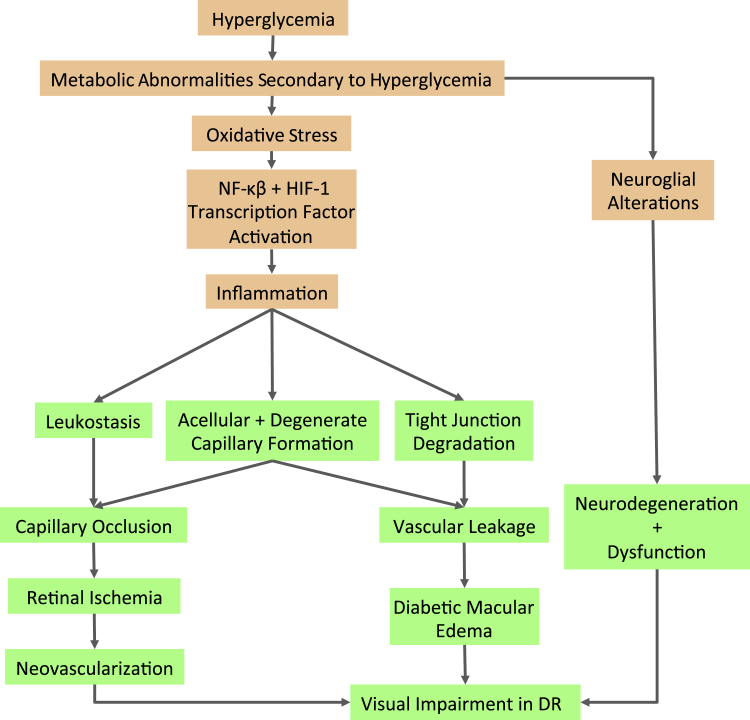
Although hyperglycemia has been demonstrated to initiate the pathology of DR, appreciable evidence suggests that oxidative stress and inflammatory changes in the retina play critical steps in the pathogenesis of the hyperglycemia-induced retinopathy (Figure 1). Recently, proinflammatory lipids,13 epigenetic and epigenomic modifications,14 insulin dysregulation,15 and β-cellulin signaling,16 which initiate and contribute to DR pathogenesis independently from hyperglycemic condition, have been identified.
Figure 1.
Effects of hyperglycemia (HG) on biochemical pathways and inflammation in diabetic retinopathy (DR) pathogenesis. In the HG condition, metabolic abnormalities secondary to hyperglycemia contribute to neuroglial alterations that can lead to neurodegeneration and dysfunction. Increased oxidative stress can promote NF-κB and hypoxia-inducible factor (HIF)-1 activation. In addition, increased activity of proinflammatory cytokine, chemokine, and adhesion molecule can result in tight junction degradation and acellular capillary formation. Leukostasis contributes to capillary occlusion and can lead to retinal ischemia. Diabetes-induced macular edema, and neovascularization, all significant pathological events in DR. The orange boxes represent stages leading to the development of retinal vascular lesions; green boxes, pathological and clinical stages leading to vision loss in DR.
Oxidative Stress
Increased production of reactive oxygen species (ROS) has been frequently identified in diabetic retinas. It appears to be causally involved in the development of the retinopathy as oral administration of antioxidants17 or genetic overexpression of superoxide dismutases18 has been shown to inhibit the diabetes-induced degeneration of retinal capillaries in animal studies. Moreover, up-regulation of ROS can lead to increased activation of NF-κB, which, in turn, increased the release of proinflammatory cytokines and nitric oxide (NO). Thus, oxidative stress might indirectly contribute to DR secondary to stimulation of inflammation or directly contribute to the retinopathy via oxidative damage to cells. Oxidative stress can be generated in multiple subcellular compartments, and each has been implicated in the pathogenesis of DR.
Mitochondrial Dysfunction
Hyperglycemia-induced electron transport chain dysfunction, resulting in electron leakage through complex I and complex III, leads to increased superoxide and ROS levels.19 In addition, mitochondrial DNA, which codes for 13 essential electron transport chain proteins, is prone to oxidative stress.20 Therefore, oxidative stress–induced damage of mitochondrial DNA often leads to impaired transcription of electron transport chain proteins, which compromises electron transport chain function and further escalates ROS production.20, 21 In addition, the inhibition of superoxides also inhibited glucose-induced release of proapoptotic cytochrome c and Bax in retinal pericytes and endothelial cells, thus further substantiating a causal link between mitochondrial oxidative stress and DR pathogenesis.22
Altered Levels of Nox
NADPH oxidase (Nox) enzymes catalyze the oxidation of NADPH to NADP in the cytosol and the reduction of oxygen across biological membranes to generate superoxide anions.23 Nox2 and Nox4 isoforms are overexpressed in retinal endothelial cells and vessels under hyperglycemic and hypoxic conditions, leading to elevated ROS production.24 Reducing several Nox isoform levels has been shown to alleviate several symptoms of DR, such as retinal neovascularization, blood retinal barrier breakdown, and leukostasis, thus connecting Nox activity to DR pathogenesis.25, 26
Uncoupled Proteins Influence NO Synthase and ROS Levels
Uncoupling of NO synthase has been shown to increase superoxide production.27 These superoxides can also react with NO to form peroxynitrite, which is a major contributor to cellular injury and oxidative stress.27 A polymorphism in the -3826A/G allele of uncoupling protein (UCP)-1 has also been reported to diminish UCP-1's protective effects against ROS, thus increasing oxidative stress in diabetic patients.28 In addition, 866G/A, Ala55Val, and 45-bp insertion/deletion polymorphisms in UCP-2 genes have been associated with the promotion of DR by also decreasing UCP-2's protective effects against ROS.29
Inflammation
Inflammation is a non-specific response by which the innate immune system of a host defends itself after exposure to an antigen or microorganism. Although acute inflammation generally yields beneficial results, such as tissue defense and repair, chronic inflammation often results in more damaging effects, such as cell death in the brain and retina.30 Typically, invading pathogens are recognized by pattern recognition receptors, such as Toll-like receptors. Binding of Toll-like receptors to pathogens is mediated by specific protein, carbohydrate, lipid, and nucleic acid sequences on the pathogen, called pathogen-associated molecular patterns.31 Activation of the Toll-like receptors facilitates the release of NF-κB, which then translocates into the nucleus to stimulate transcription of proinflammatory chemokines, such as IL-6, tumor necrosis factor-α, IL-1β, and monocyte chemoattractant protein-1.31 These proinflammatory chemokines play a major role in the recruitment and activation of leukocytes and the subsequent inflammatory responses, as discussed in Diabetes-Mediated Inflammation in Retinal Cell Types and Pathways Involved in Retinal Inflammation and Oxidative Stress in DR.
Inflammation and Diabetic Retinopathy
Increasing evidence implicates inflammation as a critical contributor to the development of DR.32 Many of the inflammatory mediators listed in the previous paragraph are activated in DR, but the signaling involved in initiating this response is less clear. Nevertheless, there is evidence that these inflammatory-like processes contribute to the pathogenesis of DR in both animal and patient studies, in that inhibition of proinflammatory enzymes or deletion of such enzymes inhibits diabetes-induced vascular pathology in animal models of DR.32 Studies also have shown that leukocytes play an important role in the structural and functional abnormalities that characterize DR.33, 34 In patients, the most compelling evidence that inflammatory processes play an important role in DR pathogenesis is the dramatic effect of corticosteroids on DME.7, 35 Therefore, the specific cell types that mediate these proinflammatory effects are of great interest.
