Abstract
AIM
To determine the prevalence of ocular demodicosis by both microscopic examination and molecular detection among patients at King Chulalongkorn Memorial Hospital, Thai Red Cross Society, Bangkok.
METHODS
One hundred individuals were enrolled in the study and were divided into five age groups. The meibomian gland dysfunction (MGD) score and qualities of cylindrical dandruff (CD) were also determined. Demodex mite infestations of eyelash samples were screened by both microscopic examination and semi-nested polymerase chain reaction (PCR).
RESULTS
The prevalence of ocular demodicosis as determined by microscopic examination was 42% [Demodex folliculorum (D. folliculorum) 41% and Demodex brevis (D. brevis) 1%]. Among patients who had ocular Demodex infestation, 69% have CD and had an average MGD score of 4; in patients without demodicosis, 15.5% had CD and had an average MGD score of 4.12. Prevalence of ocular demodicosis as determined by semi-nested PCR was 79% (D. folliculorum 78% and D. brevis 1%).
CONCLUSION
This is the first report on the prevalence of ocular demodicosis in Thailand. Patients with CD also had Demodex mites present. Semi-nested PCR is better than microscopy for Demodex infestation detection. An extensive survey with more representative samples is required to determine the prevalence in the country.
Keywords: prevalence, ocular demodicosis, Demodex folliculorum, Demodex brevis, Thailand
INTRODUCTION
The word Demodex is of Greek origin, demos means wax or fat, and dex means insect[1]. Demodex mites are elongated ectoparasites found on human body surfaces, such as the face, cheeks, forehead, nose, or eyelids[2]. The parasites are classified in the class Arachnida, superorder Acariforme. Several species of Demodex have been described, but only Demodex folliculorum (D. folliculorum) and Demodex brevis (D. brevis) are claimed to be etiologic pathogens of human demodicosis[3]. D. folliculorum is approximately 0.35-0.4 mm long and is commonly found in the infundibular part of lash follicles, whereas D. brevis is approximately 0.15-0.2 mm long and live deeper in lash sebaceous glands and meibomian glands[4].
Clinical manifestations of ocular Demodex infestation vary from asymptomatic to cylindrical dandruff (CD), eyelash disorders, lid margin inflammation, meibomian gland dysfunction (MGD), blepharoconjunctivitis, blepharokeratitis or even rare reported cases of vesiculopustular rash[5]. Previous studies reported that the prevalence of Demodex eyelash infestation was higher in lashes with CD[4]. Türk et al[6] and Zhao et al[7] stated that the incidence of Demodex infestation was high in patients with blepharitis. Moreover, Liang et al[8] reported a higher prevalence of D. brevis in chalazion patients by using lash sampling and microscopic counting methods. However, the prevalence of eyelash demodicosis in a population varies from 20.0%-90.0%[3],[7],[9].
Demodex mites can be detected by a skin scraping examination, standard skin surface biopsy, commedo-extraction, or adhesive tape, cellophane tape, and squeezing methods[10], while ocular demodicosis can be determined by eyelash removal. Detection of Demodex mites and differentiation between D. folliculorum and D. brevis can be performed using microscopic examinations based on their morphological characteristics. However, microscopic examination for the detection and identification of Demodex mites has difficulties, especially in the immature stages of the Demodex mites. Several reports described using molecular techniques for detection of Demodex mites, such as polymerase chain reaction (PCR) that targets several genes for the detection and identification of Demodex mites[11]–[14]. For example, Zhao et al[14] revealed that PCR of the mitochondrial 16S ribosomal DNA (16SrDNA) and sequencing analysis has been developed to identify Demodex spp.
Prevalence of ocular demodicosis in Thailand has never been fully evaluated; therefore, the aim of this study is to determine the prevalence of Demodex infestation in eyelashes among Thai patients in different age groups who visited the outpatient department at King Chulalongkorn Memorial Hospital. Moreover, we compared the prevalence of ocular demodicosis between microscopic examination and semi-nested PCR methods. Data obtained from the study provide fundamental data regarding the prevalence of ocular Demodex infestation and identification of the human Demodex mite species in Thailand.
