Abstract
AIM
To determine the association between retinal vasculature changes and stroke.
METHODS
MEDLINE and EMBASE were searched for relevant human studies to September 2015 that investigated the association between retinal vasculature changes and the prevalence or incidence of stroke; the studies were independently examined for their qualities. Data on clinical characteristics and calculated summary odds ratios (ORs) were extracted for associations between retinal microvascular abnormalities and stroke, including stroke subtypes where possible, and adjusted for key variables.
RESULTS
Nine cases were included in the study comprising 20 659 patients, 1178 of whom were stroke patients. The retinal microvascular morphological markers used were hemorrhage, microaneurysm, vessel caliber, arteriovenous nicking, and fractal dimension. OR of retinal arteriole narrowing and retinal arteriovenous nicking and stroke was 1.42 and 1.91, respectively, indicating that a small-caliber retinal arteriole and retinal arteriovenous nicking were associated with stroke. OR of retinal hemorrhage and retinal microaneurysm and stroke was 3.21 and 3.83, respectively, indicating that retinal microvascular lesions were highly associated with stroke. Results also showed that retinal fractal dimension reduction was associated with stroke (OR: 2.28 for arteriole network, OR: 1.80 for venular network).
CONCLUSION
Retinal vasculature changes have a specific relationship to stroke, which is promising evidence for the prediction of stroke using computerized retinal vessel analysis.
Keywords: retinal image, vasculature, stroke, Meta-analysis
INTRODUCTION
There is increasing evidence that small-vessel disease is a systemic vascular disorder that can be a major cause of stroke[1]. Retinal vasculature is a circulatory system in the eye that can been observed without invasive procedures and provides useful information about the microcirculation system in the body[2]–[4]. The retinal blood vessels are of the size and physiology similar to that of the cerebral small vessels[5]. Retinal image computing methods are being developed to assess retinal microvascular characteristics and the presence and severity of any changes in the vasculature system. The quantified retinal microvascular abnormalities might be useful as risk indicators for cerebrovascular diseases[6]–[7]. Some studies have shown that certain retinal microvascular abnormalities are associated with stroke and might act as surrogate markers for cerebral small-vessel diseases[8]–[9].
In this study, we aimed to identify the association between retinal microvascular changes, including retinal vessel caliber and pathologic lesions, and the risk of stroke, thus striving to clarify whether some correlation exists between retinal vasculature changes and stroke.
MATERIALS AND METHODS
Eligibility Criteria
The patients of stroke were defined as those having a rapid onset of a new neurological deficit lasting more than 24h when the cause of the deficit was unclear. Herein, “stroke” was either a clinically diagnosed stroke, transient ischemic attack, or a cerebral infarct identified by brain imaging without a definite associated clinical feature having been documented. We defined “incident stroke” as that which occurred after the patient had been enrolled in the study, and “prevalent stroke” as that which preceded patient enrolment in the study. Retinal vasculature changes were regarded as retinopathy, hemorrhage, microaneurysms, arteriovenous nicking, or narrowing of the retinal arterioles as well as fractal dimensional changes of the retinal vessel network. In these changes, focal arteriolar narrowing (AN) and arteriovenous nicking were defined by their definite or probable presence in any of four quadrants. Retinal hemorrhage is a breeding disorder of the eye. Retinopathy was defined as the definite or probable presence of any of the following lesions in any of the four quadrants of the retina: microaneurysms, hard exudates, macular edema, novel vessels, blot or flame-shaped hemorrhages, or soft exudates or cotton wool spots. In our research, the signs of age-related maculopathy, such as exudative maculopathy, and retinal artery or vein occlusions were excluded for retinopathy. This study was approved by Ethics Committee of Nantong University and Declaration of Helsinki (2008).
