Abstract
We are currently on the cusp of exponential growth in the understanding of the molecular landscape of bladder cancer. Emerging data regarding the mutational burden and targetable genomic and protein alterations in bladder cancer have allowed us to tap into treatments directed toward specific molecular characteristics of bladder cancer. In parallel, these developments will enable us to better select patients for existing treatments of bladder cancer in a step toward personalized therapy. The present article reviews select discoveries that have advanced our understanding of bladder cancer and gives a glimpse of the exciting opportunities on the not-so-distant horizon.
Keywords: bladder, genomics, carcinoma, sequencing
Introduction
Over the last decade, new understandings in molecular carcinogenesis as well as advances in genomic sequencing technologies have ushered in a new era of targeted cancer therapy. Rational therapeutic targets have been identified and new drugs successfully deployed in the treatment of various solid tumors. For example, patients with breast and lung cancers are routinely subjected to genotypic/phenotypic assessment, and specific targeted treatments are available for several permutations 1, 2. For bladder cancer, however, prognostication and treatment selection still depend primarily on clinical and pathologic characteristics.
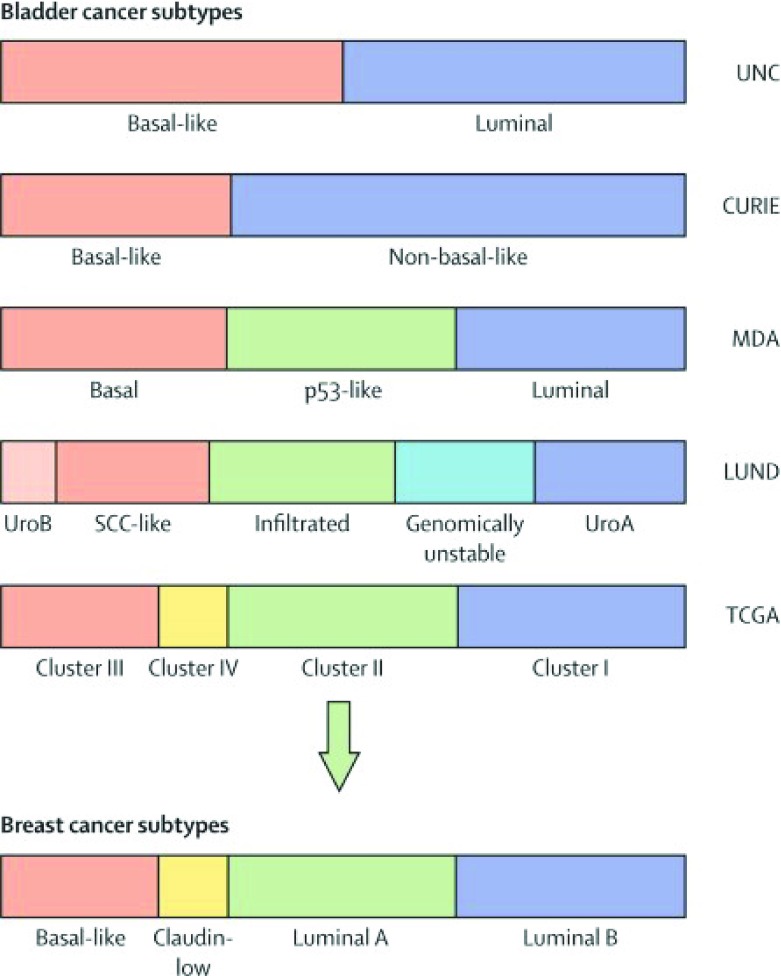
In 2014, the comprehensive molecular characterization of muscle-invasive bladder cancer (MIBC) by The Cancer Genome Atlas (TCGA) Research Network produced many new insights into the genetic makeup of MIBC 3. Integrated analysis of mRNA, microRNA, and protein expression in 129 muscle-invasive tumors yielded four distinct clusters resembling the intrinsic subtypes identified in breast cancer 4. Clusters I and II were similar to luminal A breast cancer and had high mRNA and protein expression of differentiation markers, including GATA3 and FOXA1. Cluster III and IV tumors were similar to basal breast cancer and had high expression of stem/progenitor cytokeratins 3. These findings indicate similar pathways of tumorigenesis despite different tissue origins. In addition, these clusters broadly corroborated with the findings of three other contemporary studies ( Figure 1), further validating its accuracy 5– 8.
Figure 1. Molecular subtype classification of bladder cancer and breast cancer.
