Abstract
Background
Low-level laser therapy (LLLT) is commonly used in medical applications, but scientific studies of its efficacy and the mechanism by which it causes loss of fat from fat cells for body contouring are lacking. This study examined the effectiveness and mechanism by which 635– 680 nm LLLT acts as a non-invasive body contouring intervention method.
Methods
Forty healthy men and women ages 18–65 years with a BMI <30 kg/m2 were randomized 1:1 to laser or control treatment. Subject's waistlines were treated 30 min twice a week for 4 weeks. Standardized waist circumference measurements and photographs were taken before and after treatments 1, 3, and 8. Subjects were asked not to change their diet or exercise habits. In vitro assays were conducted to determine cell lysis, glycerol, and triglyceride release.
Results
Data were analyzed for those with body weight fluctuations within 1.5 kg during 4 weeks of the study. Each treatment gave a 0.4–0.5 cm loss in waist girth. Cumulative girth loss after 4 weeks was −2.15 cm (−0.78 ± 2.82 vs. 1.35 ± 2.64 cm for the control group, p < 0.05). A blinded evaluation of standardized pictures showed statistically significant cosmetic improvement after 4 weeks of laser treatment. In vitro studies suggested that laser treatment increases fat loss from adipocytes by release of triglycerides, without inducing lipolysis or cell lysis.
Conclusions
LLLT achieved safe and significant girth loss sustained over repeated treatments and cumulative over 4 weeks of eight treatments. The girth loss from the waist gave clinically and statistically significant cosmetic improvement.
Keywords: Cold laser, Fat reduction, Low-level laser, therapy, Non-invasive laser
Background
Laser-based devices are used in a broad array of medical and surgical applications and their biological effects have been documented for over 20 years. More recently low-level laser (LLL) devices have been used to facilitate tissue repair and healing processes. Although physiological methods responsible for augmented cell proliferation and pain relief are unknown, well-controlled clinical trials have demonstrated that low-level lasers provide therapeutic relief of pain. Low-level laser therapy is defined as management with a dose rate that causes no immediate demonstrable temperature rise of the treated tissue and no macroscopically visible change in tissue structure [1]. The dosage is a magnitude used to define the laser beam energy applied to a particular area of the body tissue measured in joules per square centimeter.
The Meridian LAPEX 2000 LipoLaser System is a semiconductor-based, low-level laser therapy device (LLLD). The LAPEX 2000 LipoLaser was originally developed and approved for the treatment of pain due to carpel tunnel syndrome. The LAPEX 2000 LipoLaser has been modified and is now being rigorously evaluated for its effectiveness in reducing areas of local fat accumulation for cosmetic purposes. The LAPEX 2000 LipoLaser emits light at 635– 680 nm. It is non-thermal and does not heat the tissues. As such, it is considered to be a non-invasive treatment.
Neira et al. [2] evaluated the effect of a 635–680 nm, 10-mW diode laser radiation with exclusive energy optics on treated fat cells in biopsy specimens. Fat cells were treated in vivo with 1.2–3.6 J/cm2 of energy from the laser for 2 to 6 min. The cells were then removed by lipectomy, examined by electron microscopy, and compared to cells removed by lipectomy that were not treated with the laser. Fat cells that were not exposed to the laser treatment looked like round grapes. Eighty percent of the fat was released from the fat cells after 4 min of laser light exposure and 99% was released after 6 min of exposure. After exposure to the laser light, pores in fat cells were visible by scanning electron microscope. It was presumed, but not demonstrated, that the fat was released from these pores, taken up in the lymphatics and reesterified in other tissues or metabolized for energy [2].
Several studies have recognized that LLL accelerates repair processes, stimulates cell proliferation, and promotes vascularization in injured tissues [3–8]. However, clinical application to body fat reduction as a minimally invasive option is an evolving field which is not well studied. We conducted a blinded clinical trial to describe the application of low-level laser therapy to local fat reduction for cosmetic purposes. As a secondary objective, we also investigated the mechanism by which the laser causes fat loss from fat cells. The mechanistic study investigated whether the fat loss induced by the laser is due to (1) the activation of the complement cascade lysing adipocytes, (2) adipocyte death, or (3) release of intact triglycerides from cells vs. the release of glycerol and fatty acids after lipolysis.
