Abstract
Chronic pancreatitis is a prominent risk factor for the development of pancreatic ductal adenocarcinoma. In both conditions, the activation of myofibroblast-like pancreatic stellate cells (PSCs) plays a predominant role in the formation of desmoplastic reaction through the synthesis of connective tissue and extracellular matrix, inducing local pancreatic fibrosis and an inflammatory response. Yet the signaling events involved in chronic pancreatitis and pancreatic cancer progression and metastasis remain poorly defined. Cadherin-11 (Cad-11, also known as OB cadherin or CDH11) is a cell-to-cell adhesion molecule implicated in many biological functions, including tissue morphogenesis and architecture, extracellular matrix-mediated tissue remodeling, cytoskeletal organization, epithelial-to-mesenchymal transition, and cellular migration. In this study, we show that, in human chronic pancreatitis and pancreatic cancer tissues, Cad-11 expression was significantly increased in PSCs and pancreatic cancer cells. In particular, an increased expression of Cad-11 can be detected on the plasma membrane of activated PSCs isolated from chronic pancreatitis tissues and in pancreatic cancer cells metastasized to the liver. Moreover, knockdown of Cad-11 in cancer cells reduced pancreatic cancer cell migration. Taken together, our data underline the potential role of Cad-11 in PSC activation and pancreatic cancer metastasis.
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive form of malignancy with a proclivity to metastasize and is commonly resistant to chemotherapy, radiotherapy, or immunotherapy.1, 2 PDAC comprises >85% of pancreatic cancer and has a survival rate of approximately 5%.3, 4 Currently, surgery is the only effective therapy, but it can only be performed in a minority of the patients because of the presence of local spread and/or metastases at the time of the diagnosis.5, 6 Chronic pancreatitis (CP), an inflammatory disease of the exocrine pancreas, is associated with a significant risk of pancreatic cancer.7, 8 The pathologic hallmarks of CP are inflammation and fibrosis, which are associated with the activation of pancreatic stellate cells (PSCs).9, 10, 11, 12 PSCs are myofibroblast-like cells that are quiescent in the normal pancreas but become activated in pancreatitis, resulting in increased proliferation, aberrant movement and attachment, and production of collagen.9, 10, 11, 12 Pancreatic cancer is characterized by a desmoplastic environment that is also associated with activated PSCs.13, 14, 15, 16 However, the precise molecular mechanisms underlying CP and pancreatic cancer remain obscure. Elucidating the signaling events involved would lay the foundation for the development of novel therapeutic approaches.
Cadherin-11 (Cad-11, also known as OB cadherin or CDH11) is a member of the cadherin superfamily, a group of transmembrane proteins that are principally located in adherens junctions and mediate homophilic cell-to-cell adhesion.17, 18, 19, 20 The intracellular domain includes binding sites for signaling molecules, such as β-catenin.21 Cad-11 is also involved in many other biological functions, including cytoskeletal organization, tissue morphogenesis, cellular migration, and invasion.22, 23, 24, 25, 26, 27 In particular, Cad-11 plays a role in epithelial-to-mesenchymal transition, which represents an inflammation-induced response that has a fundamental role in the progression from chronic inflammation to cancer.28, 29 Dysregulation of Cad-11 expression has been associated with many pathologic processes such as inflammation (eg, rheumatoid arthritis18), fibrosis (eg, kidney,30 pulmonary,31 dermal20), and cancer (eg, prostatic,32 breast,33 gastric,34 bladder,35 renal carcinoma,34 and glioblastoma36). Specifically, Cad-11 is a mesenchymal cadherin that is associated with more undifferentiated and aggressive cancer.37
In the present study, we examined Cad-11 expression levels in both normal and diseased human pancreatic tissues and determined the role of Cad-11 in cancer cell migration and proliferation. We found an association between the up-regulation of Cad-11 and the activation of PSCs associated with CP and PDAC. We provide evidence that Cad-11 is involved in PSC activation and pancreatic cancer cell migration.
Materials and Methods
Cell Culture
Human pancreatic cancer cell lines BxPC-3, HPAF-II, and MIA PaCa-2 were acquired from ATCC (Rockville, MD). Human primary PSCs were isolated from normal and CP tissues (PSC-N and PSC-CP, respectively) using a modified protocol.38 Briefly, the tissues were digested in Gey's Balanced Salt Solution (Sigma-Aldrich, St. Louis, MO) supplemented with 1.3 mg/mL collagenase P (Roche Diagnostic, Indianapolis, IN), 1 mg/mL pronase (Sigma-Aldrich), and 0.01 mg/mL deoxyribonuclease (Roche Diagnostic), followed by separation using Nycodenz-based gradient centrifugation. The immortalized human pancreatic ductal epithelial cell line (HPDE6)39, 40 was propagated in Keratinocyte-serum-free medium supplemented with epidermal growth factor and bovine pituitary extract (Gibco, Carlsbad, CA).
Antibodies
The following antibodies were used: anti–Cad-11 antibodies, 5B2H5 (Life Technologies, Carlsbad, CA), 23C6, or 3H1018; anti–glyceraldehyde-3-phosphate dehydrogenase antibody (Cell Signaling, Danvers, MA); anti–α-smooth muscle actin (α-SMA; ab5694) and Fluor 647-conjugated anti-mouse IgG antibody (ab150103; Abcam, Cambridge, MA); horseradish peroxidase-conjugated anti-mouse or -rabbit IgG antibodies (Bio-Rad, Hercules, CA); Alexa 488 or cyanine 3-conjugated anti-mouse or -rabbit IgG antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA).
RT-PCR and Quantitative PCR
RNA was extracted using TRIzol reagent (Life Technologies). cDNA was generated using iScript (Bio-Rad). For the initial screen of cDNA quality, PCR was performed using the following primers: β-actin forward sequence, 5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′; β-actin reverse sequence, 5′-CTAGAAGCATTTGCGGTGGACGATGGAGGG-3′; Cad-11 forward sequence, 5′-ACCAGATGTCTGTGTCAGA-3′; Cad-11 reverse sequence, 5′-GTCATCCTTGTCATCTGCA-3′ as described in a previous study.37 The amplification of a 661-bp β-actin fragment and a 192-base Cad-11 fragment rules out genomic contamination (Supplemental Figure S1).
Real-time quantitative PCR (qPCR) was performed using SYBR green mix (Bio-Rad) on CFX Connect Thermocycler (Bio-Rad). The following primers were used: Cad-11 forward sequence, 5′-GGTCTGGAACCAGTTCTTCG-3′; Cad-11 reverse sequence, 5′-TCTCGATCCAACGTCTTGGT-3′; GAPDH forward sequence, 5′-GAAGGTGAAGGTCGGAGTCA-3′; GAPDH reverse sequence, 5′-GACAAGCTTCCCGTTCTCAG-3′. Ct values were normalized to GAPDH, and relative expression levels are presented as fold change against control. Representative reactions were resolved on 1.5% agarose gel.
Western Blot Analysis
Cell lysate preparation and Western blot analysis were performed as described previously.41 Membranes were probed with antibodies as indicated, followed by chemiluminescence imaging (Syngene PXi, Federick, MD). Bands were quantified using ImageJ Fiji software (NIH, Bethesda, MD) and normalized to glyceraldehyde-3-phosphate dehydrogenase.
Flow Cytometry
PSC-N (passage 2), PSC-CP (passage 2), or BxPC-3 cells were stained with Cad-11 antibodies 3H10 or 23C6 and were analyzed using FACSCanto II (Becton Dickinson, San Jose, CA).
siRNA Knockdown
BxPC-3 cells were transfected with either control siRNA (antisense sequence, 5′-UUGGUGCUCUUCAUCUUGUUGUU-3′) or with Cadherin-11 targeting siRNA H3 (antisense sequence, 5′-UACUGUACACUAACUUGGCGCUU-3′) or H4 (antisense sequence, 5′-AAUUGGCUGGUUGGAAAGUGGUU-3′) using Lipofectamine RNAiMAX (Life Technologies), and harvested for further analysis 48 hours after transfection.