Search Strategy
MEDLINE's EMBASE, the most extensive biomedical database in the world, was comprehensively searched for relevant citations up to June 2016. While conducting the searches, no restrictions were imposed for time and language. Two subsets of citations were enhanced-an indexing stroke or cerebral stroke-the other indexing were retinal vasculature and retinal microvascular morphology, including fractals. For developing these subsets, we used the following combination of subject headings and text terms used in medical literature: 1) exp retinal diseases/, (retina or retinal).tw., microaneurysm.tw., soft exudates.tw., haemorrhage.tw., arteriovenous nicking.tw., macular edema.tw., cotton wool spots.tw., focal arteriolar narrowing.tw., fract$.tw., text*.,tw., dimension*.tw.; 2) stroke/, infarct*, palsy.tw., apoplexy.tw., brain.tw., cerebral.tw., lacunar, cort*, ischemi?.tw.,; 3) incidence/, exp mortality/, exp epidemiologic studies/, prognos$.tw., predict$.mp., course.tw., observ$.mp., risk:.mp., between group:.tw.; and 4) photography/, photomicrography/, photo$.tw., image$.tw., retinopathy.tw., and fundus.tw. We combined the terms to generate a subset of citations that addressed the objective of our research study. We also hand searched the reference lists of relevant articles for eligible studies. We examined the reference lists of all known primary and review articles to identify additional articles not captured by the electronic searches. The detailed search strategy is available from the authors. Two reviewers (Wu HQ and Wu H) independently examined the electronic searches and obtained full reports of all citations that were likely to meet the predefined selection criteria. Disagreements were resolved by consensus and after discussion with a third reviewer (Yu LY).
Data Extraction and Analysis
Those studies meeting the quality standards, which were assessed using Grading of Recommendations Assessment, Development and Evaluation (GRADE) were included. GRADE was designed to evaluate the quality and validity of studies by assessing the quality of their directness, precision, publication bias, risk of bias, consistency of results, and magnitude of the effect. To be of good quality and included in our Meta-analysis, a study was based on prospective consecutive recruitment, such as randomized controlled trial (RCT) and cohort, or an adequate description of the study population was evaluated and independently interpreted. In each of the included studies, three individual researchers (Wu HQ, Wu H and Yu LY) independently extracted the raw data associated with age, sex, stroke numbers, retinal vasculature changes and total study numbers. In instances in which the raw data could not be extracted or calculated, we obtained the information by contacting the authors of the manuscripts. We computed the measurements of retinal microvascular abnormalities after adjusting for different risk factors, such as age, sex, and systolic blood pressure, in patients with stroke and compared these parameters to those of normal individuals who participated in this study as the control group. For improving the accuracy of these tests, subgroup analyses were used to identify the test-related or other factors responsible for heterogeneity. In this study, RevMan ver. 5.3 (http://community archive.cochrane.org/editorial and publishing policy resource/review manager revman) was used to perform the Meta-analysis. The odds ratio (OR) and its 95% confidence interval (CI), or log OR and its standard error (SE) were calculated for statistical analyses. Heterogeneity was established using the Chi-squared test and quantified by I2. In general, I2<25% indicates that the heterogeneity of research studies is low. When I2 is between 25% and 50%, the heterogeneity of research studies is moderate. I2>50% indicates that heterogeneity of research studies has the capacity impact the results[9]. If significant heterogeneity was detected in our Meta-analysis, the random effects model was used to pool the measurements. P<0.05 was regarded as statistically significant.
RESULTS
The literature search yielded 2126 references, 9 articles[10]–[18] of which were eligible for inclusion in the study. Figure 1 outlines the study selection.
Figure 1. Flow chart of study selection.
Summary Characteristics of Included Studies
Nine trials were retrieved for detailed data extraction (Table 1). The nine studies included in the analysis were from Europe, America, Australia, and Asia. There were 20 659 patients, including 1178 who had strokes with subtypes such as large artery, cardioembolic stroke, lacunar stroke, ischemic stroke, and cortical stroke, using brain magnetic resonance imaging (MRI) as the gold standard for diagnosis. All studies were assessed based on the inclusion and exclusion criteria [14]. The average age of the study population was comparable (range, 48.8 to 79y), and the fundus images taken were all optic-disk (OD) centered. Meta-analyses were performed on all studies after adjusting for different risk factors.
