Abstract
AIM
To investigate the retinal photoreceptor differentiation potential of human orbital adipose tissue-derived stem cells (ADSCs) generated by enzyme (EN) and explant (EX) culture methods.
METHODS
We investigated potentials of human orbital ADSCs to differentiate into photoreceptors through EN and EX culture methods. EN and EX orbital ADSCs were obtained from the same donor during rehabilitative orbital decompression, and then were subject to a 3-step induction using Noggin, DKK-1, IGF-1 and b-FGF at different time points for 38d. Stem cell, eye-field and photoreceptor-related gene and protein markers were measured by reverse transcription-polymerase chain reaction (RT-PCR) and immunofluorescent (IMF) staining.
RESULTS
Both EX and EN orbital ADSCs expressed CD133, a marker of cell differentiation. Moreover, PAX6 and rhodopsin, markers of the retinal progenitor cells, were detected from EX and EN orbital ADSCs. In EX orbital ADSCs, PAX6 mRNA was detected on the 17th day and then the rhodopsin mRNA was detected on the 24th day. In contrast, the EN orbital ADSCs expressed PAX6 and rhodopsin mRNA on the 31st day. EX orbital ADSCs expressed rhodopsin protein on the 24th day, while EN orbital ADSCs expressed rhodopsin protein on the 31st day.
CONCLUSION
Orbital ADSCs isolated by direct explants culture show earlier and stronger expressions of markers towards eye field and retinal photoreceptor differentiation than those generated by conventional EN method.
Keywords: photoreceptor cells, cell differentiation, adult stem cells, tissue engineering, explants culture, enzymatic digestion
INTRODUCTION
Retinal dystrophies and degeneration include a wide range of genetic or age-related disorders which are characterized by the progressive and irreversible loss of retinal photoreceptor cells and underlying retinal pigment epithelium (RPE). Retinitis pigmentosa (RP), age-related macular degeneration (AMD), myopic maculopathy and Stargardt disease are common examples which could lead to permanent visual loss, for which there is no treatment to date[1]. As a critical component of the mammalian central nervous system (CNS), the human postnatal neurosensory retina shows poor regenerative potential[2]. Therefore, stem cell and cell-based replacement therapies may offer potential therapeutic strategies[3]. Adipose tissue-derived stromal/stem cells (ADSCs) have been shown to demonstrate similar cellular properties and regenerative potential comparable to the prototypic mesenchymal stromal/stem cells (MSC) - bone marrow mesenchymal stromal/stem cells (BMSC)[4]. In comparison with BMSC, ADSC can be obtained in much larger quantities and can be harvested by less invasive methods. ADSCs have been frequently used for ophthalmic complications in the retina and the cornea involving various ocular cells[5]–[8]. In orbital adipose depots obtained from patients undergoing surgery, the pluripotential stem cells have been cultured and shown to possess the potential to differentiate into different cell lineages[9]. Orbital ADSCs may offer additional advantages to differentiate into specific retinal cell types for tissue engineering, as these cells share a common embryological origin from neural crest[10]. Consequently, human orbital adipose tissue may be a potential source of stem cells for cell therapy.
Isolation of ADSCs have been described as an alternative source to mesenchymal stem cells because adipose tissue stroma can be readily obtainable[11]. The isolation method, involving collagenase digestion of minced adipose tissues followed by serial culturing, has been shown to be uncomplicated and robust[11]. Interestingly, there are differences between the fresh stromal vascular fraction (SVF) cells derived from human adipose tissues and the cells obtained after serial culturing in terms of cellular properties[12]. Cells with plastic adherent properties, a defining characteristic of MSC[13], are identified as ADSCs.
In several studies to isolate stromal or mesenchymal cells from various tissues, primary (explant) EX culture from direct, unperturbed outgrowth of cells is always used[14]–[18]. Cells are expected to migrate from the edge of the EX. Therefore, in the present study, we compared the differentiate potential towards photoreceptor between the orbital ADSC generated by enzyme (EN) digestion and EX culture methods. We found that both EN and EX methods were successful in inducing the orbital ADSC. However, the mRNA and protein expression examinations suggested that the EX ADSCs exhibited stronger potentials to different into photoreceptor than the EN ADSCs.