Diabetes-Mediated Inflammation in Retinal Cell Types
Inflammatory cytokine release and leukocyte adhesion to the retinal vasculature are two hallmarks of early inflammation-mediated events in DR.36, 37 Such events may lead to retinal vascular cells and tight junctions becoming compromised,38, 39 which can lead to vascular leakage. Inflammatory molecules, such as tumor necrosis factor-α, IL-1β, IL-6, IL-8, intracellular adhesion molecule-1, vascular cell adhesion molecule 1, integrin β-2 (CD-18), and monocyte chemoattractant protein-1, play pivotal roles in the pathogenesis of DR lesions.
High glucose-induced glial cell dysfunction is known to be a facilitator of inflammatory molecule production and release. Müller cells exposed to hyperglycemic conditions produce increased levels of proinflammatory molecules, IL-8,40 NO, and cyclooxygenase-2.41 Furthermore, one study reported that the density of Müller cells increases significantly under hyperglycemic conditions,42 thus potentially leading to escalated production of proinflammatory cytokines. In addition, activated microglia have been observed to up-regulate proinflammatory mediators tumor necrosis factor-α, IL-1β, IL-6, and macrophage inflammatory protein-1.43 The resulting glial cell–activated neuroinflammation can thereby contribute to vascular breakdown, while promoting further glial and neuronal cell dysfunction.
Pathways Involved in Retinal Inflammation and Oxidative Stress in DR
p38 MAPK Pathway
Studies have shown that the p38 mitogen-activated protein kinase (MAPK) pathway controls other proinflammatory cytokine and chemokine gene expression by regulating NF-κB activity.44, 45 MAPK plays a significant role in the development of inflammation in the diabetic retina through up-regulation of IL-6. In retinal Müller cells, activation of the p38 MAPK/NF-κB signaling pathway increases the production of IL-6 via IL-1β.44 However, IL-6 possesses proinflammatory, anti-inflammatory, and apoptotic function in the retina.46 Pharmacological inhibition of the p38 MAPK pathway significantly inhibited diabetes-induced increases of several lesions characteristic of DR, such as acellular capillaries, pericyte ghosts, and adherent leukocytes in the retinal vasculature.47 Inhibition also reduced levels of proinflammatory mediators, such as intracellular adhesion molecule-1, inducible NO synthase, and superoxides.47 Therefore, targeting the p38 MAPK pathway may reduce the effects of inflammation in the pathogenesis of DR.
Polyol Pathway
Under hyperglycemic conditions, AR up-regulates reduction of glucose to sorbital.48 Sorbital dehydrogenase oxidizes the excess sorbital to fructose, which concurrently reduces NAD+ to NADH. The resultant increase in the intracellular NADH/NAD+ ratio leads to the inhibition of glyceraldehyde-3-phosphate dehydrogenase, which then contributes to increased triose phosphate production. Triose phosphate prompts the activation of advanced glycation end-products and protein kinase C (PKC) pathways by inducing formation of methylglyoxal, a precursor of diacylglycerol and advanced glycation end-products.49 In addition to an increase in sorbital expression, AR up-regulation results in excess oxidation of NADPH to NADP+.23 The oxidation of NADPH leaves less NADPH available for the production of the intracellular antioxidant, glutathione, thus facilitating the proliferation of ROS.
Although AR inhibitors were not previously considered as treatments for anti-inflammatory effects, studies demonstrated that the inhibitors actually possessed anti-inflammatory effects50, 51 through potential blocking of glutathione-lipid alcohol species formation. However, a recent clinical trial using AR inhibitor, epalrestat, prevented the progression of diabetic neuropathy, retinopathy, and nephropathy.52 A recent study indicated that diabetic mice lacking AR were protected from diabetes-induced reductions in visual function (namely, contrast sensitivity and spatial frequency threshold).53
PKC Pathway
A hyperglycemia-induced increase in ROS and diacylglycerol contributes to the activation of PKC. Up-regulation of PKC results in increased vascular permeability, neovascularization, and capillary apoptosis characteristic of DR.23 In addition, PKC increases the activity of Nox, leading to further ROS production, NF-κB activation, and inflammation, as demonstrated in animal studies.54 However, clinical trials failed to validate that inhibition of the PKC pathway adequately inhibited the progression of DR55 but did have effects on visual function.56
Novel Therapeutic Approaches and Targets to Inhibit DR
Recent studies examining the pathogenesis of DR are shedding light into new pharmacological approaches for inhibiting molecular alterations underlying aspects of the disease.57, 58, 59 Furthermore, studies have also identified new cellular contributors in the retinopathy beyond the common focus solely on the vasculature.58, 60, 61 Several recent lines of evidence have also identified potential new targets for inhibition of DR, and several Food and Drug Administration–approved drugs (against other diseases) have been found to inhibit the diabetes-induced degeneration of retinal capillaries characteristic of DR in diabetic rodents. Some of these repurposed drugs were initially identified as protective against DR in clinical trials, whereas others are being tested now only in animals. Such examples are discussed below.
Anti-Inflammatory Agents
One anti-inflammatory approach that has been investigated with regard to experimental treatment of experimental DR in animal models involves the inhibition of tumor necrosis factor-α with soluble receptor fusion proteins etanercept and pegsunercept.62 This treatment has been shown to down-regulate intracellular adhesion molecule-1 production, lower intercellular NF-κB concentrations, and decrease leukostasis and acellular capillary and pericyte ghost formation.62
Nonsteroidal anti-inflammatory drugs are a class of drugs that elicit anti-inflammatory effects by inhibiting cyclooxygenase enzyme-mediated prostaglandin formation.63, 64 At high doses, cyclooxygenase-2 inhibitor meloxicam has been shown to reduce endothelial NO synthase concentrations,62 NF-κB activation levels,37 and leukocyte adhesion62 in diabetic retinas. In addition, our study indicates that fenofibrate can reduce high glucose-induced cyclooxygenase-2 up-regulation to exhibit anti-inflammatory effects.65 Aspirin has also been shown to significantly reduce the adhesiveness of leukocytes62 and, at high doses, to minimize the development of microvascular lesions in patients in non-proliferative DR.64, 66, 67
Leukocytes have been shown to play a major role in the degeneration of retinal capillaries in DR.33, 34 Leukocyte integrin αmβ2 (alias CD11b/CD18 or MAC1), a protein mediating adhesion between leukocytes and endothelial cells, has been shown to facilitate such damage to endothelial cells by activating leukocytes.33 Diabetes causes a significant increase in leukocyte adhesion to the retinal microvasculature (leukostasis), and selective antagonism of that adhesion by expression of neutrophil inhibitory factor has been shown to inhibit the diabetes-induced degeneration of retinal capillaries.60 Leukocytes normally play an important role in immune function, so it is significant that the study also provided evidence that neutrophil inhibitory factor did not inhibit normal immune surveillance. Thus, inhibition of leukocyte adhesion to retinal endothelial cells is a potential therapeutic target to inhibit DR.
