Abstract
AIM
To compare of lens oxidative damage induced by vitrectomy and/or hyperoxia in rabbit.
METHODS
Sixteen New Zealand rabbits (2.4-2.5 kg) were randomly divided into two groups (Group A, n=12; Group B, n=4). In Group A, the right eyes were treated with vitrectomy and systemic hyperoxia (oxygen concentration: 80%-85%, 1 ATA, 4h/d) (Group A-right), and the left eyes were treated with hyperoxia without vitrectomy surgery (Group A-left). Four rabbits in group B (eight eyes) were untreated as the controls. Lens transparency was monitored with a slit lamp and recorded before and after vitrectomy. After hyperoxic treatment for 6mo, the eyeballs were removed and the lens cortices (containing the capsules) and nuclei were separated for further morphological and biochemical evaluation.
RESULTS
Six months after treatments, there were no significant morphological changes in the lenses in any experimental group when observed with a slit lamp. However, the levels of water-soluble proteins and ascorbate, and the activities of catalase and Na+-K+-ATPase were significantly reduced, whereas the levels of malondialdehyde and transforming growth factor β2 (TGF-β2) were significantly elevated, in both the cortices and nuclei of eyes treated with vitrectomy and hyperoxia. The increase in protein-glutathione mixed disulfides and the reduction in water-soluble proteins were more obvious in the lens nuclei. The levels of ascorbate in the vitreous fluid were also reduced after vitrectomy, whereas TGF-β2 increased after vitrectomy and hyperoxia. Systemic hyperoxia exposure increased these effects.
CONCLUSION
Removal of the intact vitreous gel with vitrectomy and exposing the lens to increased oxygen from the retina induce lens oxidation and aggregation. Thus, an intact vitreous gel structure may protect the lens from oxidative insult and maintain lens transparency.
Keywords: nuclear sclerotic cataract, vitrectomy, hyperoxia, lens, oxidative insult
INTRODUCTION
Increased exposure of the lens to molecular oxygen has been implicated in the pathogenesis of nuclear sclerotic cataract, the most common type of age-related cataract[1]–[2]. Recent studies have increased our understanding of the vitreous gel and the vitreoretinal influences on lens function and cataract formation[3]–[4].
It has been known for many years that treating patients with hyperbaric oxygen (HBO) increases the myopic shift and the development of nuclear cataract[5]. In an albino guinea pig model treated with HBO in vivo, light scattering by the lens nuclei was increased[6]. Long-term and systemic exposure to hyperoxia can also cause cataracts. Similarly, cultured intact lenses treated with hyperoxia showed severe cortical opacification on the 7th day compared with lenses cultured at lower oxygen levels. This opacification was prevented with an antioxidant (N-acetylcysteine)[7], suggesting that it may be associated with oxidation[8].
The lens has an unusual structure and is an avascular organ, composed of a thin metabolically active outer layer of nucleated fiber cells. Under normal conditions, the lens exists in a relatively hypoxic environment. This may be important for the metabolic balance and transparency of the lens[9]–[10]. The hypoxic environment around lens is largely maintained by the posterior vitreous gel[2]. Previous studies have found that oxygen diffuses from the retina to the vitreous chamber, creating an oxygen gradient. An intact vitreous gel is critical to maintaining this oxygen gradient within the eye[6],[11]–[12]. Consequently, when the integrity and ingredients of the vitreous gel are destroyed, such as during vitrectomy and/or vitreous liquefaction, oxygen is distributed fairly uniformly throughout the vitreous cavity[2]–[4].
In animal and human studies, the vitreous gel was replaced by irrigating solution during vitrectomy, which allowed the homogeneous distribution of oxygen[13]–[14]. As a direct result of vitrectomy, the lens is exposed to relatively high levels of oxygen and this high oxygen can remain adjacent to the lens for weeks, months, or years after the operation in rabbits[6] and humans[2]. These elevated oxygen levels in the vitreous cavity may also move into the lens, leading to elevated oxygen levels in the lens nucleus.
Our previous study[15] of a rabbit model of vitrectomy suggested that vitrectomy is associated with long-term reductions in the activities of antioxidative enzymes in the lens, although there are no significant morphological changes in the transparency of the lens. A further study of the lens after pharmacological vitreolysis and exposure to hyperoxia in rats indicated that exposure to excess molecular oxygen causes significant oxidative damage to the lens, especially the lens nucleus, which is enhanced by pharmacological vitreolysis[16].
Therefore, in the present study, we developed a model of systemic hyperoxia after vitrectomy in the rabbit and investigated how the lens is exposed to oxygen from the retina, and the importance of the gel vitreous in protecting the lens from oxidation.
MATERIALS AND METHODS
Experimental Animals and Materials
Five-month-old New Zealand rabbits (2.4-2.5 kg) were provided by the Animal Laboratories of the Fourth Military Medical University, Xi'an, China. All experimentation conformed to the criteria of the Institutional Animal Ethics Committee and was in full compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Enzyme quantification kits were obtained from the Jiancheng Biology Company (Nanjing, China). Protein quantification kits were obtained from the Biosynthesis Biotechnology Company (Beijing, China). Rabbit anti-glutathione antibody and goat anti-mouse IgG antibody were obtained from Millipore and Santa Cruz Biotechnology (Beijing, China), respectively. All other chemicals and solvents were obtained from local companies.
Vitrectomy
The vitrectomy procedure was performed in the rabbits by the corresponding author (Yan H), as described previously[15]. All the animals were examined with a slitlamp with dilated pupils before surgery, and any rabbit with a defect in the lens or cornea was excluded. Sixteen rabbits were randomly divided into two groups (Group A, n=12; Group B, n=4). The right eyes in group A (Group A-right; 12 eyes) underwent vitrectomy and treatment with hyperoxia (oxygen concentration: 80%-85%, 1 ATA, 4h/d), and the left eyes (Group A-left; 12 eyes) were exposed to hyperoxia without vitrectomy. The four rabbits (eight eyes) in group B were untreated and used as the controls. The vitrectomy without hyperoxia group from our previous study[15] was used as the comparison group in this study. The animals were anesthetized with an intramuscular injection of xylazine (5 mg/kg) and ketamine (45 mg/kg). The surgical technique used was a standard three-port pars plana vitrectomy, with the removal of all visible vitreous gel. The vitreous was removed with a Megatron vitrector for 10min with the removal of approximately 20 mL/time. The irrigation solution was provided by Xijing Hospital Pharmacy (Xi'an, China). Eyes with surgical complications, such as retinal detachment or cataract arising from “lens touch”, were excluded from the study. Three rabbits in group A died before the end of the experiment from hyperoxia-generated lung insult, on days 42, 56, or 60 after treatment with hyperoxia. Ultimately, nine rabbits in group A and four rabbits in group B were used for the analysis.
Lens transparency was monitored with a slitlamp (Haag-Streit BQ 900) before and weekly in the first month after vitrectomy, and then monthly.The pupils of all the rabbit eyes were dilated with a drop of 1% tropicamide. The grading of lens opacification was performed according to the system of the Nuffield Laboratory of Ophthalmology, University of Oxford[17].
