Abstract
AIM
To evaluate the clinical efficacy and safety of ranibizumab for wet age-related macular degeneration (wAMD) in Chinese patients and to determine the mean number of injections administered over one year of follow-up.
METHODS
This single centre, retrospective observational case series study included data from 121 patients with wAMD (121 eyes) who were diagnosed by indirect ophthalmoscopy, fluorescence fundus angiography (FFA), indocyanine green angiography, and optical coherence tomography. Ranibizumab was injected into the vitreous cavities once per month for 3mo and as needed afterwards. Changes in visual acuity and central foveal thickness (CFT) during the follow-up period were compared, and the mean number of injections over the year was calculated. Patients with one or more adverse events related to the drugs and injections were recorded for further adverse events analysis.
RESULTS
The study population included 70 males and 51 females aged between 50 and 87y (mean: 71.32±9.41y). The mean number of injections over the first year was 5±1 (range: 3-9). The mean best-corrected visual acuity by Early Treatment Diabetic Retinopathy Study increased from 43.2±19.3 (95%CI: 39.8-46.7) at baseline to 51.7±20.1 (95%CI: 48.1-55.3), and central foveal thickness (CFT) decreased from 526.5±277.0 µm (95%CI: 476.6-576.4) to 258.2±161.6 µm (95%CI: 229.2-287.3) at 12mo. The differences were statistically significant (P<0.001). Visual acuity significantly improved in 34.1% of the patients (38 eyes), stabilized in 66.1% of the patients (80 eyes), and significantly decreased in 2.5% of the patients (3 eyes). CFT at baseline was an independent risk factor of decreased CFT and increased visual acuity. None of the patients had severe adverse events during the follow-up period.
CONCLUSION
Ranibizumab can effectively control disease progression and improve visual acuity in patients with wAMD. The disease conditions of most patients stabilized after a one-year treatment with an average of 5 injections.
Keywords: antiangiogenic drug, ranibizumab, wet age-related macular degeneration, fluorescence fundus angiography, indocyanine green angiography, optical coherence tomography, visual acuity, central foveal thickness
INTRODUCTION
Age-related macular degeneration (AMD) is usually complicated by progressive visual impairment. It seriously affects patient quality of life, especially in aged individuals. It is an irreversible disease that causes blindness. It is the third most common cause worldwide and the most common cause in Western countries for irreversible blindness in elderly. AMD morbidity in Asia has also increased. Although AMD is not the leading cause of vision loss in China, AMD morbidity is rising as the Chinese population ages. An epidemiological study found the rate of AMD morbidity to be as high as 15.5% in developed cities in China[1]. Choroidal neovascularization (CNV) is an important pathological characteristic of wet age-related macular degeneration (wAMD). Lack of timely intervention may result in a very poor prognosis; visual acuity may decrease by 3 lines within 1y and by 4 lines within 2y[2]–[3]. Therefore, the appropriate diagnosis and treatment of this disease requires more attention. The pathogenesis of CNV has not yet been fully revealed, but vascular endothelial growth factors (VEGFs) have been found to play an important role in its development[4]. VEGF-A can promote the division and proliferation of vascular endothelial cells and neovascularization; additionally, it maintains the survival of new vessels. VEGF-A is an inflammatory cell chemotactic factor[5]–[6]. It also increases vascular permeability[7]. High VEGF-A expression has been detected in surgically isolated samples of newly formed vessel membranes associated with wAMD[8]–[9]. VEGF suppression is an ideal treatment for wAMD.
Ranibizumab is a recombinant humanized monoclonal antibody. Its receptor binding site is VEGF-A, which is known to promote vascular generation and leakage and to cause wAMD. The binding of ranibizumab prevents and hinders the interaction of vascular receptors (VEGFR1 and VEGFR2) on the surfaces of vascular endothelial cells, inhibits vascular endothelial hyperplasia, and reduces both vascular leakage into the macular region and the development of CNV. In 2006, the Food and Drug Administration (FDA) approved the use of intravitreal ranibizumab injections for the treatment of wAMD. The State Food and Drug Authority of China approved its clinical use in December 2011. A randomized controlled trial with a 6-month follow-up is the only available study on the use of ranibizumab in Chinese patients[3], and there is a great need for obtaining more clinical data on the use of this novel drug in treating wAMD. Hence, the present study aimed to evaluate the clinical efficacy and safety of ranibizumab for the treatment of wAMD in Chinese patients and to determine the mean number of injections administered over one year of follow-up.