Fibrates
Fenofibrate is known as a cholesterol-lowering agent and lipid-modifying drug that regulates the expression of many different genes, with a range of beneficial effects on inflammation, angiogenesis, extracellular matrix overexpression, and cell apoptosis. Results from several clinical trials have demonstrated unexpected benefits also on DR. The Fenofibrate Intervention and Event Lowering in Diabetes study, conducted in Australia, Finland, and New Zealand, reported a 37% reduction in the need for laser surgery to prevent vision loss, and a 79% slowing in a two-step progression on the Early Treatment Diabetic Retinopathy Study score in subjects with existing retinopathy.12 The Action to Control Cardiovascular Risk in Diabetes eye trial confirmed the beneficial effects of fenofibrate on DR,68 showing a similar 40% reduction in progression of retinopathy with fenofibrate. Fenofibrate recently has been approved for use in Australia to slow the progression of existing DR in patients with type 2 diabetes. Originally considered a lipid-modifying drug, it now appears that multiple mechanisms may underpin the benefit of fenofibrate on diabetic microvascular end points.69
G-Protein–Coupled Receptor Agonists and Antagonists
Guanine nucleotide triphosphate-binding protein (G protein)–coupled receptors form a large diverse superfamily of membrane proteins encoded by >800 genes in the human genome.70 They respond to a variety of extracellular signals, including photons, ions, small organic molecules, and proteins, that lead to conformational changes and cause activation of cytosolic signaling through activation of one or several G proteins. Subsequently, these G-protein–coupled receptors regulate effector molecules, such as calcium, potassium channels, adenylate cyclase, phospholipase C, and protein kinases.
Recently, two classes of extracellular G-protein–coupled receptors (adrenergic and serotonergic receptors) have been shown to participate also in the pathogenesis of early stages of DR, including the diabetes-induced increase in retinal oxidative stress and expression of proinflammatory proteins.58 Moreover, pharmacological inhibition of either the α1-adrenergic receptor or downstream NADPH oxidase (both components of the Gq-regulated signaling pathway) with doxazosin or apocyinin, respectively, lowered the diabetes-induced increase in retinal oxidative stress, expression of proinflammatory proteins by the retina, and the resulting degeneration of retinal capillaries.58 The results show that overactive Gq signaling plays a causal role in the development of DR.
Likewise, there is also evidence for a role of the β-adrenergic receptor in DR pathogenesis. β-Adrenergic receptors signal via the Gs pathway, leading to the accumulation of cyclic adenosine monophosphate. Loss of adrenergic input is common in diabetes, and treatment with β-adrenergic receptor antagonists71 or deletion of β2-adrenergic receptors in mice72 replicated features of DR, even in the absence of diabetes. Compound 49b, a β1/β2-adrenergic receptor agonist, inhibited the diabetes-induced degeneration of retinal capillaries and loss of capillary pericytes and ganglion cells.73 In addition, daily administration of the β-adrenergic receptor agonist, isoproterenol, significantly reduced the diabetes-induced loss of electroretinographic amplitudes, inhibited apoptosis of retinal neural cells, and decreased the numbers of degenerate capillaries.71, 74, 75
Photoreceptors and Modulation of the Visual Cycle
Photoreceptors and retinal pigment epithelium (RPE) of the outer retina form a functional unit inasmuch as both cells are needed to complete the visual cycle for vision. However, neither of these cells has been commonly regarded as important in the pathogenesis of DR. Nevertheless, accumulating evidence raises a possibility that the unique susceptibility of the retina to injury in diabetes may, in fact, be because of the effects of photoreceptors and/or RPE.
Arden76 sent a survey to a group of diabetic patients who also had retinitis pigmentosa, and found that DR seemed less severe in diabetics who also had retinitis pigmentosa (and therefore, photoreceptor degeneration). de Gooyer et al77 subsequently reported that diabetes did not cause the expected decrease in density of the retinal microvasculature in mice lacking most photoreceptors secondary to deficiency of opsin (Rho−/−). We recently confirmed this using opsin-deficient mice and in mice having a P23H mutant opsin knocked in, and extended this observation by showing that the stress in photoreceptors undergoing degeneration greatly exacerbated capillary degeneration, but that this stress is reduced after the photoreceptors have degenerated. Photoreceptors do not characteristically degenerate because of diabetes,78 so they manifest a continuous stress that includes increased oxidative stress60 and affects the vasculature.
Hypotheses that might help explain how photoreceptors influence the development of DR include hypoxia79, 80 and oxidative stress.60 These hypotheses are not mutually exclusive.
RPE Data
The RPE is a specialized epithelium that affects photoreceptor (and thus, retinal) function and integrity. Main functions of the RPE include transport of nutrients, ions, and water, absorption of light, protection against photo-oxidation, reisomerization of all-trans-retinal into 11-cis-retinal, which is crucial for the visual cycle, phagocytosis of shed photoreceptor membranes, and secretion of essential factors for the structural integrity of the retina.81 Diabetes has been found to affect many of these functions, but the contribution of the RPE to the pathogenesis of DR remained largely unknown. Nevertheless, several abnormalities induced in RPE in diabetes (excessive production of vascular endothelial growth factor, subnormal production of pigment epithelium-derived factor,82 and increased permeability across the RPE,83, 84 potentially allowing excessive water influx into the retina83) have a clear relationship with the retinopathy.
The role of RPE in early retinal vascular abnormalities of diabetes was recently tested.59 Retinylamine is an analog of vitamin A that is selectively retained by only two tissues in the body, RPE and liver. It is an effective trap for reactive aldehydes, and is known to inhibit the RPE-specific protein 65 kDa,85, 86 an enzyme of the visual cycle that converts all-trans-retinol esters back to 11-cis-retinal.87, 88 Administration of retinylamine once per week to diabetic mice significantly inhibited the diabetes-induced increase in retinal production of superoxide and expression of proinflammatory proteins. More important, this therapy, which acts in the RPE, also inhibited retinal lesions that are clinically relevant in DR (degeneration of retinal capillaries and increased permeability).59 The available data suggest that RPE cells and possibly also the visual cycle contribute to the development of DR. Neither the RPE nor enzymes of the visual cycle have previously been identified as potential contributors to the pathogenesis of DR or as potential targets for therapeutic inhibition of DR.
Photobiomodulation
Photobiomodulation is the application of low-level light that has a biological effect. Numerous studies have shown that light in the far-red to near-infrared region of the spectrum (630 to 1000 nm) can have beneficial effects in vitro and in vivo to heal existing tissue damage and to inhibit the development of tissue pathology.89, 90, 91, 92 Studies related to the retina likewise have demonstrated that the low-intensity far-red (670 nm) light treatment mitigates pathology in retinal degeneration models93, 94 and, recently, also in DR.95, 96 In both diabetic albino rats96 and pigmented mice,97 whole-body exposure to far-red light (670 nm) for only 4 minutes per day from the onset of diabetes or as an intervention mitigated abnormalities that are believed to contribute to DR, including increased generation of superoxide, induction of a local proinflammatory environment, and dysfunction or degeneration of retinal neurons. Consistent with this, daily photobiomodulation administered to a small series of diabetic patients having non–center-involved macular edema was associated with the gradual reduction in retinal thickness.95
Arden et al98 likewise used light to exert a beneficial effect on DME. In one study, they used trans-eyelid retinal illumination of one eye in patients with DR during sleep throughout a 3-month period, and found a reduction in the number of hemorrhages and microaneurysms compared to the contralateral eyes.98 In another study by the same group, diabetic patients with early (non–sight-threatening) DME wore a mask that illuminated one closed eye with 505-nm light over 6 months while they slept.57 This treatment likewise showed regression of DME and improved visual function. They postulated that avoiding periods of darkness by providing weak nocturnal illumination reduced metabolic activity of rods, thereby reducing the hypoxia that develops in the retina, especially when the vasculature is compromised (such as in diabetes).