MATERIALS AND METHODS
Subjects
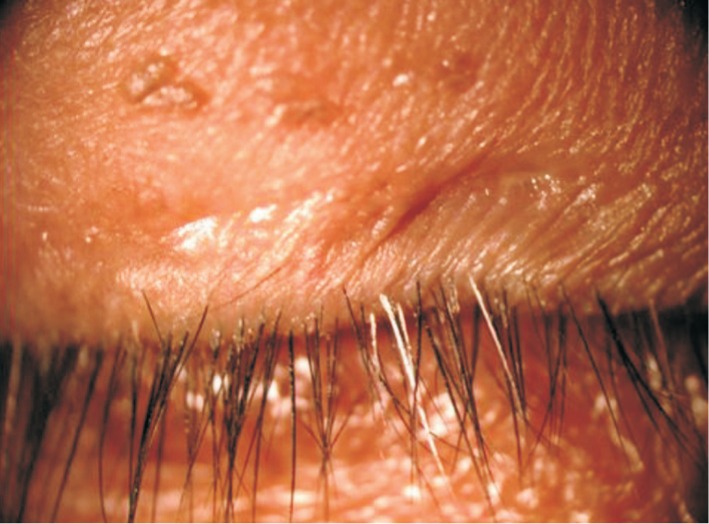
The study was ethically approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (IRB 073/56). Patients were informed, and the consent was obtained from all patients. One hundred patients were included in this study. The patients were categorized into five age groups (20 patients per each group; the age groups were divided as follows: 0-20, 21-40, 41-60, 61-80 and >80y). All patients were randomly selected from the Outpatient Department at King Chulalongkorn Memorial Hospital from January to August 2012. Then, routine complete eye examinations, including the examination of eyelids, were performed. The MGD score (0-10; normal = 0, mild = 0.1-3.3, moderate = 3.4-6.6, severe = 6.7-10) was evaluated. CD was defined as scales that form clear cuffs collaring the lash root; CD was distinguished from greasy scales that do not rest on the root of the lash and are identified in Figure 1.
Figure 1. Characteristic of CD.
Eyelash Sampling and Microscopic Detection of Demodex spp.
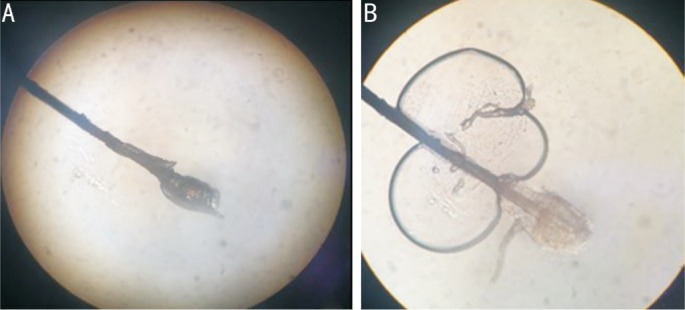
Eyelash sampling was performed for each case; lashes with CD were considered (Figure 2A) under examination by slit-lamp microscopy (Model SL-D7; Topcon, Tokyo, Japan) at a magnification of 16×. Eight lashes were removed from each case by fine forceps, four from the left eye and four from the right eye. For each eye, two lashes were removed from the upper lid, and two were removed from the lower lid; a lash from each of the upper and lower lids was placed separately on a glass slide. The coverslip was mounted onto each lash before slowly pipetting a drop of normal saline solution to the edge of the coverslip; each slide was examined under a microscope at 10× and 40× magnifications (Figure 2B). All slides were sent to the Department of Parasitology, Faculty of Medicine, Chulalongkorn University for further semi-nested PCR analysis.
Figure 2. An eyelash with CD on dry slide (A) and an eyelash with CD in normal saline (B).
Molecular Detection of Demodex spp.
