Abstract
MicroRNAs (miRNAs) are a class of evolutionarily conserved, 18–25 nucleotide non-coding sequences that post-transcriptionally regulate gene expression. Recent studies implicated their roles in the regulation of neuronal functions, such as learning, cognition and memory formation. Here we report that miR-218 inhibits heroin-induced behavioral plasticity. First, network propagation-based method was used to predict candidate miRNAs that played potential key roles in regulating drug addiction-related genes. Microarray screening was also carried out to identify miRNAs responding to chronic heroin administration in the nucleus accumbens (NAc). Among the collapsed miRNAs, top-ranked miR-218 was decreased after chronic exposure to heroin. Lentiviral overexpression of miR-218 in NAc could inhibit heroin-induced reinforcement in both conditioned place preference (CPP) test and heroin self-administration experiments. Luciferase activity assay indicated that miR-218 could regulate 3′ untranslated regions (3′ UTR) of multiple neuroplasticity-related genes and directly target methyl CpG binding protein 2 (Mecp2). Consistently, Mecp2308/y mice exhibited reduced heroin seeking behavior in CPP test. These data reveal a functional role of miR-218 and its target, MeCP2, in the regulation of heroin-induced behavioral plasticity.
Drug addiction is a psychiatric disorder characterized by loss of control over drug consumption and compulsive drug taking despite serious negative consequences1. Addictive drugs mediate their reinforcing properties by targeting the mesocorticolimbic dopaminergic (DA) circuitry, which contain the nucleus accumbens (NAc), the ventral tegmental area (VTA), prefrontal cortex (PFC) and hippocampus2. Drugs of abuse induce long-term adaptions in neuronal plasticity3, which is regulated by persistent alterations in gene expression. Extensive studies support that signaling cascades that regulate gene expression play fundamental roles in drug-induced neuroadaptions4,5,6,7. However, post-transcriptional regulation processes involved in drug addiction are largely unknown.
Recent studies indicate that epigenetic regulation of gene expression plays an important role in neurogenesis, synaptic plasticity and neurological disorders8,9,10. MicroRNAs (miRNAs) are a class of evolutionarily conserved, 18–25 nucleotide non-coding sequences that post-transcriptionally regulate gene expression. A miRNA may modulate the expression of hundreds of genes, either by translational suppression, or by degrading mRNAs that contains complementary sequences in the 3′ UTR11,12,13. Bioinformatic approaches indicate that miRNAs are likely to form miRNA-regulated gene networks, by preferentially targeting genes of certain pathways14. Recent studies indicate that several psychostimulants regulate miRNA expression in the NAc as well as other regions of mesocorticolimbic DA system, and manipulations of some specific miRNAs could alter the drug related behaviors and drug induced neuroplasticity15,16,17,18. But the role of miRNAs in heroin seeking behaviors, and the specific targets of key regulatory miRNAs remain unexplored.
In this study, we utilized bioinformatic approaches to predict potential key regulators in drug addiction. Among the top-ranked miRNAs, we found that miR-218 is down-regulated in response to chronic heroin administration. Lentiviral-mediated miR-218 overexpression significantly attenuated heroin-induced reinforcement in both conditioned place preference (CPP) and self-administration (SA) model. These effects were proposed to be mediated by suppression of target genes such as Mecp2, that participates in epigenetic control of gene transcription. Our observation provides a possible miRNA-mediated epigenetic regulatory mechanism in heroin addiction.
Results
Prediction of addiction-related miRNAs using network propagation-based strategy
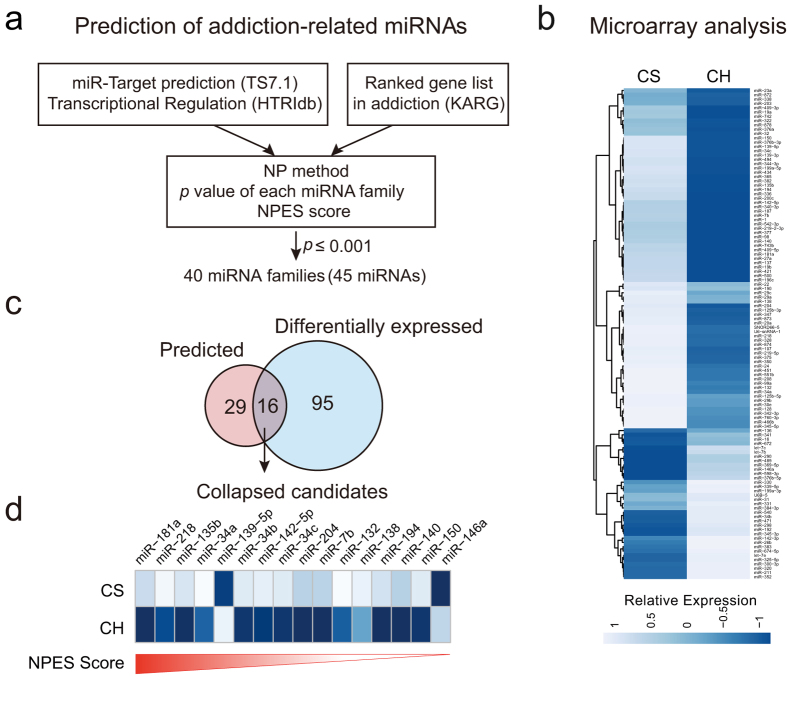
First, we tried to screen for miRNAs involved in drug addiction-related regulatory networks. A miRNA could regulate gene expression either by directly targeting drug addiction-related genes, or by targeting regulatory elements (e.g. transcription factors) whose impact may propagate across the whole regulatory network. Thus we utilized a network propagation-based model (NP-method)19 to predict miRNAs, whose target genes may contribute to major alterations in addiction-related pathways. We used miRNA-target regulation information (TargetScan 7.1) and the transcription regulatory database (HTRIdb) to model network effects of the miRNA perturbation, normalized reliability score from Knowledgebase for Addiction-Related Genes (KARG) to assess the potential involvement of addiction-related genes. The correlation between network effects of the miRNA perturbation and gene ranking was evaluated (Fig. 1a), which revealed 40 addiction-related miRNA families significantly enriched in regulation of addiction-related process (Table S1), among which, miR-132/212, miR-9, miR-181 etc. were top-ranked and previously reported17,20,21,22, indicating that NP-method is efficient in identifying miRNAs involved in addiction-related process.
