Abstract
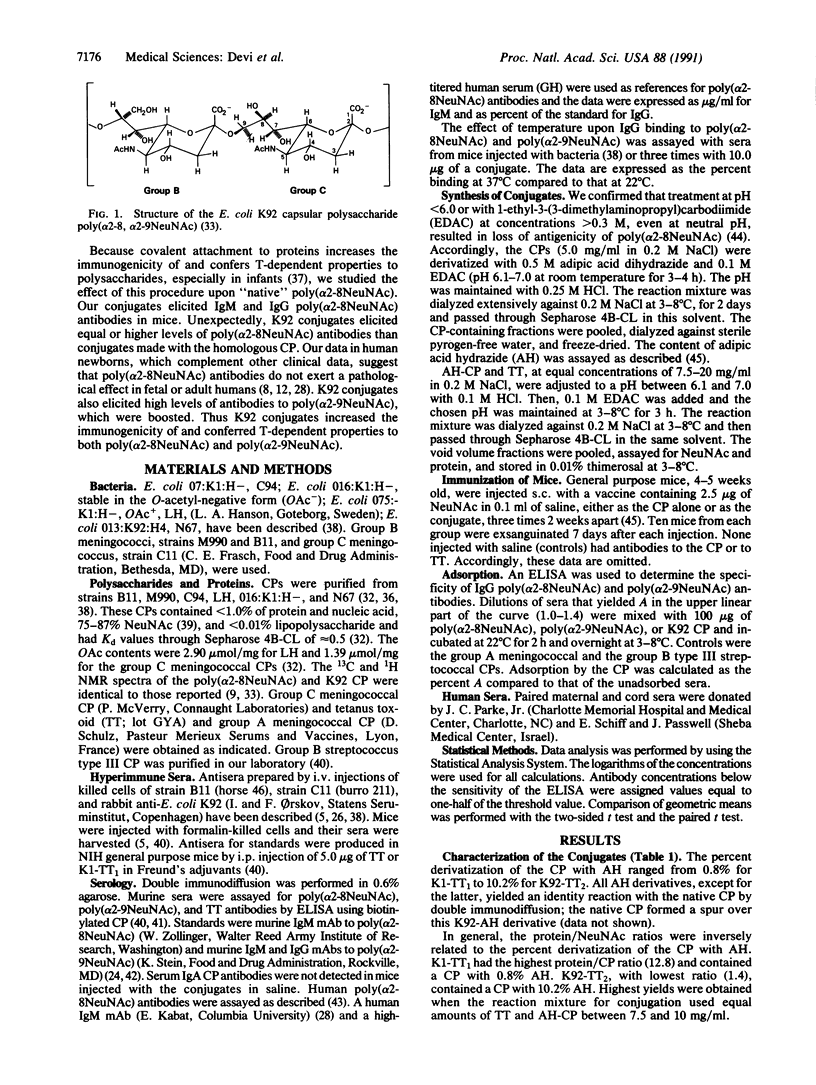
Meningitis and other systemic infections caused by group B Neisseria meningitidis and Escherichia coli K1 remain important problems. The capsular polysaccharides (CPs) of these pathogens (poly[(2----8)-alpha-N-acetylneuraminic acid] or poly(alpha 2-8NeuNAc] are identical and are virulence factors and protective antigens for both. CP vaccines for these pathogens are not available because poly(alpha 2-8NeuNAc) alone, as a complex or a conjugate, is poorly immunogenic. Because oligomers of poly(alpha 2-8NeuNAc) in fetal brain and other tissues bind antibodies in vitro, it has been suggested that antibodies to this CP might be pathologic. We synthesized conjugates of this CP with tetanus toxoid under conditions that avoid lactone formation. Using this scheme, we also synthesized conjugates of group C meningococcal CP (poly[(2----9)-alpha-N-acetylneuraminic acid] or poly(alpha 2-9NeuNAc] and of E. coli K92 CP [poly(alpha 2-8, alpha 2-9NeuNAc)]. When injected s.c. in saline into mice, conjugates of poly(alpha 2-8NeuNAc) or poly(alpha 2-9NeuNAc) elicited homologous antibodies. E. coli K92 conjugates elicited both poly(alpha 2-8NeuNAc) and poly(alpha 2-9NeuNAc) antibodies. Both components of the conjugates expressed T-dependent immunologic properties under conditions and dosages acceptable for clinical evaluation. Poly(alpha 2-8NeuNAc) antibodies elicited by the homologous or the K92 conjugates had lower binding activities at 37 degrees C than at 22 degrees C. "Natural" poly(alpha 2-8NeuNAc) antibodies were present in almost all matched pairs of human maternal and cord sera; most cord levels were higher than in corresponding maternal sera. These findings suggest that increased levels of poly(alpha 2-8NeuNAc) IgG antibodies elicited by our conjugates will confer protective immunity to group B meningococci and E. coli K1 and will not be pathologic.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. Z., Glode M., Schneerson R., Robbins J. B. Identification of immunoglobulin heavy-chain isotypes of specific antibodies of horse 46 group B meningococcal antiserum. J Clin Microbiol. 1982 Feb;15(2):324–329. doi: 10.1128/jcm.15.2.324-329.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P. W., Pichichero M. E., Insel R. A., Betts R., Eby R., Smith D. H. Vaccines consisting of periodate-cleaved oligosaccharides from the capsule of Haemophilus influenzae type b coupled to a protein carrier: structural and temporal requirements for priming in the human infant. J Immunol. 1986 Aug 15;137(4):1181–1186. [PubMed] [Google Scholar]
- Beuvery E. C., van Delft R. W., Miedema F., Kanhai V., Nagel J. Immunological evaluation of meningococcal group C polysaccharide-tetanus toxoid conjugate in mice. Infect Immun. 1983 Aug;41(2):609–617. doi: 10.1128/iai.41.2.609-617.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A. K., Jennings H. J., Kenny C. P., Martin A., Smith I. C. Structural determination of the sialic acid polysaccharide antigens of Neisseria meningitidis serogroups B and C with carbon 13 nuclear magnetic resonance. J Biol Chem. 1975 Mar 10;250(5):1926–1932. [PubMed] [Google Scholar]