Methods
Clinical Trial
Forty healthy men and women between the ages of 18– 65 years, inclusive, and body mass index (BMI) no greater than 29.9 kg/m2 were randomized in a 1:1 ratio to an experimental laser treatment or to a control laser treatment. Randomization was created from random number tables and the treatment codes were stored in sealed envelopes during the study. Subjects could not be using light sensitizing agents, diuretics, or undergoing photodynamic therapy. Subjects were required to have a stable weight, gaining or losing no more than 2.5 kg in 6 months prior to the trial. Subjects could not be on a weight reduction regimen, and they were asked not to change their diet or exercise habits during the trial. This study was performed in accordance with the Declaration of Helsinki and approved by the Argus Institutional Review Board. Written informed consent was obtained from all participants prior to study participation.
The laser therapy device consisted of a console housing most electronics, the controls for the device, and two multiprobes that housed four lasers emitting visible laser light at a wavelength of 635–680 nm. Each subject had two treatments per week for a total of eight treatments over 4 weeks. Each treatment session lasted approximately 30 min. The two multiprobes were placed over the waist bilaterally in three positions as well as two enhancement probes that were placed to both sides of the inguinal region and the laser was activated for 10 min in each of these positions to encompass the waist from the back to the front. The control arm of the trial utilized the device, but the multiprobes of the device were inactivated during the treatment session.
Two individuals conducted the study. One administered the treatment, and the other, who was blinded to treatment allocation, obtained measurements and photographs. The individual administering the treatment remained blinded to photographic and girth measurements. Each subject was advised about the rules of blinding, and the individual taking photographs and measurements could not relay this information to the subject. The individual administering the treatment did not enter the room where the photographs and measurements were obtained. A case report form was used for each measurement session and these forms were placed in a sealed envelope until data was analyzed at the end of the study. Two separate people who were not involved in other aspects of the study did the blinded evaluations of the photographs.
All subjects had photographs taken at a standardized distance with a standard background and lighting. Girth measurements of the waist were obtained in the manner recommended by the United States National Institutes of Health (NIH) guidance at the iliac crest using a tape measure with standardized tension and oriented parallel to the floor [9]. A reference point on the body for the pictures and measurements was relocated at each evaluation by measuring a distance from the floor that was determined in the first measurement at baseline. The specified measured distance was used to ensure all measurements and photographs were obtained in the same location. The camera was placed on a tripod at a fixed distance from the floor but was adjusted to the specific height of each individual participant. Standardized waist measurements were taken at baseline, treatment 3, and treatment 8. Standardized photographs were taken before and after the initial treatment, treatment 3, and treatment 8. Weight was measured and BMI was calculated at baseline and at treatment 8 (week 4). Blood pressure was measured at baseline, treatment 3, and treatment 8. All adverse events were recorded in the case report forms.
Statistics
The waist circumference measurements were compared between the control and laser-treated group using a t test. The data were analyzed using completers and the more conservative intent to treat analysis. The blinded observers judged improvement on a 0–3 scale. Zero on this qualitative scale represented no improvement, 1 represented mild improvement, 2 represented moderate improvement, and 3 represented marked improvement. The results of the two observers were averaged and compared by t test.
In Vitro Studies Using Human Fat Cells
Experiment 1 Does the laser activate the complement cascade?
Human adipose-derived stem cells obtained from subcutaneous fat during abdominal surgery were plated and differentiated to form adipocytes as described by Bunnel et al. [10]. Human adipocytes were differentiated in 12-well plates. Three of the wells in the plates were left as a control. Fresh plasma replaced one third of the cell culture media in another three wells. The next three wells had one third of the media replaced with plasma that was heat-inactivated to destroy complement. The final three wells in each plate had one third of the media replaced by a combination of fresh human plasma and white blood cells. One experimental plate was irradiated with the LAPEX 2000 LipoLaser for 10 min and the other was left as a non-irradiated control. The cells were then evaluated for evidence of lysis under the microscope.
Experiment 2 Does the laser kill adipocytes?