SUBJECTS AND METHODS
The study was in compliance with the policies described in the DRCR.net Policies document (www.drcr.net), with the ethical principles of the Declaration of Helsinki and with the standards of Good Clinical Practice.
Subjects
It was a single centre, retrospective observational case series study. Male and female Chinese patients were included if they met the following inclusion criteria: were ≥50 years of age, had CNV secondary to AMD (including classic, minimally classic, and occult CNV), had not accepted other treatments (including laser therapy, photodynamic therapy, and intravitreal injections of triamcinolone and bevacizumab), had no history of recent onset short-term active arrhythmia or apoplexy, were willing and able to sign the informed consent form; and were followed for the entire 12-month period.
Initially, a total of 220 patients who were treated for wAMD at the Department of Ophthalmology, People's Hospital, Peking University of China between February 2012 and June 2013 were considered for enrolment into the study. The total number of injections was 956. Of the patients, 50 were excluded because they were observed for less than one year, and 49 were excluded because they had polypoid choroidal vasculopathy (PCV). Hence, data from only 121 patients (70 eyes from 70 males and 51 eyes from 51 females; 121 eyes total) were included in the study.
Intervention and Observation Procedures
All patients had vision testing, intraocular tension measurement, slit-lamp microscopy, indirect ophthalmoscopy, colour fundus photography, fundus fluorescein angiography (FFA), and indocyanine green choroidal angiography (ICGA). Central retinal thickness was measured by optical coherence tomography (OCT).
Three types of CNV were observed among the patients: classic, minimally classic, and occult.
Before the operation, the patients routinely received Levofloxacin eye drops for 3d. The injection was performed using a strictly aseptic technique in an operating room. Following surface anaesthesia with 0.4% oxybuprocaine eye drops, the needle was inserted into the eye to be operated on, 3.5 mm behind the corneoscleral junction below the nose or the temple, and Ranibizumab 0.5 mg (Lucentis; produced in Switzerland; supported by Beijing Novartis Pharma Co., Ltd.) was injected into the vitreous cavities. Injections were performed once per month for 3mo and as needed afterwards (PRN). Vision testing and OCT were conducted each month. FFA and ICGA were performed when necessary. Follow-up was performed on day 1, day 3, and each month until 12mo after the operation. Levofloxacin eye drops were given for 3d after the operation. The subsidence of macular edema and CNV leakage was considered to be indicative of recovery from the events. FFA and ICGA were performed during the treatment if necessary. If a patient developed PCV, he/she was removed from the study and was recommended for PDT and transpupillary thermotherapy.
Main Outcome Measures
Significant visual acuity improvement, significant visual acuity decrease, and stable visual acuity were defined as follows: visual acuity increase ≥15 Early Treatment Diabetic Retinopathy Study (ETDRS) letters, visual acuity decrease <15 ETDRS letters, and visual acuity change within 15 letters, respectively. Responses were defined as subsidence and reduction in FFA leakage. The use of the PrONTO criteria was recommended for continued and repeated treatments.
The following findings were compared to previous follow-up results: intraretinal fluid/cyst persistence on OCT, visual acuity decrease ≥5 ETDRS letters and macular edema on OCT, central retinal thickness increase ≥100 µm in any of the scanning lines in the 6 directions on OCT, new macular bleeding, new type of CNV foci, any organic change in OCT indicating the recurrence of macular edema (including cystoid retinal edema), subretinal edema, and increased retinal pigment epithelium.
Central foveal thickness (CFT) was measured using the Humphrey 2000 OCT system, scanning line length: 4000 µm (Carl Zeiss Opthalmic System, Humphrey Division, Dubin, Califormia,USA). CFT and visual acuity were recorded at baseline and during each month of the 12-month follow-up period. Visual acuity and OCT morphology were established as the two primary indicators for assessing treatment efficacy.