- Brandt B. L., Wyle F. A., Artenstein M. S. A radioactive antigen-binding assay for Neisseria meningitidis polysaccharide antibody. J Immunol. 1972 Apr;108(4):913–920. [PubMed] [Google Scholar]
- Brandtzaeg P., Kierulf P., Gaustad P., Skulberg A., Bruun J. N., Halvorsen S., Sørensen E. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J Infect Dis. 1989 Feb;159(2):195–204. doi: 10.1093/infdis/159.2.195. [DOI] [PubMed] [Google Scholar]
- Bøvre K., Bryn K., Closs O., Hagen N., Frøholm L. O. Surface polysaccharide of Moraxella non-liquefaciens identical to Neisseria meningitidis group B capsular polysaccharide. A chemical and immunological investigation. NIPH Ann. 1983 Jun;6(1):65–73. [PubMed] [Google Scholar]
- Claesson B. A., Trollfors B., Lagergard T., Taranger J., Bryla D., Otterman G., Cramton T., Yang Y., Reimer C. B., Robbins J. B. Clinical and immunologic responses to the capsular polysaccharide of Haemophilus influenzae type b alone or conjugated to tetanus toxoid in 18- to 23-month-old children. J Pediatr. 1988 May;112(5):695–702. doi: 10.1016/s0022-3476(88)80684-x. [DOI] [PubMed] [Google Scholar]
- Egan W., Liu T. Y., Dorow D., Cohen J. S., Robbins J. D., Gotschlich E. C., Robbins J. B. Structural studies on the sialic acid polysaccharide antigen of Escherichia coli strain Bos-12. Biochemistry. 1977 Aug 9;16(16):3687–3692. doi: 10.1021/bi00635a028. [DOI] [PubMed] [Google Scholar]
- Finne J., Leinonen M., Mäkelä P. H. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet. 1983 Aug 13;2(8346):355–357. doi: 10.1016/s0140-6736(83)90340-9. [DOI] [PubMed] [Google Scholar]
- Finne J., Mäkelä P. H. Cleavage of the polysialosyl units of brain glycoproteins by a bacteriophage endosialidase. Involvement of a long oligosaccharide segment in molecular interactions of polysialic acid. J Biol Chem. 1985 Jan 25;260(2):1265–1270. [PubMed] [Google Scholar]
- Frasch C. E., Zahradnik J. M., Wang L. Y., Mocca L. F., Tsai C. M. Antibody response of adults to an aluminum hydroxide-adsorbed Neisseria meningitidis serotype 2b protein-group B polysaccharide vaccine. J Infect Dis. 1988 Oct;158(4):710–718. doi: 10.1093/infdis/158.4.710. [DOI] [PubMed] [Google Scholar]
- Frosch M., Görgen I., Boulnois G. J., Timmis K. N., Bitter-Suermann D. NZB mouse system for production of monoclonal antibodies to weak bacterial antigens: isolation of an IgG antibody to the polysaccharide capsules of Escherichia coli K1 and group B meningococci. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1194–1198. doi: 10.1073/pnas.82.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glode M. P., Lewin E. B., Sutton A., Le C. T., Gotschlich E. C., Robbins J. B. Comparative immunogenicity of vaccines prepared from capsular polysaccharides of group C Neisseria meningitidis O-acetyl-positive and O-acetyl-negative variants and Escherichia coli K92 in adult volunteers. J Infect Dis. 1979 Jan;139(1):52–59. doi: 10.1093/infdis/139.1.52. [DOI] [PubMed] [Google Scholar]
- Glode M. P., Robbins J. B., Liu T. Y., Gotschlich E. C., Orskov I., Orskov F. Cross-antigenicity and immunogenicity between capsular polysaccharides of group C Neisseria meningitidis and of Escherichia coli K92. J Infect Dis. 1977 Jan;135(1):94–104. doi: 10.1093/infdis/135.1.94. [DOI] [PubMed] [Google Scholar]