Hyperoxia Treatment
On day 2 after surgery, the rabbits in group A were placed in an airtight poly (methyl methacrylate) box with an air inlet and an air outlet, balanced at 5 L/min. The oxygen concentration exceeded 80% within 10min, and then oxygen input was stopped for approximately 40min. The final oxygen concentration inside the box was 80% and stayed above 80% for 4h. The oxygen input was usually checked four times during this procedure, as has been described previously[16]. All the rabbits in group B were kept in room air.
Lens Preparation
After six months, the rabbits were killed by anesthesia overdose and their eyeballs were removed for morphological and biochemical evaluation. The lenses in all the groups were separated into two parts: the cortex, including the capsule, and the nucleus. The lenses were removed from storage at -70°C and placed in room temperature for 10min. After the lens capsule around the equatorial region was sliced to a depth of 0.5 mm with a scalpel, a fissure was seen, the outside of which is the cortex and the inside the nucleus. The water-soluble protein concentrations, the levels of malondialdehyde (MDA), reduced glutathione (GSH), protein-bound GSH, transforming growth factor β2 (TGF-β2), and ascorbate (AsA), and the activities of catalase (CAT), Na+-K+-ATPase, and glutathione reductase (GR) were measured in both portions of the lenses. All parts of the cortices and nuclei were homogenized with a hand-held homogenizer on ice for 10min with 9 mL of 0.9% neutral normal saline per gram of lens weight, and then centrifuged at 3000 r/min for 10min in Eppendorf tubes.
Analysis of the Other Parts of the Eyeball
The aqueous humor (100 mL) and the vitreous fluid (500 mL) were collected immediately after the rabbits were killed. The TGF-β2 content in the aqueous humor, lens, and vitreous fluid, and the AsA levels in the lens and vitreous fluid were determined in all experimental groups at the same time.
Water-soluble Protein Contents
The clear supernatants were used for protein determination. The protein concentrations were determined with Coomassie Brilliant Blue staining using a protein assay kit from Jiancheng Company (Nanjing, China).
Glutathione Concentrations
The concentration of GSH in each lens (cortical and nuclear portions) were determined with 5,5′-dithiobis-(2-nitrobenzoic acid) after centrifugation (3500 r/min) in 2 mL of 10% trichloroacetic acid for 10min, and then measured with a colorimetric method at 25°C and 412 nm[18].
Assay of Na+-K+-ATPase Activity
Na+-K+-ATPase activity was determined as described by Akagawa and Tsukada[19]. The reaction mixture contained 100 mmol/L NaCl, 20 mmol/L KCl, 5 mmol/L MgCl2, 3 mmol/L ATP, and 50 mmol/L Tris (pH 7.4). The clear supernatant of the homogenate was incubated with the reaction mixture at 37°C for 15min. ATP was used as the substrate and the liberated inorganic phosphate was estimated with spectrophotometry. Ouabain was used as a specific blocker of Na+-K+-ATPase activity and ouabain-sensitive ATPase activity was determined and expressed as micromoles of inorganic phosphate released per milligram of protein per hour.
Assay of Catalase Activity
CAT activity was measured spectrophotometrically with the method of Beers and Sizer[20], recording the cleavage of H2O2 at 240 nm. The reaction mixture contained 0.023 mol/L H2O2 in 0.05 mol/L phosphate buffer (pH 7.0). One unit of enzyme activity was defined as 1 mmol/L of H2O2 cleaved per minute at 37°C.
Assay of Glutathione Reductase Activity
GR activity was measured with the procedure of Linetsky et al[21]. The reaction was initiated by the addition of 20 mL of lens homogenate. Oxidized glutathione (GSSG) was reduced to GSH with catalysis by GR, with NADPH as the cofactor. The reduction in optical density at 340 nm was recorded at 25°C for 2min. The units of enzymatic activity were calculated using an extinction coefficient of 6.22 mmol/L/cm for NADPH. One unit was equivalent to the oxidation of 1 mmol of NADPH per minute.
Protein-bound Glutathione Concentrations
The procedures used to analyze the protein-bound glutathione mixed disulfide (PSSG) in the lenses with Western blotting, including the water-soluble proteins in the lenses (cortices and nuclei) from each group, were separated by electrophoresis on a 12% SDS-polyacrylamide gel. The proteins were electroblotted onto a nitrocellulose membrane, which was then blocked for 1h with 0.5% nonfat dry milk in Tris-buffered saline with Tween 20 (TBST), and then incubated for 2h with a primary rabbit anti-GSH antibody (diluted 1:800). The membrane was washed three times in TBST for 5min each and incubated with alkaline-phosphatase-conjugated goat anti-rabbit IgG secondary antibody (diluted 1:15 000) for 1h. After three washes in TBST for 5min each, an enhanced chemiluminescence substrate (Pierce) was applied to the nitrocellulose membrane for 1min, and the proteins were visualized with exposure to X-ray film.
Ascorbate Concentrations
The concentrations of AsA in each lens (cortex and nucleus) and vitreous fluid were determined with 2,2′-dipyridyl and ferric chloride, after centrifugation (4000 rpm, 15min) in 15 mL of 10% trichloroacetic acid, and measured with a colorimetric method at 42°C and 525 nm, as described by Okamura[22].
Malondialdehyde Concentration
The easy and sensitive methods available to quantify MDA have led to the routine use of MDA to detect and measure lipid peroxidation in a wide array of sample types, as described by Halliwell and Chirico[23]. The samples (cortices and nuclei) were heated with 2-thiobarbituruic at low pH, and the absorbance of the resulting pink chromogen was measured at 532 nm.
Analysis of TGF-β2 with an Enzyme-linked Immunosorbent Assay
A double-antibody “sandwich” Enzyme-linked immunosorbent assay (ELISA; R&D Systems, Wiesbaden, Germany) was used to determine the TGF-β2 concentrations in the aqueous humor, lens, and vitreous of the rabbit eyes. The aqueous humor and homogenates of the lens and vitreous were acidified by the addition of 1 mol/L HCl to activate the latent TGF-β2 to its immunoreactive form. This mixture was incubated for 10min at room temperature and neutralized with 1.2 mol/L NaOH/0.5 mol/L HEPES. After 100 mL of the assay diluent was added to each well of a microtiter ELSIA plate, which were coated with a murine monoclonal antibody directed against TGF-β2, the probe or the standard provided was pipetted into the plate. After incubation for 2h at room temperature, each well was washed three times with 400 mL of washing buffer, and 200 mL of horseradish-peroxidase-conjugated polyclonal antibody directed against TGF-β2 was added and incubated for 2h. After the samples were washed three times with washing buffer, 200 mL of substrate solution (a mixture of H2O2 and tetramethylbenzidine) was added to each well and the plate was incubated for 20min at room temperature. Color development was stopped by the addition of 50 mL of stop solution to each well. The optical density was determined with a Molecular Devices ELISA reader (MWG Biotech, Ebersberg, Germany) at 450 nm.