Adverse Events
Patients with one or more adverse events related to the drugs and injections were recorded for further adverse events analysis. The adverse events included the following: endophthalmitis, severe uveitis, retinal detachment, vitreous haemorrhage, related thromboembolism, suspected sensitivity to Lucentis, permanent foveal injury, and poor visual acuity without edema.
Statistical Methods
Statistical analysis was performed using Stata 11 (StataCorp, College Station, TX, United States). Paired Student's t-test and the paired Wilcoxon signed-rank test were performed to compare visual acuity and OCT-measured CFT before and after the injections. Spearman's correlation was used to assess the relationship between the baseline CFTs and CFT changes after treatment. Multiple linear regression was used to assess whether CFT at baseline and CFT at baseline after adjusting for age, gender, and number of injections were associated with the decrease in CFT or the increase in visual acuity. Statistical significance was defined as P<0.05.
RESULTS
Baseline and Demographic Characteristics
The patient population comprised 70 (57.85%) males and 51 (42.15%) females who were between the ages of 50 and 87y (mean: 71.32±9.41y). The course of disease ranged from 10d to 1y.
Number of injections over one year
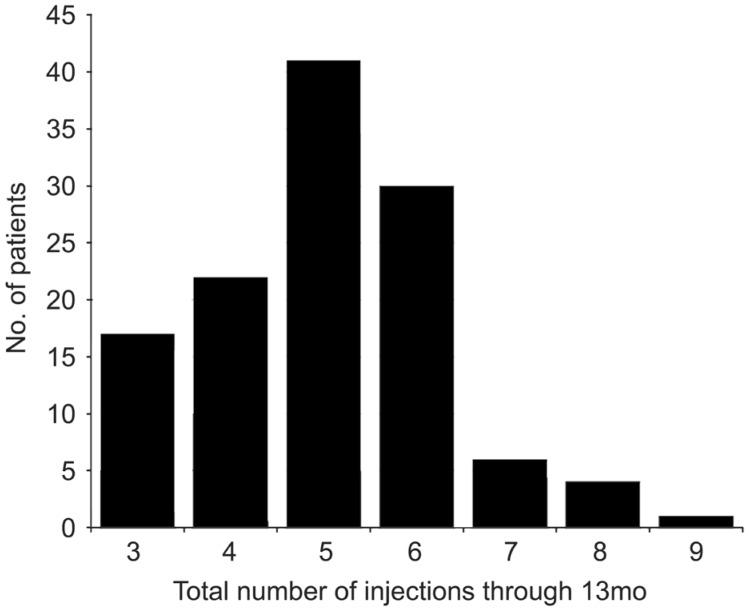
The mean number of injections over the first year was 5±1 (range: 3 to 9) (Figure 1).
Figure 1. Distribution of the total number of injections of ranibizumab administered over 12mo.
Patients were enrolled in the study based on specific study criteria.
