Abstract
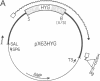
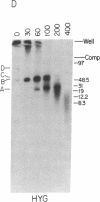
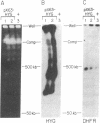
We have used double gene targeting to create homozygous gene replacements in the protozoan parasite Leishmania major, an asexual diploid. This method uses two independent selectable markers in successive rounds of gene targeting to replace both alleles of an endogenous gene. We developed an improved hygromycin B-resistance cassette encoding hygromycin phosphotransferase (HYG) for use as a selectable marker for Leishmania. HYG-containing vectors functioned equivalently to those containing the neomycin phosphotransferase (NEO) cassette previously used for extrachromosomal transformation or gene targeting. Drug resistances conferred by the NEO and HYG markers were independent, allowing simultaneous selection for both markers. A HYG targeting vector was utilized to replace the single dihydrofolate reductase-thymidylate synthase (DHFR-TS) gene remaining in a line heterozygous for a NEO replacement at the dhfr-ts locus (+/neo), with a targeting efficiency comparable to that seen with wild-type recipients. The resultant dhfr-ts- line (hyg/neo) was auxotrophic for thymidine. The double targeted replacement method will enable functional genetic testing in a variety of asexual diploids, including cultured mammalian cells and fungi such as Candida albicans. Additionally, it may be possible to use Leishmania bearing conditionally auxotrophic gene replacements as safe, improved live vaccines for leishmaniasis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beverley S. M. Characterization of the 'unusual' mobility of large circular DNAs in pulsed field-gradient electrophoresis. Nucleic Acids Res. 1988 Feb 11;16(3):925–939. doi: 10.1093/nar/16.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley S. M. Estimation of circular DNA size using gamma-irradiation and pulsed-field gel electrophoresis. Anal Biochem. 1989 Feb 15;177(1):110–114. doi: 10.1016/0003-2697(89)90023-7. [DOI] [PubMed] [Google Scholar]
- Blochlinger K., Diggelmann H. Hygromycin B phosphotransferase as a selectable marker for DNA transfer experiments with higher eucaryotic cells. Mol Cell Biol. 1984 Dec;4(12):2929–2931. doi: 10.1128/mcb.4.12.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R. Altering the genome by homologous recombination. Science. 1989 Jun 16;244(4910):1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Charron J., Malynn B. A., Robertson E. J., Goff S. P., Alt F. W. High-frequency disruption of the N-myc gene in embryonic stem and pre-B cell lines by homologous recombination. Mol Cell Biol. 1990 Apr;10(4):1799–1804. doi: 10.1128/mcb.10.4.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Clayton C. E. The molecular biology of the Kinetoplastidae. Genet Eng. 1988;(7):1–56. [PubMed] [Google Scholar]
- Coburn C. M., Otteman K. M., McNeely T., Turco S. J., Beverley S. M. Stable DNA transfection of a wide range of trypanosomatids. Mol Biochem Parasitol. 1991 May;46(1):169–179. doi: 10.1016/0166-6851(91)90210-w. [DOI] [PubMed] [Google Scholar]
- Cruz A., Beverley S. M. Gene replacement in parasitic protozoa. Nature. 1990 Nov 8;348(6297):171–173. doi: 10.1038/348171a0. [DOI] [PubMed] [Google Scholar]
- Doetschman T., Gregg R. G., Maeda N., Hooper M. L., Melton D. W., Thompson S., Smithies O. Targetted correction of a mutant HPRT gene in mouse embryonic stem cells. Nature. 1987 Dec 10;330(6148):576–578. doi: 10.1038/330576a0. [DOI] [PubMed] [Google Scholar]
- Egelhoff T. T., Brown S. S., Manstein D. J., Spudich J. A. Hygromycin resistance as a selectable marker in Dictyostelium discoideum. Mol Cell Biol. 1989 May;9(5):1965–1968. doi: 10.1128/mcb.9.5.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger T. E., Beverley S. M. Multiple drug resistance and conservative amplification of the H region in Leishmania major. J Biol Chem. 1989 Sep 5;264(25):15094–15103. [PubMed] [Google Scholar]
- Ellenberger T. E., Beverley S. M. Reductions in methotrexate and folate influx in methotrexate-resistant lines of Leishmania major are independent of R or H region amplification. J Biol Chem. 1987 Oct 5;262(28):13501–13506. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Garvey E. P., Coderre J. A., Santi D. V. Selection and properties of Leishmania tropica resistant to 10-propargyl-5,8-dideazafolate, an inhibitor of thymidylate synthetase. Mol Biochem Parasitol. 1985 Oct;17(1):79–91. doi: 10.1016/0166-6851(85)90129-x. [DOI] [PubMed] [Google Scholar]
- Green M. S., Kark J. D., Witztum E., Greenblatt C. L., Spira D. T. Frozen stored Leishmania tropica vaccine: the effects of dose, route of administration and storage on the evolution of the clinical lesion. Two field trials in the Israel Defense Forces. Trans R Soc Trop Med Hyg. 1983;77(2):152–159. doi: 10.1016/0035-9203(83)90054-8. [DOI] [PubMed] [Google Scholar]