DNA extraction
Eye lash samples (from both upper and lower lids) were transferred into 1.5 mL microcentrifuge tubes containing 100 µL of lysis buffer. Samples were then processed for total DNA extraction by a tissue DNA extraction kit (Invisorb® Spin Tissue Mini Kit, Invitek, Germany) according to the manufacturer's instructions. DNA was eluted in 50 mL of elution buffer; quantity and quality was determined by a Nanodrop 2000c apparatus (Thermo Scientific, USA). The extracted DNA samples were kept at -80°C for long-term storage.
Primer design
Primers were designed based on the 18S ribosomal RNA (18SrRNA) gene of Demodex mites. The gene sequences of D. folliculorum (accession no JF783994 and JF783996) and D. brevis (accession no JF783998, JF783999, and HQ844220) were obtained from the GenBank database. Three primers were used in this study: the forward primer Dm2F-(5′-TAACAGGTGACGGGGAATC-3′) and the reverse primer Dmm2R-(5′-TAGTGGTTGACCCAATAACA-3′) were used for detection of D. folliculorum with expected PCR products with a size of 382 bp; the reverse primer DDm2R-(5′-AACACYCGGTAAAGAGC-3′) was used for detection of D. brevis with expected PCR products with a size of 317 bp. The primers were synthesized by 1st BASE Oligonucleotide Synthesis Services (1st BASE Laboratories, Malaysia).
Semi-nested polymerase chain reaction amplification
The PCR reaction was composed of 9.3 µL of double distilled water, 2.5 µL of 10× Taq buffer, 2 mmol/L of dNTPs, 25 mmol/L of MgCl2, 1 µL of each primer, and 1 unit of Taq DNA polymerase (Fermentas, USA). Thermal cycle conditions were initial denaturation for 5min at 95°C, followed by 35 cycles of denaturation at 95°C for 30s, annealing at 54.7°C for 1s, extension at 72°C for 1min and the final extension at 72°C for 5min. The PCR products were detected on a 1.5% agarose gel stained with 0.5 µg/mL of ethidium bromide and were visualized with Quantity One Quantification Analysis Software version 4.5.2 (Gel Doc EQ System; Bio-Rad, CA, USA).
DNA cloning and sequencing
To confirm that PCR products were amplified from the Demodex gene, PCR products obtained from semi-nested PCR were ligated into a pGEM-T Easy Vector (Promega, USA). The ligations were then used to transform Escherichia coli DH5α strain competent cells. The recombinant DNA was screened using the blue-white colonies system. The colonies containing the inserted gene were cultured, and the purified plasmid DNA was extracted by using the Invisorb® Spin Plasmid Mini kit (STRATEC molecular GmbH, Germany) according to the manufacturer's instructions. The purified plasmids were sequenced by 1st Base laboratories in Malaysia.
Statistical Analysis
The prevalence of Demodex was recorded for each age group in both microscopic and PCR techniques. The correlation between Demodex and the non-Demodex group was analyzed as a percentage.
RESULTS
One hundred individuals were enrolled in the study that included 34 males and 66 females. The mean age was 48y (ranging from 6-87 years old). The incidence of ocular Demodex infestation determined by microscopic examination in this study was 42%. The prevalence of each age group was 25% (5 cases), 15% (3 cases), 40% (8 cases), 60% (12 cases), and as much as 70% (14 cases) in the 0-20, 21-40, 41-60, 61-80, and >80 years age groups, respectively (Table 1). Among the subjects who had Demodex, 69% also had CD; in subjects who did not have Demodex, CD was found in only 15.5% (Table 2). In individuals with Demodex, the MGD score was 4.0 (range 0-10); in individuals without Demodex, the MGD score was 4.12 (range 0-10) (Table 2). The prevalence of D. folliculorum and D. brevis from microscopic examination was 41% and 1%, respectively (Table 3).
