Abstract
Acute Achilles tendon rupture is one of the most common tendon injuries in adults. We hypothesized that Platelet-Rich Plasma (PRP) can be used as biological augmentation for surgical treatment of acute Achilles tendon rupture. Our study is a prospective randomized controlled trial. Patients with acute Achilles tendon rupture undergoing surgical repair were randomly assigned into either control group or PRP group. End-to-end modified Krackow suture was performed in both groups. In the PRP group, PRP was injected into the paratenon sheath and around the ruptured tissue after the tendon was repaired. Postoperatively we evaluated isokinetic muscle strength at 3, 6, 12, and 24 months. In addition, ankle ROM, calf circumference, Leppilahti score, and the SF-36 score were evaluated at 6, 12, and 24 months after operation. At 3 months, the PRP group had better isokinetic muscle. The PRP group also achieved higher SF-36 and Leppilahti scores at 6 and 12 months. At 24 months, the PRP group had an improved ankle range of motion compared to the control group. Our study results suggest that PRP can serve as a biological augmentation to acute Achilles tendon rupture repair and improves both short and midterm functional outcomes.
1. Introduction
The acute Achilles tendon rupture is one of the most common tendon injuries in adults. The incidence of Achilles tendon injury is higher among the middle-aged population because of their participation in high-level sports [1–3]. Surgical treatment will accelerate the healing process, reduce rerupture, and improve the quality of life.
The use of Platelet-Rich Plasma (PRP) in the treatment of orthopedic injuries has been widely reported in recent years [4–7]. PRP is defined as a high concentration of platelets in plasma after special processing. Platelets are known to contain more than 300 bioactive proteins, including vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), fibroblast growth factor, platelet-derived growth factor (PDGF), platelet-derived epidermal growth factor (PD-EGF), transforming growth factor beta (TGFb), and epidermal growth factor (EGF) [8–10]. Thus, using PRP promotes postoperative healing and can potentially improve functional outcomes by concentrating these platelet-derived bioactive proteins at the side of injury.
The objective of this study was to evaluate clinical outcomes and calf muscle strength recovery after surgical repair of acute Achilles tendon repair with PRP augmentation.
2. Materials and Methods
2.1. Patient Recruitment
The current study was approved by Shanghai Sixth People's Hospital Ethics review board. All participants signed informed consent prior to entering the study.
Patients who presented with acute Achilles tendon rupture and underwent surgical repair at the Department of Orthopaedics, Shanghai Sixth People's Hospital, from January 2013 to January 2014, were recruited in the study. These patients were diagnosed with acute Achilles tendon rupture by the presence of a palpable gap, a positive Thompson squeeze test and ultrasonography were included. Included patients were aged 18–45 years, with a confirmed closed acute Achilles tendon rupture due to noncontact sport injuries. Major exclusion criteria included previously incurred Achilles tendinopathy, prior Achilles tendon rupture, patients with previous surgical procedures on the affected or contralateral side, open Achilles tendon rupture, rupture more than 3 weeks prior to presentation, pathological rupture, smokers, and polytrauma. In addition, patients with diabetes, paraplegia, or any peripheral neuropathy that could impair healing were also excluded. Our study is a prospective randomized controlled trial. Patients diagnosed with acute Achilles tendon rupture were randomly assigned to control group or PRP group. Computer generated a blinded randomization number. If generated number was an odd number, the patient was assigned to PRP group. If generated number was an even number, the patient was assigned to control group. RICE principle (rest, ice, compression, and elevation) was used to decrease leg swelling in all patients before the surgery.
2.2. PRP Preparation
The PRP was prepared using the WEGO Platelet-Rich Plasma Preparation Kits (WEGO Ltd., Shandong, China) [11]. Approximately 40 mL of blood was taken from patient via venipuncture and then placed in Kits and spun in a potable centrifuge (WEGO LTD, Shandong, China) at 2000 rpm for 10 minutes twice. This standard process produces 3 to 4 ml of plasma that has 6 times higher platelet concentration than normal physiological value. The leukocytes in PRP are also 4 times higher in concentration than normal blood.
2.3. Operation Procedure
All patients were operated on under epidural anesthesia. They were placed in prone position with thigh tourniquet applied. In both groups, end-to-end modified Krackow suture technique [12] was performed after longitudinal incision of the fascia and paratenon. To maintain the Achilles length in PRP group, only blood clots were removed. The ruptured tissue was preserved at both ends. The ruptured Achilles tendon was repaired with a number 2 nonabsorbable Ethibond suture (Ethicon, Somerville, NJ) (Figures 1 and 2). PRP was injected into the paratenon sheath and the surrounding lacerated tissue (Figures 3 and 4). Then, the paratenon was carefully closed with number 3-0 braided polyglycolic absorbable suture (Vicryl, Ethicon). The skin was closed with number 4-0 Prolene suture.