- Glode M. P., Sutton A., Moxon E. R., Robbins J. B. Pathogenesis of neonatal Escherichia coli meningitis: induction of bacteremia and meningitis in infant rats fed E. coli K1. Infect Immun. 1977 Apr;16(1):75–80. doi: 10.1128/iai.16.1.75-80.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969 Jun 1;129(6):1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings H. J., Gamian A., Michon F., Ashton F. E. Unique intermolecular bactericidal epitope involving the homosialopolysaccharide capsule on the cell surface of group B Neisseria meningitidis and Escherichia coli K1. J Immunol. 1989 May 15;142(10):3585–3591. [PubMed] [Google Scholar]
- Jennings H. J., Lugowski C., Ashton F. E. Conjugation of meningococcal lipopolysaccharide R-type oligosaccharides to tetanus toxoid as route to a potential vaccine against group B Neisseria meningitidis. Infect Immun. 1984 Jan;43(1):407–412. doi: 10.1128/iai.43.1.407-412.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings H. J., Lugowski C. Immunochemistry of groups A, B, and C meningococcal polysaccharide-tetanus toxoid conjugates. J Immunol. 1981 Sep;127(3):1011–1018. [PubMed] [Google Scholar]
- Jennings H. J., Roy R., Gamian A. Induction of meningococcal group B polysaccharide-specific IgG antibodies in mice by using an N-propionylated B polysaccharide-tetanus toxoid conjugate vaccine. J Immunol. 1986 Sep 1;137(5):1708–1713. [PubMed] [Google Scholar]
- Jennings H. J., Roy R., Michon F. Determinant specificities of the groups B and C polysaccharides of Neisseria meningitidis. J Immunol. 1985 Apr;134(4):2651–2657. [PubMed] [Google Scholar]
- Kabat E. A., Nickerson K. G., Liao J., Grossbard L., Osserman E. F., Glickman E., Chess L., Robbins J. B., Schneerson R., Yang Y. H. A human monoclonal macroglobulin with specificity for alpha(2----8)-linked poly-N-acetyl neuraminic acid, the capsular polysaccharide of group B meningococci and Escherichia coli K1, which crossreacts with polynucleotides and with denatured DNA. J Exp Med. 1986 Aug 1;164(2):642–654. doi: 10.1084/jem.164.2.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper D. L., Winkelhake J. L., Zollinger W. D., Brandt B. L., Artenstein M. S. Immunochemical similarity between polysaccharide antigens of Escherichia coli 07: K1(L):NM and group B Neisseria meningitidis. J Immunol. 1973 Jan;110(1):262–268. [PubMed] [Google Scholar]
- Kim K. S., Cross A. S., Zollinger W., Sadoff J. Prevention and therapy of experimental Escherichia coli infection with monoclonal antibody. Infect Immun. 1985 Dec;50(3):734–737. doi: 10.1128/iai.50.3.734-737.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagergard T., Shiloach J., Robbins J. B., Schneerson R. Synthesis and immunological properties of conjugates composed of group B streptococcus type III capsular polysaccharide covalently bound to tetanus toxoid. Infect Immun. 1990 Mar;58(3):687–694. doi: 10.1128/iai.58.3.687-694.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen M., Frasch C. E. Class-specific antibody response to group B Neisseria meningitidis capsular polysaccharide: use of polylysine precoating in an enzyme-linked immunosorbent assay. Infect Immun. 1982 Dec;38(3):1203–1207. doi: 10.1128/iai.38.3.1203-1207.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifely M. R., Gilbert A. S., Moreno C. Sialic acid polysaccharide antigens of Neisseria meningitidis and Escherichia coli: esterification between adjacent residues. Carbohydr Res. 1981 Aug 1;94(2):193–203. doi: 10.1016/s0008-6215(00)80717-x. [DOI] [PubMed] [Google Scholar]