The amounts of TGF-β2 in the aqueous humor, lens, and vitreous were calculated from a standard curve generated from the absorbances of a serial dilution of rabbit recombinant TGF-β2. These data were linearized with a log/log scale. The correlation coefficient of the standard curve was 0.998.
Statistical Analysis
The results are expressed as mean values ± standard errors of the means (SE). One-way analysis of variance (ANOVA) was used to test the statistical significance of differences among groups. The statistical analyses were performed with the SPSS 13.0 statistical package. P<0.05 was deemed to indicate statistical significance.
RESULTS
Effects on Lens Transparency
No significant morphological changes or opacification of the lens was detected in rabbits with a slitlamp examination after hyperoxic treatment for 6mo, in eyes that were treated with or without vitrectomy (data not shown).
Concentrations of Water-soluble Proteins
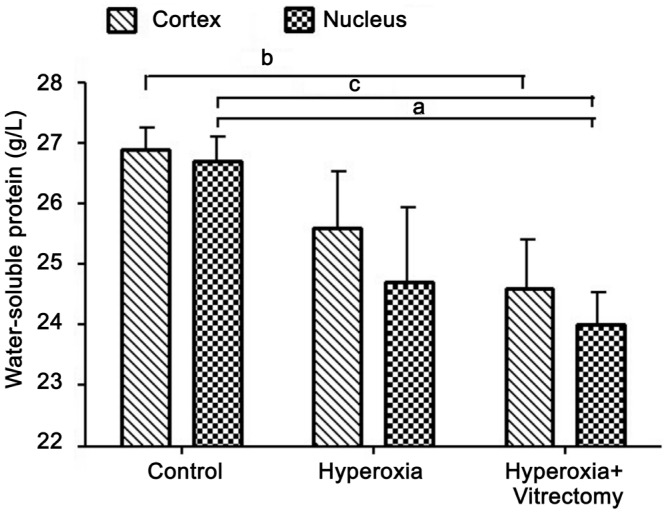
The treatment of rabbits with hyperoxia alone (Group A-left) produced a significant reduction in the water-soluble protein concentration in the lens nucleus (7.74%; P=0.02), but not in the cortex, compared with the controls, which were kept in a room-air environment and in which the vitreous was intact. However, hyperoxia and vitrectomy (Group A-right) caused a greater reduction (8.27%, P=0.004) in the water-soluble protein concentration in the lens cortex and in the lens nucleus (10.09%, P<0.001). No statistically significant change was detected after treatment with hyperoxia alone (Group A-left) (P>0.05) (Figure 1).
Figure 1. Water-soluble proteins in rabbit lenses.
aP =0.02, bP =0.004, cP <0.001.
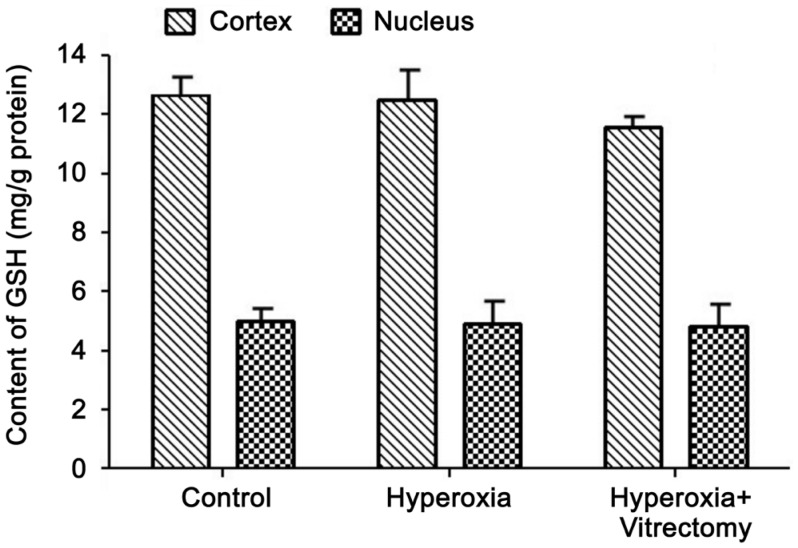
Concentrations of Glutathione
The concentration of GSH was always higher in the lens cortex than in the nucleus, in both the control and experimental lenses. Compared with the lens cortex, the GSH levels in the lens nuclei of the control group were relatively low, at about 42.56% of the cortical values (Figure 2). After treatment with hyperoxia only for 6mo, the GSH levels in the lens cortex (including the capsule) and nucleus decreased slightly but not statistically significantly (P>0.05). Exposure to hyperoxia after vitrectomy (Group A-right) produced no further reduction in the GSH levels, in either the cortex or the nucleus (P>0.05).
Figure 2. GSH levels in rabbit lenses.
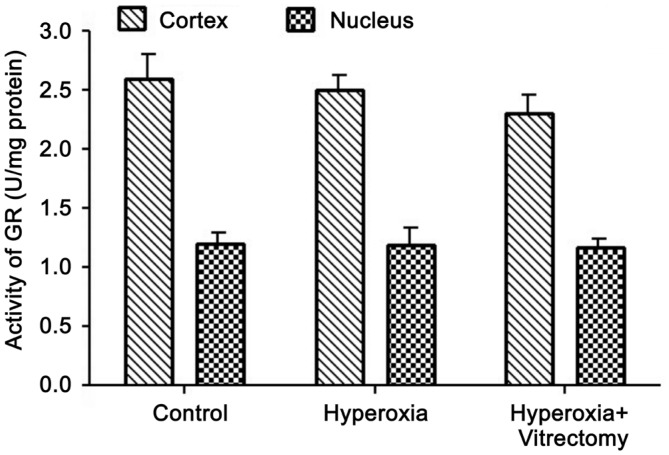
Activity of Glutathione Reductase
A similar pattern was observed for GR activity (Figure 3). Compared with the control group, GR activity was reduced in the lens cortices and nuclei of Group A-right, but the levels in the cortices and nuclei were not significantly different. The GR activity in the two parts of the lenses in the vitrectomy and hyperoxia group (Group A-right) was slightly lower than in the hyperoxia treated group, but the differences were not statistically significant. The GR activity in the two parts of the lenses were reduced after treatment with hyperoxia alone (Group A-left) compared with the control group, but the changes were not statistically significant (P>0.05).
Figure 3. Activity of GR in rabbit lenses.