Figure 1.
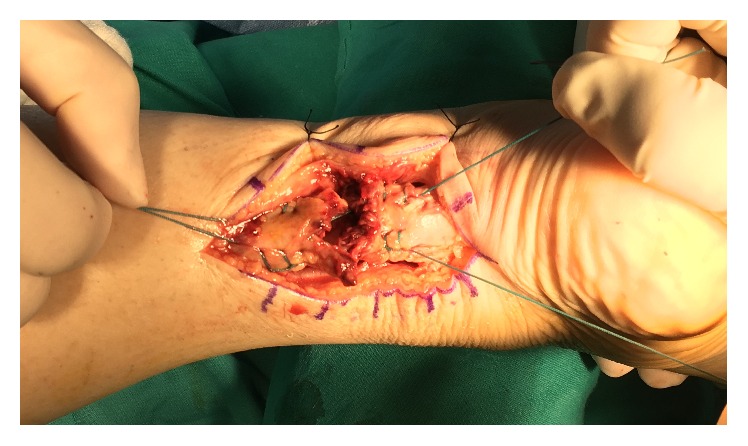
Modified Krackow suture technique was performed.
Figure 2.
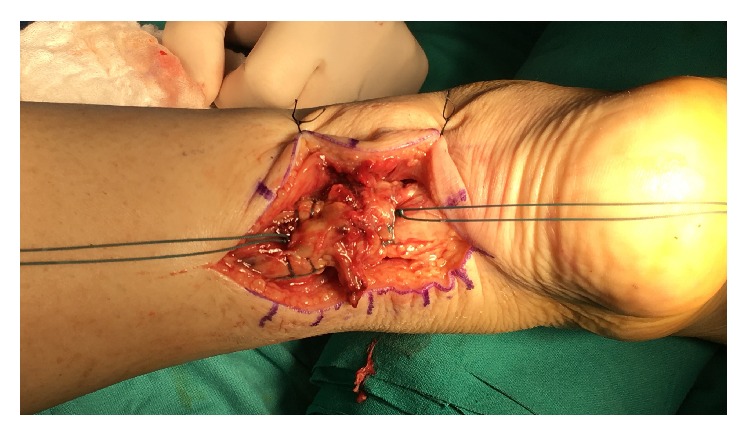
Modified Krackow suture technique was performed and maintained lacerated tissue.
Figure 3.
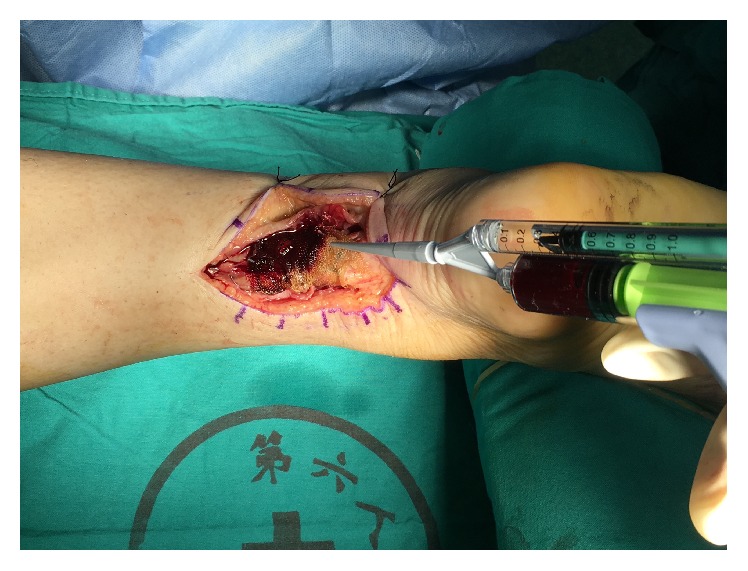
PRP was injected in rupture ends and paratenon sheath.
Figure 4.
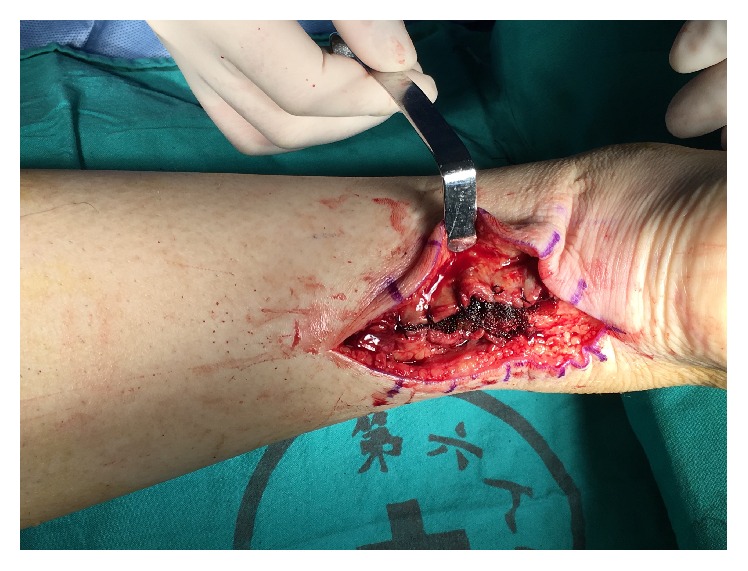
Close paratenon sheath and preserve PRP in it.