- Gritz L., Davies J. Plasmid-encoded hygromycin B resistance: the sequence of hygromycin B phosphotransferase gene and its expression in Escherichia coli and Saccharomyces cerevisiae. Gene. 1983 Nov;25(2-3):179–188. doi: 10.1016/0378-1119(83)90223-8. [DOI] [PubMed] [Google Scholar]
- Iovannisci D. M., Beverley S. M. Structural alterations of chromosome 2 in Leishmania major as evidence for diploidy, including spontaneous amplification of the mini-exon array. Mol Biochem Parasitol. 1989 May 1;34(2):177–188. doi: 10.1016/0166-6851(89)90009-1. [DOI] [PubMed] [Google Scholar]
- Iovannisci D. M., Goebel D., Allen K., Kaur K., Ullman B. Genetic analysis of adenine metabolism in Leishmania donovani promastigotes. Evidence for diploidy at the adenine phosphoribosyltransferase locus. J Biol Chem. 1984 Dec 10;259(23):14617–14623. [PubMed] [Google Scholar]
- Johnson R. S., Sheng M., Greenberg M. E., Kolodner R. D., Papaioannou V. E., Spiegelman B. M. Targeting of nonexpressed genes in embryonic stem cells via homologous recombination. Science. 1989 Sep 15;245(4923):1234–1236. doi: 10.1126/science.2506639. [DOI] [PubMed] [Google Scholar]
- Kapler G. M., Beverley S. M. Transcriptional mapping of the amplified region encoding the dihydrofolate reductase-thymidylate synthase of Leishmania major reveals a high density of transcripts, including overlapping and antisense RNAs. Mol Cell Biol. 1989 Sep;9(9):3959–3972. doi: 10.1128/mcb.9.9.3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapler G. M., Coburn C. M., Beverley S. M. Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol Cell Biol. 1990 Mar;10(3):1084–1094. doi: 10.1128/mcb.10.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur K., Coons T., Emmett K., Ullman B. Methotrexate-resistant Leishmania donovani genetically deficient in the folate-methotrexate transporter. J Biol Chem. 1988 May 25;263(15):7020–7028. [PubMed] [Google Scholar]
- Kelley S. L., Basu A., Teicher B. A., Hacker M. P., Hamer D. H., Lazo J. S. Overexpression of metallothionein confers resistance to anticancer drugs. Science. 1988 Sep 30;241(4874):1813–1815. doi: 10.1126/science.3175622. [DOI] [PubMed] [Google Scholar]
- Laban A., Tobin J. F., Curotto de Lafaille M. A., Wirth D. F. Stable expression of the bacterial neor gene in Leishmania enriettii. Nature. 1990 Feb 8;343(6258):572–574. doi: 10.1038/343572a0. [DOI] [PubMed] [Google Scholar]
- LeBowitz J. H., Coburn C. M., McMahon-Pratt D., Beverley S. M. Development of a stable Leishmania expression vector and application to the study of parasite surface antigen genes. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9736–9740. doi: 10.1073/pnas.87.24.9736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon W., Fouts D. L., Manning J. Sequence arrangement of the 16S and 26S rRNA genes in the pathogenic haemoflagellate Leishmania donovani. Nucleic Acids Res. 1978 Feb;5(2):491–504. doi: 10.1093/nar/5.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Marsh J. L., Erfle M., Wykes E. J. The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene. 1984 Dec;32(3):481–485. doi: 10.1016/0378-1119(84)90022-2. [DOI] [PubMed] [Google Scholar]
- Panton L. J., Tesh R. B., Nadeau K. C., Beverley S. M. A test for genetic exchange in mixed infections of Leishmania major in the sand fly Phlebotomus papatasi. J Protozool. 1991 May-Jun;38(3):224–228. doi: 10.1111/j.1550-7408.1991.tb04433.x. [DOI] [PubMed] [Google Scholar]
- Peixoto M. P., Beverley S. M. In vitro activity of sulfonamides and sulfones against Leishmania major promastigotes. Antimicrob Agents Chemother. 1987 Oct;31(10):1575–1578. doi: 10.1128/aac.31.10.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Shulman M. J., Nissen L., Collins C. Homologous recombination in hybridoma cells: dependence on time and fragment length. Mol Cell Biol. 1990 Sep;10(9):4466–4472. doi: 10.1128/mcb.10.9.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait A. Sexual processes in the kinetoplastida. Parasitology. 1983 Apr;86(Pt 4):29–57. doi: 10.1017/s0031182000050836. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Tibayrenc M., Kjellberg F., Ayala F. J. A clonal theory of parasitic protozoa: the population structures of Entamoeba, Giardia, Leishmania, Naegleria, Plasmodium, Trichomonas, and Trypanosoma and their medical and taxonomical consequences. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2414–2418. doi: 10.1073/pnas.87.7.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan W. L. The genetics of medically important fungi. Crit Rev Microbiol. 1987;14(2):99–170. doi: 10.3109/10408418709104437. [DOI] [PubMed] [Google Scholar]
- te Riele H., Maandag E. R., Clarke A., Hooper M., Berns A. Consecutive inactivation of both alleles of the pim-1 proto-oncogene by homologous recombination in embryonic stem cells. Nature. 1990 Dec 13;348(6302):649–651. doi: 10.1038/348649a0. [DOI] [PubMed] [Google Scholar]