MATERIALS AND METHODS
Sample Collection and Processing
Orbital adipose tissues were obtained as surgical specimen after informed consent from a single, male donor, aged 52, with stable thyroid associated orbitopathy undergoing rehabilitative orbital decompression at the hospital of Hong Kong Eye Hospital. He was euthyroid for more than 6mo and did not receive any prior systemic steroid, radioactive iodine or orbital radiotherapy. This study was approved by the Research Ethics Committee of the Chinese University of Hong Kong (CUHK) and followed the tenets of Helsinki with informed consent. Orbital adipose tissues were aliquoted into 2 halves for ADSC isolation.
Cell Culture Media and Materials
Dulbecco's modified Eagle's medium/Nutrient Mixture F-12 Ham (DF), fetal bovine serum (FBS), 1% penicillin-streptomycin (PS) were obtained from Invitrogen (Carlsbad, CA, USA). Collagenase IV was from Worthington (Lakewood, NJ, USA). Noggin, Dkk-1, N2, Vitamin B27, IGF-1, b-FGF were purchased from Sigma (St. Louis, MO, USA). Anti-CD133 Antibody (Ab), Anti-Rhodopsin Ab were from Thermo Fisher (Waltham, MA, USA).
Isolation of Human Orbital ADSCs by Enzymatic Digestion Method
Orbital ADSCs were isolated as described by Korn et al[9] with modification. In brief, after removal of all fibrous tissues and visible blood vessels, the orbital adipose tissues were minced into small pieces of 2 to 5 mm in diameter for digestion in pre-warmed collagenase IV working solution (DF, 0.075% collagenase IV, 1% FBS, 1% PS) for 2h by gentle agitation in a shaking water bath at 37°C. The digested tissues mixture were then centrifuged at 1500 rpm for 10min at room temperature. The cell pellets obtained were washed and plated in 5 mL ADSC culture medium (DF with 10% FBS, 1% PS and 1% fungizone) in a 100 mm dish at a density of 10[5] cells/mL and incubated at 37°C in 5% CO2. The medium was exchanged once every three days. The cells isolated by this enzymatic digestion procedure were named EN.
Isolation of Human Orbital ADSCs by Explants Culture Method
Orbital ADSCs were isolated as described with minor modifications[14]–[15]. The other half of the same adipose tissue specimen was used for EX culture. The tissue was rinsed with phosphate buffered saline (PBS) and minced into pieces about 1×1 mm2 after trimming of the fibrous tissues and visible blood vessels. Tissues were allowed to attach to the culture dish by gentle smearing. EX cultured orbital tissues were kept in DF with 20% FBS, 1% PS and 1% fungizone at 37°C, 5% CO2 for 10d (or until significant out-growth of cells from the tissues). The medium was changed once every three days. The explant tissues were then discarded and the cells were harvested by trypsinization. The ADSCs isolated by this explants culture procedure were named EX.
Orbital ADSC Culture, Expansion and Differentiation towards Photoreceptors
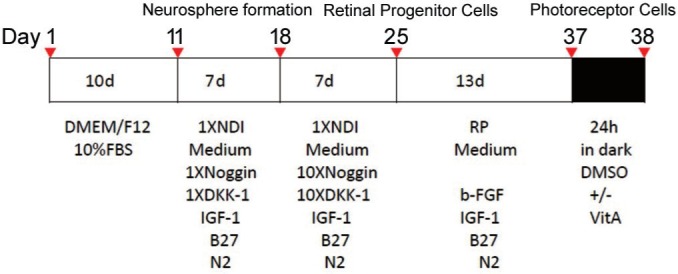
Orbital ADSCs were subcultured at 80% of confluence by brief trypsinization. The cells were harvested from the second to the fifth passage for the following induction experiments. This study protocol was modified from published methods[12],[19]. In the induction protocol (Figure 1), 1.0×106 cells were seeded in each well of the 6-well plates and allowed to grow for 10d until about 80% confluent. Then cells were treated by 1.5 mL of 1×NDI medium (1 ng/mL Noggin, 1 ng/mL DKK-1, 10 ng/mL IGF-1, 1% N2, 2% Vitamin B27) for 7d. On the 18th day, 10×NDI medium (10 ng/mL Noggin, 10 ng/mL DKK-1, 10 ng/mL IGF-1, 1% N2, 2% Vitamin B27) was given. On 25th day, RP medium (5 ng/mL b-FGF, 5 ng/mL IGF-1, 1% N2, 2% Vitamin B27) was added. On 37th day, 100 µmol/L Vitamin A dissolved in dimethyl sulfoxide (DMSO) or DMSO solvent control (less than 0.1% of final DMSO concentration in culture medium) was added and the mixtures were kept in dark for 24h followed by 5min of light exposure. Cells were kept in humidified incubator (37°C, 95% O2 and 5% CO2) and the media was changed every three days. Cells were harvested for analysis before the addition of each reagent (Figure 1).