Table 1. Prevalence of Demodex categorized in age group.
| Age (a) | No. of cases | Individuals with Demodex | Ocular demodicosis (%) |
| 0-20 | 20 | 5 | 25 |
| 21-40 | 20 | 3 | 15 |
| 41-60 | 20 | 8 | 40 |
| 61-80 | 20 | 12 | 60 |
| >80 | 20 | 14 | 70 |
| Total | 100 | 42 | 42 |
Table 2. Percentage of cases with CD and average MGD score in individual with and without Demodex determined by microscopic examination.
| Microscopic examination | No. of case with CD | Average MGD score |
| Individual with Demodex (42) | 29 (69) | 4.0 |
| Individual without Demodex (58) | 9 (15.5) | 4.12 |
CD: Cylindrical dandruff; MGD: Meibomian gland dysfunction.
n (%)
Table 3. Prevalence of ocular demodicosis determined by microscopic examination and semi-nested PCR.
| Parameters | Prevalence |
| Microscopic examination | |
| D. folliculorum | 41 |
| D. brevis | 1 |
| Semi-nested PCR | |
| D. folliculorum | 78 |
| D. brevis | 1 |
PCR: Polymerase chain reaction.
%
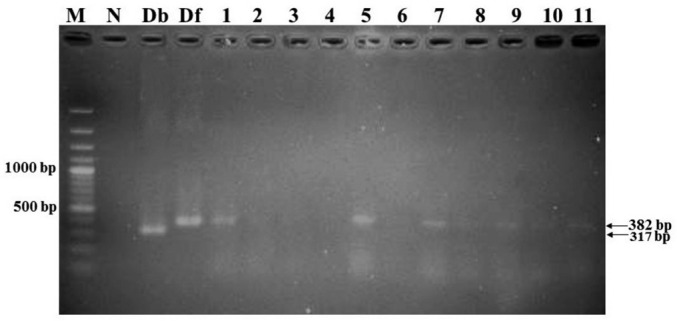
Semi-nested PCR was performed with total DNA extracted from 100 eye lash samples. PCR was able to amplify the 18SrRNA gene from both human Demodex mite species with approximately 382 bp for D. folliculorum and 317 bp for D. brevis (Figure 3). Amplified sequences of D. folliculorum and D. brevis were submitted to GenBank and were assigned to accession numbers KT449878 and KT449882, respectively. Nucleotide sequences of both Demodex species compared with the GenBank database revealed that the percentage of D. folliculorum and D. brevis is approximately 99.04% and 100%, respectively. In the semi-nested PCR techniques, D. folliculorum can be detected more easily than in microscopic examination, but D. brevis showed the same result with both techniques. The prevalence of D. folliculorum was 78%, while the prevalence of D. brevis was 1% when determined by semi-nested PCR (Table 3). All individuals who had CD (38 cases) were positive for Demodex mites when analyzed with semi-nested PCR; some of these patients did not have CD. In individuals with Demodex determined by semi-nested PCR, the MGD score was 4.04, whereas in individuals without Demodex, the MGD score was 4.02 (Table 4).
Figure 3. A 1.5% gel electrophoresis showed PCR products amplified by semi-nested PCR.
Lane 1:100 bp DNA standard marker; Lane N: Negative control; Lane Db: Positive control (D. brevis); Lane Df: Positive control (D. folliculorum); Lane 1-11: Eyelash samples.
Table 4. Percentage of cases with CD and average MGD score in individual with and without Demodex determined by semi-nested PCR.
| Semi-nested PCR | No. of case with CD | Average MGD score |
| Individual with Demodex (79) | 38 (48.1) | 4.04 |
| Individual without Demodex (21) | 0 (0) | 4.02 |
PCR: Polymerase chain reaction; CD: Cylindrical dandruff; MGD: Meibomian gland dysfunction.
n (%)
DISCUSSION
Demodex mites are widespread parasites that live on hair follicles and in the pilosebaceous gland of the eyelids. Several studies reported that ocular demodicosis is associated with conditions such as blepharitis[6], rosacea[15], oily skin surface[16], uncontrolled blood sugar in diabetic patients[17], pregnant with gestational diabetes[18], malignancy, malnutrition and children with a low socioeconomic status[19]. Most studies have focused on skin demodicosis; few have studied the prevalence of Demodex in eyelashes of the general population.