Figure 1. Screen for miRNAs regulated by chronic heroin administration.
(a) Identification of addiction-related miRNA candidates. Network propagation based method (NP method) was used to predict potentially perturbed miRNAs in addiction-related process. Based on significance of NPES score, 40 miRNA families (45 miRNAs) were identified as putative candidates. (b) Heatmap of differentially expressed miRNAs in microarray. Rats were treated with saline or heroin (1 mg/kg, i.p., b.i.d.) for 7 days. MiRNAs that exhibit ≥25% alterations were shown. (c) NP method-based prediction and altered miRNAs in response to chronic heroin exposure was collapsed, resulting in 16 intersected miRNAs. (d) Heatmap of the 16 intersected miRNAs arranged by prediction scores (CS, Chronic Saline; CH, Chronic Heroin).
MiR-218 is down-regulated in response to chronic heroin administration
Microarray screening was carried out to identify miRNAs responding to chronic heroin administration. As shown in Fig. 1b, chronic treatment of heroin (CH, 1 mg/kg heroin, twice daily intraperitoneal injections for 7 days) resulted in differential expression (≥25%) of 111 miRNAs in the NAc, as compared with chronic saline group (CS, equivalent volume of saline, twice daily intraperitoneal injections for 7 days). The results obtained using NP-method and microarray screening were collapsed, resulting in 16 miRNAs from 14 families (Fig. 1d), which are likely important regulators of heroin-induced reinforcement.
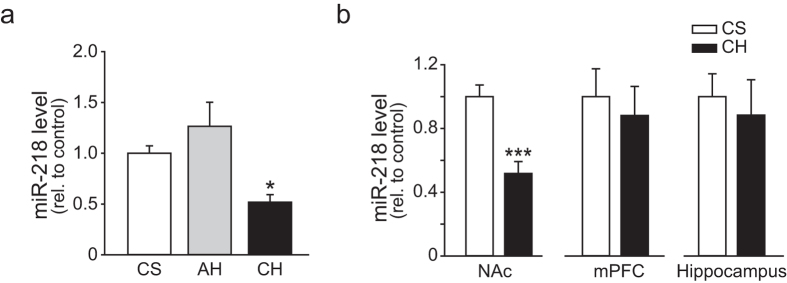
Among these 16 candidate miRNAs, miR-181a, a miRNA previously implicated in cocaine-induced plasticity17, was top-ranked. MiR-218, whose role in drug addiction has not been reported, was second highest scored, and microarray analysis indicated that miR-218 was down regulated in response to chronic heroin exposure (Fig. 1d). Quantitative real-time PCR also confirmed that chronic, but not acute heroin administration (AH, saline twice daily intraperitoneal injections for 7 days, then one injection of heroin, 1 mg/kg), significantly reduced the expression level of miR-218 in NAc (Fig. 2a). Moreover, the decrease of miR-218 level induced by chronic heroin exposure was restricted to NAc, since no significant change of miR-218 level was observed in mPFC or hippocampus (Fig. 2b).
Figure 2. Chronic heroin administration downregulates miR-218 expression level in NAc.
(a) Relative miR-218 level in NAc after saline, acute heroin or chronic heroin administration. miR-218 was significantly downregulated by chronic heroin exposure (CS, Chronic Saline, n = 10; AH, Acute Heroin, n = 6; CH, Chronic Heroin, n = 6; One-way ANOVA, *P < 0.05 vs. Saline group). (b) Relative miR-218 level in NAc, mPFC and hippocampus after saline or chronic heroin administration. MiR-218 level was decreased in NAc, but not in mPFC or hippocampus after chronic heroin administration (CS, Chronic Saline, n = 10; CH, Chronic Heroin, n = 6, Student’s t-test, ***P < 0.001). Data are expressed as mean ± s.e.m.
MiR-218 regulates heroin reinforcement
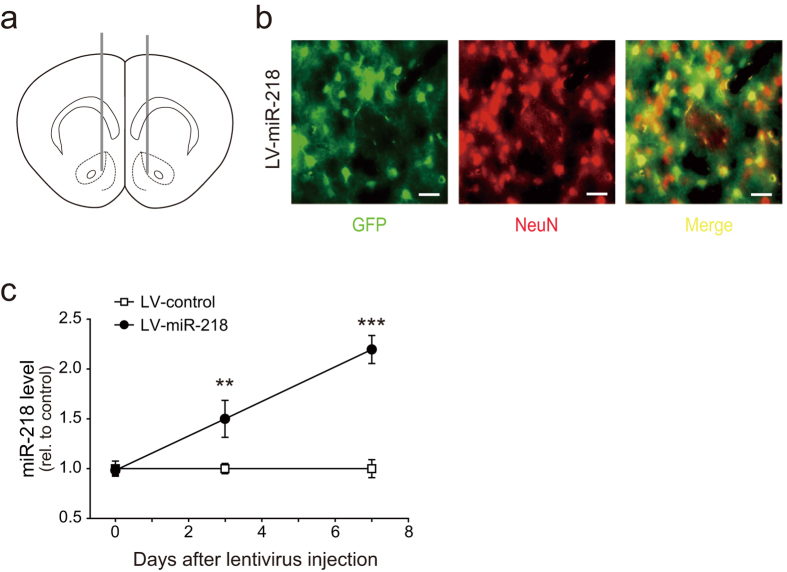
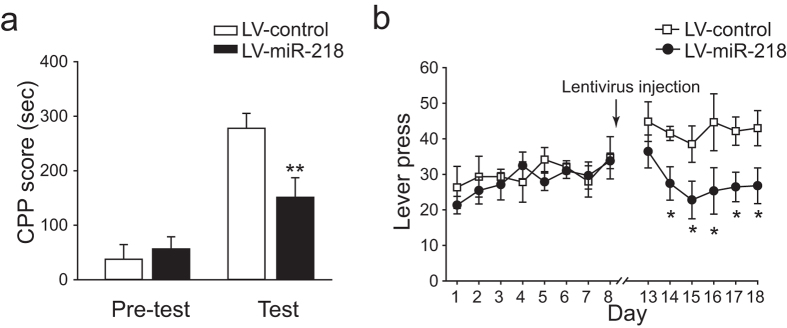
To explore the role of miR-218 in heroin-induced behavioral plasticity, we delivered recombinant lentivirus that express miR-218 (LV-miR-218) or scramble control sequence (LV-control) into the NAc region (Fig. 3a). The expression of virus-derived reporter GFP and its co-localization with neuronal marker NeuN were observed (Fig. 3b). Quantitative PCR analysis revealed that significant increase of miR-218 level could be detected 3 days after lentivirus injection and a 2-fold increase was observed 7 days after LV-miR-218 injection in NAc (Fig. 3c). To determine if miR-218 played a role in heroin-induced reinforcement, the effect of miR-218 overexpression in NAc was examined on CPP. One week after NAc infusion of LV-miR-218 or LV-control, rats were given repeated heroin injections paired with a contextually-distinct chamber in CPP apparatus, and their performance in the subsequent CPP test was scored. We found a significant effect of treatment ×test phase interaction (F(1,17) = 11.80, p = 0.003). Moreover, Bonferroni’s post hoc analysis revealed that rats with miR-218 overexpression in the NAc exhibited reduced preference to the heroin-paired chamber, compared with LV-control injected rats (Fig. 4a).