- Lifely M. R., Roberts S. C., Shepherd W. M., Esdaile J., Wang Z., Cleverly A., Aulaqi A. A., Moreno C. Immunogenicity in adult males of a Neisseria meningitidis group B vaccine composed of polysaccharide complexed with outer membrane proteins. Vaccine. 1991 Jan;9(1):60–66. doi: 10.1016/0264-410x(91)90318-z. [DOI] [PubMed] [Google Scholar]
- Mandrell R. E., Zollinger W. D. Measurement of antibodies to meningococcal group B polysaccharide: low avidity binding and equilibrium binding constants. J Immunol. 1982 Nov;129(5):2172–2178. [PubMed] [Google Scholar]
- McCracken G. H., Jr, Sarff L. D., Glode M. P., Mize S. G., Schiffer M. S., Robbins J. B., Gotschlich E. C., Orskov I., Orskov F. Relation between Escherichia coli K1 capsular polysaccharide antigen and clinical outcome in neonatal meningitis. Lancet. 1974 Aug 3;2(7875):246–250. doi: 10.1016/s0140-6736(74)91413-5. [DOI] [PubMed] [Google Scholar]
- Mendelman P. M., Caugant D. A., Kalaitzoglou G., Wedege E., Chaffin D. O., Campos J., Saez-Nieto J. A., Viñas M., Selander R. K. Genetic diversity of penicillin G-resistant Neisseria meningitidis from Spain. Infect Immun. 1989 Apr;57(4):1025–1029. doi: 10.1128/iai.57.4.1025-1029.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michon F., Brisson J. R., Jennings H. J. Conformational differences between linear alpha (2----8)-linked homosialooligosaccharides and the epitope of the group B meningococcal polysaccharide. Biochemistry. 1987 Dec 15;26(25):8399–8405. doi: 10.1021/bi00399a055. [DOI] [PubMed] [Google Scholar]
- Nedelec J., Boucraut J., Garnier J. M., Bernard D., Rougon G. Evidence for autoimmune antibodies directed against embryonic neural cell adhesion molecules (N-CAM) in patients with group B meningitis. J Neuroimmunol. 1990 Sep-Oct;29(1-3):49–56. doi: 10.1016/0165-5728(90)90146-e. [DOI] [PubMed] [Google Scholar]
- Orskov F., Orskov I., Sutton A., Schneerson R., Lin W., Egan W., Hoff G. E., Robbins J. B. Form variation in Escherichia coli K1: determined by O-acetylation of the capsular polysaccharide. J Exp Med. 1979 Mar 1;149(3):669–685. doi: 10.1084/jem.149.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F. Escherichia coli in extra-intestinal infections. J Hyg (Lond) 1985 Dec;95(3):551–575. doi: 10.1017/s0022172400060678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola H. Meningococcal disease: still with us. Rev Infect Dis. 1983 Jan-Feb;5(1):71–91. doi: 10.1093/clinids/5.1.71. [DOI] [PubMed] [Google Scholar]
- Poolman J. T., Lind I., Jónsdóttir K., Frøholm L. O., Jones D. M., Zanen H. C. Meningococcal serotypes and serogroup B disease in north-west Europe. Lancet. 1986 Sep 6;2(8506):555–558. doi: 10.1016/s0140-6736(86)90123-6. [DOI] [PubMed] [Google Scholar]
- Raff H. V., Devereux D., Shuford W., Abbott-Brown D., Maloney G. Human monoclonal antibody with protective activity for Escherichia coli K1 and Neisseria meningitidis group B infections. J Infect Dis. 1988 Jan;157(1):118–126. doi: 10.1093/infdis/157.1.118. [DOI] [PubMed] [Google Scholar]