Wesolowska et al[9] studied 290 individuals who were divided into groups (inpatients, drug abusers, health professionals, medical students) and reported that the overall prevalence was 41%. They found that the 70-79 years old drug abuser group had the highest prevalence rate of 75%; this group may be prone to HIV infection, but the prevalence was not significantly higher than other groups. In contrast with previous studies, some suggested that the prevalence of demodicosis was higher in immunocompromised hosts, such as those with diabetes, pregnancy, leukemia and human immunodeficiency virus (HIV)[18],[20]–[22]. Another study suggested that immunosuppression did not seem to increase the rate of Demodex spp. infection[23]. One reason that could explain this discrepancy is that they might not be susceptible to infection if the immune system was not very deficient; additionally, in the era of anti-retroviral therapy, the prevalence may increase in severely immunocompromised hosts.
The average prevalence of eyelash Demodex infestation that was determined by microscopic examination in this study was 42%. The highest prevalence was found in people over the age of 80 (70%). The result was similar to previous reports that described an increase in prevalence in elderly groups. Vargas-Arzola et al[3] studied 1010 individuals and found that the average percentage of Demodex in eyelashes was 20% (male 57%, female 43%). The number of affected cases increased with age: 64% in 76-85 years old, 75% in 86-95 years old, and 100% in ages over 95y. In our study, the prevalence increased up to 70% in ages over 80y. The reasons may include that elderly patients have a tendency for meibomian gland obstruction and poorer immune systems in addition to a decrease in healthy hygiene habits that could provide a good environment for Demodex growth.
We found that 69% of individuals with Demodex infestation as determined by microscopy had CD, while only 15.5% of individuals without Demodex had CD. This result was consistent with a previous study that suggested a high prevalence of Demodex in eyelashes with CD. Gao et al[4] found the Demodex in all cases with CD (n=32), but it was found in only in 22% in individuals with clean eyelashes (n=23); they explained that this result was reasonable because even in the group with CD, cases with diffuse CD still had more positive Demodex prevalence rates compared with cases with sporadic CD. Furthermore, the MGD score was 4.0 in individuals with Demodex but was higher in individuals without Demodex (4.12). Wesolowska et al[9] also reported the prevalence of Demodex spp. in subjects with and without eye complaints, suggesting that blepharitis was similar (41.6% vs 40.2%, respectively, P=0.9). This may be because many eye conditions also present with eye discomfort, and many patients with ocular demodicosis are asymptomatic.
Türk et al[6] conducted a prospective study with 96 eyes from 48 blepharitis patients and 96 eyes from 48 normal subjects. D. follicullorum was found in 11 of 37 (29.72%) patients with blepharitis, it was found in 1 of 11 (9.09%) patients with blepharoconjunctivitis, and it was found in 2 of 48 (4.16%) persons in the healthy control group. Although many studies have reported that a Demodex infection is associated with blepharitis, Kaya et al[19] reported a prospective study of 500 individuals, 170 individuals had seborrheic blepharitis and 330 individuals were from the normal population[19]. Twelve eyelashes were taken from each individual. D. folliculorum was found in 28.8% (49/170) of patients with blepharitis and in 26.7% (88/330) of control patients; the difference was not statistically significant. Cengiz et al[24] studied 67 patients and determined that 53.1% of Demodex spp. positive patients had eritemato telangiectatic rosacea, and 21.9% had papulo-pustular type rosacea.
The results from almost all of the studies show an increasing prevalence trend for ocular Demodex in elderly patients. Our study and Wesolowska et al's[9] study were conducted on a hospital-based population; the results were quite similar (overall prevalence was 41% and 42%, respectively, and increased prevalence in ages over 70y to approximately 70% in both studies)[9]. Compared with the study of Vargas-Arzola et al[3] that was conducted on a normal population, the prevalence of ocular Demodex was lower. One reason that could explain the result was that in hospital-based studies, cases were selected from people who came to the ophthalmology clinic; thus, these subjects tended to have an ocular condition as well as blepharitis.