Connexins
Studies have demonstrated that the gap junction protein, connexin 43 (Cx43), plays a critical role in regulating intracellular communication and vascular homeostasis in the retina.99, 100 Down-regulation of Cx43 expression subsequently decreases cell-cell communication to promote vascular cell death and vascular permeability under high glucose conditions.100 However, the role of high glucose–induced changes in Cx43 expression in human DR, and the potential application of this knowledge in the treatment of DR, is only beginning to be understood. Our findings demonstrated that high glucose–induced Cx43 up-regulation may have protective effects on retinal Müller cells,99 whereas a decreased level of Cx43 alone is sufficient to induce retinal vascular cell apoptosis.100 Likewise, reduced levels of Cx43 are associated with retinal vascular lesions in human DR.61
Neuroprotectants
Although there is evidence that the death of retinal neurons develops before that of vascular cells in both animal and human models,101 suggesting the importance of neurodegeneration in the pathogenesis of DR, it is not yet clear that cell death is the culprit, as opposed to less obvious metabolic or functional defects within remaining neurons. Nonetheless, neuroprotective treatment strategies have been shown to treat retinopathy and accompanying visual impairment or loss.102 Pigment epithelium-derived factor injections in diabetic rats have been shown to suppress induction of glial fibrillary acidic protein, reduce oxidative stress–mediated neurodegeneration, and inhibit blood retinal barrier breakdown in diabetes.103 In addition, intravitreal injections of pigment epithelium-derived factor in diabetic rats protected retinal neurons from glutamate toxicity, likely by decreasing IL-1β levels.104 Intravitreal injections of exogenous erythropoietin inhibit retinal cell death and protect blood retinal barrier structure in diabetic rats105 and improve visual acuity in patients with chronic DME.106 Administration of a somatostatin analog had a direct antiangiogenic effect in the retina of patients with proliferative diabetic retinopathy.107
Are Laboratory Rodent Models of DR Suitable for the Assessment of Potential Therapies?
Rodent models of diabetes have played a critical role in improving our understanding of the pathogenesis of diabetic retinopathy. These animal models were used for identification of biochemical and structural changes associated with structural or functional defects of the retina in diabetes.24, 49, 80, 108 Investigators have made significant progress in identifying changes associated with gene expression in different retinal cell types, and biochemical sequelae triggered by hyperglycemia, which have contributed to improved understanding of the development of molecular and cellular events that occur in DR.100, 109, 110, 111, 112
Despite the enormous popularity of rodent models in DR research, the applicability and suitability of rodent models for the assessment of potential therapies remains an open question. Rodent models have certainly proved useful in providing much of our current understanding regarding the response of the retinal cells to the diabetic milieu, and it is expected that these models would continue to provide insight into the molecular mechanisms contributing to specific abnormalities, including capillary degeneration, vascular permeability, and neovascularization. However, the rodent model has its limitations for the study of DR, because it lacks the macula, the region responsible for vision acuity, which is severely affected in diabetes. This makes it inherently difficult to study mechanisms underlying the development and progression of DME as well as development of new therapies directed for macula-related diseases. It is certain that while rodent models will retain their usefulness and applicability in the area of research, other animal models would offer the ability to study unique areas presently lacking in the rodent models.
Primates
Primates are evolutionarily closer to humans than rodents and, therefore, knowledge gained from studying primate models of diseases is likely to be more physiologically relevant and applicable toward treatment strategies for humans. Significant impediments in using the primate model for DR research currently are the long durations for retinopathy to develop in primates, and the cost involved in maintaining these primates in institutional animal research facilities over that interval. The common marmoset, a new world primate, has been shown to be an excellent model for studying diseases, including ocular diseases such as exudative age-related macular degeneration with choroidal neovascularization, glaucoma, and myopia.113, 114, 115, 116
Experimentally galactosemic marmosets recently have been shown to develop lesions that are characteristic of nonproliferative DR in patients (including degenerate capillaries, microaneurysms, and vascular permeability) within 2 years of disease onset.117, 118 There are two significant additional features of this primate model. The first is that these animals are small, and so are well suited for the space limitations of most research facilities. The second is that, in addition to the lesions of early DR, galactose-fed marmosets develop also DME, which cannot be studied in any rodent models of diabetes (rodents and other laboratory animals lack a macula). Therefore, this model offers a unique platform for studying vascular and macular changes in diabetic-like retinopathy as well as testing novel drugs in an animal model that is a step closer to human DR.
Conclusions
Ongoing research continues to offer new insights into the pathogenesis of DR. Vascular lesions seem to account for most clinically meaningful vision loss in DR, but accumulating evidence shows that multiple different cell types (from inside and outside the eye) actively contribute to the development of those structural and functional changes that compose the retinopathy. Each of these different cell types and molecular alterations identified offer new potential therapeutic sites at which to inhibit the retinopathy. Studies to date have offered statistical insight on the efficacy of a given therapy toward a population of patients or animals, but biomarkers or other ways to predict which patients are most at risk of developing sight-threatening DR, or who will respond to a particular therapy, are needed in the future.
Footnotes
Supported by the National Eye Institute grants NIH EY018218 and EY025528 (S.R.), the Boston University Undergraduate Research Opportunities Program award (B.S.), and a Merit grant from the Department of Veteran Affairs (T.S.K.).
Disclosures: None declared.
References
- 1.Centers for Disease Control and Prevention: National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. Atlanta, GA, CDC, 2014. Available at https://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf (accessed January 4, 2016)
- 2.The Diabetic Retinopathy Study Research Group Photocoagulation treatment of proliferative diabetic retinopathy: clinical application of Diabetic Retinopathy Study (DRS) findings, DRS report number 8. Ophthalmology. 1981;88:583–600. [PubMed] [Google Scholar]