Table 1. The characteristics of included studies.
| Studies | Year | Retinal feature | Control | Stroke | Mean age | Female ratio | Stroke types | Cigarette smoker |
| El-Asrar et al[10] | 2002 | DR | 640 | 8 | 48.8 | 47.5% | Prevalent stroke | / |
| Petitti and Bhatt[11] | 1995 | DR | 52 | 52 | 67 | 48.0% | Prevalent stroke | 63.5% |
| Wong et al[12] | 2003 | AN | 1610 | 94 | 78.5 | 61.1% | Prevalent stroke | 56.8% |
| Longstreth et al[13] | 2007 | AN | 897 | 496 | 78.3 | 60.3% | Prevalent stroke | 13.0% |
| AVN | 939 | 496 | 78.3 | 60.3% | Prevalent stroke | 13.0% | ||
| Cooper et al[14] | 2006 | AN | 1428 | 164 | 62.2 | 59.8% | Prevalent stroke | 20.6% |
| AVN | 1462 | 173 | 62.2 | 59.8% | Prevalent stroke | 20.6% | ||
| Hemorrhage | 1422 | 169 | 62.2 | 59.8% | Prevalent stroke | 20.6% | ||
| Microaneurysm | 1311 | 151 | 62.2 | 59.8% | Prevalent stroke | 20.6% | ||
| Kwa et al[15] | 2002 | AN | 71 | 108 | 61.9 | 62.0% | Prevalent stroke | 6.1% |
| Kawasaki[16] | 2011 | SFD | 184 | 101 | 74 | 58.0% | Incident stroke | 7.3% |
| Cheung et al[17] | 2007 | DR | 1471 | 75 | 60.1 | 47.0% | Incident stroke | / |
| Hemorrhage | 1511 | 79 | 60.1 | 47.0% | Incident stroke | / | ||
| Microaneurysm | 1474 | 75 | 60.1 | 47.0% | Incident stroke | / | ||
| Wong et al[18] | 2001 | AN | 10358 | 110 | 53.6 | 55.7% | Incident ischemic stroke | 32.6% |
| AVN | 10264 | 94 | 53.6 | 55.7% | Incident ischemic stroke | 32.6% | ||
| Hemorrhage | 10095 | 92 | 53.6 | 55.7% | Incident ischemic stroke | 32.6% | ||
| Microaneurysm | 9866 | 89 | 53.6 | 55.7% | Incident ischemic stroke | 32.6% |
/: Not given in paper and unable to calculate from data; DR: Diabetic retinopathy; AN: Arteriolar narrowing; AVN: Arterio-venous nicking; SFD: Spectrum fractal dimension.
Retinal Arteriole Narrowing and Stroke
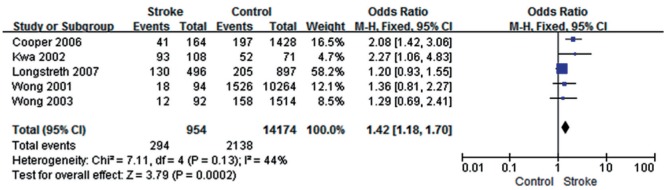
Five studies[12]–[15],[18] showed moderate heterogeneity (P=0.13, I2=44%); the total effect size OR in this study was 1.42 (95% CI: 1.18, 1.70) and Z value was 3.79 (P<0.05), suggesting that retinal artery narrowing might be associated with stroke (Figure 2).
Figure 2. Retinal arteriole narrowing and stroke.
Diabetic Retinopathy and Stroke
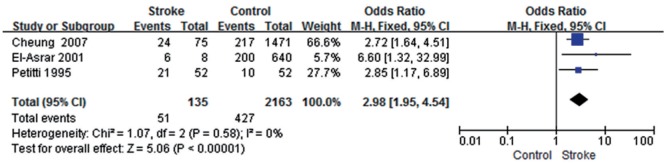
Three studies[10]–[11],[17] showed low heterogeneity in this study (P=0.58, I2=0);the total effect size OR was 2.98 (95% CI: 1.95, 4.54) and Z value was 5.06 (P<0.05), suggesting that the presence of diabetic retinopathy (DR) might be associated with stroke (Figure 3).
Figure 3. DR and stroke.
Retinal Arteriovenous Nicking and Stroke
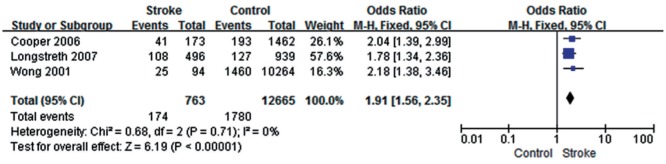
Three studies[13]–[14],[18] showed low heterogeneity in this study (P=0.71, I2=0); the total effect size OR was 1.91 (95% CI: 1.56, 2.35) and Z value was 6.19 (P<0.05), suggesting that retinal arteriovenous nicking might be associated with stroke (Figure 4).