Figure 1. Timeline of stepwise induction procedure.
Immunofluorescence Staining
The culture medium was aspirated and the cells were washed twice with PBS. They were then fixed in 4% paraformaldehyde (Sigma, St. Louis, USA) in PBS for 10min at 4°C. Afterwards the cells were washed three times with PBS prior to incubation with Anti- CD133 Ab (in 1:100 from Santa Cruz Biotechnologies, Dallas, TX, USA) or Anti- Rhodopsin Ab (in 1:200 from NeoMarkers, Fremont, CA,USA) according to the manufacturers' instructions. The nuclei were stained with 4′, 6-diamidino-2-phenylindole (DAPI) and cells were visualized with 100× Oil 1.25NA plan objective using a fluorescent microscope (Leica DMRB, Wetzlar, Germany Germany). Images were captured by Spot v3.5.0 for windows software (Lecia).
Reverse Transcription-Polymerase Chain Reaction Analysis (RT-PCR) of ADSCs
Total RNA was extracted using Total RNA Extraction Kit (Qiagen, Düsseldorf, Germany). cDNAs were synthesized from extracted RNA with PrimeScript RT Reagent Kit (Invitrogen, Carlsbad, CA, USA). Target mRNA was amplified by the PCR kit (Invitrogen) using PCR oligonucleotide primers as listed in Table 1. Amplification of d-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a control for assessing PCR efficiency. The products were electrophoresed on 1.5% agarose gels and stained with ethidium bromide.
Table 1. Primers for RT-PCR.
| Gene | Primers |
| PAX6 | F:5′-CGGAGTGAATCAGCTCGGTG-3′ R:5′-GCAAAATAGCCCAGTATAAGCGG-3′ |
| RHODOPSIN | F:5′-CGGTACGTGGTGGTGTGTAA-3′ R:5′-AGGTGTAGGGGATGGGAGAC-3′ |
| GAPDH | F:5′-TCAACGGCACAGTCAAGG-3′ R:5′-ACCAGTGGATGCAGGGAT-3′ |
RESULTS
Primary Culture of Human Adipose Tissue by Explant Culture Method
Minced adipose tissue fragments were adhered onto the culture dish shortly after plating and smearing. After 48h in vitro, the spindle-shaped cells began to migrate from the edge of the EX and reached 80% confluence after 2wk of culture.
Primary Culture of Human Adipose Tissue by Enzyme Digestion Method
Some of the suspended SVF cells began to attach onto the culture dish 3h after plating. They resumed their spindle shaped and fibroblast-like morphology, and reached 80% confluence after 2wk of culture.
Morphological Changes during the Induction Process
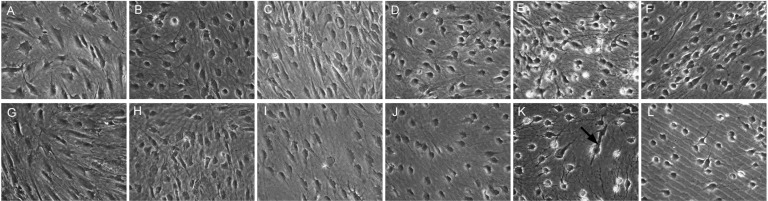
The orbital ADSCs isolated by either the EX culture method or EN digestion method were uniform in size of 15 to 20 µm and exhibited spindle shaped, fibroblast-like morphology. None of these cells demonstrated any intracellular lipid droplets (Figure 2A and 2G). Cells isolated by either method reached 80% confluence in 10d. After 3-staged induction for 28d and treatment with 100 µmol/L vitamin A, both EX and EN orbital ADSCs showed synapsis-like structure resembling early neuronal differentiation (Figure 3A and 3B). In addition, a few EX orbital ADSCs exhibited tubular, rod-like structure (red arrow in Figure 2K and 3B) but none of these structures was found in EN ADSCs (Figure 2E and 3A). Cytotoxic effects were evident in both EX and EN orbital ADSCs after Vitamin A treatment, but not in the DMSO, which served as the solvent control.