In the control group, the same modified Krackow suture technique was performed after the rupture ends of the tendon were debrided without PRP injection.
2.4. Postoperative Care
Patients in both groups underwent the same postoperative protocol. All patients had postoperation follow-up assessment at 3 weeks and 3, 6, 12, and 24 months. The surgical site was examined at 3 weeks postop. If there was no evidence of infection, skin sutures were removed. Postoperatively, the foot was immobilized in an anterior splint for 3 weeks, followed by nonweight-bearing walking boot with heel lifts and daily active range of motion for the first 6 weeks. Then the heal lift height was decreased by 1 mm every day. The patient was allowed gradual return to full weight bearing walking over another 6 weeks. Full weight bearing was permitted in 3 months, and full activities were permitted as tolerated in 6 months. Patients were reassessed at 12 and 24 months after surgery.
2.5. Outcome Measures
The primary outcome measures were the Leppilahti score at 6, 12, and 24 months after surgery. The secondary outcome measures including the Short Form (36) Survey (SF-36) score, ankle range of motion (ROM), and calf circumference were evaluated at 6, 12, and 24 months after surgery. In addition, Isokinetic evaluation was performed at 60 degrees per second, 120 degrees per second and 240 degrees per second angular speeds with the Multi-Joint Isokinetic Dynamometer Prima DOC (Firenze, Italy) at 3, 6, 12, and 24 months. The calf circumference and the isokinetic calf muscle strength were measured and compared with the contralateral normal side. An independent clinical observer performed all evaluations.
2.6. Leppilahti Score
The Leppilahti score includes subjective factors (pain, stiffness, muscle weakness, footwear restriction, and subjective outcome) and objective factors (range of ankle active motion and isokinetic calf muscle strength score). The isokinetic muscle strength score is calculated from the plantar flexion and dorsiflexion peak torques of 3 different ankle test speeds as described by Leppilahti et al. [13]. The maximum Leppilahti score is 102 points. A total score of 87 to 102 points was graded as excellent, 72 to 86 as good, 57 to 71 as fair, and 56 or less as poor. Patients completed the subjective part of the Leppilahti score independently without any supervision or instruction.
2.7. Ankle Range of Motion (ROM)
Bilateral ankle dorsal flexion was assessed at 6, 12, and 24 months after operation. The physiotherapist calculated an average of three readings with a hand-held goniometer that measured the maximum ankle dorsiflexion.
2.8. Strength Measurements
The isokinetic strength of both ankles in plantar flexion and dorsiflexion was assessed 3, 6, 12, and 24 months after surgery. An independent athletic therapist, who is blinded to both groups, used isokinetic dynamometer (CSMI HUMAC Norm, Stoughton, Massachusetts, USA) to measure muscle strength. During the measurements as patients sat on the positioning chair their feet were fixed with Velcro straps. All patients were encouraged to achieve a maximal effort during testing. Five isokinetic plantar flexion and dorsiflexion cycles were performed at a speed of 60 degrees per second, 120 degrees per second, and 240 degrees per second. The peak torque of the injured leg as well as uninjured leg was measured. The relative performance of the injured limb was calculated as follows: injured side/healthy side ∗ 100%.
2.9. Complications
The complication rates included the rerupture rates, superficial and deep infection rates, and sural nerve injury. All complications were recorded during the follow-up assessment.
2.10. Statistical Methods
Descriptive statistics included mean ± standard deviation, median (interquartile, IQR), frequency distribution, and percentage. Pearson's chi-square test and Fisher's exact test were used to evaluate the categorical variables. The fitness of the variables to normal distribution was evaluated using visual (histogram and probability graphs) and analytic methods (Shapiro-Wilk Test). In the presence of a significant difference between two independent groups, Student's t-test was used for normal distributed variables. For variables not distributed normally, Mann–Whitney U test was used to compare two independent groups. Repeated-measures analysis of variance (ANOVA) was employed to determine if there were any significant differences between the time points (3, 6, 12, and 24 months after surgery) or between the groups. Post hoc Bonferroni analysis was performed to identify the mean differences within the existence of a statistically significant P value (P < 0.05) in time or between groups. Two-tailed tests of significance are reported and P value <0.05 were considered statistically significant. Statistical analyses were performed using SPSS statistical software program (version 11.0.0, SPSS, IBM).