Figure 3. Lentiviral-mediated overexpression of miR-218 in NAc.
(a) Schematic representation of lentivirus injection sites. (b) Cell localization of LV-miR-218 infected cells (green), neurons using NeuN antibody (red), scale bar = 20 μm. (c) Relative miR-218 level in NAc with LV-control or LV-miR-218 injection on day 0, 3, 7. Enhanced expression level of miR-218 after LV-miR-218 injection in NAc in day 3 and day 7 (LV-control, n = 5, LV-miR-218, n = 5 on each day, **P < 0.01; ***P < 0.001). Data are shown as mean ± s.e.m.
Figure 4. MiR-218 inhibits heroin seeking behavior.
(a) CPP score of LV-control and LV-miR-218 group in pre-test and test session. Rats were subjected to LV-control or LV-miR-218 injection. Seven days later, they were subjected to pre-test, three conditioning sessions and CPP test. Heroin induced CPP is decreased in LV-miR-218 group (LV-control, n = 10, LV-miR-218, n = 9, **P < 0.01 vs. LV control during test session). (b) Lever presses of LV-control and LV-miR-218 rats in heroin self-administration. Rats were subjected to heroin self-administration procedure for eight days and then randomly grouped to receive NAc LV-control or LV-miR-218 injection. Three days later, daily SA sessions were resumed. LV-miR-218 group showed decreased heroin consumption after lentivirus injection (LV-control, n = 6, LV-miR-218, n = 9, Two-way RM ANOVA. *P < 0.05 vs. LV-control on the same day). Data are shown as mean ± s.e.m.
Next, we carried out heroin self-administration tests to further validate the role of miR-218 in heroin seeking behavior. Rats were trained to self-administer heroin for 8 consecutive days, then subjected to LV-miR-218 or LV-control injection. Consistent with CPP results, miR-218 overexpression significantly reduced heroin acquisition compared with the control group (Fig. 4b).
MiR-218 regulates drug addiction network and directly targets MeCP2
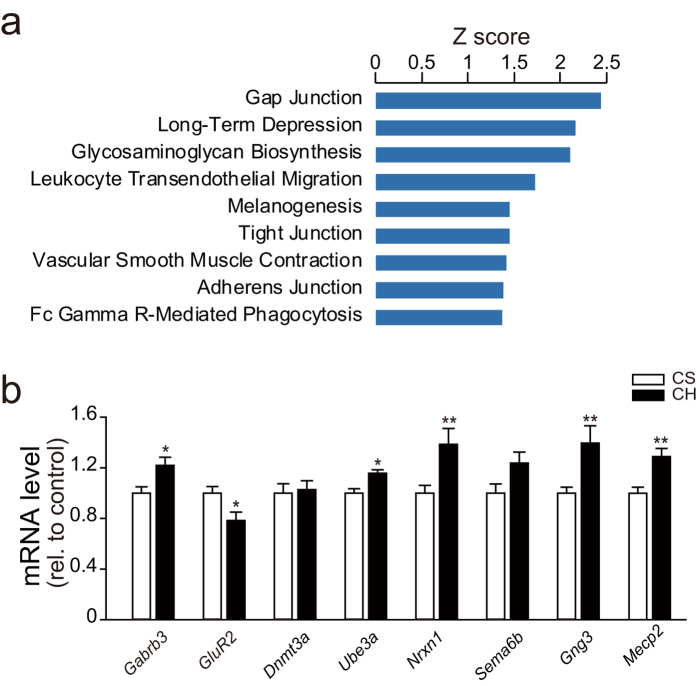
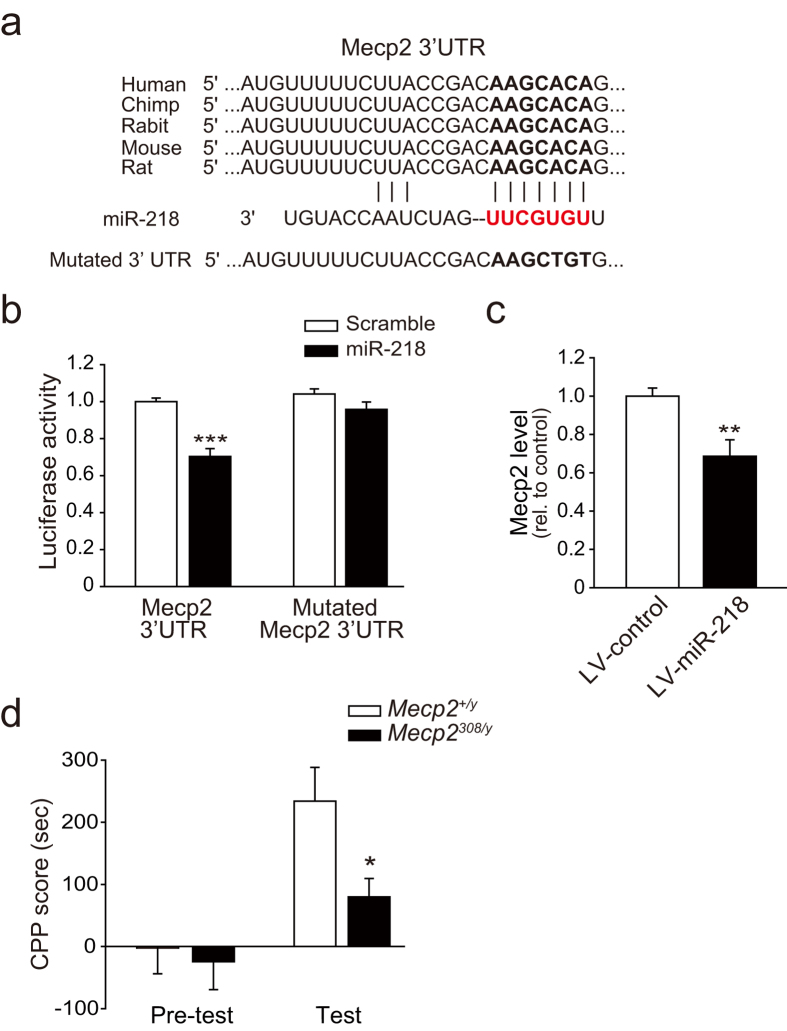
To infer biological roles and network communities of miR-218, miRSystem database23 was used for characterizing enriched functions and pathways of miR-218 targets. MiR-218 targets, co-predicted by at least two canonical databases (PicTar, TargetScan, DIANA, miRanda), were enriched in KEGG pathways highly relevant to neuronal functions, such as gap junction and long-term depression (Fig. 5a), suggesting its potential involvement in regulation of neuronal plasticity. We selected a few predicted targets of miR-218, which are indicated by both KARG database and recent literature to contribute to drug-induced neuroplasticity, and tested the validity of the miRNA-gene regulatory relationship. Indeed, Gabrb3, and Mecp2, which play critical roles in drug induced synaptic plasticity22,24,25,26, Nrxn1, Gng3, and