Figure 2. Phase-contrast microscopic changes (×200) in morphology during induction.
A-F: EN derived orbital ADSCs; G-L: EX derived orbital ADSCs. A&G: Before induction, the cells are spindle shape. Connections between cells were scarcely detected; B&H: After 7d in 1×NDI medium; C&I: After 7d in 10×NDI medium; D&J: After 7d in RP medium, the cells lost the spindle shape and formed round shape gradually; E&K: After 14d in RP medium with 100mM Vitamin A for 24h; synapses were formed widely between cells. Cytotoxic effect was detected at this point. K: Black arrow pointed to an EX orbital ADSC showing tubular, rod-like structure. F&L: After 14d in RP medium with 100mM DMSO for 24h. These significant changes of morphology were not detected.
Figure 3. Morphological changes at the end of induction after Vitamin A for 24h.
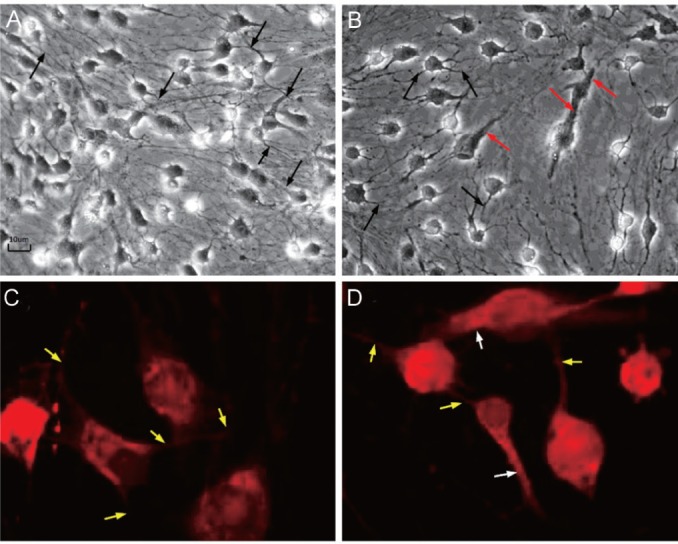
A, B: Phase-contrast microscopic pictures (×200); A: Some EN orbital ADSCs showed synapsis-like structure (the black arrow) with evidence of cell-to-cell contact; B: Besides synapsis-like structure (the black arrow), a few EX orbital ADSCs exhibited tubular, rod-like structure (the red arrow) resembling outer segment of rod photoreceptors; C, D: Synapse and rhodopsin staining by immunofluorescence (IMF) staining. These images show synapse structure and rhodopsin expression (at the end of induction after vitamin A for 24h); C: EN orbital ADSCs. The red fluorescence is rhodopsin expression. Some cells showed synapsis-like structure (the yellow arrow) with evidence of cell-to-cell contact; D: EX orbital ADSCs. The red fluorescence is rhodopsin expression. Besides synapsis-like structure (the yellow arrow), a few EX orbital ADSCs exhibited tubular, rod-like structure (the white arrow) resembling outer segment of rod photoreceptors.
Immunofluorescence Staining of Neural/hematopoietic Stem Cell Marker CD133
We detected the CD133, a hematopoietic, neural and cancer stem cell marker, from both EX and EN orbital ADSC before the induction protocol[20]. In greater detail, the fluorescence intensity of CD133 in the EX ADSC appeared to be stronger than that in the EN ADSC. Subsequently, with the developing of the induced differentiation, the CD133 expression became weaker in both the EX and EN ADSC.