3. Results
3.1. Demographic and Anatomical Characteristics of Achilles Tendon Rupture
A total number of 52 cases of acute Achilles ruptures were recruited during the study period. However, 9 patients refused operative treatment and 7 patients refused postoperative assessment. Therefore, only 36 patients were included in the study. There were 16 cases in the PRP group and 20 cases in the control group. Most of patients were males and had sports related tendon rupture. All ruptures happened at middle portion of the tendon. There were no significant differences in demographic data or pattern of tendon rupture between two groups (Table 1).
Table 1.
The basic information of two groups.
| PRP group (n = 16) | Control group (n = 20) | |
|---|---|---|
| Age (yrs) | 30.2 ± 5.8 | 28.9 ± 5.7 |
| Sex | ||
| Male | 16 | 19 |
| Female | 0 | 1 |
| Affected leg | ||
| Left | 6 | 7 |
| Right | 10 | 13 |
| Causes of rupture | ||
| Sport | 15 | 19 |
| Others | 1 | 1 |
| Location of rupture | ||
| Insertion | 0 | 0 |
| Midtendon | 16 | 20 |
| Enthesis | 0 | 0 |
3.2. Postoperative Complications
In control group, 18 cases healed within 3 months. Among them, there were 2 superficial infections and that resolved with antibiotic treatment. One had deep infection and one had a rerupture. The deep infection was because of allergic reaction to nonabsorbable suture. After debridement, the wound healed at 4 months from the first operation. The rerupture was caused due to a mechanical fall and it was managed successfully nonoperatively with walking boots for one month. In the PRP group, all 16 cases healed uneventfully in 3 months after surgery. There was neither infection nor rerupture. For both groups, there was no sural nerve injury or skin adhesion.
3.3. Patient Reported Outcome Measures
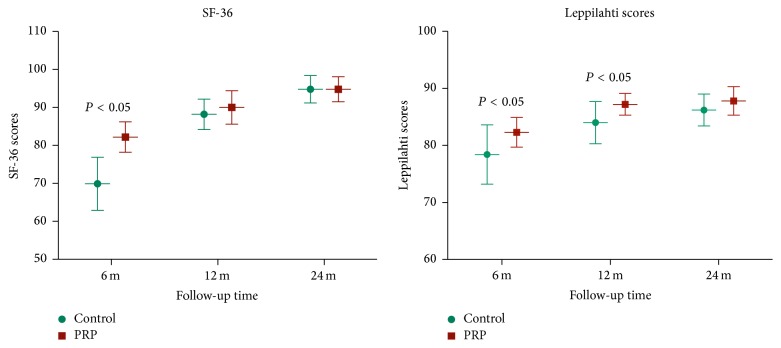
Both the Leppilahti scores and SF-36 scores were evaluated at 6 months, one year, and two years after the operation. For the SF-36 scores, although there were no significant differences between two groups at one and two years of follow-up, the scores in the PRP group were significantly higher than in control group at 6 months postop. Similar results were seen in Leppilahti scores with significantly higher scores in PRP group at 6 months and 1 year after the surgery. Finally, both the groups had similar Leppilahti scores at 2-year postoperative follow-up (Figure 5). The results were excellent for 12 patients (60%), good for 7 patients (35%), and fair for 1 patient (5%) in control group. In PRP group, scores were excellent for 13 patients (72%) and good for 5 patients (28%) at 2-year postoperative follow-up.
Figure 5.
The SF-36 scores and Leppilahti scores were evaluated at 6, 12, and 24 months after operation. PRP group had higher scores in SF-36 and Leppilahti scores than control group for up to postoperative 6 and 12 months, respectively.
3.4. Ankle and Leg Function Tests
The ankle ROM was measured for plantar flexion and dorsiflexion at 6-month, one-year, and two-year postoperative follow-up. Patients in the PRP group had significantly better range of motion at all time points (Table 2).
Table 2.
Ankle range of motion (ROM).
| Control group (mean ± SD) (°) | PRP group (mean ± SD) (°) | P value | |
|---|---|---|---|
| Plantar flexion | |||
| 6 months | −4.5 ± 0.5 | −3.0 ± 0.3 | <0.001 |
| 12 months | −2.2 ± 0.4 | −0.9 ± 0.4 | <0.001 |
| 24 months | −2.0 ± 0.4 | −1.1 ± 0.3 | <0.001 |
| Dorsiflexion | |||
| 6 months | −4.4 ± 0.4 | −2.6 ± 0.4 | <0.001 |
| 12 months | −2.2 ± 0.3 | −1.1 ± 0.5 | <0.001 |
| 24 months | −1.9 ± 0.4 | −1.0 ± 0.4 | <0.001 |
When compared to the normal (contralateral) side of calf circumferences, calf circumferences at the injured side were smaller, but there were no significant differences in the calf circumferences between two groups at all time periods (Table 3).