Changes in visual acuity and central foveal thickness
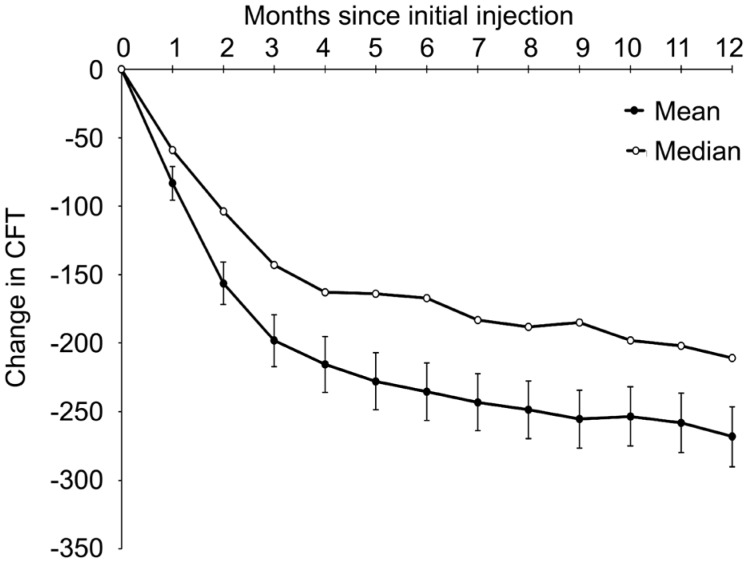
After ranibizumab treatment, the patients' mean best-corrected visual acuity (number of ETDRS letters) increased from 43.2±19.3 (95%CI: 39.8-46.7) at baseline to 51.7±20.1 (95%CI: 48.1-55.3) at 12mo (P<0.001). The mean CFT decreased from 526.5±277.0 µm (95%CI: 476.6-576.4) at baseline to 258.2±161.6 µm (95%CI: 229.2-287.3) at 12mo (P<0.001) (Table 1). Visual acuity significantly improved in 31.4% of the patients (38 eyes), stabilized in 66.1% of the patients (80 eyes), and significantly decreased in only 2.5% of the patients (3 eyes). The improvement in patients' visual acuity was particularly pronounced at the first two follow-up visits after treatment. The visual acuity variation curves were similar to those reported in previous literature (Figure 2). This study also found that CFT decreased with prolonged treatment duration (Figure 3). In addition, the relationship between baseline CFT and the decrease in CFT after treatment was analysed using Spearman's correlation. The correlation coefficient was 0.748 (P<0.001). This corresponded to the clinical phenomenon that the patients with more serious central foveal edema improved to a greater extent after treatment (Figure 4).
Table 1. Comparison of visual acuity and mean CFT before and after ranibizumab treatment: baseline and outcome measures at 12mo.
| Patients' studied eyes (n=121) | Baseline | Month 12 | Change from baseline to month 12 | P |
| Visual acuity letters (ETDRS letters) | ||||
| x±s | 43.2±19.3 | 51.7±20.1 | 8.5±0.97 | <0.001 |
| Median (IQR) | 44 (34-60) | 55 (35-66) | 10 (0-15) | <0.001 |
| CFT (µm) | ||||
| x±s | 526.5±277.0 | 258.2±161.6 | -268.3±241.1 | <0.001 |
| Median (IQR) | 440.0 (344.5, 628.5) | 205.0 (164.5, 294.0) | -211.0 (-344.0, -115.0) | <0.001 |
CFT: Central foveal thickness; IQR: Interquartile range.
Figure 2. Mean and median change in visual acuity over 12mo in patients with neovascular AMD treated with a variable intravitreal ranibizumab dosing regimen.
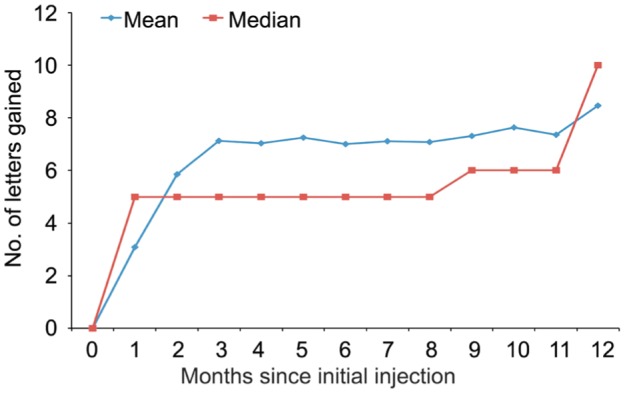
Vertical lines are within 1 standard error of the means.
Figure 3. Mean and median change in CFT (measured by OCT) over 12mo in patients with neovascular AMD treated with a variable intravitreal ranibizumab dosing regimen.
Figure 4. Association between CFT at baseline and the decrease in CFT.