Cell Migration Assay
BxPC-3 cells seeded in Transwell inserts (Costar, NY) were exposed to serum-free RPMI supplemented with or without 5 ng/mL transforming growth factor (TGF)-β in 12-well plates. The cells that migrated to the bottom of the inserts after 24 hours were imaged using Olympus CX41 microscope (Center Valley, PA) or were stained with 0.1% crystal violet, followed by extraction with methanol. Absorbance at 590 nm wavelength was recorded on a spectrophotometer (SpectraMax; Microdevices, Sunnyvale, CA).
Cell Proliferation and Viability Assay
BxPC-3 cells transfected with siRNA oligos were cultured in RPMI supplemented with 0.5% fetal bovine serum and analyzed by MTT assay as described previously.42 Absorbance at 560 nm and 700 nm was measured on spectrophotometer (SpectraMax; Microdevices).
Human Specimens
Formalin-fixed, paraffin-embedded human tissues of individuals with pancreatic cancer, CP, and normal pancreas were acquired from the biorepository at Cedars-Sinai Medical Center and analyzed under a protocol approved by the Cedars-Sinai Internal Review Board (protocol 34086). Patients with histologically proven CP or PDAC were included in the study. Patients who had undergone chemotherapy or radiotherapy or patients with pancreatic adenocarcinomas who have CP-like morphologic changes adjacent to the tumor were excluded. The Oncomine analysis tool (Thermo Fisher Scientific, Waltham, MA)43 was used to analyze the microarray data sets of Badea et al44 and Pei et al.45
IHC and IF
Immunohistochemistry (IHC) and immunofluorescence (IF) were performed as described previously.46 Briefly, formalin-fixed, paraffin-embedded sections (4 μm) were subjected to de-paraffin treatment and heat-induced antigen retrieval, blocked in Animal-free Blocker (Vector Laboratories, Burlingame, CA), and stained with antibodies as indicated or an isotype-matched IgG as negative control. For IHC, the Diaminobenzidine Peroxidase substrate kit (SK4100; Vector Laboratories) was used. The images were captured using Aperio Imagescope (Leica, Buffalo Grove, IL) or Leica TCS SP5 Confocal microscope (Leica).
Statistical Analysis
All data were collected from three or more independent experiments, and values were expressed as means ± SD. Statistical significance was assessed using t-test or Fisher's exact test (for gene coexpression analysis in the IF study). Results with P < 0.05 were considered statistically significant.
Results
Cah-11 Expression in Normal and Diseased Human Pancreatic Tissues
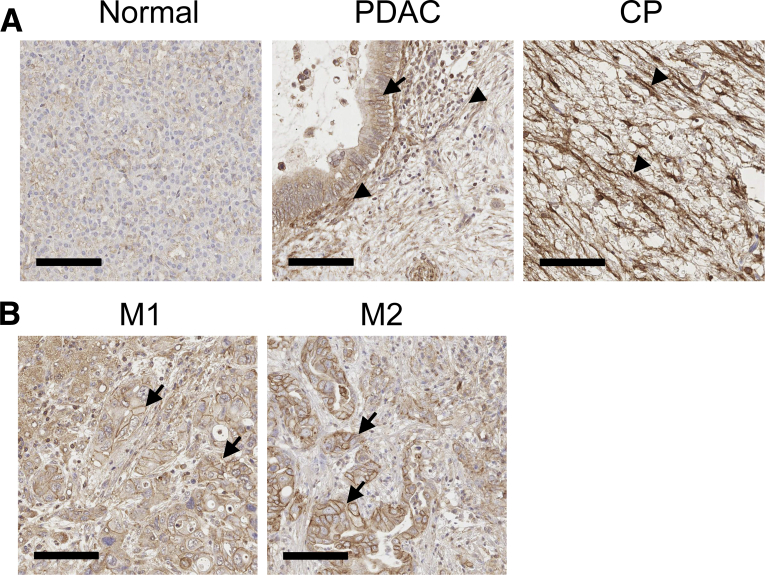
To evaluate Cad-11 expression levels in clinical specimens, we performed IHC on normal pancreas, CP, and pancreatic cancer tissues obtained from eight individuals for each condition. In normal pancreas, Cad-11 can be detected at low levels in acinar cells, with a membrane localization pattern that is consistent with its known role in establishing cell-to-cell contact (Figure 1A). Among tissues of both CP and pancreatic cancer, significantly elevated levels of Cad-11 were observed in stromal cells that resemble the morphologic characteristics of activated PSCs (Figure 1). In addition, Cad-11 can be detected on the plasma membrane of cancer cells of primary PDAC tissues (Figure 1A) and of the cancer cells disseminated to the liver (Figure 1B).
Figure 1.
Cad-11 expression in normal and diseased pancreatic tissues. A: Immunohistochemistry analysis of Cad-11 expression in normal pancreas and tissues of CP and PDAC. B: Immunohistochemistry analysis of Cad-11 expression in two cases of pancreatic cancer metastasis to the liver (M1 and M2). Note that Cad-11 can be detected in cancer cells (arrows) and stellate cells (arrowheads). Scale bars = 100 μm. Original magnification, ×20. Cad-11, cadherin-11; CP, chronic pancreatitis; N, normal pancreas; PDAC, pancreatic ductal adenocarcinoma.
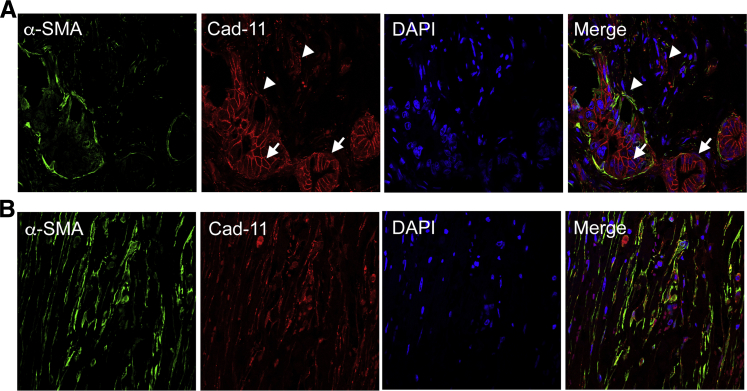
By IF, Cad-11 shows a membranous expression pattern in the cancer cells (Figure 2A). In the stromal regions, almost all of the cells that are stained positively for Cad-11 also express α-SMA (Figure 2B and Supplemental Table S1), an established marker of activated PSCs.16 These results indicate that Cad-11 is expressed in activated PSCs that are closely associated with CP and pancreatic cancer.
Figure 2.
Cad-11 expression in activated pancreatic stellate cells. A: Immunofluorescence staining of PDAC tissue using anti–α-SMA, Cad-11 antibody, or DAPI. α-SMA (green), Cad-11 (red), and DAPI (blue). Note that Cad-11 can be detected on the membrane of the cancer cells (arrows) and in the α-SMA+, stellate cells (arrowheads). B: Images of PDAC tissue stained in the same way as in A, showing a field predominantly of stellate cells. Note that the α-SMA+, stellate cells also express Cad-11. Original magnification, ×63. Cad-11, cadherin-11; PDAC, pancreatic ductal adenocarcinoma; α-SMA, α-smooth muscle actin.