Reverse Transcription-Polymerase Chain Reaction Analysis of Explant and Enzyme Orbital ADSCs during Induction
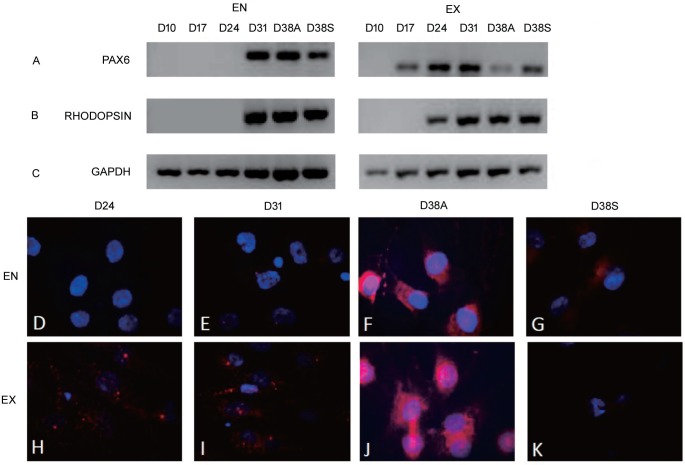
The expression levels of the eye field and photoreceptor-related genes (PAX6 and RHODOPSIN) were detected at different time points during the induction period (Figure 4). The PAX6 expression was first detected in the EX orbital ADSCs on the 17th day, while first detected in the EN orbital ADSCs on the 31st day. The RHODOPSIN expression was first detected in EX ADSCs on the 24th day, while first detected in the EN orbital ADSCs on the 31st day. Thus PAX6 was expressed earlier than RHODOPSIN in the EX ADSCs. In EN ADSCs. However, the PAX6 and RHODOPSIN expressions were simultaneously first detected on the 31th day (Figure 4). Subsequently, the RHODOPSIN IMF staining was first detected in EX orbital ADSCs on the 24th day, while first detected in the EN orbital ADSCs on the 31th day, (Figure 4). These findings were consistent with the aforementioned RT-PCR results. Moreover, the RHODOPSIN staining was stronger in EX orbital ADSC (Figure 4F) than the EN orbital ADSCs (Figure 4J) and was further enhanced after Vitamin A treatment.
Figure 4. Eye field and photoreceptor-related genes and protein expression (PAX6 and RHODOPSIN).
D: denotes time point (day) at which orbital ADSCs were analyzed during the induction period. D10: After basal medium×10d; D17: After 1×NDI medium×7d; D24: After 10×NDI medium×7d; D31: After RP medium×7d; D38A: After RP medium induction×14d and with 100 mmol/L Vitamin A×24h; D38S: After RP medium induction×14d and with 100 mmol/L DMSO×24h. EN: Enzymatic; EX: Explant culture. Exposure time=5000ms.
Figure 4A to Figure 4C shows RT-PCR analyses of eye field and photoreceptor-related genes (PAX6 and RHODOPSIN). EN orbital ADSCs were detected to express PAX6 and RHODOPSIN on 31d. EX orbital ADSCs were detected to express PAX6 and RHODOPSIN on 17d and 24d separately.
Figure 4D to Figure 4K shows RHODOPSIN expression by IMF staining. RHODOPSIN is in red and DAPI in blue. RHODOPSIN was detected in EX after 24d of induction (Figure 4H) while the positive signal was not detected in EN at the same time (Figure 4D). RHODOPSIN was detected in both of EN and EX ADSCs after 31d of induction (Figure 4E and 4I), the fluorescence was stronger in EX ADSCs. After treated with 100 mmol/L vitamin A, expression of RHODOPSIN was much stronger than before (Figure 4F and 4J) and the fluorescence was still stronger in EX ADSCs. After treated with 100mmol/L DMSO, expression of RHODOPSIN was also detected in both EX and EN cells (Figure 4G and 4K), but it was much weaker than Figure 4F and 4J.
DISCUSSION
EX culture have been universally used and reported as an easy, simple and cost-effective method to isolate mesenchymal stem/stromal cells from various tissues, including synovium and dental pulp[21]–[22]. Particularly, the EX method is regarded as more efficient than the EN method for isolation of MSCs from the umbilical cord[23]. The EX culture procedure is comparatively short and simple in comparison with the enzymatic dissociation process. Meanwhile, complicated isolation protocols involving enzymatic dissociation may introduce errors, such as microbial contaminations. Additionally, the enzymatic digestion procedure, involving the use of collagenase and other reagents, may have unwanted effects on the isolated cells.
CD133 is closely associated with neuron and astrocyte differentiation[20]. The retinal photoreceptors is a type of neuron intrinsically. In this study, we sought to explore the differentiate potentials of the ADSCs into photoreceptors and the CD133 was used as a differentiate marker. It was found that the CD133 was expressed in the ADSCs, and the expression level gradually degraded with the development of the differentiation. We found the differentiation course towards photoreceptor lasted for 38d and could be divided into two stages. The first stage lasted for 10d using the basic culture medium without induction chemicals. The second stage lasted for 28d (11th to 38th day) and involved a 3-step induction: the 1×NDI and 10×NDI and RP medium were sequentially used to induce orbital ADSCs towards photoreceptor differentiation[19].