Figure 4. Retinal arteriovenous nicking and stroke.
Retinal Hemorrhage and Stroke
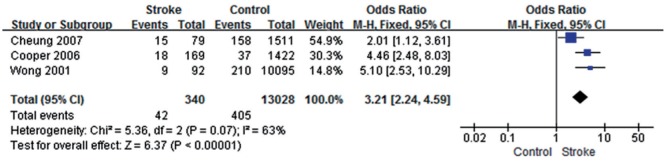
Three studies[14],[17]–[18] showed some heterogeneity in this study (P=0.07, I2=63%); the total effect size OR was 3.21 (95% CI: 2.24, 4.59) and Z value was 6.37 (P<0.05), suggesting that the occurrence of a retinal hemorrhage might be associated with stroke (Figure 5).
Figure 5. Retinal hemorrhage and stroke.
Retinal Microaneurysm and Stroke
Three studies[14],[17]–[18] showed some heterogeneity in this study (P=0.08, I2=61%); the total effect size OR was 3.83 (95% CI: 2.75, 5.34) and Z value was 7.92 (P<0.05), suggesting that a retinal microaneurysm might be associated with stroke (Figure 6).
Figure 6. Retinal microaneurysm and stroke.
Retinal Fractals and Stroke
One study[16] showed that decreased spectrum fractal dimension (SFN; 95% CI: -2.03, -1.46) was associated with stroke, while another two studies[19]–[20] indicated that OR was 1.85 and 2.28 for arteriole network and 1.80 for venular network (Table 2).
Table 2. Retinal fractals and stroke.
DISCUSSION
Cerebral stroke, one of the most common of all brain diseases, often results in cerebral infarction or cerebral hemorrhage, thus seriously affecting a patient's health. During the last decade, the number of stroke-related deaths increased by 26% (95% CI: 14%-32%) and disability-adjusted life-years by 19% (5.0%-26%), making stroke the second leading worldwide cause of death[21]–[22]. For stroke diagnosis, MRI is an appropriate powerful tool for observing the pathological changes in an artery in vivo and subtyping stroke, and thus could be the gold standard imaging technique for cerebral stroke diagnosis; however, the high cost of MRIs becomes an obstacle that limits its assessment on a large number of subjects. Recently, retinal imaging techniques have been widely used for their convenience and low cost to investigate small cerebral infarcts detected by cerebral computerized tomography (CT) or MRI[14]–[15]. The reason that retinal blood vessels are such biomarkers for cerebral microvascular diseases diagnosis and monitoring lies with the fact that they share common anatomical and physiological features with cerebral arterioles[14]–[15],[23]–[24]. Retinal microvascular abnormalities, such as microaneurysms and arteriovenous nicking on fundus during stroke, can be photographed with a fundus camera. In addition, the quality of retinal vessel assessment might be more objective with the aid of computer image-processing techniques.
As indicated by studies on hypertensive retinopathy, retinal vasculature changes are associated with hypertension[25]–[26]. These findings are supported by data from epidemiological studies using less-quantitative clinical assessments of retinal images[27]. More recent studies have described the association between retinal arterial sclerosis and small infarcts detected by cerebral CT or MRI[15],[28].
In this review, we summarized the association between retinal vessel vasculature changes and stroke by investigating relevant clinical studies. We found significant associations between cerebral stroke and the degree of retinal vasculature changes, such as arteriole narrowing and arteriovenous nicking. The findings were consistent with the biological mechanisms that, during the process of stroke, would cause a series of degenerative changes to small blood vessels, such as fibrinoid degeneration, fibrous nodules, fibrohyalinoid thickening, and calcification[29]. It is logical that retinopathy should predict stroke accompanied by diffuse microvascular changes[4],[14],[30]. Our analysis is also consistent with recent findings on the association between retinal microvascular signs and clinical stroke[26], and we grouped various studies on certain retinal vessel changes and stroke outcomes. It has been shown that the presence of DR is also associated with stroke. In addition, stroke is associated with hemorrhage, fractals, microaneurysms, arteriovenous nicking, and retinal arteriole narrowing. Based on the results of our study, decreased retinal fractal dimension might represent a diminishing physiological measure in the cerebral and microcirculatory vasculature, which is more vulnerable to damage from risk factors associated with stroke. Interestingly, some investigations have shown that retinal vessels go through physiological changes with aging[18],[31], which is consistent with findings of the studies included in our Meta-analysis that older populations with stroke will have more retinal abnormalities.