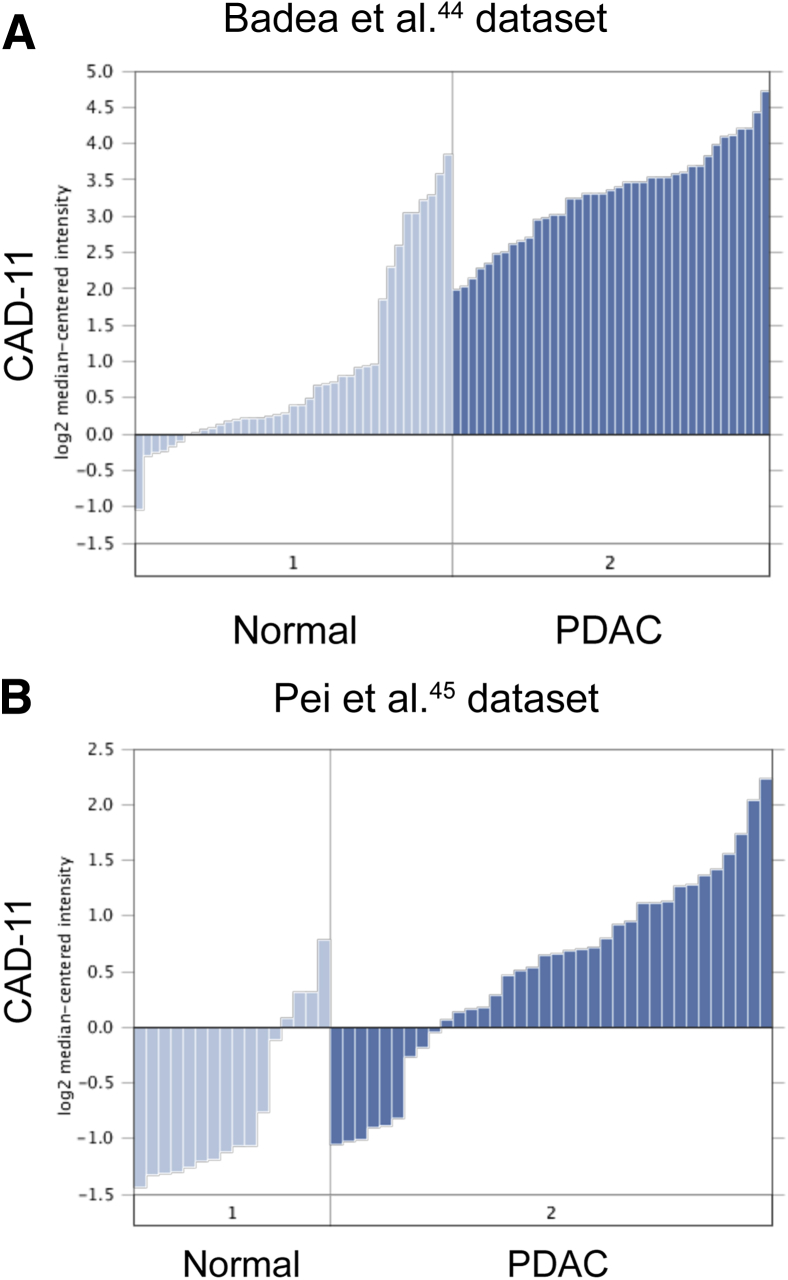
We compared Cad-11 mRNA levels between normal and PDAC tissues of a larger sampling size using publically available microarray data sets.44, 45 The results indicate that PDAC is associated with a significant increase of Cad-11 expression (Figure 3 and Supplemental Figure S2). We also used the Oncomine coexpression tool to identify genes coexpressed with Cad-11. Interestingly, many of the top genes identified represent stromal signature genes (Tables 1 and 2 and Supplemental Figure S2). These include genes that encode proteins known for playing a role in fibrosis or stellate cell functions (eg, various forms of collagens, FN1, FAP, LOXL1, LOX, MXRA5, TIMP1, SPARC, and POSTN) (Tables 1 and 2). A search using other mesenchymal cadherins, such as N-cadherin, does not produce similar results, indicating that the coexpression pattern is unique to Cad-11.
Figure 3.
Cad-11 levels are elevated in human pancreatic cancer. A: Oncomine analysis of Cad-11 mRNA levels using the Badea et al44 microarray. Cad-11 mRNA levels are elevated in PDAC compared with normal tissues. B: Oncomine analysis of Cad-11 mRNA levels in pancreatic cancer and normal tissues, using the Pei et al45 microarray data set. n = 39 normal tissues (A); n = 39 PDAC tissues (A); n = 16 normal tissues (B); n = 36 pancreatic cancer tissues (B). P < 2.23 × 10−15 (A); P < 3.77 × 10−6 (B). Cad-11, cadherin-11; PDAC, pancreatic ductal adenocarcinoma.
Table 1.
Correlation Coefficient for Selected Genes Coexpressed with Cad-11 Identified in the Badea et al44 Data Set
| Gene symbol | Full name | Reporter ID | Correlation |
|---|---|---|---|
| EDNRA∗ | Endothelin receptor type A | 204464_s_at | 0.962 |
| SPARC∗ | Secreted protein acidic and cysteine rich | 200665_s_at | 0.962 |
| F2R∗ | Coagulation factor II (thrombin) receptor | 203989_x_at | 0.949 |
| NREP | Neuronal regeneration related protein | 201309_x_at | 0.939 |
| COL4A2 | Collagen type IV α 2 | 211966_at | 0.933 |
| COL4A1 | Collagen type IV α 1 | 211980_at | 0.933 |
| COL1A1∗ | Collagen type I α 1 | 202311_s_at | 0.910 |
| COL1A2∗ | Collagen type I α 2 | 229218_at | 0.910 |
| COL3A1∗ | Collagen type III α 1 | 232458_at | 0.910 |
| COL5A1∗ | Collagen type V α 1 | 203325_s_at | 0.910 |
| FN1 | Fibronectin 1 | 210495_x_at | 0.910 |
| LOXL1 | Lysyl oxidase-like 1 | 203570_at | 0.910 |
| RUNX1∗ | Runt-related transcription factor 1 | 209360_s_at | 0.910 |
| SRPX2∗ | Sushi-repeat-containing protein, X-linked 2 | 205499_at | 0.910 |
| SH3PXD2A∗ | SH3 and PX domains 2A | 224817_at | 0.910 |
| Thy-1∗ | Thy-1 cell surface antigen | 213869_x_at | 0.910 |
| TIMP1∗ | TIMP metallopeptidase inhibitor 1 | 201666_at | 0.910 |
| TPBG | Trophoblast glycoprotein | 203476_at | 0.910 |
| THBS2∗ | Thrombospondin 2 | 203083_at | 0.910 |
| VCAN∗ | Versican | 211571_s_at | 0.910 |
| GPNMB | Glycoprotein (transmembrane) nmb | 201141_at | 0.910 |
| PLXDC2 | Plexin domain containing 2 | 227276_at | 0.910 |
| MYOF | Myoferlin | 211864_s_at | 0.910 |
| DACT1∗ | Dapper, antagonist of β-catenin, homolog 1 | 219179_at | 0.910 |
| AEBP1∗ | AE binding protein 1 | 201792_at | 0.910 |
| CTHRC1∗ | Collagen triple helix repeat containing 1 | 225681_at | 0.910 |
| SULF1∗ | Sulfatase 1 | 212344_at | 0.910 |
| MXRA5∗ | Matrix-remodelling associated 5 | 209596_at | 0.902 |
| VGLL4 | Vestigial like 4 | 212399_s_at | 0.895 |
| RASAL2 | RAS protein activator like 2 | 222810_s_at | 0.895 |
| MRC2∗ | Mannose receptor, C type 2 | 37408_at | 0.885 |
| PDGFRB∗ | Platelet-derived growth factor receptor, β | 202273_at | 0.885 |
| NOTCH3 | Notch homolog 3 | 203238_s_at | 0.885 |
| LOX | Lysyl oxidase | 204298_s_at | 0.861 |
| SNAI2 | Snail homolog 2 | 213139_at | 0.861 |
| NUAK1 | NUAK family kinase 1 | 204589_at | 0.856 |
| POSTN∗ | Periostin | 1555778_a_at | 0.844 |
| FAP∗ | Fibroblast activation protein, α | 209955_s_at | 0.838 |
Cad-11, cadherin-11; ID, identification.