Table 3.
Calf circumference (%).
| 6 months | 1 year | 2 years | |
|---|---|---|---|
| Control | 85.3 ± 4.0 | 95.8 ± 3.3 | 96.2 ± 3.1 |
| PRP | 88.1 ± 4.9 | 95.5 ± 3.0 | 96.8 ± 2.2 |
| t | 1.9 | 0.3 | 0.6 |
| P | 0.07 | 0.73 | 0.64 |
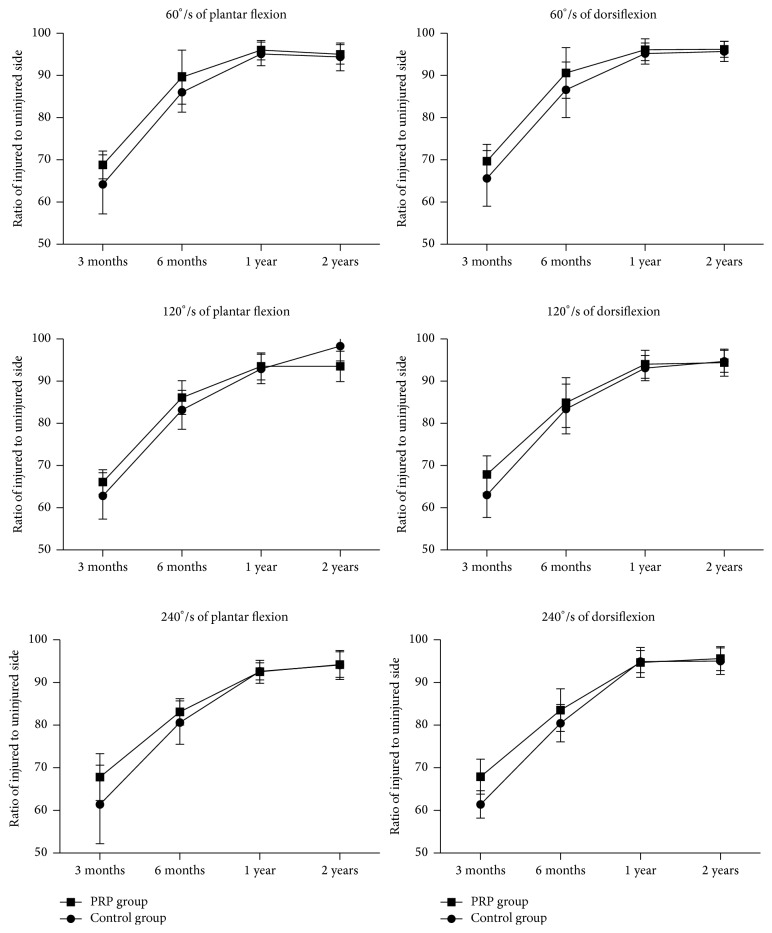
The isokinetic calf muscle strengths were evaluated in percentage of plantar flexion strength and the percentage of dorsiflexion strength for 60 degrees, 120 degrees, and 240 degrees at 3-month, 6-month, one-year, and two-year postoperative follow-up. At 3-month postoperative follow-up, PRP group had significantly higher percentage in all measured degrees than those in the control group. However, after 6-month follow-up both groups were similar (Tables 4 and 5 and Figure 6).
Table 4.
Percentage of plantar flexion strength (%).
| 3 months | 6 months | 1 year | 2 years | |
|---|---|---|---|---|
| 60°/s | ||||
| Control | 64.2 ± 7.0 | 86.0 ± 4.7 | 95.1 ± 2.8 | 94.4 ± 3.3 |
| PRP | 68.8 ± 3.3 | 89.6 ± 6.4 | 96.0 ± 2.3 | 95.0 ± 2.3 |
| t | 3.6 | 2.3 | 0.6 | 1.1 |
| P | 0.022 | 0.059 | 0.28 | 0.54 |
| 120°/s | ||||
| Control | 62.8 ± 5.5 | 83.2 ± 4.6 | 92.9 ± 3.5 | 93.8 ± 3.5 |
| PRP | 66.1 ± 2.9 | 86.1 ± 4.0 | 93.5 ± 3.2 | 93.5 ± 3.6 |
| t | 2.103 | 1.996 | 0.4899 | 0.2953 |
| P | 0.043 | 0.054 | 0.63 | 0.77 |
| 240°/s | ||||
| Control | 61.4 ± 9.2 | 80.6 ± 5.1 | 92.6 ± 2.0 | 94.1 ± 3.4 |
| PRP | 67.8 ± 5.5 | 83.1 ± 3.1 | 92.5 ± 2.7 | 94.2 ± 3.0 |
| t | 2.4 | 3.9 | 0.7 | 0.2 |
| P | 0.021 | 0.091 | 0.9 | 0.94 |
Table 5.