Color bars represent subtype classifications made by each institution. Subtype groupings were made independently, and associations were assigned on the basis of the MD Anderson Cancer Center (MDA) classifier. CURIE, Institut Curie; UNC, University of North Carolina. Adapted from Kamat et al. 8.
Another significant finding of TCGA was the large number of somatic DNA alterations found in MIBC. Mean and median somatic mutation rates were respectively 7.7 and 5.5 per megabase, more than those found in any adult malignancy other than lung cancer and melanoma 3. Importantly, actionable therapeutic targets were found in 69% of the tumors, converging on three main pathways: cell cycle regulation, kinase and phosphatidylinositol-3-OH kinase (PI[3]K) signaling, and chromatin remodeling. Specifically, mutations and somatic copy number alteration were frequently found in histone-modifying genes (89%), components of the SWI/SNF nucleosome remodeling complex (64%), PI(3)K/AKT/mTOR pathway (42%), and the RTK/RAS pathway (44%) 3.
Each of these three major findings in TCGA has major implications for the prognostication and treatment design for MIBC. Here, we will review advances in the understanding of MIBC since the landmark publication of TCGA and the new therapeutic strategies they have fostered. Furthermore, the use of high-throughput next-generation sequencing (NGS) has only recently been extended to non-MIBC (NMIBC). Undoubtedly, the experience garnered from studying MIBC using these methods can be readily transferred to help answer many unresolved questions for NMIBC.
Sensitivity to neoadjuvant chemotherapy
Since the 1980s, cisplatin-based chemotherapy has been recognized as the standard of care for metastatic urothelial carcinoma (UC), specifically with M-VAC (methotrexate, vinblastine, doxorubicin, and cisplatin) established as the superior combination and GC (gemcitabine and cisplatin) as an alternative 9– 13. Subsequently, neoadjuvant chemotherapy (NAC) prior to surgical or radiation treatment of MIBC has demonstrated survival benefit in separate phase 3 trials and meta-analyses 14– 16. Despite its proven efficacy, NAC rendered only 38% of patients pT0, conferring a modest 5% improvement in 5-year survival. Additionally, toxicity associated with NAC is not insignificant 14, 15. In light of this marginal risk-benefit ratio, attempts have been made using clinicopathologic features to refine patient selection for NAC 17. However, no clear prediction model for response to NAC exists to date, leading to ongoing debate regarding who should undergo NAC 18, 19.
Drawing on the success correlating intrinsic subtypes with NAC response in breast cancer 20, Choi et al. from MD Anderson Cancer Center used their tripartite molecular subtyping of MIBC (basal, luminal, and p53-like) to predict chemosensitivity 5. They found a marked increase in chemoresistance associated with the p53-like tumors compared with the other subtypes. Similar to luminal breast cancers, this chemoresistance was associated with lower levels of proliferation and cell cycle biomarkers in the p53-like subtype 21. Interestingly, activation of wild-type p53-like gene expression signature was observed in post-chemotherapy tumor specimens, leading to a larger percentage of p53-like tumors in the post-chemotherapy cohort. In conjunction, these findings suggest that NAC may selectively decimate non-p53-like tumor cells, thus enriching the p53-like gene expression signature that dominates after treatment 22. In a subsequent study, the authors showed that basal tumors were associated with better survival outcomes after NAC 23. As only M-VAC was used to treat the cohort of tumors analyzed, it is unknown whether this chemoresistant feature of p53-like tumors can be generalized to other therapy combinations.
On the other hand, NGS studies have also linked platinum chemosensitivity to specific genomic alterations. Activating mutations of ERBB2 (tyrosine kinase receptor) were exclusively found in the pre-treatment tumors resected from patients with complete pathologic response (pT0) 24. In addition, mutations in the DNA repair genes are linked with increased chemosensitivity. Mutations in ERCC2, a nucleotide excision repair gene, were found to be enriched in patients responding to NAC 25. In line with this finding, Plimack et al. discovered that alterations in one or more of the DNA repair genes ATM, RB1, and FANCC were enriched in patients downstaged to ≤pT1N0M0 after chemotherapy, leading to improved overall survival 26. The authors postulated that the deleterious defects in the DNA repair genes represent a fatal flaw for the associated tumors, making it impossible to recover from the DNA damage incurred by the alkylating agent cisplatin.