This Meta-analysis had some limitations. First, included populations were not from the same region and the sex distribution was not uniform in among the research groups. Second, differential errors could be attributed to the diverse photographic procedures and retinal vessel caliber. Third, despite the relatively large population size from which cases were derived, the number of stroke patients was small. In addition, there were missing data, which might have had unpredictable effects on multivariate estimates of risk. The indicators we included in the final analysis are the most common pathological changes, such as hemorrhage and aneurysm, but other important indicators, such as tortuosity and exudates, were not summarized because of the lack of data.
Despite the study limitations, the strength of the association between retinal vasculature changes and stroke is encouraging, especially given the consistency among the studies. This suggests that retinal examination offers an excellent way by which to non-invasively investigate the effects of common vascular risk factors on small vessels and gain a better understanding of the pathophysiological processes involved in cerebral small-vessel disease. Retinal vasculature changes have specific relationships with stroke, which is a promising evidence for further computerized retinal vessel analysis. We encourage more studies on these relationships and other important indicators, such as tortuosity and exudates.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81501559; No.81271668); Natural Science Foundation of the Higher Education Institutions of Jiangsu Province (No.15KJB310015); Pre-research project for Natural Science Foundation of Nantong University (No.14ZY021); Science and Technology Project of Nantong City (No.MS12015105); Graduate Research and Innovation Plan Project of Nantong University (No.YKC14048; No.YKC15056).
Conflicts of Interest: Wu HQ, None; Wu H, None; Shi LL, None; Yu LY, None; Wang LY, None; Chen YL, None; Geng JS, None; Shi J, None; Jiang K, None; Dong JC, None.
REFERENCES
- 1.Hägg S, Thorn LM, Putaala J, Liebkind R, Harjutsalo V, Forsblom CM, Gordin D, Tatlisumak T, Groop PH, FinnDiane Study Group Incidence of stroke according to presence of diabetic nephropathy and severe diabetic retinopathy in patients with type 1 diabetes. Diabetes Care. 2013;36(12):4140–4146. doi: 10.2337/dc13-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frydkjaer-Olsen U, Soegaard Hansen R, Simó R, Cunha-Vaz J, Peto T, Grauslund J, EUROCONDOR Correlation between retinal vessel valibre and neurodegeneration in patients with type 2 diabetes mellitus in the European consortium for the early treatment of diabetic retinopathy (EUROCONDOR) Ophthalmic Res. 2016;56(1):10–16. doi: 10.1159/000444396. [DOI] [PubMed] [Google Scholar]
- 3.Phan K, Mitchell P, Liew G, Plant AJ, Wang SB, Au C, Chiha J, Kovoor P, Thiagalingam A, Burlutsky G, Gopinath B. Association between retinal arteriolar and venule calibre with prevalent heart failure: a cross-sectional study. PLoS One. 2015;10(12):e0144850. doi: 10.1371/journal.pone.0144850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gopinath B, Chiha J, Plant AJ, Thiagalingam A, Burlutsky G, Kovoor P, Liew G, Mitchell P. Associations between retinal microvascular structure and the severity and extent of coronary artery disease. Atherosclerosis. 2014;236(1):25–30. doi: 10.1016/j.atherosclerosis.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Patton N, Aslam T, Macgillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat. 2005;206(4):319–348. doi: 10.1111/j.1469-7580.2005.