Percentage of dorsiflexion strength (%).
| 3 months | 6 months | 1 year | 2 years | |
|---|---|---|---|---|
| 60°/s | ||||
| Control | 65.6 ± 6.6 | 86.6 ± 6.6 | 95.5 ± 2.5 | 95.7 ± 2.4 |
| PRP | 69.7 ± 4.0 | 90.6 ± 6.0 | 96.1 ± 2.6 | 96.2 ± 1.9 |
| t | 2.2 | 1.8 | 0.6 | 0.7 |
| P | 0.035 | 0.072 | 0.52 | 0.46 |
| 120°/s | ||||
| Control | 63.0 ± 5.3 | 83.4 ± 5.9 | 93.1 ± 3.0 | 94.7 ± 2.6 |
| PRP | 67.9 ± 4.4 | 84.9 ± 5.9 | 94.0 ± 3.3 | 94.4 ± 3.2 |
| t | 3.2 | 3.1 | 0.2 | 0.1 |
| P | 0.006 | 0.44 | 0.39 | 0.74 |
| 240°/s | ||||
| Control | 61.4 ± 3.2 | 80.45 ± 4.4 | 94.9 ± 2.6 | 95.0 ± 3.1 |
| PRP | 67.9 ± 4.1 | 83.5 ± 5.0 | 94.7 ± 3.5 | 95.6 ± 2.8 |
| t | 5.3 | 1.9 | 0.2 | 0.5 |
| P | <0.001 | 0.061 | 0.83 | 0.61 |
Figure 6.
Demography of isokinetic calf muscle strength.
4. Discussion
PRP has been used to enhance bone and soft tissue healing for several years, previously being mainly used in oral and maxillofacial surgery [4, 14]. The use of PRP in orthopedics and sports medicine is a novel concept. It has been used as an agent to improve the healing of muscle, bone, cartilage, tendon, and ligaments [15]. Previous laboratory studies have shown PRP as an activator of circulation-derived cells for the improvement of the initial tendon healing process. The major findings of our study were to find out the role of PRP in short and midterm outcome of surgical repair of acute Achilles tendon rupture. Both subjective patients reported scores and objective functional tests results demonstrated that PRP group has better early-midterm outcomes. In addition, the PRP group showed better ankle ROM for up to two years after operation.
Our results were consistent with other studies. Sánchez et al. [16] demonstrated an early recovery of ROM and running after the application of PRP in Achilles tendon suture. The functional performances in isokinetic calf muscle strength were better in the PRP group. In our study, at postoperative 3-month follow-up, we also found that the calf circumference proportion was similar in both groups. This implicated the similar levels of leg muscle decrease after the injury and operation between two groups. However, the muscle performance was statistically different between two groups for up to 6-month postoperative follow-up. This could be due to the biological augmentation effect of PRP on early-midterm tendon healing. The underlying mechanism is likely increased vascular activity after direct administration of PRP into tendon body.
However, one potential limitation of our study is the different surgical preparation of the rupture ends of the tendon in the two groups. In the control group, the ruptured tendon was debrided before end-to-end repair. This may have caused shortening of the Achilles tendon, while in the PRP group, there was no tendon debridement performed; therefore, tendon length could be maintained. We need further studies to evaluate whether the difference in final tendon length influenced our current study results.
The functional performances in isokinetic calf muscle strengths were better in the PRP group at postoperative 3-month follow-up. This result was also similar to Sánchez et al.'s study [16], where they proved that PRP injection after surgical repair of Achilles tendon rupture could facilitate patients' return to sports activities and training faster than others.
Apart from ankle ROM, other midterm outcomes of the PRP injection as an adjuvant of acute Achilles tendon surgical repair were similar to control group in our study. Those results were consistent with other studies as well. De Carli et al. [17] showed a substantial equivalent structural and functional results in Achilles tendon ruptures surgically treated with and without PRP at 6 months and 24 months after surgery.
When measuring patient reported outcome, we used both SF-36 and the Leppilahti scores. The SF-36 score is a validated, commonly used tool in postoperative recovery. It contains both physical and psychological evaluation. In our study, patients who had PRP injection had higher scores in SF-36 at 3 months after the surgery when compared to patients in control group. The Leppilahti scores is the measurement for subjective report outcome after ankle, tendon treatment. The scores were higher at 3- and 6-month postoperative follow-up in PRP group than in control group. These subjective outcomes were consistent with our functional (objective) outcomes but were different from other studies. De Carli et al. [17] found that the Foot and Ankle Outcome Score (FAOS) and VISA-A showed no difference between the PRP and control groups at 1, 3, 6, and 24 months after operation of acute Achilles tendon rupture. The Leppilahti score system has been used in our hospital for years. Because of the different scales, it would be difficult to compare our results with others.