Ube3a, which are involved in axon guidance and dendritic morphogenesis27,28,29, were increased in NAc after chronic heroin administration (Fig. 5b). Using luciferase reporter system, we first cloned each predicted target site into the 3′ UTR of Renilla luciferase and co-transfected the constructs with miR-218 mimic or scramble control in HEK293T cells. Reduced luciferase activity indicated that these genes were the directly regulated by miR-218 (Fig. S1). Among the targets identified, we focused on MeCP2 for its crucial role in psychostimulus-induced plasticity22 and broad transcription regulatory activity30. As shown in Fig. 6a, the predicted miR-218 target sequences of Mecp2 were highly conserved among mammals. Target sequence of Mecp2 3′ UTR and a seed region-mutated sequence were subjected to luciferase assay. We found that the expression of luciferase with mutant Mecp2 3′ UTR was not affected by miR-218 mimic transfection, in contrast to significantly attenuated activity of luciferase with wild type Mecp2 3′ UTR (Fig. 6b). Furthermore, overexpression of miR-218 in C6 cells downregulated Mecp2 expression level (Fig. 6c), which further confirmed that Mecp2 is a target of miR-218. Coincident with miR-218 overexpression result, heroin CPP was significantly blocked in Mecp2308/y mice (Fig. 6d). These data support the notion that miR-218 directly targets Mecp2 in a sequence-specific manner and thus inhibits heroin seeking behavior.
Figure 5. MiR-218 regulates synaptic plasticity-related genes.
(a) MirSystem analysis of miR-218 target genes revealed significant enrichment in neuronal plasticity-related pathways. (c) Relative expression level of Gabrb3, GluR2, Dnmt3a, Ube3a, Nrxn1, Sema6b, Gng3 and Mecp2 in NAc of rats treated with saline or chronic heroin (1 mg/kg, i.p., b.i.d, CS, Chronic Saline, n = 10; CH, Chronic Heroin, n = 6; Student’s t-test *P < 0.05, **P < 0.01). Data are shown as mean ± s.e.m.
Figure 6. MiR-218 inhibits heroin-induced CPP by directly targeting MeCP2.
(a) Schematic representation of miR-218 sequence and its target sequences within the 3′ UTR of Mecp2. The seed sequence is indicated by bold letters, while the pairing sequence of miR-218 is in red bold letters. (b) Relative luciferase activity with the Mecp2 3′ UTR or mutated 3′ UTR was determined in 293 T cells co-transfected with scramble or miR-218 mimic. (n = 6 for each group, ***P < 0.001 vs. scramble with Mecp2 3′ UTR). (c) Relative Mecp2 level with LV-control or LV-miR-218 infection in C6 cell (n = 6 for each group, **P < 0.01 vs LV-control). (d) CPP score of Mecp2+/y and Mecp2308/y mice in pre-test and test sessions (Mecp2+/y, n = 9, Mecp2308/y, n = 7, *P < 0.05 vs. Mecp2+/y mice during test session). Data are shown as mean ± s.e.m.