Genes also identified in the Pei et al45 data set.
Table 2.
Correlation Coefficient for Selected Genes Coexpressed with Cad-11 Identified in the Pei et al45 Data Set
| Gene symbol | Full name | Reporter ID | Correlation |
|---|---|---|---|
| THBS2∗ | Thrombospondin 2 | 203083_at | 0.957 |
| VCAN∗ | Versican | 204619_s_at | 0.954 |
| SPARC∗ | Secreted protein acidic and cysteine rich | 200665_s_at | 0.945 |
| COL3A1∗ | Collagen type III α 1 | 215076_s_at | 0.945 |
| COL1A2∗ | Collagen type I α 2 | 202403_s_at | 0.945 |
| COL5A1∗ | Collagen type V α1 | 212489_at | 0.945 |
| CTHRC1∗ | Collagen triple helix repeat containing 1 | 225681_at | 0.945 |
| FAP∗ | Fibroblast activation protein, α | 209955_s_at | 0.932 |
| COL6A3 | Collagen type VI α3 | 201438_at | 0.924 |
| Thy-1∗ | Thy-1 cell surface antigen | 213869_x_at | 0.924 |
| SULF1∗ | Sulfatase 1 | 212344_at | 0.908 |
| LEF1 | Lymphoid enhancer-binding factor 1 | 221558_s_at | 0.908 |
| RUNX2 | Runt-related transcription factor 2 | 232231_at | 0.908 |
| POSTN∗ | Periostin | 1555778_a_at | 0.908 |
| SRPX2∗ | Sushi-repeat-containing protein, X-linked 2 | 205499_at | 0.908 |
| EDNRA∗ | Endothelin receptor type A | 201666_at | 0.893 |
| DACT1∗ | Dapper, antagonist of β-catenin, homolog 1 | 219179_at | 0.884 |
| AXL | AXL receptor tyrosine kinase | 202686_s_at | 0.871 |
| BMPR2 | Bone morphogenetic protein receptor, type II | 225144_at | 0.871 |
| PALLD | Palladin | 200906_s_at | 0.871 |
| TIMP1∗ | TIMP metallopeptidase inhibitor 1 | 201666_at | 0.856 |
| COL1A1∗ | Collagen type I α1 | 202311_s_at | 0.856 |
| FBN1 | Fibrillin 1 | 235318_at | 0.855 |
| CTSK | Cathepsin K | 202450_s_at | 0.855 |
| RUNX1∗ | Runt-related transcription factor 1 | 209360_s_at | 0.842 |
| MXRA5∗ | Matrix-remodelling associated 5 | 209596_at | 0.832 |
| MRC2∗ | Mannose receptor, C type 2 | 37408_at | 0.819 |
| SH3PXD2A∗ | SH3 and PX domains 2A | 231823_s_at | 0.819 |
| PDGFRB∗ | Platelet-derived growth factor receptor, β | 202273_at | 0.819 |
| F2R∗ | Coagulation factor II (thrombin) receptor | 203989_x_at | 0.819 |
| AEBP1∗ | AE binding protein 1 | 201792_at | 0.819 |
| Gli2 | GLI family zinc finger 2 | 228537_at | 0.819 |
Cad-11, cadherin-11; ID, identification.
Genes also identified in the Badea et al44 data set.
Up-Regulation of Cah-11 in Activated PSCs and Pancreatic Cancer Cell Lines
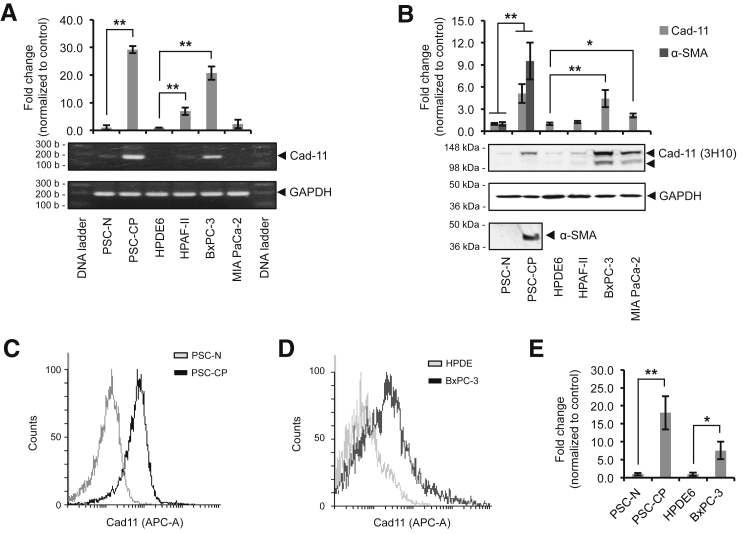
We examined Cad-11 expression in human PSCs isolated from CP and in human pancreatic cancer cells by qPCR. The early passages of PSCs isolated from a CP patient (ie, PSC-CP) are activated, as indicated by high expression levels of α-SMA compared with those isolated from the adjacent normal pancreatic tissue in the same patient (ie, PSC-N) (Figure 4B). Interestingly, the mRNA levels of Cad-11 were 29.2- ± 1.3-fold greater in the PSCs isolated from CP tissue than PSCs isolated from normal pancreatic tissue (Figure 4A). We noted that HPDE6, an immortalized human pancreatic ductal epithelial cell line, shows the lowest levels of Cad-11 mRNA levels (Figure 4A). In comparison, Cad-11 levels are higher among the pancreatic cancer cell lines that we tested, with the HPAFII and MIA PaCa 2 cells exhibiting 6.9- ± 1.3-fold and 2.3- ± 1.5-fold higher levels of Cad-11 mRNA expression, respectively, and BxPC-3 cells exhibiting a 20.7- ± 2.5-fold higher level of Cad-11 over that of HPDE6 (Figure 4A).
Figure 4.
Cad-11 expression in isolated human primary PSCs and pancreatic cell lines. A: Quantitative PCR analysis of Cad-11 mRNA levels expressed as fold change compared with PSC-N or HPDE6 control. B: Western blot analysis of Cad-11 protein levels. Densitometric quantification of data is shown in the graph as fold change using PSC-N or HPDE6, respectively, as control. The arrowheads indicate the bands that represent the pro- and mature forms of the Cad-11 protein. C: Flow cytometric analysis of Cad-11 in PSC-N and PSC-CP. Overlay of the histogram is shown. D: Flow cytometric analysis of Cad-11 in BxPC-3 and HDPE6 cells. E: Flow cytometric comparison of Cad-11 between PSC-N and PSC-CP or HPDE6 and BxPC-3. Data are expressed as fold change compared with PSC-N or HPDE6 control, and the error bars represent SD (A, B, and E). n = 3 separate experiments (A, B, and E). ∗P < 0.05, ∗∗P < 0.01. APC-A, allophycocyanin area; Cad-11, cadherin-11; CP, chronic pancreatitis; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HDPE6, immortalized human pancreatic epithelial cell; PSC, pancreatic stellate cell; PSC-CP, PSC isolated from CP tissue; PSC-N, primary PSC isolated from normal pancreas; α-SMA, α-smooth muscle actin.
Next, we evaluated overall Cad-11 protein levels by Western blot analysis using two previously established Cad-11–specific monoclonal antibodies, which are reactive with an intracellular (5B2H547) and extracellular (3H1048, 49) epitope, respectively. Consistent with the qPCR data, our results showed that the PSCs from CP samples expressed 5.1- ± 1.3-fold higher levels of Cad-11 protein than those isolated from normal tissue (Figure 4B). Of note, Cad-11 protein was detected at low levels in the immortalized, but untransformed, HPDE6 cells (Figure 4B). Among the human pancreatic cell lines we examined, BxPC-3 exhibited the highest levels of Cad-11 at 4.4- ± 1.2-fold above the HPDE6 control (Figure 4B).