In 1×NDI medium, the concentration of Noggin and DKK-1 was 1 ng/mL respectively and was stepped up to 10ng/ml in NDI×10 medium. Noggin binds and inactivates members of the transforming growth factor-beta (TGF-β) super-family signaling proteins, bone morphogenic protein 4 (BMP4). By diffusing through extracellular matrices more efficiently than members of the TGF-β superfamily, Noggin creates a morphogenic gradient which promotes neuronal morphogenesis in the absence of BMP4 signaling. DKK-1, a member of the Dickkopf family, is a secreted protein with two cysteine-rich regions. It is closely related to the embryonic development through the inhibition of the Wnt signaling pathway. When used together with Noggin, which inhibits the BMP pathway in the culture medium, the DKK1 will enable IGF-1 to drive the differentiation of the ADSC towards rhodopsin producing cells. Our results indicate that antagonizing the Wnt and BMP signaling pathways is essential for the specification of the eye field.
The third step lasted for 13d in RP medium without Vitamin A supplementation. However, the IGF-1 and b-FGF supplementation could promote the retinal photoreceptor differentiation of this step. IGF-1 has been shown to be involved in cell proliferation, differentiation, survival, growth, apoptosis and regeneration. IGF-1 is a ligand for the IGF-1R which mediates numerous functions after activation. It has been found that worms with “knockout” IGF-1R live twice longer but with significantly lower metabolic rates[24]. Meanwhile, basic Fibroblast Growth Factor (b-FGF) is a potent mitogenic agent which plays important roles in cell proliferation and differentiation associated with embryogenesis, tissue regeneration, CNS development, wound healing and angiogenesis[25]. In the present study, the b-FGF was used in the final step of differentiation (a component of the PR medium) as it could regulate neurogenesis. During the last 24h of induction, Vitamin A or DMSO was added in the RP medium and the cells were kept in dark, followed by light exposure for 5min before the final harvesting. After that, the retinal progenitor cells were stimulated by Vitamin A to differentiate into photoreceptor cells at the final stage of induction. Vitamin A is a precursor to retinal which binds to rhodopsin (G-protein coupled receptor) to trigger off light transmission in the photo-receptor cells. Upon stimulation by retinoic acid, the neural progenitor cells would differentiate into photoreceptor cells.
On the other hand, the DMSO is solvent for all the reagents used in the experiments, thus it is used as a control. It was found that the cytotoxic effects towards EX and EN orbital ADSCs were evident after treatment with 100 µmol/L Vitamin A rather than treatment with DMSO. It is highly possible that the visual cycle was uncompleted. Absorption of light by rhodopsin's bound ligand, 11-cis retinal will isomerize to the all-trans isomer. Interruptions in the clearing of all-trans-retinal in the photoreceptors can cause the accumulation of these toxic products[26]. It is plausible that the induced ADSCs did not acquire complete function of retinal photoreceptor or the availability of RPE to recycle all-trans-RAL after light exposure. Meanwhile, if differentiation has not completed within the 38d of culture, the addition of vitamin A would be too hazardous. This issue should be addressed by further experiments.
PAX6, a master transcription factor which is essential for normal development of the eye, could be sufficient to induce proliferation, depigmentation, and neuronal gene expression in chick RPE cells[27]. The expression of PAX6 molecule implies that the orbital ADSCs are redirected toward eye field development after induction. Rhodopsin is expressed only in the rod photoreceptor cells. Absorption of light by rhodopsin's bound ligand[28], 11-cis retinal, leads to isomerization to the all-trans isomer, and this activation process occurs within milliseconds
Further experiments are needed to verify the expression level of the all-trans retinal isomer. In EX orbital ADSCs, PAX6 mRNA was detected on the 17th day and then the RHODOPSIN mRNA was detected on the 24th day. This is consistent with the regulated pattern of retinal development. In contrast, the EN orbital ADSC expressed PAX6 and RHODOPSIN on the 31th day. Since the sampling interval lasted for seven days, it was still possible that PAX6 was expressed between the 24th and the 31th day. The sampling intervals should be examined in further details.