- 3.Elman M.J., Aiello L.P., Beck R.W., Bressler N.M., Bressler S.B., Edwards A.R., Ferris F.L., 3rd, Friedman S.M., Glassman A.R., Miller K.M., Scott I.U., Stockdale C.R., Sun J.K., Diabetic Retinopathy Clinical Research Network Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–1077e35. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen Q.D., Brown D.M., Marcus D.M., Boyer D.S., Patel S., Feiner L., Gibson A., Sy J., Rundle A.C., Hopkins J.J., Rubio R.G., Ehrlich J.S., RISE and RIDE Research Group Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 5.Jardeleza M.S., Miller J.W. Review of anti-VEGF therapy in proliferative diabetic retinopathy. Semin Ophthalmol. 2009;24:87–92. doi: 10.1080/08820530902800330. [DOI] [PubMed] [Google Scholar]
- 6.Grover D., Li T.J., Chong C.C. Intravitreal steroids for macular edema in diabetes. Cochrane Database Syst Rev. 2008;(1):CD005656. doi: 10.1002/14651858.CD005656.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva P.S., Sun J.K., Aiello L.P. Role of steroids in the management of diabetic macular edema and proliferative diabetic retinopathy. Semin Ophthalmol. 2009;24:93–99. doi: 10.1080/08820530902800355. [DOI] [PubMed] [Google Scholar]
- 8.Erol N., Topba S. Complications of intravitreal triamcinolone acetonide. Surv Ophthalmol. 2009;54:427. doi: 10.1016/j.survophthal.2009.02.012. author reply-8. [DOI] [PubMed] [Google Scholar]
- 9.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 10.UK Prospective Diabetes Study Group Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ. 1998;317:713–720. [PMC free article] [PubMed] [Google Scholar]
- 11.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 12.Keech A.C., Mitchell P., Summanen P.A., O'Day J., Davis T.M., Moffitt M.S., Taskinen M.R., Simes R.J., Tse D., Williamson E., Merrifield A., Laatikainen L.T., d'Emden M.C., Crimet D.C., O'Connell R.L., Colman P.G. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370:1687–1697. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 13.Crosby-Nwaobi R., Chatziralli I., Sergentanis T., Dew T., Forbes A., Sivaprasad S. Cross talk between lipid metabolism and inflammatory markers in patients with diabetic retinopathy. J Diabetes Res. 2015;2015:191382. doi: 10.1155/2015/191382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kowluru R.A., Mishra M. Contribution of epigenetics in diabetic retinopathy. Sci China Life Sci. 2015;58:556–563. doi: 10.1007/s11427-015-4853-0. [DOI] [PubMed] [Google Scholar]
- 15.Gardner T.W., Abcouwer S.F., Barber A.J., Jackson G.R. An integrated approach to diabetic retinopathy research. Arch Opthalmol. 2011;129:230–235. doi: 10.1001/archophthalmol.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anand-Apte B., Ebrahem Q., Cutler A., Farage E., Sugimoto M., Hollyfield J., Folkman J. Betacellulin induces increased retinal vascular permeability in mice. PLoS One. 2010;5:e13444. doi: 10.1371/journal.pone.0013444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kowluru R.A., Tang J., Kern T.S. Abnormalities of retinal metabolism in diabetes and experimental galactosemia, VII: effect of long-term administration of antioxidants on the development of retinopathy. Diabetes. 2001;50:1938–1942. doi: 10.2337/diabetes.50.8.1938. [DOI] [PubMed] [Google Scholar]
- 18.Kanwar M., Chan P.S., Kern T.S., Kowluru R.A. Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Invest Ophthalmol Vis Sci. 2007;48:3805–3811. doi: 10.1167/iovs.06-1280. [DOI] [PubMed] [Google Scholar]
- 19.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scarpulla R.C. Nucleus-encoded regulators of mitochondrial function: integration of respiratory chain expression, nutrient sensing and metabolic stress. Biochim Biophys Acta. 2012;1819:1088–1097. doi: 10.1016/j.bbagrm.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madsen-Bouterse S.A., Zhong Q., Mohammad G., Ho Y.S., Kowluru R.A. Oxidative damage of mitochondrial DNA in diabetes and its protection by manganese superoxide dismutase. Free Radic Res. 2010;44:313–321. doi: 10.3109/10715760903494168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowluru R.A., Abbas S.N. Diabetes-induced mitochondrial dysfunction in the retina. Invest Ophthalmol Vis Sci. 2003;44:5327–5334. doi: 10.1167/iovs.03-0353. [DOI] [PubMed] [Google Scholar]
- 23.Kowluru R.A., Mishra M. Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim Biophys Acta. 2015;1852:2474–2483. doi: 10.1016/j.bbadis.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Coucha M., Elshaer S.L., Eldahshan W.S., Mysona B.A., El-Remessy A.B. Molecular mechanisms of diabetic retinopathy: potential therapeutic targets. Middle East Afr J Ophthalmol. 2015;22:135–144. doi: 10.4103/0974-9233.154386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Shabrawey M., Bartoli M., El-Remessy A., Ma G., Matragoon S., Lemtalsi T., Caldwell R.W., Caldwell R.B. Role of NADPH oxidase and STAT3 in statin-mediated protection against diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49:3231–3238. doi: 10.1167/iovs.08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Shabrawey M., Bartoli M., El-Remessy A.B., Platt D.H., Matragoon S., Behzadian M.A., Caldwell R.W., Caldwell R.B. Inhibition of NAD(P)H oxidase activity blocks vascular endothelial growth factor overexpression and neovascularization during ischemic retinopathy. Am J Pathol. 2005;167:599–607. doi: 10.1016/S0002-9440(10)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narayanan S.P., Rojas M., Suwanpradid J., Toque H.A., Caldwell R.W., Caldwell R.B. Arginase in retinopathy. Prog Retin Eye Res. 2013;36:260–280. doi: 10.1016/j.preteyeres.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brondani L.A., de Souza B.M., Duarte G.C., Kliemann L.M., Esteves J.F., Marcon A.S., Gross J.L., Canani L.H., Crispim D. The UCP1 -3826A/G polymorphism is associated with diabetic retinopathy and increased UCP1 and MnSOD2 gene expression in human retina. Invest Ophthalmol Vis Sci. 2012;53:7449–7457. doi: 10.1167/iovs.12-10660. [DOI] [PubMed] [Google Scholar]
- 29.Crispim D., Fagundes N.J., dos Santos K.G., Rheinheimer J., Boucas A.P., de Souza B.M., Macedo G.S., Leiria L.B., Gross J.L., Canani L.H. Polymorphisms of the UCP2 gene are associated with proliferative diabetic retinopathy in patients with diabetes mellitus. Clin Endocrinol. 2010;72:612–619. doi: 10.1111/j.1365-2265.2009.03684.x. [DOI] [PubMed] [Google Scholar]
- 30.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 31.Jialal I., Kaur H. The role of Toll-like receptors in diabetes-induced inflammation: implications for vascular complications. Curr Diab Rep. 2012;12:172–179. doi: 10.1007/s11892-012-0258-7. [DOI] [PubMed] [Google Scholar]
- 32.Tang J., Kern T.S. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30:343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joussen A.M., Murata T., Tsujikawa A., Kirchhof B., Bursell S.E., Adamis A.P. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol. 2001;158:147–152. doi: 10.1016/S0002-9440(10)63952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talahalli R., Zarini S., Tang J., Li G., Murphy R., Kern T.S., Gubitosi-Klug R.A. Leukocytes regulate retinal capillary degeneration in the diabetic mouse via generation of leukotrienes. J Leukoc Biol. 2013;93:135–143. doi: 10.1189/jlb.0112025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomez-Ulla F., Marticorena J., Alfaro D.V., 3rd, Fernandez M., Mendez E.R., Rothen M. Intravitreal triamcinolone for the treatment of diabetic macular edema. Curr Diabetes Rev. 2006;2:99–112. doi: 10.2174/157339906775473572. [DOI] [PubMed] [Google Scholar]