The prevalence of ocular Demodex was 52% in blepharitis cases and 28.7% in a normal population in Germany, which was as high as many reported studies from developing countries. We can assume that socioeconomic status may not influence the prevalence of ocular Demodex.
Because of the anatomy of the face, eyelids are not accessible for routine cleansing, and this situation provides a favorable environment for Demodex mites to flourish. Rosacea patients have a high risk of developing blepharitis because rosacea creates a skin environment that congests all the oil-producing glands necessary for healthy dermis and epidermis. Furthermore, Demodex mites can cause blepharitis by carrying bacteria, including Streptococcus and Staphylococcus, on their surface. Superantigens produced by bacteria are also implicated in the induction of rosacea. One study discussed a strong correlation among positive serum immunoreactivity to the 83-kDa and 62-kDa bacillus proteins, ocular Demodex infestation, facial rosacea, and blepharitis[25].
Szkaradkiewicz et al[26] reported that some bacteria may be a co-pathogen of Demodex severe blepharitis. They studied the eyelashes of chronic blepharitis individuals and healthy individuals and found that Bacillus olerenius, which is a gram negative, rod-shaped, endospore-forming bacterium, was significantly different between each group. This corresponds with the previous study that noted that B. olerenius is associated with rosacea. Furthermore, Szkaradkiewicz et al[26] suggested that all the strains were sensitive to ciprofloxacin, doxycycline and gentamicin, and most strains showed a sensitivity to clindamycin. Treatment of ocular Demodex is still controversial; salicylic acid, selenium sulfide, metronidazole, crotamitone, lindane, tea tree oil, ivermectin and topical permethrin have been suggested[1].
In our study, we first examined eyelashes with a light microscope; we found just one case of D. brevis in a total of 42 cases. Examination with semi-nested PCR showed that D. brevis was also positive for one case. We can assume that microscopic evaluation can differentiate both species. The total prevalence was 42% in microscopic evaluation but was as high as 79% with PCR results. The prevalence of Demodex from PCR was nearly twice higher than with morphological detection. Interestingly, all cases that had CD were positive for Demodex mites by semi-nested PCR; however, some cases that were positive for PCR had no CD.
There are several limitations of this study. It was conducted with a small sample size (20 individuals in each age group), and the population was only patients at King Chulalongkorn Memorial Hospital, who might not represent the normal population in Thailand. This study could not analyze risk factors for developing demodicosis. However, this study was the first study to identify the prevalence of ocular Demodex infection in Thailand by microscopic examination, and we developed a semi-nested PCR method for Demodex DNA detection.
In conclusion, we reported a Demodex prevalence in 79% of the eyelashes examined by semi-nested PCR in all age groups. The percentage of D. folliculorum was 41% when examined by microscopic examination, which was the same as the PCR results, whereas D. brevis was found in only 1% of the sample examined. Prevalence increased with age and was found to be 70% in ages older than 80. CD is an important sign of ocular Demodex infection. Blepharitis symptoms cannot differentiate the causes of disease in patients with or without ocular Demodex.
Acknowledgments
Foundation: Supported by National Science and Technology Development Agency (Thailand) for a Research Chair Grant.
Conflicts of Interest: Kasetsuwan N, None; Kositphipat K, None; Busayarat M, None; Threekhan P, None; Preativatanyou K, None; Phumee A, None; Siriyasatien P, None.