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding J, Wai KL, McGeechan K, Ikram MK, Kawasaki R, Xie J, Klein R, Klein BB, Cotch MF, Wang JJ, Mitchell P, Shaw JE, Takamasa K, Sharrett AR, Wong TY, Meta-Eye Study Group Retinal vascular caliber and the development of hypertension: a meta-analysis of individual participant data. J Hypertens. 2014;32(2):207–215. doi: 10.1097/HJH.0b013e32836586f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Virdis A, Savoia C, Grassi G, Lembo G, Vecchione C, Seravalle G, Taddei S, Volpe M, Rosei EA, Rizzoni D. Evaluation of microvascular structure in humans: a ‘state-of-the-art’ document of the Working Group on Macrovascular and Microvascular Alterations of the Italian Society of Arterial Hypertension. J Hypertens. 2014;32(11):2120–2129. doi: 10.1097/HJH.0000000000000322. [DOI] [PubMed] [Google Scholar]
- 8.Williams MA, McGowan AJ, Cardwell CR, Cheung CY, Craig D, Passmore P, Silvestri G, Maxwell AP, McKay GJ. Retinal microvascular network attenuation in Alzheimer's disease. Alzheimers Dement (Amst) 2015;1(2):229–235. doi: 10.1016/j.dadm.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung HW, Kim SW, Yoon SJ, Choi JY, Kim KI, Kim CH. Associations between frailty, retinal microvascular changes, and cerebral white matter abnormalities in Korean older adults. J Am Geriatr Soc. 2014;62(11):2209–2210. doi: 10.1111/jgs.13114. [DOI] [PubMed] [Google Scholar]
- 10.El-Asrar AM, Al-Rubeaan KA, Al-Amro SA, Moharram OA, Kangave D. Retinopathy as a predictor of other diabetic complications. Int Ophthalmol. 2002;24(1):1–11. doi: 10.1023/a:1014409829614. [DOI] [PubMed] [Google Scholar]
- 11.Petitti DB, Bhatt H. Retinopathy as a risk factor for nonembolic stroke in diabetic subjects. Stroke. 1995;26(4):593–596. doi: 10.1161/01.str.26.4.593. [DOI] [PubMed] [Google Scholar]
- 12.Wong TY, Klein R, Sharrett AR, Manolio TA, Hubbard LD, Marino EK, Kuller L, Burke G, Tracy RP, Polak JF, Gottdiener JS, Siscovick DS. The prevalence and risk factors of retinal microvascular abnormalities in older persons: the cardiovascular health study. Ophthalmology. 2003;110(4):658–666. doi: 10.1016/S0161-6420(02)01931-0. [DOI] [PubMed] [Google Scholar]
- 13.Longstreth W, Larsen EK, Klein R, Wong TY, Sharrett AR, Lefkowitz D, Manolio TA. Associations between findings on cranial magnetic resonance imaging and retinal photography in the elderly: the Cardiovascular Health Study. Am J Epidemiol. 2007;165(1):78–84. doi: 10.1093/aje/kwj350. [DOI] [PubMed] [Google Scholar]
- 14.Cooper LS, Wong TY, Klein R, Sharrett AR, Bryan RN, Hubbard LD, Couper DJ, Heiss G, Sorlie PD. Retinal microvascular abnormalities and MRI-defined subclinical cerebral infarction: the Atherosclerosis Risk in Communities Study. Stroke. 2006;37(1):82–86. doi: 10.1161/01.STR.0000195134.04355.e5. [DOI] [PubMed] [Google Scholar]
- 15.Kwa VI, van der Sande JJ, Stam J, Tijmes N, Vrooland JL. Retinal arterial changes correlate with cerebral small-vessel disease. Neurology. 2002;59(10):1536–1540. doi: 10.1212/01.wnl.0000033093.16450.5c. [DOI] [PubMed] [Google Scholar]
- 16.Kawasaki R, Che Azemin MZ, Kumar DK, Tan AG, Liew G, Wong TY, Mitchell P, Wang JJ. Fractal dimension of the retinal vasculature and risk of stroke: a nested case-control study. Neurology. 2011;76(20):1766–1767. doi: 10.1212/WNL.0b013e31821a7d7d. [DOI] [PubMed] [Google Scholar]