To evaluate influence of LAPEX 2000 LipoLaser on adipose cell death and viability, we used LIVE/DEAD® Cell Viability Assays (Invitrogen). Human adipocytes were differentiated in 96-well plates. The experimental plate was irradiated with the LAPEX 2000 LipoLaser for 10 min and the other was left as a non-irradiated control. The cells were then probed with cell viability assay reagent using the manufacturer's protocol. Calcein and propidium iodide emissions were then analyzed using a fluorescent plate reader. Images were acquired on a Zeiss Axiovert 40 CFL using a 10× (Zeiss Achroplan objective) and a 20× (LD plan NeoFluor objective), and a Zeiss Axiocam HRc camera [11].
Experiment 3 Does the laser increase triglyceride release or lipolysis from adipocytes?
This experiment used human adipocytes in eight 6-well plates. Two wells in each plate were used as a control with media containing 10% fetal bovine serum (FBS). Two other wells had 25% of the media with 10% FBS replaced with human serum with 10% FBS. The last two wells had 25% of the media with 10% FBS replaced with heat-inactivated human serum with 10% FBS. Four of the plates were irradiated for 10 min with the laser and the other four plates served as a non-irradiated control. Media from the eight replicates of each of the three conditions in the laser irradiated plates and the non-irradiated control plates were used for glycerol and triglyceride determination.
Results
Clinical Trial
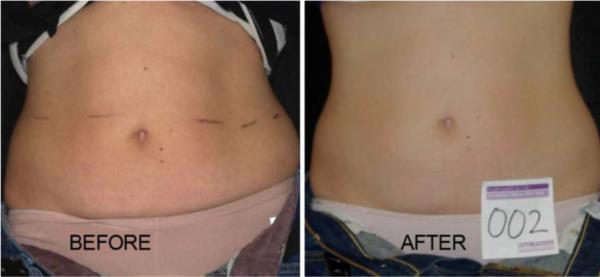
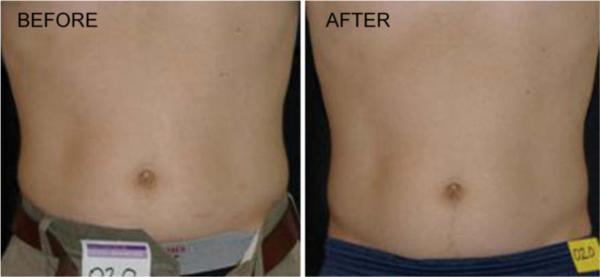
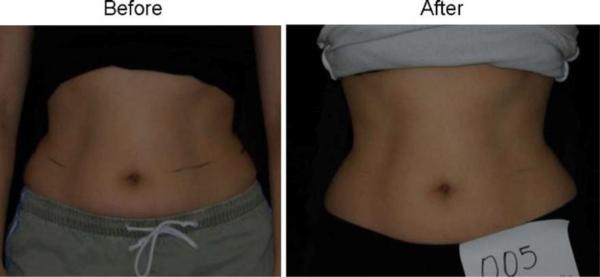
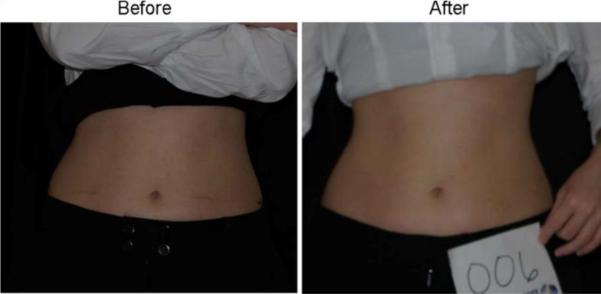
Forty subjects participated in the clinical trial. Twenty were treated with the LAPEX 2000 LipoLaser and 20 were treated with an inactive version of the device. One subject in the treatment group did not complete the study due to scheduling conflicts. There were no adverse events in either group during the trial. The groups were well balanced at baseline, and the group characteristics are illustrated in Table 1. Mean weight and BMI did not change significantly over the eight treatments and 4 weeks. Blood pressure did not change significantly from baseline to treatment 3, from treatment 3 to treatment 8, or from baseline to treatment 8. The mean placebo subtracted reductions in waist girth at treatments 1, 3, and 8 with the LAPEX 2000 LipoLaser were 0.49, 0.41, and 0.40 cm, respectively. This single treatment difference, 0.41 cm (laser −0.59 ± 0.71 cm vs. placebo −0.19 ± 0.47 cm) (mean±SD), was significant (p < 0.05) on the third treatment done during week 2 in the completers analysis, but was not statistically significant by the intent to treat analysis. The cumulative girth loss at treatment 3 on week 2 was a significant 1.74 cm (laser −1.89 ± 2.97 cm vs. placebo −0.16 ± 2.46 cm, p < 0.05) on both the completers' analysis and by intent to treat analysis. Cumulative