Rhodopsin protein staining was detected in EX ADSCs on the 24th day and in EN ADSCs on the 31th day. Rhodopsin is retinal photoreceptor specific protein, and thus it was used as the only marker in the present study. The expression levels of Rhodopsin agreed well with RHODOPSIN mRNA expression levels in the present study. Both mRNA and protein expression results suggested that the EX ADSCs exhibited stronger potentials to differentiate into photoreceptor than the EN ADSCs. The Rhodopsin protein expression was further enhanced after treated with 100 µmol/L Vitamin A on the 38th day, indicating that the induced orbital ADSC can synthesize rhodopsin protein from Vitamin A, which is ascribed as a specific function of rod photoreceptors.
In conclusion, both EN and EX methods were successful in inducing the orbital ADSC. However, orbital ADSCs isolated by direct EX culture showed earlier and stronger differentiated markers towards eye-field and photoreceptor than those generated by conventional EN method.
Acknowledgments
Conflicts of Interest: Xu WW, None; Huang L, None; Chong KKL, None; Leung DSY, None; Li BFL, None; Yin ZQ, None; Huang YF, None; Pang CP, None.
REFERENCES
- 1.Ramsden CM, Powner MB, Carr AJ, Smart MJ, da Cruz L, Coffey PJ. Stem cells in retinal regeneration: past, present and future. Development. 2013;140(12):2576–2585. doi: 10.1242/dev.092270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones BW, Watt CB, Frederick JM, Baehr W, Chen CK, Levine EM, Milam AH, Lavail MM, Marc RE. Retinal remodeling triggered by photoreceptor degenerations. J Comp Neurol. 2003;464(1):1–16. doi: 10.1002/cne.10703. [DOI] [PubMed] [Google Scholar]
- 3.Baker PS, Brown GC. Stem-cell therapy in retinal disease. Curr Opin Ophthalmol. 2009;20(3):175–181. doi: 10.1097/icu.0b013e328329b5f2. [DOI] [PubMed] [Google Scholar]
- 4.Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21(14):2724–2752. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 5.Vossmerbaeumer U, Ohnesorge S, Kuehl S, Haapalahti M, Kluter H, Jonas JB, Thierse HJ, Bieback K. Retinal pigment epithelial phenotype induced in human adipose tissue-derived mesenchymal stromal cells. Cytotherapy. 2009;11(2):177–188. doi: 10.1080/14653240802714819. [DOI] [PubMed] [Google Scholar]
- 6.Zeppieri M, Salvetat ML, Beltrami AP, Cesselli D, Bergamin N, Russo R, Cavaliere F, Varano GP, Alcalde I, Merayo J, Brusini P, Beltrami CA, Parodi PC. Human adipose-derived stem cells for the treatment of chemically burned rat cornea: preliminary results. Curr Eye Res. 2013;38(4):451–463. doi: 10.3109/02713683.2012.763100. [DOI] [PubMed] [Google Scholar]
- 7.Rajashekhar G, Ramadan A, Abburi C, Callaghan B, Traktuev DO, Evans-Molina C, Maturi R, Harris A, Kern TS, March KL. Regenerative therapeutic potential of adipose stromal cells in early stage diabetic retinopathy. PLoS One. 2014;9(1):e84671. doi: 10.1371/journal.pone.0084671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsuruma K, Yamauchi M, Sugitani S, Otsuka T, Ohno Y, Nagahara Y, Ikegame Y, Shimazawa M, Yoshimura S, Iwama T, Hara H. Progranulin, a major secreted protein of mouse adipose-derived stem cells, inhibits light-induced retinal degeneration. Stem Cells Transl Med. 2014;3(1):42–53. doi: 10.5966/sctm.2013-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korn BS, Kikkawa DO, Hicok KC. Identification and characterization of adult stem cells from human orbital adipose tissue. Ophthal Plast Reconstr Surg. 2009;25(1):27–32. doi: 10.1097/IOP.0b013e3181912292. [DOI] [PubMed] [Google Scholar]
- 10.Langenberg T, Kahana A, Wszalek JA, Halloran MC. The eye organizes neural crest cell migration. Dev Dyn. 2008;237(6):1645–1652. doi: 10.1002/dvdy.21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24(2):376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 13.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 14.Jing W, Xiao J, Xiong Z, Yang X, Huang Y, Zhou M, Chen S, Lin Y, Tian W. Explant culture: an efficient method to isolate adipose-derived stromal cells for tissue engineering. Artif Organs. 2011;35(2):105–112. doi: 10.1111/j.1525-1594.2010.01054.x. [DOI] [PubMed] [Google Scholar]