- 36.Adamiec-Mroczek J., Oficjalska-Mlynczak J., Misiuk-Hojlo M. Roles of endothelin-1 and selected proinflammatory cytokines in the pathogenesis of proliferative diabetic retinopathy: analysis of vitreous samples. Cytokine. 2010;49:269–274. doi: 10.1016/j.cyto.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Kern T.S. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res. 2007;2007:95103. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Behl Y., Krothapalli P., Desta T., DiPiazza A., Roy S., Graves D.T. Diabetes-enhanced tumor necrosis factor-alpha production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy. Am J Pathol. 2008;172:1411–1418. doi: 10.2353/ajpath.2008.071070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aveleira C.A., Lin C.M., Abcouwer S.F., Ambrosio A.F., Antonetti D.A. TNF-alpha signals through PKCzeta/NF-kappaB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes. 2010;59:2872–2882. doi: 10.2337/db09-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X., Ye F., Xiong H., Hu D., Limb G.A., Xie T., Peng L., Yang W., Sun Y., Zhou M., Song E., Zhang D.Y. IL-1beta upregulates IL-8 production in human Muller cells through activation of the p38 MAPK and ERK1/2 signaling pathways. Inflammation. 2014;37:1486–1495. doi: 10.1007/s10753-014-9874-5. [DOI] [PubMed] [Google Scholar]
- 41.Du Y., Sarthy V.P., Kern T.S. Interaction between NO and COX pathways in retinal cells exposed to elevated glucose and retina of diabetic rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R735–R741. doi: 10.1152/ajpregu.00080.2003. [DOI] [PubMed] [Google Scholar]
- 42.Rungger-Brandle E., Dosso A.A., Leuenberger P.M. Glial reactivity, an early feature of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2000;41:1971–1980. [PubMed] [Google Scholar]
- 43.Grigsby J.G., Cardona S.M., Pouw C.E., Muniz A., Mendiola A.S., Tsin A.T., Allen D.M., Cardona A.E. The role of microglia in diabetic retinopathy. J Ophthalmol. 2014;2014:705783. doi: 10.1155/2014/705783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu X., Ye F., Xiong H., Hu D.N., Limb G.A., Xie T., Peng L., Zhang P., Wei Y., Zhang W., Wang J., Wu H., Lee P., Song E., Zhang D.Y. IL-1beta induces IL-6 production in retinal Muller cells predominantly through the activation of p38 MAPK/NF-kappaB signaling pathway. Exp Cell Res. 2015;331:223–231. doi: 10.1016/j.yexcr.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 45.Saccani S., Pantano S., Natoli G. p38-Dependent marking of inflammatory genes for increased NF-kappa B recruitment. Nat Immunol. 2002;3:69–75. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- 46.Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 47.Du Y., Tang J., Li G., Berti-Mattera L., Lee C.A., Bartkowski D., Gale D., Monahan J., Niesman M.R., Alton G., Kern T.S. Effects of p38 MAPK inhibition on early stages of diabetic retinopathy and sensory nerve function. Invest Ophthalmol Vis Sci. 2010;51:2158–2164. doi: 10.1167/iovs.09-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kowluru R.A., Chan P.S. Oxidative stress and diabetic retinopathy. Exp Diabetes Res. 2007;2007:43603. doi: 10.1155/2007/43603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 50.Ramana K.V., Srivastava S.K. Aldose reductase: a novel therapeutic target for inflammatory pathologies. Int J Biochem Cell Biol. 2010;42:17–20. doi: 10.1016/j.biocel.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Srivastava S.K., Yadav U.C., Reddy A.B., Saxena A., Tammali R., Shoeb M., Ansari N.H., Bhatnagar A., Petrash M.J., Srivastava S., Ramana K.V. Aldose reductase inhibition suppresses oxidative stress-induced inflammatory disorders. Chem Biol Interact. 2011;191:330–338. doi: 10.1016/j.cbi.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hotta N., Kawamori R., Fukuda M., Shigeta Y., Aldose Reductase Inhibitor-Diabetes Complications Trial Study Group Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on progression of diabetic neuropathy and other microvascular complications: multivariate epidemiological analysis based on patient background factors and severity of diabetic neuropathy. Diabet Med. 2012;29:1529–1533. doi: 10.1111/j.1464-5491.2012.03684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee C.A., Li G., Patel M.D., Petrash J.M., Benetz B.A., Veenstra A., Amengual J., Von Lintig J., Burant C., Tang J., Kern T.S. Diabetes-induced impairment in visual function in mice: contributions of p38 MAPK, RAGE, leukocytes, and aldose reductase. Invest Ophthalmol Vis Sci. 2013;93:135–143. doi: 10.1167/iovs.13-11659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zong H., Ward M., Stitt A.W. AGEs, RAGE, and diabetic retinopathy. Curr Diab Rep. 2011;11:244–252. doi: 10.1007/s11892-011-0198-7. [DOI] [PubMed] [Google Scholar]
- 55.PKC-DRS Study Group The effect of ruboxistaurin on visual loss in patients with moderately severe to very severe nonproliferative diabetic retinopathy: initial results of the Protein Kinase C beta Inhibitor Diabetic Retinopathy Study (PKC-DRS) multicenter randomized clinical trial. Diabetes. 2005;54:2188–2197. doi: 10.2337/diabetes.54.7.2188. [DOI] [PubMed] [Google Scholar]
- 56.Aiello L.P., Davis M.D., Girach A., Kles K.A., Milton R.C., Sheetz M.J., Vignati L., Zhi X.E. Effect of ruboxistaurin on visual loss in patients with diabetic retinopathy. Ophthalmology. 2006;113:2221–2230. doi: 10.1016/j.ophtha.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 57.Arden G.B., Jyothi S., Hogg C.H., Lee Y.F., Sivaprasad S. Regression of early diabetic macular oedema is associated with prevention of dark adaptation. Eye (London, England) 2011;25:1546–1554. doi: 10.1038/eye.2011.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Du Y., Cramer M., Lee C., Tang J., Muthusamy A., Antonetti D., Jin H., Palczewski K., Kern T. Adrenergic and serotonin receptors affect retinal superoxide generation in diabetic mice: relationship to capillary degeneration and permeability. FASEB J. 2015;29:2194–2204. doi: 10.1096/fj.14-269431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu H., Tang J., Du Y., Lee C.A., Golczak M., Muthusamy A., Antonetti D.A., Veenstra A.A., Amengual J., von Lintig J., Palczewski K., Kern T.S. Retinylamine benefits early diabetic retinopathy in mice. J Biol Chem. 2015;290:21568–21579. doi: 10.1074/jbc.M115.655555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Du Y., Veenstra A., Palczewski K., Kern T.S. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proc Natl Acad Sci U S A. 2013;110:16586–16591. doi: 10.1073/pnas.1314575110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tien T., Muto T., Zhang J., Sohn E.H., Mullins R.F., Roy S. Association of reduced connexin 43 expression with retinal vascular lesions in human diabetic retinopathy. Exp Eye Res. 2016;146:103–106. doi: 10.1016/j.exer.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 62.Joussen A.M., Poulaki V., Mitsiades N., Kirchhof B., Koizumi K., Dohmen S., Adamis A.P. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB J. 2002;16:438–440. doi: 10.1096/fj.01-0707fje. [DOI] [PubMed] [Google Scholar]