girth loss at treatment 8 (4 weeks of treatment) was 2.15 cm with 15 subjects in the laser group and 16 subjects in the placebo group (laser −0.78 ± 2.82 cm vs. placebo 1.35 ± 2.64 cm) in those who maintained their weight within 1.5 kg of their baseline weight (p < 0.05). Cumulative girth loss at treatment 8 (4 weeks of treatment) was 1.33 cm with 19 subjects completing in the placebo group and 20 subjects completing in the laser group (laser −0.87 ± 2.65 cm vs. placebo 0.47 ± 3.19 cm) regardless of weight change (p = NS). The standardized pictures of the participants showed a significant 1.21 difference (laser 1.21 ± 0.42 vs. placebo 0 ± 0) in appearance on a 0–3 scale favoring the LAPEX 2000 LipoLaser group comparing baseline to week 4 (treatment 8) pictures (p < 0.001). When only those participants that remained within 1.5 kg of their baseline weight (N = 31) were considered, the improvement in appearance increased to 1.25 (laser 1.25 ± 0.45 vs. placebo 0 ± 0) on a 0–3 scale comparing baseline to week 4 (treatment 8) pictures (p < 0.001). The girth difference in the laser group compared to the placebo group is illustrated in Fig. 1. The differences in appearance from baseline to week 4 (treatment 8) in the whole group and the subjects who remained within 1.5 kg of their baseline weight are illustrated in Figs. 2, 3, and 4. Fig. 5 shows a placebo subject at baseline and 4 weeks (treatment 8) who remained within 1.5 kg of her baseline weight. Figs. 6 and 7 show a subject who lost 2.2 kg and 2.7 kg in the laser and placebo treatment groups, respectively.
Table 1.
Baseline demographic characteristics of study subjects in the LAPEX 2000 LipoLaser study
| Variable | Active | Placebo | P value |
|---|---|---|---|
| No. enrolled | 20 | 20 | |
| Gender | NS | ||
| Female | 19 | 15 | |
| Male | 1 | 5 | |
| Age (years) | 35.1 | 38.35 | NS |
| SD | 9.11 | 11.55 | |
| Weight (kg) | 63.97 | 67.31 | NS |
| SD | 8.23 | 14.31 | |
| Height (cm) | 164.12 | 165.68 | NS |
| SD | 5.99 | 9.32 | |
| Waist circumference (cm) | 83.5 | 84.1 | NS |
| SD | 6.1 | 10.7 | |
| Body mass index (kg/m2) | 23.77 | 24.35 | NS |
| SD | 2.02 | 2.87 | |
| Systolic blood pressure | 120.15 | 121.40 | NS |
| SD | 11.98 | 11.00 | |
| Diastolic blood pressure | 75.35 | 75.00 | NS |
| SD | 8.23 | 8.00 |
Values are means and standard deviation (SD).
Fig. 1.
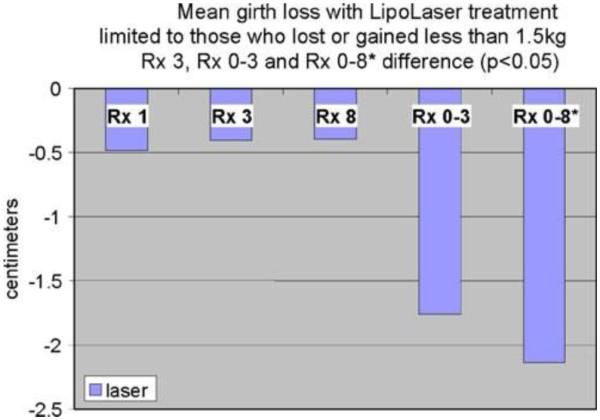
The difference in girth loss between placebo and the LAPEX 2000 LipoLaser at treatments 1, 3, and 8 were all 0.4 to 0.5 cm, and the difference in girth loss at treatment 3 was statistically significant (p < 0.05). The difference in cumulative girth loss compared from treatment 1 to 3 was statistically significant by LOCF or completer's analysis (p < 0.05). The difference in cumulative girth loss at treatment 8 was significant in subjects who remained within 1.5 kg of their baseline weights (p < 0.05)
Fig. 2.