Though intriguing, these findings need to be validated in larger studies encompassing diverse patient demographics and chemotherapy regimens. Fortunately, this effort is currently under way, as are efforts to assess the benefit of combining genomic signatures with clinicopathologic characteristics for the prognostication of response to NAC. The knowledge gained will aid in the development of novel chemotherapy/targeted therapy agents in the future.
Immune checkpoint blockade for metastatic bladder cancer
Overall survival with cisplatin-based chemotherapy, despite its firmly established efficacy in the treatment of metastatic UC, remains rather poor 27, 28. The prognosis is especially dismal for patients with relapse after chemotherapy, and median survival ranges from 5 to 7 months 29. Moreover, no new drug therapy has been found to be effective in the four decades since the adoption of cisplatin-based chemotherapy. As such, the recent introduction of anti-PD-L1 (programmed death ligand 1) treatment for metastatic UC was met with great enthusiasm 30, 31. PD-L1 negatively regulates T-cell function by binding to its receptors programmed death 1 (PD-1 or B7-1) on activated T lymphocytes and other immune cells. The overexpression of PD-L1 in the tumor microenvironment is thought to be the mechanism by which tumor evasion of the host immune system occurs. Blockade of the PD-L1 pathway with a high-affinity engineered human anti-PD-L1 monoclonal immunoglobulin-G1 antibody (atezolizumab) was shown to improve objective response rate in a heavily pre-treated population with poor prognostic features 32, 33. Extended median overall survival ranging from 7.9 to 11.4 months was observed. One-year overall survival ranged between 36% and 48% compared with the historic rate of 20% from a pooled analysis 34.
Objective response rate, progression-free survival, and overall survival were found to be directly related to PD-L1 expression status on the infiltrating immune cells (ICs). In addition, treatment response correlated with mutation load and was found to be the highest in the luminal tumors subtyped within cluster II of the TCGA scheme 33. The finding that PD-L1 was more efficacious in tumors with higher mutation load was consistent with patterns recognized in other malignancies 35, 36. Non-synonymous somatic mutations are thought to increase tumor neoantigen burden, leading to increased T-cell recognition and more potent tumoricidal activities unleashed by PD-L1 treatment.
Interestingly, TCGA subtyping was found to have prognostic implications independent of the PD-L1 expression levels in ICs. Despite having lower IC PD-L1 expression, cluster II tumors responded to treatment at a higher rate than the basal cluster III and IV tumors. Their higher response rate to PD-L1 treatment may be attributed to the intrinsic biology of the cluster II tumors. Alternatively, additional immunosuppressive pathways may be used by basal tumors to prevent effective immune activation. Of course, these tumors were previously treated with cisplatinum, which itself has effects of subtype migration as elucidated earlier. Nonetheless, the differential response to PD-L1 therapy in the different tumor subtypes highlights the need for further understanding of their associated immunobiology.
Targeted therapy: lessons learned and future strategies
Genetic alterations in the mTOR, FGFR, EGFR, and HER2 pathways have long been recognized in subsets of bladder cancer. TCGA and other studies have identified actionable drug targets in over 60% of the tumors interrogated 3, 37. Disappointingly, no trial to date has proven efficacy for any rationally designed targeted agent in advanced UC 38. The conundrum of futility against the preponderance of potential therapeutic targets can partially be explained by the highly variable genomic landscape of UC uncovered by recent studies. A key finding in a multi-platform analysis of 12 cancer types was the genetic diversity of bladder cancer, splitting into three pan-cancer subtypes 39. Such heterogeneity found in advanced UC renders ineffective the one-size-fits-all approach undertaken by most previous trials.
Instead, careful patient selection is needed for targeted drug trials to increase the signal-to-noise ratio derived from effective treatment. Alternatively, in-depth retrospective analysis of exceptional responders may provide insight for refining trial design to yield meaningful outcomes. One such example was illustrated by the phase II study of everolimus in a metastatic UC trial 40, 41. Although the trial as a whole failed to achieve the predetermined progression-free survival end point, whole genome sequencing in an exceptional responder revealed mutations that enhanced therapeutic efficacy. Subsequently, by using the newly discovered mutation as a biomarker, the authors were able to demonstrate treatment efficacy in a smaller pre-selected subset 40. This prompted the Exceptional Responders Initiative launched by the National Cancer Institute to identify molecular indicators in malignant tissue from exceptional responders using NGS (ClinicalTrials.gov identifier: NCT02243592). With this strategy, afatinib (tyrosine kinase inhibitor of the ErbB receptor family) was found to have efficacy in the subpopulation of patients with platinum-refractory UC with somatic ERBB family alterations 42.