Discussion
MiRNA functions are executed by the multiprotein RNA-induced silencing complex containing Argonaute (Ago)31. The expression level of Ago2 is decreased in the VTA of morphine-dependent rats32. Ablation of Ago2 in dopamine 2 receptor-expressing neurons attenuates the reinforcing effects of cocaine and cocaine induced CPP, implicating miRNA may contribute to addictive behaviors16. Hollander et al. reported cocaine SA increase miR-212 in striatum and increasing miR-212 in this region decrease cocaine reward18. Chronic cocaine exposure also regulates miR-124 and miR-181a expression in brain, and overexpression of miR-124 in NAc reduced cocaine CPP while miR-181a has the opposite effect33. He et al. reported let-7 targets the 3′ UTR of MOR, and chronic morphine treatment increased let-7 expression in brain, while let-7 inhibition causes increased levels of MOR and partially attenuated opioid antinociceptive tolerance34. MiR-382 modulated the expression of ΔFosB which had been linked directly to several addiction-related behaviors, and overexpression of miR-382 in NAc decreased voluntary intake and preference for alcohol in rats35. Our research found that miR-218 in NAc decreased by heroin administration and overexpression of miR-218 inhibited heroin seeking behavior, providing a direct evidence of miR-218 involved in heroin induced behavioral plasticity.
In the present study, we found that miR-218 was downregulated by chronic heroin use in NAc. Consistently, bioinformatic analysis indicated the predicted targets of miR-218 was enriched in addiction-related genes and involved in neuroplasticity. MiR-218 is encoded by an intron of the Slit gene and inhibits the expression of Robo1 which play important roles in axonal growth36,37. Recent studies indicated that miR-218 plays a crucial role in motor neuron differentiation and loss of miR-218 cause systemic neuromuscular failure38,39. Recent large-scale miRNA expression profiling showed that miR-218 exhibits ubiquitous enrichment in hippocampus, cortex and cerebellum, but exhibits lower expression in olfactory bulb and brain stem40. Hippocampal cell-type-based analysis of miRNAs revealed that miR-218 is specifically enriched in neurons, compared with microglia, astrocytes and oligodendrocytes41. And more specifically, it is enriched in parvalbumin (PV)-positive, but not neuropeptide somatostatin expressing GABAergic interneurons. And its expression in CaMKIIα positive neurons exhibits three-fold higher expression than Gad2-expressing neurons42. Together with the predicted pathway of its target genes, we postulate that miR-218 is essential to the regulation of neuronal plasticity.
MeCP2 is a transcription factor binding to methylated cytosine residues in DNA and recruits histone deacetylases and other transcriptional repressors to silence target genes43. Previous studies reported a critical role of Mecp2 in normal neurological function. Hippocampal glutamatergic neurons that lack MeCP2 display a reduction in synaptic response whereas neurons with doubling of Mecp2 exhibit a two-fold enhancement in synapse number44. Mutations in MeCP2 results in nonsyndromic mental retardation, mild learning disability, and classic autism45,46,47. Recent studies reveal the role of MeCP2 in psychostimulant induced behavioral plasticity. Extended cocaine access increased Mecp2 expression in dorsal striatum and striatal Mecp2 knockdown decrease cocaine intake22. Coincidently, we found reduced heroin conditioned preference in Mecp2308/y mice. However, Deng et al. found that Mecp2308/y mutant mice showed enhanced locomotor response to acute amphetamine administration and impaired CPP, but lentivirus mediated knockdown or overexpression of MeCP2 in NAc enhanced or inhibited amphetamine-induced locomotion and CPP respectively48. Later, the same group found Mecp2 Ser421Ala knock-in mice display both a reduced threshold for amphetamine sensitization and enhanced behavioral sensitivity to the reinforcing properties in cocaine self-administration, indicating that MeCP2 in NAc limited behavioral plasticity upon repeated psychostimulant exposure49. Functionally, early studies found that MeCP2 interacts specifically with methylated cytosine in the CG context (mCG). However, recent evidence suggest that MeCP2 regulates expression of genes in a spatial- and temporal- distinct manner and binds to non-CG methylation30,50,51. These result implicated that the role of MeCP2 played in drug addiction seems much more complicated, and more detail understanding the molecular mechanism of MeCP2 in drug induced plasticity still need to be experimental verified.
Taken together, our results reveal that miR-218 is involved in heroin induced behavioral plasticity possibly by regulating Mecp2. Overexpression of miR-218 in NAc decreased heroin conditioned preference and heroin self-administration. MiR-218 is likely to contribute to heroin related modifications, both transcriptionally and epigenetically. Our results provide a promising and novel approach to the treatment of heroin addiction. However, the role of miR-218 in other drug induced plasticity and the downstream signaling pathways regulated by miR-218 in addiction are remained to be elucidated.
Methods
Subjects
Male Sprague-Dawley rats were purchased from the Shanghai SLAC Laboratory Animal Co. Ltd. and housed with a 12 h reverse dark/light cycle at 23 °C. All subjects were allowed free access to food and water. Rats used for experiments were 8–10 weeks old. Mecp2308/y mice and littermates were maintained by mating heterozygotes with C57BL/6 J. All animal treatments were in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by Animal Care and Use Committee of Shanghai Medical College of Fudan University.
Lentivirus Construction and Packaging
The lentivirus construct and packaging were performed as we described previously6. Briefly, human U6 promoter and small hairpin RNA that overexpress miR-218 (LV-miR-218, TTGTGCTTGATCTAACCATGT) or control sequences (LV-control, GCAGTTATCACGTCTATGTTT) were cloned into FG12 plasmid and co-transfected into HEK293T cells with pMDLg/RRE, pRSV/rev and pHCMV-G for lentivirus packaging. Forty-eight hours after transfection, the culture medium was collected and concentrated by ultracentrifugation, titrated, aliquoted, and stored at −80 °C, the final virus titer was estimated to be 1 × 108 IU/ml.