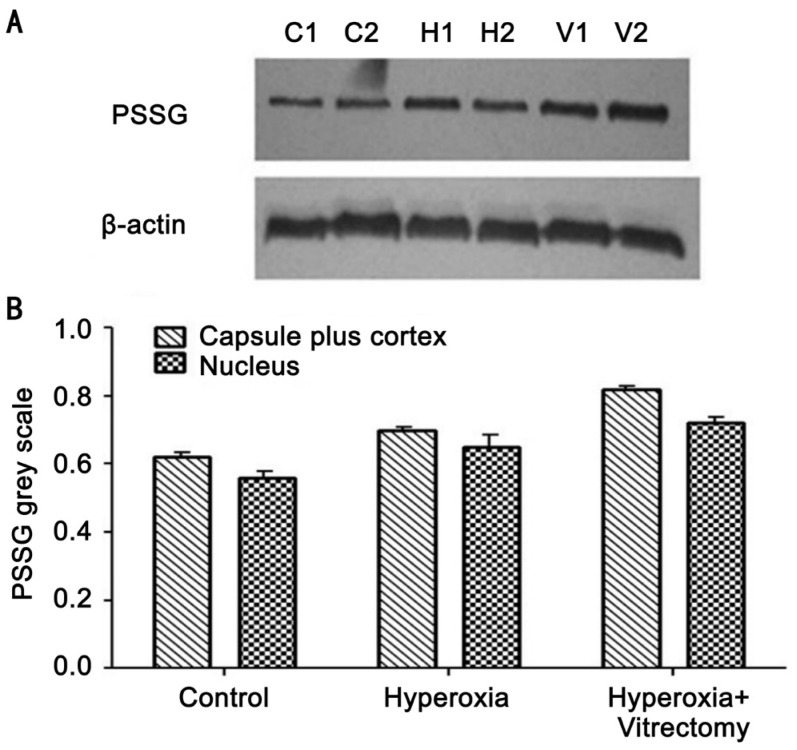
Concentrations of Protein-bound Glutathione Mixed Disulfides
The analysis of PSSG in the lens cortices and nuclei, detected with Western blotting, is shown in Figure 4. The amount of PSSG was lower in the lens nucleus than in the cortex in all lenses (Figure 4A: C2, H2 and V2 compared with C1, H1 and V1, respectively). The combined vitrectomy and hyperoxia treatment produced significantly higher PSSG levels in the lens cortex than hyperoxia alone or than was observed in the control group (Figure 4B).
Figure 4. PSSG expression detected with Western blotting (A) and the integrated volumes of PSSG of the lenses (n=3) (B).
C1: Capsule plus cortex of a lens in the control group; C2: Nucleus of a lens in the control group; H1: Capsule plus cortex of a lens in the hyperoxia group; H2: Nucleus in a hyperoxia treated lens; V1: Capsule plus cortex of a lens in the vitrectomy and hyperoxia group; V2: Lens nucleus in the vitrectomy+hyperoxia group.
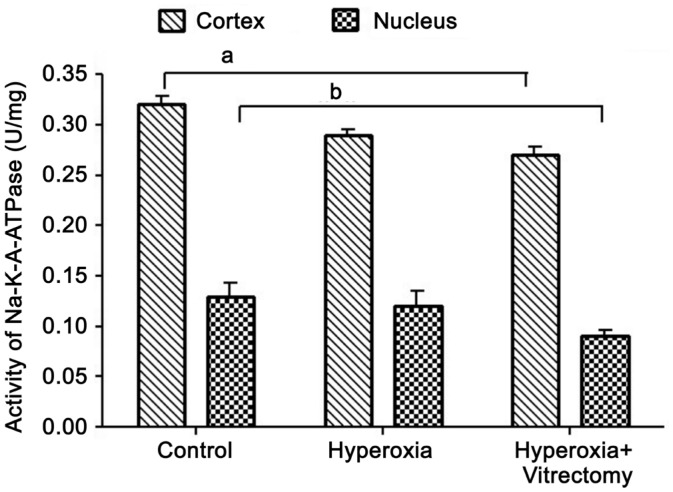
Activity of Na+-K+-ATPase
The Na+-K+-ATPase activity in the lens cortex was high in the eyes of all the groups. Compared with the cortices, the Na+-K+-ATPase activity in the nuclei of the control lenses was relatively low, at about 40.87% of the cortical value (Figure 5). After the vitrectomy and hyperoxia treatment (Group A-right) for 6mo, the Na+-K+-ATPase activity in the cortex and nucleus had decreased significantly, by 9.06% and 19.66%, respectively (P=0.002 and 0.012, respectively) compared with those in the control group (eyes without surgery). When compared with our previous report of rabbits treated with vitrectomy alone[15], the vitrectomy and hyperoxia treatment reduced Na+-K+-ATPase activity. However, there was no significant difference in the activity of this enzyme between the hyperoxia group and the control group, either in the cortex or the nucleus. No statistical difference was observed in the two parts of the lenses in the vitrectomy and hyperoxia treatment group (Group A-right) and the group treated with hyperoxia alone (Group A-left) (P>0.05).
Figure 5. Na+-K+-ATPase activity in rabbit lenses.
aP=0.002, bP=0.012.
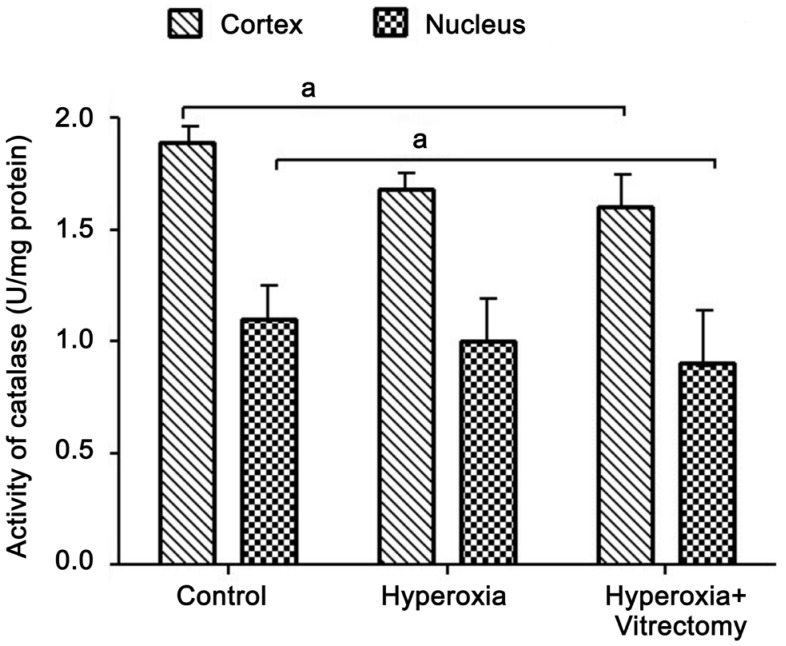
Catalase Activity
Consistent with our previous finding that CAT activity decreases 17% in the whole lens after vitrectomy in rabbit eyes[15], in the present study, the CAT activity in the lens cortex of the eyes treated with hyperoxia and vitrectomy was reduced by 14.6% (P=0.042) and 19.32% (P=0.029) in the nucleus, compared with those in the control group (Figure 6). It seems that the greatest reduction in activity was in the lens nucleus, whereas extra exposure to hyperoxia after vitrectomy did not cause any further marked reduction in CAT activity. Moreover, when compared with the lenses treated with hyperoxia alone, there was no significant difference in CAT activity in the cortex or nucleus after treatment with vitrectomy and hyperoxia (Group A-right) (P>0.05). Similarly, in the Group A-left eyes, the CAT activity in the two parts of the lens was reduced compared with that in the control group, but the changes were not statistically significant.