REFERENCES
- 1.Holzchuh FG, Hida RY, Moscovici BK, Villa Albers MB, Santo RM, Kara-Jose N, Holzchuh R. Clinical treatment of ocular Demodex folliculorum by systemic ivermectin. Am J Ophthalmol. 2011;151(6):1030–1034.e1. doi: 10.1016/j.ajo.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 2.Kim JH, Chun YS, Kim JC. Clinical and immunological responses in ocular demodicosis. J Korean Med Sci. 2011;26(9):1231–1237. doi: 10.3346/jkms.2011.26.9.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vargas-Arzola J, Reyes-Velasco L, Segura-Salvador A, Marquez-Navarro A, Diaz-Chiguer DL, Nogueda-Torres B. Prevalence of Demodex mites in eyelashes among people of Oaxaca, Mexico. Acta Microbiol Immunol Hung. 2012;59(2):257–262. doi: 10.1556/AMicr.59.2012.2.10. [DOI] [PubMed] [Google Scholar]
- 4.Gao YY, Di Pascuale MA, Li W, Liu DT, Baradaran-Rafii A, Elizondo A, Kawakita T, Raju VK, Tseng SC. High prevalence of Demodex in eyelashes with cylindrical dandruff. Invest Ophthalmol Vis Sci. 2005;46(9):3089–3094. doi: 10.1167/iovs.05-0275. [DOI] [PubMed] [Google Scholar]
- 5.Yun SH, Levin F, Servat J. Demodex folliculitis presenting as periocular vesiculopustular rash. Orbit. 2013;32(6):370–371. doi: 10.3109/01676830.2013.812124. [DOI] [PubMed] [Google Scholar]
- 6.Türk M, Oztürk I, Sener AG, Küçükbay S, Afşar I, Maden A. Comparison of incidence of Demodex folliculorum on the eyelash follicule in normal people and blepharitis patients. Turkiye Parazitol Derg. 2007;31(4):296–297. [PubMed] [Google Scholar]
- 7.Zhao YE, Wu LP, Hu L, Xu JR. Association of blepharitis with Demodex: a meta-analysis. Ophthalmic Epidemiol. 2012;19(2):95–102. doi: 10.3109/09286586.2011.642052. [DOI] [PubMed] [Google Scholar]
- 8.Liang L, Ding X, Tseng SC. High prevalence of demodex brevis infestation in chalazia. Am J Ophthalmol. 2014;157(2):342–348.e1. doi: 10.1016/j.ajo.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 9.Wesolowska M, Knysz B, Reich A, Blazejewska D, Czarnecki M, Gladysz A, Pozowski A, Misiuk-Hojlo M. Prevalence of Demodex spp. in eyelash follicles in different populations. Arch Med Sci. 2014;10(2):319–324. doi: 10.5114/aoms.2014.42585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao YE, Guo N, Xun M, Xu JR, Wang M, Wang DL. Sociodemographic characteristics and risk factor analysis of Demodex infestation (Acari: Demodicidae) J Zhejiang Univ Sci B. 2011;12(12):998–1007. doi: 10.1631/jzus.B1100079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sastre N, Ravera I, Ferreira D, Altet L, Sanchez A, Bardagi M, Francino O, Ferrer L. Development of a PCR technique specific for Demodex injai in biological specimens. Parasitol Res. 2013;112(9):3369–3372. doi: 10.1007/s00436-013-3531-z. [DOI] [PubMed] [Google Scholar]
- 12.Zhao YE, Ma JX, Hu L, Wu LP, De Rojas M. Discrimination between Demodex folliculorum (Acari: Demodicidae) isolates from China and Spain based on mitochondrial cox1 sequences. J Zhejiang Univ Sci B. 2013;14(9):829–836. doi: 10.1631/jzus.B1200363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu L, Zhao YE, Cheng J, Ma JX. Molecular identification of four phenotypes of human Demodex in China. Exp Parasitol. 2014;142:38–42. doi: 10.1016/j.exppara.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Zhao YE, Hu L, Ma JX. Molecular identification of four phenotypes of human Demodex mites (Acari: Demodicidae) based on mitochondrial 16S rDNA. Parasitol Res. 2013;112(11):3703–3711. doi: 10.1007/s00436-013-3558-1. [DOI] [PubMed] [Google Scholar]