- Robbins J. B., McCracken G. H., Jr, Gotschlich E. C., Orskov F., Orskov I., Hanson L. A. Escherichia coli K1 capsular polysaccharide associated with neonatal meningitis. N Engl J Med. 1974 May 30;290(22):1216–1220. doi: 10.1056/NEJM197405302902202. [DOI] [PubMed] [Google Scholar]
- Robbins J. B., Myerowitz L., Whisnant J. K., Argaman M., Schneerson R., Handzel Z. T., Gotschlich E. C. Enteric bacteria cross-reactive with Neisseria meningitidis groups A and C and Diplococcus pneumoniae types I and 3. Infect Immun. 1972 Nov;6(5):651–656. doi: 10.1128/iai.6.5.651-656.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. B., Schneerson R. Polysaccharide-protein conjugates: a new generation of vaccines. J Infect Dis. 1990 May;161(5):821–832. doi: 10.1093/infdis/161.5.821. [DOI] [PubMed] [Google Scholar]
- Rubinstein L. J., Stein K. E. Murine immune response to the Neisseria meningitidis group C capsular polysaccharide. II. Specificity. J Immunol. 1988 Dec 15;141(12):4357–4362. [PubMed] [Google Scholar]
- Sarff L. D., McCracken G. H., Schiffer M. S., Glode M. P., Robbins J. B., Orskov I., Orskov F. Epidemiology of Escherichia coli K1 in healthy and diseased newborns. Lancet. 1975 May 17;1(7916):1099–1104. doi: 10.1016/s0140-6736(75)92496-4. [DOI] [PubMed] [Google Scholar]
- Saukkonen K., Haltia M., Frosch M., Bitter-Süerman D., Leinonen M. Antibodies to the capsular polysaccharide of Neisseria meningitidis group B or E. coli K1 bind to the brains of infant rats in vitro but not in vivo. Microb Pathog. 1986 Feb;1(1):101–105. doi: 10.1016/0882-4010(86)90036-7. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Barrera O., Sutton A., Robbins J. B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980 Aug 1;152(2):361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skevakis L., Frasch C. E., Zahradnik J. M., Dolin R. Class-specific human bactericidal antibodies to capsular and noncapsular surface antigens of Neisseria meningitidis. J Infect Dis. 1984 Mar;149(3):387–396. doi: 10.1093/infdis/149.3.387. [DOI] [PubMed] [Google Scholar]
- Sutton A., Vann W. F., Karpas A. B., Stein K. E., Schneerson R. An avidin-biotin based ELISA for quantitation of antibody to bacterial polysaccharides. J Immunol Methods. 1985 Oct 10;82(2):215–224. doi: 10.1016/0022-1759(85)90353-9. [DOI] [PubMed] [Google Scholar]
- Wyle F. A., Artenstein M. S., Brandt B. L., Tramont E. C., Kasper D. L., Altieri P. L., Berman S. L., Lowenthal J. P. Immunologic response of man to group B meningococcal polysaccharide vaccines. J Infect Dis. 1972 Nov;126(5):514–521. doi: 10.1093/infdis/126.5.514. [DOI] [PubMed] [Google Scholar]
- Yao K., Ubuka T. Determination of sialic acids by acidic ninhydrin reaction. Acta Med Okayama. 1987 Dec;41(6):237–241. doi: 10.18926/AMO/31741. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E., Griffiss J. M., Altieri P., Berman S. Complex of meningococcal group B polysaccharide and type 2 outer membrane protein immunogenic in man. J Clin Invest. 1979 May;63(5):836–848. doi: 10.1172/JCI109383. [DOI] [PMC free article] [PubMed] [Google Scholar]