Figure 6. Catalase activity in rabbit lenses.
aP=0.042.
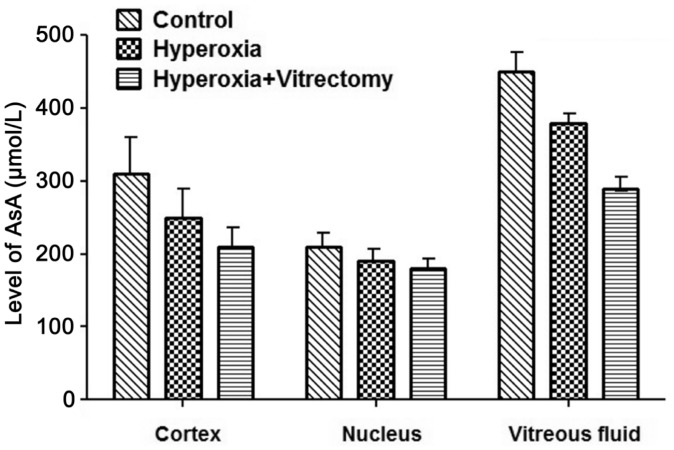
Ascorbate Concentrations
After treatment for 6mo, the levels of AsA in the cortex, the nucleus, and the vitreous fluid in the Group A-right eyes were significantly reduced, by 29.68% (P=0.007), 26.53% (P=0.027), and 37.66% (P<0.001), respectively (Figure 7), whereas they were reduced by 24.2% (P=0.015), 18.6% (P=0.0502), and 15.96% (P=0.007) in the eyes of Group A-left, compared with the corresponding parts of the lenses in the control group. However, no significant differences were noted between the lens cortex or nucleus after vitrectomy and hyperoxia (Group A-right), but reductions of 25.82% (P<0.001) were observed in the vitreous fluid compared with that in the group treated with hyperoxia alone (Group A-left).
Figure 7. Levels of AsA in rabbit lenses and vitreous fluid.
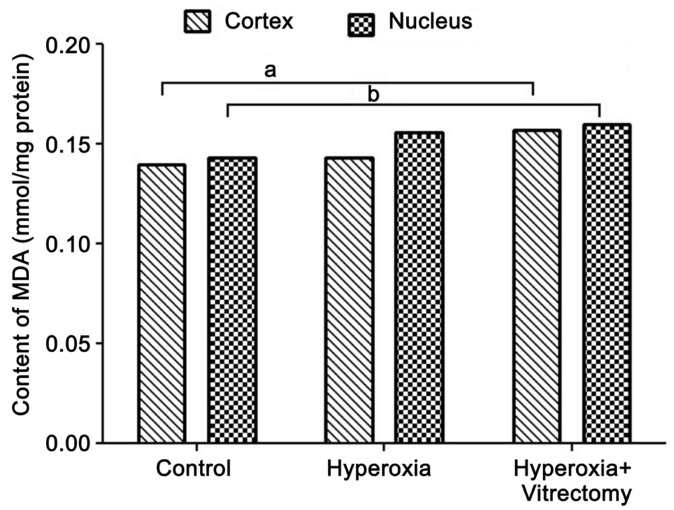
Concentrations of Malondialdehyde
Exposure to hyperoxia after vitrectomy in rabbit eyes produced a significant increase in the levels of MDA in the lens cortex (by 15.11%; P=0.029) and nucleus (16.20%; P=0.011) compared with those in the control. Systemic exposure to hyperoxia alone caused a minimal increase in the MDA contents of the two parts of the lens, but the changes were not statistically significant (Figure 8).
Figure 8. Levels of MDA in rabbit lenses.
aP=0.029, bP=0.011.
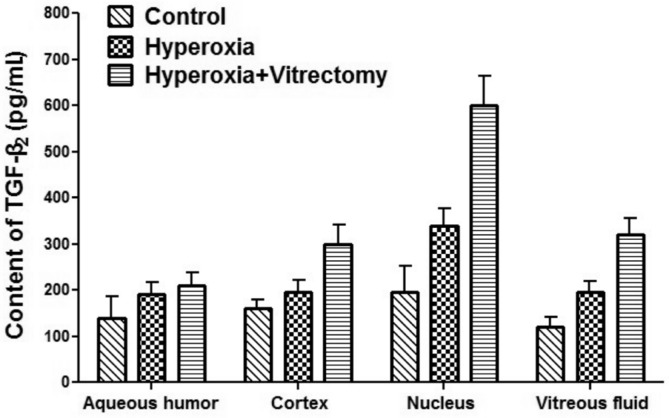
Concentrations of TGF-β2
Six months after hyperoxic exposure, the levels of TGF-β2 in the lens nuclei of the group treated with hyperoxia alone (Group A-left) increased by 75.88% (P=0.043) compared with the control group (Figure 9). However, in the other part of the eyeball, the levels of TGF-β2 increased, but not statistically significantly (P>0.05). The TGF-β2 levels in the lens cortex, nucleus, and regenerated vitreous fluid in the vitrectomy and hyperoxia group (Group A-right) increased by 90.47% (P=0.016), 217.94% (P=0.002), and 148.40% (P=0.002), respectively, compared with those in the control (Group B). The TGF-β2 levelin the aqueous humor also increased, but not statistically significantly. When the group treated with hyperoxia alone (Group A-left) was compared with the group treated with vitrectomy and hyperoxia (Group A-right), the TGF-β2 levels in the cortices, nuclei, and regenerated vitreous fluid increased in the former by 54.81% (P=0.048), 80.87% (P=0.007), and 70.34% (P=0.039), respectively. The TGF-β2 level in the aqueous humor increased slightly but not statistically significantly.
Figure 9. TGF-β2 levels in the rabbit aqueous humor, lens, and vitreous samples.
DISCUSSION
The formation of cataract, followed by the oxidative damage of the crystallins and the loss of protein sulfhydryl groups, is the end result of an ongoing process of the nuclear cataract formation[1],[24]–[26]. In our previous study, we showed that there was no significant difference in the water-soluble protein concentrations in vitrectomy-treated and untreated eyes 5mo after vitrectomy[15]. However, in the present study, extra exposure to hyperoxia for 6mo caused a significant reduction in the water-soluble protein concentrations, particularly in the lens nuclei. This indicates that the lens nucleus has a weaker antioxidant defense system than the cortex, so the proteins in the center of the lens are more susceptible to oxidation. Vitrectomy may cause extensive diffusion of oxygen into the central vitreous and close to the lens[2],[12]. Treatment with hyperoxia increased the susceptibility of the lens to oxidative injury. Our data also imply that the intact vitreous gel protects the lens proteins against oxidative stress by inhibiting the diffusion of oxygen from the surfaces of the retinal vessels.