The complication after surgical treatment of acute Achilles tendon rupture could delay the healing process. The common complications include infection and rerupture. Bartel et al. [18] conducted a systematic review showing that the wound site infection rate was 4% in open surgery and the risk of rerupture was 3.4%. Among our participants, the rerupture rate was only 1% in control group and none in PRP group. The infection rate was 1.5% in control group. Despite there being no statistical difference between the two groups, our small sample size may give rise to a false negative result. Recent research [5, 19] suggests that PRP has significantly high serum growth factor levels, and it also has potential anti-infection effects when applied to tissue. The concentration of PRP obtained in our study using the WEGO apparatus is 6 times the concentration of platelets in baseline blood and 4 times the white cells of normal value. This concentration has been found to be effective both in vitro and in vivo studies in proliferation and promote angiogenesis of the tissue [20, 21].
PRP is most effective in the treatment of chronic tendon injuries, such as Tennis elbow. But the effectiveness in treating chronic noninsertional Achilles tendinopathy is controversial. Gaweda et al. [22] treated Achilles tendinopathy with local injection of autologous PRP. They found that PRP was effective in elimination of clinical symptoms, normalization of tendon thickness in the region of intrasubstance tears, decreased peritendineum and tendon thickening, and resolution of hypoechogenic lesions. On the contrary, De Vos et al. [6] reported that a PRP injection did not have improvement in pain and function of patients with chronic Achilles tendinopathy. De Jonge et al. [23] also showed no clinical or ultrasonographic superiority of Platelet-Rich Plasma injection over placebo injection in chronic Achilles tendinopathy at 1-year follow-up.
There are several limitations in our study. Firstly, the number of patients in our study was relatively small and so the power is limited. This could result in either false positive or false negative results. Secondly, the two groups had different surgical preparation of the ruptured tendon that caused difference in tendon length. This may be responsible in differences in ankle ROM. Thirdly, the time interval between follow-up times may be too long, especially during early and midtime period after the surgery. If we could evaluate our patients more frequently, we may be able to find out how quick the PRP effect and how long it could last. Such results may be more useful for the clinical use of PRP.
5. Conclusion
PRP is safe and effective as a biological augmentation agent for surgical repair of acute Achilles tendon rupture. It may improve early-midterm postoperative functional recovery. Further studies to determine the long-term effects of PRP on functional outcomes after Achilles tendon repair and the cost-benefit analysis of using PRP are recommended.
Acknowledgments
Selected data from this study were presented at the Shanghai Sixth People's Hospital, Shanghai, China.
Competing Interests
All authors state that they have no conflict of interests.
Authors' Contributions
Jian Zou and Xiaolian Mo contributed equally to this study and should be regarded as first authors.
References
- 1.Olsson N., Petzold M., Brorsson A., Karlsson J., Eriksson B. I., Silbernagel K. G. Predictors of clinical outcome after acute achilles tendon ruptures. The American Journal of Sports Medicine. 2014;42(6):1448–1455. doi: 10.1177/0363546514527409. [DOI] [PubMed] [Google Scholar]
- 2.Keating J. F., Will E. M. Operative versus non-operative treatment of acute rupture of tendo Achillis: a prospective randomised evaluation of functional outcome. The Journal of Bone & Joint Surgery—British Volume. 2011;93(8):1071–1078. doi: 10.1302/0301-620x.93b8.25998. [DOI] [PubMed] [Google Scholar]
- 3.Neumayer F., Mouhsine E., Arlettaz Y., Gremion G., Wettstein M., Crevoisier X. A new conservative-dynamic treatment for the acute ruptured achilles tendon. Archives of Orthopaedic and Trauma Surgery. 2010;130(3):363–368. doi: 10.1007/s00402-009-0865-1. [DOI] [PubMed] [Google Scholar]
- 4.Choi B.-H., Zhu S.-J., Kim B.-Y., Huh J.-Y., Lee S.-H., Jung J.-H. Effect of platelet-rich plasma (PRP) concentration on the viability and proliferation of alveolar bone cells: an in vitro study. International Journal of Oral and Maxillofacial Surgery. 2005;34(4):420–424. doi: 10.1016/j.ijom.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Creaney L., Wallace A., Curtis M., Connell D. Growth factor-based therapies provide additional benefit beyond physical therapy in resistant elbow tendinopathy: a prospective, single-blind, randomised trial of autologous blood injections versus platelet-rich plasma injections. British Journal of Sports Medicine. 2011;45(12):966–971. doi: 10.1136/bjsm.2010.082503. [DOI] [PubMed] [Google Scholar]