To demonstrate expression of Cad-11 on the plasma membrane, we conducted flow cytometric analysis using anti–Cad-11 antibody 3H10. We found that the intensity of Cad-11 staining on the cell surface of PSCs isolated from CP tissue was 18.1- ± 4.6-fold greater than that from normal tissue (Figure 4, C and E, and Supplemental Figure S3). Similarly, BxPC-3 cells also exhibited a 7.6- ± 2.4-fold greater level of Cad-11 expression on the plasma membrane than HPDE6 cells (Figure 4, D and E, and Supplemental Figure S3).
Effect of Cad-11 Knockdown on BxPC3 Cell Migration and Proliferation
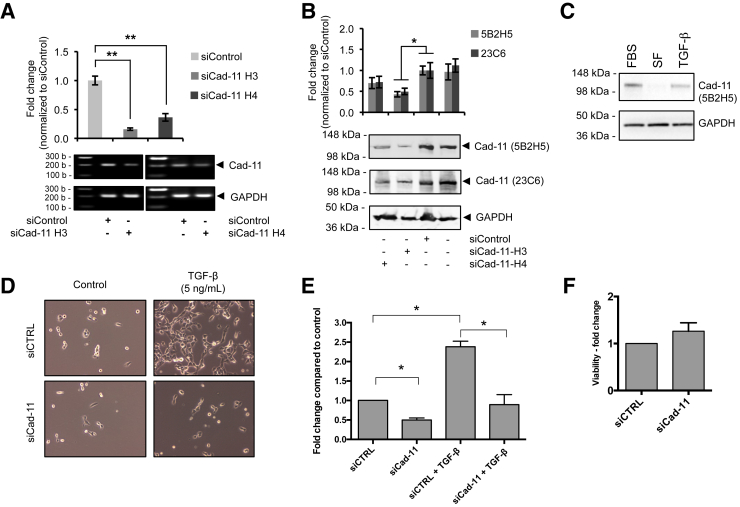
Using two different siRNAs specific for Cad-11 (siCad-11-H3 and siCad-11-H4), we were able to suppress cad-11 expression levels in BxPC-3 cells, as documented by both Western blot analysis and qPCR (Figure 5, A and B). We then used the Transwell assay to evaluate the effect of Cad-11 silencing on cancer cell migration. As shown in previous studies, TGF-β can enhance BxPC-3 cell migration.50, 51 We found that TGF-β, as well as fetal bovine serum, can up-regulate Cad-11 expression in BxPC-3 cells cultured in serum-free medium (Figure 5C). The migratory capacity of BxPC-3 was significantly suppressed after Cad-11 silencing, both in the presence and absence of TGF-β (Figure 5, D and E). However, ablation of Cad-11 expression did not affect BxPC-3 cell proliferation (Figure 5F). Moreover, the migratory behavior of the pancreatic cancer cell lines we tested correlates with their Cad-11 expression levels (Figure 4 and Supplemental Figure S4). Together, these results indicate that cad-11 exerts a role in the migration, but not proliferation, of the pancreatic cancer cell line BxPC3 in the in vitro setting.
Figure 5.
Knockdown of cad-11 reduced pancreatic cancer cell migration. A: Quantitative PCR analysis of Cad-11 mRNA levels in BxPC-3 cells transfected with control (siControl) or Cad-11–specific siRNAs (siCad-11 H3 and H4). B: Western blot analysis of Cad-11 protein levels in BxPC-3 cells transfected with control or Cad-11–specific siRNAs. The graph shows densitometric quantification of Cad-11 levels normalized to GAPDH. C: Western blot analysis of Cad-11 and GAPDH protein levels in BxPC-3 cells after 24-hour incubation in medium containing 10% FBS, SF, or SF containing 5 ng/mL TGF-β. D: Microscopic image of migrated BxPC-3 cells in Transwell. BxPC-3 cells were transfected with control or Cad-11–specific siRNA (siCad-11 H3). The concentration of TGF-β is indicated. E: Quantification of Transwell migration as shown in D by crystal blue staining. F: MTT assay of BxPC-3 cell viability 72 hours after transfection with control or Cad-11–specific siRNA (siCad-11 H3). Data are expressed as fold change compared with siControl, and the error bars represent SD (A, B, E, and F). n = 3 separate experiments (A, B, E, and F). ∗P < 0.05, ∗∗P < 0.01. Cad-11, cadherin-11; CTRL, control; FBS, fetal bovine serum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; SF, serum-free medium; TGF-β, transforming growth factor β.
Discussion
In this study we examined Cad-11 expression levels in both normal and diseased pancreatic tissues. Our data indicate that, although Cad-11 is expressed at low levels on the membrane of the parenchymal cells in normal pancreas, its expression is significantly increased in CP and pancreatic cancer tissues. The association between increased expression of Cad-11 levels and PDAC was further corroborated by our analysis of gene microarray data sets. At the cellular level, up-regulation of Cad-11 has been found in both cancer cells and PSCs associated with PDAC or CP. In addition, we showed an increased Cad-11 expression in pancreatic cancer cell lines BxPC-3 and MIA PaCa-2 compared with the untransformed pancreatic ductal cell line HPDE. These findings in pancreatic cancer are consistent with previous reports that implicated Cad-11 signaling in other forms of human malignancy.32, 33, 34, 35, 36
Our results indicate that Cad-11 may be involved in pancreatic cancer cell migration. Expression of Cad-11 on the surface of cancer cells circulating in the blood has been reported.52, 53 Indeed, Cad-11–mediated cell adhesion has been shown to facilitate breast and prostate cancer cell migration to distant sites, particularly to the bone.34, 54, 55, 56, 57 Of note, we showed that TGF-β treatment leads to up-regulation of Cad-11 expression in BxPC3 cells, similar to previous studies using a lung cancer cell line31 or cardiac valve myofibroblasts.58 This accounts for, as least in part, our finding that silencing Cad-11 can reduce TGF-β–induced cancer cell migration. Downstream of TGF-β signaling, the epithelial-to-mesenchymal transition–associated transcriptional factor ZEB2 and Sp1 appear to be involved in up-regulation of Cad-11 expression, as shown by studies using colon, gastric, or liver cancer cells.59 Conversely, Cad-11 may also mediate TGF-β production31, 60 and constitute a feed-forward mechanism in TGF-β signaling. Clearly, additional work is needed to decipher the crosstalk between Cad-11 and TGF-β signaling pathways that mediates the aberrant migratory behavior of pancreatic cancer cells.
Our finding of elevated levels of Cad-11 in the PSCs associated with CP and PDAC suggests that Cad-11 could potentially represent a cell surface marker for stellate cell activation. In this regard, Cad-11 signaling may play an important role in mediating the function of activated stellate cells, which act as a central player in defining the fibrotic and inflammatory microenvironment in both pancreatitis and cancer.16, 61 The PSCs associated with PDAC, by producing growth factors and cytokines that promote cell proliferation and inflammation,16 can conceivably contribute to the progression of malignancy. However, the fibrotic environment generated by the stellate cells may also constrain the growth of the tumor. Notably, several recent studies showed that depletion of stellate cells lead to undifferentiated and more aggressive malignancy, suggesting that the stellate cells may play a role in restricting pancreatic cancer progression.62, 63 It is also possible that the cancer-associated stellate cells consist of distinct subpopulations that play opposing roles in cancer progression.61 It would be of interest to determine the role of Cad-11–expressing PSCs in the progression of pancreatic malignancy.