The degree of protein thiolation, predominantly disulfide linkages to GSH, also increases as cataract formation progresses[24]–[25]. GSH plays a role in reversing this thiolation in the lens areas, where it remains active[27]. It also inhibitsfree-radical-mediated lens injury by eliminating reactive oxygen species, and protects the protein thiol groups and lipids of the lens from oxidative damage by acting as a biological redox agent[28]. The oxidation of GSH in the nuclear region of the lens may be a crucial event preceding cataract formation[25]. GR is an enzyme that reduces glutathione disulfide (GSSG) to the sulfhydryl form, GSH, which is an important cellular antioxidant[29]. GR was previously suggested by Srivastava and Beutler[30] to be a dethiolase enzyme that cleaves lens protein-GSH mixed disulfides. PSSG mixed disulfides are a species of thiols bound to proteins, and their formation is considered to be a possible mechanism of protein aggregation in the process of cataractogenesis[31]. The data presented here suggest that high oxygen levels that induced oxidative stress after treatment with vitrectomy and hyperoxia produced slight reductions GSH and GR in the lens. Systemic hyperoxia exposure, even in eyes from which the intact vitreous gel had been removed (vitrectomy), showed no significant reductionin GSH levels or GR activity in the lens, suggesting that GSH and GR were resistant to oxidative stress. This may be attributable to the young age of the rabbits and the short period of observation. The most probable reason is that rabbit and human lenses differ in their susceptibility to the effects of hyperoxia.
High levels of GSH may contribute to the resistance to oxidative injury, such as that induced by the high oxygen stress after vitrectomy. However, after the hyperoxia and vitrectomy treatment, the presence of PSSG was much higher in the rabbit lens cortex than in the nucleus, suggesting that most oxygen stress is consumed by the cortex (including the epithelium) before it reaches the nucleus, where it subsequently induces mixed disulfide formation. With a normal vitreous gel (without vitrectomy), the PSSG levels were lower than they were after hyperoxia + vitrectomy, even in rabbits systemically exposed to hyperoxia, suggesting the importance of the vitreous gel in preventing oxidative stress to the lens.
The GSH system plays a key role in protecting the eye from oxidative stress. The rabbit lens has an unusually high concentration of reduced GSH compared with the human lens[32], and GSH is quantitatively, the most important endogenous rechargeable antioxidant. GSH functions as an essential antioxidant, vital for the maintenance of the tissue's transparency. Reduced GSH is oxidized to form GSSG, which reacts with proteins to generate mixed disulfides. Similar results were demonstrated by Lou and Dickerson[31] in 1992. These data indicate that an increase in PSSG in the lens reflects the extent of oxidative insult to the lens.
Na+-K+-ATPase, a species of active sodium-potassium transport enzyme, is very important in maintaining the ion gradient between the interiors of lens cells and the extracellular environment[33]–[35]. The present results suggest that the relatively low Na+-K+-ATPase activity in the nucleus of the lens makes the nucleus especially vulnerable to oxidative stress. A possible reason involves the phosphorylation of Na+-K+-ATPase. The degree of endogenous tyrosine phosphorylation of the Na+-K+-ATPase α1 subunit is greater in lens fibers than in the lens epithelium[35]. Treatment with hyperoxia alone had no significant effect on the Na+-K+-ATPase activity in the lens, which decreased in the vitrectomy and hyperoxia treatment group, indicating that an intact vitreous gel protects against a reduction in Na+-K+-ATPase activity. In our previous study, we found that Na+-K+-ATPase activity decreased significantly in the lens after vitrectomy, and that treatment with N-acetylcysteine enhanced and restored the Na+-K+-ATPase activity in the lens[15]. Based on the results of those two experiments, we believe that the biochemical changes that occur after vitrectomy are associated with the oxidative insult caused by oxygen diffusing into the vitreous cavity and around the lens. N-Acetylcysteine, as a supplement to GSH in cells, could impede these biochemical changes.
CAT decomposes hydrogen peroxide (H2O2) in reactions catalyzed by itsenzymatic activity. The role of CAT in defending cells and tissues from oxidative stress has been extensively studied. CAT is a very important antioxidative enzyme in the lens, protecting the lens from peroxide injury[36]. Our previous study showed that the CAT activity in the lens was reduced after vitrectomy compared with that in the untreated eyes, whereas N-acetylcysteine treatment alleviated these changes[15]. In the present study, the eyes treated with vitrectomy and hyperoxia showed clearly impaired CAT activity, suggesting that the intact vitreous gel has a protective effect on maintaining the lens CAT activity against oxidative insult.
AsA, an antioxidant, scavenges free radicals and consumes oxygen to protect ocular tissues from oxidative or photo-oxidative damage[37]. Oxygen consumption by AsA in the vitreous may maintain lower levels of molecular oxygen near the lens, protecting the lens from nuclear cataract[38]. The data presented here suggest that the levels of AsA in the two parts of the lens, and especially the vitreous fluid, were clearly and markedly reduced in the rabbits treated with vitrectomy and hyperoxia. This may indicate that much of the oxygen diffusing from the retinal vessels generates free radicals and consequently causes a decline in AsA in the lens and vitreous fluid. This reduction is more significant in the vitreous fluid, suggesting that the relatively high concentration of AsA in the intact vitreous gel consumes more oxygen and stops the oxygen inthe vitreous cavity reaching the lens[4],[38]. These data also support the hypothesis that the vitreous gel provides a good “sink” for oxygen, which naturally protects the lens from oxidative insult.
Our increasing appreciation of the causative role of oxidative injury in nuclear cataract means that the reliable assessment of lipid peroxidationis extremely important[39]. Polyunsaturated fatty acids are readily attacked by free radicals, being oxidized to lipid peroxides. Lipid peroxides are toxic and capable of damaging most body cells. MDA is one of several low-molecular-weight end products formed by the decomposition of certain primary and secondary lipid peroxidation products[40]. It has been shown that peroxide damage to the lens fiber membranes may be the initial cause of cataract[41]. Therefore, the levels of lipid peroxidation products, such as MDA, in the lens probably correlate with the degree of lens opacity, although the damage to the lens by lipid peroxides has not become visible. The present results clearly show that high-level oxygen exposure aggravates the production of lipid peroxides in the lens, especially in the lens nucleus. With a normal vitreous gel (without vitrectomy), the MDA levelsdid not change significantly, even after systemic hyperoxia exposure, demonstrating the protection afforded by an intact vitreous against oxidative insult.
TGF-β2 is considered to be a potent stimulator of the formation of extracellular matrix components. It activates gene transcription and increases the synthesis and secretion of matrix proteins, fibronectin, elastin, and proteoglycans in various tissues, whereas it decreases the synthesis of proteolytic enzymes, degrading these proteins[42]. After the rabbits were treated with hyperoxia for 6mo after vitrectomy, the level of TGF-β2 increased dramatically in the lens and vitreous fluid, especially in the lens nucleus, suggesting the extreme oxidative stress in the nucleus. Following vitrectomy, the increased TGF-β2 in the lens and the vitreous fluid promotes the formation of extracellular matrix components and the transdifferentiation to myofibroblasts, which is believed to be associated with post-vitrectomy cataract formation.