- 6.De Vos R. J., Weir A., Van Schie H. T. M., et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144–149. doi: 10.1001/jama.2009.1986. [DOI] [PubMed] [Google Scholar]
- 7.Hall M. P., Band P. A., Meislin R. T., Jazrawi L. M., Cardone D. A. Platelet-rich plasma: current concepts and application in sports medicine. Journal of the American Academy of Orthopaedic Surgeons. 2009;17(10):602–608. doi: 10.5435/00124635-200910000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Alsousou J., Thompson M., Harrison P., Willett K., Franklin S. Effect of platelet-rich plasma on healing tissues in acute ruptured Achilles tendon: a human immunohistochemistry study. The Lancet. 2015;385(supplement 1):p. S19. doi: 10.1016/s0140-6736(15)60334-8. [DOI] [PubMed] [Google Scholar]
- 9.Rajabi H., Sheikhani Shahin H., Norouzian M., Mehrabani D., Dehghani Nazhvani S. The healing effects of aquatic activities and allogenic injection of Platelet-Rich Plasma (PRP) on injuries of achilles tendon in experimental rat. World Journal of Plastic Surgery. 2015;4(1):66–73. [PMC free article] [PubMed] [Google Scholar]
- 10.Rubio-Azpeitia E., Sánchez P., Delgado D., Andia I. Three-dimensional platelet-rich plasma hydrogel model to study early tendon healing. Cells Tissues Organs. 2015;200(6):394–404. doi: 10.1159/000441053. [DOI] [PubMed] [Google Scholar]
- 11.Yuan T., Guo S.-C., Han P., Zhang C.-Q., Zeng B.-F. Applications of leukocyte- and platelet-rich plasma (L-PRP) in trauma surgery. Current Pharmaceutical Biotechnology. 2012;13(7):1173–1184. doi: 10.2174/138920112800624445. [DOI] [PubMed] [Google Scholar]
- 12.Labib S. A., Rolf R., Dacus R., Hutton W. C. The “Giftbox” repair of the Achilles tendon: a modification of the Krackow technique. Foot & Ankle International. 2009;30(5):410–414. doi: 10.3113/fai.2009.0410. [DOI] [PubMed] [Google Scholar]
- 13.Leppilahti J., Siira P., Vanharanta H., Orava S. Isokinetic evaluation of calf muscle performance after Achilles rupture repair. International Journal of Sports Medicine. 1996;17(08):619–623. doi: 10.1055/s-2007-972905. [DOI] [PubMed] [Google Scholar]
- 14.Mishra A., Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. American Journal of Sports Medicine. 2006;34(11):1774–1778. doi: 10.1177/0363546506288850. [DOI] [PubMed] [Google Scholar]
- 15.Franceschi F., Papalia R., Franceschetti E., Paciotti M., Maffulli N., Denaro V. Platelet-rich plasma injections for chronic plantar fasciopathy: a systematic review. British Medical Bulletin. 2014;112(1):83–95. doi: 10.1093/bmb/ldu025. [DOI] [PubMed] [Google Scholar]
- 16.Sánchez M., Anitua E., Azofra J., Andía I., Padilla S., Mujika I. Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. The American Journal of Sports Medicine. 2007;35(2):245–251. doi: 10.1177/0363546506294078. [DOI] [PubMed] [Google Scholar]
- 17.De Carli A., Lanzetti R. M., Ciompi A., et al. Can platelet-rich plasma have a role in Achilles tendon surgical repair? Knee Surgery, Sports Traumatology, Arthroscopy. 2016;24(7):2231–2237. doi: 10.1007/s00167-015-3580-1. [DOI] [PubMed] [Google Scholar]
- 18.Bartel A. F. P., Elliott A. D., Roukis T. S. Incidence of complications after achillon® mini-open suture system for repair of acute midsubstance achilles tendon ruptures: a systematic review. Journal of Foot and Ankle Surgery. 2014;53(6):744–746. doi: 10.1053/j.jfas.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Gosens T., Peerbooms J. C., Van Laar W., Den Oudsten B. L. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. The American Journal of Sports Medicine. 2011;39(6):1200–1208. doi: 10.1177/0363546510397173. [DOI] [PubMed] [Google Scholar]
- 20.Aspenberg P., Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthopaedica Scandinavica. 2004;75(1):93–99. doi: 10.1080/00016470410001708190. [DOI] [PubMed] [Google Scholar]
- 21.Weibrich G., Hansen T., Kleis W., Buch R., Hitzler W. E. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone. 2004;34(4):665–671. doi: 10.1016/j.bone.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Gaweda K., Tarczynska M., Krzyzanowski W. Treatment of Achilles tendinopathy with platelet-rich plasma. International Journal of Sports Medicine. 2010;31(8):577–583. doi: 10.1055/s-0030-1255028. [DOI] [PubMed] [Google Scholar]
- 23.De Jonge S., De Vos R. J., Weir A., et al. One-year follow-up of platelet-rich plasma treatment in chronic achilles tendinopathy: a double-blind randomized placebo-controlled trial. The American Journal of Sports Medicine. 2011;39(8):1623–1629. doi: 10.1177/0363546511404877. [DOI] [PubMed] [Google Scholar]