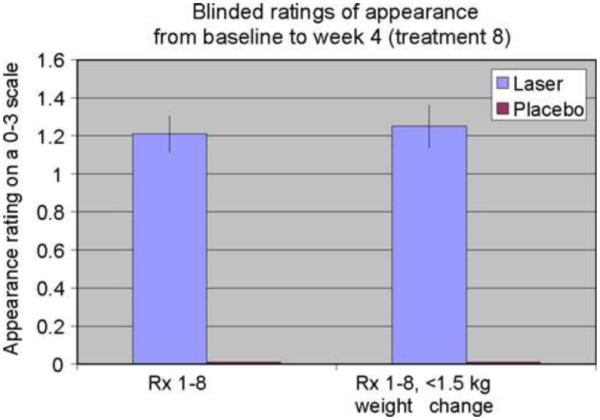
Blinded appearance ratings on a 0–3 scale over 4 weeks and eight treatments favored the LAPEX 2000 LipoLaser treatment compared to the placebo treatment (p < 0.001)
Fig. 3.
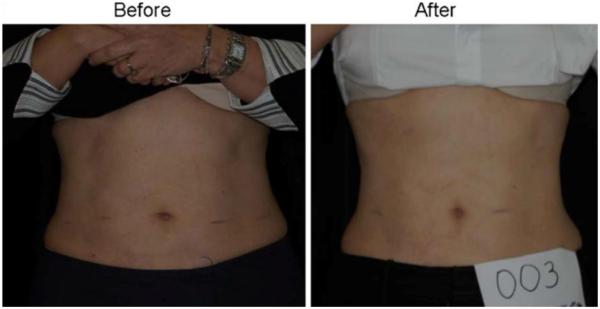
Woman before and after 4 weeks and eight treatments with the LAPEX 2000 LipoLaser
Fig. 4.
Man before and after 4 weeks and eight treatments with the LAPEX 2000 LipoLaser
Fig. 5.
Woman before and after 4 weeks and eight treatments with the placebo LAPEX 2000 LipoLaser who maintained her weight within 1.5 kg of starting weight
Fig. 6.
Woman who lost 2.2 kg before and after 4 weeks and eight treatments with the LAPEX 2000 LipoLaser
Fig. 7.
Woman who lost 2.7 kg before and after 4 weeks and eight treatments with the placebo LAPEX 2000 LipoLaser
In Vitro Study Using Human Fat Cells
Experiment 1 The laser does not activate the complement cascade
The fat cells that came into contact with plasma or plasma with white blood cells were lysed in both the laser treated and the control plate, but cells in the control wells or in wells with heat-inactivated plasma were not lysed. This indicates that serum complement does lyse fat cells, but that the laser does not activate complement. This is consistent with the mechanism shown by Niera [2] in which the laser created pores through which the fat leaked from the fat cells into the interstitial space.
Experiment 2 The laser does not kill adipocytes
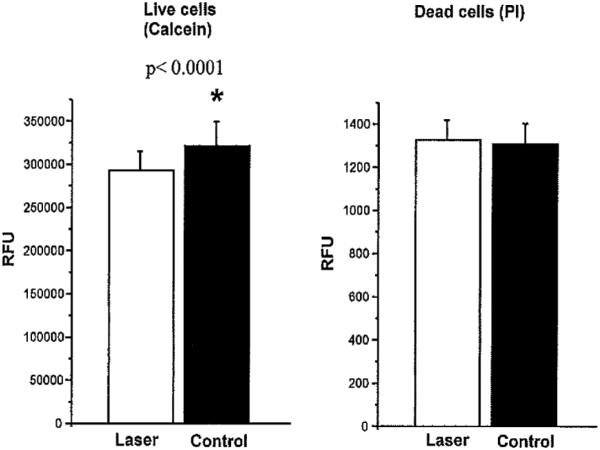
The number of viable cells in the laser-treated or untreated group as determined by the propidium iodide assay were similar, but calcein levels were lower in the laser-treated cells (Fig. 8). Calcein, a non-fluorescent dye, gets transported through the cell membrane, becomes fluorescent due to cleavage with cellular esterases, and gets trapped intracellularly. Normally functioning cells can extrude the entrapped dye. Considering the equal cell viability in the two groups, lower calcein levels in the laser-treated group suggests either intact metabolic functioning of cells and/or reduction of cell-trapped calcein, perhaps by leakage.