- 63.Agrawal N.K., Kant S. Targeting inflammation in diabetes: newer therapeutic options. World J Diabetes. 2014;5:697–710. doi: 10.4239/wjd.v5.i5.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kastelan S., Tomic M., Gverovic Antunica A., Salopek Rabatic J., Ljubic S. Inflammation and pharmacological treatment in diabetic retinopathy. Mediators Inflamm. 2013;2013:213130. doi: 10.1155/2013/213130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roy S., Kim D., Hernandez C., Simo R., Roy S. Beneficial effects of fenofibric acid on overexpression of extracellular matrix components, COX-2, and impairment of endothelial permeability associated with diabetic retinopathy. Exp Eye Res. 2015;140:124–129. doi: 10.1016/j.exer.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.The DAMAD Study Group Effect of aspirin alone and aspirin plus dipyridamole in early diabetic retinopathy: a multicenter randomized controlled clinical trial. Diabetes. 1989;38:491–498. [PubMed] [Google Scholar]
- 67.Early Treatment Diabetic Retinopathy Study Research Group Effects of aspirin treatment on diabetic retinopathy: ETDRS report number 8. Ophthalmology. 1991;98:757–765. [PubMed] [Google Scholar]
- 68.Chew E.Y., Ambrosius W.T., Davis M.D., Danis R.P., Gangaputra S., Greven C.M., Hubbard L., Esser B.A., Lovato J.F., Perdue L.H., Goff D.C., Jr., Cushman W.C., Ginsberg H.N., Elam M.B., Genuth S., Gerstein H.C., Schubart U., Fine L.J. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Noonan J.E., Jenkins A.J., Ma J.X., Keech A.C., Wang J.J., Lamoureux E.L. An update on the molecular actions of fenofibrate and its clinical effects on diabetic retinopathy and other microvascular end points in patients with diabetes. Diabetes. 2013;62:3968–3975. doi: 10.2337/db13-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fredriksson R., Lagerstrom M.C., Lundin L.G., Schioth H.B. The G-protein-coupled receptors in the human genome form five main families: phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 71.Jiang Y., Walker R.J., Kern T.S., Steinle J.J. Application of isoproterenol inhibits diabetic-like changes in the rat retina. Exp Eye Res. 2010;91:171–179. doi: 10.1016/j.exer.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang Y., Zhang Q., Liu L., Tang J., Kern T.S., Steinle J.J. Beta2-adrenergic receptor knockout mice exhibit a diabetic retinopathy phenotype. PLoS One. 2013;8:e70555. doi: 10.1371/journal.pone.0070555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Q., Guy K., Pagadala J., Jiang Y., Walker R.J., Liu L., Soderland C., Kern T.S., Ferry R., Jr., He H., Yates C.R., Miller D.D., Steinle J.J. Compound 49b prevents diabetes-induced apoptosis through increased IGFBP-3 levels. Invest Ophthalmol Vis Sci. 2012;53:3004–3013. doi: 10.1167/iovs.11-8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Steinle J.J., Chin V.C., Williams K.P., Panjala S.R. Beta-adrenergic receptor stimulation modulates iNOS protein levels through p38 and ERK1/2 signaling in human retinal endothelial cells. Exp Eye Res. 2008;87:30–34. doi: 10.1016/j.exer.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 75.Walker R.J., Steinle J.J. Role of beta-adrenergic receptors in inflammatory marker expression in Muller cells. Invest Ophthalmol Vis Sci. 2007;48:5276–5281. doi: 10.1167/iovs.07-0129. [DOI] [PubMed] [Google Scholar]
- 76.Arden G.B. The absence of diabetic retinopathy in patients with retinitis pigmentosa: implications for pathophysiology and possible treatment. Br J Ophthalmol. 2001;85:366–370. doi: 10.1136/bjo.85.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Gooyer T.E., Stevenson K.A., Humphries P., Simpson D.A., Gardiner T.A., Stitt A.W. Retinopathy is reduced during experimental diabetes in a mouse model of outer retinal degeneration. Invest Ophthalmol Vis Sci. 2006;47:5561–5568. doi: 10.1167/iovs.06-0647. [DOI] [PubMed] [Google Scholar]
- 78.Kern T.S., Berkowitz B.A. Photoreceptors in diabetic retinopathy. J Diabetes Investig. 2015;6:371–380. doi: 10.1111/jdi.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arden G.B., Sivaprasad S. Hypoxia and oxidative stress in the causation of diabetic retinopathy. Curr Diabetes Rev. 2011;7:291–304. doi: 10.2174/157339911797415620. [DOI] [PubMed] [Google Scholar]
- 80.Arden G.B., Sivaprasad S. The pathogenesis of early retinal changes of diabetic retinopathy. Doc Ophthalmol. 2012;124:15–26. doi: 10.1007/s10633-011-9305-y. [DOI] [PubMed] [Google Scholar]
- 81.Simo R., Villarroel M., Corraliza L., Hernandez C., Garcia-Ramirez M. The retinal pigment epithelium: something more than a constituent of the blood-retinal barrier–implications for the pathogenesis of diabetic retinopathy. J Biomed Biotechnol. 2010;2010:190724. doi: 10.1155/2010/190724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chmielewska K., Robaszkiewicz J., Kosatka M. Role of the retinal pigment epithelium (RPE) in the pathogenesis and treatment of diabetic macular edema (DME) Klin Oczna. 2008;110:318–320. [PubMed] [Google Scholar]
- 83.Xu H.Z., Song Z., Fu S., Zhu M., Le Y.Z. RPE barrier breakdown in diabetic retinopathy: seeing is believing. J Ocul Biol Dis Infor. 2011;4:83–92. doi: 10.1007/s12177-011-9068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trudeau K., Roy S., Guo W., Hernandez C., Villarroel M., Simo R., Roy S. Fenofibric acid reduces fibronectin and collagen type IV overexpression in human retinal pigment epithelial cells grown in conditions mimicking the diabetic milieu: functional implications in retinal permeability. Invest Ophthalmol Vis Sci. 2011;52:6348–6354. doi: 10.1167/iovs.11-7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Golczak M., Kuksa V., Maeda T., Moise A.R., Palczewski K. Positively charged retinoids are potent and selective inhibitors of the trans-cis isomerization in the retinoid (visual) cycle. Proc Natl Acad Sci U S A. 2005;102:8162–8167. doi: 10.1073/pnas.0503318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Golczak M., Imanishi Y., Kuksa V., Maeda T., Kubota R., Palczewski K. Lecithin:retinol acyltransferase is responsible for amidation of retinylamine, a potent inhibitor of the retinoid cycle. J Biol Chem. 2005;280:42263–42273. doi: 10.1074/jbc.M509351200. [DOI] [PubMed] [Google Scholar]
- 87.Redmond T.M., Poliakov E., Yu S., Tsai J.Y., Lu Z., Gentleman S. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc Natl Acad Sci U S A. 2005;102:13658–13663. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jin M., Li S., Moghrabi W.N., Sun H., Travis G.H. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122:449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Desmet K.D., Paz D.A., Corry J.J., Eells J.T., Wong-Riley M.T., Henry M.M., Buchmann E.V., Connelly M.P., Dovi J.V., Liang H.L., Henshel D.S., Yeager R.L., Millsap D.S., Lim J., Gould L.J., Das R., Jett M., Hodgson B.D., Margolis D., Whelan H.T. Clinical and experimental applications of NIR-LED photobiomodulation. Photomed Laser Surg. 2006;24:121–128. doi: 10.1089/pho.2006.24.121. [DOI] [PubMed] [Google Scholar]
- 90.Johnstone D.M., el Massri N., Moro C., Spana S., Wang X.S., Torres N., Chabrol C., De Jaeger X., Reinhart F., Purushothuman S., Benabid A.L., Stone J., Mitrofanis J. Indirect application of near infrared light induces neuroprotection in a mouse model of parkinsonism: an abscopal neuroprotective effect. Neuroscience. 2014;274:93–101. doi: 10.1016/j.neuroscience.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 91.Lim J., Sanders R.A., Snyder A.C., Eells J.T., Henshel D.S., Watkins J.B., 3rd Effects of low-level light therapy on streptozotocin-induced diabetic kidney. J Photochem Photobiol B. 2010;99:105–110. doi: 10.1016/j.jphotobiol.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 92.Yeager R.L., Franzosa J.A., Millsap D.S., Angell-Yeager J.L., Heise S.S., Wakhungu P., Lim J., Whelan H.T., Eells J.T., Henshel D.S. Effects of 670-nm phototherapy on development. Photomed Laser Surg. 2005;23:268–272. doi: 10.1089/pho.2005.23.268. [DOI] [PubMed] [Google Scholar]