- 15.Priya N, Sarcar S, Majumdar AS, SundarRaj S. Explant culture: a simple, reproducible, efficient and economic technique for isolation of mesenchymal stromal cells from human adipose tissue and lipoaspirate. J Tissue Eng Regen Med. 2014;8(9):706–716. doi: 10.1002/term.1569. [DOI] [PubMed] [Google Scholar]
- 16.Ghorbani A, Jalali SA, Varedi M. Isolation of adipose tissue mesenchymal stem cells without tissue destruction: a non-enzymatic method. Tissue Cell. 2014;46(1):54–58. doi: 10.1016/j.tice.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Busser H, De Bruyn C, Urbain F, Najar M, Pieters K, Raicevic G, Meuleman N, Bron D, Lagneaux L. Isolation of adipose-derived stromal cells without enzymatic treatment: expansion, phenotypical, and functional characterization. Stem Cells Dev. 2014;23(19):2390–2400. doi: 10.1089/scd.2014.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raposio E, Caruana G, Bonomini S, Libondi G. A novel and effective strategy for the isolation of adipose-derived stem cells: minimally manipulated adipose-derived stem cells for more rapid and safe stem cell therapy. Plast Reconstr Surg. 2014;133(6):1406–1409. doi: 10.1097/PRS.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 19.Huang L, Liang J, Geng Y, Tsang WM, Yao X, Jhanji V, Zhang M, Cheung HS, Pang CP, Yam GH. Directing adult human periodontal ligament-derived stem cells to retinal fate. Invest Ophthalmol Vis Sci. 2013;54(6):3965–3974. doi: 10.1167/iovs.13-11910. [DOI] [PubMed] [Google Scholar]
- 20.Mizrak D, Brittan M, Alison M. CD133: molecule of the moment. J Pathol. 2008;214(1):3–9. doi: 10.1002/path.2283. [DOI] [PubMed] [Google Scholar]
- 21.Lee DH, Joo SD, Han SB, Im J, Lee SH, Sonn CH, Lee KM. Isolation and expansion of synovial CD34(-)CD44(+)CD90(+) mesenchymal stem cells: comparison of an enzymatic method and a direct explant technique. Connect Tissue Res. 2011;52(3):226–234. doi: 10.3109/03008207.2010.516850. [DOI] [PubMed] [Google Scholar]
- 22.Hilkens P, Gervois P, Fanton Y, Vanormelingen J, Martens W, Struys T, Politis C, Lambrichts I, Bronckaers A. Effect of isolation methodology on stem cell properties and multilineage differentiation potential of human dental pulp stem cells. Cell Tissue Res. 2013;353(1):65–78. doi: 10.1007/s00441-013-1630-x. [DOI] [PubMed] [Google Scholar]
- 23.Ishige I, Nagamura-Inoue T, Honda MJ, Harnprasopwat R, Kido M, Sugimoto M, Nakauchi H, Tojo A. Comparison of mesenchymal stem cells derived from arterial, venous, and Wharton's jelly explants of human umbilical cord. Int J Hematol. 2009;90(2):261–269. doi: 10.1007/s12185-009-0377-3. [DOI] [PubMed] [Google Scholar]
- 24.Barbieri M, Bonafè M, Franceschi C, Paolisso G. Insulin/IGF-I-signaling pathway: an evolutionarily conserved mechanism of longevity from yeast to humans. Am J Physiol Endocrinol Metab. 2003;285(5):E1064–E1071. doi: 10.1152/ajpendo.00296.2003. [DOI] [PubMed] [Google Scholar]
- 25.Mellough CB, Sernagor E, Moreno-Gimeno I, Steel DH, Lako M. Efficient stage-specific differentiation of human pluripotent stem cells toward retinal photoreceptor cells. Stem Cells. 2012;30(4):673–686. doi: 10.1002/stem.1037. [DOI] [PubMed] [Google Scholar]
- 26.Maeda T, Golczak M, Maeda A. Retinal photodamage mediated by all-trans-retinal. Photochem Photobiol. 2012;88(6):1309–1319. doi: 10.1111/j.1751-1097.2012.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsonis PA, Fuentes EJ. Focus on molecules: Pax-6, the eye master. Exp Eye Res. 2006;83(2):233–234. doi: 10.1016/j.exer.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Zhou XE, Melcher K, Xu HE. Structure and activation of rhodopsin. Acta Pharmacol Sin. 2012;33(3):291–299. doi: 10.1038/aps.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]