- 17.Cheung N, Rogers S, Couper DJ, Klein R, Sharrett AR, Wong TY. Is diabetic retinopathy an independent risk factor for ischemic stroke. Stroke. 2007;38(2):398–401. doi: 10.1161/01.STR.0000254547.91276.50. [DOI] [PubMed] [Google Scholar]
- 18.Wong TY, Klein R, Couper DJ, Cooper LS, Shahar E, Hubbard LD, Wofford MR, Sharrett AR. Retinal microvascular abnormalities incident stroke: the Atherosclerosis Risk in Communities Study. Lancet. 2001;358(9288):1134–1140. doi: 10.1016/S0140-6736(01)06253-5. [DOI] [PubMed] [Google Scholar]
- 19.Cheung N, Liew G, Lindley RI, Liu EY, Wang JJ, Hand P, Baker M, Mitchell P, Wong TY. Retinal fractals and acute lacunar stroke. Ann Neurol. 2010;68(1):107–111. doi: 10.1002/ana.22011. [DOI] [PubMed] [Google Scholar]
- 20.Ong YT, De Silva DA, Chang HM, Chen CP, Wong MC, Wong TY, Ikram MK. Microvascular structure and network in the retina of patients with ischemic stroke. Stroke. 2013;44(8):2121–2127. doi: 10.1161/STROKEAHA.113.001741. [DOI] [PubMed] [Google Scholar]
- 21.Doubal FN, MacGillivray TJ, Dhillon B, Dennis MS, Wardlaw JM. Differences in retinal vessels support a distinct vasculopathy causing lacunar stroke. Neurology. 2009;72(20):1773–1778. doi: 10.1212/WNL.0b013e3181a60a71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung CY, Ong YT, Ikram MK, Chen C, Wong TY. Retinal microvasculature in Alzheimer's disease. J Alzheimers Dis. 2014;42 Suppl. 4:S339–S352. doi: 10.3233/JAD-141596. [DOI] [PubMed] [Google Scholar]
- 24.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: systematic a analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9867):2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 25.Aissopou EK, Argyris AA, Nasothimiou EG, Konstantonis GD, Tampakis K, Tentolouris N, Papathanassiou M, Theodossiadis PG, Papaioannou TG, Stehouwer CD, Sfikakis PP, Protogerou AD. Ambulatory aortic stiffness is associated with narrow retinal arteriolar caliber in hypertensives: the SAFAR study. Am J Hypertens. 2016;29(5):626–633. doi: 10.1093/ajh/hpv145. [DOI] [PubMed] [Google Scholar]
- 26.Zhu P, Huang F, Lin F, Li Q, Yuan Y, Gao Z, Chen F. The relationship of retinal vessel diameters and fractal dimensions with blood pressure and cardiovascular risk factors. PLoS One. 2014;9(9):e106551. doi: 10.1371/journal.pone.0106551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aoki N. Epidemiological evaluation of funduscopic findings in cerebrovascular diseases. II. A multivariate analysis of funduscopic findings. Jpn Circ J. 1975;39(2):271–282. doi: 10.1253/jcj.39.271. [DOI] [PubMed] [Google Scholar]
- 28.Rim TH, Han J, Choi YS, Hwang SS, Lee CS, Lee SC, Kim SS. Retinal artery occlusion and the risk of stroke development: Twelve-year Nationwide Cohort Study. Stroke. 2016;47(2):376–382. doi: 10.1161/STROKEAHA.115.010828. [DOI] [PubMed] [Google Scholar]
- 29.Goto I, Katsuki S, Ikui H, Kimoto K, Mimatsu T. Pathological studies on the intracerebral and retinal arteries in cerebrovascular and noncerebrovascular diseases. Stroke. 1975;6(3):263–269. doi: 10.1161/01.str.6.3.263. [DOI] [PubMed] [Google Scholar]
- 30.Cheung CY, Tay WT, Ikram MK, Ong YT, De Silva DA, Chow KY, Wong TY. Retinal microvascular changes and risk of stroke: the Singapore Malay Eye Study. Stroke. 2013;44(9):2402–2408. doi: 10.1161/STROKEAHA.113.001738. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto Y, Yamamoto Y, Marugame A, Ogura M, Saito A, Ohta K, Fukumoto M, Murata T, Fukumoto M. Age-related decrease of the retinal vasculature area identified with a novel computer-aided analysis system. Tohoku J Exp Med. 2012;228(3):229–237. doi: 10.1620/tjem.228.229. [DOI] [PubMed] [Google Scholar]