Above all, the oxygen that diffuses from the retinal vessels is probably consumed by the intact vitreous gel and abundant AsA. Under hyperoxic conditions, the lens still functions because there were no obvious changes in the lens or the vitreous fluid when treated with hyperoxia alone. However, when the vitreous gel is removed (vitrectomy), oxygen easily approaches the lens. Therefore, the lens is already exposed to a relatively high-oxygen environment. In this situation, extra hyperoxia aggravates this effect. The reduced contents of water-soluble proteins and AsA, the reduced activities of catalase and Na+-K+-ATPase, and the increasesin MDA and TGF-β2 together indicate that oxygen-induced lens damage occurs with the oxygen levels around the lens increase after treatment with vitrectomy and hyperoxia (Figure 10). This study clearly demonstrates that the state of the vitreous gel plays an important role in protecting the lens proteins from oxidative stress, which is associated with nuclear sclerosis and cataract formation after vitrectomy.
Figure 10. Diagrams illustrating the changes in the distribution of oxygen in the eye and oxygen-induced lens damage following vitrectomy and hyperoxia.
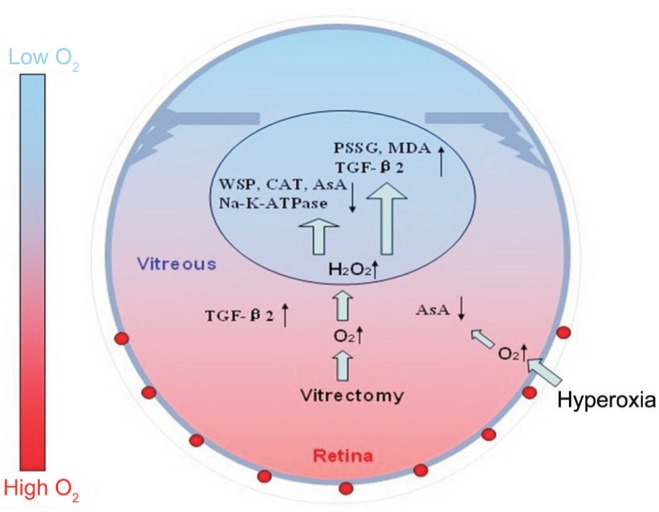
AsA: Ascorbate; CAT: Catalase; H2O2: Hydrogen peroxide; MDA: Malondialdehyde; PSSG: Protein-bound glutathione mixed disulfide; TGF-β2: Transforming growth factor β2; WSP: Water-soluble protein.
Acknowledgments
We are grateful to Ying-Bo Shui and David C. Beebe (Department of Ophthalmology and Visual Sciences, Washington University School of Medicine, St. Louis, MO, USA) for their great help and valuable discussions.
This study was presented orally at the 8th Asian Cataract Research Conference (8th ACRC) and Chinese Cataract Research Conference (CCRC), 17-20 June 2010, Hangzhou, China.
Foundation: Supported by National Natural Science Foundation of China (No.30872837).
Conflicts of Interest: Yan H, None; Wang D, None; Ding TB, None; Zhou HY, None; Yan WJ, None; Wang XC, None.
REFERENCES
- 1.Truscott RJ, Augusteyn RC. Oxidative changes in human lens proteins during senile nuclear cataract formation. Biochim Biophys Acta. 1977;492(1):43–52. doi: 10.1016/0005-2795(77)90212-4. [DOI] [PubMed] [Google Scholar]
- 2.Holekamp NM, Shui YB, Beebe DC. Vitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formation. Am J Ophthalmol. 2005;139(2):302–310. doi: 10.1016/j.ajo.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 3.Beebe DC, Holekamp NM, Shui YB. Oxidative damage and the prevention of age-related cataracts. Ophthalmic Res. 2010;44(3):155–165. doi: 10.1159/000316481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beebe DC, Holekamp NM, Siegfried C, Shui YB. Vitreoretinal influences on lens function and cataract. Philos Trans R Soc Lond B Biol Sci. 2011;366(1568):1293–1300. doi: 10.1098/rstb.2010.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmquist BM, Philipson B, Barr PO. Nuclear cataract and myopia during hyperbaric oxygen therapy. Br J Ophthalmol. 1984;68(2):113–117. doi: 10.1136/bjo.68.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang AJ, Shui YB, Han YP, Bai F, Siegfried CJ, Beebe DC. Impact of corneal endothelial dysfunctions on intraocular oxygen levels in human eyes. Invest Ophthalmol Vis Sci. 2015;56(11):6483–6488. doi: 10.1167/iovs.15-17191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang P, Liu XC, Yan H, Li MY. Hyperoxia-induced lens damage in rabbit: protective effects of N-acetylcysteine. Mol Vis. 2009;15:2945–2952. [PMC free article] [PubMed] [Google Scholar]
- 8.Truscott RJ. Age-related nuclear cataract-oxidation is the key. Exp Eye Res. 2005;80(5):709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Ma N, Siegfried C, Kubota M, Huang J, Liu Y, Liu M, Dana B, Huang A, Beebe D, Yan H, Shui YB. Expression profiling of ascorbic acid-related transporters in human and mouse eyes. Invest Ophthalmol Vis Sci. 2016;57(7):3440–3450. doi: 10.1167/iovs.16-19162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bassnett S, Winzenburger PA. Morphometric analysis of fibre cell growth in the developing chicken lens. Exp Eye Res. 2003;76(3):291–302. doi: 10.1016/s0014-4835(02)00315-9. [DOI] [PubMed] [Google Scholar]
- 11.Shui YB, Holekamp NM, Beebe DC. Oxygen levels in human eyes before and after vitrectomy. Invest Ophthalmol Vis Sci. 2003;44(2):34–36. [Google Scholar]
- 12.Shui YB, Fu JJ, Garcia C, Dattilo LK, Rajagopal R, McMillan S, Mak G, Holekamp NM, Lewis A, Beebe DC. Oxygen distribution in the rabbit eye and oxygen consumption by the lens. Invest Ophthalmol Vis Sci. 2006;47(4):1571–1580. doi: 10.1167/iovs.05-1475. [DOI] [PubMed] [Google Scholar]
- 13.Stefansson E, Novack RL, Hatchell DL. Vitrectomy prevents retinal hypoxia in branch retinal vein occlusion. Invest Ophthalmol Vis Sci. 1990;31(2):284–289. [PubMed] [Google Scholar]
- 14.Harocopos GJ, Shui YB, McKinnon M, Holekamp NM, Gordon MO, Beebe DC. Importance of vitreous liquefaction in age-related cataract. Invest Ophthalmol Vis Sci. 2004;45(1):77–85. doi: 10.1167/iovs.03-0820. [DOI] [PubMed] [Google Scholar]