In summary, our study demonstrated the expression patterns of Cad-11 in both normal and diseased human pancreas. The elevated expression levels of Cad-11 in PSC associated with CP and pancreatic cancer implicate a role in maintaining the activated phenotype of PSCs. Moreover, our data support the notion that Cad-11–mediated cell adhesion and signaling events participate in cancer cell migration involved in the spread and metastasis of the tumor.
Acknowledgments
We thank members of the pancreatic research group for helpful discussion and Dr. Kolja Wawrowsky for assistance in microscopy.
Footnotes
Supported by the Cedars-Sinai Medical Center Samuel Oschin Comprehensive Cancer Institute Developmental Funds for Liver Metastasis Team Grant Research Award “Colon, pancreas, and prostate cancer engraftment in the liver metastatic niche”; the National Center for Advancing Translational Sciences UCLA CTSI grant UL1TR000124 (Q.W.); the Department of Veterans Affairs grant I01BX001484 (S.J.P.); and NIH grants P01CA163200, P50 AA11999, and P01DK098108 (S.J.P.).
Disclosures: M.B.B. is a consultant to Roche (Basel, Switzerland).
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2016.09.012.
Contributor Information
Qiang Wang, Email: qiang.wang@cshs.org.
Stephen J. Pandol, Email: stephen.pandol@cshs.org.
Supplemental Data
PCR analysis of Cad-11 mRNA levels in human pancreatic cells. BxPC-3, MIA PaCa-2, and HPAF-II are human pancreatic cancer cell lines. The isolated RNA was converted to cDNA and screened for Cad-11 and β-actin. Representative reactions are shown. Cad-11, cadherin-11; HDPE6, immortalized human pancreatic epithelial cell; PSC-CP, pancreatic stellate cell isolated from chronic pancreatitis tissue; PSC-N, pancreatic stellate cell isolated from normal pancreas.
Oncomine analysis of genes coexpressed with Cad-11. A and B: Box plot and heat map of the top genes coexpressed with Cad-11 in the Badea et al44 data set. C and D: Box plot and heat map of genes coexpressed with Cad-11 in the Pei et al45 microarray data set. In the box plot, the dark bar represents the median; the box shows the first and third quartile; the top and bottom bars indicate the 90 and 10 percentile respectively; the dots represent the maximum and minimum values. P < 2.23 × 10−15 (A and B); P < 3.77 × 10−6 (C and D). Cad-11, cadherin-11; PDAC, pancreatic ductal adenocarcinoma.
Negative control for flow cytometric analysis of Cad-11 levels. The procedure was performed under the same conditions as those shown in Figure 3, C and D. The histograms of cells unstained or stained with secondary antibody only are shown. Note that the secondary antibody alone does not generate any signal in flow cytometry. APC-A, allophycocyanin area; Cad-11, cadherin-11; HDPE6, immortalized human pancreatic epithelial cell; PSC-CP, primary pancreatic stellate cell isolated from chronic pancreatitis tissue; PSC-N: primary pancreatic stellate cell isolated from normal pancreas.
Relative migratory capacity of human pancreatic cancer cells. The cells were seeded in Transwell inserts in serum-free medium and evaluated for migration 24 hours later. Data are expressed as relative migratory capacity compared with BxPC-3. n = 3 separate experiments. ∗P < 0.05.
References
- 1.Buchler M., Friess H., Schultheiss K.H., Gebhardt C., Kubel R., Muhrer K.H., Winkelmann M., Wagener T., Klapdor R., Kaul M., Muller G., Schulz G., Beger H.G. A randomized controlled trial of adjuvant immunotherapy (murine monoclonal antibody 494/32) in resectable pancreatic cancer. Cancer. 1991;68:1507–1512. doi: 10.1002/1097-0142(19911001)68:7<1507::aid-cncr2820680707>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Friess H., Buchler M., Kruger M., Beger H.G. Treatment of duct carcinoma of the pancreas with the LH-RH analogue buserelin. Pancreas. 1992;7:516–521. doi: 10.1097/00006676-199209000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Vincent A., Herman J., Schulick R., Hruban R.H., Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan D.P., Hong T.S., Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:2140–2141. doi: 10.1056/NEJMc1412266. [DOI] [PubMed] [Google Scholar]
- 5.Hsueh C.T. Pancreatic cancer: current standards, research updates and future directions. J Gastrointest Oncol. 2011;2:123–125. doi: 10.3978/j.issn.2078-6891.2011.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konstantinidis I.T., Warshaw A.L., Allen J.N., Blaszkowsky L.S., Castillo C.F., Deshpande V., Hong T.S., Kwak E.L., Lauwers G.Y., Ryan D.P., Wargo J.A., Lillemoe K.D., Ferrone C.R. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a “true” R0 resection? Ann Surg. 2013;257:731–736. doi: 10.1097/SLA.0b013e318263da2f. [DOI] [PubMed] [Google Scholar]
- 7.Yadav D., Lowenfels A.B. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–1261. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malka D., Hammel P., Maire F., Rufat P., Madeira I., Pessione F., Levy P., Ruszniewski P. Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut. 2002;51:849–852. doi: 10.1136/gut.51.6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omary M.B., Lugea A., Lowe A.W., Pandol S.J. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 2007;117:50–59. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conwell D.L., Lee L.S., Yadav D., Longnecker D.S., Miller F.H., Mortele K.J., Levy M.J., Kwon R., Lieb J.G., Stevens T., Toskes P.P., Gardner T.B., Gelrud A., Wu B.U., Forsmark C.E., Vege S.S. American Pancreatic Association Practice Guidelines in Chronic Pancreatitis: evidence-based report on diagnostic guidelines. Pancreas. 2014;43:1143–1162. doi: 10.1097/MPA.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erkan M., Kleeff J., Gorbachevski A., Reiser C., Mitkus T., Esposito I., Giese T., Buchler M.W., Giese N.A., Friess H. Periostin creates a tumor-supportive microenvironment in the pancreas by sustaining fibrogenic stellate cell activity. Gastroenterology. 2007;132:1447–1464. doi: 10.1053/j.gastro.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 12.Mews P., Phillips P., Fahmy R., Korsten M., Pirola R., Wilson J., Apte M. Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut. 2002;50:535–541. doi: 10.1136/gut.50.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinho A.V., Chantrill L., Rooman I. Chronic pancreatitis: a path to pancreatic cancer. Cancer Lett. 2014;345:203–209. doi: 10.1016/j.canlet.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 14.McKay C.J., Glen P., McMillan D.C. Chronic inflammation and pancreatic cancer. Best Pract Res Clin Gastroenterol. 2008;22:65–73. doi: 10.1016/j.bpg.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Lowenfels A.B., Maisonneuve P., Cavallini G., Ammann R.W., Lankisch P.G., Andersen J.R., Dimagno E.P., Andren-Sandberg A., Domellof L. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433–1437. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 16.Apte M.V., Wilson J.S., Lugea A., Pandol S.J. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology. 2013;144:1210–1219. doi: 10.1053/j.gastro.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiener H.P., Brenner M.B. Building the synovium: cadherin-11 mediates fibroblast-like synoviocyte cell-to-cell adhesion. Arthritis Res Ther. 2005;7:49–54. doi: 10.1186/ar1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee D.M., Kiener H.P., Agarwal S.K., Noss E.H., Watts G.F., Chisaka O., Takeichi M., Brenner M.B. Cadherin-11 in synovial lining formation and pathology in arthritis. Science. 2007;315:1006–1010. doi: 10.1126/science.1137306. [DOI] [PubMed] [Google Scholar]