Intravenous heroin self-administration
To facilitate subsequent heroin self-administration, rats were trained to press an active lever for food pellets until they self-administered 100 pellets for 3 consecutive days on fixed-ratio 1 program. Then the rats were anesthetized, and a silastic catheter (0.04 cm interior diameter) was inserted 3 cm into the jugular vein, and the other end of the catheter was attached to a stainless steel pedestal and mounted on the skull. Bilateral 26-gauge guide cannulae were implanted in NAc (1.7 mm A/P, ±1.6 mm M/L, and −5 mm D/V). After surgery, the rats were allowed to recover for 7 days and the catheters were flushed daily with saline supplemented with heparin (30 IU/ml) and gentamycin (5 mg/ml). Next the rats were allowed to self-administer heroin (0.05 mg/kg/injection, FR1 program) during a daily 4 h session. After the 8th session, the rats were anesthetized and subjected to lentivirus infusion with needle (Plastics One, Inc.) that extend 1.5 mm beyond the guide cannulae. The self-administration procedure was resumed 3 days after lentivirus injection and tested on FR1 schedule for six consecutive days.
Conditioned place preference
The apparatus was three-chambered (Med Associates), with two large side chambers with distinct floors and decorations and one small center chamber. In pretest, the rat was first confined in the center chamber for adaption for 5 min, and then allowed to freely explore the chambers for 15 min. Unbiased rats were subjected to lentiviral injection into NAc (1.7 mm A/P, ± 1.6 mm M/L, and −6.5 mm D/V). The rats were allowed to recover for 7 days. Then the rats were alternatively confined in each large chamber for 30 min after injection of heroin (1 mg/kg, i.p., days 1, 3, 5) or saline (days 2, 4, 6). On the test session (day 8), the rats were adapted in center chamber for 5 min and allowed to explore all chambers unrestrictedly for 15 min. The time which the rat spent in each large chamber was recorded and CPP score was defined as the time spent in the heroin-paired chamber minus the time spent in the saline-paired chamber during the CPP test. The CPP pretest, conditioning and test procedure of Mecp2308/y and Mecp2+/y mice followed the same procedure.
MiRNA microarray and data analysis
Total RNA are harvested using TRIzol (Invitrogen) and RNeasy mini kit (QIAGEN) according to manufacturer’s instructions. The RNA samples are labeled using the miRCURY™ Hy3™/Hy5™ Power labeling kit and hybridized on the miRCURY™ LNA Array (v.11.0). Scanning was performed with the Axon GenePix 4000B microarray scanner. GenePix pro V6.0 is used to read the raw intensity of the image. Median normalized signal of each miRNA was used to represent expression level. MiRNAs that exhibit 1.25-fold up-regulation or 0.75-fold down-regulation was chosen for further analysis.
Network propagation based model was generated as described19. Briefly, miRNA families that are highly conserved (conservation score ≥2) were chosen from TargetScan Human 7.1, and their predicted targets were used. Curated human transcriptional regulation interactions database (HTRIdb) was provided by the software. Genes from the KARG database was used, and their corresponding Reliability score was log transformed and used as candidate list for correlation (i.e. NPES) calculation with the algorism. Leading edge genes of each miRNA was extracted, with NPES score at or before the point of maximum NPES score from the ranked list. Pathway analysis was performed with miRSystem database with default settings. Microarray data have been deposited into the Gene Expression Ominibus with accession number GSE89202.
Quantitative real-time PCR
TaqMan small RNA assay (Applied Biosystems) for miRNAs of interest were performed. U6 was used for normalization. Primers used in RT-PCR were Mecp2 [CCTATGTATGATGACCCCACCT (forward), GAAAGGCATCTTGACGAGAAGT (reverse)], Gabrb3 [ATGGAACAGTGCTGTACGGG (forward), ACCTGTGGCGAAGACAACAT (reverse)], GluR2 [CCTTTATGCGGCAAGGATGC (forward), AGGGCTCTGCACTCCTCATA (reverse)], Dnmt3a [TGGTGTGTGTCGAGAAGCTC (forward), TTCGTAGATGGCTTTGCGGT (reverse)], Ube3a [ACCCTGATGTCACCGAATGG (forward), TCATTCGTGCAGGCCTCATT (reverse)], Nrxn1 [GCATCATCACAGAACGACGC (forward), GGATCCGCGATGATGTTCCT (reverse)], Sema6b [ATGGGATGCTCTTCACAGCC (forward), ATTCTTGCATACACGGGCCA (reverse)], Gng3 [CAACGCCTGTGTTAGCGTTC (forward), GCTTTCATTGCACGCTCGTT (reverse)], β-actin [CAACCTTCTTGCAGCTCCTCCGT (forward), AGGGTCAGGATGCCTCTCTTGCTC (reverse)]. All samples were run in duplicate. The relative expression level of mRNA was calculated by 2−ΔΔCt method.
Luciferase assay
Predicted target sequences of miR-218 were cloned to the 3′ UTR of Renilla luciferase of psiCHECK-2 vector. The target sequences were, Gabrb3 [CCTTTATTTCTGTACTAACTTATCTCATAAGCACACCCAATTCCTCCTAG], GluR2 [TATTGTTAGTCTCTTGATTCATAATGACTTAAGCACACTTGACATCAACT], Dnmt3a [TTGGTTGTCTCTAGCCTGATCAGATAGGAGCACAAACAGGAACAGAATAG], Ube3a [TCTTTGTAGCTGGACAGCACAATGTTTATGATTTATTTAATCTGTAGTTT], Nrxn1 [AAACTTATTTACTTTCCTTTTTATGAAGCACATACAAAAGAAGACAGGGA], Sema6b [CGGGTGGGGATCTCCTCGCCACAGGGAAGCACAAGAGCCCCCTCCATCCC], Gng3 [CGCACTTATCCTGAGATTATCTGAAGCACAAGGCCCTCCTTACCCACCTC]. Mecp2 [TTGGGATGTTTTTCTTACCGACAAGCACAGTCAGGTTGAAGACCTAACCA]. We transfected 500 ng recombinant vector with 10 pmol miRNA mimic or scramble (miR-218, TTGTGCTTGATCTAACCATGT, Scramble, GCAGTTATCACGTCTATGTTT) into HEK293T cells with Lipofectamine 2000 (Invitrogen). After 24 h, dual-luciferase report assay system (Promega) was performed to measure Renilla and Firefly luciferase activity, then Renilla activity was normalized to the Firefly luciferase activity. All the experiments were repeated for at least three times.