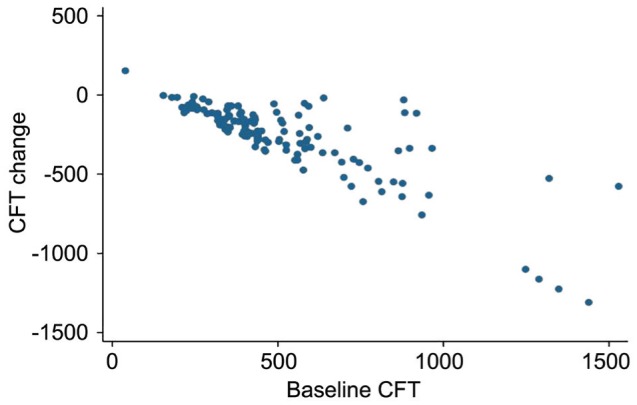
Predictors of decreased CFT and increased visual acuity after adjusting for gender, age, and number of injections. Baseline CFT had a positively associated with the decrease in CFT (P<0.001) and negatively associated with the increase in visual acuity (P=0.002). Additionally, visual acuity at baseline was negatively associated with the increase in visual acuity (P=0.004), while the association between decreased CFT and increased visual acuity was not statistically significant. The percent of total variance of the decrease in CFT explained by baseline CFT was greater than that of the total variance of the increase in visual acuity explained by baseline CFT and visual acuity (adjusted R2: 0.659 vs 0.083; Table 2).
Table 2. Predictors of the decrease in CFT and increase in visual acuity after adjusting for gender, age, and number of injections.
| Variables | Decrease in CFT |
Increase in visual acuity |
||||||
| β | 95%CI | P | Adj R2 | β | 95%CI | P | Adj R2 | |
| Female vs Male | -40.02 | -92.24, 12.19 | 0.132 | 0.659 | 0.21 | -3.62, 4.04 | 0.913 | 0.083 |
| Age (a) | 0.55 | -2.33, 3.44 | 0.706 | 0.09 | -0.12, 0.30 | 0.374 | ||
| No. of injections | 0.65 | -19.60, 20.91 | 0.949 | -0.09 | -1.56, 1.38 | 0.906 | ||
| Visual acuity at baseline | -0.52 | -2.01, 0.95 | 0.484 | -0.16 | -0.26, -0.05 | 0.005 | ||
| CFT at baseline | 0.69 | 0.59, 0.80 | <0.001 | -0.01 | -0.03, -0.003 | 0.013 | ||
| Decrease in CFT | - | - | - | 0.005 | -3.19, 0.02 | 0.468 | ||
FFA: Based on FFA, 70.2% of the patients (85 eyes) showed controlled leakage; CFT: Central foveal thickness; Adj: Adjusted.
Complications
No generalized complications occurred in the study population. None of out patients had the adverse events during the follow-up period as listed: endophthalmitis, severe uveitis, retinal detachment, vitreous haemorrhage, related thromboembolism, suspected sensitivity to Lucentis, permanent foveal injury, and poor visual acuity without edema. Small-area subconjunctival haemorrhage occurred in 20 of the patients; however, no other ophthalmic complications were reported (Figures 5, 6).
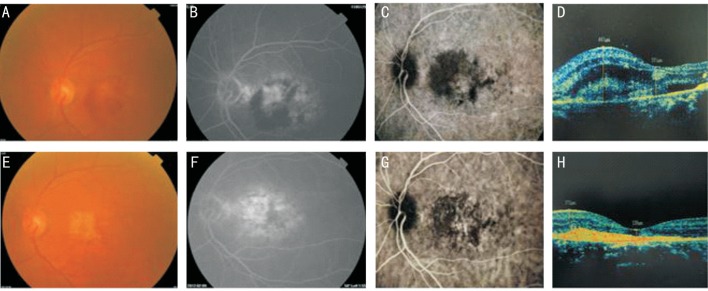
Figure 5. A 77-year-old female patient had decreased visual acuity of the left eye and dysmorphopsia for 20d.
Central subfoveal minimally classic CNV was found in the left eye, and colour fundus images along with FFA, ICG, and OCT were obtained before the operation and at 12mo after five injections. Before the operation, the patient's visual acuity was 35 letters; macular haemorrhage was present; CNV leakage was present; and OCT showed that the CFT was 565 µm. After five injections, visual acuity increased to 55 letters; haemorrhage was absorbed; FFA showed subsidence of CNV leakage and only scar staining; and OCT showed that the CFT decreased to 139 µm. These effects were stable until month 12. A, B, C, and D: Preoperative; E, F, G and H: Postoperative.