Furthermore, the recently published National Comprehensive Cancer Network (NCCN) Bladder Cancer Guidelines recommend broadening the scope of molecular profiling for advanced UC 43 in an effort to identify more patients with specific mutations as candidates for various ongoing clinical trials. In addition, those with higher mutational burden or specific tumor subtypes may be selected for immune checkpoint blockade.
Future outlook
The NGS technology that has brought such major advances in the understanding of MIBC has only recently been used in the study of NMIBC. The traditional dichotomization of bladder cancer into low-grade papillary tumors and invasive carcinoma that arise from flat dysplasia and carcinoma in situ 44 has been challenged by a proposed subtyping scheme spanning both MIBC and NMIBC 7. Others have found NMIBC to exhibit markedly different gene expression profiles than MIBC, leading to its own assigned cluster group on unsupervised hierarchical cluster analysis 45.
A recent multi-institutional study of 460 early stage UC identified three major subgroups of NMIBC with basal- and luminal-like characteristics, each associated with different clinicopathologic features and progression-free survival 46. However, the authors found imperfect reconciliation between these subtypes to the basal and luminal subtypes found in MIBC. They hypothesized that the three subgroups represented three different developmental pathways of NMIBC.
Further studies using NGS technology in NMIBC are warranted to characterize this group of diverse tumors. Along the way, new insights into the biology of development and progression are likely to be uncovered. Conceivably, biomarkers or subtypes may be identified for susceptibility to intravesical treatment with chemotherapy or bacillus Calmette-Guérin. On the other hand, molecular characteristics of progressive tumor may be collected early on to select patients for early radical treatment to avoid metastatic spread. In fact, evidence is already emerging from transgenic mouse models demonstrating the loss of sonic hedgehog ( SHH) expression as an important molecular switch on the path to MIBC 47. These are fascinating times both for us in the scientific community and for our patients, and there is hope on the horizon to truly advance the needle in the quest for cure.
Abbreviations
IC, infiltrating immune cell; MIBC, muscle-invasive bladder cancer; M-VAC, methotrexate, vinblastine, doxorubicin, cisplatin; NAC, neoadjuvant chemotherapy; NGS, next-generation sequencing; NMIBC, non-muscle-invasive bladder cancer; PD-L1, programmed death ligand 1; PI(3)K, phosphatidylinositol-3-OH kinase; TCGA, The Cancer Genome Atlas; UC, urothelial carcinoma.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Ellen Zwarthoff, Department of Pathology, Erasmus Medical Centre, Rotterdam, Netherlands
Joseph Liao, Department of Urology, Stanford University School of Medicine, Stanford, CA, USA; Urology Section, Veterans Affairs Palo Alto Health Care System, Palo Alto, CA, USA
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. Ettinger DS, Wood DE, Akerley W, et al. : Non-Small Cell Lung Cancer, Version 6.2015. J Natl Compr Canc Netw. 2015;13(5):515–24. [DOI] [PubMed] [Google Scholar]
- 2. Gradishar WJ, Anderson BO, Balassanian R, et al. : Breast Cancer Version 2.2015. J Natl Compr Canc Netw. 2015;13(4):448–75. [DOI] [PubMed] [Google Scholar]
- 3. Cancer Genome Atlas Research Network: Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507(7492):315–22. 10.1038/nature12965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McConkey DJ, Choi W, Dinney CP: New insights into subtypes of invasive bladder cancer: considerations of the clinician. Eur Urol. 2014;66(4):609–10. 10.1016/j.eururo.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 5. Choi W, Porten S, Kim S, et al. : Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25(2):152–65. 10.1016/j.ccr.2014.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Damrauer JS, Hoadley KA, Chism DD, et al. : Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci U S A. 2014;111(8):3110–5. 10.1073/pnas.1318376111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sjödahl G, Lauss M, Lövgren K, et al. : A molecular taxonomy for urothelial carcinoma. Clin Cancer Res. 2012;18(12):3377–86. 10.1158/1078-0432.CCR-12-0077-T [DOI] [PubMed] [Google Scholar]
- 8. Kamat AM, Hahn NM, Efstathiou JA, et al. : Bladder cancer. Lancet. 2016;388(10061):2796–2810. 10.1016/S0140-6736(16)30512-8 [DOI] [PubMed] [Google Scholar]