- 15.Liu XC, Wang P, Yan H. A rabbit model to study biochemical damage to the lens after vitrectomy: effects of N-acetylcysteine. Exp Eye Res. 2009;88(6):1165–1170. doi: 10.1016/j.exer.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Li Q, Yan H, Ding TB, Han J, Shui YB, Beebe DC. Oxidative responses induced by pharmacologic vitreolysis and/or long-term hyperoxia treatment in rat lenses. Curr Eye Res. 2013;38(6):639–648. doi: 10.3109/02713683.2012.760741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blakytny R, Harding JJ. Prevention of cataract in diabetic rats by aspirin, paracetamol (acetaminophen) and ibuprofen. Exp Eye Res. 1992;54(4):509–518. doi: 10.1016/0014-4835(92)90129-g. [DOI] [PubMed] [Google Scholar]
- 18.Harding JJ. Free and protein-bound glutathione in normal and cataractous human lenses. Biochem J. 1970;117(5):957–960. doi: 10.1042/bj1170957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akagawa K, Tsukada Y. Presence and characteristics of catecholamine-sensitive Na-K ATPase in rat striatum. J Neurochem. 1979;32(1):269–271. doi: 10.1111/j.1471-4159.1979.tb04544.x. [DOI] [PubMed] [Google Scholar]
- 20.Beers RF, Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195(1):133–140. [PubMed] [Google Scholar]
- 21.Linetsky MD, Shipova EV, Legrand RD, Argirov OO. Glucose-derived Amadori compounds of glutathione. Biochim Biophys Acta. 2005;1724(1–2):181–193. doi: 10.1016/j.bbagen.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Okamura M. An improved method for determination of L-ascorbic acid and L-dehydroascorbic acid in blood plasma. Clin Chim Acta. 1980;103(3):259–268. doi: 10.1016/0009-8981(80)90144-8. [DOI] [PubMed] [Google Scholar]
- 23.Halliwell B, Chirico S. Lipid peroxidation: its mechanism, measurement, and significance. Am J Clin Nutr-; discussion. 1993;57(5 Suppl) doi: 10.1093/ajcn/57.5.715S. [DOI] [PubMed] [Google Scholar]
- 24.Lou MF, Dickerson JE, Jr, Tung WH, Wolfe JK, Chylack LT., Jr Correlation of nuclear color and opalescence with protein S-thiolation in human lenses. Exp Eye Res. 1999;68(5):547–552. doi: 10.1006/exer.1998.0638. [DOI] [PubMed] [Google Scholar]
- 25.Spector A. Oxidative stress-induced cataract: mechanism of action. FASEB J. 1995;9(12):1173–1182. [PubMed] [Google Scholar]
- 26.Giblin FJ. Glutathione: a vital lens antioxidant. J Ocul Pharmacol Ther. 2000;16(2):121–135. doi: 10.1089/jop.2000.16.121. [DOI] [PubMed] [Google Scholar]
- 27.Lou MF. Thiol regulation in the lens. J Ocul Pharmacol Ther. 2000;16(2):137–148. doi: 10.1089/jop.2000.16.137. [DOI] [PubMed] [Google Scholar]
- 28.Harding JJ, Blakytny R, Ganea E. Glutathione in disease. Biochem Soc Trans. 1996;24(3):881–884. doi: 10.1042/bst0240881. [DOI] [PubMed] [Google Scholar]
- 29.Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988;263(33):17205–17208. [PubMed] [Google Scholar]
- 30.Srivastava SK, Beutler E. Cleavage of lens protein-GSH mixed disulfide by glutathione reductase. Exp. Eye Res. 1973;17(1):33–42. doi: 10.1016/0014-4835(73)90165-6. [DOI] [PubMed] [Google Scholar]
- 31.Lou MF, Dickerson JE., Jr Protein-thiol mixed disulfides in human lens. Exp Eye Res. 1992;55(6):889–896. doi: 10.1016/0014-4835(92)90015-k. [DOI] [PubMed] [Google Scholar]
- 32.Padgaonkar VA, Leverenz VR, Fowler KE, Reddy VN, Giblin FJ. The effects of hyperbaric oxygen on the crystallins of cultured rabbit lenses: a possible catalytic role for copper. Exp Eye Res. 2000;71(4):371–383. doi: 10.1006/exer.2000.0887. [DOI] [PubMed] [Google Scholar]
- 33.Duncan G, Bushell AR. Ion analyses of human cataractous lenses. Exp Eye Res. 1975;20(3):223–230. doi: 10.1016/0014-4835(75)90136-0. [DOI] [PubMed] [Google Scholar]
- 34.Moseley AE, Dean WL, Delamere NA. Isoforms of Na, K-ATPase in rat lens epithelium and fiber cells. Invest Ophthalmol Vis Sci. 1996;37(8):1502–1508. [PubMed] [Google Scholar]
- 35.Delamere NA, Tamiya S. Lens ion transport: from basic concepts to regulation of Na,K-ATPase activity. Exp Eye Res. 2009;88(2):140–143. doi: 10.1016/j.exer.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma W, Nunes I, Young CS, Spector A. Catalase enrichment using recombinant adenovirus protects alphaTN4-1 cells from H(2)O(2) Free Radic Biol Med. 2006;40(2):335–340. doi: 10.1016/j.freeradbiomed.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 37.Rose RC. Transport of ascorbic acid and other water-soluble vitamins. Biochim Biophys Acta. 1988;947(2):335–366. doi: 10.1016/0304-4157(88)90014-7. [DOI] [PubMed] [Google Scholar]
- 38.Shui YB, Holekamp NM, Kramer BC, Crowley JR, Wilkins MA, Chu F, Malone PE, Mangers SJ, Hou JH, Siegfried CJ, Beebe DC. The gel state of the vitreous and ascorbate-dependent oxygen consumption: relationship to the etiology of nuclear cataracts. Arch Ophthalmol. 2009;127(4):475–482. doi: 10.1001/archophthalmol.2008.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990;9(6):515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 40.Babizhayev MA, Deyev AI, Yermakova VN, Brikman IV, Bours J. Lipid peroxidation and cataracts: N-acetylcarnosine as a therapeutic tool to manage age-related cataracts in human and in canine eyes. Drugs R D. 2004;5(3):125–139. doi: 10.2165/00126839-200405030-00001. [DOI] [PubMed] [Google Scholar]
- 41.Micelli-Ferrari T, Vendemiale G, Grattagliano I, Boscia F, Arnese L, Altomare E, Cardia L. Role of lipid peroxidation in the pathogenesis of myopic and senile cataract. Br J Ophthalmol. 1996;80(9):840–843. doi: 10.1136/bjo.80.9.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozcan AA, Ozdemir N, Canataroglu A. The aqueous levels of TGF-beta2 in patients with glaucoma. Int Ophthalmol. 2004;25(1):19–22. doi: 10.1023/b:inte.0000018524.48581.79. [DOI] [PubMed] [Google Scholar]