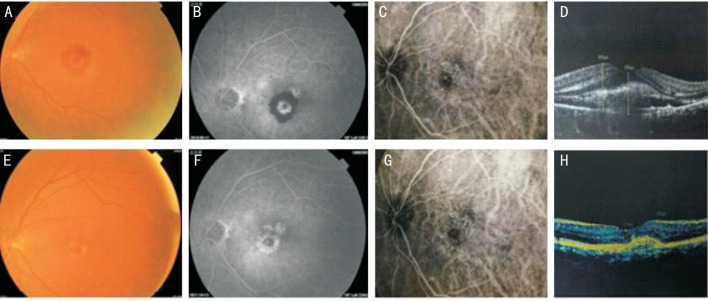
Figure 6. A 70-year-old male patient had decreased visual acuity of the left eye and dysmorphopsia for half a month.
Central subfoveal classic CNV was found in the left eye; colour fundus images along with FFA, ICG, and OCT were obtained before the operation and at 12mo after seven injections. Before the operation, the patient's visual acuity was 35 letters; macular haemorrhage was present; CNV leakage was present; and OCT showed that the CFT was 543 µm. After seven injections, visual acuity increased to 75 letters; haemorrhage was absorbed; FFA showed subsidence of CNV leakage and only scar staining; and OCT showed that the CFT decreased to 234 µm. The effects were stable until month 12. A, B, C, and D: Preoperative; E, F, G and H: Postoperative.
DISCUSSION
Morbidity related to wAMD has increased from year to year as the life expectancy of the Chinese population has risen. The disease prognosis is very poor if wAMD is not treated in a timely manner. VEGFs are recognized as the most important angiogenic stimulating factors and play important roles in the generation and development of new vessels[4]. Ranibizumab, which was designed as a Fab fragment, has a high binding affinity and low molecular weight, which are optimal for bioactivity; additionally, it binds with high affinity to the VEGF-A receptor binding sites to inhibit cascade reactions that would occur following the binding of VEGF-A to its receptors. It inhibits wAMD through the following mechanisms: inhibiting endothelial cell proliferation to hinder the development of CNV and inhibiting vascular permeation and inflammatory reactions to reduce leakage and edema[10].
Monthly injections of ranibizumab may prevent visual acuity loss in patients with wAMD. This has been reported in the MARINA and ANCHOR studies[11]–[12]. Since its official release date in China, ranibizumab has become the only legal anti-VEGF product in the country. However, considering its administration method and high price, it is imperative to investigate how many injections are needed to cure wAMD in Chinese patients.
Recent studies indicate that injections given monthly and PRN afterwards are equivalent in therapeutic effect[13]–[14]. A 3+PRN treatment modality was recommended by PrONTO[15]. The results showed that the efficacy of 3+PRN is similar to that of monthly injection, despite the fact that it requires a significantly lower number of injections; consecutive injections over the first 3mo rapidly improved visual acuity to a plateau, and monthly maintenance afterwards preserved but did not further improve visual acuity. The Chinese Ophthalmological Society 2013 guidelines for AMD recommend VEGF therapy as the first-line treatment for wAMD; the therapy involves 3 consecutive injections at the beginning and treatment PRN afterwards[16]. The criteria for retreatment in this study were based on noninvasive OCT for follow-up monitoring and judgment, and the PrONTO[15] study was referenced. However, considering the high PCV morbidity in China, FFA and ICGA were performed, as appropriate, and patients with established PCV were excluded. The data of patients who received ranibizumab treatment and were followed for more than one year were summarized. The results showed that, after one year of treatment, visual acuity stabilized or improved in 97.1% of the patients (118 eyes); the visual acuity at one year improved compared to the baseline, and the difference was statistically significant. The CFT also significantly decreased compared to the baseline value, and the difference was also statistically significant. The mean number of injections over one year was 5.1, which is very similar to the findings of studies conducted in European countries and America (Table 3).