- 93.Albarracin R., Eells J., Valter K. Photobiomodulation protects the retina from light-induced photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2011;52:3582–3592. doi: 10.1167/iovs.10-6664. [DOI] [PubMed] [Google Scholar]
- 94.Liang H.L., Whelan H.T., Eells J.T., Meng H., Buchmann E., Lerch-Gaggl A., Wong-Riley M. Photobiomodulation partially rescues visual cortical neurons from cyanide-induced apoptosis. Neuroscience. 2006;139:639–649. doi: 10.1016/j.neuroscience.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 95.Tang J., Herda A.A., Kern T.S. Photobiomodulation in the treatment of patients with non-center-involving diabetic macular oedema. Br J Ophthalmol. 2014;98:1013–1015. doi: 10.1136/bjophthalmol-2013-304477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tang J., Du Y., Lee C.A., Talahalli R., Eells J.T., Kern T.S. Low-intensity far-red light inhibits early lesions that contribute to diabetic retinopathy: in vivo and in vitro. Invest Ophthalmol Vis Sci. 2013;54:3681–3690. doi: 10.1167/iovs.12-11018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saliba A., Du Y., Liu H., Patel S., Roberts R., Berkowitz B.A., Kern T.S. Photobiomodulation mitigates diabetes-induced retinopathy by direct and indirect mechanisms: evidence from intervention studies in pigmented mice. PLoS One. 2015;10:e0139003. doi: 10.1371/journal.pone.0139003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arden G.B., Gunduz M.K., Kurtenbach A., Volker M., Zrenner E., Gunduz S.B., Kamis U., Ozturk B.T., Okudan S. A preliminary trial to determine whether prevention of dark adaptation affects the course of early diabetic retinopathy. Eye (London, England) 2010;24:1149–1155. doi: 10.1038/eye.2009.328. [DOI] [PubMed] [Google Scholar]
- 99.Muto T., Tien T., Kim D., Sarthy V.P., Roy S. High glucose alters Cx43 expression and gap junction intercellular communication in retinal Muller cells: promotes Muller cell and pericyte apoptosis. Invest Ophthalmol Vis Sci. 2014;55:4327–4337. doi: 10.1167/iovs.14-14606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tien T., Muto T., Barrette K., Challyandra L., Roy S. Downregulation of Connexin 43 promotes vascular cell loss and excess permeability associated with the development of vascular lesions in the diabetic retina. Mol Vis. 2014;20:732–741. [PMC free article] [PubMed] [Google Scholar]
- 101.Barber A.J., Lieth E., Khin S.A., Antonetti D.A., Buchanan A.G., Gardner T.W. Neural apoptosis in the retina during experimental and human diabetes: early onset and effect of insulin. J Clin Invest. 1998;102:783–791. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hernandez C., Dal Monte M., Simo R., Casini G. Neuroprotection as a therapeutic target for diabetic retinopathy. J Diabetes Res. 2016;2016:9508541. doi: 10.1155/2016/9508541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yoshida Y., Yamagishi S., Matsui T., Jinnouchi Y., Fukami K., Imaizumi T., Yamakawa R. Protective role of pigment epithelium-derived factor (PEDF) in early phase of experimental diabetic retinopathy. Diabetes Metab Res Rev. 2009;25:678–686. doi: 10.1002/dmrr.1007. [DOI] [PubMed] [Google Scholar]
- 104.Shen X., Xie B., Cheng Y., Jiao Q., Zhong Y. Effect of pigment epithelium derived factor on the expression of glutamine synthetase in early phase of experimental diabetic retinopathy. Ocul Immunol Inflamm. 2011;19:246–254. doi: 10.3109/09273948.2011.580073. [DOI] [PubMed] [Google Scholar]
- 105.Zhang J., Wu Y., Jin Y., Ji F., Sinclair S.H., Luo Y., Xu G., Lu L., Dai W., Yanoff M., Li W., Xu G.T. Intravitreal injection of erythropoietin protects both retinal vascular and neuronal cells in early diabetes. Invest Ophthalmol Vis Sci. 2008;49:732–742. doi: 10.1167/iovs.07-0721. [DOI] [PubMed] [Google Scholar]
- 106.Li W., Sinclair S.H., Xu G.T. Effects of intravitreal erythropoietin therapy for patients with chronic and progressive diabetic macular edema. Ophthalmic Surg Lasers Imaging. 2010;41:18–25. doi: 10.3928/15428877-20091230-03. [DOI] [PubMed] [Google Scholar]
- 107.Hernandez C., Simo R. Strategies for blocking angiogenesis in diabetic retinopathy: from basic science to clinical practice. Expert Opin Investig Drugs. 2007;16:1209–1226. doi: 10.1517/13543784.16.8.1209. [DOI] [PubMed] [Google Scholar]
- 108.Ciulla T.A., Amador A.G., Zinman B. Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care. 2003;26:2653–2664. doi: 10.2337/diacare.26.9.2653. [DOI] [PubMed] [Google Scholar]
- 109.Beltramo E., Porta M. Pericyte loss in diabetic retinopathy: mechanisms and consequences. Curr Med Chem. 2013;20:3218–3225. doi: 10.2174/09298673113209990022. [DOI] [PubMed] [Google Scholar]
- 110.Chronopoulos A., Trudeau K., Roy S., Huang H., Vinores S.A., Roy S. High glucose-induced altered basement membrane composition and structure increases trans-endothelial permeability: implications for diabetic retinopathy. Curr Eye Res. 2011;36:747–753. doi: 10.3109/02713683.2011.585735. [DOI] [PubMed] [Google Scholar]
- 111.Feit-Leichman R.A., Kinouchi R., Takeda M., Fan Z., Mohr S., Kern T.S., Chen D.F. Vascular damage in a mouse model of diabetic retinopathy: relation to neuronal and glial changes. Invest Ophthalmol Vis Sci. 2005;46:4281–4287. doi: 10.1167/iovs.04-1361. [DOI] [PubMed] [Google Scholar]
- 112.Wisniewska-Kruk J., Klaassen I., Vogels I.M., Magno A.L., Lai C.M., Van Noorden C.J., Schlingemann R.O., Rakoczy E.P. Molecular analysis of blood-retinal barrier loss in the Akimba mouse, a model of advanced diabetic retinopathy. Exp Eye Res. 2014;122:123–131. doi: 10.1016/j.exer.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 113.Rada J.A., Nickla D.L., Troilo D. Decreased proteoglycan synthesis associated with form deprivation myopia in mature primate eyes. Invest Ophthalmol Vis Sci. 2000;41:2050–2058. [PubMed] [Google Scholar]
- 114.Shimazawa M., Masuda T., Nakamura S., Miwa M., Nakamura K., Hara H. An experimental model for exudative age-related macular degeneration with choroidal neovascularization using the common marmoset. Curr Neovasc Res. 2015;12:128–134. doi: 10.2174/1567202612666150311105814. [DOI] [PubMed] [Google Scholar]
- 115.Shimazawa M., Nakamura S., Miwa M., Tsuruma K., Aihara M., Nakamura K., Hara H. Establishment of the ocular hypertension model using the common marmoset. Exp Eye Res. 2013;111:1–8. doi: 10.1016/j.exer.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 116.Troilo D., Judge S.J. Ocular development and visual deprivation myopia in the common marmoset (Callithrix jacchus) Vision Res. 1993;33:1311–1324. doi: 10.1016/0042-6989(93)90039-y. [DOI] [PubMed] [Google Scholar]
- 117.Chronopoulos A., Roy S., Beglova E., Mansfield K., Wachtman L., Roy S. Hyperhexosemia-induced retinal vascular pathology in a novel primate model of diabetic retinopathy. Diabetes. 2015;64:2603–2608. doi: 10.2337/db14-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tardif S.D., Mansfield K.G., Ratnam R., Ross C.N., Ziegler T.E. The marmoset as a model of aging and age-related diseases. ILAR J. 2011;52:54–65. doi: 10.1093/ilar.52.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]