Fig. 8.
The number of live and dead cells measured by propidium iodide and the cellular metabolism measured by calcein with and without laser treatment
These findings are also consistent with the studies by Niera [2] in which the laser-treated cells showed micropores in the membrane, which presumably contributed to the leakage of fat from those cells.
Experiment 3 The laser increases triglyceride release, but not lipolysis from adipocytes
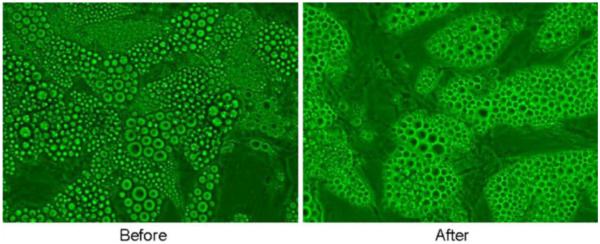
Baseline triglycerides in the control wells were undetectable and were increased, as expected, in the wells with serum. The control wells did not increase triglycerides or glycerol in the media in response to laser irradiation. The laser-irradiated wells containing serum had significantly greater increases in triglycerides than the non-irradiated wells containing serum (69 ± 1.7 vs. 66.7 ± 1.5 mg/dL, p = 0.004). The laser-irradiated wells containing heat-inactivated serum had a significantly greater increase in triglycerides than the non-irradiated wells containing heat-inactivated serum (72.6 ± 1.8 vs. 70.1 ± 1.6 mg/dL, p = 0.008). Baseline glycerol levels were not different in the laser-treated or the non-irradiated groups (0.11 ± 0.01 vs. 011 ± 0.01 mmol/L, p = 0.44). The laser-irradiated wells with serum had significantly lower glycerol levels than the non-irradiated group (0.14 ± 0.01 vs. 0.17 ± 0.03 mmol/L, p = 0.01). The glycerol levels in the laser-irradiated wells containing heat-inactivated serum were not different from the non-irradiated wells with heat-inactivated serum (0.14 ± 0.01 vs. 0.15 ± 0.02 mmol/L, p = 0.3). Before and after laser irradiation in the presence of serum in which triglycerides were released, the cells continued to appear intact without evidence of lysis (Fig. 9). Laser treatment did not release glycerol into the media in the presence of heat-inactivated or normal serum suggesting that any fat loss from adipocytes in response to the laser treatment is not due to a stimulation of lipolysis. On the other hand, the increase of triglyceride into the media in response to laser irradiation in the presence of normal or heat-inactivated plasma suggests leakage of intact triglycerides from cells, a possible mechanism to explain the observations of Niera [2], which showed reduction in lipid content and the appearance of micropores in laser-treated adipocytes. These findings suggest that human serum is necessary for the laser to release triglycerides from the fat cell and that the action is not complement activation-dependent.
Fig. 9.
Human adipocytes in culture before and after LAPEX 2000 LipoLaser irradiation for 10 min in the presence of serum in which triglycerides were released—cells remain intact without evidence of lysis
Discussion
Low-level laser therapy is a light source treatment that generates light of a single wavelength. Low-level laser therapy emits no heat, sound, or vibration. Instead of producing a thermal effect, low-level laser therapy acts via nonthermal or photochemical reactions in the cells, also referred to as photobiological or biostimulatory [12].
A single LAPEX 2000 LipoLaser treatment yielded girth loss, and repeated treatments remained effective giving approximately a 0.4 to 0.5 cm girth loss per treatment. This difference was statistically significant at treatment 3, demonstrating that the effect of the LAPEX 2000 LipoLaser does not appear to diminish with repeated treatments through time. The 1.74 cm girth loss at treatment 3 suggests that the LAPEX 2000 LipoLaser treatments twice a week are cumulative in their effect on girth loss.