Table 3. Visual acuity outcomes and mean number of injections in different clinical studies[17].
| Author and study | Region | No. of eyes | Mean No. of injections | Mean increase in the number of ETDRS letters over one year |
| CATT (Martin et al, 2012)[14] | Multicentre | 285 | 6.9 | 6.9 |
| PrONTO (Philip J. Rosenfeld et al, 2009)[15] | America | 40 | 5.6 | 5.6 |
| Muniraju et al, 2013 [17] | Britain | 174 | 4.8 | 3.0 |
| SUSTAIN (Holz et al, 2011)[18] | Multicentre | 513 | 5.6 | 5.6 |
| Arias et al, 2011[19] | Spain | 90 | 4.4 | 4.4 |
| Bandukwala et al, 2010[20] | Canada | 94 | 5.1 | 5.1 |
| Hjelmqvist et al, 2011[21] | Sweden | 370 | 4.7 | 4.7 |
| Kumar et al, 2011[22] | Britain | 81 | 5.6 | 5.6 |
| Williams and Blyth, 2011[23] | Britain | 615 | 5.6 | 5.6 |
| Present study | China | 91 | 5.1 | 7.4 |
Over the past decade, a number of multicentre clinical studies on the treatment of AMD in foreign countries with high-level evidence have been carried out. However, many uncertain problems concerning AMD treatment need to be explored; in China, this exploration is still in its initial period. Due to the short marketing history of ranibizumab in China, data on longer treatment durations and larger patient populations are not available. Therefore, it is imperative to continue observation in future research to improve the levels of AMD diagnosis and treatment, which would benefit more patients. Based on the findings of this study and other studies in various countries, it is clear that wAMD is manageable and that a specific treatment duration can produce positive results is not endless. Nevertheless, the process is long and requires the confidence of patients and oculists so that the quality of life of the elderly population in China may be improved.
The retrospective nature of this study and the lack of a control group are the most critical study limitations. Based on the currently available data, it can be concluded that intravitreal injection of ranibizumab is safe and effective for treating wAMD in Chinese people; additionally, the mean number of injections over the first year was 5.1. Prospective studies of longer duration and with larger sample sizes are needed to further verify the efficacy and safety of ranibizumab.
Acknowledgments
The authors thank Dr. Yuan-Zhi Yuan (Department of Ophthalmology, Zhongshan Hospital, Affiliated to Fudan University and the Center for Evidence-based Medicine at Fudan University) for his assistance in writing and proofreading the manuscript.
Conflicts of Interest: Qi HJ, None; Li XX, None; Zhang JY, None; Zhao MW, None.
REFERENCES
- 1.Zou HD, Zhang X, Xu X, Wang FH, Zhang SJ. Prevalence study of age-related macular degeneration in Caojiadu Blocks, Jing'an District of Shanghai. Zhonghua Yan Ke Za Zhi. 2005;41(1):15–19. [PubMed] [Google Scholar]
- 2.Wong TY, Chakravarthy U, Klein R, Mitchell P, Zlateva G, Buggage R, Fahrbach K, Probst C, Sledge I. The natural history and prognosis of neovascular age-related macular degeneration. Ophthalmology. 2008;115(1):115–126. doi: 10.1016/j.ophtha.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Jun Li, Zhang H, Sun P, Gu F, Liu ZL. Bevacizumab vs ranibizumab for neovascular age-related macular degeneration in Chinese patients. Int J Ophthalmol. 2013;6(2):169–173. doi: 10.3980/j.issn.2222-3959.2013.02.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campochiaro PA. Retinal and choroidal neovascularization. J Cell Physiol. 2000;184(3):301–310. doi: 10.1002/1097-4652(200009)184:3<301::AID-JCP3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N, Gerber HP. The role of vascular endothelial growth factor in angiogenesis. Acta Haematol. 2001;106(4):148–156. doi: 10.1159/000046610. [DOI] [PubMed] [Google Scholar]
- 6.Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D'Amore PA, Dana MR, Wiegand SJ, Streilein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113(7):1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adamis AP, Shima DT, Yeo KT, Yeo TK, Brown LF, Berse B, D'Amore PA, Folkman J. Synthesis and secretion of vascular permeability factor/vascular endothelial growth factor by human retinal pigment epithelial cells. Biochem Biophys Res Commun. 1993;193(2):631–638. doi: 10.1006/bbrc.1993.1671. [DOI] [PubMed] [Google Scholar]