Immunofluorescence staining
The rats were anesthetized and transcardially perfused with saline, followed by 4% paraformaldehyde in PBS. Then the brains were quickly removed and post fixed with 4% paraformaldehyde at 4 °C for about 4 h and stored in 30% PBS-buffered sucrose solution for 72 h. The brains were sectioned (40 μm) with a cryostat (Leica) and the sections were washed in PBS, blocked with blocking buffer (10% donkey serum in PBS containing 0.3% Triton X-100) for 1 h, then incubated with NeuN antibody (1:500, rabbit polyclonal, Upstate Biotechnology) at 4 °C overnight. Sections were subsequently rinsed with PBS and incubated for 1 h at room temperature with CY3 goat anti-rabbit antibody (1:1000, Vector Laboratories). The images were captured with a microscopic CDD camera (Olympus).
Statistical analysis
Data are presented as mean ± s.e.m. For quantitative PCR results, data were analyzed by student’s t-test or one-way analysis of variance (ANOVA). For all the behavior results, data were analyzed by two-way repeated measures ANOVA followed by Bonferroni’s post hoc test. Luciferase activity data were analyzed by student’s t-test or two-way ANOVA followed by Bonferroni’s post hoc test. P < 0.05 are defined as statistically significant.
Additional Information
How to cite this article: Yan, B. et al. MiR-218 targets MeCP2 and inhibits heroin seeking behavior. Sci. Rep. 7, 40413; doi: 10.1038/srep40413 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We thank Dr. Zilong Qiu for providing Mecp2308/y mice (Mecp2tm1Hzo/J strain). This work was supported by the National Natural Science Foundation of China (31430033, 91232307, 31421091, and 81272295) and the Ministry of Science and Technology (2015CB553500, 2014CB942801, 2013CB835102).
Footnotes
Author Contributions B.Y., Z.H. and L.M. conceived and designed this study. B.Y., Z.H., W.Y., Q.L. and B.X. performed the experiments. B.Y., W.Y. and Q.L. conducted statistical analysis. B.Y., Q.L., Z.H., X.L. and L.M. wrote the manuscript.
References
- Nestler E. J. Molecular mechanisms of drug addiction. Neuropharmacology 47, 24–32, doi: 10.1016/j.neuropharm.2004.06.031 (2004). [DOI] [PubMed] [Google Scholar]
- Lüscher C. & Malenka Robert C. Drug-Evoked Synaptic Plasticity in Addiction: From Molecular Changes to Circuit Remodeling. Neuron 69, 650–663, doi: 10.1016/j.neuron.2011.01.017 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuown S. C. & Wood M. A. Epigenetic Regulation in Substance Use Disorders. Current Psychiatry Reports 12, 145–153, doi: 10.1007/s11920-010-0099-5 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison A. J. & Nestler E. J. Transcriptional and epigenetic mechanisms of addiction. Nature Reviews Neuroscience 12, 623–637, doi: 10.1038/nrn3111 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M. et al. Histone Deacetylase 5 Limits Cocaine Reward through cAMP-Induced Nuclear Import. Neuron 73, 108–120, doi: 10.1016/j.neuron.2011.10.032 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. et al. Chronic Cocaine-Induced H3 Acetylation and Transcriptional Activation of CaMKII alpha in the Nucleus Accumbens Is Critical for Motivation for Drug Reinforcement. Neuropsychopharmacology 35, 913–928, doi: 10.1038/Npp.2009.193 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E. et al. Plasticity-associated gene Krox24/Zif268 is required for long-lasting behavioral effects of cocaine. Journal of Neuroscience 26, 4956–4960, doi: 10.1523/Jneurosci.4601-05.2006 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson E. et al. Environmentally induced epigenetic transgenerational inheritance of ovarian disease. PLoS One 7, e36129, doi: 10.1371/journal.pone.0036129 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert S. et al. The schizophrenia risk gene product miR-137 alters presynaptic plasticity. Nat Neurosci 18, 1008–1016, doi: 10.1038/nn.4023 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J. et al. miR-17-92 Cluster Regulates Adult Hippocampal Neurogenesis, Anxiety, and Depression. Cell Rep, doi: 10.1016/j.celrep.2016.06.101 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D. P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297, doi: S0092867404000455 [pii] (2004). [DOI] [PubMed] [Google Scholar]
- Guo H., Ingolia N. T., Weissman J. S. & Bartel D. P. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466, 835–840, doi: 10.1038/nature09267 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas S. & Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat Rev Genet 16, 421–433, doi: 10.1038/nrg3965 (2015). [DOI] [PubMed] [Google Scholar]
- Kawulok J. & Deorowicz S. CoMeta: classification of metagenomes using k-mers. PLoS One 10, e0121453, doi: 10.1371/journal.pone.0121453 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper-Mains J. E. et al. microRNA-Seq reveals cocaine-regulated expression of striatal microRNAs. RNA 17, 1529–1543, doi: 10.1261/rna.2775511 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A. et al. Argonaute 2 in dopamine 2 receptor-expressing neurons regulates cocaine addiction. Journal of Experimental Medicine 207, 1843–1851, doi: 10.1084/jem.20100451 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekar V. & Dreyer J.-L. microRNAs miR-124, let-7d and miR-181a regulate Cocaine-induced Plasticity. Molecular and Cellular Neuroscience 42, 350–362, doi: 10.1016/j.mcn.2009.08.009 (2009). [DOI] [PubMed] [Google Scholar]