- 19.Chang S.K., Noss E.H., Chen M., Gu Z., Townsend K., Grenha R., Leon L., Lee S.Y., Lee D.M., Brenner M.B. Cadherin-11 regulates fibroblast inflammation. Proc Natl Acad Sci U S A. 2011;108:8402–8407. doi: 10.1073/pnas.1019437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu M., Pedroza M., Lafyatis R., George A.T., Mayes M.D., Assassi S., Tan F.K., Brenner M.B., Agarwal S.K. Identification of cadherin 11 as a mediator of dermal fibrosis and possible role in systemic sclerosis. Arthritis Rheumatol. 2014;66:1010–1021. doi: 10.1002/art.38275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- 22.Angst B.D., Marcozzi C., Magee A.I. The cadherin superfamily: diversity in form and function. J Cell Sci. 2001;114:629–641. doi: 10.1242/jcs.114.4.629. [DOI] [PubMed] [Google Scholar]
- 23.Christofori G., Semb H. The role of the cell-adhesion molecule E-cadherin as a tumour-suppressor gene. Trends Biochem Sci. 1999;24:73–76. doi: 10.1016/s0968-0004(98)01343-7. [DOI] [PubMed] [Google Scholar]
- 24.Goodwin M., Yap A.S. Classical cadherin adhesion molecules: coordinating cell adhesion, signaling and the cytoskeleton. J Mol Histol. 2004;35:839–844. doi: 10.1007/s10735-004-1833-2. [DOI] [PubMed] [Google Scholar]
- 25.Perl A.K., Wilgenbus P., Dahl U., Semb H., Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 26.Anastasiadis P.Z. p120-ctn: a nexus for contextual signaling via Rho GTPases. Biochim Biophys Acta. 2007;1773:34–46. doi: 10.1016/j.bbamcr.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 27.Gumbiner B.M. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 28.Li L., Ying J., Li H., Zhang Y., Shu X., Fan Y., Tan J., Cao Y., Tsao S.W., Srivastava G., Chan A.T., Tao Q. The human cadherin 11 is a pro-apoptotic tumor suppressor modulating cell stemness through Wnt/[beta]-catenin signaling and silenced in common carcinomas. Oncogene. 2012;31:3901–3912. doi: 10.1038/onc.2011.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ortiz A., Lee Y.C., Yu G., Liu H.C., Lin S.C., Bilen M.A., Cho H., Yu-Lee L.Y., Lin S.H. Angiomotin is a novel component of cadherin-11/[beta]-catenin/p120 complex and is critical for cadherin-11-mediated cell migration. FASEB J. 2015;29:1080–1091. doi: 10.1096/fj.14-261594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Craciun F.L., Bijol V., Ajay A.K., Rao P., Kumar R.K., Hutchinson J., Hofmann O., Joshi N., Luyendyk J.P., Kusebauch U., Moss C.L., Srivastava A., Himmelfarb J., Waikar S.S., Moritz R.L., Vaidya V.S. RNA sequencing identifies novel translational biomarkers of kidney fibrosis. J Am Soc Nephrol. 2016;27:1702–1713. doi: 10.1681/ASN.2015020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider D.J., Wu M., Le T.T., Cho S.H., Brenner M.B., Blackburn M.R., Agarwal S.K. Cadherin-11 contributes to pulmonary fibrosis: potential role in TGF-beta production and epithelial to mesenchymal transition. FASEB J. 2012;26:503–512. doi: 10.1096/fj.11-186098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shibata T., Ochiai A., Gotoh M., Machinami R., Hirohashi S. Simultaneous expression of cadherin-11 in signet-ring cell carcinoma and stromal cells of diffuse-type gastric cancer. Cancer Lett. 1996;99:147–153. doi: 10.1016/0304-3835(95)04047-1. [DOI] [PubMed] [Google Scholar]
- 33.Tamura D., Hiraga T., Myoui A., Yoshikawa H., Yoneda T. Cadherin-11-mediated interactions with bone marrow stromal/osteoblastic cells support selective colonization of breast cancer cells in bone. Int J Oncol. 2008;33:17–24. [PubMed] [Google Scholar]
- 34.Satcher R.L., Pan T., Cheng C.J., Lee Y.C., Lin S.C., Yu G., Li X., Hoang A.G., Tamboli P., Jonasch E., Gallick G.E., Lin S.H. Cadherin-11 in renal cell carcinoma bone metastasis. PLoS One. 2014;9:e89880. doi: 10.1371/journal.pone.0089880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin Y.L., Gui S.L., Ma J.G. Aberrant methylation of CDH11 predicts a poor outcome for patients with bladder cancer. Oncol Lett. 2015;10:647–652. doi: 10.3892/ol.2015.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaur H., Phillips-Mason P.J., Burden-Gulley S.M., Kerstetter-Fogle A.E., Basilion J.P., Sloan A.E., Brady-Kalnay S.M. Cadherin-11, a marker of the mesenchymal phenotype, regulates glioblastoma cell migration and survival in vivo. Mol Cancer Res. 2012;10:293–304. doi: 10.1158/1541-7786.MCR-11-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pishvaian M.J., Feltes C.M., Thompson P., Bussemakers M.J., Schalken J.A., Byers S.W. Cadherin-11 is expressed in invasive breast cancer cell lines. Cancer Res. 1999;59:947–952. [PubMed] [Google Scholar]
- 38.Apte M.V., Haber P.S., Applegate T.L., Norton I.D., McCaughan G.W., Korsten M.A., Pirola R.C., Wilson J.S. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43:128–133. doi: 10.1136/gut.43.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furukawa T., Duguid W.P., Rosenberg L., Viallet J., Galloway D.A., Tsao M.S. Long-term culture and immortalization of epithelial cells from normal adult human pancreatic ducts transfected by the E6E7 gene of human papilloma virus 16. Am J Pathol. 1996;148:1763–1770. [PMC free article] [PubMed] [Google Scholar]
- 40.Ouyang H., Mou L., Luk C., Liu N., Karaskova J., Squire J., Tsao M.S. Immortal human pancreatic duct epithelial cell lines with near normal genotype and phenotype. Am J Pathol. 2000;157:1623–1631. doi: 10.1016/S0002-9440(10)64800-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang J.M., Nagatomo I., Suzuki E., Mizuno T., Kumagai T., Berezov A., Zhang H., Karlan B., Greene M.I., Wang Q. YAP modifies cancer cell sensitivity to EGFR and survivin inhibitors and is negatively regulated by the non-receptor type protein tyrosine phosphatase 14. Oncogene. 2013;32:2220–2229. doi: 10.1038/onc.2012.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berezov A., Cai Z., Freudenberg J.A., Zhang H., Cheng X., Thompson T., Murali R., Greene M.I., Wang Q. Disabling the mitotic spindle and tumor growth by targeting a cavity-induced allosteric site of survivin. Oncogene. 2012;31:1938–1948. doi: 10.1038/onc.2011.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rhodes D.R., Kalyana-Sundaram S., Mahavisno V., Varambally R., Yu J., Briggs B.B., Barrette T.R., Anstet M.J., Kincead-Beal C., Kulkarni P., Varambally S., Ghosh D., Chinnaiyan A.M. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9:166–180. doi: 10.1593/neo.07112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Badea L., Herlea V., Dima S.O., Dumitrascu T., Popescu I. Combined gene expression analysis of whole-tissue and microdissected pancreatic ductal adenocarcinoma identifies genes specifically overexpressed in tumor epithelia. Hepatogastroenterology. 2008;55:2016–2027. [PubMed] [Google Scholar]