- 8.Das A, McGuire PG. Retinal and choroidal angiogenesis: pathophysiology and strategies for inhibition. Prog Retin Eye Res. 2003;22(6):721–748. doi: 10.1016/j.preteyeres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 2003;22(1):1–29. doi: 10.1016/s1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 10.Krzystolik MG, Afshari MA, Adamis AP, Gaudreault J, Gragoudas ES, Michaud NA, Li W, Connolly E, O'Neill CA, Miller JW. Prevention of experimental choroidal neovascularization with intravitreal anti-vascular endothelial growth factor antibody fragment. Arch Ophthalmol. 2002;120(3):338–346. doi: 10.1001/archopht.120.3.338. [DOI] [PubMed] [Google Scholar]
- 11.Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T, ANCHOR Study Group Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology. 2009;116(1):57–65. doi: 10.1016/j.ophtha.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;335(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 13.Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, Reeves BC. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomizedtrial. Ophthalmology. 2012;119(7):1399–1411. doi: 10.1016/j.ophtha.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth C, Redford M, Ferris FL., 3rd Comparison of age-related macular degeneration treatments trials research group: ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–1398. [Google Scholar]
- 15.Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W, Davis JL, Flynn HW, Jr, Esquiabro M. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO study. Am J Ophthalmol. 2009;148(1):43–58. doi: 10.1016/j.ajo.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 16.Xiao-Xin Li, et al. China clinical guide and clinical pathway committee for age-related macular degeneration china clinical diagnosis and treatment pathways of age-related macular degeneration. Chin J Ophthalmol. 2013;29:343–345. [Google Scholar]
- 17.Muniraju R, Ramu J, Sivaprasad S. Three-year visual outcome and injection frequency of intravitreal ranibizumab therapy for neovascular age-related macular degeneration. Ophthamologica. 2013;230(1):27–33. doi: 10.1159/000350238. [DOI] [PubMed] [Google Scholar]
- 18.Holz FG, Amoaku W, Donate J, Guymer RH, Kellner U, Schlingemann RO, Weichselberger A, Staurenghi G, SUSTAIN Study Group Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the SUSTAIN study. Ophthalmology. 2011;118(4):663–671. doi: 10.1016/j.ophtha.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Arias L, Roman I, Masuet-Aumatell C, Rubio MJ, Caminal JM, Catala J, Pujol O. One-year results of a flexible regimen with ranibizumab therapy in macular degeneration: relationship with the number of injections. Retina. 2011;31(7):1261–1267. doi: 10.1097/IAE.0b013e318207d152. [DOI] [PubMed] [Google Scholar]
- 20.Bandukwala T, Muni RH, Schwartz C, Eng KT, Kertes PJ. Effectiveness of intravitreal ranibizumab for the treatment of neovascular age-related macular degeneration in a Canadian retina practice: a retrospective review. Can J Ophthalmol. 2010;45(6):590–595. doi: 10.3129/i10-082. [DOI] [PubMed] [Google Scholar]
- 21.Hjelmqvist L, Lindberg C, Kanulf P, Dahlgren H, Johansson I, Siewert A. One-year outcomes using ranibizumab for neovascular age-related macular degeneration: results of a prospective and retrospective observational multicentre study. J Ophthalmol. 2011;2011:405724. doi: 10.1155/2011/405724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar A, Sahni JN, Stangos AN, Campa C, Harding SP. Effectiveness of ranibizumab for neovascular age-related macular degeneration using clinician-determined retreatment strategy. Br J Ophthalmol. 2011;95(4):530–533. doi: 10.1136/bjo.2009.171868. [DOI] [PubMed] [Google Scholar]
- 23.Williams TA, Blyth CP. Outcome of ranibizumab treatment in neovascular age related macular degeneration in eyes with baseline visual acuity better than 6/12. Eye(Lond) 2011;25(12):1617–1621. doi: 10.1038/eye.2011.224. [DOI] [PMC free article] [PubMed] [Google Scholar]