- Hollander J. A. et al. Striatal microRNA controls cocaine intake through CREB signalling. Nature 466, 197–U166, doi: 10.1038/Nature09202 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Gu J. & Li Y. Inferring the perturbed microRNA regulatory networks from gene expression data using a network propagation based method. BMC Bioinformatics 15, 255, doi: 10.1186/1471-2105-15-255 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most D., Workman E. & Harris R. A. Synaptic adaptations by alcohol and drugs of abuse: changes in microRNA expression and mRNA regulation. Front Mol Neurosci 7, 85, doi: 10.3389/fnmol.2014.00085 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzykowski A. Z. et al. Posttranscriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron 59, 274–287, doi: 10.1016/j.neuron.2008.05.032 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im H.-I., Hollander J. A., Bali P. & Kenny P. J. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nature Neuroscience 13, 1120–1127, doi: 10.1038/nn.2615 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T. P. et al. miRSystem: an integrated system for characterizing enriched functions and pathways of microRNA targets. PLoS One 7, e42390, doi: 10.1371/journal.pone.0042390 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C. J., Eisinger B. E., Driessen T. M. & Gammie S. C. Addiction and reward-related genes show altered expression in the postpartum nucleus accumbens. Front Behav Neurosci 8, doi: 10.3389/Fnbeh.2014.00388 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad K. L. et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454, 118–U119, doi: 10.1038/nature06995 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPlant Q. et al. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nature Neuroscience 13, 1137–1143, doi: 10.1038/nn.2619 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andermatt I. et al. Semaphorin 6B acts as a receptor in post-crossing commissural axon guidance. Development 141, 3709–3720, doi: 10.1242/dev.112185 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrosa E., Kaushik S. & Lachman H. M. ChIP-chip analysis of neurexins and other candidate genes for addiction and neuropsychiatric disorders. Journal of neurogenetics 24, 5–17, doi: 10.3109/01677060903305658 (2010). [DOI] [PubMed] [Google Scholar]
- Judson M. C. et al. GABAergic Neuron-Specific Loss of Ube3a Causes Angelman Syndrome-Like EEG Abnormalities and Enhances Seizure Susceptibility. Neuron 90, 56–69, doi: 10.1016/j.neuron.2016.02.040 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. et al. MeCP2 binds to non-CG methylated DNA as neurons mature, influencing transcription and the timing of onset for Rett syndrome. Proc Natl Acad Sci USA 112, 5509–5514, doi: 10.1073/pnas.1505909112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister G. et al. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell 15, 185–197, doi: 10.1016/j.molcel.2004.07.007 (2004). [DOI] [PubMed] [Google Scholar]
- Garcia-Perez D. et al. Morphine regulates Argonaute 2 and TH expression and activity but not miR-133b in midbrain dopaminergic neurons. Addict Biol 20, 104–119, doi: 10.1111/adb.12083 (2015). [DOI] [PubMed] [Google Scholar]
- Chandrasekar V. & Dreyer J. L. Regulation of MiR-124, Let-7d, and MiR-181a in the accumbens affects the expression, extinction, and reinstatement of cocaine-induced conditioned place preference. Neuropsychopharmacology 36, 1149–1164, doi: 10.1038/npp.2010.250 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Yang C., Kirkmire C. M. & Wang Z. J. Regulation of Opioid Tolerance by let-7 Family MicroRNA Targeting the mu Opioid Receptor. Journal of Neuroscience 30, 10251–10258, doi: 10.1523/Jneurosci.2419-10.2010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. et al. MicroRNA expression profile and functional analysis reveal that miR-382 is a critical novel gene of alcohol addiction. EMBO Mol Med 5, 1402–1414, doi: 10.1002/emmm.201201900 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpar A. et al. Endocannabinoids modulate cortical development by configuring Slit2/Robo1 signalling. Nat Commun 5, 4421, doi: 10.1038/ncomms5421 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small E. M., Sutherland L. B., Rajagopalan K. N., Wang S. & Olson E. N. MicroRNA-218 regulates vascular patterning by modulation of Slit-Robo signaling. Circulation research 107, 1336–1344, doi: 10.1161/CIRCRESAHA.110.227926 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin N. D. et al. Loss of motoneuron-specific microRNA-218 causes systemic neuromuscular failure. Science 350, 1525–1529, doi: 10.1126/science.aad2509 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebes K. P. et al. miR-218 is essential to establish motor neuron fate as a downstream effector of Isl1-Lhx3. Nat Commun 6, 7718, doi: 10.1038/ncomms8718 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichardo-Casas I. et al. Expression profiling of synaptic microRNAs from the adult rat brain identifies regional differences and seizure-induced dynamic modulation. Brain Res 1436, 20–33, doi: 10.1016/j.brainres.2011.12.001 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O. et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci 17, 131–143, doi: 10.1038/nn.3599 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M. et al. Cell-type-based analysis of microRNA profiles in the mouse brain. Neuron 73, 35–48, doi: 10.1016/j.neuron.2011.11.010 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. L. et al. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet 19, 187–191, doi: 10.1038/561 (1998). [DOI] [PubMed] [Google Scholar]
- Chao H. T., Zoghbi H. Y. & Rosenmund C. MeCP2 controls excitatory synaptic strength by regulating glutamatergic synapse number. Neuron 56, 58–65, doi: 10.1016/j.neuron.2007.08.018 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney R. M. et al. Identification of MeCP2 mutations in a series of females with autistic disorder. Pediatr Neurol 28, 205–211 (2003). [DOI] [PubMed] [Google Scholar]
- Couvert P. et al. MECP2 is highly mutated in X-linked mental retardation. Hum Mol Genet 10, 941–946 (2001). [DOI] [PubMed] [Google Scholar]
- Meloni I. et al. A mutation in the rett syndrome gene, MECP2, causes X-linked mental retardation and progressive spasticity in males. Am J Hum Genet 67, 982–985, doi: 10.1086/303078 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J. V. et al. MeCP2 in the nucleus accumbens contributes to neural and behavioral responses to psychostimulants. Nature Neuroscience 13, 1128–1136, doi: 10.1038/nn.2614 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J. V. et al. MeCP2 phosphorylation limits psychostimulant-induced behavioral and neuronal plasticity. J Neurosci 34, 4519–4527, doi: 10.1523/JNEUROSCI.2821-13.2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui D. H. et al. MeCP2 modulates gene expression pathways in astrocytes. Mol Autism 4, 3, doi: 10.1186/2040-2392-4-3 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. et al. Genome-wide activity-dependent MeCP2 phosphorylation regulates nervous system development and function. Neuron 72, 72–85, doi: 10.1016/j.neuron.2011.08.022 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.