- 45.Pei H., Li L., Fridley B.L., Jenkins G.D., Kalari K.R., Lingle W., Petersen G., Lou Z., Wang L. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell. 2009;16:259–266. doi: 10.1016/j.ccr.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morvaridi S., Dhall D., Greene M.I., Pandol S.J., Wang Q. Role of YAP and TAZ in pancreatic ductal adenocarcinoma and in stellate cells associated with cancer and chronic pancreatitis. Sci Rep. 2015;5:16759. doi: 10.1038/srep16759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feltes C.M., Kudo A., Blaschuk O., Byers S.W. An alternatively spliced cadherin-11 enhances human breast cancer cell invasion. Cancer Res. 2002;62:6688–6697. [PubMed] [Google Scholar]
- 48.Valencia X., Higgins J.M., Kiener H.P., Lee D.M., Podrebarac T.A., Dascher C.C., Watts G.F., Mizoguchi E., Simmons B., Patel D.D., Bhan A.K., Brenner M.B. Cadherin-11 provides specific cellular adhesion between fibroblast-like synoviocytes. J Exp Med. 2004;200:1673–1679. doi: 10.1084/jem.20041545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noss E.H., Watts G.F., Zocco D., Keller T.L., Whitman M., Blobel C.P., Lee D.M., Brenner M.B. Evidence for cadherin-11 cleavage in the synovium and partial characterization of its mechanism. Arthritis Res Ther. 2015;17:126. doi: 10.1186/s13075-015-0647-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao S., Ammanamanchi S., Brattain M., Cao L., Thangasamy A., Wang J., Freeman J.W. Smad4-dependent TGF-beta signaling suppresses RON receptor tyrosine kinase-dependent motility and invasion of pancreatic cancer cells. J Biol Chem. 2008;283:11293–11301. doi: 10.1074/jbc.M800154200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vogelmann R., Nguyen-Tat M.D., Giehl K., Adler G., Wedlich D., Menke A. TGFbeta-induced downregulation of E-cadherin-based cell-cell adhesion depends on PI3-kinase and PTEN. J Cell Sci. 2005;118:4901–4912. doi: 10.1242/jcs.02594. [DOI] [PubMed] [Google Scholar]
- 52.Aceto N., Bardia A., Miyamoto D.T., Donaldson M.C., Wittner B.S., Spencer J.A., Yu M., Pely A., Engstrom A., Zhu H., Brannigan B.W., Kapur R., Stott S.L., Shioda T., Ramaswamy S., Ting D.T., Lin C.P., Toner M., Haber D.A., Maheswaran S. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158:1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bitting R.L., Boominathan R., Rao C., Kemeny G., Foulk B., Garcia-Blanco M.A., Connelly M., Armstrong A.J. Development of a method to isolate circulating tumor cells using mesenchymal-based capture. Methods. 2013;64:129–136. doi: 10.1016/j.ymeth.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee Y.C., Bilen M.A., Yu G., Lin S.C., Huang C.F., Ortiz A., Cho H., Song J.H., Satcher R.L., Kuang J., Gallick G.E., Yu-Lee L.Y., Huang W., Lin S.H. Inhibition of cell adhesion by a cadherin-11 antibody thwarts bone metastasis. Mol Cancer Res. 2013;11:1401–1411. doi: 10.1158/1541-7786.MCR-13-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chu K., Cheng C.J., Ye X., Lee Y.C., Zurita A.J., Chen D.T., Yu-Lee L.Y., Zhang S., Yeh E.T., Hu M.C., Logothetis C.J., Lin S.H. Cadherin-11 promotes the metastasis of prostate cancer cells to bone. Mol Cancer Res. 2008;6:1259–1267. doi: 10.1158/1541-7786.MCR-08-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Assefnia S., Dakshanamurthy S., Guidry Auvil J.M., Hampel C., Anastasiadis P.Z., Kallakury B., Uren A., Foley D.W., Brown M.L., Shapiro L., Brenner M., Haigh D., Byers S.W. Cadherin-11 in poor prognosis malignancies and rheumatoid arthritis: common target, common therapies. Oncotarget. 2014;5:1458–1474. doi: 10.18632/oncotarget.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang C.F., Lira C., Chu K., Bilen M.A., Lee Y.C., Ye X., Kim S.M., Ortiz A., Wu F.L., Logothetis C.J., Yu-Lee L.Y., Lin S.H. Cadherin-11 increases migration and invasion of prostate cancer cells and enhances their interaction with osteoblasts. Cancer Res. 2010;70:4580–4589. doi: 10.1158/0008-5472.CAN-09-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang H., Leinwand L.A., Anseth K.S. Roles of transforming growth factor-beta1 and OB-cadherin in porcine cardiac valve myofibroblast differentiation. FASEB J. 2014;28:4551–4562. doi: 10.1096/fj.14-254623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nam E.H., Lee Y., Zhao X.F., Park Y.K., Lee J.W., Kim S. ZEB2-Sp1 cooperation induces invasion by upregulating cadherin-11 and integrin alpha5 expression. Carcinogenesis. 2014;35:302–314. doi: 10.1093/carcin/bgt340. [DOI] [PubMed] [Google Scholar]
- 60.Alimperti S., You H., George T., Agarwal S.K., Andreadis S.T. Cadherin-11 regulates both mesenchymal stem cell differentiation into smooth muscle cells and the development of contractile function in vivo. J Cell Sci. 2014;127:2627–2638. doi: 10.1242/jcs.134833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pandol S.J., Edderkaoui M. What are the macrophages and stellate cells doing in pancreatic adenocarcinoma? Front Physiol. 2015;6:125. doi: 10.3389/fphys.2015.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ozdemir B.C., Pentcheva-Hoang T., Carstens J.L., Zheng X., Wu C.C., Simpson T.R., Laklai H., Sugimoto H., Kahlert C., Novitskiy S.V., De Jesus-Acosta A., Sharma P., Heidari P., Mahmood U., Chin L., Moses H.L., Weaver V.M., Maitra A., Allison J.P., LeBleu V.S., Kalluri R. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rhim A.D., Oberstein P.E., Thomas D.H., Mirek E.T., Palermo C.F., Sastra S.A., Dekleva E.N., Saunders T., Becerra C.P., Tattersall I.W., Westphalen C.B., Kitajewski J., Fernandez-Barrena M.G., Fernandez-Zapico M.E., Iacobuzio-Donahue C., Olive K.P., Stanger B.Z. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PCR analysis of Cad-11 mRNA levels in human pancreatic cells. BxPC-3, MIA PaCa-2, and HPAF-II are human pancreatic cancer cell lines. The isolated RNA was converted to cDNA and screened for Cad-11 and β-actin. Representative reactions are shown. Cad-11, cadherin-11; HDPE6, immortalized human pancreatic epithelial cell; PSC-CP, pancreatic stellate cell isolated from chronic pancreatitis tissue; PSC-N, pancreatic stellate cell isolated from normal pancreas.
Oncomine analysis of genes coexpressed with Cad-11. A and B: Box plot and heat map of the top genes coexpressed with Cad-11 in the Badea et al44 data set. C and D: Box plot and heat map of genes coexpressed with Cad-11 in the Pei et al45 microarray data set. In the box plot, the dark bar represents the median; the box shows the first and third quartile; the top and bottom bars indicate the 90 and 10 percentile respectively; the dots represent the maximum and minimum values. P < 2.23 × 10−15 (A and B); P < 3.77 × 10−6 (C and D). Cad-11, cadherin-11; PDAC, pancreatic ductal adenocarcinoma.
Negative control for flow cytometric analysis of Cad-11 levels. The procedure was performed under the same conditions as those shown in Figure 3, C and D. The histograms of cells unstained or stained with secondary antibody only are shown. Note that the secondary antibody alone does not generate any signal in flow cytometry. APC-A, allophycocyanin area; Cad-11, cadherin-11; HDPE6, immortalized human pancreatic epithelial cell; PSC-CP, primary pancreatic stellate cell isolated from chronic pancreatitis tissue; PSC-N: primary pancreatic stellate cell isolated from normal pancreas.
Relative migratory capacity of human pancreatic cancer cells. The cells were seeded in Transwell inserts in serum-free medium and evaluated for migration 24 hours later. Data are expressed as relative migratory capacity compared with BxPC-3. n = 3 separate experiments. ∗P < 0.05.