- 9. von der Maase H, Hansen SW, Roberts JT, et al. : Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18(17):3068–77. [DOI] [PubMed] [Google Scholar]
- 10. Loehrer PJ, Sr, Einhorn LH, Elson PJ, et al. : A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol. 1992;10(7):1066–73. [DOI] [PubMed] [Google Scholar]
- 11. Logothetis CJ, Dexeus FH, Finn L, et al. : A prospective randomized trial comparing MVAC and CISCA chemotherapy for patients with metastatic urothelial tumors. J Clin Oncol. 1990;8(6):1050–5. [DOI] [PubMed] [Google Scholar]
- 12. Saxman SB, Propert KJ, Einhorn LH, et al. : Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol. 1997;15(7):2564–9. [DOI] [PubMed] [Google Scholar]
- 13. Sternberg CN, Yagoda A, Scher HI, et al. : M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for advanced transitional cell carcinoma of the urothelium. J Urol. 1988;139(3):461–9. [DOI] [PubMed] [Google Scholar]
- 14. Grossman HB, Natale RB, Tangen CM, et al. : Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349(9):859–66. 10.1056/NEJMoa022148 [DOI] [PubMed] [Google Scholar]
- 15. Advanced Bladder Cancer (ABC) Meta-analysis Collaboration: Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48(2):202–5. discussion 205–6. 10.1016/j.eururo.2005.04.006 [DOI] [PubMed] [Google Scholar]
- 16. International Collaboration of Trialists; Medical Research Council Advanced Bladder Cancer Working Party (now the National Cancer Research Institute Bladder Cancer Clinical Studies Group); European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group, et al. : International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29(16):2171–7. 10.1200/JCO.2010.32.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Culp SH, Dickstein RJ, Grossman HB, et al. : Refining patient selection for neoadjuvant chemotherapy before radical cystectomy. J Urol. 2014;191(1):40–7. 10.1016/j.juro.2013.07.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Black P, Kassouf W: Re: Günter Niegisch, Anja Lorch, Michael J. Droller, Hugh J. Lavery, Kristian D. Stensland, Peter Albers. Neoadjuvant chemotherapy in patients with muscle-invasive bladder cancer: which patients benefit? Eur Urol 2013;64:355–7. Eur Urol. 2014;65(1):e8–9. 10.1016/j.eururo.2013.08.016 [DOI] [PubMed] [Google Scholar]
- 19. Niegisch G, Lorch A, Droller MJ, et al. : Neoadjuvant chemotherapy in patients with muscle-invasive bladder cancer: which patients benefit? Eur Urol. 2013;64(3):355–7. 10.1016/j.eururo.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 20. Esserman LJ, Berry DA, Demichele A, et al. : Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL--CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30(26):3242–9. 10.1200/JCO.2011.39.2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brown JR, DiGiovanna MP, Killelea B, et al. : Quantitative assessment Ki-67 score for prediction of response to neoadjuvant chemotherapy in breast cancer. Lab Invest. 2014;94(1):98–106. 10.1038/labinvest.2013.128 [DOI] [PubMed] [Google Scholar]
- 22. Choi W, Czerniak B, Ochoa A, et al. : Intrinsic basal and luminal subtypes of muscle-invasive bladder cancer. Nat Rev Urol. 2014;11(7):400–10. 10.1038/nrurol.2014.129 [DOI] [PubMed] [Google Scholar]
- 23. McConkey DJ, Choi W, Shen Y, et al. : A Prognostic Gene Expression Signature in the Molecular Classification of Chemotherapy-naïve Urothelial Cancer is Predictive of Clinical Outcomes from Neoadjuvant Chemotherapy: A Phase 2 Trial of Dose-dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin with Bevacizumab in Urothelial Cancer. Eur Urol. 2016;69(5):855–62. 10.1016/j.eururo.2015.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Groenendijk FH, de Jong J, Fransen van de Putte EE, et al. : ERBB2 Mutations Characterize a Subgroup of Muscle-invasive Bladder Cancers with Excellent Response to Neoadjuvant Chemotherapy. Eur Urol. 2016;69(3):384–8. 10.1016/j.eururo.2015.01.014 [DOI] [PubMed] [Google Scholar]
- 25. van Allen EM, Mouw KW, Kim P, et al. : Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov. 2014;4(10):1140–53. 10.1158/2159-8290.CD-14-0623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Plimack ER, Dunbrack RL, Brennan TA, et al. : Defects in DNA Repair Genes Predict Response to Neoadjuvant Cisplatin-based Chemotherapy in Muscle-invasive Bladder Cancer. Eur Urol. 2015;68(6):959–67. 10.1016/j.eururo.2015.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. De Santis M, Bellmunt J, Mead G, et al. : Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012;30(2):191–9. 10.1200/JCO.2011.37.3571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. von der Maase H, Sengelov L, Roberts JT, et al. : Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23(21):4602–8. 10.1200/JCO.2005.07.757 [DOI] [PubMed] [Google Scholar]