It is likely that weight change over the course of treatment would change waist circumference and confound the results. The subjects selected for the study were asked not to lose or gain weight over the course of the study. Since some subjects did gain or lose a significant amount of weight over the 4-week study, the cumulative fat loss was analyzed only on those subjects whose weight was within 1.5 kg of their baseline weight. The 1.5-kg weight fluctuation was to accommodate the effect of menstruation-related fluid shifts in women while representing a more conservative value in one man with a weight fluctuation of this magnitude [13].
Girth loss over the course of the study was greater than 2 cm and statistically significant. The subjects in this study were not obese and an approximate 1 inch (2.54 cm) reduction in waist girth over the course of 8 treatments and 4 weeks was clinically significant and cosmetically relevant. The blinded ratings of the baseline pictures compared to treatment 8 (week 4) pictures taken in a standardized way demonstrated an improvement in appearance that was highly statistically significant. As expected, the improvement was greater when limiting the comparison to only those subjects that remained within 1.5 kg of their baseline weight.
The mechanism by which the laser reduces fat from fat cells observed by Neira et al. [2] was unclear. Fat cell lysis, lipolysis, followed by glycerol and fatty acid release, or leakage of fat from fat cells are some possible explanations. First, we determined if the laser-induced cell lysis by a complement-mediated process. Gay-Crosier et al. found that a pulsed dye laser activated complement in normal skin and confirmed this phenomenon by measuring a rise in membrane attack complex of complement [14]. Our experiments revealed that plasma with complement lysed cells with or without the laser and that heat-inactivated serum without complement did not. Confirming the findings of Niera [2], we found that the cells were not killed by laser treatment, but had increased clearance of the dye, consistent with pores being present in the membranes of the cells. Interestingly, laser treatment of human fat cells without serum present did not result in the release of triglycerides, but in the presence of normal serum or heat-inactivated serum, triglyceride in the media was increased by laser irradiation. Presence of serum along with fat cells simulates in vivo environment to release triglycerides in the presence of laser irradiation and further confirms the ability of the laser to influence fat loss. There was no increase of glycerol in the media, confirming that the laser did not stimulate lipolysis.
Fat that is mobilized by the laser presumably enters the blood stream via the lymphatics in fat tissue much like fat in food enters the body from the intestinal lymphatics into the blood stream. The amount of fat mobilized with a single lipolaser treatment, based on the average circumference changes, is a mean of about 52 grams. This amount of fat can be consumed in a large meal and is less than one third the amount of fat that is administered intravenously when people cannot use their intestinal tract. If weight is stable, the mobilized fat from the lipolaser treatment will either be burned for food in the body or be distributed into fat depots typical of that person's fat distribution. Redistribution of fat using the laser does not change the body's lipolytic thresholds. Thus, without periodic treatments, the body will redistribute fat in its normal pattern. The laser, therefore, should add no more risk of developing atherosclerosis than the routine eating of meals.
Thus, the LAPEX 2000 LipoLaser gives a significant waist girth loss that is sustained over repeated treatments and is cumulative over 4 weeks of eight treatments. This waist girth loss was almost 1 inch (2.54 cm) in magnitude. Therefore, the LAPEX 2000 LipoLaser gave a clinically meaningful, a cosmetically detectable, and a statistically significant improvement in appearance. The fat loss was probably a consequence of the laser creating temporary pores in the fat cells through which triglycerides were leaked, a process that requires serum, but is not complement-mediated.
Current options for cosmetic body contouring include surgery or cream application [9, 15]. Although low-level laser therapy appears to offer a non-surgical option to mobilize subcutaneous fat for body contouring without weight loss, future investigations should involve larger samples and explore the application of this technique to other body parts for cosmetic contouring.
Acknowledgements
This study was supported by a grant from Meridian Medical, Inc. Mary Katherine Caruso-Davis received support for her assistantship through the Bissoon Mesotherapy Foundation. The mechanistic in vitro studies were partially supported by a CNRU Center Grant # 1P30 DK072476 entitled “Nutritional Programming: Environmental and Molecular Interactions”, sponsored by NIDDK with the assistance of Jeffrey Gimble, M.D., Ph.D. and Ying Yu, MS. The authors wish to thank Eleanor Meador for coordinating the study and performing the LAPEX 2000 LipoLaser treatments, Lindsay Southard and Canaan Heard, undergraduates working on the study, and Mary Beth Burnett who assisted in manuscript preparation.