- 15.Murillo N, Aubert J, Raoult D. Microbiota of Demodex mites from rosacea patients and controls. Microb Pathog. 2014;71–72:37–40. doi: 10.1016/j.micpath.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Porta Guardia CA. Demodex folliculorum: its association with oily skin surface rather than rosacea lesions. Int J Dermatol. 2015;54(1):e14–e17. doi: 10.1111/ijd.12398. [DOI] [PubMed] [Google Scholar]
- 17.Gokce C, Aycan-Kaya O, Yula E, Ustun I, Yengil E, Sefil F, Rizaoglu H, Gultepe B, Bayram F. The effect of blood glucose regulation on the presence of opportunistic Demodex folliculorum mites in patients with type 2 diabetes mellitus. J Int Med Res. 2013;41(5):1752–1758. doi: 10.1177/0300060513494730. [DOI] [PubMed] [Google Scholar]
- 18.Keskin Kurt R, Aycan Kaya O, Karateke A, Silfeler DB, Soylu Karapinar O, Akkoca AN, Hakverdi AU. Increased density of Demodex folliculorum mites in pregnancies with gestational diabetes. Med Princ Pract. 2014;23(4):369–372. doi: 10.1159/000363244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaya S, Selimoglu MA, Kaya OA, Ozgen U. Prevalence of Demodex folliculorum and Demodex brevis in childhood malnutrition and malignancy. Pediatr Int. 2013;55(1):85–89. doi: 10.1111/j.1442-200X.2012.03740.x. [DOI] [PubMed] [Google Scholar]
- 20.Damian D, Rogers M. Demodex infestation in a child with leukaemia: treatment with ivermectin and permethrin. Int J Dermatol. 2003;42(9):724–726. doi: 10.1046/j.1365-4362.2003.01916.x. [DOI] [PubMed] [Google Scholar]
- 21.Vithayasai P, Vithayasai V. Clinical manifestations of 174 AIDS cases in Maharaj Nakorn Chiang Mai Hospital. J Dermatol. 1993;20(7):389–393. doi: 10.1111/j.1346-8138.1993.tb01305.x. [DOI] [PubMed] [Google Scholar]
- 22.Inci M, Kaya OA, Inci M, Yula E, Gokce H, Rifaioglu MM, Demirtaş O, Yengil E. Investigating Demodex folliculorum in patients with urological cancer. Turkiye Parazitol Derg. 2012;36(4):208–210. doi: 10.5152/tpd.2012.50. [DOI] [PubMed] [Google Scholar]
- 23.Kosik-Bogacka DI, Lanocha N, Lanocha A, Czepita D, Grobelny A, Zdziarska B, Kalisińska E. Demodex folliculorum and Demodex brevis in healthy and immunocompromised patients. Ophthalmic Epidemiol. 2013;20(3):159–163. doi: 10.3109/09286586.2013.789532. [DOI] [PubMed] [Google Scholar]
- 24.Cengiz ZT, Yilmaz H, Ozkol HU, Ekici A, Odemis N. The prevalence of Demodex sp. in patients admitted to the parasitology laboratory of the Dursun Odabas Medical Center in Yuzuncu Yil University, Van. Turkiye Parazitol Derg. 2014;38(1):9–11. doi: 10.5152/tpd.2014.3407. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Sheha H, Tseng SC. Pathogenic role of Demodex mites in blepharitis. Curr Opin Allergy Clin Immunol. 2010;10(5):505–510. doi: 10.1097/ACI.0b013e32833df9f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szkaradkiewicz A, Chudzicka-Strugala I, Karpinski TM, Goslinska-Pawlowska O, Tulecka T, Chudzicki W, Szkaradkiewicz AK, Zaba R. Bacillus oleronius and Demodex mite infestation in patients with chronic blepharitis. Clin Microbiol Infect. 2012;18(10):1020–1025. doi: 10.1111/j.1469-0691.2011.03704.x. [DOI] [PubMed] [Google Scholar]