- 29. Bellmunt J, Théodore C, Demkov T, et al. : Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27(27):4454–61. 10.1200/JCO.2008.20.5534 [DOI] [PubMed] [Google Scholar]
- 30. Dong H, Strome SE, Salomao DR, et al. : Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. 10.1038/nm730 [DOI] [PubMed] [Google Scholar]
- 31. Iwai Y, Ishida M, Tanaka Y, et al. : Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99(19):12293–7. 10.1073/pnas.192461099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Powles T, Eder JP, Fine GD, et al. : MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–62. 10.1038/nature13904 [DOI] [PubMed] [Google Scholar]
- 33. Rosenberg JE, Hoffman-Censits J, Powles T, et al. : Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10030):1909–20. 10.1016/S0140-6736(16)00561-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Agarwal N, Bellmunt J, Maughan BL, et al. : Six-month progression-free survival as the primary endpoint to evaluate the activity of new agents as second-line therapy for advanced urothelial carcinoma. Clin Genitourin Cancer. 2014;12(2):130–7. 10.1016/j.clgc.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rizvi NA, Hellmann MD, Snyder A, et al. : Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–8. 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yadav M, Jhunjhunwala S, Phung QT, et al. : Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515(7528):572–6. 10.1038/nature14001 [DOI] [PubMed] [Google Scholar]
- 37. Iyer G, Al-Ahmadie H, Schultz N, et al. : Prevalence and co-occurrence of actionable genomic alterations in high-grade bladder cancer. J Clin Oncol. 2013;31(25):3133–40. 10.1200/JCO.2012.46.5740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Plimack ER, Geynisman DM: Targeted Therapy for Metastatic Urothelial Cancer: A Work in Progress. J Clin Oncol. 2016;34(18):2088–92. 10.1200/JCO.2016.67.1420 [DOI] [PubMed] [Google Scholar]
- 39. Hoadley KA, Yau C, Wolf DM, et al. : Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell. 2014;158(4):929–44. 10.1016/j.cell.2014.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Iyer G, Hanrahan AJ, Milowsky MI, et al. : Genome sequencing identifies a basis for everolimus sensitivity. Science. 2012;338(6104):221. 10.1126/science.1226344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Milowsky MI, Iyer G, Regazzi AM, et al. : Phase II study of everolimus in metastatic urothelial cancer. BJU Int. 2013;112(4):462–70. 10.1111/j.1464-410X.2012.11720.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Choudhury NJ, Campanile A, Antic T, et al. : Afatinib Activity in Platinum-Refractory Metastatic Urothelial Carcinoma in Patients With ERBB Alterations. J Clin Oncol. 2016;34(18):2165–71. 10.1200/JCO.2015.66.3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Clark PE, Spiess PE, Agarwal N, et al. : NCCN Guidelines Insights: Bladder Cancer, Version 2.2016. J Natl Compr Canc Netw. 2016;14(10):1213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dinney CP, McConkey DJ, Millikan RE, et al. : Focus on bladder cancer. Cancer Cell. 2004;6(2):111–6. 10.1016/j.ccr.2004.08.002 [DOI] [PubMed] [Google Scholar]
- 45. McConkey D, Choi W, Dinney C: Reply to Mattias Aine, Fredrik Liedberg, Gottfrid Sjödahl, and Mattias Höglund's Letter to the Editor re: David J. McConkey, Woonyoung Choi, Colin P.N. Dinney. New insights into subtypes of invasive bladder cancer: considerations of the clinician. Eur Urol 2014;66:609–10. Eur Urol. 2015;67(4):e76–8. 10.1016/j.eururo.2014.08.064 [DOI] [PubMed] [Google Scholar]
- 46. Hedegaard J, Lamy P, Nordentoft I, et al. : Comprehensive Transcriptional Analysis of Early-Stage Urothelial Carcinoma. Cancer Cell. 2016;30(1):27–42. 10.1016/j.ccell.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 47. Shin K, Lim A, Zhao C, et al. : Hedgehog signaling restrains bladder cancer progression by eliciting stromal production of urothelial differentiation factors. Cancer Cell. 2014;26(4):521–33. 10.1016/j.ccell.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]