This study was supported by Meridian Medical, Inc., Vancouver, BC, Canada V6K 4L9.
References
- 1.King PR. Low level laser therapy: a review. Lasers Med Sci. 1989;4:141. [Google Scholar]
- 2.Neira R, Arroyave J, Ramirez H, et al. Fat liquefaction: effect oflow-level laser energy on adipose tissue. Plast Reconstr Surg. 2002;110:912–22. doi: 10.1097/00006534-200209010-00030. discussion 923–5. [DOI] [PubMed] [Google Scholar]
- 3.Benedicenti A, Verrando M, Cherlone F, et al. Effect of a 904 nm laser on microcirculation and arteriovenous circulation as evaluated using telethermographic imaging. Parodontol Stomatol (Nuova) 1984;23:167–78. [PubMed] [Google Scholar]
- 4.Dortbudak O, Haas R, Mallath-Pokorny G. Biostimulation of bone marrow cells with a diode soft laser. Clin Oral Implants Res. 2000;11:540–5. doi: 10.1034/j.1600-0501.2000.011006540.x. [DOI] [PubMed] [Google Scholar]
- 5.Garavello-Freitas I, Baranauskas V, Joazeiro PP, et al. Low-power laser irradiation improves histomorphometrical parameters and bone matrix organization during tibia wound healing in rats. J Photochem Photobiol B. 2003;70:81–9. doi: 10.1016/s1011-1344(03)00058-7. [DOI] [PubMed] [Google Scholar]
- 6.Hall G, Anneroth G, Schennings T, et al. Effect of low level energy laser irradiation on wound healing. An experimental study in rats. Swed Dent J. 1994;18:29–34. [PubMed] [Google Scholar]
- 7.Mester E, Mester AF, Mester A. The biomedical effects of laser application. Lasers Surg Med. 1985;5:31–9. doi: 10.1002/lsm.1900050105. [DOI] [PubMed] [Google Scholar]
- 8.Campana V, Moya M, Gavotto A, et al. Effects of diclofenac sodium and He:Ne laser irradiation on plasmatic fibrinogen levels in inflammatory processes. J Clin Laser Med Surg. 1998;16:317–20. doi: 10.1089/clm.1998.16.317. [DOI] [PubMed] [Google Scholar]
- 9.Caruso MK, Pekarovic S, Raum WJ, et al. Topical fat reduction from the waist. Diabetes Obes Metab. 2007;9:300–3. doi: 10.1111/j.1463-1326.2006.00600.x. [DOI] [PubMed] [Google Scholar]
- 10.Bunnell BA, Estes BT, Guilak F, et al. Differentiation of adipose stem cells. Methods Mol Biol. 2008;456:155–71. doi: 10.1007/978-1-59745-245-8_12. [DOI] [PubMed] [Google Scholar]
- 11.Rogers PM, Fusinski KA, Rathod MA, et al. Human adenovirusAd-36 induces adipogenesis via its E4 orf-1 gene. Int J Obes (Lond) 2008;32:397–406. doi: 10.1038/sj.ijo.0803748. [DOI] [PubMed] [Google Scholar]
- 12.Karu T. Photobiological fundamentals of low power laser therapy. IEEE J Quantum Electron. 1987;23:1703–18. [Google Scholar]
- 13.Robinson MF, Watson PE. Day-to-day variations in body-weight of young women. Br J Nutr. 1965;19:225–35. doi: 10.1079/bjn19650022. [DOI] [PubMed] [Google Scholar]
- 14.Gay-Crosier F, Polla LL, Tschopp J, et al. Complement activation by pulsed tunable dye laser in normal skin and hemangioma. J Invest Dermatol. 1990;94:426–31. doi: 10.1111/1523-1747.ep12874510. [DOI] [PubMed] [Google Scholar]
- 15.Dhami LD, Agarwal M. Safe total corporal contouring with large volume liposuction for the obese patient. Aesthetic Plast Surg. 2006;30:574–88. doi: 10.1007/s00266-006-0050-7. [DOI] [PubMed] [Google Scholar]